Tumor-Associated Macrophages and Collagen Remodeling in Mammary Carcinomas: A Comparative Analysis in Dogs and Humans
Abstract
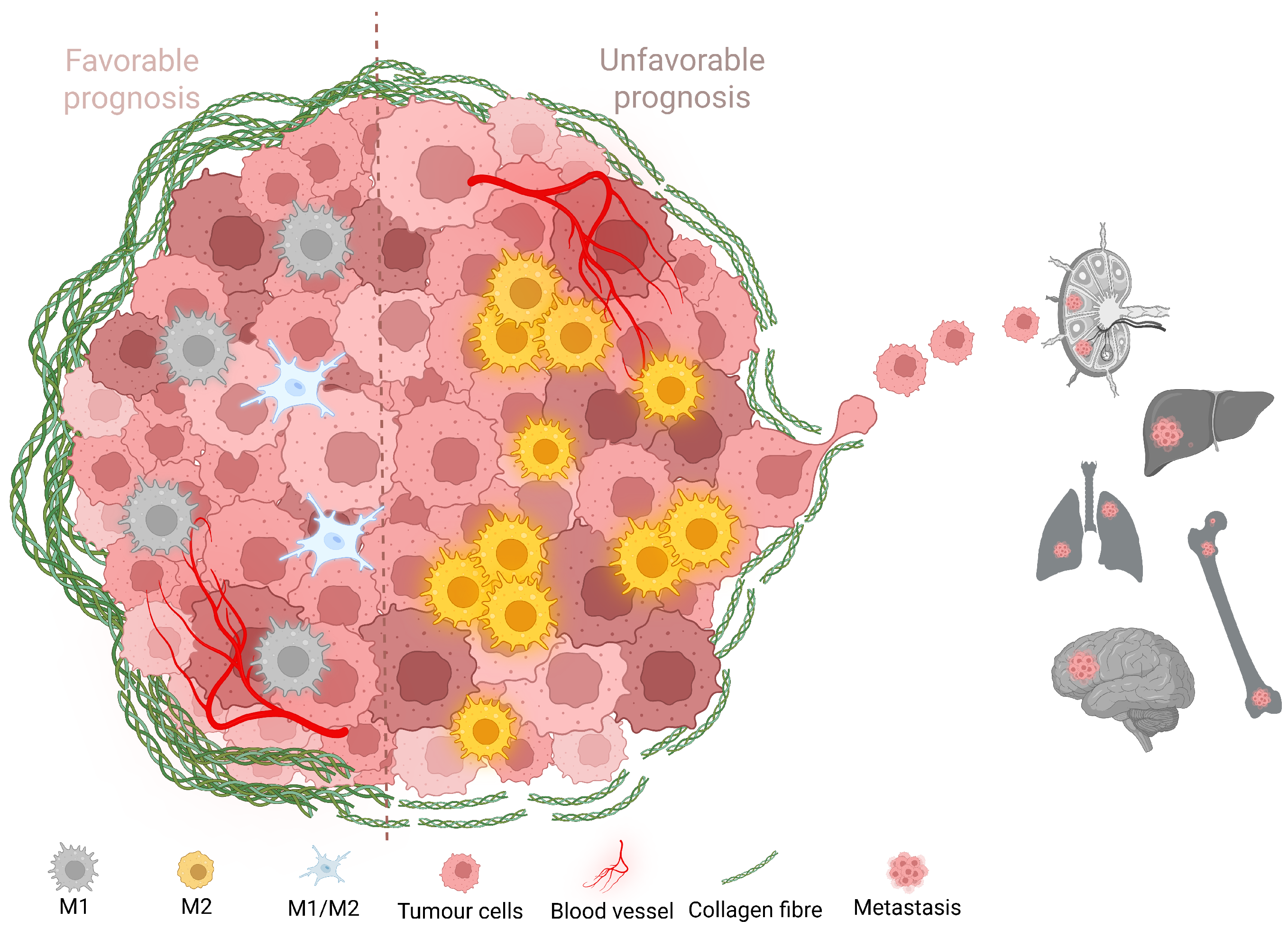
1. Introduction
2. Results
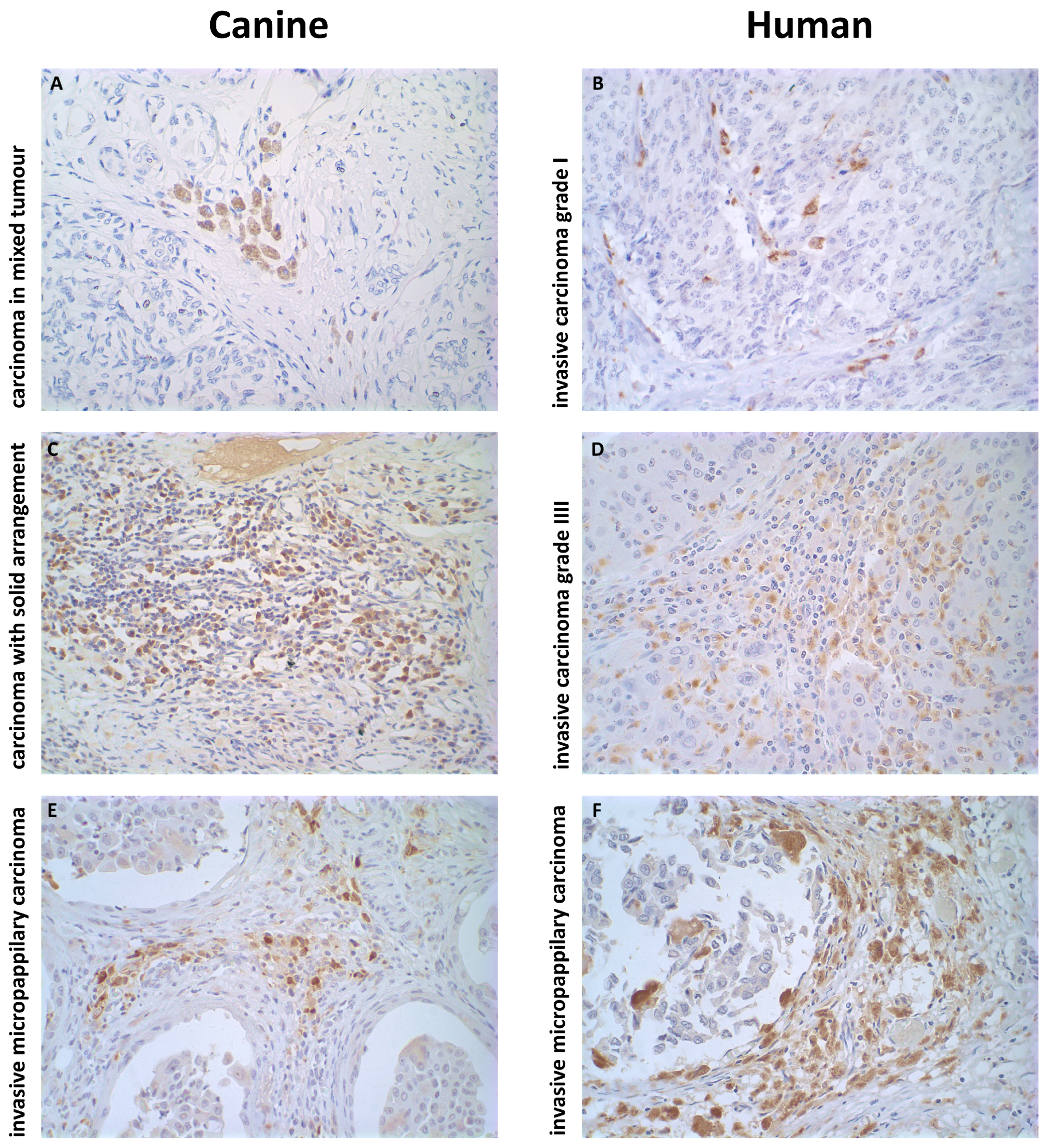
2.1. Characterization of the Analyzed Samples
2.2. Histopathological Profiles of the Mammary Carcinomas Evaluated
| Species | Histological Type | n | Age (years) | Staging | Grade | Subtype | Survival a (mo/days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | I | II | III | HR+/Ki67 < 20% | HR+/Ki67 > 20% | HER2+ | HR-/HER2- | |||||
| Human | tubular carcinoma (TC) | 4 | 71.3 | 2 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 0 | 69.0 |
| Human | invasive carcinoma grade I (ICgI) | 16 | 68.4 | 5 | 7 | 0 | 3 | 1 | 16 | 0 | 0 | 13 | 3 | 0 | 0 | 76.3 |
| Human | invasive carcinoma grade III (ICgIII) | 21 | 59.6 | 5 | 2 | 2 | 10 | 2 | 0 | 0 | 21 | 2 | 12 | 4 | 3 | 62.1 |
| Human | invasive micropapillary carcinoma (IMC) | 17 | 52.3 | 4 | 3 | 1 | 9 | 0 | 3 | 8 | 2 | 3 | 8 | 6 | 0 | 52.3 |
| Canine | carcinoma in mixed tumors (CMT) | 14 | 11.0 | 7 | 3 | 4 | 0 | 0 | 11 | 2 | 1 | 13 | 1 | 0 | 0 | 1116.2 |
| Canine | carcinosarcoma (CS) | 6 | 8.5 | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 6 | 2 | 4 | 0 | 0 | 321.8 |
| Canine | invasive micropapillary carcinoma (IMC) | 16 | 10.8 | 0 | 1 | 2 | 10 | 3 | 2 | 9 | 5 | 1 | 7 | 3 | 5 | 293.9 |
| Canine | carcinoma with solid arrangement (CSA) | 23 | 11.8 | 5 | 4 | 4 | 9 | 1 | 9 | 10 | 4 | 3 | 17 | 2 | 1 | 279.5 |
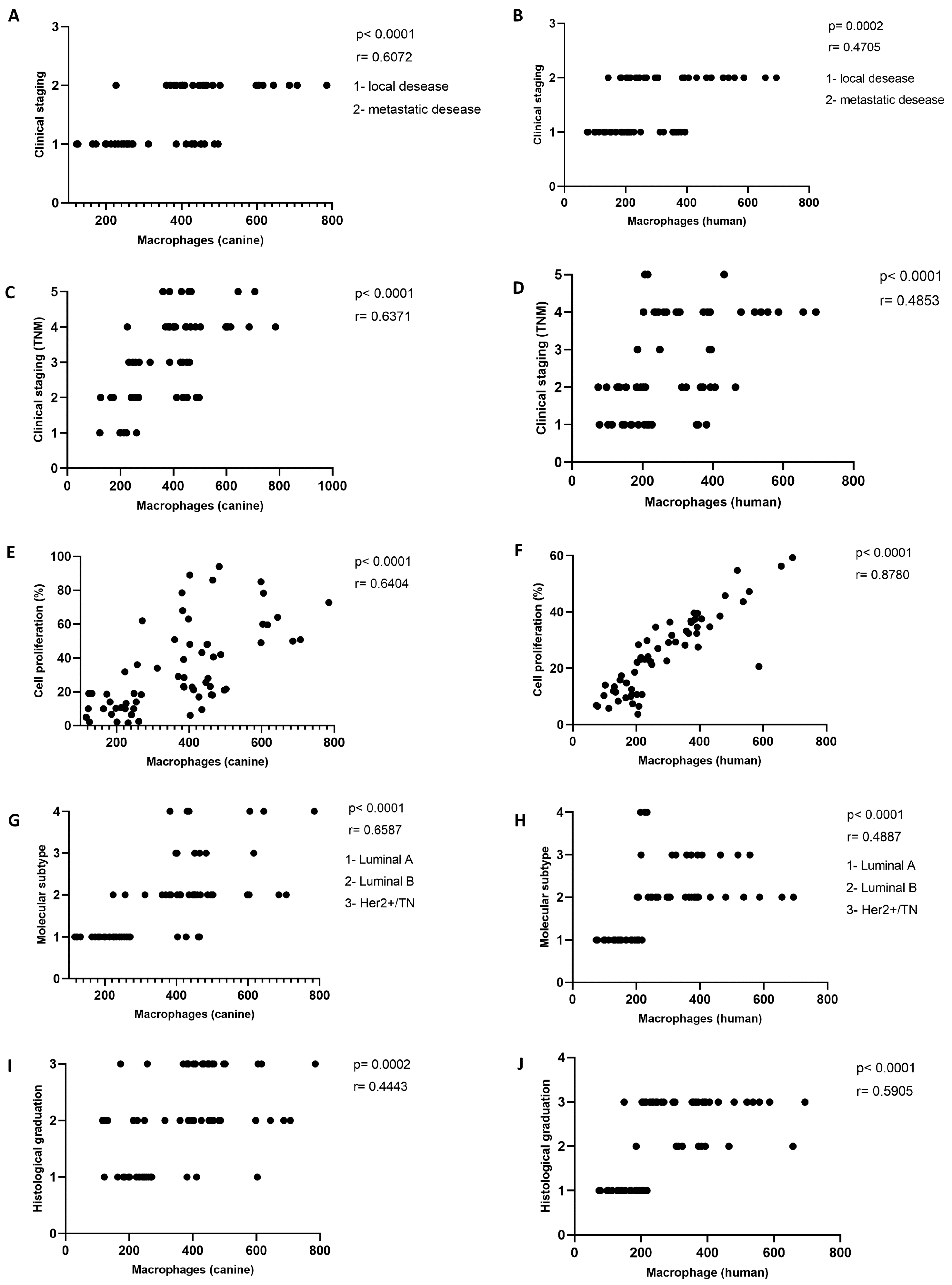
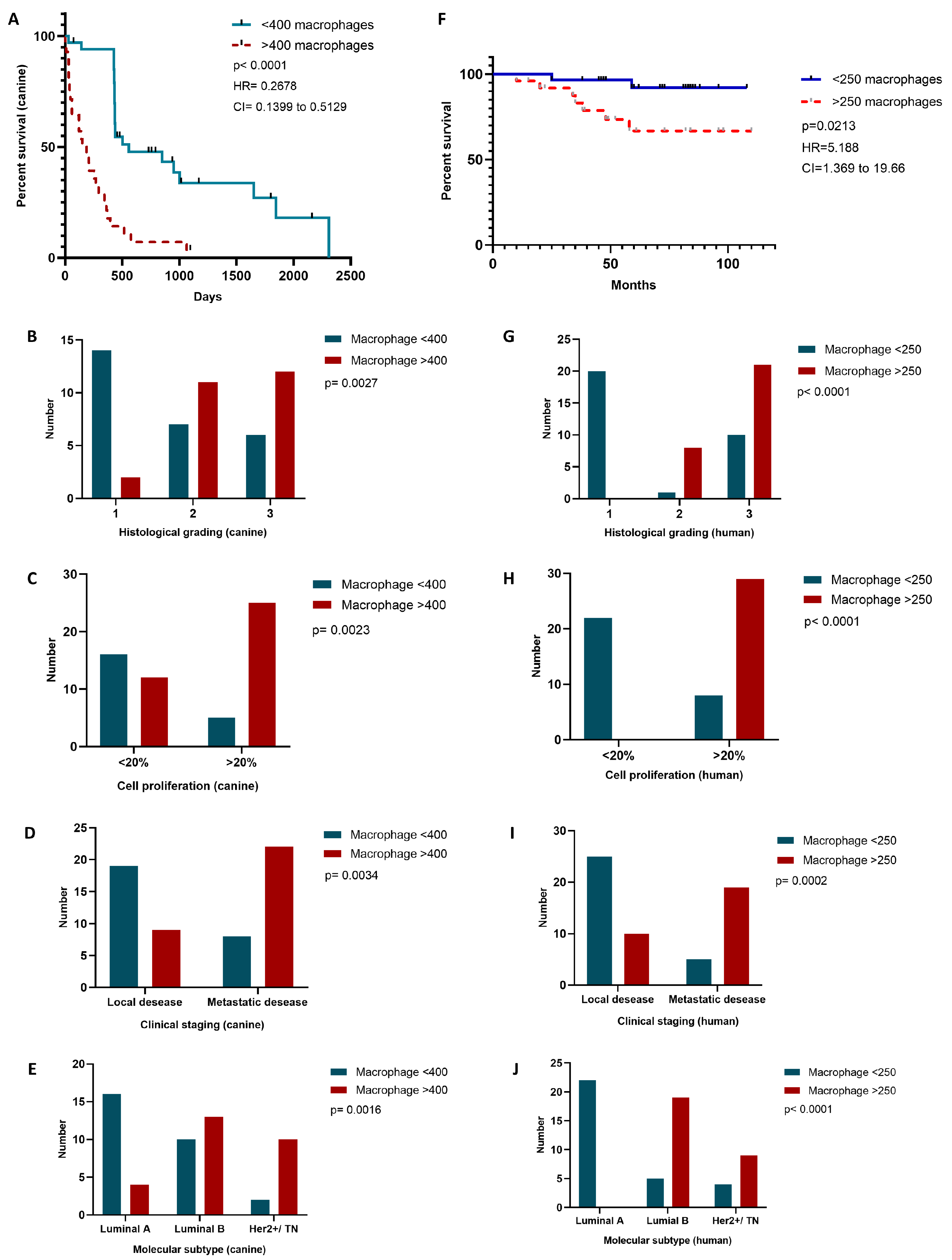
2.3. Increased TAM Infiltration Is Associated with Tumors of Poor Prognosis
2.4. Carcinomas with Shorter Collagen Fibers Have the Highest TAM Infiltration
2.5. More Aggressive Carcinomas Exhibit Higher TAM Infiltration
2.6. TAM Infiltration Stratifies Survival in Canine and Human Mammary Carcinomas
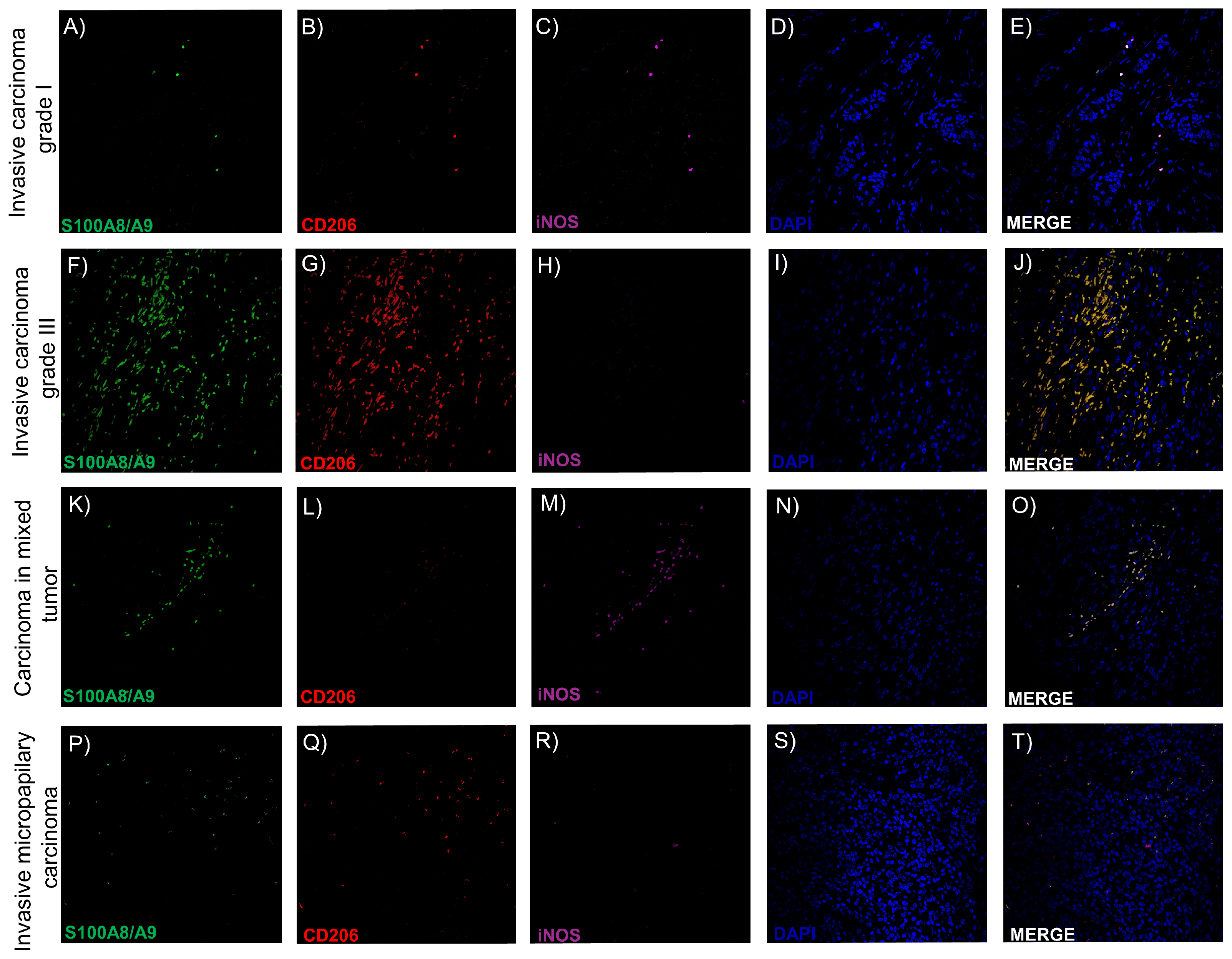
2.7. Intratumoral TAMs Express M1 and M2 Markers Concurrently
3. Discussion
4. Materials and Methods
4.1. Ethical Aspects
4.2. Case Selection
4.3. Immunohistochemistry Analysis
4.4. Immunofluorescence Analysis
