Design, Synthesis, and Testing of 1,2,3-Triazolo-Quinobenzothiazine Hybrids for Cytotoxic and Immunomodulatory Activity
Abstract
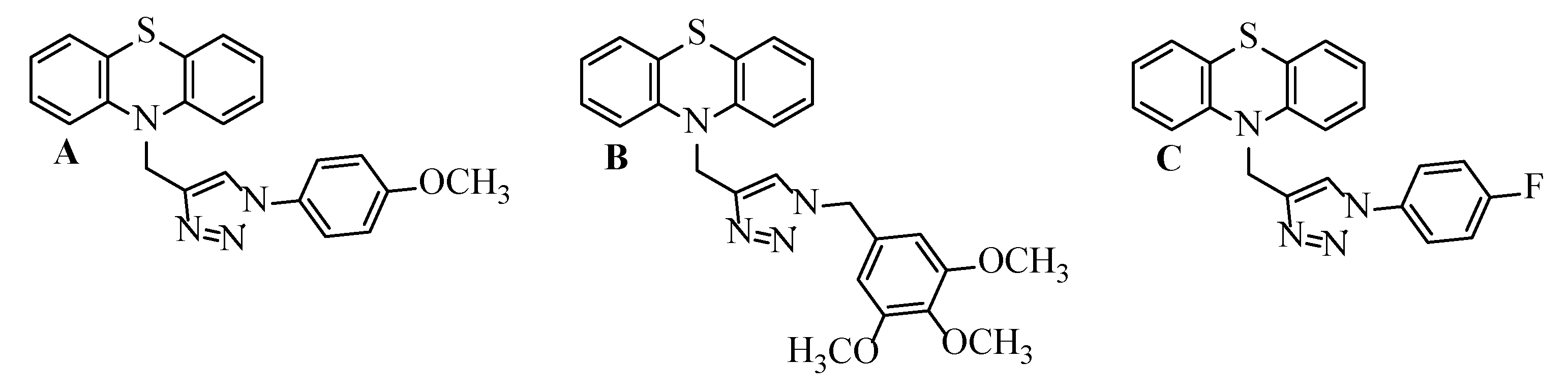
1. Introduction
2. Results and Discussion
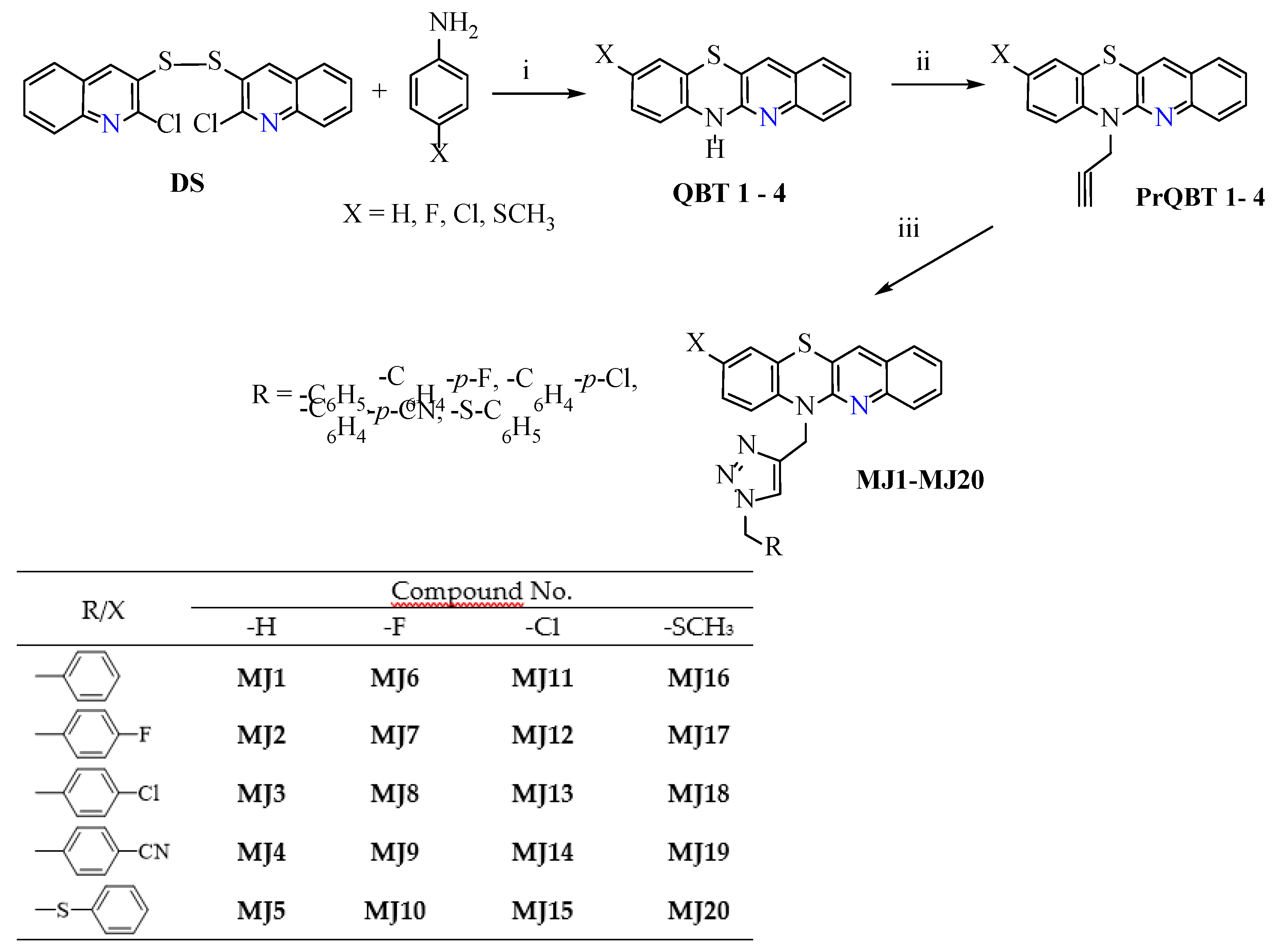
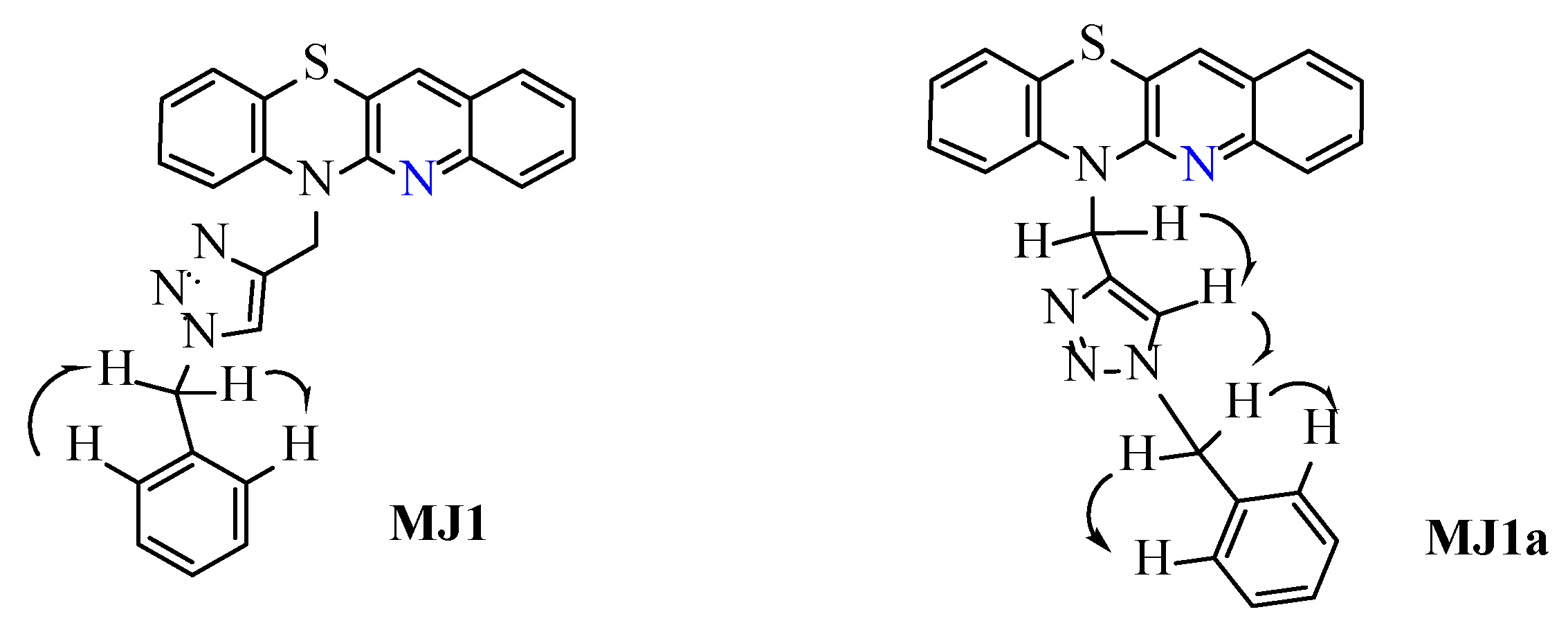
2.1. Synthesis of 1,2,3-Triazolo-Quinobenzothiazine Hybrids
2.2. Prediction of Biological Activity Using the PASS Web Platform
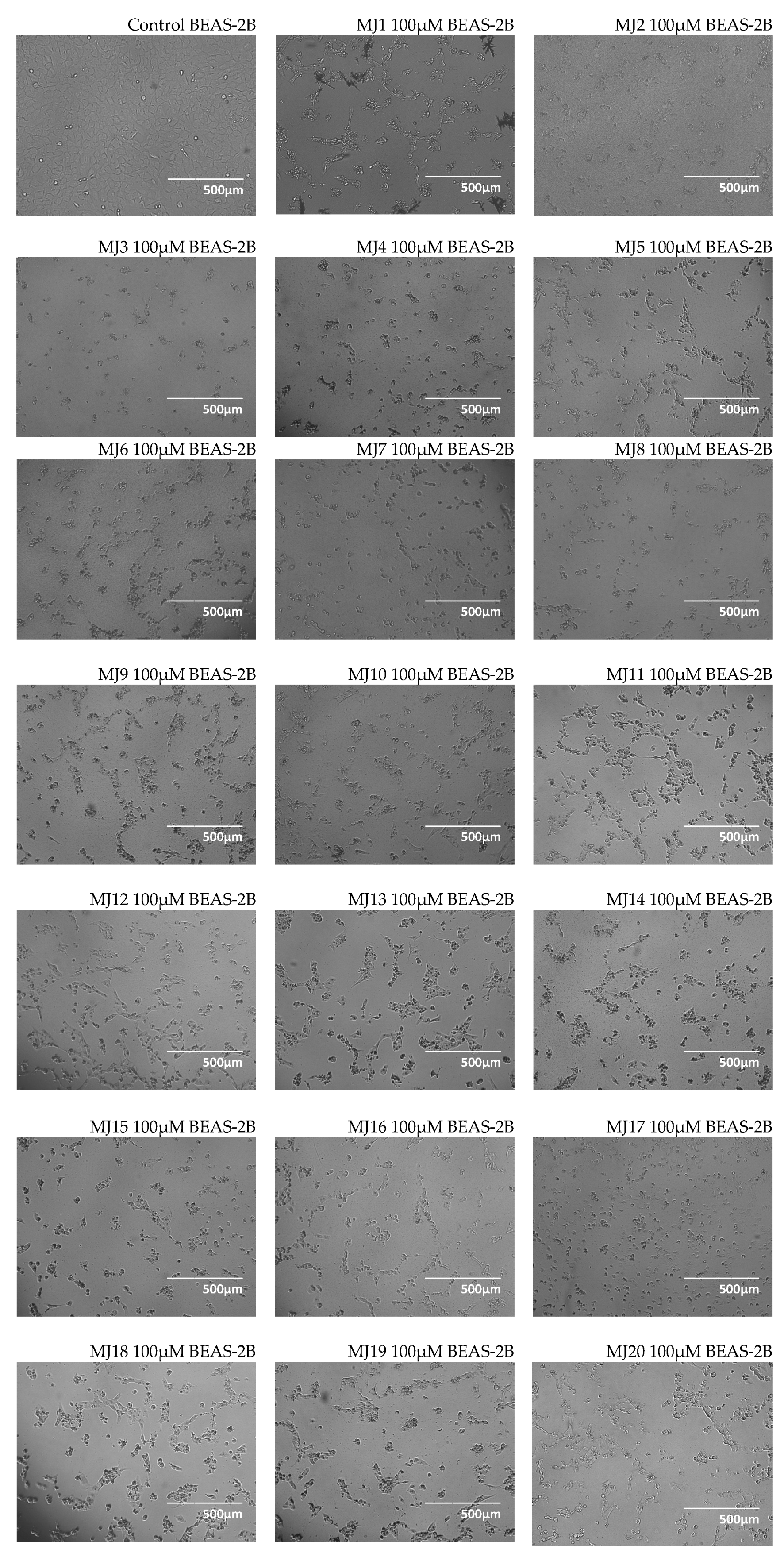
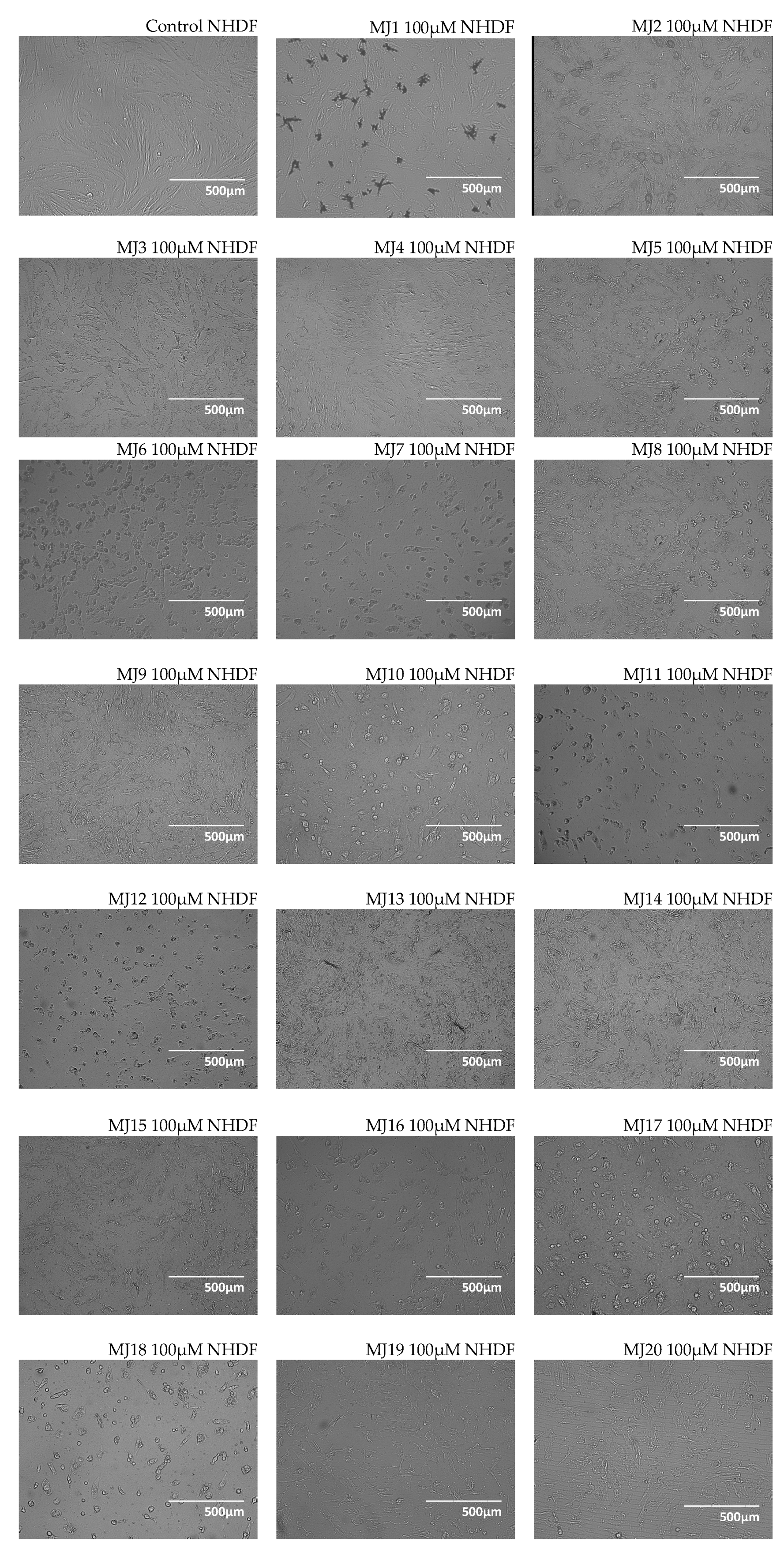
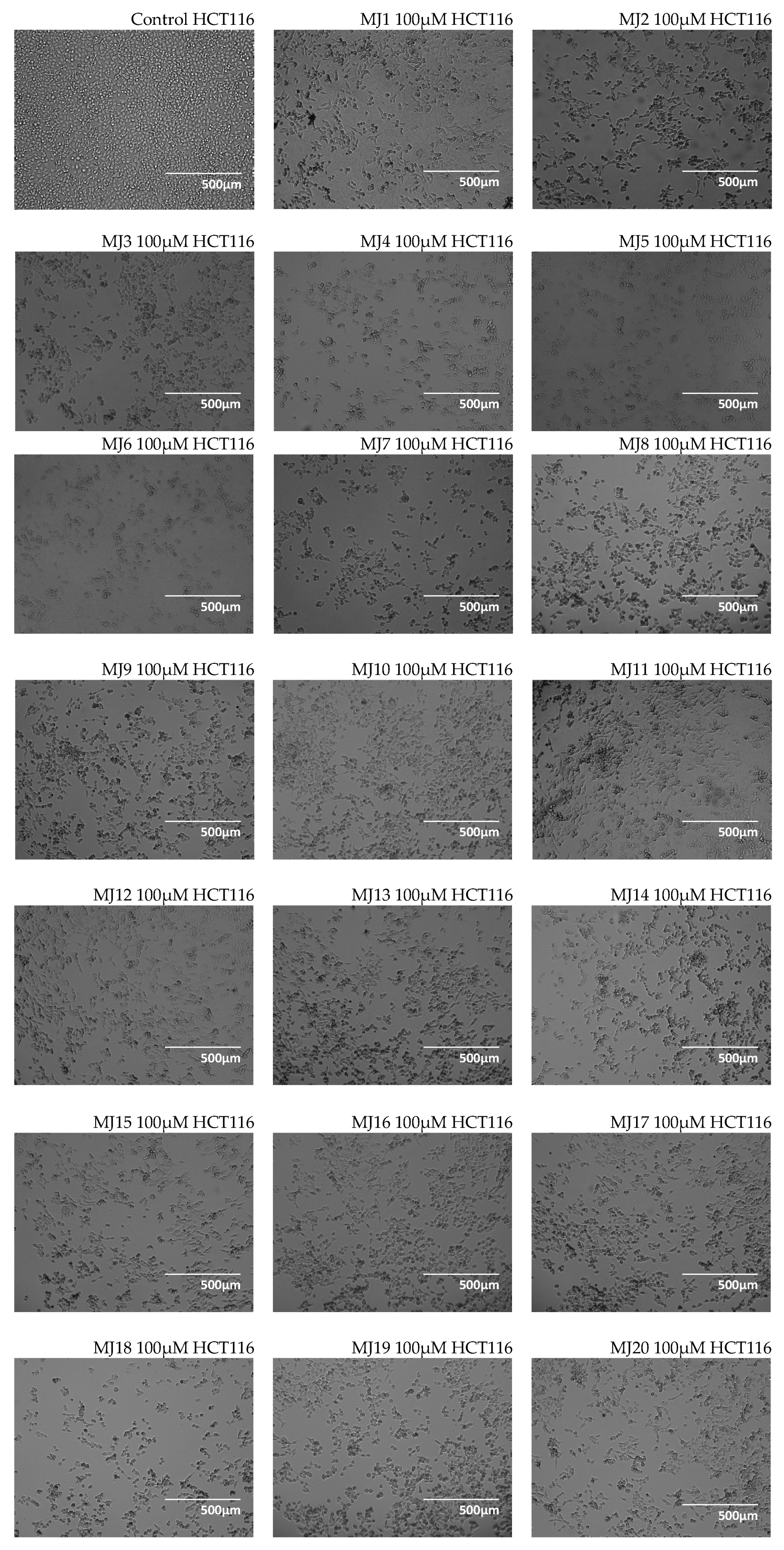
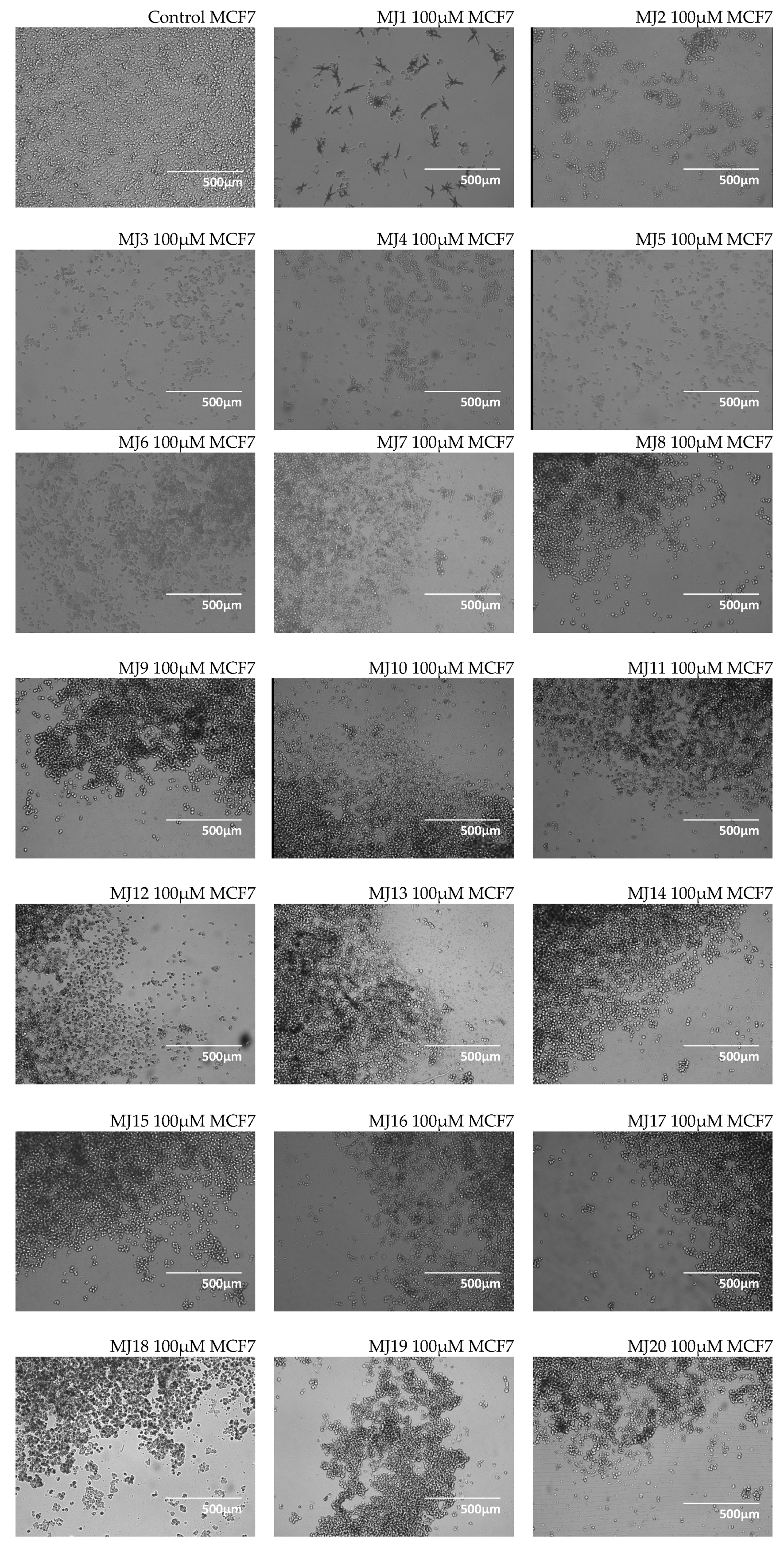
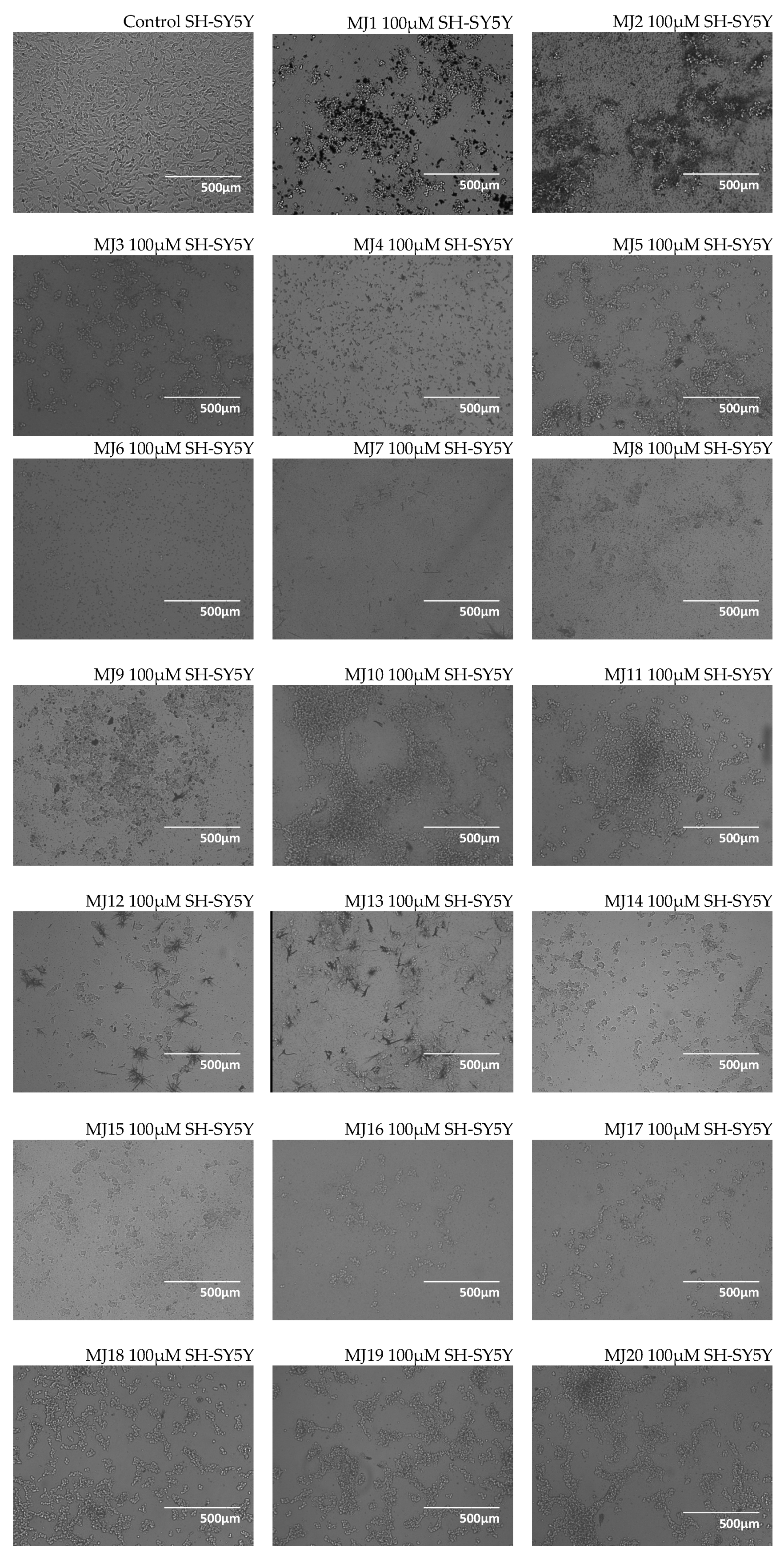
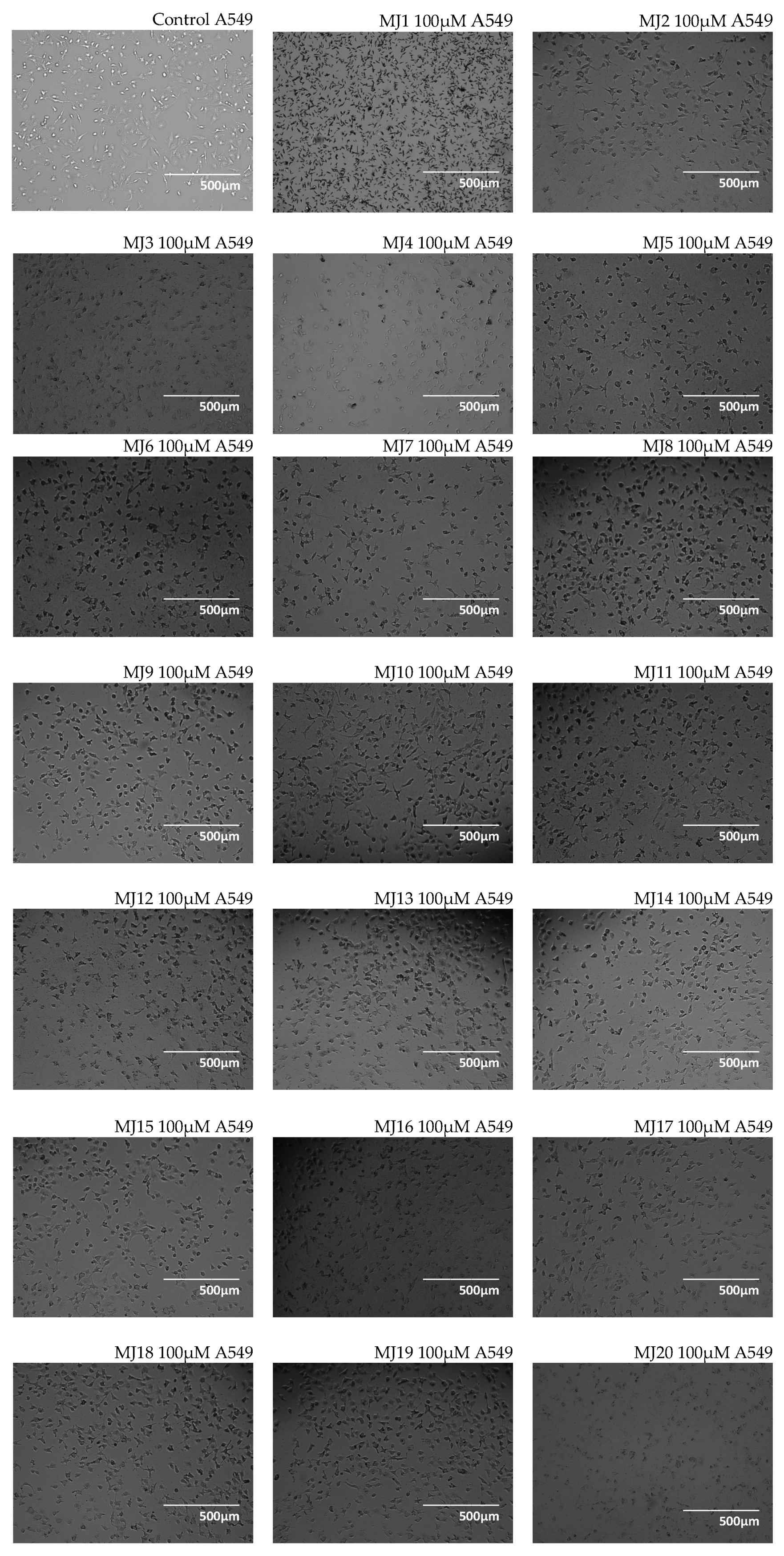
2.3. Microscopic Observations
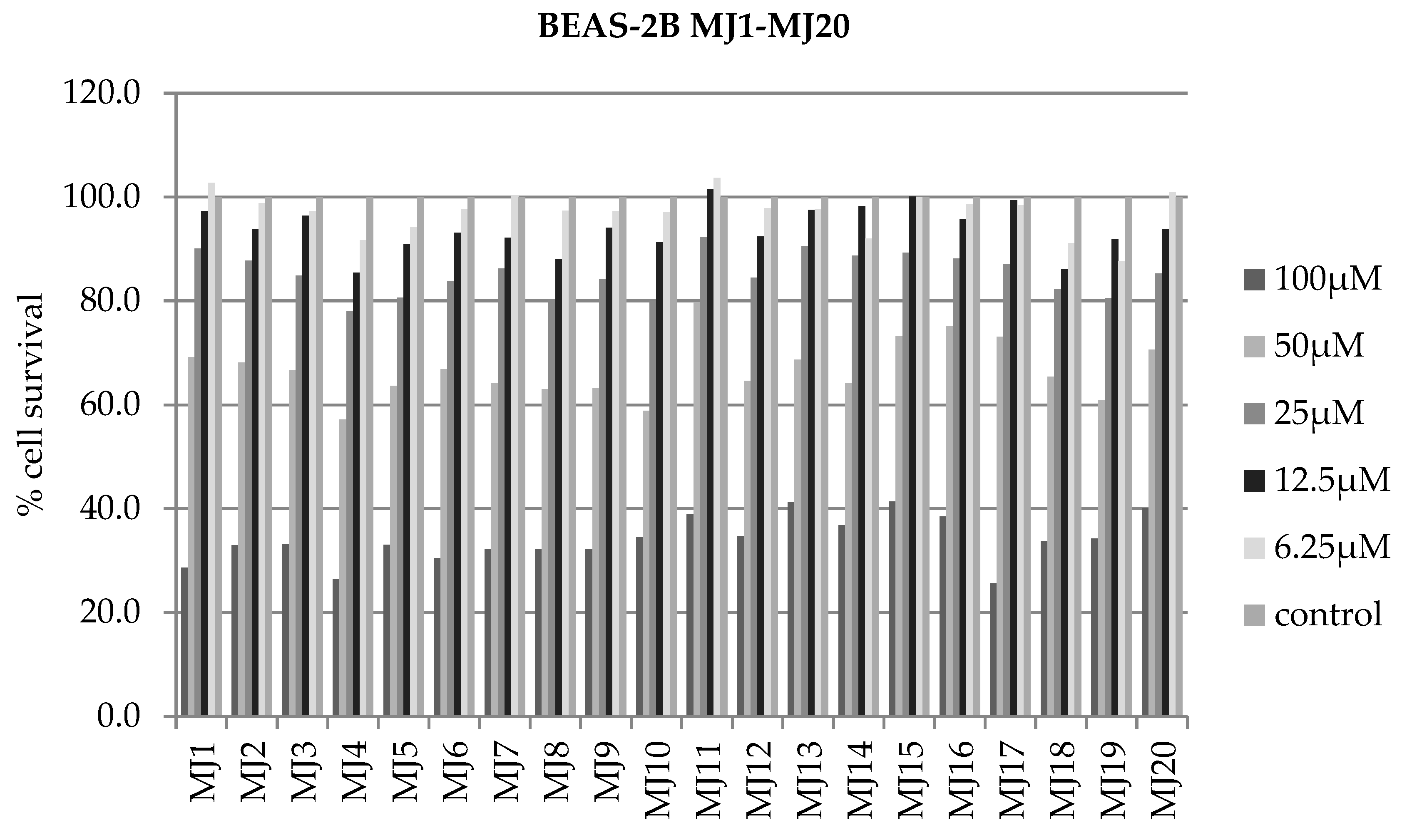
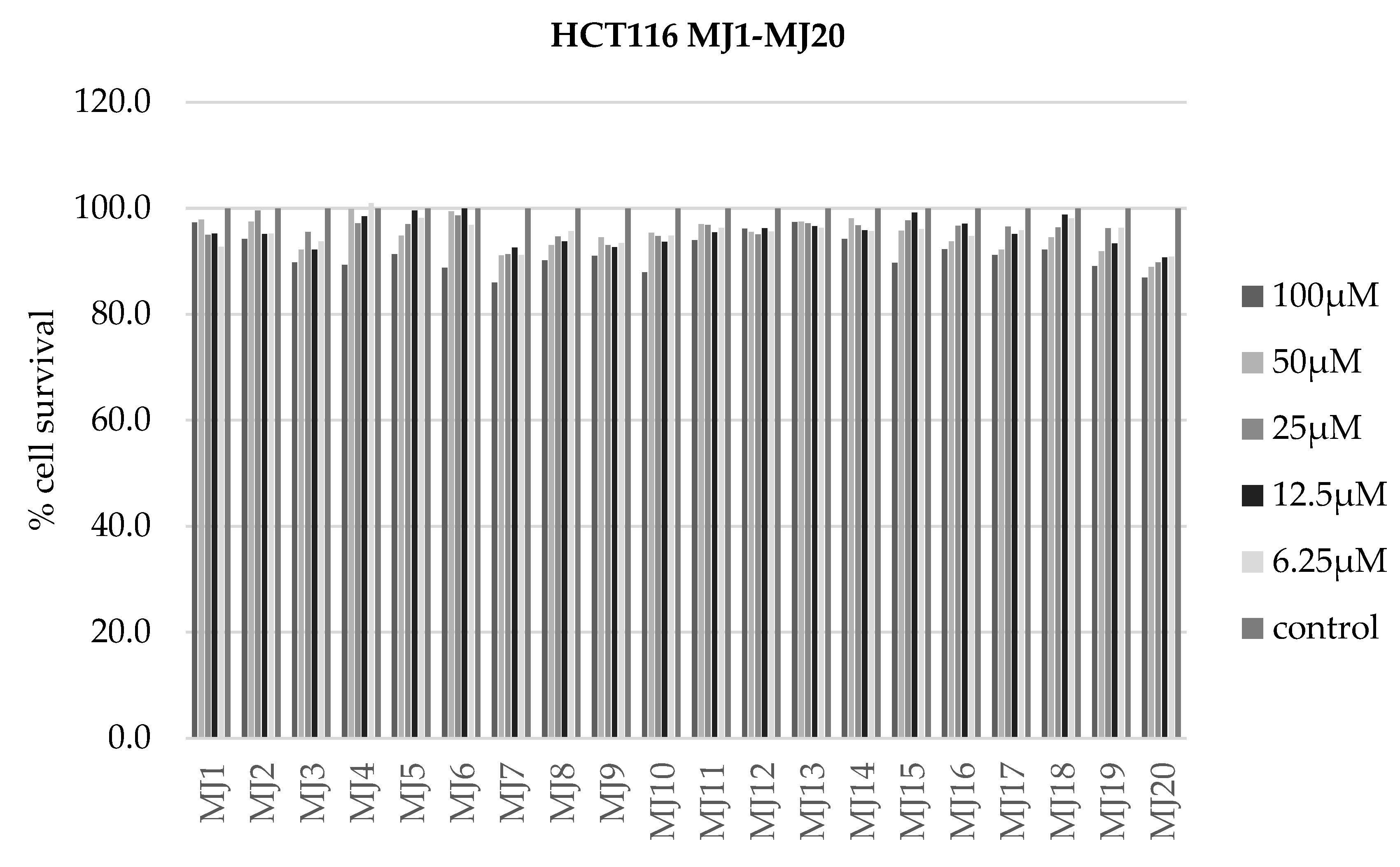
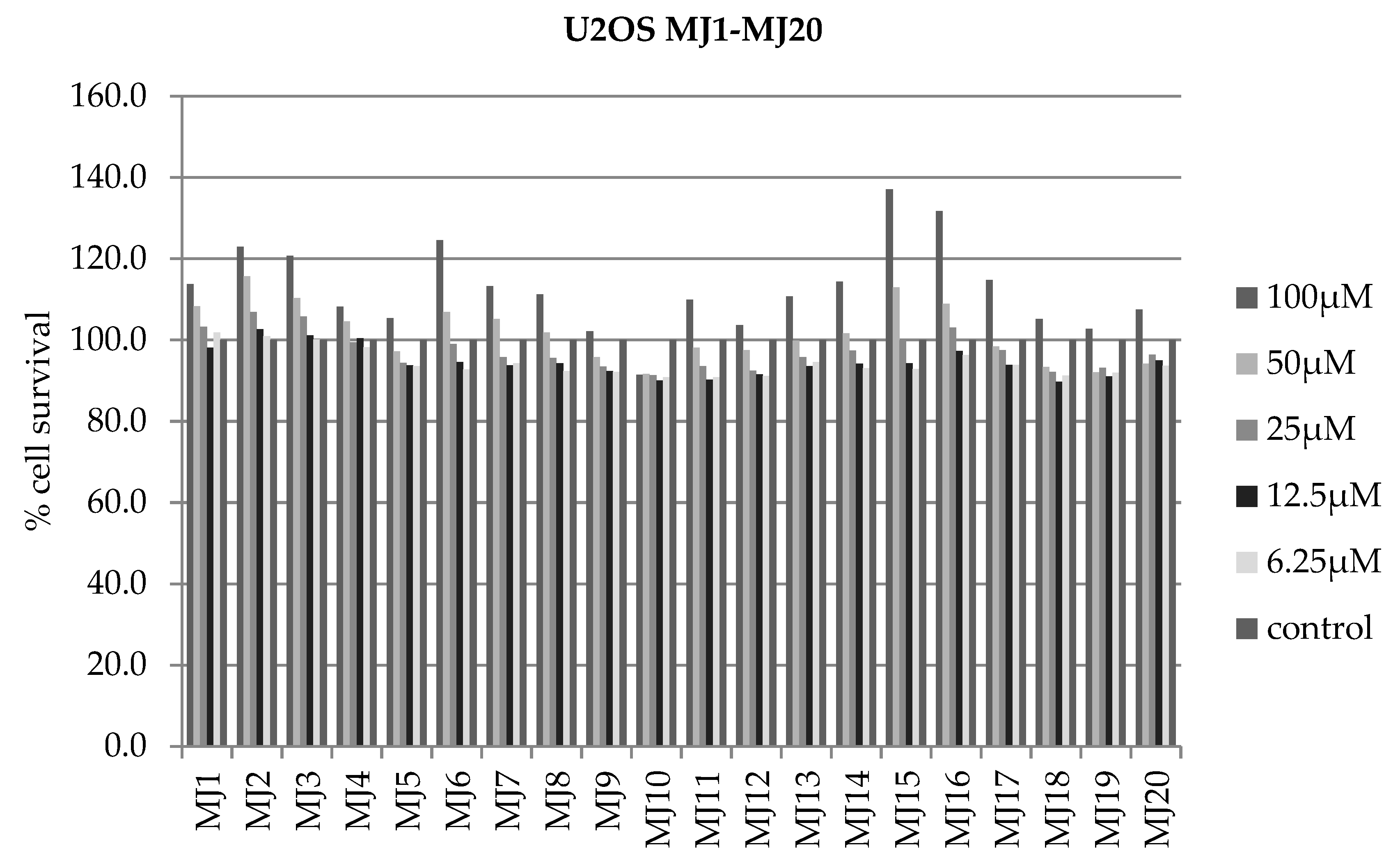
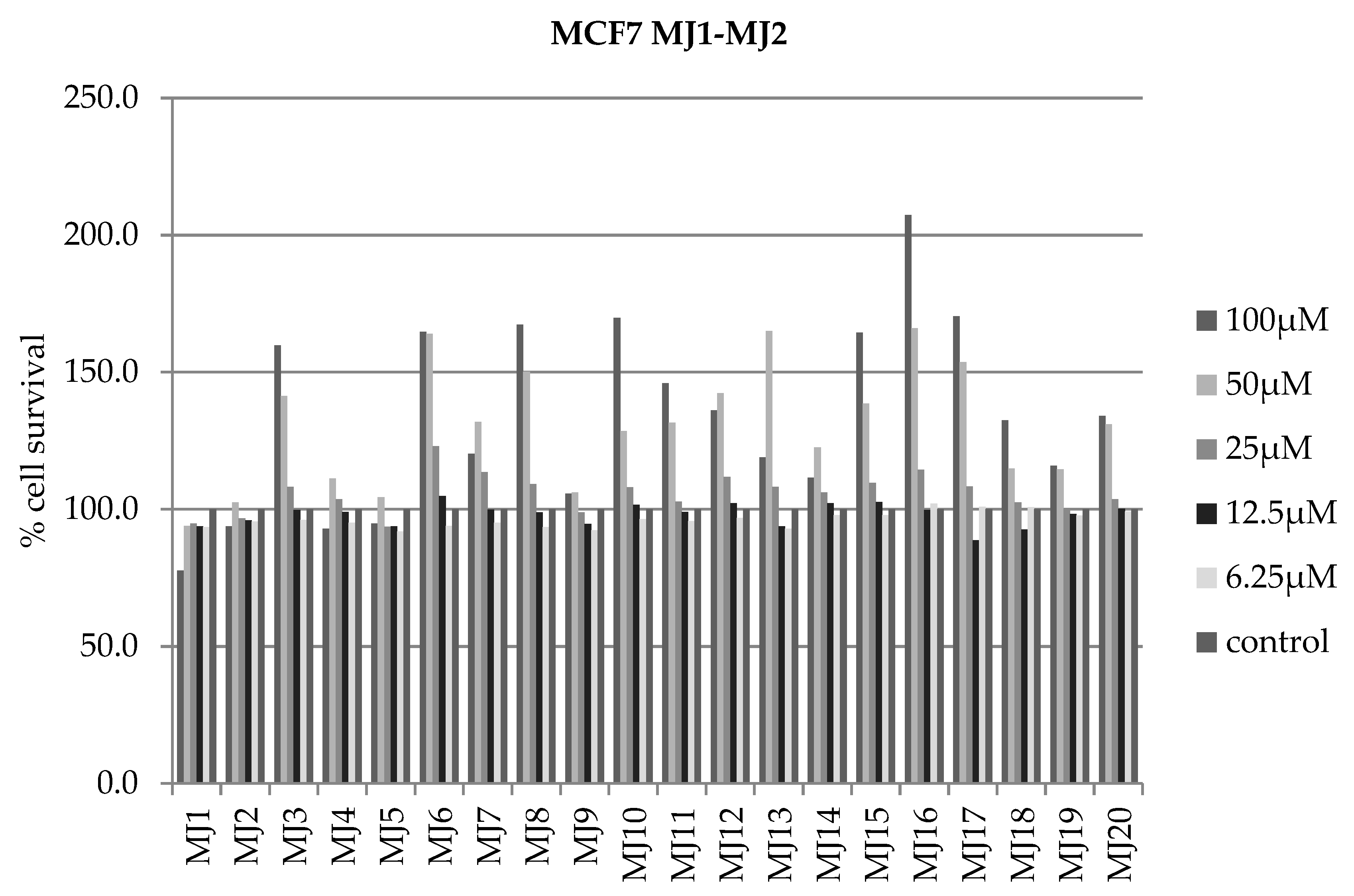
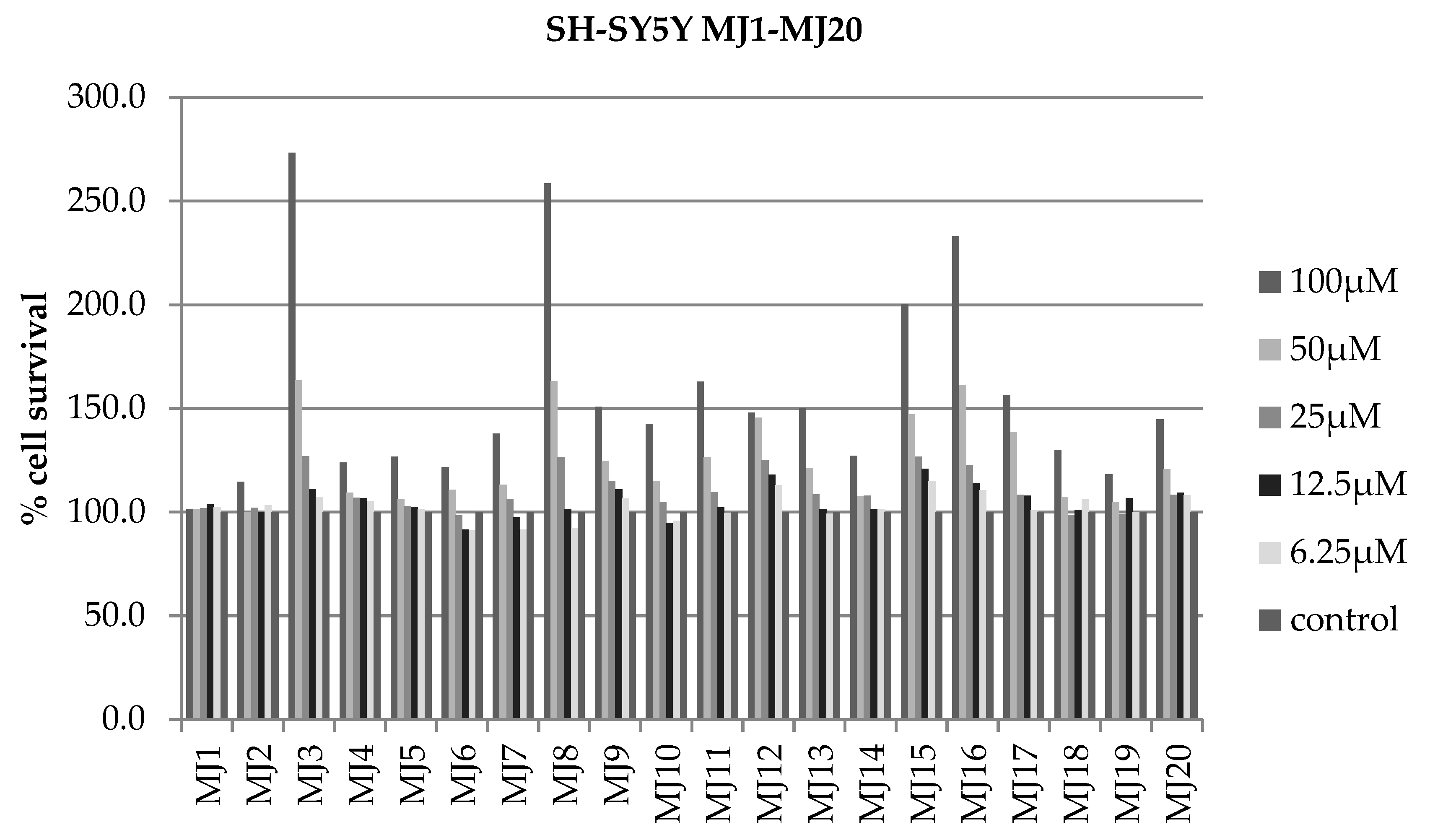
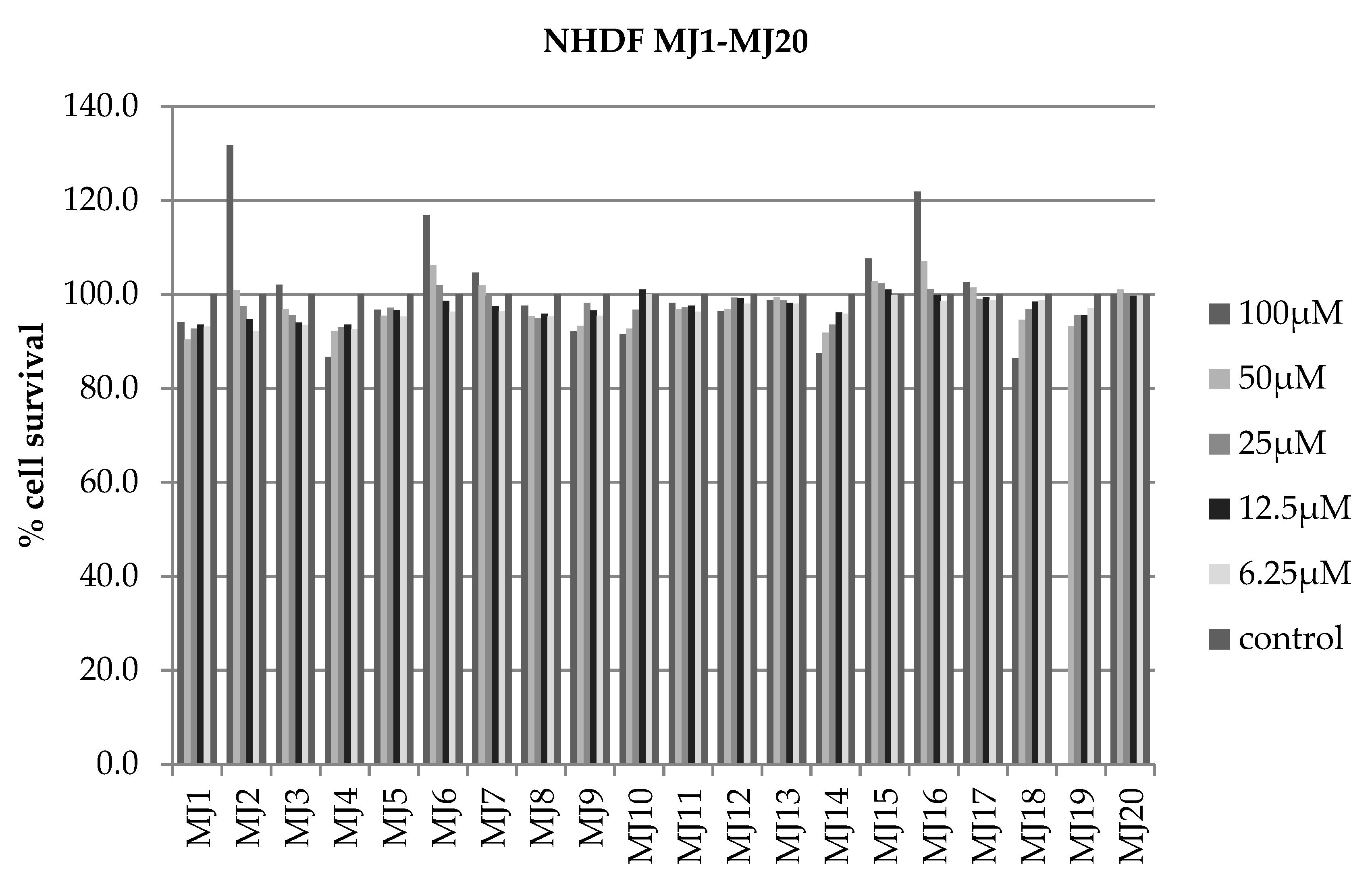
2.4. Alamar Blue Test
2.5. RT-qPCR
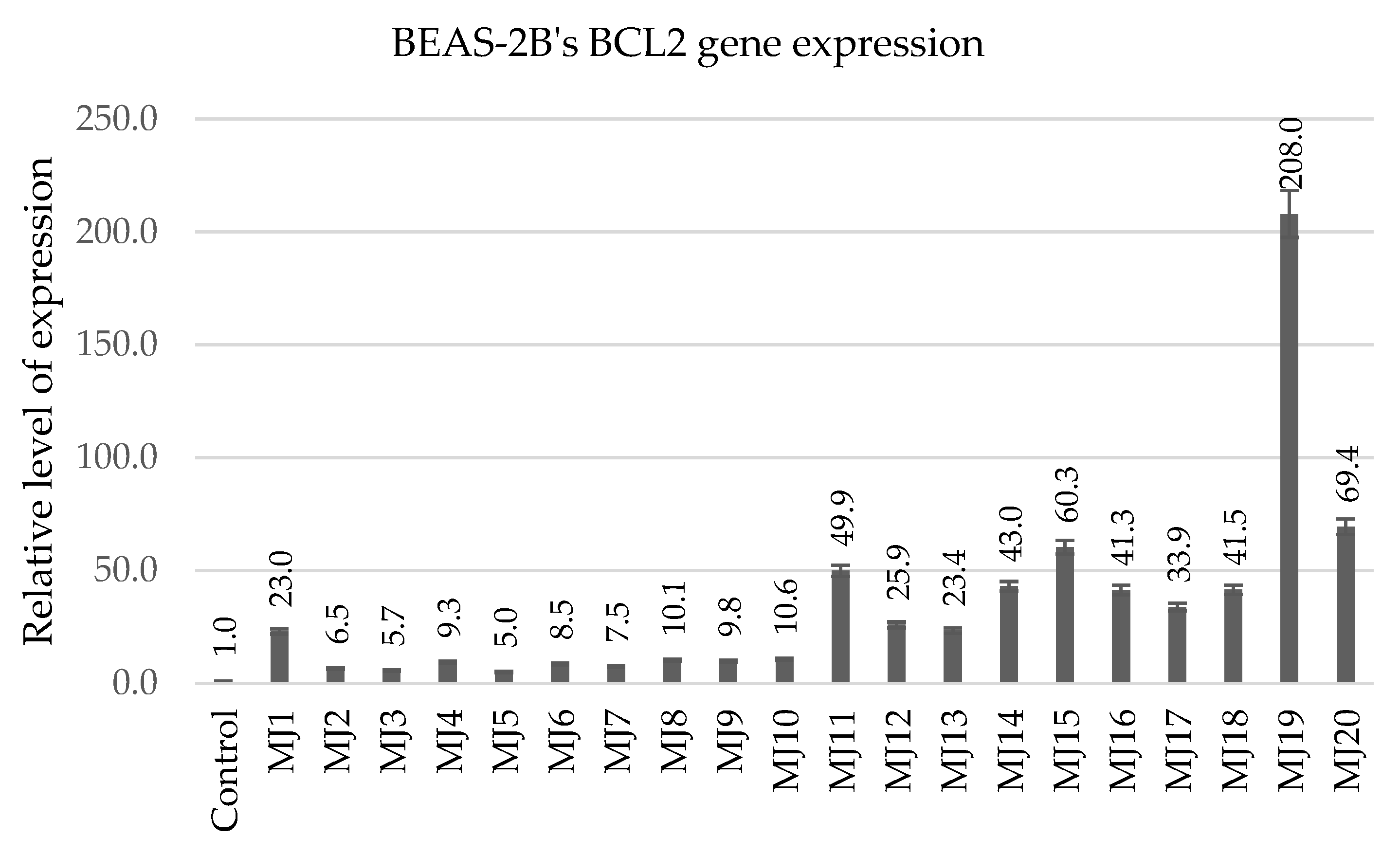
2.5.1. BEAS-2B’s BCL2 Gene Expression
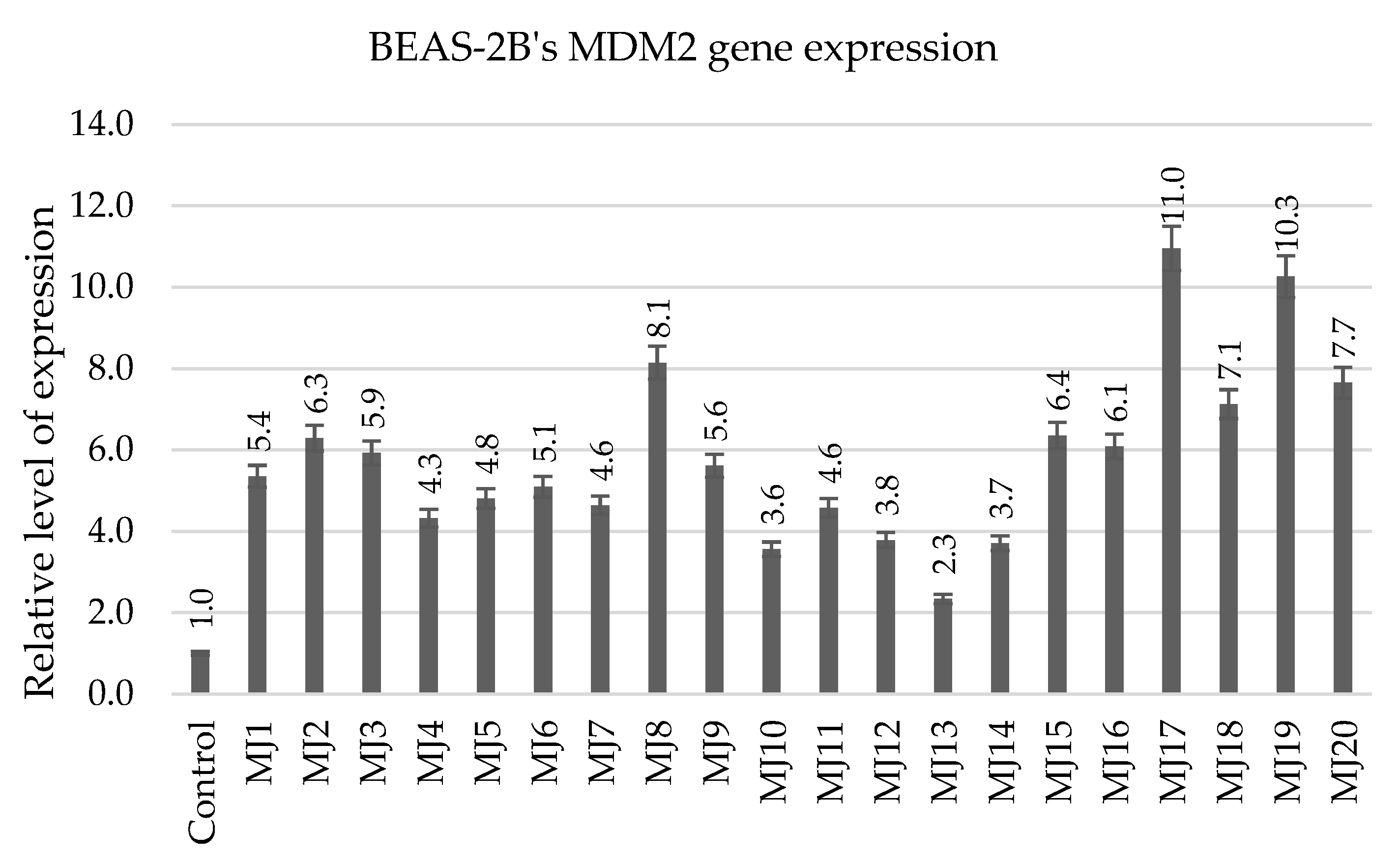
2.5.2. BEAS-2B’s MDM2 Gene Expression
2.5.3. BEAS-2B’s AIFM2 Gene Expression
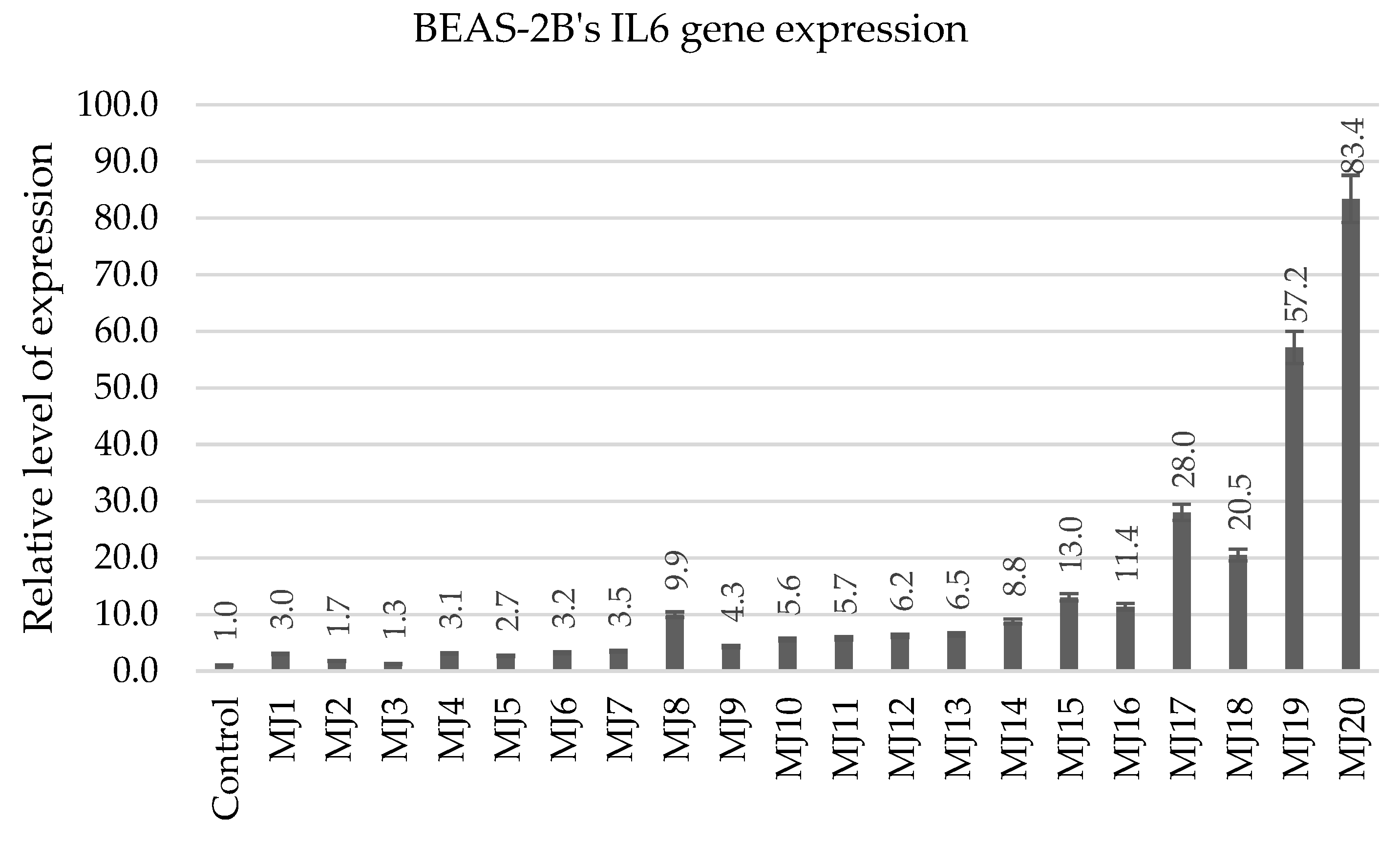
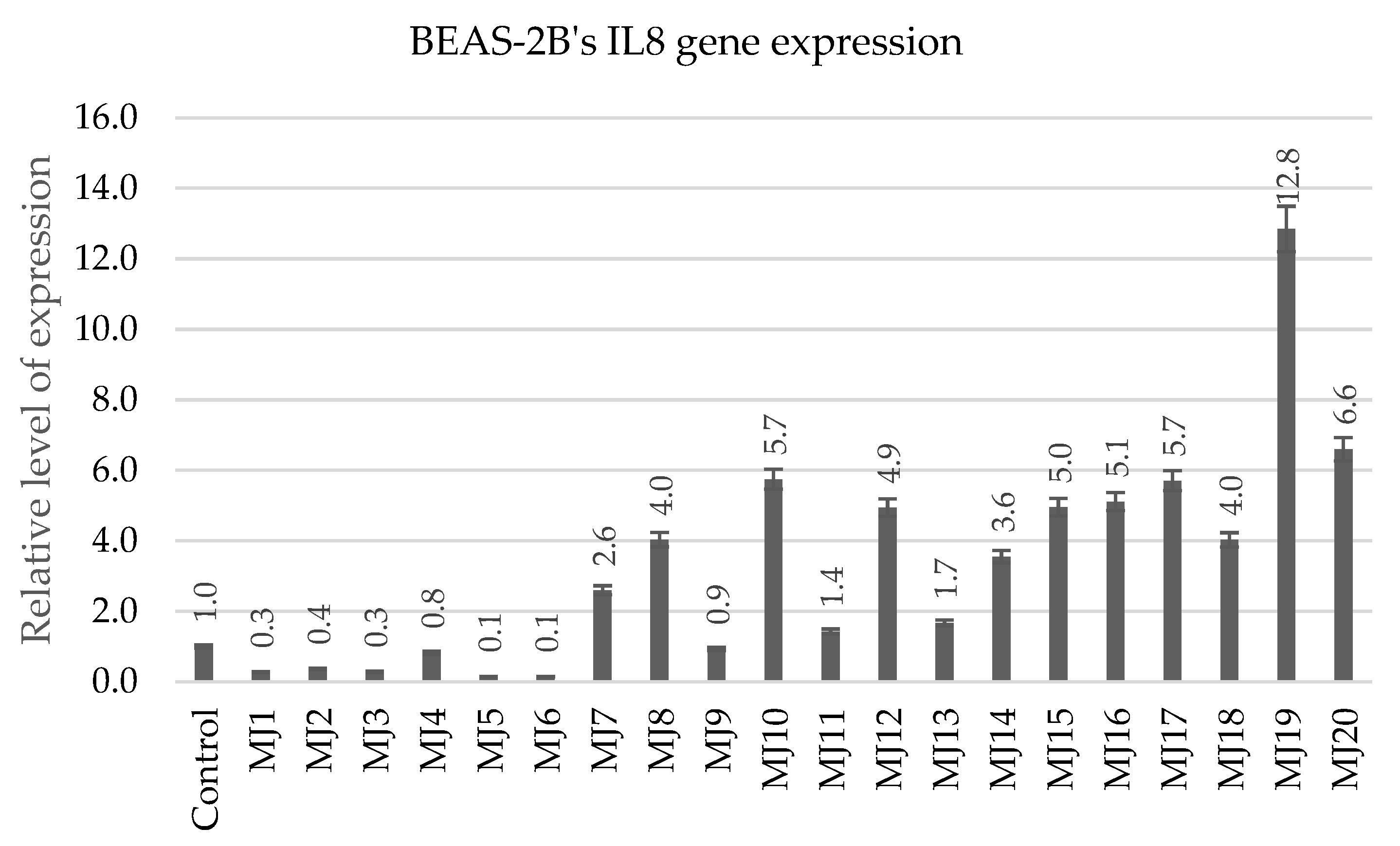
2.5.4. BEAS-2B’s IL6 and IL8 Gene Expression
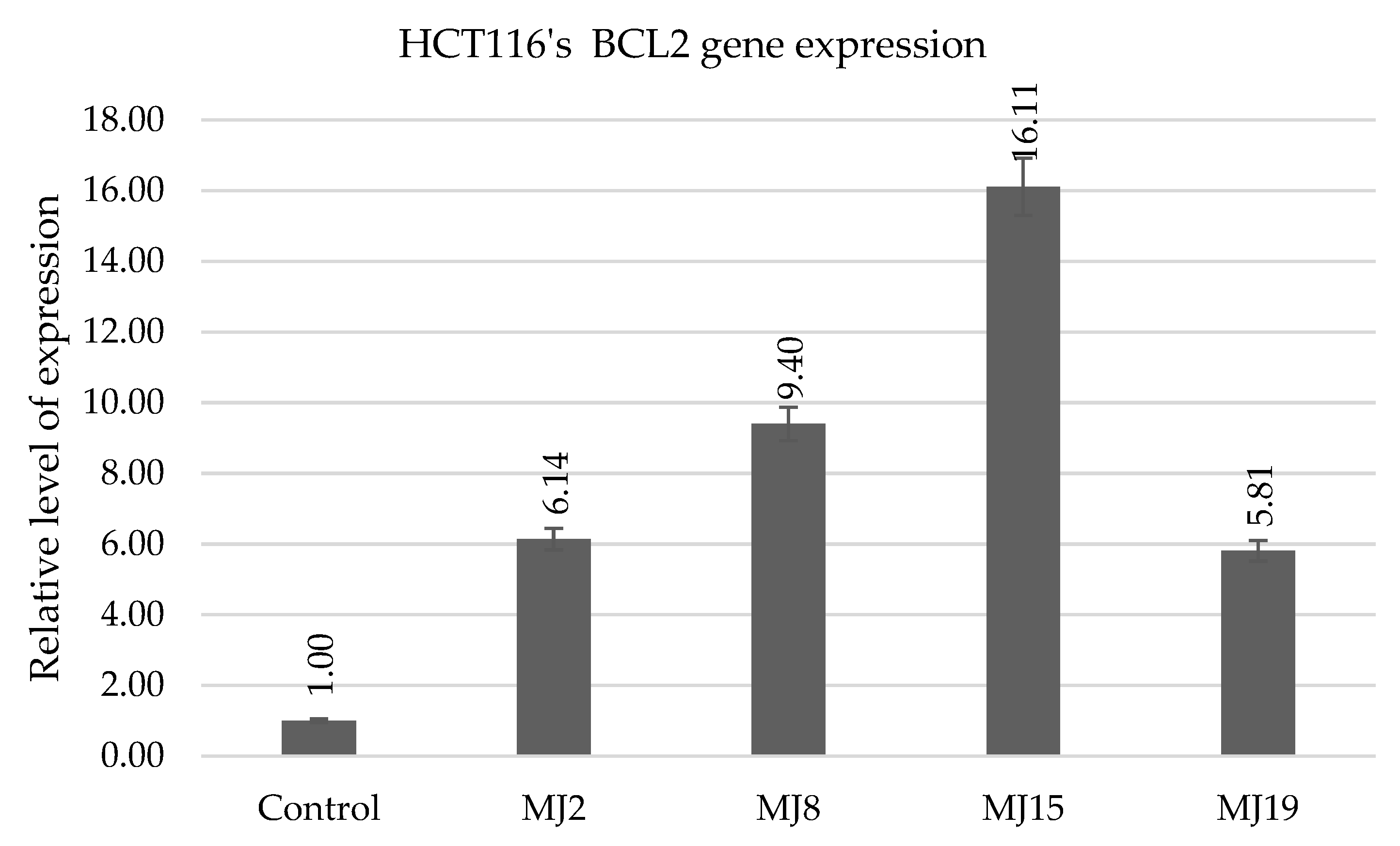
2.5.5. HCT116′s BCL2 Gene Expression
| MJ1–MJ5 | MJ6–MJ10 | MJ11–MJ15 | MJ16–MJ20 | |
|---|---|---|---|---|
| IC50 | MJ4 (64.526 µM) | MJ8 (71.858 µM) | MJ12 (75.636 µM) | MJ17 (72.595 µM) |
| BCL2 | MJ1 (23.0) | MJ10 (10.6) | MJ15 (60.3) | MJ19 (208.0) |
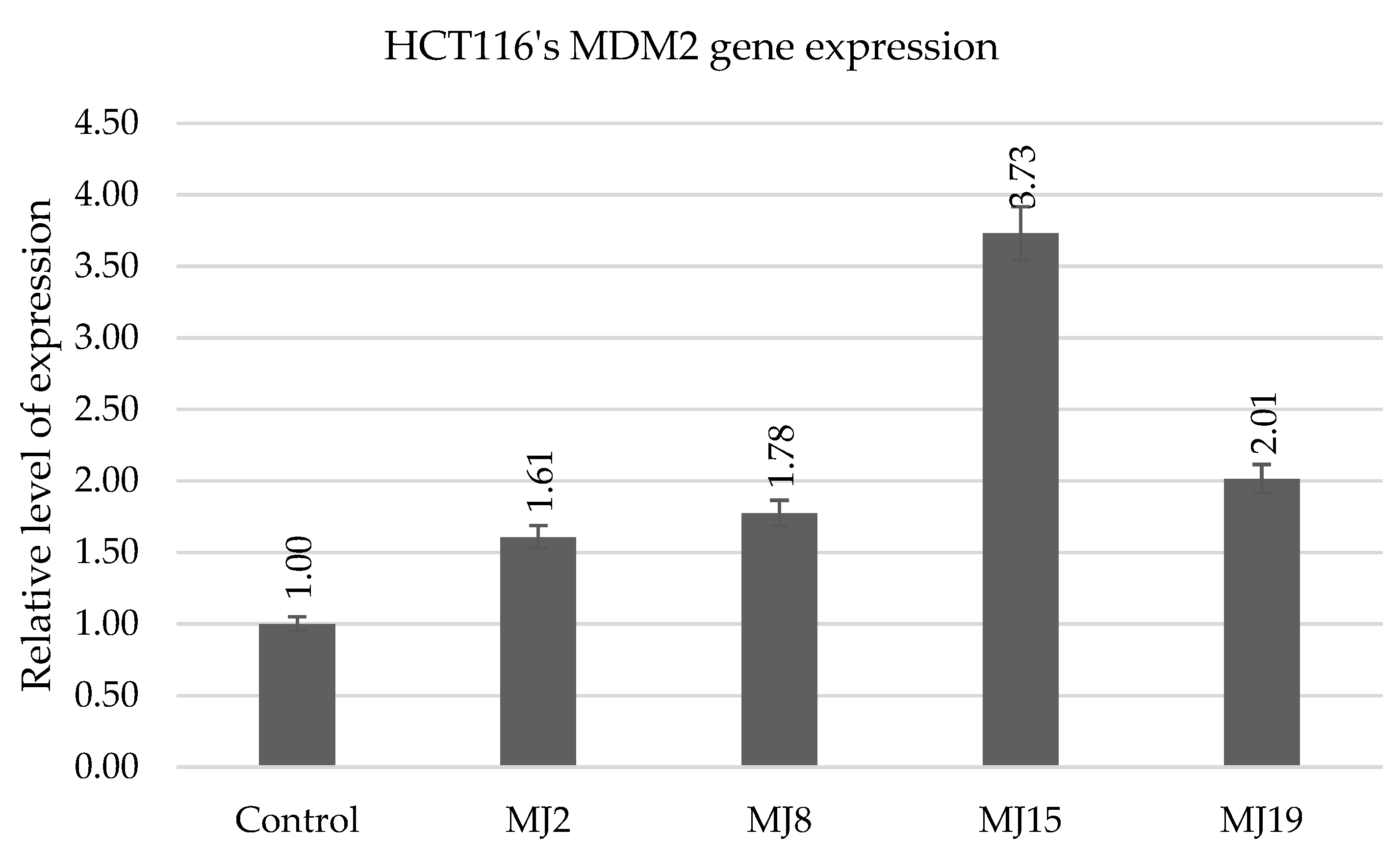
| MDM2 | MJ2 (6.3) | MJ8 (8.1) | MJ15 (6.4) | MJ17 (11.0) |
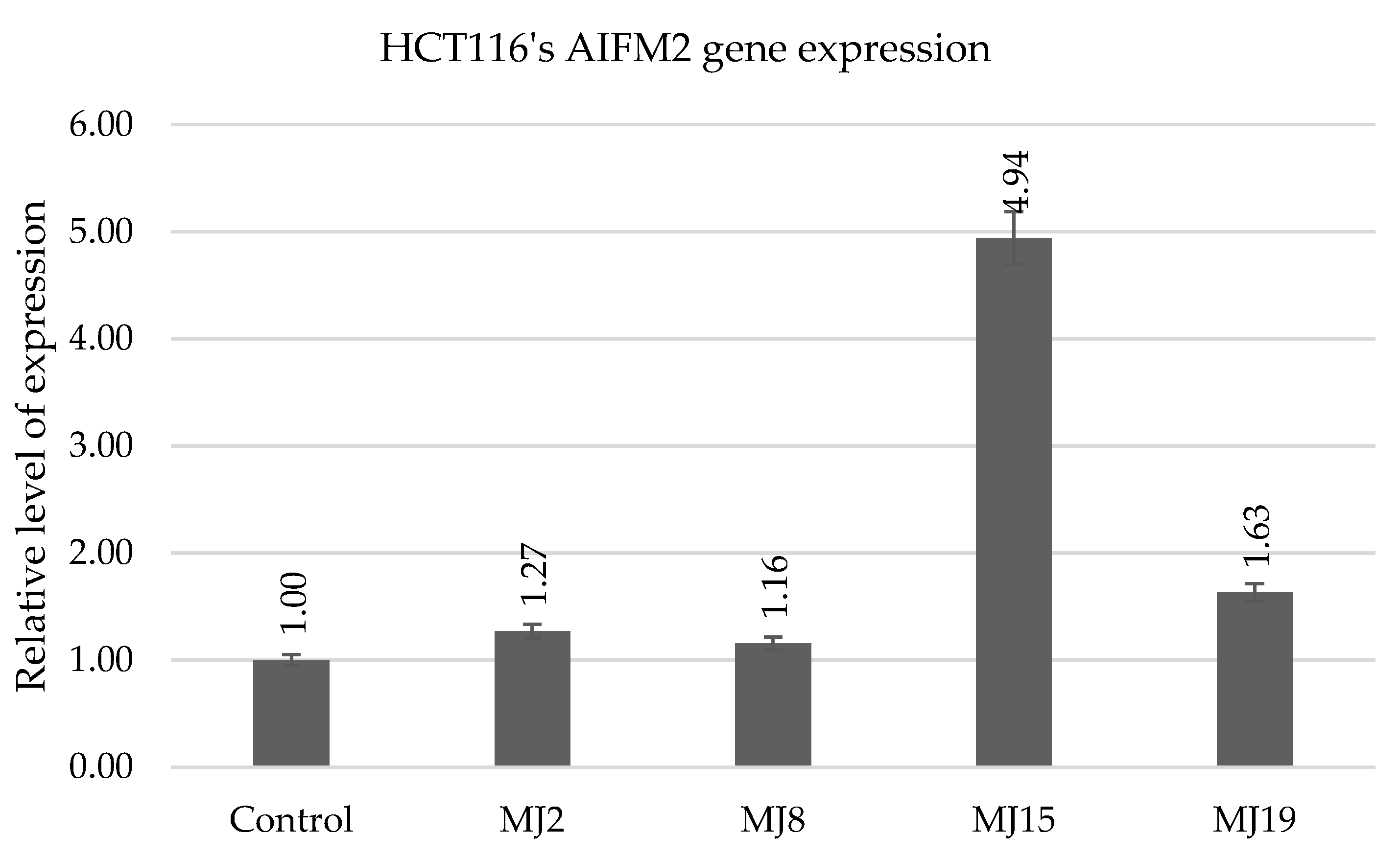
| AIFM2 | MJ2 (11.4) | MJ9 (4.5) | MJ15 (2.6) | MJ19 (14.9) |
| IL6 | MJ1 (3.0) | MJ8 (9.9) | MJ15 (13.0) | MJ20 (83.4) |
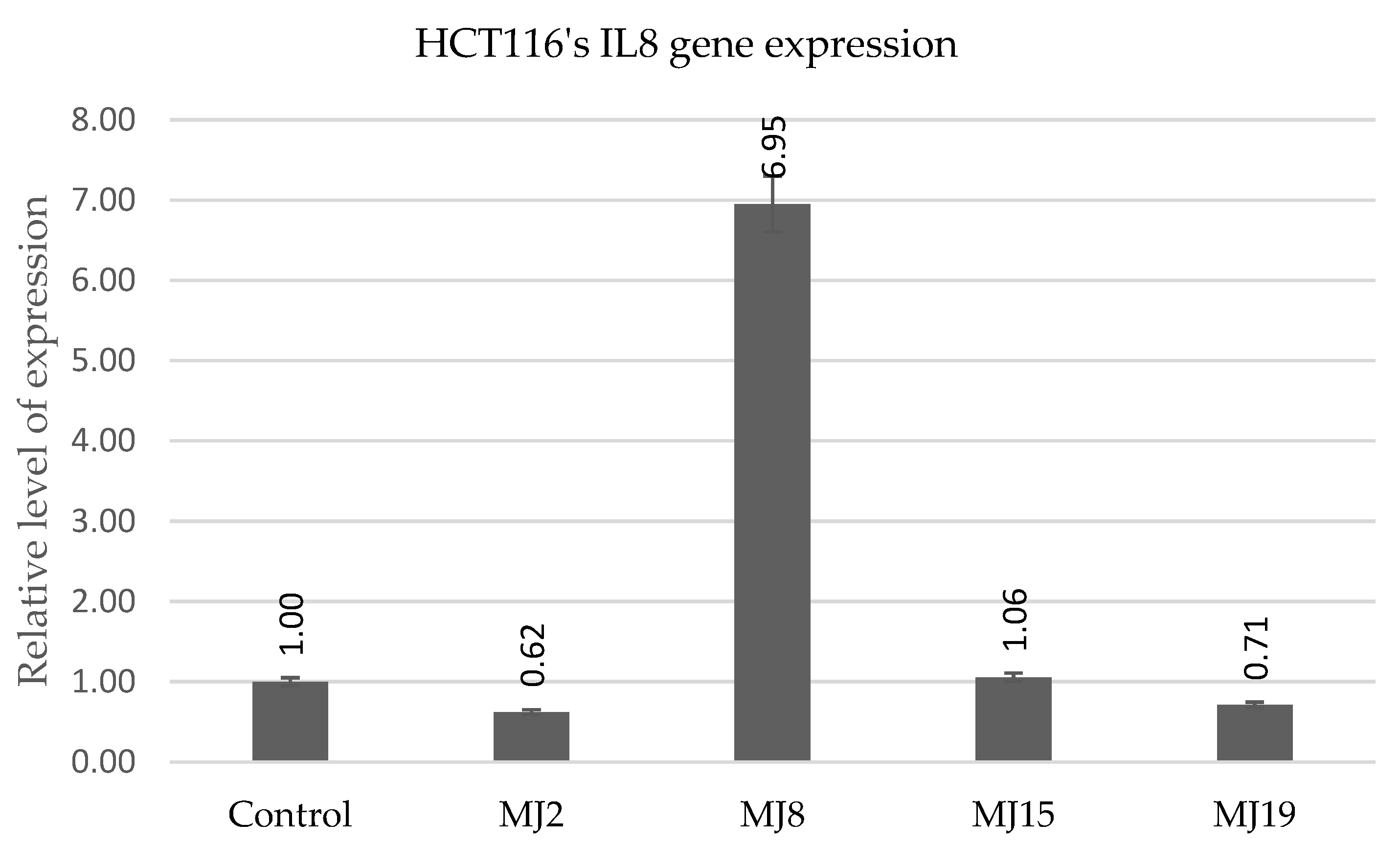
