Integrating Redox Proteomics and Computational Modeling to Decipher Thiol-Based Oxidative Post-Translational Modifications (oxiPTMs) in Plant Stress Physiology
Abstract
1. Introduction
2. Redox Proteomics in Plant Biology
2.1. Methodological Advances in Redox Proteomics
2.2. Redox-Driven Signaling Pathways and Stress Responses
2.3. Implications for Plant Development and Seed Germination
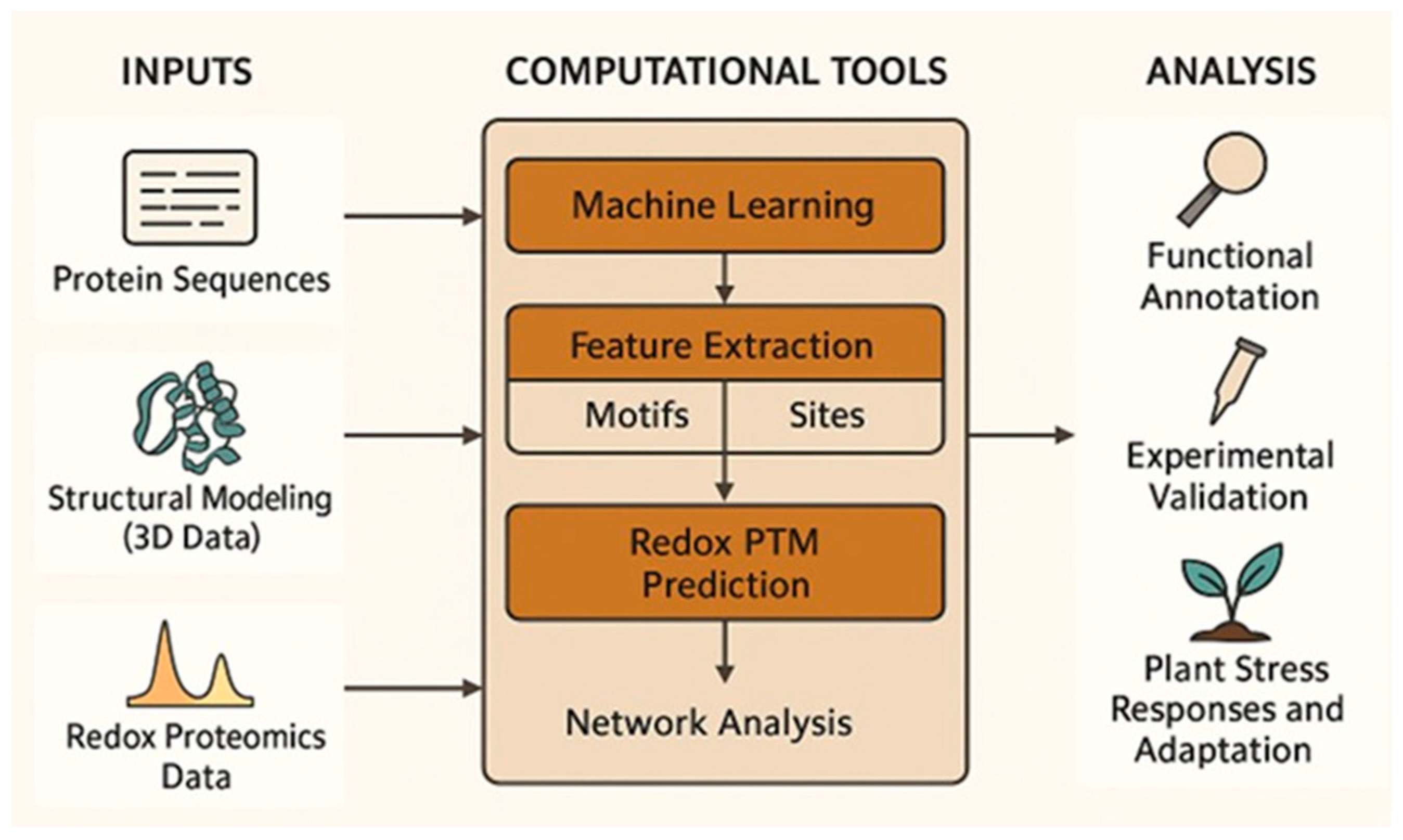
3. Computational Prediction Tools for Redox-Dependent PTMs
3.1. Computational Approaches for Cysteine-Targeting Redox PTMs
3.1.1. Gasotransmitter-Mediated Modifications
S-Nitrosation
Persulfidation
3.1.2. Redox Buffering-Dependent Modifications (GSH and ROS)
S-Glutathionylation
S-Sulfenylation and S-Sulfinylation
Cysteine Oxidation
3.1.3. Structural and Regulatory Thiol Modifications
Reversible Disulfide Bonds
Multiple Cysteine Modifications
Redox-Sensitive Cysteines
4. Future Prospects
- Development of real-time redox sensors to monitor dynamic thiol modifications in living plant tissues.
- Integration of AI-enhanced biosensors for in vivo detection of redox PTMs under stress conditions.
- Application of deep learning and natural language processing to automate redox proteomics data analysis and annotation.
- Utilization of graph neural networks to model redox interaction networks and predict protein function within signaling cascades.
- Expansion of multi-species training datasets to improve the generalizability of redox PTM prediction tools across diverse plant systems.
- Advancement of single-cell redox proteomics to uncover cell-specific redox regulation and signaling heterogeneity.