4.5. Multiphoton Imaging and SHG Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Soerjomataram, I. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Toss, M.S.; Miligy, I.M.; Gorringe, K.L.; AlKawaz, A.; Mittal, K.; Aneja, R.; Ellis, I.O.; Green, A.R.; Roxanis, I.; Rakha, E.A. Geometric characteristics of collagen have independent prognostic significance in breast ductal carcinoma in situ: An image analysis study. Mod. Pathol. 2019, 32, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.; Weaver, V. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J. Macrophages define the invasive microenvironment in breast cancer. J. Leukoc. Biol. 2008, 84, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.; Pollard, J. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, E. Know thy model: Charting molecular homology in stromal reprogramming between canine and human mammary tumors. Front. Cell Dev. Biol. 2019, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Ajeti, V.; Nadiarnykh, O.; Ponik, S.; Keely, P.; Eliceiri, K.; Campagnola, P. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: Implications for probing stromal alterations in human breast cancer. Biomed. Opt. Express 2011, 2, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Almici, E.; Arshakyan, M.; Carrasco, J.L.; Martínez, A.; Ramírez, J.; Enguita, A.B.; Monsó, E.; Montero, J.; Samitier, J.; Alcaraz, J. Quantitative Image Analysis of Fibrillar Collagens Reveals Novel Diagnostic and Prognostic Biomarkers and Histotype-Dependent Aberrant Mechanobiology in Lung Cancer. Mod. Pathol. 2023, 36, 100155. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.; Smid, M.; Dawes, R.; Timmermans, M.A.; Salzman, P.; van Deurzen, C.H.M.; Beer, D.G.; Foekens, J.A.; Brown, E. Using second harmonic generation to predict patient outcome in solid tumors. BMC Cancer 2015, 15, 929. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.; O’Leary, K.; Brockman, J.; Rugowski, D.E.; Liu, Y.; Garcia, N.; Yu, M.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumour cells. Breast Cancer Res. 2017, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Hompland, T.; Erikson, A.; Lindgren, M.; Lindmo, T.; de Lange Davies, C. Second-harmonic generation in collagen as a potential cancer diagnostic parameter. J. Biomed. Opt. 2008, 13, 054050. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.; Tang, P.; Brown, E. Second harmonic generation reveals matrix alterations during breast tumor progression. J. Biomed. Opt. 2012, 18, 031106. [Google Scholar] [CrossRef] [PubMed]
