Innovative In Situ Interfacial Co-Assembled Lignin/Chitosan Nanoparticles—Green Synthesis, Physicochemical Characterization, In Vitro Release, and Intermolecular Interactions
Abstract
1. Introduction
2. Results and Discussion
2.1. Potentiometric Titration
2.2. ξ-Potential
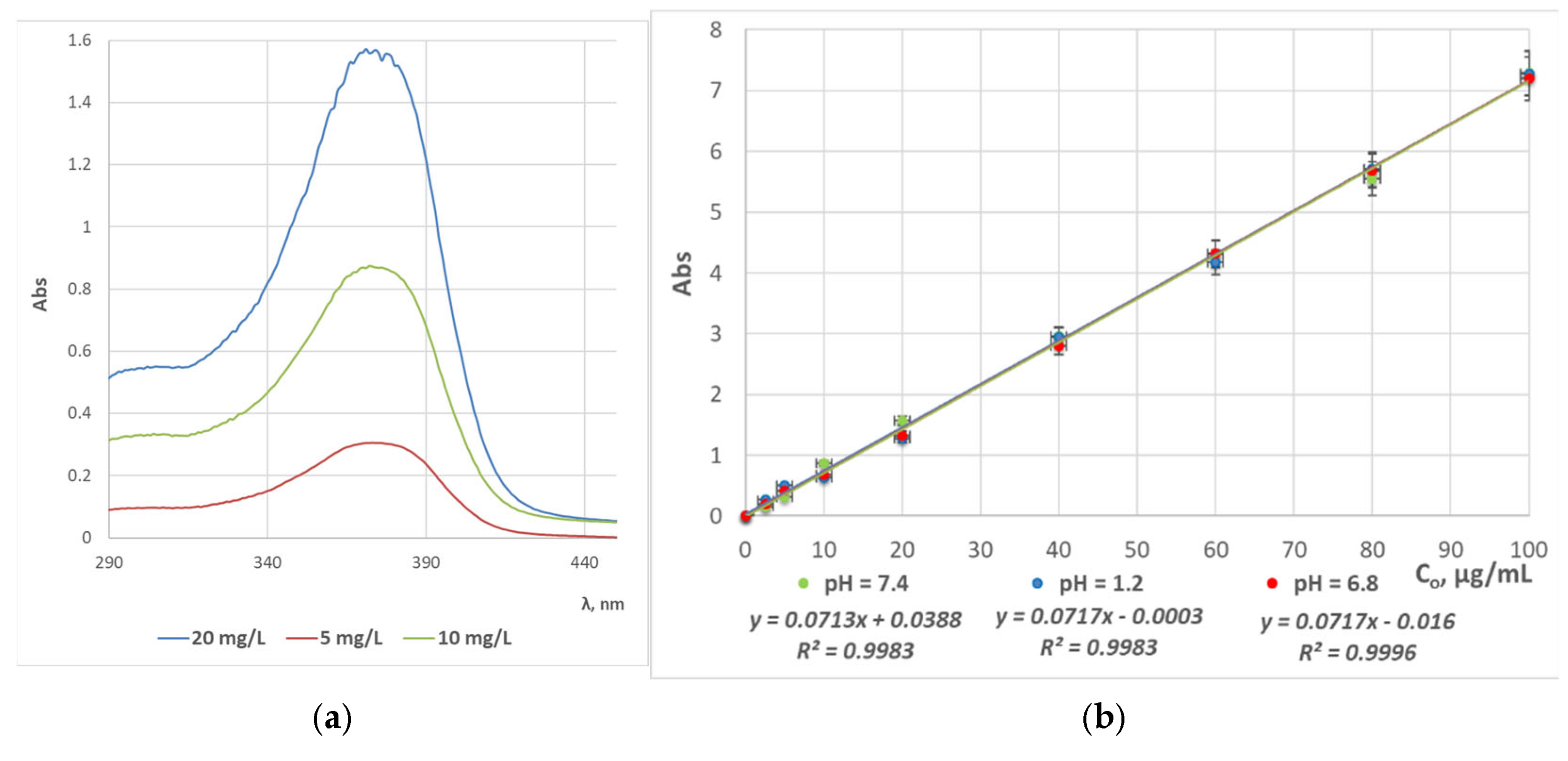
2.3. UV/Vis Spectrophotometric Analyses
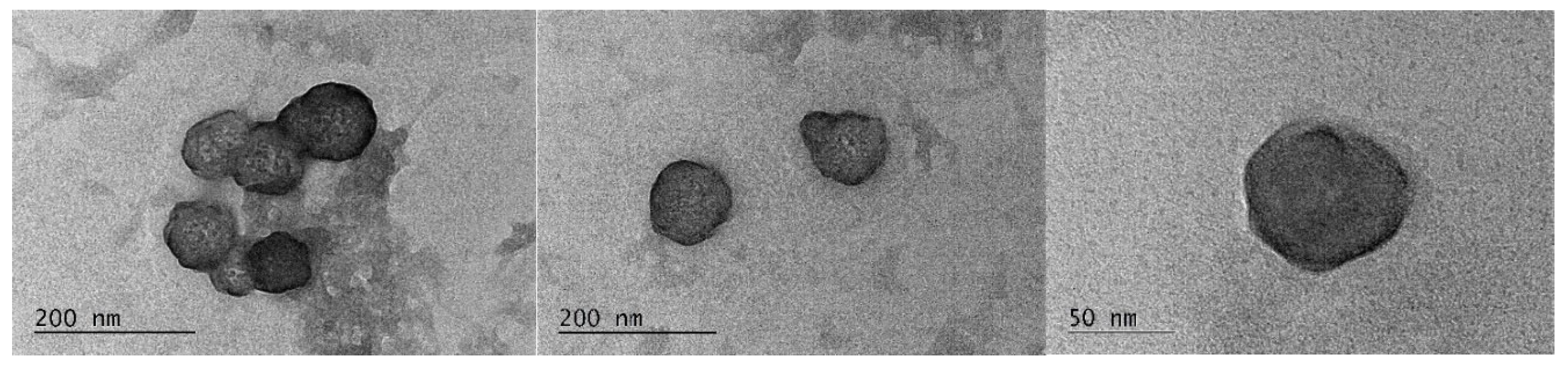
2.4. TEM Analyses
2.5. XRD Analyses
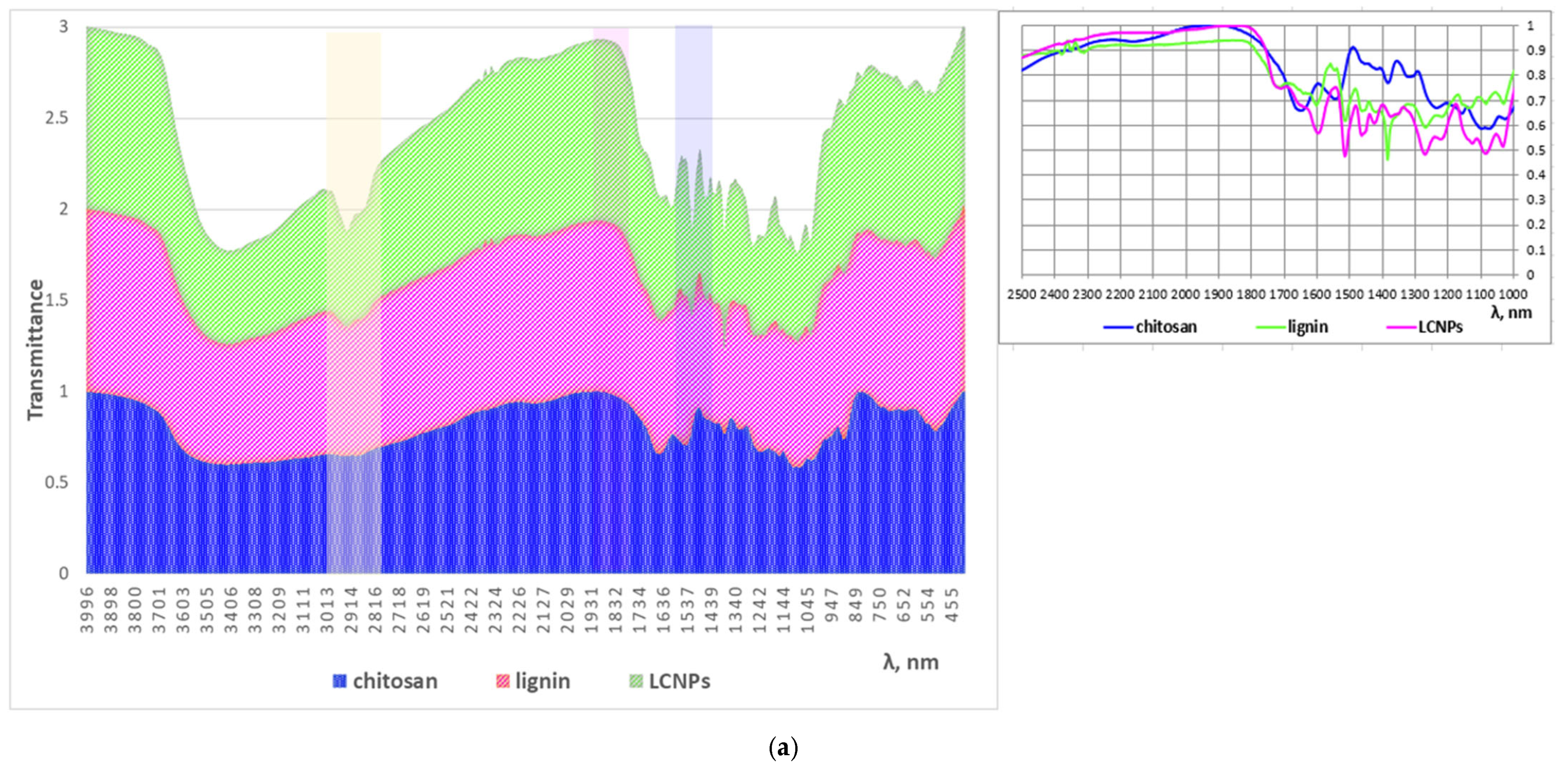
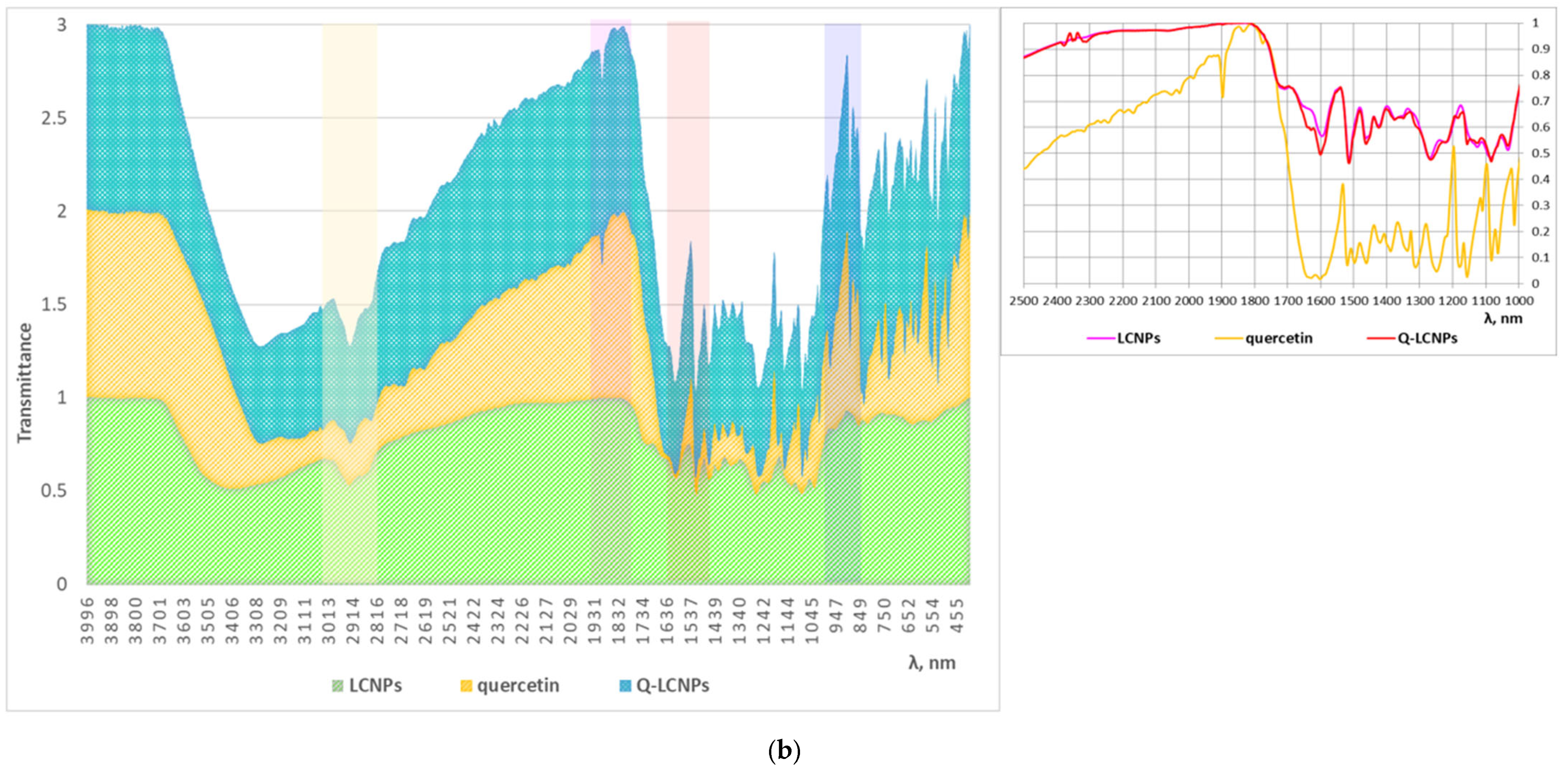
2.6. FTIR Analysis
2.7. Encapsulation Efficiency
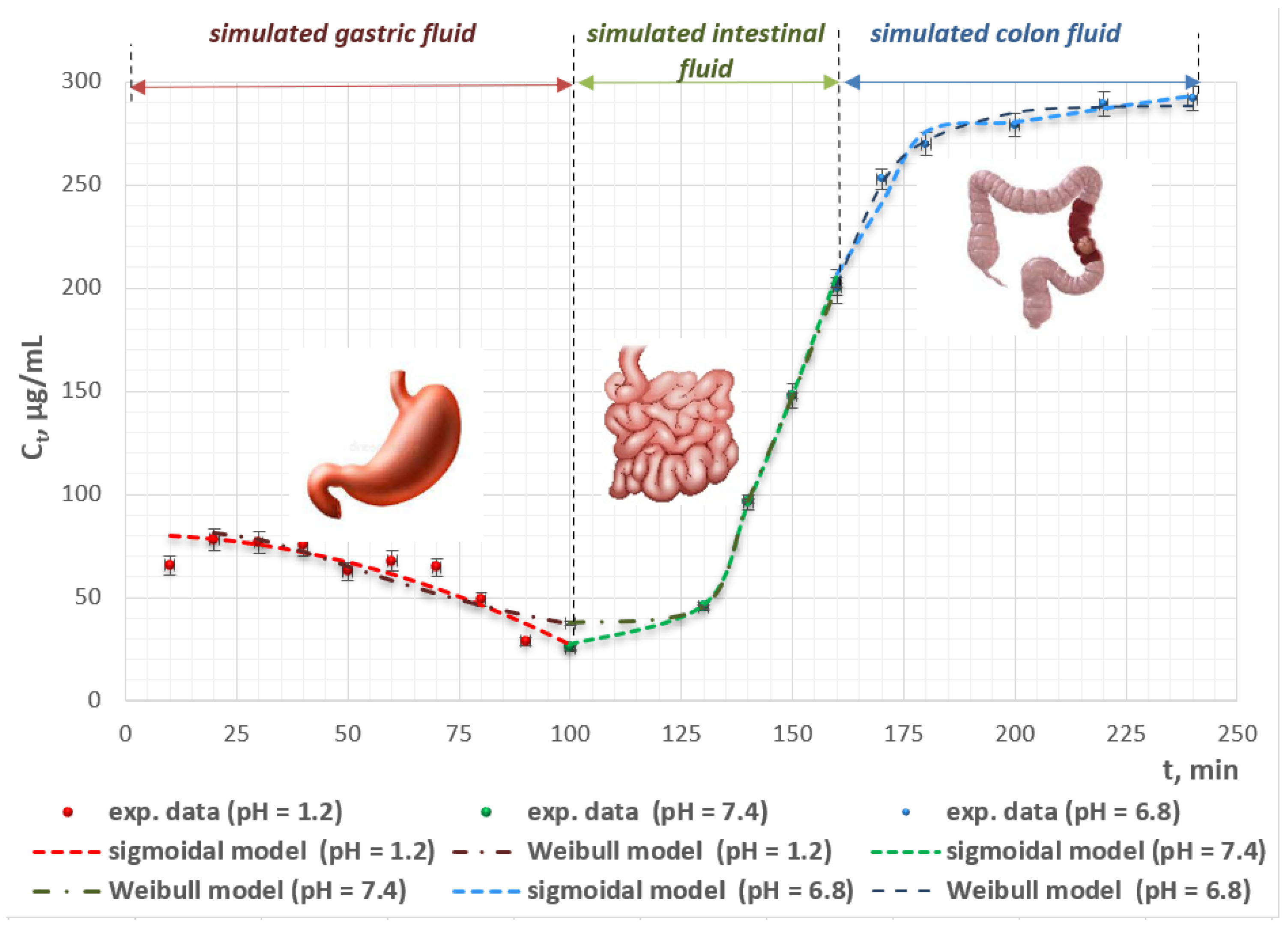
2.8. In Vitro Release Study
2.9. In Vitro Kinetics Modeling
