Evidence for the Prognostic Value of CDH17 Expression in Colorectal Carcinoma
Abstract
1. Introduction
2. Results
2.1. CDH17 Immunohistochemical Expression and Clinicopathological Characteristics
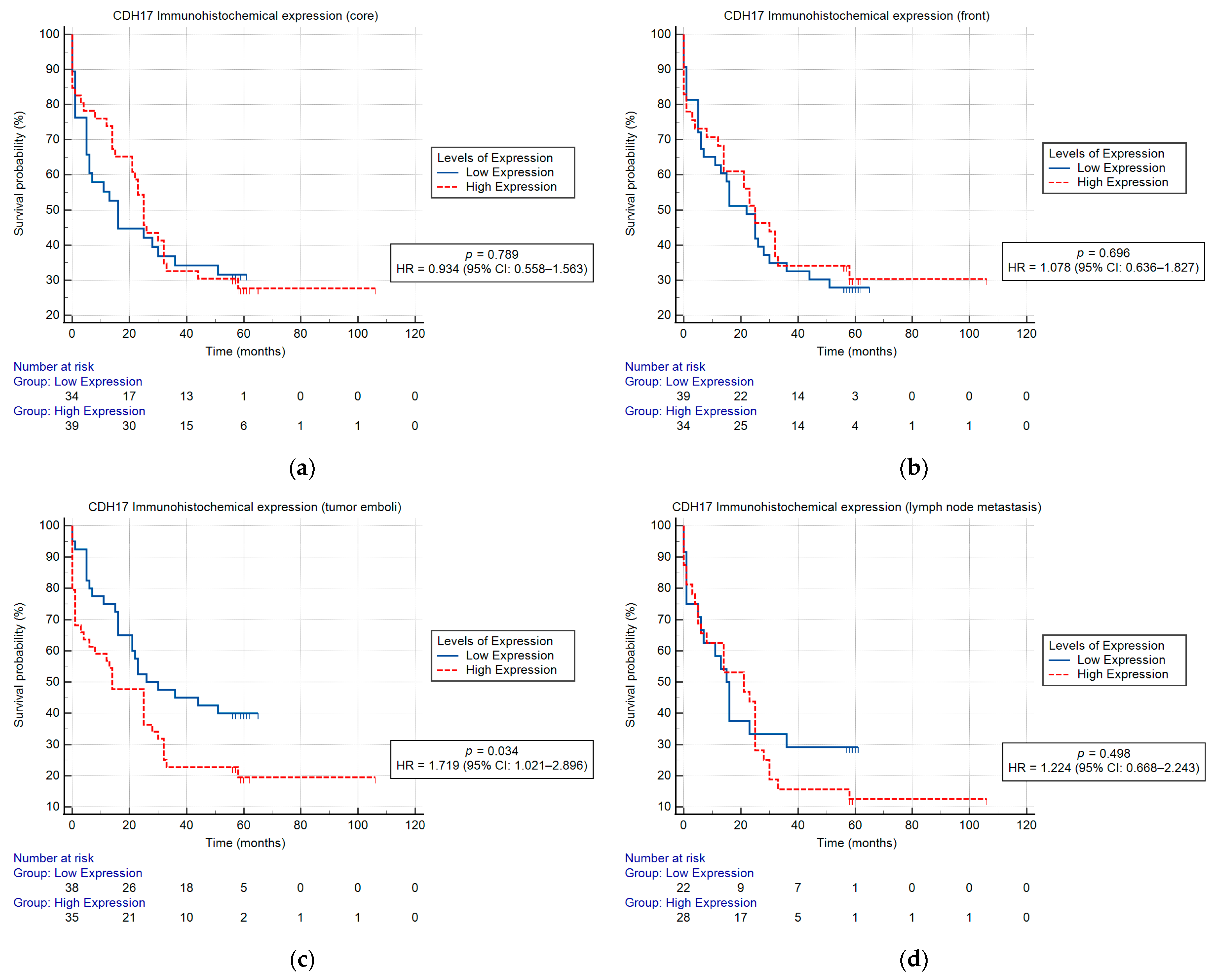
2.2. CDH17 Immunohistochemical Expression and Survival
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Pathological Reassessment
4.3. Immunohistochemistry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 1 June 2025).
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef]
- Brown, K.G.M.; Koh, C.E. Surgical management of recurrent colon cancer. J. Gastrointest. Oncol. 2020, 11, 513–525. [Google Scholar] [CrossRef]
- Nomura, M.; Takahashi, H.; Fujii, M.; Miyoshi, N.; Haraguchi, N.; Hata, T.; Matsuda, C.; Yamamoto, H.; Mizushima, T.; Mori, M.; et al. Clinical significance of invasion distance relative to prognosis in pathological T3 colorectal cancer. Oncol. Lett. 2019, 18, 5614–5620. [Google Scholar] [CrossRef]
- Mathonnet, M.; Perraud, A.; Christou, N.; Akil, H.; Melin, C.; Battu, S.; Jauberteau, M.O.; Denizot, Y. Hallmarks in colorectal cancer: Angiogenesis and cancer stem-like cells. World J. Gastroenterol. 2014, 20, 4189–4196. [Google Scholar] [CrossRef]
- Zhou, M.; Fu, L.; Zhang, J. Who will benefit more from maintenance therapy of metastatic colorectal cancer? Oncotarget 2017, 9, 12479–12486. [Google Scholar] [CrossRef] [PubMed]
- Gloushankova, N.A.; Rubtsova, S.N.; Zhitnyak, I.Y. Cadherin-mediated cell-cell interactions in normal and cancer cells. Tissue Barriers 2017, 5, e1356900. [Google Scholar] [CrossRef] [PubMed]
- Takamura, M.; Yamagiwa, S.; Matsuda, Y.; Ichida, T.; Aoyagi, Y. Involvement of liver-intestine cadherin in cancer progression. Med. Mol. Morphol. 2013, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W. Possible roles of LI-Cadherin in the formation and maintenance of the intestinal epithelial barrier. Tissue Barriers 2013, 1, e23815. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, K.H.; Oh, T.Y.; Hong, S.P.; Jeon, T.J.; Kim, C.H.; Park, S.W.; Chung, J.B.; Song, S.Y.; Bang, S. Up-regulated claudin 7 expression in intestinal-type gastric carcinoma. Oncol. Rep. 2007, 18, 377–382. [Google Scholar] [CrossRef]