| IL8 | MJ4 (0.8) | MJ10 (5.7) | MJ15 (5.0) | MJ19 (12.8) |
2.5.6. HCT116′s MDM2 Gene Expression
2.5.7. HCT116 AIFM2
2.5.8. HCT116′s IL6 andIL8 Gene Expression
2.6. DAPI Staining for Nuclear Morphology Assessment and Apoptosis Observation
3. Materials and Methods
3.1. In Silico Study
3.2. General Procedure for the Synthesis of Compounds MJ1–MJ20
3.3. Microscopic Observations
3.4. Alamar Blue Test
3.5. RT-qPCR
3.6. DAPI Staining for Nuclear Morphology Assessment
4. Summary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIFM2 | Apoptosis-Inducing Factor Mitochondria-Associated 2 |
| A549 | Human lung adenocarcinoma cell line |
| BEAS-2B | Human bronchial epithelial cell line |
| BCL2 | B-cell CLL/lymphoma 2 |
| COSY | COrrelation SpectroscopY |
| CuAAC | Copper(I)-catalyzed Azide-Alkyne Cycloaddition |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| HCT116 | Human colon cancer cell line |
| IL6 | Interleukin 6 |
| IL8 | Interleukin 8 |
| MCF7 | Human breast cancer cell line |
| MDM2 | Mouse Double Minute 2 homolog |
| NHDF | Normal Human Dermal Fibroblasts |
| NOESY | Nuclear Overhauser effect Spectroscopy |
| PASS | Prediction of Activity Spectra for Substances |
| PDE3 | Phosphodiesterase 3 |
| RPL41 | Ribosomal Protein L41 |
| RT-qPCR | quantitative Real-Time Polymerase Chain Reaction |
| SH-SY5Y | Human neuroblastoma cell line |
| U2OS | Human osteosarcoma cell line |
References
- Posso, M.C.; Domingues, F.C.; Ferreira, S.; Silvestre, S. Development of Phenothiazine Hybrids with Potential Medicinal Interest: A Review. Molecules 2022, 27, 276. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.J.; Moosmann, B. Phenothiazine: The Seven Lives of Pharmacology’s First Lead Structure. Drug Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S. Phenothiazine: The Parent Molecule. Curr. Drug Targets 2006, 7, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Csonka, Á.; Csonka, A.; Molnár, J.; Amaral, L.; Spengler, G. Possible Biological and Clinical Applications of Phenothiazines. Anticancer. Res. 2017, 37, 5983–5993. [Google Scholar] [CrossRef]