2.10. Intermolecular Biopolymer–Biopolymer Interactions in the Structure of LCNPs
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of LCNPs
3.3. Encapsulation Study
3.4. Surface Chemistry Characterization
3.5. In Vitro Release Studies
3.6. Mathematical Modeling; Statistical and Error Function Analyses
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Sustainable biodegradable biopolymer-based nanoparticles for healthcare applications. Int. J. Mol. Sci. 2023, 24, 3188. [Google Scholar] [CrossRef]
- Pathak, N.; Singh, P.; Singh, P.K.; Sharma, S.; Singh, R.P.; Gupta, A.; Mishra, R.; Mishra, V.K.; Tripathi, M. Biopolymeric nanoparticles based effective delivery of bioactive compounds toward the sustainable development of anticancerous therapeutics. Front. Nutr. 2022, 9, 963413. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen nanoparticles in drug delivery systems and tissue engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Van Bavel, N.; Lewrenz, A.-M.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. Synthesis of alginate nanoparticles using hydrolyzed and enzyme-digested alginate using the ionic gelation and water-in-oil emulsion method. Polymers 2023, 15, 1319. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric nanoparticles–Multifunctional materials of the future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef]
- Suresh, D.; Suresh, A.; Kannan, R. Engineering biomolecular systems: Controlling the self-assembly of gelatin to form ultra-small bioactive nanomaterials. Bioact. Mater. 2022, 18, 321–336. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-based nanoparticles: A review on their preparations and applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y.; Dai, L.; Chuanling, S. Micro/nanosized lignin for biomedical application. Pap. Biomater. 2021, 6, 77–89. Available online: https://www.sciopen.com/article/10.1213/j.issn.2096-2355.2021.03.008 (accessed on 20 May 2025).