- Su, M.C.; Yuan, R.H.; Lin, C.Y.; Jeng, Y.M. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod. Pathol. 2008, 21, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Fang, X.J.; Wang, J.X.; Xue, J.F.; Zhang, Y. Expression of liver-intestine cadherin and its significance in hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi 2011, 91, 2546–2548. [Google Scholar] [PubMed]
- Panarelli, N.C.; Yantiss, R.K.; Yeh, M.M.; Liu, Y.; Chen, Y.T. Tissue-specific cadherin CDH17 is a useful marker of gastrointestinal adenocarcinomas with higher sensitivity than CDX2. Am. J. Clin. Pathol. 2012, 138, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Oue, N.; Sentani, K.; Anami, K.; Uraoka, N.; Naito, Y.; Oo, H.Z.; Hinoi, T.; Ohdan, H.; Yanagihara, K.; et al. Liver-intestine cadherin induction by epidermal growth factor receptor is associated with intestinal differentiation of gastric cancer. Cancer Sci. 2012, 103, 1744–1750. [Google Scholar] [CrossRef]
- Long, Z.W.; Zhou, M.L.; Fu, J.W.; Chu, X.Q.; Wang, Y.N. Association between cadherin-17 expression and pathological characteristics of gastric cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 3694–3705. [Google Scholar] [CrossRef]
- Altree-Tacha, D.; Tyrrell, J.; Haas, T. CDH17 is a more sensitive marker for gastric adenocarcinoma than CK20 and CDX2. Arch. Pathol. Lab. Med. 2017, 141, 144–150. [Google Scholar] [CrossRef]
- Casal, J.I.; Bartolomé, R.A. Beyond N-Cadherin, Relevance of cadherins 5, 6 and 17 in cancer progression and metastasis. Int. J. Mol. Sci. 2019, 20, 3373. [Google Scholar] [CrossRef]
- Yu, Q.F.; Dong, W.G.; Ren, J.L. Knockdown of LI-cadherin increases metastatic behaviors of LoVo cells. J. Cancer Res. Clin. Oncol. 2010, 136, 1641–1649. [Google Scholar] [CrossRef]
- Yu, Q.; Shen, W.; Zhou, H.; Dong, W.; Gao, D. Knockdown of LI-cadherin alters expression of matrix metalloproteinase- 2 and -9 and galectin-3. Mol. Med. Rep. 2016, 13, 4469–4474. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Yu, L.C.; Yu, I.S.; Jhuang, Y.L.; Huang, W.J.; Yang, C.Y.; Jeng, Y.M. Deletion of cadherin-17 enhances intestinal permeability and susceptibility to intestinal tumour formation. J. Pathol. 2018, 246, 289–299. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Winberg, J.O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Mokrowiecka, A.; Zonnur, S.; Veits, L.; Musial, J.; Kordek, R.; Lochowski, M.; Kozak, J.; Malecka-Panas, E.; Vieth, M.; Hartmann, A. Liver-intestine-cadherin is a sensitive marker of intestinal differentiation during Barrett’s carcinogenesis. Dig. Dis. Sci. 2013, 58, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Takamura, M.; Ichida, T.; Matsuda, Y.; Kobayashi, M.; Yamagiwa, S.; Genda, T.; Shioji, K.; Hashimoto, S.; Nomoto, M.; Hatakeyama, K.; et al. Reduced expression of liver-intestine cadherin is associated with progression and lymph node metastasis of human colorectal carcinoma. Cancer Lett. 2004, 212, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Sun, S.; Shan, W.Y.; Leung, P.; Luk, J.M.; Foo, D.C.C. Tissue cadherin 17 (CDH17): A favorable prognostic determinant of colorectal cancer using digital image analysis. J. Clin. Oncol. 2023, 41, e14651. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Fu, J.; Xu, W.; Li, Z. Diagnostic value and prognostic significance of LI-cadherin and miR-378e in colorectal cancer. Oncol. Lett. 2020, 20, 2456–2464. [Google Scholar] [CrossRef]
- Chen, R.Y.; Cao, J.J.; Chen, J.; Yang, J.P.; Liu, X.B.; Zhao, G.Q.; Zhang, Y.F. Single nucleotide polymorphisms in the CDH17 gene of colorectal carcinoma. World J. Gastroenterol. 2012, 18, 7251–7261. [Google Scholar] [CrossRef]
- Yui, A.; Kuroda, D.; Maruno, T.; Nakakido, M.; Nagatoishi, S.; Uchiyama, S.; Tsumoto, K. Molecular mechanism underlying the increased risk of colorectal cancer metastasis caused by single nucleotide polymorphisms in LI-cadherin gene. Sci. Rep. 2023, 13, 6493. [Google Scholar] [CrossRef]
- Pei, X.M.; Wong, H.T.; Ng, S.S.M.; Leung, W.W.; Wong, Y.N.; Tsang, H.F.; Chan, A.K.C.; Wong, Y.K.E.; Yu, A.C.S.; Yim, A.K.Y.; et al. The diagnostic significance of CDH17-positive circulating tumor cells in patients with colorectal cancer. Expert Rev. Mol. Diagn. 2023, 23, 171–179. [Google Scholar] [CrossRef]
- Ng, L.; Yu, W.S.; Aung, N.M.; Leung, P.; Luk, J.M.; Wong, D.A.; Sun, S.; Foo, D.C. Tissue Cadherin 17 (CDH17): An Important Prognostic Determinant of Colorectal Cancer Using Digital Image Analysis. Cancer Rep. 2024, 7, e70069. [Google Scholar] [CrossRef]