- Golaraei, A.; Kontenis, L.; Cisek, R.; Tokarz, D.; Done, S.J.; Wilson, B.C.; Barzda, V. Changes of collagen ultrastructure in breast cancer tissue determined by second-harmonic generation double Stokes-Mueller polarimetric microscopy. Biomed. Opt. Express 2016, 7, 4054–4068. [Google Scholar] [CrossRef] [PubMed]
- Case, A.; Brisson, B.; Durham, A.; Rosen, S.; Monslow, J.; Buza, E.; Salah, P.; Gillem, J.; Ruthel, G.; Veluvolu, S.; et al. Identification of prognostic collagen signatures and potential therapeutic stromal targets in canine mammary gland carcinoma. PLoS ONE 2017, 12, e0176824. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Gangnon, R.E.; Sprague, B.L.; Van Germert, L.; Hampton, J.M.; Eliceiri, K.W.; Bredfeldt, J.S.; Liu, Y.; Surachaicharn, N.; Newcomb, P.A.; et al. Collagen Alignment as a Predictor of Recurrence after Ductal Carcinoma in situ. Cancer Epidemiol. Biomark. Prev. 2018, 27, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.A.; Garcia, A.P.V.; Gomes, E.F.A.; Longford, F.G.J.; Frey, J.G.; Cassali, G.D.; de Paula, A.M. Canine mammary cancer diagnosis from quantitative properties of nonlinear optical images. Biomed. Opt. Express 2020, 11, 6413–6427. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.P.V.; Reis, L.A.; Nunes, F.C.; Longford, F.G.J.; Frey, J.G.; de Paula, A.M.; Cassali, G.D. Canine mammary cancer tumour behaviour and patient survival time are associated with collagen fibre characteristics. Sci. Rep. 2021, 11, 5668. [Google Scholar] [CrossRef] [PubMed]
- Natal, R.A.; Vassallo, J.; Paiva, G.R.; Pelegati, V.B.; Barbosa, G.O.; Mendonça, G.R.; Bondarik, C.; Derchain, S.F.; Carvalho, H.F.; Lima, C.S.; et al. Collagen analysis by second-harmonic generation microscopy predicts outcome of luminal breast cancer. Tumor Biol. 2018, 40, 1010428318770953. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Taborda, D.; Reis, L.; de Paula, A.; Cassali, G. Collagen modifications predictive of lymph node metastasis in dogs with carcinoma in mixed tumours. Front. Vet. Sci. 2024, 11, 1362693. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Luo, Y. Collagen and cancer progression. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188316. [Google Scholar] [CrossRef]