- Ten, E.; Ling, C.; Wang, Y.; Srivastava, A.; Dempere, L.A.; Vermerris, W. Lignin nanotubes as vehicles for gene delivery into human cells. Biomacromolecules 2014, 15, 327–338. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Shi, Q.-S.; Zhong, X.-Q.; Guo, Y.; Xie, X.-B. High value valorization of lignin as environmental benign antimicrobial. Mater. Today Bio 2023, 18, 100520. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial properties and mechanisms of action of sonoenzymatically synthesized lignin-based nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-structured lignin as green antioxidant and UV shielding ingredient for sunscreen applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Wang, Y.; Li, B.; Qi, W.; Su, R.; He, Z. Microfluidic synthesis of lignin/chitosan nanoparticles for the pH-responsive delivery of anticancer drugs. Langmuir 2021, 37, 7219–7226. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Tran, M.T.; Mai, H.D.; Nguyen, T.; Le, G.; Can, M.V.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Surendran, V.; Palei, N.N. Formulation and characterization of rutin loaded chitosan-alginate nanoparticles: Antidiabetic and cytotoxicity studies. Curr. Drug Deliv. 2022, 19, 379–394. [Google Scholar] [CrossRef]
- Rosova, E.; Smirnova, N.; Dresvyanina, E.; Smirnova, V.; Vlasova, E.; Ivan’kova, E.; Sokolova, M.; Maslennikova, T.; Malafeev, K.; Kolbe, K.; et al. Biocomposite materials based on chitosan and lignin: Preparation and characterization. Cosmetics 2021, 8, 24. [Google Scholar] [CrossRef]
- Sohni, S.; Hashim, R.; Nidaullah, H.; Lamaming, J.; Sulaiman, O. Chitosan/nano-lignin based composite as a new sorbent for enhanced removal of dye pollution from aqueous solutions. Int. J. Biol. Macromol. 2019, 132, 1304–1317. [Google Scholar] [CrossRef]
- Zhang, Z.; Argenziano, R.; Konate, A.; Shi, X.; Salazar, S.A.; Cerruti, P.; Panzella, L.; Terrasson, V.; Guénin, E. Preparation of chitosan/lignin nanoparticles-based nanocomposite films with high-performance and improved physicochemical properties for food packaging applications. Int. J. Biol. Macromol. 2025, 293, 139079. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D.; Toneva, M. Green synthesis, characterization, encapsulation, and measurement of the release potential of novel alkali lignin micro-/submicron particles. J. Vis. Exp. 2024, 2024, e66216. [Google Scholar] [CrossRef]
- Sharma, S.; Kachhia, P.; Shukla, V.; Patel, A.; Rathod, V.; Narra, M. Valorization of lignin into nanoparticles and nanogel: Characterization and application. Bioresour. Technol. Rep. 2022, 18, 101041. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Tardy, B.L.; Richardson, J.J.; Guo, J.; Lehtonen, J.; Agoa, M.; Rojas, O.J. Lignin nano- and microparticles as template for nanostructured materials: Formation of hollow metal-phenolic capsules. Green Chem. 2018, 20, 1335–1344. [Google Scholar] [CrossRef]
- Tse, H.Y.; Yeung, C.S.; Lau, C.Y.; Cheung, M.Y.; Guan, J.Y.; Islam, M.K.; Anastas, P.T.; Leu, S.-Y. One-pot synthesis to prepare lignin/photoacid nanohybrids for multifunctional biosensors and photo-triggered singlet oxygen generation. Green Chem. 2022, 24, 2904–2918. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, A.; Kumar, S.; Pathania, K.; Kumar, K.; Arora, A.; Mehta, S.K.; Petrovsky, N.; Sah, S.P.; Salunke, D.; et al. Lignin nanoparticles as a novel carrier for efficacious delivery of toll like receptor 7/8 agonist: Physicochemical and in-vitro evaluation. J. Indian Chem. Soc. 2023, 100, 101008. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Oxford, UK, 1998. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- Cheng, L.; Deng, B.; Luo, W.; Nie, S.; Liu, X.; Yin, Y.; Liu, S.; Wu, Z.; Zhan, P.; Zhang, L.; et al. pH-Responsive lignin-based nanomicelles for oral drug delivery. J. Agric. Food Chem. 2020, 68, 5249–5258. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Jassal, A.; Pathania, K.; Kumar, P.; Kaushik, D.; Dhingra, S.; Salunke, D.B.; Pawar, S.V. Lignin-chitosan-based biocomposite film for the localized delivery of TLR7 agonist imiquimod. Futur. J. Pharm. Sci. 2024, 10, 162. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. Green synthesis of chitosan nanoparticles, optimization, characterization and antibacterial efficacy against multi drug resistant biofilm-forming Acinetobacter baumannii. Sci. Rep. 2022, 12, 19869. [Google Scholar] [CrossRef]