- Lum, Y.L.; Luk, J.M.; Staunton, D.E.; Ng, D.K.P.; Fong, W.P. Cadherin-17 targeted near-infrared photoimmunotherapy for treatment of gastrointestinal cancer. Mol. Pharm. 2020, 17, 3941–3951. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Wang, S.; Weishaeupl, C.; Wernitznig, A.; Chetta, P.; Pinto, C.; Ho, J.; Dutcher, D.; Gorman, P.N.; Kroe-Barrett, R.; et al. Selective tumor cell apoptosis and tumor regression in CDH17-positive colorectal cancer models using BI 905711, a novel liver-sparing TRAILR2 agonist. Mol. Cancer Ther. 2021, 20, 96–108. [Google Scholar] [CrossRef]
- Wong, D.A.; Luk, M.C.; Wong, T.K.; Staunton, D.E.; Harlan, J.M. Efficacy and preclinical safety of ARB202, a potential first-in- class anti-CDH17/CD3 bispecific T-cell engager, for treatment of pancreatic and colorectal cancers. J. Clin. Oncol. 2021, 39, 405. [Google Scholar] [CrossRef]
- Wong, K.K. CDH17 as a cell surface immunotherapy target in colorectal cancer: An in silico analysis. Res. Sq. 2022. [Google Scholar] [CrossRef]
- O’Brien, N.A.; McDermott, M.S.; Zhang, J.; Lu, M.; Gong, K.W.; Hoffstrom, B.; Jia, W.P.; Luo, T.; Madrid, A.M.; Liang, M.; et al. Abstract 1900: TORL-3-600, a novel antibody drug conjugate directed against cadherin 17 (CDH17), has preclinical efficacy in colorectal, gastric, and pancreatic cancer. Cancer Res. 2024, 84, 1900. [Google Scholar] [CrossRef]
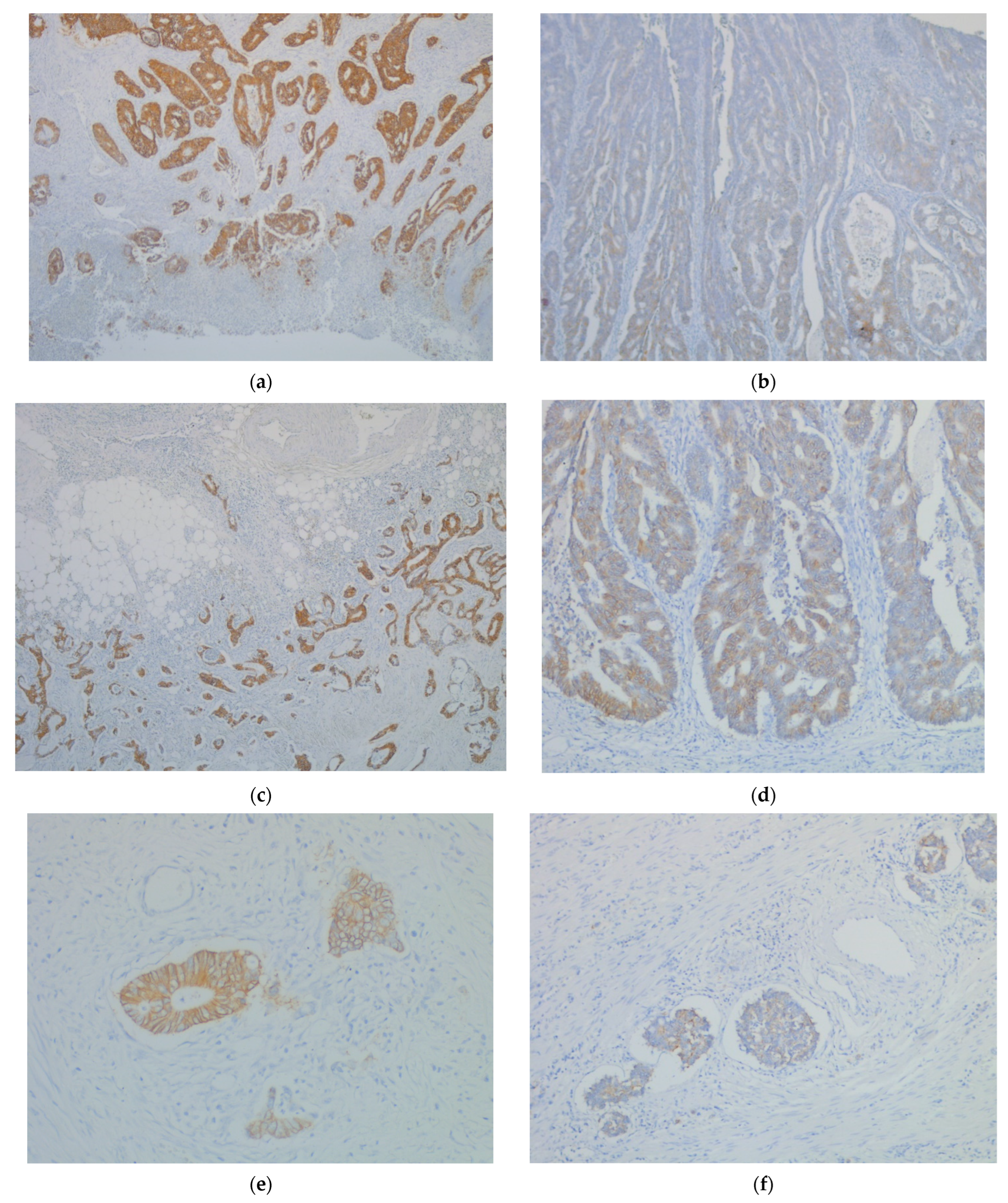

| Clinicopathological Parameters | n 84 | CDH17 (Core) Expression | CDH17 (Front) Expression | CDH17 (Emboli) Expression | ||||
|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | |||
| Gender | male | 43 | 16 (19%) | 27 (32.1%) | 19 (22.6%) | 24 (28.6%) | 20 (23.8%) | 23 (27.4%) |
| female | 41 | 22 (26.2%) | 19 (22.6%) | 24 (28.6%) | 17 (20.2%) | 20 (23.8%) | 21 (25%) | |
| p = 0.130 | p = 0.188 | p = 0.835 | ||||||
| ADK NOS | 64 | 23 (27.4%) | 41 (48.8%) | 29 (34.5%) | 35 (41.7%) | 25 (29.8%) | 39 (46.4%) | |
| ADK with mucinous component | 13 | 11 (13.1%) | 2 (2.4%) | 9 (10.7%) | 4 (4.8%) | 10 (11.9%) | 3 (3.6%) | |
| Histological type | ADK with signet ring cell component | 2 | 2 (2.4%) | 0 (0%) | 2 (2.4%) | 0 (0%) | 1 (1.2%) | 1 (1.2%) |
