Hemodialysis Intensifies NLRP3 Inflammasome Expression and Oxidative Stress in Patients with Chronic Kidney Disease
Abstract
1. Introduction
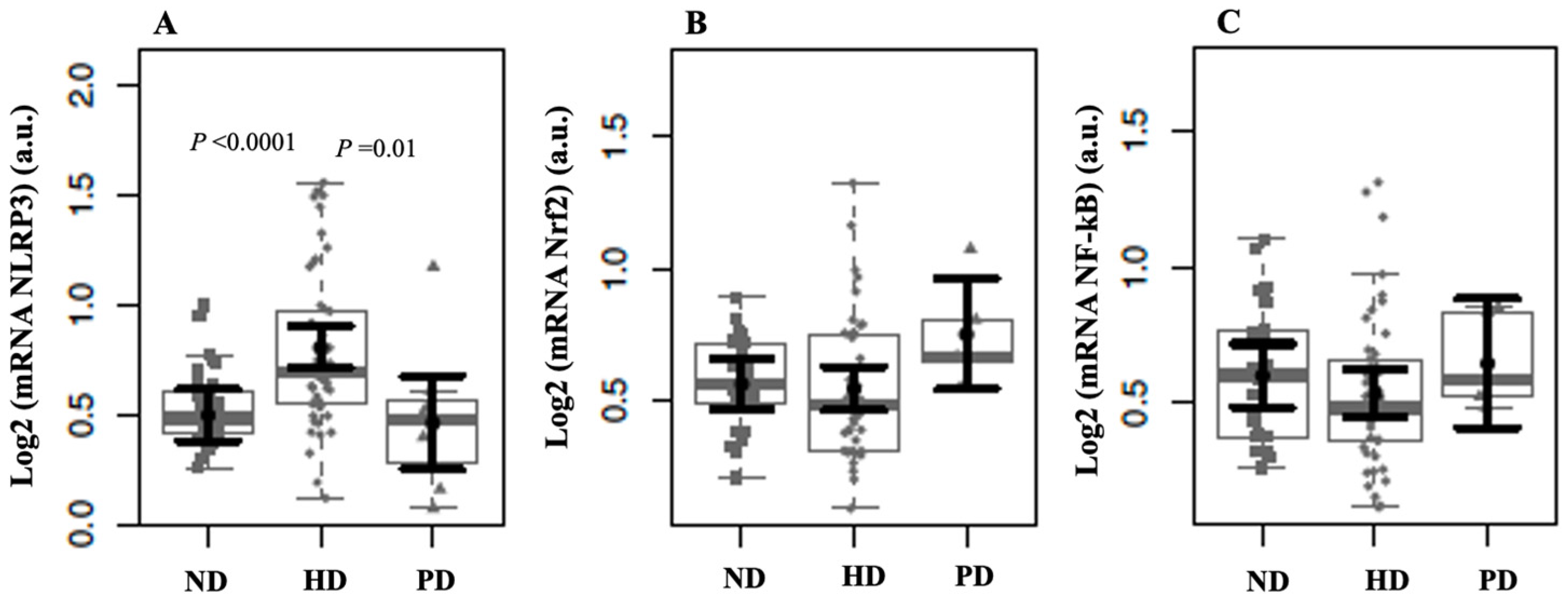
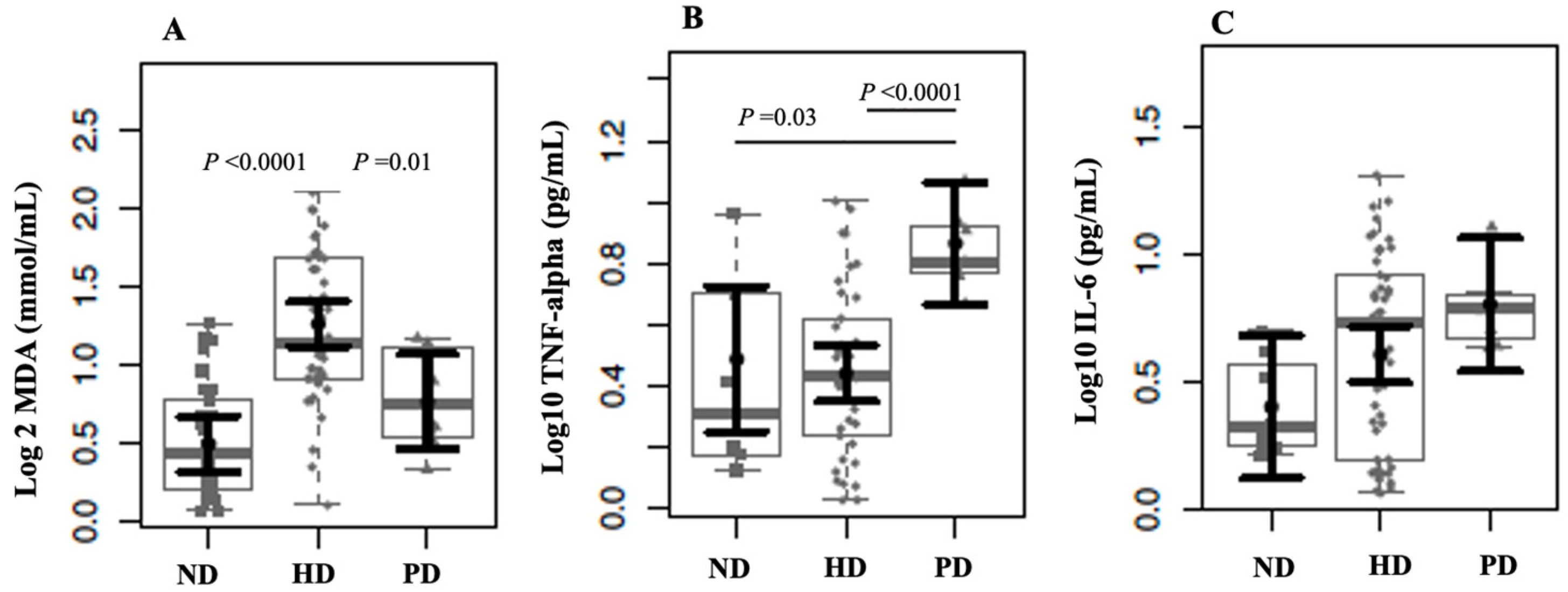
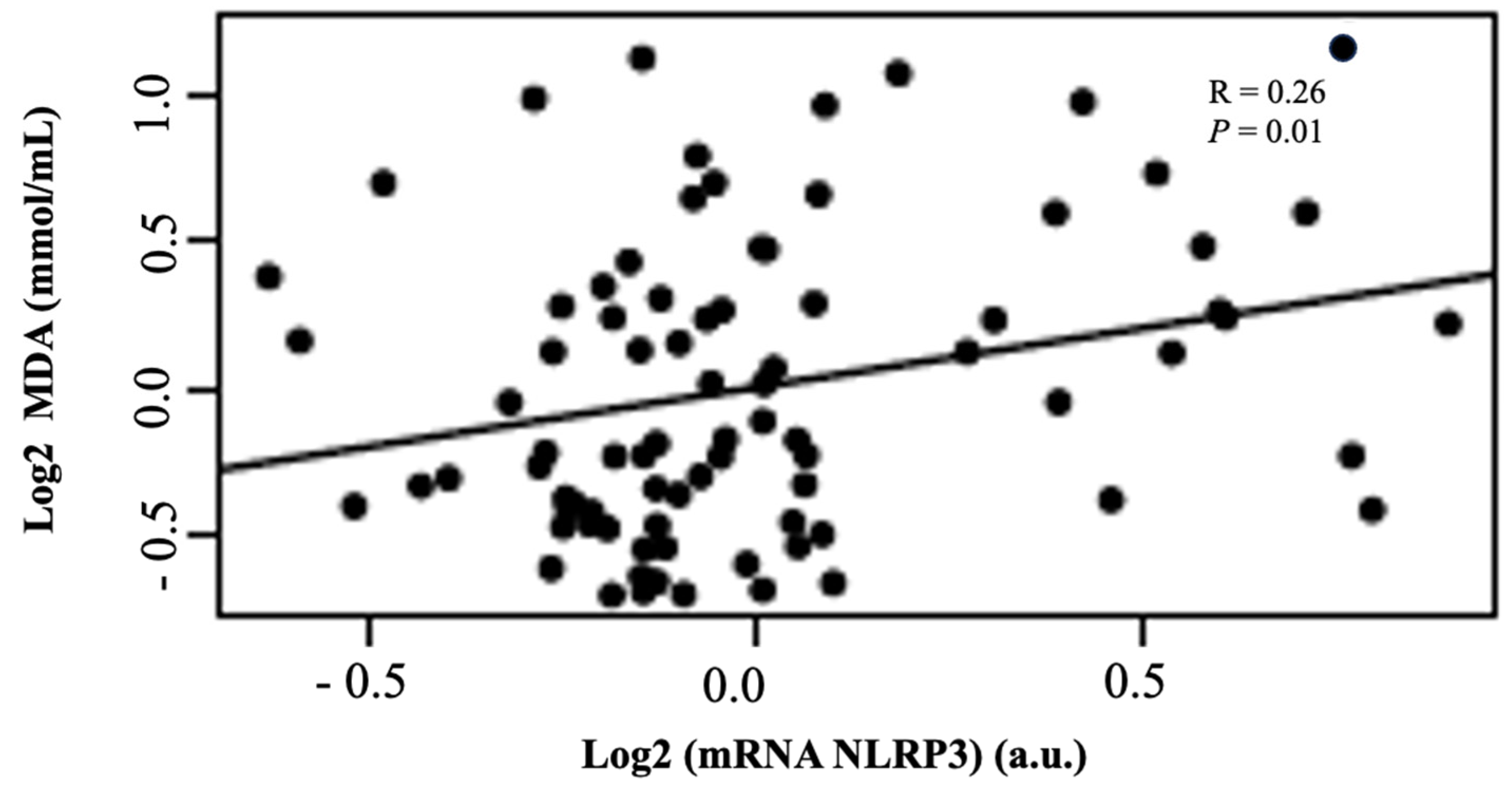
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Patients
4.2. Analytic Procedures and Sample Processing
4.3. Quantitative Real-Time PCR Analyses
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| ND | non-dialysis |
| HD | hemodialysis |
| PD | peritoneal dialysis |
| MDA | malondialdehyde |
| NBD | nucleotide-binding domain |
| LRRs | leucine-rich repeats |
| GSDMD | gasdermin D |
| PRRs | pattern recognition receptors |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| GFR | glomerular filtration rate |
| RRT | renal replacement therapy |
| NLRP3 | nod-like receptor pyrin domain containing |
| NF-κB | nuclear factor kappa-B |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| ROS | reactive oxygen species |
| TBARSs | thiobarbituric acid-reactive substances |
References
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Pandey, A.; Li, Z.; Gautam, M.; Ghosh, A.; Man, S.M. Molecular mechanisms of emerging inflammasome complexes and their activation and signaling in inflammation and pyroptosis. Immunol. Rev. 2025, 329, e13406. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Hornung, V. The NLRP3 Inflammasome Renders Cell Death Pro-inflammatory. J. Mol. Biol. 2018, 430, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Akbal, A.; Dernst, A.; Lovotti, M.; Mangan, M.S.J.; McManus, R.M.; Latz, E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell. Mol. Immunol. 2022, 19, 1201–1214. [Google Scholar] [CrossRef]