- Mosnaim, A.D.; Ranade, V.V.; Wolf, M.E.; Puente, J.; Antonieta Valenzuela, M. Phenothiazine Molecule Provides the Basic Chemical Structure for Various Classes of Pharmacotherapeutic Agents. Am. J. Ther. 2006, 13, 261–273. [Google Scholar] [CrossRef]
- Sudeshna, G.; Parimal, K. Multiple Non-Psychiatric Effects of Phenothiazines: A Review. Eur. J. Pharmacol. 2010, 648, 6–14. [Google Scholar] [CrossRef]
- Spengler, G.; Csonka, Á.; Molnár, J.; Amaral, L. The Anticancer Activity of the Old Neuroleptic Phenothiazine-Type Drug Thioridazine. Anticancer. Res. 2016, 36, 5701–5706. [Google Scholar] [CrossRef]
- Amaral, L. Thioridazine: An Old Neuroleptic Effective against Totally Drug Resistant Tuberculosis. Acta Med. Port. 2012, 25, 118–121. [Google Scholar]
- Abbruzzese, C.; Matteoni, S.; Persico, M.; Villani, V.; Paggi, M.G. Repurposing Chlorpromazine in the Treatment of Glioblastoma Multiforme: Analysis of Literature and Forthcoming Steps. J. Exp. Clin. Cancer Res. 2020, 39, 26. [Google Scholar] [CrossRef]
- Grobs, Y.; Awada, C.; Lemay, S.-E.; Romanet, C.; Bourgeois, A.; Toro, V.; Nadeau, V.; Shimauchi, K.; Orcholski, M.; Breuils-Bonnet, S.; et al. Preclinical Investigation of Trifluoperazine as a Novel Therapeutic Agent for the Treatment of Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 2919. [Google Scholar] [CrossRef]
- Rácz, B.; Spengler, G. Repurposing Antidepressants and Phenothiazine Antipsychotics as Efflux Pump Inhibitors in Cancer and Infectious Diseases. Antibiotics 2023, 12, 137. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Antipsychotic Drug Fluphenazine against Human Cancer Cells. Biomolecules 2022, 12, 1360. [Google Scholar] [CrossRef] [PubMed]