| medullary ADK | 1 | 1 (1.2%) | 0 (0%) | 1 (1.2%) | 0 (0%) | 1 (1.2%) | 0 (0%) | |
| mucinous ADK | 4 | 1 (1.2%) | 3 (3.6%) | 2 (2.4%) | 2 (2.4%) | 3 (3.6%) | 1 (1.2%) | |
| p = 0.001 | p = 0.227 | p = 0.023 | ||||||
| Grade | high-grade | 12 | 10 (11.9%) | 2 (2.4%) | 9 (10.7%) | 3 (3.6%) | 9 (10.7%) | 3 (3.6%) |
| low-grade | 72 | 28 (33.3%) | 44 (52.4%) | 34 (40.5%) | 38 (45.2%) | 31 (36.9%) | 41 (48.8%) | |
| p = 0.004 | p = 0.075 | p = 0.040 | ||||||
| 1 | 1 | 0 (0%) | 1 (1.2%) | 0 (0%) | 1 (1.2%) | 0 (0%) | 1 (1.2%) | |
| 2 | 2 | 1 (1.2%) | 1 (1.2%) | 2 (2.4%) | 0 (0%) | 2 (2.4%) | 0 (0%) | |
| T stage | ||||||||
| 3 | 43 | 16 (19%) | 27 (32.1%) | 18 (21.4) | 25 (29.8%) | 16 (19%) | 27 (32.1%) | |
| 4 | 38 | 21 (25%) | 17 (20.2%) | 23 (27.4%) | 15 (17.9%) | 22 (26.2%) | 16 (19%) | |
| p = 0.240 | p = 0.083 | p = 0.046 | ||||||
| negative | 28 | 10 (11.9%) | 18 (21.4%) | 14 (16.7%) | 14 (16.7%) | 16 (19%) | 12 (14.3%) | |
| N status | ||||||||
| positive | 56 | 28 (33.3%) | 28 (33.3%) | 29 (34.5%) | 27 (32.1%) | 24 (28.6%) | 32 (38.1%) | |
| p = 0.215 | p = 1.000 | p = 0.217 | ||||||
| LVI2 | 27 | 13 (15.5%) | 14 (16.7%) | 12 (14.3%) | 15 (17.9%) | 17 (20.2%) | 10 (11.9%) | |
| LVI | ||||||||
| LVI4 | 57 | 25 (29.8%) | 32 (38.1%) | 31 (36.9%) | 26 (31%) | 23 (27.4) | 34 (40.5%) | |
| p = 0.712 | p = 0.395 | p = 0.053 | ||||||
| IMVI | absent | 61 | 28 (33.3%) | 33 (39.3%) | 28 (33.3%) | 33 (39.3%) | 31 (36.9%) | 30 (35.7%) |
| present | 23 | 10 (11.9%) | 13 (15.5%) | 15 (17.9%) | 8 (9.5%) | 9 (10.7%) | 14 (16.7%) | |
| p = 0.842 | p = 0.114 | p = 0.339 | ||||||
| EMVI | absent | 37 | 16 (19%) | 21 (25%) | 20 (23.8%) | 17 (20.2%) | 22 (26.2%) | 15 (17.9%) |
| present | 47 | 22 (26.2%) | 25 (29.8%) | 23 (27.4%) | 24 (28.6%) | 18 (21.4%) | 29 (34.5%) | |
| p = 0.744 | p = 0.641 | p = 0.054 | ||||||
| IPNI | absent | 52 | 22 (26.2%) | 30 (35.7%) | 23 (27.4%) | 29 (34.5%) | 25 (29.8%) | 27 (32.1%) |
| present | 32 | 16 (19%) | 16 (19%) | 20 (23.8%) | 12 (14.3%) | 15 (17.9%) | 17 (20.2%) | |
| p = 0.492 | p = 0.104 | p = 0.915 | ||||||
| EPNI | absent | 39 | 13 (15.5%) | 26 (31%) | 21 (25%) | 18 (21.4%) | 19 (22.6%) | 20 (23.8%) |
| present | 45 | 25 (29.8%) | 20 (23.8%) | 22 (26.2%) | 23 (27.4%) | 21 (25%) | 24 (28.6%) | |