- Miao, N.; Yin, F.; Xie, H.; Wang, Y.; Xu, Y.; Shen, Y.; Xu, D.; Yin, J.; Wang, B.; Zhou, Z.; et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 2019, 96, 1105–1120. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, R.D.; Morgan, A.R.; Whyte, M.B.; Hanif, W.; Bain, S.C.; Davies, S.; Dashora, U.; Yousef, Z.; Patel, D.C.; et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv. Ther. 2022, 39, 33–43. [Google Scholar] [CrossRef]
- Rispoli, R.M.; Popolo, A.; de Fabrizio, V.; Bianca, R.D.d.V.; Autore, G.; Dalli, J.; Marzocco, S. Targeting Inflammatory Imbalance in Chronic Kidney Disease: Focus on Anti-Inflammatory and Resolution Mediators. Int. J. Mol. Sci. 2025, 26, 3072. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Zhang, Y.; Ma, Y. Chronic kidney disease and NLRP3 inflammasome: Pathogenesis, development and targeted therapeutic strategies. Biochem. Biophys. Rep. 2022, 33, 101417. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Dong, Y.; Chen, Z.; Eckols, T.K.; Kasembeli, M.M.; Tweardy, D.J.; Mitch, W.E. Pharmacokinetics and pharmacodynamics of TTI-101, a STAT3 inhibitor that blocks muscle proteolysis in rats with chronic kidney disease. Am. J. Physiol. Renal Physiol. 2020, 319, F84–F92. [Google Scholar] [CrossRef]
- Leal, V.O.; Saldanha, J.F.; Stockler-Pinto, M.B.; Cardozo, L.F.; Santos, F.R.; Albuquerque, A.S.; Leite, M., Jr.; Mafra, D. NRF2 and NF-κB mRNA expression in chronic kidney disease: A focus on nondialysis patients. Int. Urol. Nephrol. 2015, 47, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Bigdeli, R.; Shahnazari, M.; Oruji, F.; Fattahi, S.; Panahnejad, E.; Ghadri, A.; Movahedi-Asl, E.; Mahdavi-Ourtakand, M.; Asgary, V.; et al. Evaluation of Inflammasome Activation in Peripheral Blood Mononuclear Cells