- Gomes, E.F.A.; Junior, E.P.; de Lima, M.F.R.; Reis, L.A.; Paranhos, G.; Mamede, M.; Longford, F.G.J.; Frey, J.G.; de Paula, A.M. Prostate cancer tissue classification by multiphoton imaging, automated image analysis and machine learning. J. Biophotonics 2023, 16, e202200382. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, J.N.; Drifka, C.R.; Pointer, K.B.; Liu, Y.; Lieberthal, T.J.; Kao, W.J.; Kuo, J.S.; Loeffler, A.G.; Eliceiri, K.W. Navigating the Collagen Jungle: The Biomedical Potential of Fiber Organization in Cancer. Bioengineering 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- DuChez, B.; Doyle, A.; Dimitriadis, E.; Yamada, K. Durotaxis by human cancer cells. Biophys. J. 2019, 116, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Brabrand, A.; Kariuki, I.I.; Engstrøm, M.J.; Haugen, O.A.; Dyrnes, L.A.; Åsvold, B.O.; Lilledahl, M.B.; Bofin, A.M. Alterations in collagen fibre patterns in breast cancer. A premise for tumour invasiveness? APMIS 2015, 123, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.; Longmore, G. Collagen Linearization within Tumors. Cancer Res. 2021, 81, 5611–5612. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Deng, C. Effect of stromal cells in tumor microenvironment on metastasis initiation. Int. J. Biol. Sci. 2018, 14, 2083. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, F.; Seo, B.; Fischbach, C.; Chen, W.; Hsu, L.; Gourdon, D. Breast cancer cells alter the dynamics of stromal fibronectin–collagen interactions. Matrix Biol. 2017, 60–61, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Gole, L.; Yeong, J.; Lim, J.C.T.; Ong, K.H.; Han, H.; Thike, A.A.; Poh, Y.C.; Yee, S.; Iqbal, J.; Hong, W.; et al. Quantitative stain-free imaging and digital profiling of collagen structure reveal diverse survival of triple negative breast cancer patients. Breast Cancer Res. 2020, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Cox, T. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Vacek, P.M.; Mulrow, S.E.; Evans, M.F.; Trentham-Dietz, A.; Herschorn, S.D.; James, T.A.; Surachaicharn, N.; Keikhosravi, A.; Eliceiri, K.W.; et al. Collagen organization in relation to ductal carcinoma in situ pathology and outcomes. Cancer Epidemiol. Biomark. Prev. 2021, 30, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Yu, Z.; Zu, X.; Li, G.; Hu, Z.; Xue, Y. Collagen Remodeling along Cancer Progression Providing a Novel Opportunity for Cancer Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 10509. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.; Schreiber, H.; Fu, Y. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyan, A.; Metcalf, K.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Pollard, J. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2020, 19, 799–820. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Coussens, L.; Kroemer, G.; Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.; Li, M. The tumor microenvironment and immune response. Cancer Lett. 2016, 380, 263–271. [Google Scholar] [CrossRef]
- Mazzone, M.; Menga, A. Macrophage plasticity and polarization in tissue repair and cancer treatment. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 225–247. [Google Scholar]
- Luo, M.; Guan, J.; Guo, X. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2018, 16, 234. [Google Scholar]
- Wang, J.; Su, H. Tumor-associated macrophages are mediators of extratumoral collagen remodeling in breast cancer metastasis. Cancer Res. 2021, 81, 1387–1399. [Google Scholar] [CrossRef]
- Afik, R.; Zigmond, E.; Vugman, M.; Klepfish, M.; Shimshoni, E.; Pasmanik-Chor, M.; Shenoy, A.; Bassat, E.; Halpern, Z.; Geiger, T.; et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J. Exp. Med. 2016, 213, 2315–2331. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; dos Reis, D.; Salgado, B.; Cassali, G. Clinical significance and prognostic role of tumor-associated macrophages infiltration according to histologic location in canine mammary carcinomas. Res. Vet. Sci. 2021, 135, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.; Raposo, T.; Carvalho, M.; Prada, J.; Pires, I. Canine mammary tumours as a model to study human breast cancer: Most recent findings. In Vivo 2011, 25, 455–465. [Google Scholar] [PubMed]