- Cibotaru, S.; Sandu, A.-I.; Nicolescu, A.; Marin, L. Antitumor Activity of PEGylated and TEGylated Phenothiazine Derivatives: Structure–Activity Relationship. Int. J. Mol. Sci. 2023, 24, 5449. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, M.; Morak-Młodawska, B.; Korlacki, R. Anticancer Activities of Tetra-, Penta-, and Hexacyclic Phenothiazines Modified with Quinoline Moiety. J. Mol. Struct. 2023, 1287, 135700. [Google Scholar] [CrossRef]
- Haidasz, E.A.; Pratt, D.A. Diazaphenoxazines and Diazaphenothiazines: Synthesis of the “Correct” Isomers Reveals They Are Highly Reactive Radical-Trapping Antioxidants. Org. Lett. 2017, 19, 1854–1857. [Google Scholar] [CrossRef]
- Kushwaha, K.; Buchanan, D.; Nkosi, A.; Jangra, J.; Kumar, R.; Singh, S.K.; Jain, S.C.; Panda, S.S. Azaphenothiazine Conjugates: A New Class of Antimicrobial Compounds. Chem. Biodivers. 2025, 22, e202403338. [Google Scholar] [CrossRef]
- Brosend, S.C.; Guin, S.; Giovine, G.; Gadalla, C.; Campos, M.A.; Mara, A.; Jentsch, N.G.; Thakellapalli, H.; Alden, K.M.; Beattie, S.R.; et al. Antifungal Structure-Activity Relationship Studies of Broad-Spectrum Phenothiazines. ACS Omega 2025, 10, 18347–18355. [Google Scholar] [CrossRef]
- Pooryaghoobi, N.; Bakavoli, M.; Alimardani, M.; Bazzazan, T.; Sadeghian, H. New Insight into the SAR of Pyrimido [4,5-b][1,4] Benzothiazines as 15-Lipoxygenase Inhibitors. Iran J. Basic Med. Sci. 2013, 16, 784–789. [Google Scholar]
- Podsiadły, R.; Strzelczyk, R. N-Substituted Quinoxalinobenzothiazine/Iodonium Salt Systems as Visible Photoinitiators for Hybrid Polymerization. Dye. Pigment. 2013, 97, 462–468. [Google Scholar] [CrossRef]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Recent Progress in Biological Activities of Synthesized Phenothiazines. Eur. J. Med. Chem. 2011, 46, 3179–3189. [Google Scholar] [CrossRef]
- Kumar, A.; Vigato, C.; Boschi, D.; Lolli, M.L.; Kumar, D. Phenothiazines as Anti-Cancer Agents: SAR Overview and Synthetic Strategies. Eur. J. Med. Chem. 2023, 254, 115337. [Google Scholar] [CrossRef]