| p = 0.041 | p = 0.650 | p = 0.851 | ||||||
| Prognostic stage group | I-II | 28 | 10 (11.9%) | 18 (21.4%) | 14 (16.7%) | 14 (16.7%) | 16 (19%) | 12 (14.3%) |
| III-IV | 56 | 28 (33.3%) | 28 (33.3%) | 29 (34.5%) | 27 (32.1%) | 24 (28.6%) | 32 (38.1%) | |
| p = 0.215 | p = 0.877 | p = 0.217 | ||||||
| Tumor growth pattern | infiltrative | 71 | 31 (36.9%) | 40 (47.6%) | 35 (41.7%) | 36 (42.9%) | 35 (41.7%) | 36 (42.9%) |
| pushing borders | 13 | 7 (8.3%) | 6 (7.1%) | 8 (9.5%) | 5 (6%) | 35 (41.7%) | 36 (42.9%) | |
| p = 0.498 | p = 0.417 | p = 0.472 | ||||||
| Bd | Bd1 | 48 | 16 (19%) | 32 (38.1%) | 20 (23.8%) | 28 (33.3%) | 21 (25%) | 27 (32.1%) |
| Bd2 | 19 | 11 (13.1%) | 8 (9.5%) | 12 (14.3%) | 7 (8.3%) | 12 (14.3%) | 7 (8.3%) | |
| Bd3 | 17 | 11 (13.1%) | 6 (7.1%) | 11 (13.1%) | 6 (7.1%) | 7 (8.3%) | 10 (11.9%) | |
| p = 0.037 | p = 0.130 | p = 0.300 | ||||||
| PDCs | Pdc1 | 54 | 21 (25%) | 33 (39.3%) | 24 (28.6%) | 30 (35.7%) | 24 (28.6%) | 30 (35.7%) |
| Pdc2 | 25 | 14 (16.7%) | 11 (13.1%) | 18 (21.4%) | 7 (8.3%) | 13 (15.5%) | 12 (14.3%) | |
| Pdc3 | 5 | 3 (3.6%) | 2 (2.4%) | 1 (1.2%) | 4 (4.8%) | 3 (3.6%) | 2 (2.4%) | |
| p = 0.309 | p = 0.018 | p = 0.710 | ||||||
| Clinicopathological Parameters | n 56 | CDH17 Expression (Lymph Node Metastasis) | ||
|---|---|---|---|---|
| Low | High | |||
| Gender | male | 27 | 9 (16.1%) | 18 (32.1%) |
| female | 29 | 15 (26.8%) | 14 (25%) | |
| p = 0.165 | ||||
| Histological Type | ADK NOS | 40 | 15 (26.8%) | 25 (44.6%) |
| ADK with mucinous component | 10 | 7 (12.5%) | 3 (5.4%) | |
| ADK with signet ring cell component | 1 | 1 (1.8%) | 0 (0%) | |
| medullary ADK | 1 | 1 (1.8%) | 0 (0%) | |
| mucinous ADK | 4 | 0 (0%) | 4 (7.1%) | |
| p = 0.022 | ||||
| Grade | high-grade | 8 | 4 (7.1%) | 4 (7.1%) |
| low-grade | 48 | 20 (35.7%) | 28 (50%) | |
| p = 0.713 | ||||
| T stage | 1 | 0 | 0 (0%) | 0 (0%) |
| 2 | 0 | 0 (0%) | 0 (0%) | |
| 3 | 25 | 8 (14.3%) | 17 (30.4%) | |
| 4 | 31 | 16 (28.6%) | 15 (26.8%) | |
| p = 0.140 | ||||
| LVI | LVI2 | 12 | 6 (10.7%) | 6 (10.7%) |
| LVI4 | 44 | 18 (31.1%) | 26 (46.4%) | |
| p = 0.573 | ||||
| IMVI | absent | 38 | 17 (30.4%) | 21 (37.5%) |
| present | 18 | 7 (12.5%) | 11 (19.6%) | |