- Uva, P.; Aurisicchio, L.; Watters, J.; Loboda, A.; Kulkarni, A.; Castle, J.; Palombo, F.; Viti, V.; Mesiti, G.; Zappulli, V.; et al. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genom. 2009, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Classification of Tumours: Breast Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer (IARC): Lyon, France, 2019; Volume 2. [Google Scholar]
- Gamba, C.; Dias, E.; Ribeiro, L.; Campos, L.; Estrela-Lima, A.; Ferreira, E.; Cassali, G. Histopathological and immunohistochemical assessment of invasive micropapillary mammary carcinoma in dogs: A retrospective study. Vet. J. 2013, 196, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Gamba, C.; Faria, J.; Ferreira, E.; Goes, A.; Gomes, D.; Cassali, G. Inner nuclear membrane localization of epidermal growth factor receptor (EGFR) in spontaneous canine model of invasive micropapillary carcinoma of the mammary gland. Pathol. Res. Pract. 2016, 212, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Cassali, G.; Jark, P.; Gamba, C.; Damasceno, K.; Estrela-Lima, A.; Nardi, A.; Ferreira, E.; Horta, R.; Firmo, B.; Sueiro, F.; et al. Consensus Regarding the Diagnosis, Prognosis and Treatment of Canine and Feline Mammary Tumors—2019. Braz. J. Vet. Pathol. 2020, 13, 555–574. [Google Scholar] [CrossRef]
- Cassali, G.; Bertagnolli, A.; Ferreira, E.; Damasceno, K.; Gamba, C.; Campos, C. Canine mammary mixed tumours: A review. Vet. Med. Int. 2012, 2012, 274608. [Google Scholar] [CrossRef]
- Damasceno, K.A.; Ferreira, E.; Estrela-Lima, A.; Bosco, Y.; Silva, L.P.; Barros, A.L.B.; Bertagnolli, A.C.; Cassali, G.D. Relationship between the expression of versican and EGFR, HER-2, HER-3 and CD44 in matrix-producing tumours in the canine mammary gland. Histol. Histopathol. 2016, 31, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, K.; Nunes, M.; Garcia, A.; Nunes, F.; Schmitt, F.; Cassali, G. Solid Carcinoma of the Canine Mammary Gland: A Histological Type or Tumour Cell Arrangement? J. Comp. Pathol. 2022, 190, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Fujiwara, Y. Hypoxia-induced macrophages: Keys to shaping tumor microenvironment and drug resistance. Int. J. Mol. Sci. 2021, 22, 2458. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Martínez-Pérez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-occurring canine mammary tumors as a translational model for human breast cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. Collagen density regulates the activity of tumor-infiltrating T cells. J. Cell Biol. 2014, 205, 625–638. [Google Scholar] [CrossRef]