- Lodarski, K.; Jończyk, J.; Guzior, N.; Bajda, M.; Gładysz, J.; Walczyk, J.; Jeleń, M.; Morak-Młodawska, B.; Pluta, K.; Malawska, B. Discovery of Butyrylcholinesterase Inhibitors among Derivatives of Azaphenothiazines. J. Enzym. Inhib. Med. Chem. 2015, 30, 98–106. [Google Scholar] [CrossRef][Green Version]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal Attributes of 1,2,3-Triazoles: Current Developments. Bioorganic Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Praveena, K.S.S.; Murthy, N.Y.S.; Pal, S. Syntheses and Biological Activities of 1,4-Disubstituted-1,2,3-Triazoles. J. Chem. Pharm. Res. 2015, 7, 506–522. [Google Scholar]
- Gonnet, L.; Baron, M.; Baltas, M. Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry. Molecules 2021, 26, 5667. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-Triazole Ring as a Bioisostere in Medicinal Chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Totobenazara, J.; Burke, A.J. New Click-Chemistry Methods for 1,2,3-Triazoles Synthesis: Recent Advances and Applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Sharma, J.; Ahmad, S.; Alam, M.S. Bioactive Triazoles: A Potential Review. J. Chem. Pharm. Res. 2012, 4, 5157–5164. [Google Scholar]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A Promising Antitubercular Agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef]
- Khandelwal, R.; Vasava, M.; Abhirami, R.B.; Karsharma, M. Recent Advances in Triazole Synthesis via Click Chemistry and Their Pharmacological Applications: A Review. Bioorganic Med. Chem. Lett. 2024, 112, 129927. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorganic Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, Z.; Zhang, J.; Jin, J. Antitumor Evaluation of Novel Phenothiazine Derivatives That Inhibit Migration and Tubulin Polymerization against Gastric Cancer MGC-803 Cells. Investig. New Drugs 2019, 37, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Guo, J.-M.; Zhang, T.-T.; Lin, H.-J.; Qi, N.-S.; Li, Z.-G.; Zhou, J.-C.; Zhang, Z.-Z. Antiproliferative Phenothiazine Hybrids as Novel Apoptosis Inducers against MCF-7 Breast Cancer. Molecules 2018, 23, 1288. [Google Scholar] [CrossRef]