| p = 0.680 | ||||
| EMVI | absent | 18 | 9 (16.1%) | 9 (16.1%) |
| present | 38 | 15 (26.8%) | 23 (41.1%) | |
| p = 0.457 | ||||
| IPNI | absent | 32 | 13 (23.2%) | 19 (33.9%) |
| present | 24 | 11 (19.6%) | 13 (23.2%) | |
| p = 0.697 | ||||
| EPNI | absent | 18 | 6 (10.7%) | 12 (21.4%) |
| present | 38 | 18 (32.1%) | 20 (35.7%) | |
| p = 0.322 | ||||
| Prognostic stage group | I-II | 0 | 0 (0%) | 0 (0%) |
| III-IV | 56 | 24 (42.9%) | 32 (57.1%) | |
| a | ||||
| Tumor growth pattern | infiltrative | 48 | 20 (35.7%) | 28 (50%) |
| pushing borders | 8 | 4 (7.1%) | 4 (7.1%) | |
| p = 0.713 | ||||
| Bd | Bd1 | 25 | 9 (16.1%) | 16 (28.6%) |
| Bd2 | 15 | 10 (17.9%) | 5 (8.9%) | |
| Bd3 | 16 | 5 (8.9%) | 11 (19.6%) | |
| p = 0.089 | ||||
| PDCs | Pdc1 | 31 | 12 (21.4%) | 19 (33.9%) |
| Pdc2 | 22 | 11 (19.6%) | 11 (19.6%) | |
| Pdc3 | 3 | 1 (1.8%) | 2 (3.6%) | |
| p = 0.747 | ||||
| Variables | Univariate Analysis | p-Value | Multivariable Analysis | p-Value |
|---|---|---|---|---|
| HR/CI (95%) | HR/CI (95%) | |||
| Gender (male/female) | 0.963 (0.577–1.607) | 0.896 | ||
| Histological type (ADK NOS/Other subtypes) | 1.502 (0.832–2.713) | 0.177 | ||
| Grade (high-grade/low-grade) | 0.757 (0.372–1.543) | 0.444 | ||
| T stage (1/2/3/4) | 1.157 (0.765–1.751) | 0.490 | ||
| N status (negative/positive) | 2.300 (1.254–4.220) | 0.007 | ||
| LVI (LVI2/LVI4) | 1.952 (1.084–3.518) | 0.026 | ||
| IMVI (absent/present) | 1.442 (0.827–2.514) | 0.197 | ||
| EMVI (absent/present) | 1.954 (1.148–3.324) | 0.014 | ||
| IPNI (absent/present) | 0.897 (0.528–1.521) | 0.686 | ||
| EPNI (absent/present) | 1.959 (1.160–3.307) | 0.012 | ||
| Prognostic stage group (I-II/III-IV) | 2.300 (1.254–4.220) | 0.007 | ||
| Tumor growth pattern (infiltrative/pushing borders) | 1.346 (0.698–2.595) | 0.376 | ||
| Bd1 | 1.000 (Reference) | |||
| Bd2 | 1.286 (0.686–2.412) | 0.433 | ||
| Bd3 | 1.299 (0.680–2.481) | 0.428 | ||
| Pdc1 | 1.000 (Reference) | |||
| Pdc2 | 1.414 (0.814–2.456) | 0.219 | ||
| Pdc3 | 1.112 (0.342–3.615) | 0.859 | ||
| CDH17 expression (core) (low/high) | 0.934 (0.558–1.563) | 0.795 | ||
| CDH17 expression (front) (low/high) | 1.078 (0.636–1.827) | 0.782 | ||