of Hemodialysis Treated Patients with Glomerulonephritis. Iran. J. Pharm Res. 2021, 20, 609–617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hishida, E.; Ito, H.; Komada, T.; Karasawa, T.; Kimura, H.; Watanabe, S.; Kamata, R.; Aizawa, E.; Kasahara, T.; Morishita, Y.; et al. Crucial Role of NLRP3 Inflammasome in the Development of Peritoneal Dialysis-related Peritoneal Fibrosis. Sci. Rep. 2019, 9, 10363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hautem, N.; Morelle, J.; Sow, A.; Corbet, C.; Feron, O.; Goffin, E.; Huaux, F.; Devuyst, O. The NLRP3 Inflammasome Has a Critical Role in Peritoneal Dialysis-Related Peritonitis. J. Am. Soc. Nephrol. 2017, 28, 2038–2052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granata, S.; Masola, V.; Zoratti, E.; Scupoli, M.T.; Baruzzi, A.; Messa, M.; Sallustio, F.; Gesualdo, L.; Lupo, A.; Zaza, G. NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS ONE 2015, 10, e0122272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahib, A.; Choudhury, C.; Wani, I.A.; Wani, M.M. Evaluation of Inflammatory Status in Chronic Kidney Disease Patients and a Comparison Between Hemodialysis and Peritoneal Dialysis Patients. Cureus 2024, 16, e69443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Zhang, X.; Liao, N.; Mi, L.; Peng, Y.; Liu, B.; Zhang, S.; Wen, F. Enhanced Expression of NLRP3 Inflammasome-Related Inflammation in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 978–985. [Google Scholar] [CrossRef]
- Ekiner, S.A.; Gęgotek, A.; Skrzydlewska, E. Inflammasome activity regulation by PUFA metabolites. Front. Immunol. 2024, 15, 1452749. [Google Scholar] [CrossRef]