- Integration of multi-omics platforms (transcriptomics, metabolomics, proteomics) to construct predictive redox regulatory networks for crop improvement.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foyer, C.H.; Baker, A.; Wright, M.; Sparkes, I.A.; Mhamdi, A.; Schippers, J.H.; Van Breusegem, F. On the move: Redox-dependent protein relocation in plants. J. Exp. Bot. 2020, 71, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Rodríguez-Ruiz, M.; Muñoz-Vargas, M.A.; Palma, J.M. Thiol-based oxidative posttranslational modifications (oxiPTMs) of plant proteins. Plant Cell Physiol. 2022, 63, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Martí, M.C.; Sevilla, F. Oxidative post-translational modifications of plant antioxidant systems under environmental stress. Physiol. Plant. 2025, 177, e70118. [Google Scholar] [CrossRef] [PubMed]
- Rinalducci, S.; Murgiano, L.; Zolla, L. Redox proteomics: Basic principles and future perspectives for the detection of protein oxidation in plants. J. Exp. Bot. 2008, 59, 3781–3801. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiao, Y.; Ming, Y.; Peng, R.; Hu, J.; Wang, H.B.; Jin, H.L. Quantitative proteomics reveals redox-based functional regulation of photosynthesis under fluctuating light in plants. J. Integr. Plant Biol. 2022, 64, 2168–2186. [Google Scholar] [CrossRef] [PubMed]
- Bykova, N.V.; Rampitsch, C. Modulating protein function through reversible oxidation: Redox-mediated processes in plants revealed through proteomics. Proteomics 2013, 13, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Day, N.J.; Gaffrey, M.J.; Qian, W.J. Stoichiometric thiol redox proteomics for quantifying cellular responses to perturbations. Antioxidants 2021, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Fermani, S.; Marchand, C.H.; Costa, A.; Sparla, F.; Rouhier, N.; Trost, P. Redox homeostasis in photosynthetic organisms: Novel and established thiol-based molecular mechanisms. Antioxid. Redox Signal. 2019, 31, 155–210. [Google Scholar] [CrossRef] [PubMed]
- Jamil, I.N.; Remali, J.; Azizan, K.A.; Nor Muhammad, N.A.; Arita, M.; Goh, H.H.; Aizat, W.M. Systematic multi-omics integration (MOI) approach in plant systems biology. Front. Plant Sci. 2020, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.E. Light-driven dynamics: Unravelling thiol-redox networks in plants through proteomics. Plant Physiol. 2024, 195, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Zhang, J.; Xie, Y.; Romero, L.C.; Gotor, C. Hydrogen sulfide signaling in plant adaptations to adverse conditions: Molecular mechanisms. J. Exp. Bot. 2021, 72, 5893–5904. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Daur, I.; Nawaz, M.A.; Ahmad, M.Q.; Chung, G. Redox and ionic homeostasis regulations against oxidative, salinity and drought stress in wheat (a systems biology approach). Front. Genet. 2017, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Gong, S.; Sun, K.; Li, J.; Zhang, H.; Fan, J.; Yan, C. Integrated proteomics and metabolomics analysis of rice leaves in response to rice straw return. Fron. Plant Sci. 2022, 13, 997557. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, K.; Wu, G.; Zhang, Q.; Wang, P.; Zheng, J.; Cheng, H. pCysMod: Prediction of multiple cysteine modifications based on deep learning framework. Front. Cell Dev. Biol. 2021, 9, 617366. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Lindermayr, C. Computational prediction of NO-dependent posttranslational modifications in plants: Current status and perspectives. Plant Physiol. Biochem. 2021, 167, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Staes, A.; Impens, F.; Demichev, V.; Van Breusegem, F.; Gevaert, K.; Willems, P. CysQuant: Simultaneous quantification of cysteine oxidation and protein abundance using data dependent or independent acquisition mass spectrometry. Redox Biol. 2023, 67, 102908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jia, J.; Chen, C.; Zhang, Y.; Yu, B. BiGRUD-SA: Protein S-sulfenylation sites prediction based on BiGRU and self-attention. Comput. Biol. Med. 2023, 163, 107145. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Li, J. DLF-Sul: A multi-module deep learning framework for prediction of S-sulfinylation sites in proteins. Brief. Bioinform. 2022, 23, bbac323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, Z.C.; Su, W.; Yang, Y.H.; Lv, H.; Yang, H.; Lin, H. iCarPS: A computational tool for identifying protein carbonylation sites by novel encoded features. Bioinformatics 2021, 37, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Keßler, M.; Wittig, I.; Ackermann, J.; Koch, I. Prediction and analysis of redox-sensitive cysteines using machine learning and statistical methods. Biol. Chem. 2021, 402, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Alam, M.A.; Shoombuatong, W.; Kurata, H. IRC-Fuse: Improved and robust prediction of redox-sensitive cysteine by fusing of multiple feature representations. J. Comput. Aided Mol. Des. 2021, 35, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gaffrey, M.J.; Li, X.; Qian, W.J. Characterization of cellular oxidative stress response by stoichiometric redox proteomics. Am. J. Physiol. Cell Physiol. 