| CDH17 expression (emboli) (low/high) | 1.719 (1.021–2.896) | 0.042 | 2.003 (1.077–3.723) | 0.028 |
| Clinicopathological Parameters | n (%) | |
|---|---|---|
| Gender | male | 43 (51.2%) |
| female | 41 (48.8%) | |
| Histological type | ADK NOS | 64 (76.2%) |
| ADK with mucinous component | 13 (15.5%) | |
| ADK with signet ring cell component | 2 (2.4%) | |
| medullary ADK | 1 (1.2%) | |
| mucinous ADK | 4 (4.8%) | |
| Grade | high-grade | 12 (14.3%) |
| low-grade | 72 (85.7%) | |
| T stage | 1 | 1 (1.2%) |
| 2 | 2 (2.4%) | |
| 3 | 43 (51.2%) | |
| 4 | 38 (45.2%) | |
| N status | negative | 28 (33.3%) |
| positive | 56 (66.7%) | |
| LVI | LVI2 | 27 (32.1%) |
| LVI4 | 57 (67.9%) | |
| IMVI | absent | 61 (72.6%) |
| present | 23 (27.4%) | |
| EMVI | absent | 37 (44%) |
| present | 47 (56%) | |
| IPNI | absent | 52 (61.9%) |
| present | 32 (38.1%) | |
| EPNI | absent | 39 (46.4%) |
| present | 45 (53.6%) | |
| Prognostic stage group | I-II | 28 (33.3%) |
| III-IV | 56 (66.7%) | |
| Tumor growth pattern | infiltrative | 71 (84.5%) |
| pushing borders | 13 (15.5%) | |
| Bd | Bd1 | 48 (57.1%) |
| Bd2 | 19 (22.6%) | |
| Bd3 | 17 (20.2%) | |
| PDCs | Pdc1 | 54 (64.3%) |
| Pdc2 | 25 (29.8%) | |
| Pdc3 | 5 (6%) | |
| Overall survival | alive | 25 (29.8%) |
| dead | 59 (70.2%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianole, V.; Giușcă, S.-E.; Căruntu, I.-D. Evidence for the Prognostic Value of CDH17 Expression in Colorectal Carcinoma. Int. J. Mol. Sci. 2025, 26, 6960. https://doi.org/10.3390/ijms26146960
Ianole V, Giușcă S-E, Căruntu I-D. Evidence for the Prognostic Value of CDH17 Expression in Colorectal Carcinoma. International Journal of Molecular Sciences. 2025; 26(14):6960. https://doi.org/10.3390/ijms26146960
Chicago/Turabian StyleIanole, Victor, Simona-Eliza Giușcă, and Irina-Draga Căruntu. 2025. "Evidence for the Prognostic Value of CDH17 Expression in Colorectal Carcinoma" International Journal of Molecular Sciences 26, no. 14: 6960. https://doi.org/10.3390/ijms26146960
APA StyleIanole, V., Giușcă, S.-E., & Căruntu, I.-D. (2025). Evidence for the Prognostic Value of CDH17 Expression in Colorectal Carcinoma. International Journal of Molecular Sciences, 26(14), 6960. https://doi.org/10.3390/ijms26146960