- Wagner, J.; Rapsomaniki, M.; Chevrier, S.; Anzeneder, T.; Langwieder, C.; Dykgers, A.; Rees, M.; Ramaswamy, A.; Muenst, S.; Soysal, S.D.; et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell 2019, 177, 1330–1345.e18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Moriarty, R.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Raftery, M.; Goyette, J.; Hsu, K. S100A8/A9 and S100A12 in inflammation: Biomarkers or therapeutic targets? Clin. Transl. Med. 2016, 5, 1–8. [Google Scholar]
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016, 167, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.; Sunderkötter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.; Ernst, M. Tumor-associated macrophages in breast cancer: Therapeutic opportunities and challenges. J. Mammary Gland. Biol. Neoplasia 2021, 26, 35–48. [Google Scholar]
- McCauley, L.; Koh, A. Tumor-associated macrophages in breast cancer as a potential target for new therapies. Oncotarget 2020, 11, 178–183. [Google Scholar]
- Zhang, Y.; Cheng, S.; Zhang, M.; Zhen, L.; Pang, D.; Zhang, Q. Remodeling of the tumor microenvironment by tumor-associated macrophages: Mechanisms and therapeutic opportunities. Cancer Lett. 2021, 506, 292–301. [Google Scholar] [CrossRef]
- Gilkes, D.; Semenza, G. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 2013, 9, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.; Wynn, T. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Carvalho, S.; Cabral, J.; Reis, C.A.; Gärtner, F. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in canine tumors: A review of the literature. Vet. Pathol. 2015, 52, 798–806. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Brett, E.; Sauter, M.; Machens, H.; Duscher, D. Tumor-associated collagen signatures: Pushing tumor boundaries. Cancer Metastasis Rev. 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Giesen, C.; Wang, H.; Schapiro, D.; Zivanovic, N.; Jacobs, A.; Hattendorf, B.; Schüffler, P.J.; Grolimund, D.; Buhmann, J.M.; Brandt, S.; et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 2014, 11, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Knop, S.; Leahy, K.; Satyamitra, M.M.; Hspel, P.; Hendrickson, R.G.; Carr, S.A. Multiplexed imaging of biomarkers in tissues using t-CyCIF and mass spectrometry. Nat. Protoc. 