2021, 320, C182–C194. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N. Exploring the unmapped cysteine redox proteoform landscape. Am. J. Physiol. Cell Physiol. 2024, 327, C844–C866. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Jones, D.P. The redox code of plants. Plant Cell Environ. 2024, 47, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Heimer, N.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Redox proteomics: Methods for the identification and enrichment of redox-modified proteins and their applications. Proteomics 2016, 16, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, M.; McComb, M.E.; Huang, H.; Huang, S.; Heibeck, T.; Costello, C.E.; Cohen, R.A. Isotope-coded affinity tag (ICAT) approach to redox proteomics: Identification and quantitation of oxidant-sensitive cysteine thiols in complex protein mixtures. J. Proteome Res. 2004, 3, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Gaffrey, M.J.; Day, N.J.; Li, X.; Qian, W.J. Resin-assisted capture coupled with isobaric tandem mass tag labeling for multiplexed quantification of protein thiol oxidation. J. Vis. Exp. 2021, 172, e63562. [Google Scholar]
- Jiang, J.; Wang, K.; Nice, E.C.; Zhang, T.; Huang, C. High-throughput screening of cellular redox sensors using modern redox proteomics approaches. Expert Rev. Proteom. 2015, 12, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wang, C.; Lei, X.; Gao, X.; Li, J.; Zhang, S.; Guo, J. IodoTMT-labeled redox proteomics reveals the involvement of oxidative post-translational modification in response to para-hydroxybenzoic acid and hydrogen peroxide stresses in poplar. Ecotoxicol. Environ. Saf. 2023, 259, 115033. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.J.; Dreyer, A.; Ugalde, J.M.; Feitosa-Araujo, E.; Dietz, K.J.; Schwarzländer, M. Shifting paradigms and novel players in Cys-based redox regulation and ROS signaling in plants-and where to go next. Biol. Chem. 2021, 402, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Gao, T.; Pan, Z.; Cheng, H.; Yang, Q.; Xue, Y. CPLM: A database of protein lysine modifications. Nucleic Acids Res. 2014, 42, D531–D536. [Google Scholar] [CrossRef] [PubMed]
- Balmant, K.M.; Lawrence, S.R., II; Duong, B.V.; Zhu, F.; Zhu, N.; Nicklay, J.; Chen, S. Guard cell redox proteomics reveals a role of lipid transfer protein in plant defense. J. Proteom. 2021, 242, 104247. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Wang, Y.; Wang, W.; Tian, S.; Qin, G. Redox proteomic analysis reveals the involvement of oxidative post-translational modification in tomato fruit ripening. Postharvest Biol. Technol. 2021, 178, 111556. [Google Scholar] [CrossRef]
- McDonagh, B.; Ogueta, S.; Lasarte, G.; Padilla, C.A.; Bárcena, J.A. Shotgun redox proteomics identifies specifically modified cysteines in key metabolic enzymes under oxidative stress in Saccharomyces cerevisiae. J. Proteom. 2009, 72, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, S.; Qin, G. NADPH oxidase is crucial for the cellular redox homeostasis in fungal pathogen Botrytis cinerea. Mol. Plant-Microbe Inter. 2019, 32, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Lemaire, S.D. Redox regulation of the Calvin–Benson cycle: Something old, something new. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, U.; Braymer, J.J.; Christ, S.; Rietzschel, N.; Uzarska, M.A.; Weiler, B.D.; Lill, R. Glutaredoxins and iron-sulfur protein biogenesis at the interface of redox biology and iron metabolism. Biol. Chem. 2020, 401, 1407–1428. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; López-Jaramillo, J.; Padilla, M.N.; Carreras, A.; Corpas, F.J.; Barroso, J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Nietzel, T.; Mostertz, J.; Ruberti, C.; Née, G.; Fuchs, P.; Wagner, S.; Schwarzländer, M. Redox-mediated kick-start of mitochondrial energy metabolism drives resource-efficient seed germination. Proc. Natl. Acad. Sci. USA 2020, 117, 741–751. [Google Scholar] [CrossRef] [PubMed]
- García-Quirós, E.; Alché, J.D.D.; Karpinska, B.; Foyer, C.H. Glutathione redox state plays a key role in flower development and pollen vigour. J. Exp. Bot. 2020, 71, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Lauri, A.; Zachgo, S. Redox regulation and flower development: A novel function for glutaredoxins. Plant Biol. 2006, 8, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Navrot, N.; Finnie, C.; Svensson, B.; Hägglund, P. Plant redox proteomics. J. Proteom. 2011, 74, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, V.A.; Sant, S.S.; Jadhav, A.R.; War, A.R.; Sharma, H.C.; Jaleel, A.; Kashikar, A.S. Label-free quantitative proteomics of Sorghum bicolor reveals the proteins strengthening plant defense against insect pest Chilo partellus. Proteome Sci. 2021, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, F.; Liu, Y.; Wang, Y.; Gao, H.; Zhao, S.; Zhu, Y.; Wang, Q.; Li, J. Quantitative proteomic and lipidomics analyses of high oil content GmDGAT1-2 transgenic soybean illustrate the regulatory mechanism of lipoxygenase and oleosin. Plant Cell Rep. 2021, 40, 2303–2323. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Y.; Qin, Z.; Guo, S.; Li, Y.; Miao, Y.; Dai, S. Plant chloroplast stress response: Insights from thiol redox proteomics. Antioxid. Redox Signal. 2020, 33, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Sun, X.; Zhai, J.; Li, X.; Pedro, G.C.; Nian, H.; Li, K.; Xu, H. SlTrxh functions downstream of SlMYB86 and positively regulates nitrate stress tolerance via S-nitrosation in tomato seedling. Hortic. Res. 2024, 11, uhae184. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Clément, C.; Rivoal, J. Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction. Int. J. Mol. Sci. 2024, 25, 9845. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, F.; Pourmirzaei, M.; Ramazi, S.; Shojaeilangari, S.; Yavari, E. A Review of Machine Learning and Algorithmic Methods for Protein Phosphorylation Site Prediction. Genom. Proteom. Bioinform. 2023, 21, 1266–1285. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.N.; Yin, T.; Zhang, T.; Im, A.K.; Cort, J.R.; Rozum, J.C.; Feng, S. Artificial Intelligence Transforming Post-Translational Modification Research. Bioengineering 2024, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Li, T. Ptm-x: Prediction of Post-Translational Modification Crosstalk within and across Proteins. In Computational Methods for Predicting Post-Translational Modification Sites; Springer: Cham, Switzerland, 2022; pp. 275–283. [Google Scholar]
- Huang, Y.X.; Liu, R. Improved Prediction of Post-Translational Modification Crosstalk within Proteins Using DeepPCT. Bioinformatics 2024, 40, btae675. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Vogel, C.; Choi, H. PTMscape: An Open Source Tool to Predict Generic Post-Translational Modifications and Map Modification Crosstalk in Protein Domains and Biological Processes. Mol. Omics 2018, 14, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, X.; Li, F.; Li, C.; Marquez-Lago, T.; Leier, A.; Song, J. Large-Scale Comparative Assessment of Computational Predictors for Lysine Post-Translational Modification Sites. Brief. Bioinform. 2019, 20, 2267–2290. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation: The prototypic redox-based signaling mechanism. Cell 2001, 106, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Parrott, A.M.; Fu, C.; Liu, T.; Marino, S.M.; Gladyshev, V.N.; Li, H. Thioredoxin 1-mediated post-translational modifications: Reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid. Redox Signal. 2011, 15, 2565–2604. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qiu, H. Post-translational S-nitrosylation of proteins in regulating cardiac oxidative stress. Antioxidants 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Lubega, J.; Umbreen, S.; Loake, G.J. Recent advances in the regulation of plant immunity by S-nitrosylation. J. Exp. Bot. 2021, 72, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Yang, Q.; Sun, X. Global proteome-wide analysis of cysteine S-nitrosylation in Toxoplasma gondii. Molecules 2023, 28, 7329. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide (NO) scaffolds the peroxisomal protein–protein interaction network in higher plants. Int. J. Mol. Sci. 2021, 22, 2444. [Google Scholar] [CrossRef] [PubMed]
- Lindermayr, C.; Saalbach, G.; Durner, J. Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2005, 137, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Lindermayr, C. Nitric oxide-dependent posttranslational modification in plants: An update. Int. J. Mol. Sci. 2012, 13, 15193–15208. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pérez, C.; Sánchez-Vicente, I.; Arteaga, N.; Gómez-Jiménez, S.; Fuentes-Terrón, A.; Oulebsir, C.S.; Lorenzo, Ó. Functions of nitric oxide-mediated post-translational modifications under abiotic stress. Front. Plant Sci. 2023, 14, 1158184. [Google Scholar] [CrossRef] [PubMed]
- Martí-Guillén, J.M.; Pardo-Hernández, M.; Martínez-Lorente, S.E.; Almagro, L.; Rivero, R.M. Redox post-translational modifications and their interplay in plant abiotic stress tolerance. Front. Plant Sci. 2022, 13, 1027730. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.I.; Lourenço, A.S.; Simão, S.; da Silva, D.M.; Santos, D.F.; de Carvalho, A.P.O.; Araújo, I.M. Identification of new targets of S-nitrosylation in neural stem cells by thiol redox proteomics. Redox Biol. 2020, 32, 101457. [Google Scholar] [CrossRef] [PubMed]