- Ma, X.-H.; Liu, N.; Lu, J.-L.; Zhao, J.; Zhang, X.-J. Design, Synthesis and Antiproliferative Activity of Novel Phenothiazine-1,2,3-Triazole Analogues. J. Chem. Res. 2017, 41, 696–698. [Google Scholar] [CrossRef]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M. Anticancer Activity of Newly Synthesized Azaphenothiazines from NCI’s Anticancer Screening Bank. Pharmacol. Rep. 2010, 62, 319–332. [Google Scholar] [CrossRef]
- Zimecki, M.; Artym, J.; Kocięba, M.; Pluta, K.; Morak-Młodawska, B.; Jeleń, M. The Immunosuppressive Activities of Newly Synthesized Azaphenothiazines in Human and Mouse Models. Cell. Mol. Biol. Lett. 2009, 14, 622–635. [Google Scholar] [CrossRef]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M. Synthesis and Selected Immunological Properties of Substituted Quino[3,2-b]Benzo[1,4]Thiazines. Eur. J. Med. Chem. 2013, 63, 444–456. [Google Scholar] [CrossRef]
- Mehrabi, S.F.; Elmi, S.; Nylandsted, J. Repurposing Phenothiazines for Cancer Therapy: Compromising Membrane Integrity in Cancer Cells. Front. Oncol. 2023, 13, 1320621. [Google Scholar] [CrossRef]
- Zou, X.; Xie, B. Therapeutic Mechanisms of Phenothiazine Drugs: A Mini-Review of Advances in Cancer Treatment and Antibiotic Resistance. Iran J. Pharm. Res. 2025, 24, e157923. [Google Scholar] [CrossRef]
- Otręba, M.; Stojko, J.; Rzepecka-Stojko, A. Phenothiazine Derivatives and Their Impact on the Necroptosis and Necrosis Processes. A Review. Toxicology 2023, 492, 153528. [Google Scholar] [CrossRef]