- Worku, L.A.; Bachheti, R.K.; Tadesse, M.G.; Bachheti, A.; Ali, D.; Kumar, G.; Chaubey, K.K.; Juyal, A.; Almarzoug, M.H.A. Synthesis of lignin nanoparticles from Oxytenanthera abyssinica by nanoprecipitation method followed by ultrasonication for the nanocomposite application. J. King Saud Univ. Sci. 2023, 35, 102793. [Google Scholar] [CrossRef]
- Liang, Q.; Sun, X.; Raza, H.; Khan, M.A.; Ma, H.; Ren, X. Fabrication and characterization of quercetin loaded casein phosphopeptides-chitosan composite nanoparticles by ultrasound treatment: Factor optimization, formation mechanism, physicochemical stability and antioxidant activity. Ultrason. Sonochemistry 2021, 80, 105830. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Li, J.; Li, F.; Wang, X.; Man, J.; Li, J.; Zhang, C.; Peng, S. A biodegradable chitosan-based composite film reinforced by ramie fibre and lignin for food packaging. Carbohydr. Polym. 2022, 281, 119078. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Mei, A.; Peng, L.; Sun, J. Novel chitosan/lignin hydrogel prepared by the Mannich reaction for Pb(II) and Cu(II) removal from aqueous solution. Int. J. Biol. Macromol. 2025, 285, 138177. [Google Scholar] [CrossRef]
- Ivanova, D.; Toneva, M.; Simeonov, E.; Nikolova, B.; Semkova, S.; Antov, G.; Yaneva, Z. Newly synthesized lignin microparticles as bioinspired oral drug-delivery vehicles: Flavonoid-carrier potential and in vitro radical-scavenging activity. Pharmaceutics 2023, 15, 1067. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Farias, F.R.; Arunatat, S.; Griffin, D.; Gerometta, M.; Rocca-Smith, J.R.; Weber, G.; Sok, N.; Karbowiaket, T. Functionalization of chitosan with lignin to produce active materials by waste valorization. Green Chem. 2019, 21, 4633–4641. [Google Scholar] [CrossRef]
- Yaneva, Z.; Georgieva, N. Physicochemical and morphological characterization of pharmaceutical nanocarriers and mathematical modeling of drug encapsulation/release mass transfer processes. In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 173–218. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Démigné, C.; Rémésy, C. Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. J. Nutr. 2001, 131, 2109–2114. [Google Scholar] [CrossRef]
- Marunaka, Y.; Niisato, N.; Zou, X.; Xiao, J.B.; Nakahari, T. Food intake targeting and improving acidity in diabetes and cancer. Food Front. 2020, 1, 9–12. [Google Scholar] [CrossRef]
- Luo, A.; Xie, M.; Li, X.; Zhou, C.; Liu, Q. Preparation, characterization, stability, and antioxidant of quercetin-loaded hyaluronic acid complexes via pH-induced co-assembly. Food Biosci. 2024, 62, 105399. [Google Scholar] [CrossRef]
- Meenu, M.; Pujari, A.K.; Kirar, S.; Mansi Thakur, A.; Garg, M.; Bhaumik, J. Development of bionanocomposite packaging films based on lignin nanoencapsulated anthocyanins extracted from agro-waste for enhancing the post-harvest shelf life of tomatoes. Sustain. Food Technol. 2025, 3, 414–424. [Google Scholar] [CrossRef]
- Mileva, R.; Petkova, T.; Yaneva, Z.; Milanova, A. Investigation of the Effect of pH on the Adsorption–Desorption of Doxycycline in Feed for Small Ruminants. Antibiotics 2023, 12, 268. [Google Scholar] [CrossRef]
- Österberg, M.; Henn, K.A.; Farooq, M.; Valle-Delgado, J.J. Biobased Nanomaterials—The role of interfacial interactions for advanced materials. Chem. Rev. 2023, 123, 2200–2241. [Google Scholar] [CrossRef]