- Shahin, W.S.; Ebed, S.O.; Tyler, S.R.; Miljkovic, B.; Choi, S.H.; Zhang, Y.; Engelhardt, J.F. Redox-dependent Igfbp2 signaling controls Brca1 DNA damage response to govern neural stem cell fate. Nat. Commun. 2023, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Soares, C.; Canuto, G.A.B.; Tavares, M.F.M.; Colli, W.; Alves, M.J.M. Down regulation of NO signaling in Trypanosoma cruzi upon parasite–extracellular matrix interaction: Changes in protein modification by nitrosylation and nitration. PLoS Negl. Trop. Dis. 2015, 9, e0003683. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Rasul, S.; Koen, E.; Manzoor, H.; Besson-Bard, A.; Lamotte, O.; Wendehenne, D. S-nitrosylation: An emerging post-translational protein modification in plants. Plant Sci. 2011, 181, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Jedelská, T.; Luhová, L.; Petřivalský, M. Thioredoxins: Emerging players in the regulation of protein S-nitrosation in plants. Plants 2020, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Rogowska-Wrzesinska, A. The challenge of detecting modifications on proteins. Essays Biochem. 2020, 64, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wu, J.; Liang, Z.; Zhang, Y.; Huang, Z. Protein S-nitrosation: Biochemistry, identification, molecular mechanisms, and therapeutic applications. J. Med. Chem. 2022, 65, 5902–5925. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, Z.; Gao, X.; Jin, C.; Wen, L.; Yao, X.; Ren, J. GPS-SNO: Computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PLoS ONE 2010, 5, e11290. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Kovacs, I.; Spannagl, M.; Lindermayr, C. Computational prediction of candidate proteins for S-nitrosylation in Arabidopsis thaliana. PLoS ONE 2014, 9, e110232. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Manavalan, B.; Khatun, M.S.; Kurata, H. Prediction of S-nitrosylation sites by integrating support vector machines and random forest. Mol. Omics 2019, 15, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.R.; Wang, Q.K.; Guan, M.Y.; Jia, J.H.; Xiao, X. Predicting S-nitrosylation proteins and sites by fusing multiple features. Math. Biosci. Eng. 2021, 18, 9132–9147. [Google Scholar] [PubMed]
- Pratyush, P.; Pokharel, S.; Saigo, H.; Kc, D.B. pLMSNOSite: An ensemble-based approach for predicting protein S-nitrosylation sites by integrating supervised word embedding and embedding from pre-trained protein language model. BMC Bioinform. 2023, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Lv, P.; Wei, X.; Qiu, W. SNO-DCA: A model for predicting S-nitrosylation sites based on densely connected convolutional networks and attention mechanism. Heliyon 2024, 10, e19949. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, A.T.; Ballot, J.G.; Vignane, T.; Li, H.; Worth, M.M.; Muller, L.; Siegler, M.A.; Kane, M.A.; Filipovic, M.R.; Goldberg, D.P.; et al. Chemoselective proteomics, zinc fingers, and a zinc(II) model for H2S mediated persulfidation. Angew. Chem. Int. Ed. 2024, 63, e202401003. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Stoltzfus, A.T.; Michel, S.L.J. Mining proteomes for zinc finger persulfidation. RSC Chem. Biol. 2024, 5, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xie, Y.; Van Breusegem, F.; Huang, J. Hydrogen Sulfide and Protein Persulfidation in Plant Stress Signaling. J. Exp. Bot. 2025, eraf100. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Jurado-Flores, A.; Filipovic, M.R.; Gotor, C.; Romero, L.C. Detection of protein persulfidation in plants by the dimedone switch method. Methods Enzymol. 2022, 676, 385–402. [Google Scholar] [PubMed]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exper. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Aroca, A.; Romero, L.C.; Gotor, C.; Palma, J.M.; Corpas, F.J. Persulfidome of sweet pepper fruits during ripening: The case study of leucine aminopeptidase that is positively modulated by H2S. Antioxidants 2024, 13, 719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Aroca, A.; Hervás, M.; Navarro, J.A.; Moreno, I.; Xie, Y.; Romero, L.C.; Gotor, C. Analysis of sulfide signaling in rice highlights specific drought responses. J. Exp. Bot. 2024, 75, 5130–5145. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, R.; Moreno, I.; Romero, L.C.; Aroca, A.; Gotor, C. Hydrogen sulfide-induced barley resilience to drought and salinity through protein persulfidation. Plant Physiol. Biochem. 2025, 221, 109644. [Google Scholar] [CrossRef] [PubMed]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Anashkina, A.A.; Poluektov, Y.M.; Dmitriev, V.A.; Kuznetsov, E.N.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. A novel approach for predicting protein S-glutathionylation. BMC Bioinform. 2020, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.W.; Singh, S.; Townsend, D.M.; Tew, K.D. An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic. Biol. Med. 2018, 120, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Musaogullari, A.; Chai, Y.C. Redox regulation by protein S-glutathionylation: From molecular mechanisms to implications in health and disease. Int. J. Mol. Sci. 2020, 21, 8113. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Chibani, K.; Jacquot, J.P.; Rouhier, N. Cysteine–based redox regulation and signaling in plants. Front. Plant Sci. 2013, 4, 105. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Cysteine modifications (oxPTM) and protein sulphenylation-mediated sulfenome expression in plants: Evolutionary conserved signaling networks? Plant Signal. Behav. 2021, 16, 1831792. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.Y.; Chou, C.C.; Hsieh, F.T.; Gao, S.; Lin, J.C.Y.; Lin, S.H.; Lin, C.H. In vivo tagging and characterization of S-glutathionylated proteins by a chemoenzymatic method. Angew. Chem. Int. Ed. 2012, 51, 5871. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, T.; Day, N.J.; Feng, S.; Gaffrey, M.J.; Qian, W.J. Defining the S-glutathionylation proteome by biochemical and mass spectrometric approaches. Antioxidants 2022, 11, 2272. [Google Scholar] [CrossRef] [PubMed]