- Nag, S.; Qin, J.; Srivenugopal, K.S.; Wang, M.; Zhang, R. The MDM2-P53 Pathway Revisited. J. Biomed. Res. 2013, 27, 254–271. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.L.; Xiang, Y.; Bai, X.Y.; Qiang, R.R.; Zhang, X.; Yang, Y.L.; Liu, X.L. Ferroptosis Inhibitors: Past, Present and Future. Front. Pharmacol. 2024, 15, 1407335. [Google Scholar] [CrossRef] [PubMed]
- De Sena Murteira Pinheiro, P.; Franco, L.S.; Montagnoli, T.L.; Fraga, C.A.M. Molecular Hybridization: A Powerful Tool for Multitarget Drug Discovery. Expert Opin. Drug Discov. 2024, 19, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, G. An Overview of Molecular Hybrids in Drug Discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Cavalli, A.; Valgimigli, L.; Bartolini, M.; Rosini, M.; Andrisano, V.; Recanatini, M.; Melchiorre, C. Multi-Target-Directed Drug Design Strategy: From a Dual Binding Site Acetylcholinesterase Inhibitor to a Trifunctional Compound against Alzheimer’s Disease. J. Med. Chem. 2007, 50, 6446–6449. [Google Scholar] [CrossRef]
- Alkhzem, A.H.; Woodman, T.J.; Blagbrough, I.S. Design and Synthesis of Hybrid Compounds as Novel Drugs and Medicines. RSC Adv. 2022, 12, 19470–19484. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Pluta, K.; Jeleń, M. Synthesis of Quinobenzo-1,4-Thiazines from Diquino-1,4-Dithiin and 2,2′-Dichloro-3,3′-Diquinolinyl Disulfide. Heterocycles 2009, 78, 2325. [Google Scholar] [CrossRef]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M.; Kochanowska, I. Synthesis and Biological Evaluation of Novel Propargylquinobenzothiazines and Their Derivatives as Potential Antiproliferative, Anti-Inflammatory, and Anticancer Agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 83–88. [Google Scholar] [CrossRef]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M. 6-Substituted 9-Fluoroquino[3,2-b]Benzo[1,4]Thiazines Display Strong Antiproliferative and Antitumor Properties. Eur. J. Med. Chem. 2015, 89, 411–420. [Google Scholar] [CrossRef]
- Way2Drug—Main. Available online: https://www.way2drug.com/PassOnline/ (accessed on 7 June 2025).