2020, 15, 3591–3624. [Google Scholar]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.-R.; Kurian, A.; Van Valen, D.; West, R.; et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 2018, 174, 1373–1387.e19. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.; Bertagnolli, A.; Lavalle, G.; Silveira, T.; Balabram, D.; Cassali, G. The prognostic significance of immunophenotypes in canine malignant mammary tumors. Arq. Bras. Med. Vet. Zootec. 2022, 74. [Google Scholar] [CrossRef]
- Monteiro, L.; Rodrigues, M.; Gomes, D.; Salgado, B.; Cassali, G. Tumour-associated macrophages: Relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours. Vet. J. 2018, 234, 119–125. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.P.V.; Salvi, M.; Reis, L.A.; Ribeiro, B.R.M.; Nunes, C.B.; de Paula, A.M.; Cassali, G.D. Tumor-Associated Macrophages and Collagen Remodeling in Mammary Carcinomas: A Comparative Analysis in Dogs and Humans. Int. J. Mol. Sci. 2025, 26, 6928. https://doi.org/10.3390/ijms26146928
Garcia APV, Salvi M, Reis LA, Ribeiro BRM, Nunes CB, de Paula AM, Cassali GD. Tumor-Associated Macrophages and Collagen Remodeling in Mammary Carcinomas: A Comparative Analysis in Dogs and Humans. International Journal of Molecular Sciences. 2025; 26(14):6928. https://doi.org/10.3390/ijms26146928
Chicago/Turabian StyleGarcia, Ana Paula Vargas, Marisa Salvi, Luana Aparecida Reis, Bárbara Regina Melo Ribeiro, Cristiana Buzelin Nunes, Ana Maria de Paula, and Geovanni Dantas Cassali. 2025. "Tumor-Associated Macrophages and Collagen Remodeling in Mammary Carcinomas: A Comparative Analysis in Dogs and Humans" International Journal of Molecular Sciences 26, no. 14: 6928. https://doi.org/10.3390/ijms26146928
APA StyleGarcia, A. P. V., Salvi, M., Reis, L. A., Ribeiro, B. R. M., Nunes, C. B., de Paula, A. M., & Cassali, G. D. (2025). Tumor-Associated Macrophages and Collagen Remodeling in Mammary Carcinomas: A Comparative Analysis in Dogs and Humans. International Journal of Molecular Sciences, 26(14), 6928. https://doi.org/10.3390/ijms26146928