- Wible, R.S.; Sutter, T.R. Soft cysteine signaling network: The functional significance of cysteine in protein function and the soft acids/bases thiol chemistry that facilitates cysteine modification. Chem. Res. Toxicol. 2017, 30, 729–762. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.M.; Salinas, G.; Gladyshev, V.N. Computational functional analysis of cysteine residues in proteins. In Redox Chemistry and Biology of Thiols; Academic Press: Cambridge, MA, USA, 2022; pp. 59–80. [Google Scholar]
- Zhao, X.; Ning, Q.; Ai, M.; Chai, H.; Yin, M. PGluS: Prediction of protein S-glutathionylation sites with multiple features and analysis. Mol. BioSyst. 2015, 11, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Lu, C.T.; Huang, K.Y.; Wu, H.Y.; Chen, Y.J.; Lee, T.Y. GSHSite: Exploiting an iteratively statistical method to identify S-glutathionylation sites with substrate specificity. PLoS ONE 2015, 10, e0118752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bibli, S.I.; Fleming, I. Oxidative post-translational modifications: A focus on cysteine S-sulfhydration and the regulation of endothelial fitness. Antioxid. Redox Signal. 2021, 35, 1494–1514. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gluth, A.; Zhang, T.; Qian, W.J. Thiol redox proteomics: Characterization of thiol-based post-translational modifications. Proteomics 2023, 23, 2200194. [Google Scholar] [CrossRef] [PubMed]
- Petushkova, A.I.; Zamyatnin, A.A., Jr. Redox-mediated post-translational modifications of proteolytic enzymes and their role in protease functioning. Biomolecules 2020, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cui, X.; Yu, B.; Chen, C.; Ma, Q.; Zhou, H. SulSite-GTB: Identification of protein S-sulfenylation sites by fusing multiple feature information and gradient tree boosting. Neural Comput. Appl. 2020, 32, 13843–13862. [Google Scholar] [CrossRef]
- Lyu, X.; Li, S.; Jiang, C.; He, N.; Chen, Z.; Zou, Y.; Li, L. DeepCSO: A deep-learning network approach to predicting cysteine S-sulphenylation sites. Front. Cell Dev. Biol. 2020, 8, 594587. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.; Le, T.Q.T.; Le, N.Q.K. Using deep neural networks and biological subwords to detect protein S-sulfenylation sites. Brief. Bioinform. 2021, 22, bbaa128. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bazán, V.; Guzmán-Ocampo, D.C.; Domínguez, L.; Aguirre, J. Filamentous actin destabilization by H2O2 favors DnmA aggregation, with crucial roles of cysteines 450 and 776 in mitochondrial and peroxisomal division in Aspergillus nidulans. mBio 2023, 14, e0282223. [Google Scholar] [CrossRef] [PubMed]
- Garrido Ruiz, D.; Sandoval-Perez, A.; Rangarajan, A.V.; Gunderson, E.L.; Jacobson, M.P. Cysteine oxidation in proteins: Structure, biophysics, and simulation. Biochemistry 2022, 61, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.V.; Templeton, D.J. Regulation of signal transduction through protein cysteine oxidation. Antioxid. Redox Signal. 2006, 8, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, H.; Shahab, U.; Rehman, S.; Rafi, Z.; Khan, M.Y.; Uddin, M. Protein oxidation: An overview of metabolism of sulphur containing amino acid, cysteine. Front. Biosci. (Schol. Ed.) 2017, 9, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Giron, P.; Dayon, L.; Sanchez, J.C. Cysteine tagging for MS-based proteomics. Mass Spectrom. Rev. 2011, 30, 366–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, Q.; Wu, G.; Wen, J.; Liao, S.; Xu, C. Molecular basis for cysteine oxidation by plant cysteine oxidases from Arabidopsis thaliana. J. Struct. Biol. 2021, 213, 107663. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Wesemann, C.; Gossmann, N.; Sahm, A.; Krüger, J.; Sczyrba, A.; Dietz, K.J. ConCysFind: A pipeline tool to predict conserved amino acids of protein sequences across the plant kingdom. BMC Bioinform. 2020, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.; Riddle, M.; Woo, J.; Momand, J. Prediction of reversibly oxidized protein cysteine thiols using protein structure properties. Protein Sci. 2008, 17, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, F.; Martí, M.C.; De Brasi-Velasco, S.; Jiménez, A. Redox regulation, thioredoxins, and glutaredoxins in retrograde signalling and gene transcription. J. Exp. Bot. 2023, 74, 5955–5969. [Google Scholar] [CrossRef] [PubMed]