- Monsinjon, T.; Gasque, P.; Chan, P.; Ischenko, A.; Brady, J.J.; Fontaine, M.C. Regulation by Complement C3a and C5a Anaphylatoxins of Cytokine Production in Human Umbilical Vein Endothelial Cells. FASEB J. 2003, 17, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Klos, A.; Tenner, A.J.; Johswich, K.-O.; Ager, R.R.; Reis, E.S.; Köhl, J. The Role of the Anaphylatoxins in Health and Disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Mastellos, D.; Papadimitriou, J.C.; Franchini, S.; Tsonis, P.A.; Lambris, J.D. A Novel Role of Complement: Mice Deficient in the Fifth Component of Complement (C5) Exhibit Impaired Liver Regeneration. J. Immunol. 2001, 166, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.-Z.; Xue, Q.; Shao, H. Inflammatory Markers Related to Innate and Adaptive Immunity in Atherosclerosis: Implications for Disease Prediction and Prospective Therapeutics. J. Inflamm. Res. 2021, 14, 379–392. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lee, S.-B.; Lee, H.-S.; Lee, S.-Y.; Baek, J.-S.; Kim, D.; Moon, T.-W.; Robyt, J.F.; Park, K.-H. Comparative Study of the Inhibition of α-Glucosidase, α-Amylase, and Cyclomaltodextrin Glucanosyltransferase by Acarbose, Isoacarbose, and Acarviosine–Glucose. Arch. Biochem. Biophys. 1999, 371, 277–283. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in Cancer Immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef]
- Kandimalla, R.; Reddy, P.H. Therapeutics of Neurotransmitters in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1049–1069. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Comp. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
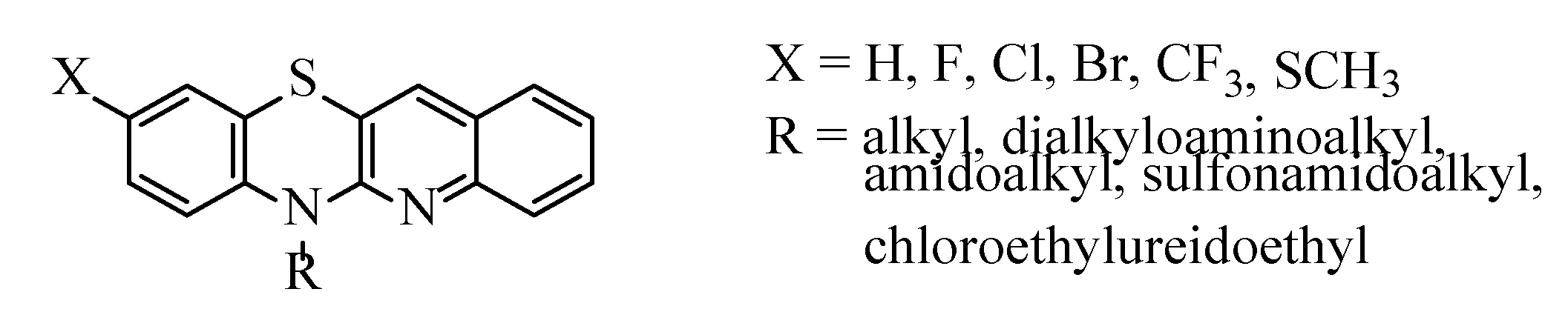



| Pa | Pi | Activity | Pa | Pi | Activity | ||
|---|---|---|---|---|---|---|---|
| MJ1 | 0.746 | 0.018 | Anaphylatoxin receptor antagonist | MJ11 | 0.702 | 0.023 | Anaphylatoxin receptor antagonist |
| 0.681 | 0.041 | Glycosylphosphatidylinositol phospholipase D inhibitor | 0.694 | 0.037 | Glycosylphosphatidylinositol phospholipase D inhibitor | ||
| 0.574 | 0.011 | Atherosclerosis treatment | 0.531 | 0.015 | Atherosclerosis treatment | ||
| 0.503 | 0.027 | Autoimmune disorders treatment | 0.484 | 0.031 | Autoimmune disorders treatment | ||
| 0.493 | 0.004 | Leukotriene synthesis inhibitor | 0.460 | 0.004 | Leukotriene synthesis inhibitor | ||
| MJ2 | 0.665 | 0.029 | Anaphylatoxin receptor antagonist | MJ12 | 0.619 | 0.036 | Anaphylatoxin receptor antagonist |
| 0.579 | 0.010 | Atherosclerosis treatment | 0.542 | 0.014 | Atherosclerosis treatment | ||
| 0.522 | 0.023 | Autoimmune disorders treatment | 0.503 | 0.027 | Autoimmune disorders treatment | ||
| 0.478 | 0.004 | Leukotriene synthesis inhibitor | 0.474 | 0.110 | Glycosylphosphatidylinositol phospholipase D inhibitor | ||
| 0.453 | 0.004 | Allergic rhinitis treatment | 0.450 | 0.004 | Leukotriene synthesis inhibitor | ||
| MJ3 | 0.757 | 0.016 | Anaphylatoxin receptor antagonist | MJ13 | 0.702 | 0.023 | Anaphylatoxin receptor antagonist |
| 0.754 | 0.022 | Glycosylphosphatidylinositol phospholipase D inhibitor | 0.694 | 0.037 | Glycosylphosphatidylinositol phospholipase D inhibitor | ||
| 0.546 | 0.013 | Atherosclerosis treatment | 0.531 | 0.015 | Atherosclerosis treatment | ||
| 0.471 | 0.034 | Autoimmune disorders treatment | 0.484 | 0.031 | Autoimmune disorders treatment | ||
| 0.466 | 0.004 | Leukotriene synthesis inhibitor | 0.460 | 0.004 | Leukotriene synthesis inhibitor | ||
| MJ4 | 0.665 | 0.029 | Anaphylatoxin receptor antagonist | MJ14 | 0.619 | 0.036 | Anaphylatoxin receptor antagonist |
| 0.542 | 0.014 | Atherosclerosis treatment | 0.495 | 0.018 | Atherosclerosis treatment | ||
| 0.550 | 0.044 | Neurotransmitter uptake inhibitor | 0.493 | 0.029 | Autoimmune disorders treatment | ||
| 0.511 | 0.025 | Autoimmune disorders treatment | 0.474 | 0.110 | Glycosylphosphatidylinositol phospholipase D inhibitor | ||
| 0.480 | 0.009 | CYP2C19 inhibitor | 0.439 | 0.013 | CYP2C19 inhibitor | ||
| MJ5 | 0.740 | 0.019 | Anaphylatoxin receptor antagonist | MJ15 | 0.696 | 0.024 | Anaphylatoxin receptor antagonist |
| 0.671 | 0.043 | Glycosylphosphatidylinositol phospholipase D inhibitor | 0.684 | 0.040 | Glycosylphosphatidylinositol phospholipase D inhibitor | ||
| 0.650 | 0.006 | Atherosclerosis treatment | 0.615 | 0.008 | Atherosclerosis treatment | ||
| 0.436 | 0.037 | Interleukin 2 agonist | 0.439 | 0.119 | Nicotinic alpha4beta4 receptor agonist | ||
| 0.414 | 0.140 | Nicotinic alpha4beta4 receptor agonist | 0.419 | 0.044 | Interleukin 2 agonist | ||
| MJ6 | 0.740 | 0.019 | Anaphylatoxin receptor antagonist | MJ16 | 0.596 | 0.041 | Anaphylatoxin receptor antagonist |
| 0.671 | 0.043 | Glycosylphosphatidylinositol phospholipase D inhibitor | 0.567 | 0.011 | Atherosclerosis treatment | ||
| 0.650 | 0.006 | Atherosclerosis treatment | 0.524 | 0.023 | Autoimmune disorders treatment | ||
| 0.436 | 0.037 | Interleukin 2 agonist | 0.430 | 0.005 | Leukotriene synthesis inhibitor | ||
| 0.414 | 0.140 | Nicotinic alpha4beta4 receptor agonist | 0.424 | 0.002 | Transglutaminase 2 inhibitor | ||
| 0.740 | 0.019 | Anaphylatoxin receptor antagonist | 0.596 | 0.041 | Anaphylatoxin receptor antagonist | ||
| MJ7 | 0.579 | 0.044 | Anaphylatoxin receptor antagonist | MJ17 | 0.572 | 0.011 | Atherosclerosis treatment |
| 0.542 | 0.014 | Atherosclerosis treatment | 0.536 | 0.021 | Autoimmune disorders treatment | ||
| 0.471 | 0.004 | Leukotriene synthesis inhibitor | 0.504 | 0.063 | Anaphylatoxin receptor antagonist | ||
| 0.470 | 0.035 | Autoimmune disorders treatment | 0.427 | 0.083 | Antiinflammatory | ||
| 0.446 | 0.004 | Allergic rhinitis treatment | 0.423 | 0.005 | Leukotriene synthesis inhibitor | ||
| MJ8 | 0.619 | 0.036 | Anaphylatoxin receptor antagonist | MJ18 | 0.632 | 0.034 | Anaphylatoxin receptor antagonist |
| 0.512 | 0.017 | Atherosclerosis treatment | 0.541 | 0.014 | Atherosclerosis treatment | ||
| 0.474 | 0.110 | Glycosylphosphatidylinositol phospholipase D inhibitor | 0.509 | 0.096 | Glycosylphosphatidylinositol phospholipase D inhibitor | ||
| 0.450 | 0.004 | Leukotriene synthesis inhibitor | 0.491 | 0.029 | Autoimmune disorders treatment | ||
| 0.444 | 0.042 | Autoimmune disorders treatment | 0.413 | 0.005 | Leukotriene synthesis inhibitor | ||
| MJ9 | 0.508 | 0.017 | Atherosclerosis treatment | MJ19 | 0.538 | 0.014 | Atherosclerosis treatment |
| 0.486 | 0.068 | Anaphylatoxin receptor antagonist | 0.528 | 0.022 | Autoimmune disorders treatment | ||
| 0.479 | 0.032 | Autoimmune disorders treatment | 0.504 | 0.063 | Anaphylatoxin receptor antagonist | ||
| 0.439 | 0.004 | Leukotriene synthesis inhibitor | 0.418 | 0.102 | Neurotransmitter uptake inhibitor | ||
| 0.430 | 0.095 | Neurotransmitter uptake inhibitor | 0.400 | 0.005 | Leukotriene synthesis inhibitor | ||
| MJ10 | 0.621 | 0.008 | Atherosclerosis treatment | MJ20 | 0.628 | 0.007 | Atherosclerosis treatment |
| 0.577 | 0.044 | Anaphylatoxin receptor antagonist | 0.596 | 0.041 | Anaphylatoxin receptor antagonist | ||
| 0.433 | 0.039 | Interleukin 2 agonist | 0.404 | 0.144 | Glycosylphosphatidylinositol phospholipase D inhibitor | ||
| 0.371 | 0.047 | Anxiolytic | 0.391 | 0.059 | Interleukin 2 agonist | ||
| 0.365 | 0.044 | Cognition disorders treatment | 0.377 | 0.038 | MAP3K5 inhibitor |
| MJ1 | MJ2 | MJ3 | MJ4 | MJ5 | MJ6 | MJ7 | MJ8 | MJ9 | MJ10 |
| 73.838 | 76.163 | 75.436 | 64.526 | 72.958 | 72.869 | 73.617 | 71.858 | 72.961 | 72.267 |
| MJ11 | MJ12 | MJ13 | MJ14 | MJ15 | MJ16 | MJ17 | MJ18 | MJ19 | MJ20 |
| 88.062 | 75.635 | 85.203 | 78.428 | 87.21 | 84.727 | 72.595 | 74.130 | 72.719 | 83.657 |
| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| BCL2 | CTTCAGGGACGGGGTGAAC | GGATCCAGGTGTGCAGGTG |
| MDM2 | GCCCTTCGTGAGAATTGGCT | CCTCAACACATGACTCTCTGG |
| AIFM2 | CTGCCCTTCTCTCATCTTATCCT | CTGCCTCACCATGTCCTCATAG |
| IL6 | GTTCTGCCAAACCAGCCTTG | AGATCACCTAGTCCACCCCC |
| IL8 | GGTGCAGTTTTGCCAAGGAG | ACCAAGGCACAGTGGAACAA |
| RPL41 | TCCTGCGTTGGGATTCCGTG | ACGGTGCAACAAGCTAGCGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giercuszkiewicz-Haśnik, K.; Skonieczna, M.; Morak-Młodawska, B.; Jeleń, M. Design, Synthesis, and Testing of 1,2,3-Triazolo-Quinobenzothiazine Hybrids for Cytotoxic and Immunomodulatory Activity. Int. J. Mol. Sci. 2025, 26, 6920. https://doi.org/10.3390/ijms26146920
Giercuszkiewicz-Haśnik K, Skonieczna M, Morak-Młodawska B, Jeleń M. Design, Synthesis, and Testing of 1,2,3-Triazolo-Quinobenzothiazine Hybrids for Cytotoxic and Immunomodulatory Activity. International Journal of Molecular Sciences. 2025; 26(14):6920. https://doi.org/10.3390/ijms26146920
Chicago/Turabian StyleGiercuszkiewicz-Haśnik, Klaudia, Magdalena Skonieczna, Beata Morak-Młodawska, and Małgorzata Jeleń. 2025. "Design, Synthesis, and Testing of 1,2,3-Triazolo-Quinobenzothiazine Hybrids for Cytotoxic and Immunomodulatory Activity" International Journal of Molecular Sciences 26, no. 14: 6920. https://doi.org/10.3390/ijms26146920
APA StyleGiercuszkiewicz-Haśnik, K., Skonieczna, M., Morak-Młodawska, B., & Jeleń, M. (2025). Design, Synthesis, and Testing of 1,2,3-Triazolo-Quinobenzothiazine Hybrids for Cytotoxic and Immunomodulatory Activity. International Journal of Molecular Sciences, 26(14), 6920. https://doi.org/10.3390/ijms26146920