- Cao, W.; Zeng, Y.; Su, Y.; Gong, H.; He, J.; Liu, Y.; Li, C. The involvement of oxidative stress and the TLR4/NF-κB/NLRP3 pathway in acute lung injury induced by high-altitude hypoxia. Immunobiology 2024, 229, 152809. [Google Scholar] [CrossRef]
- Capusa, C.; Stoian, I.; Rus, E.; Lixandru, D.; Barbulescu, C.; Mircescu, G. Does dialysis modality influence the oxidative stress of uremic patients? Kidney Blood Press. Res. 2012, 35, 220–225. [Google Scholar] [CrossRef]
- Ajala, M.O.; Ogunro, P.S.; Odun, A. Effect of hemodialysis on total antioxidant status of chronic renal failure patients in government hospitals in Lagos Nigeria. Niger. J. Clin. Pract. 2011, 14, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef] [PubMed]
- Tbahriti, H.F.; Kaddous, A.; Bouchenak, M.; Mekki, K. Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients. Biochem. Res. Int. 2013, 2013, 358985. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Oliva, C.; Yuste, C.; Ceprián, N.; Caro, P.J.; Valera, G.; de Pablos, I.G.; Morales, E.; Carracedo, J. Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes. Antioxidants 2021, 10, 1155. [Google Scholar] [CrossRef]
- Chermut, T.R.; Fonseca, L.; Figueiredo, N.; Leal, V.d.O.; Borges, N.A.; Cardozo, L.F.; Correa Leite, P.E.; Alvarenga, L.; Regis, B.; Delgado, A.; et al. Effects of propolis on inflammation markers in patients undergoing hemodialysis: A randomized, double-blind controlled clinical trial. Complement. Ther. Clin. Pract. 2023, 51, 101732. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.C.M.V.; Alvarenga, L.; Cardozo, L.F.M.F.; Baptista, B.G.; Fanton, S.; Paiva, B.R.; Ribeiro-Alves, M.; Fortunato, R.S.; Vasconcelos, A.L.; Nakao, L.S.; et al. Can curcumin supplementation break the vicious cycle of inflammation, oxidative stress, and uremia in patients undergoing peritoneal dialysis? Clin. Nutr. ESPEN 2024, 59, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