- Cremers, C.M.; Jakob, U. Oxidant sensing by reversible disulfide bond formation. J. Biol. Chem. 2013, 288, 26489–26496. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.; Novichkova, M. Glutathione in protein redox modulation through S-glutathionylation and S-nitrosylation. Molecules 2021, 26, 435. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, T.J.; Weerapana, E. From structure to redox: The diverse functional roles of disulfides and implications in disease. Proteomics 2017, 17, 1600391. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.A.; Wang, Y.; Zhang, Q.; Xia, Y.; Ge, W.; Guo, D. Prediction of reversible disulfide based on features from local structural signatures. BMC Genomics 2017, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kabir, M.W.U.; Hoque, M.T. diSBPred: A machine learning based approach for disulfide bond prediction. Comput. Biol. Chem. 2021, 91, 107436. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, F.; Clote, P. DiANNA 1.1: An extension of the DiANNA web server for ternary cysteine classification. Nucleic Acids Res. 2006, 34, W182–W185. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Akter, S.; Jacques, S.; Huang, J.; Messens, J.; Van Breusegem, F. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 2015, 66, 2923–2934. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Fermani, S.; Calvaresi, M.; Orrù, R.; Iommarini, L.; Sparla, F.; Trost, P. Tuning cysteine reactivity and sulfenic acid stability by protein microenvironment in glyceraldehyde-3-phosphate dehydrogenases of Arabidopsis thaliana. Antioxid. Redox Signal. 2016, 24, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, L.; Zhang, L.; Wang, Z.; Wang, X.; Li, C.; Li, L. CysModDB: A comprehensive platform with the integration of manually curated resources and analysis tools for cysteine posttranslational modifications. Brief. Bioinform. 2022, 23, bbac460. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Huang, J.; Waszczak, C.; Jacques, S.; Gevaert, K.; Van Breusegem, F.; Messens, J. Cysteines under ROS attack in plants: A proteomics view. J. Exp. Bot. 2015, 66, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Chicco, D.; Jurman, G. The Matthews Correlation Coefficient (MCC) Should Replace the ROC AUC as the Standard Metric for Assessing Binary Classification. BioData Min. 2023, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, W.; Jin, J.; Zheng, Z.; Zhou, B. Graph Neural Network for Protein–Protein Interaction Prediction: A Comparative Study. Molecules 2022, 27, 6135. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qian, P.; Zhao, Z.; Zeng, Z.; Chen, J.; Liu, W.; Yang, X. Graph Neural Networks for Protein–Protein Interactions—A Short Survey. arXiv 2024, arXiv:2404.10450. [Google Scholar]
- Willems, P.; Horne, A.; Van Parys, T.; Goormachtig, S.; De Smet, I.; Botzki, A.; Van Breusegem, F.; Gevaert, K. The Plant PTM Viewer, a central resource for exploring plant protein modifications. Plant J. 2019, 99, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.; Sterck, L.; Dard, A.; Huang, J.; De Smet, I.; Gevaert, K.; Van Breusegem, F. The Plant PTM Viewer 2.0: In-depth exploration of plant protein modification landscapes. J. Exp. Bot. 2024, 75, 4611–4624. [Google Scholar] [CrossRef] [PubMed]
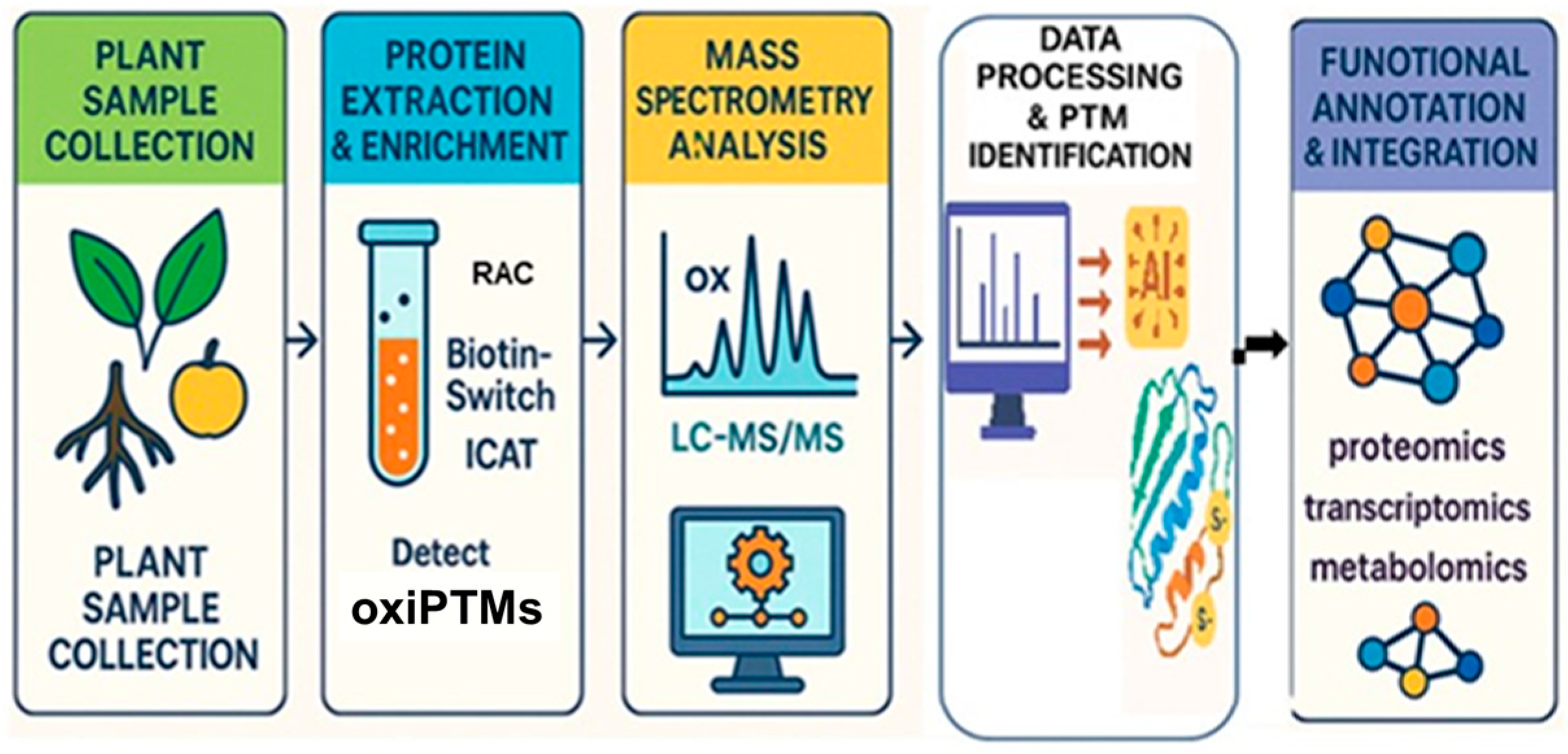
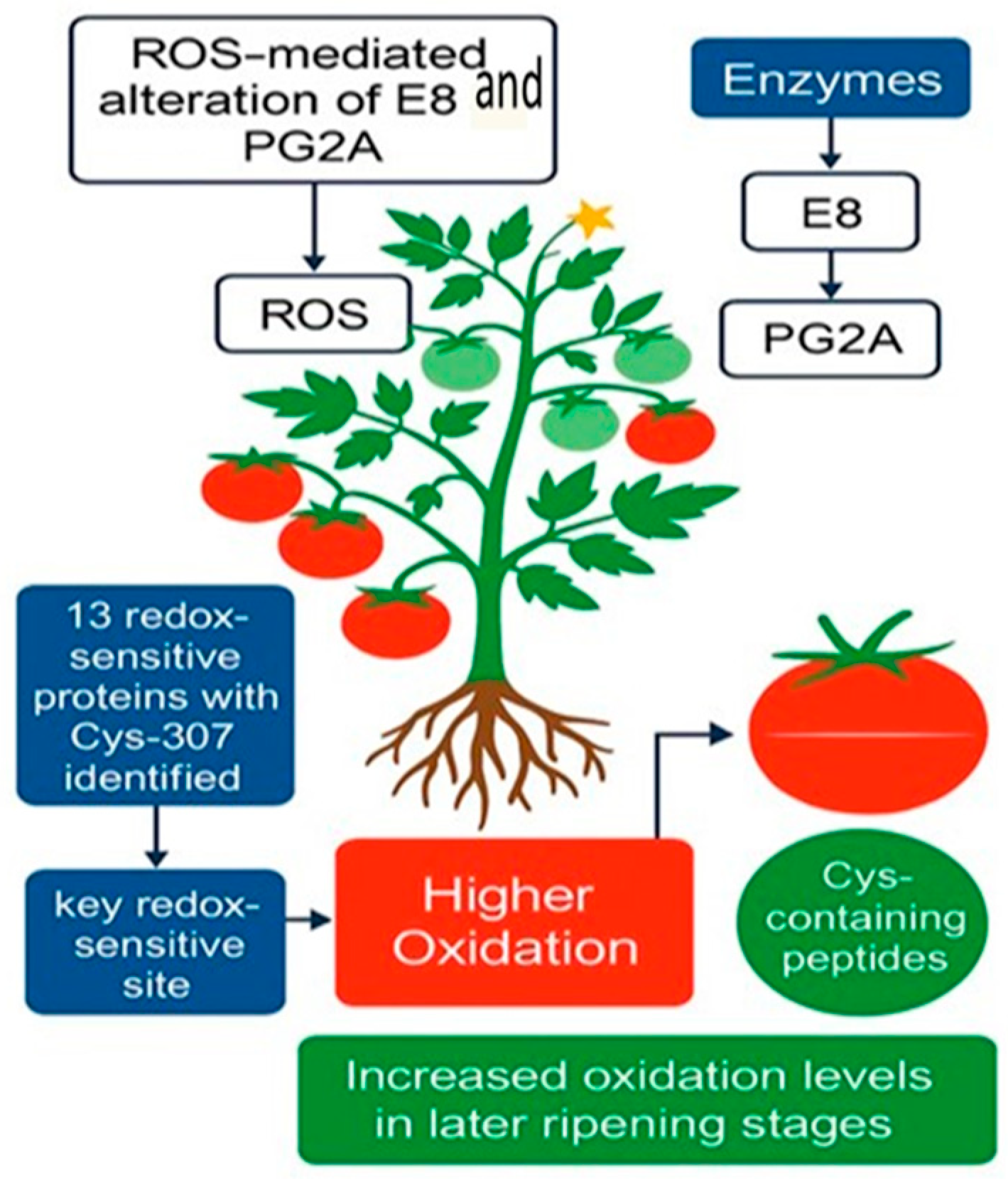
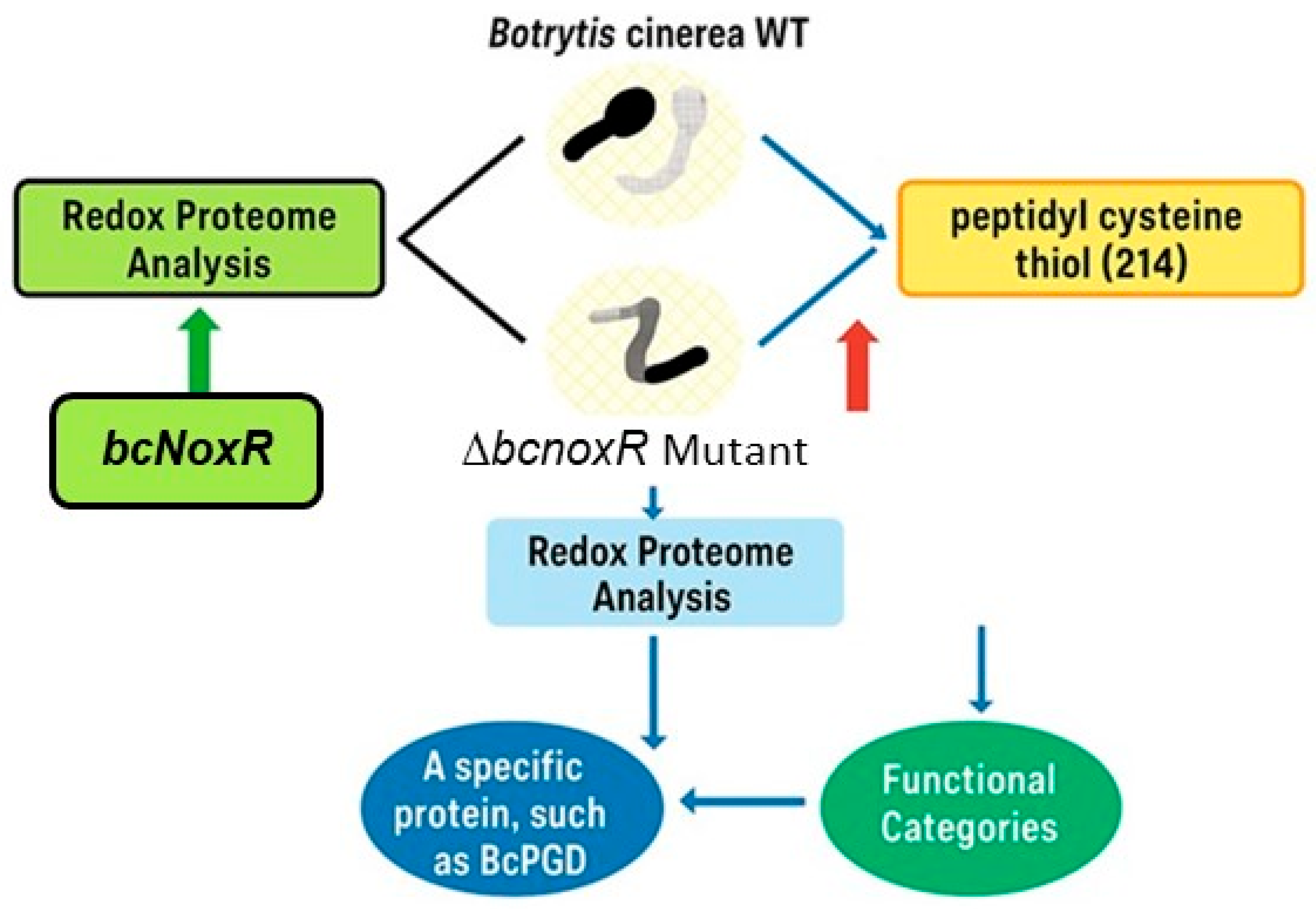
| Protein(s)/Process | Plant/System | Stress or Trigger | Proteomic Method | Key Finding | Citation |
|---|---|---|---|---|---|
| PSI assembly factor PSA3 (Cys199/200 redox switch) | Arabidopsis thaliana | Fluctuating light | Label-free redox proteomics | Identified thiol switches regulating PSI stability | [6] |
| Lipid transfer protein LTP-II | Brassica napus guard cells | flg22 exposure (bacterial peptide) | cysTMTRAQ + TMT redox proteomics | LTP-II is redox-responsive and required for pathogen defense | [34] |
| Fruit ripening enzymes (e.g., E8, PG2A) | Tomato fruit | Ripening (ROS fluctuations) | IodoTMT-based redox proteomics | Identified 70 Cys-peptides from 51 proteins; Cys-307 in E8 pinpointed as redox-sensitive site | [35] |
| 214 peptidyl cysteine thiols from 168 proteins | Botrytis cinerea (fungal pathogen) | ΔbcnoxR mutant (NOX regulatory subunit deletion) | IodoTMT-based redox proteomics | Mutant showed increased oxidation of 214 Cys sites; highlights NOXR’s role in redox homeostasis and pathogenesis | [37] |
| Mitochondrial thiol redox switches driving seed germination | Arabidopsis thaliana seeds | Seed imbibition (imbibition stage) | Targeted redox proteomics | Demonstrated redox kick-start of mitochondrial metabolism via thiol switches during early germination | [42] |
| Global cysteine redox landscape (~84 proteins) | Arabidopsis thaliana | H2O2 treatment | Isotope affinity labeling + MS | Mapped proteome-wide redox-responsive thiols in metabolism and signaling | [45] |
| 967 total proteins; differential abundance linked to defense enzymes (endochitinases, peroxidases, GST, LTP) | Sorghum bicolor leaves | Infestation by Chilo partellus | Label-free quantitative proteomics | Resistant genotypes maintain photosynthesis and stress-response proteins; 68 proteins showed differential expression | [46] |
| Lipoxygenases (down-regulated), oleosins (up-regulated) | Glycine max (soybean pods) | Overexpression of GmDGAT1-2 | Quantitative proteomics + lipidomics | 436 DEPs and 180 DEMs identified; lipoxygenases down, oleosins up—linked to increased total oil and oleic acid | [47] |