- Yaneva, Z.; Beev, G.; Rusenova, N.; Ivanova, D.; Tzanova, M.; Stoeva, D.; Toneva, M. Antimicrobial potential of conjugated lignin/morin/chitosan combinations as a function of system complexity. Antibiotics 2022, 11, 650. [Google Scholar] [CrossRef]
- Han, X.; Li, R.; Miao, P.; Gao, J.; Hu, G.; Zhao, Y.; Chen, T. Design, synthesis and adsorption evaluation of bio-based lignin/chitosan beads for Congo red removal. Materials 2022, 15, 2310. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, Z.; Ivanova, D.; Nikolova, G.; Karamalakova, Y.; Rusenova, N.; Georgieva, E.; Petkova-Parlapanska, K. Nanoparticle Compositions. GB Patent Application No: GB2415240.7, 16 October 2024. [Google Scholar]
- Geißler, D.; Nirmalananthan-Budau, N.; Scholtz, L.; Tavernaro, I.; Resch-Genger, U. Analyzing the surface of functional nanomaterials-how to quantify the total and derivatizable number of functional groups and ligands. Mikrochim. Acta 2021, 188, 321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Flaherty, D.W. Modified potentiometric titration method to distinguish and quantify oxygenated functional groups on carbon materials by pKa and chemical reactivity. Carbon 2020, 166, 436–445. [Google Scholar] [CrossRef]
- Breen, R.; Goggin, C.; Holmes, J.D.; Collins, J. A collaborative cocurricular undergraduate research experience on sustainable materials: Analysis of biochar using the Boehm titration and spectroscopic techniques. J. Chem. Educ. 2025, 102, 1323–1332. [Google Scholar] [CrossRef]




| Nanoparticles | Size, nm | ξ-Potential, mV | pH |
|---|---|---|---|
| LCNPs | 56.3–72.8 | −39.51 | 9.15 |
| Q-LCNPs | 61.5–85.7 | −31.68 | 8.57 |
| Wavenumber, cm−1 | 3700–3200 | 2950–2800 | 1700–1600 | 1590–1470 | 1500–1350 | 1350–1200 | 1200–1100 | 1100–1000 | 900–600 |
|---|---|---|---|---|---|---|---|---|---|
| Lignin | 3390 Phenolic hydroxyl groups | 2935 C–H bond stretching; C=O bond vibrations of -COOH | 1595 Aromatic ring vibrations and C–H, O–H, C=O bonds | 1479; 1384 C=C–C ring bonding; C–H bonds of -CH3 groups | 1301 Guaiacyl ring and methoxy C–O stretching; O–H bonds of phenolic and non-ether groups 1234 Syringyl units | 1166 Guaiacyl unit band 1160–1130 -OH bonds of secondary alcohols; condensed aromatic rings | 1058 Band of guaiacyl unit; -OH bonds of primary alcohols | 893 Guaiacyl unit band | |
| Chitosan | 3200 N-H and O-H stretching; intramolecular hydrogen bonds. | 2921; 2877 C-H symmetric and asymmetric stretching | 1652 C=O stretching of amide I | 1589 N-H bending of the primary amine 1550 N-H bending of amide II | 1423; 1375 -CH2 bending; -CH3 symmetrical deformations | 1325 C-N stretching of amide III | 1153 Asymmetric stretching of the C-O-C bridge | 1066; 1028 C-O stretching | |
| LCNPs | 3413 Phenolic hydroxyl groups; N-H and O-H stretching; intramolecular hydrogen bonds. | 2925 C–H symmetric and asymmetric stretching; C=O bond vibrations of -COOH | 1600 C=O stretching of amide I C=C stretching | 1549 Aromatic ring vibrations and C–H, O–H, C=O bonds; N-H bending of the primary amine; 1515 N-H bending of amide II; | 1384 C=C–C ring bonding; C–H bonds of -CH3 groups bending; -CH3 symmetrical deformations | 1323 Guaiacyl ring and methoxy C–O stretching; O–H bonds of phenolic and non-ether groups 1263; 1234 Syringyl units; C-N stretching of amide III 1261 syringyl units | 1166 Guaiacyl unit band; -OH bonds of secondary alcohols; condensed aromatic rings; asymmetric stretching of the C-O-C bridge | 1028 Band of guaiacyl unit; -OH bonds of primary alcohols C-O stretching | |
| Quercetin | 3280 O–H stretching band; dimeric hydroxyl O–H stretching | 2910 C–H bond stretching | 1615 -C=O stretching vibration; double-bond alkenyl C=C stretching | 1533 Aromatic ring vibrations and C–H, O–H, C=O bonds | 1471 C=C–C ring bonding; C–H bonds of -CH3 groups | 1238 C-H stretching; -C−OH deformation vibrations; -C-O-C bending | 1153 Asymmetric stretching of the C-O-C bridge | 1082 C-O-C stretching; −C−OH stretching | 889 C–H vibration of aromatic ring 553–528 Bending vibration of -OH alcoholic groups |