| Parameters | ND (N = 32) | HD (N = 50) | PD (N = 8) | p Values |
|---|---|---|---|---|
| Sex (male/female) | 14/18 | 20/30 | 5/3 | 0.490 |
| Age (years) | 63 (11.2) | 48.5 (16.5) | 56.5 (8.5) | 0.000 |
| Time on dialysis (months) | - | 60.5 (50) | 40.5 (41.2) | 0.187 |
| eGFR (mL/min/1.72 m2) | 43.5 (22.0) | - | - | - |
| BMI (kg/m2) | 29.5 (10.0) | 24.2 (4.9) | 28.8 (2.6) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.; Cardozo, L.F.M.F.; Coutinho-Wolino, K.S.; Ribeiro-Alves, M.; Mafra, D. Hemodialysis Intensifies NLRP3 Inflammasome Expression and Oxidative Stress in Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2025, 26, 6933. https://doi.org/10.3390/ijms26146933
Ribeiro M, Cardozo LFMF, Coutinho-Wolino KS, Ribeiro-Alves M, Mafra D. Hemodialysis Intensifies NLRP3 Inflammasome Expression and Oxidative Stress in Patients with Chronic Kidney Disease. International Journal of Molecular Sciences. 2025; 26(14):6933. https://doi.org/10.3390/ijms26146933
Chicago/Turabian StyleRibeiro, Marcia, Ludmila F. M. F. Cardozo, Karen Salve Coutinho-Wolino, Marcelo Ribeiro-Alves, and Denise Mafra. 2025. "Hemodialysis Intensifies NLRP3 Inflammasome Expression and Oxidative Stress in Patients with Chronic Kidney Disease" International Journal of Molecular Sciences 26, no. 14: 6933. https://doi.org/10.3390/ijms26146933
APA StyleRibeiro, M., Cardozo, L. F. M. F., Coutinho-Wolino, K. S., Ribeiro-Alves, M., & Mafra, D. (2025). Hemodialysis Intensifies NLRP3 Inflammasome Expression and Oxidative Stress in Patients with Chronic Kidney Disease. International Journal of Molecular Sciences, 26(14), 6933. https://doi.org/10.3390/ijms26146933