| Chloroplast stress-responsive proteins (e.g., elongation factors, chaperones) | Plants (chloroplasts) | Oxidative stress | Thiol redox proteomics (review + MS) | Highlighted key redox-regulated circuits in chloroplast signaling and PSII repair | [48] |
| oxiPTM | Tool | Methodology | Performance | Web | Citations |
|---|---|---|---|---|---|
| Multiple Cys PTMs | pCysMod | Deep learning with sequence features, optimized via PSO | AUC: 0.793–0.876 across five PTMs | http://pcysmod.omicsbio.info (accessed on 25 June 2025) | [16] |
| Cysteine Oxidation | CysQuant | MS (DDA/DIA) with isotopologue labeling | Quantified avg. 18% cysteine oxidation in Arabidopsis | https://github.com/patrick-willems/CysQuant (accessed on 25 June 2025) | [18] |
| S-sulfenylation | BiGRUD-SA | BiGRU + self-attention | Acc: 95.91% (test) | Not specified | [19] |
| S-sulfinylation | DLF-Sul | Deep learning: BiLSTM + attention + CNN | Acc: 92.08%; MCC: 0.8416; AUC: 96.4% | https://github.com/ningq669/DLF-Sul (accessed on 25 June 2025) | [20] |
| S-Nitrosylation | GPS-SNO 1.0 | Group-based prediction system (GPS) | Predicted 31,900 sites in Arabidopsis proteome | http://mapman.gabipd.org/web/guest/mapman (accessed on 25 June 2025) | [76] |
| S-Nitrosylation | RF-SNOPS | ML-based feature extraction and fusion | Accuracy: 81.84% | Specified but not accessible | [78] |
| S-Nitrosylation | pLMSNOSite | Protein language model (ProtT5), deep learning | Sensitivity: 0.735; specificity: 0.773 | https://github.com/KCLabMTU/pLMSNOSite (accessed on 25 June 2025) | [79] |
| S-Nitrosylation | SNO-DCA | Deep learning (CNN, attention module) | Outperforms previous models | https://github.com/peanono/SNO-DCA (accessed on 25 June 2025) | [80] |
| S-glutathionylation | PGluS | SVM with multiple features | 71.41% accuracy (train), 71.25% (test) | Specified but not accessible | [99] |
| S-glutathionylation | GSHSite | SVM using motifs and ASA | High performance; validated experimentally | Specified but not accessible | [100] |
| S-sulfenylation | SulSite-GTB | GTB + SMOTE + LASSO | Acc: 92.86% (train); 88.53% (test); AUC: 0.97/0.94 | https://github.com/QUST-AIBBDRC/SulSite-GTB/ (accessed on 25 June 2025) | [104] |
| S-sulfenylation | DeepCSO | LSTM with word embedding | AUC: 0.82–0.85 across species | http://www.bioinfogo.org/DeepCSO (accessed on 25 June 2025) | [105] |
| S-sulfenylation | fastSulf-DNN | DNN using protein sequences as “biological language” | Acc: 77.09%; MCC: 0.5554; AUC: 0.833 | https://github.com/khanhlee/fastSulf-DNN (accessed on 25 June 2025) Not specified | [106] |
| Conserved Redox PTMs | ConCysFind | Phylogenetic conservation analysis | Validated with redox proteins | https://bibiserv.cebitec.uni-bielefeld.de/concysfind (accessed on 25 June 2025) | [113] |
| Thiol Oxidation | COPA | Decision tree (J48) with 12 features | 80.1% accuracy (LOO CV) | Not specified | [114] |
| Reversible Disulfides | RevssPred | SVM based on structural features | Acc: 75%; AUC: 0.751 (CV) | Specified but not accessible | [119] |
| Disulfide Bond | diSBPred | Stacked ML with sequence and structure features | Acc: 94.2% (cys-pair), 82.29% (cys-site), 43.25% over NNA | Specified but not accessible | [120] |
| Disulfide Connectivity | DiANNA | ANN (v1.0), SVM (v1.1) for oxidation state and disulfide connectivity | Not quantified | http://bioinformatics.bc.edu/clotelab/DiANNA/ (accessed on 25 June 2025) | [121] |
| Multiple Cys PTMs | CysModDB | Integrated database and tools for CysPTMs | Resource/tool integration | https://cysmoddb.bioinfogo.org/ (accessed on 25 June 2025) | [124] |
| Multiple PTMs (19 types in 2019; 33 types in 2024) | Plant PTM Viewer | Integrative plant PTM database; includes sequence overview, confidence scoring, BLAST (accessed on 25 June 2025) and search tools | ~370,000 PTM sites (2019); +112,000 modified peptides in update (2024); includes 8 species total | http://www.psb.ugent.be/PlantPTMViewer (accessed on 25 June 2025) | [129,130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, C.; Corpas, F.J. Integrating Redox Proteomics and Computational Modeling to Decipher Thiol-Based Oxidative Post-Translational Modifications (oxiPTMs) in Plant Stress Physiology. Int. J. Mol. Sci. 2025, 26, 6925. https://doi.org/10.3390/ijms26146925
Kaya C, Corpas FJ. Integrating Redox Proteomics and Computational Modeling to Decipher Thiol-Based Oxidative Post-Translational Modifications (oxiPTMs) in Plant Stress Physiology. International Journal of Molecular Sciences. 2025; 26(14):6925. https://doi.org/10.3390/ijms26146925
Chicago/Turabian StyleKaya, Cengiz, and Francisco J. Corpas. 2025. "Integrating Redox Proteomics and Computational Modeling to Decipher Thiol-Based Oxidative Post-Translational Modifications (oxiPTMs) in Plant Stress Physiology" International Journal of Molecular Sciences 26, no. 14: 6925. https://doi.org/10.3390/ijms26146925
APA StyleKaya, C., & Corpas, F. J. (2025). Integrating Redox Proteomics and Computational Modeling to Decipher Thiol-Based Oxidative Post-Translational Modifications (oxiPTMs) in Plant Stress Physiology. International Journal of Molecular Sciences, 26(14), 6925. https://doi.org/10.3390/ijms26146925