| Q-LCNPs | 3280 O–H stretching; dimeric hydroxyl O–H stretching; N-H and O-H stretching; intramolecular hydrogen bonds. phenolic hydroxyl groups | 2906 C–H bond stretching; C=O bond vibrations of -COOH; C–H symmetric and asymmetric stretching | 1600 C=O stretching of amide I; -C=O stretching vibration; double-bond alkenyl C=C stretching | 1533 Aromatic ring vibrations and C–H, O–H, C=O bonds; N-H bending of the primary amine; 1515 N-H bending of amide II; | 1384 C=C–C ring bonding; C–H bonds of -CH3 groups bending; -CH3 symmetrical deformations | 1323 Guaiacyl ring and methoxy C–O stretching; O–H bonds of phenolic and non-ether groups 1261 Syringyl units; C-H stretching; -C−OH deformation vibrations; -C-O-C bending | 1191 Guaiacyl unit band; phenolic C–O stretching vibration 1160–1130 -OH bonds of secondary alcohols; condensed aromatic rings; asymmetric stretching of the C-O-C bridge | 1082 Band of guaiacyl unit; -OH bonds of primary alcohols; C-O-C stretching; −C−OH stretching | 889 C–H vibration of aromatic ring 553–528 bending vibration of -OH alcoholic groups |
| Model | Gastric Medium (pH = 1.2) | Small Intestinal Medium (pH = 7.4) | Colonic Medium (pH = 6.8) | |
|---|---|---|---|---|
| Korsmeyer–Peppas | Model parameters | a = 330.808 n = −0.439 | a = 8.461 × 10−15 n = 7.467 | a = 7.623 n = 0.673 |
| Error functions | R2 = 0.629 SSE = 1185.901 MSE = 169.414 RMSE = 13.016 | R2 = 0.985 SSE = 79.788 MSE = 79.788 RMSE = 8.932 | R2 = 0.704 SSE = 1712.195 MSE = 428.049 RMSE = 20.689 | |
| Weibull | Model parameters | b = −1.5 Co = 81.809 T = 5.767 a = 0.001 | b = 1 Co = 8.97 × 106 T = 121.092 a = 1754770.820 | b = 1 Co = 288.244 T = 145.210 a = 12.304 |
| Error functions | R2 = 0.830 SSE = 580.262 MSE = 96.710 RMSE = 9.834 | R2 = 1.000 SSE = 0.342 | R2 = 0.989 SSE = 61.188 MSE = 20.396 RMSE = 4.516 | |
| Sigmoidal + | Model parameters | ks1 = 0.277 ns1 = −0.001 ks2 = 0.024 ns2 = −0.001 | ks1 = 0.855 ns1 = 0.010 ks2 = 0.006 ns2 = 0.010 | ks1 = 0.173 ns1 = 0.811 ks2 = 0.310 ns2 = 0.682 |
| Error functions | R2 = 0.858 SSE = 0.005 MSE = 0.001 RMSE = 0.028 | R2 = 1.000 SSE = 1.054 × 10−5 | R2 = 0.919 SSE = 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanev, Z.; Georgieva, D.; Hristova, S.; Tzanova, M.; Nicheva, D.; Andonova-Lilova, B.; Zagorcheva, T.; Vladova, D.; Grozeva, N.; Yaneva, Z. Innovative In Situ Interfacial Co-Assembled Lignin/Chitosan Nanoparticles—Green Synthesis, Physicochemical Characterization, In Vitro Release, and Intermolecular Interactions. Int. J. Mol. Sci. 2025, 26, 6883. https://doi.org/10.3390/ijms26146883
Yanev Z, Georgieva D, Hristova S, Tzanova M, Nicheva D, Andonova-Lilova B, Zagorcheva T, Vladova D, Grozeva N, Yaneva Z. Innovative In Situ Interfacial Co-Assembled Lignin/Chitosan Nanoparticles—Green Synthesis, Physicochemical Characterization, In Vitro Release, and Intermolecular Interactions. International Journal of Molecular Sciences. 2025; 26(14):6883. https://doi.org/10.3390/ijms26146883
Chicago/Turabian StyleYanev, Zhani, Denitsa Georgieva, Silviya Hristova, Milena Tzanova, Denitsa Nicheva, Boika Andonova-Lilova, Tzvetelina Zagorcheva, Diyana Vladova, Neli Grozeva, and Zvezdelina Yaneva. 2025. "Innovative In Situ Interfacial Co-Assembled Lignin/Chitosan Nanoparticles—Green Synthesis, Physicochemical Characterization, In Vitro Release, and Intermolecular Interactions" International Journal of Molecular Sciences 26, no. 14: 6883. https://doi.org/10.3390/ijms26146883
APA StyleYanev, Z., Georgieva, D., Hristova, S., Tzanova, M., Nicheva, D., Andonova-Lilova, B., Zagorcheva, T., Vladova, D., Grozeva, N., & Yaneva, Z. (2025). Innovative In Situ Interfacial Co-Assembled Lignin/Chitosan Nanoparticles—Green Synthesis, Physicochemical Characterization, In Vitro Release, and Intermolecular Interactions. International Journal of Molecular Sciences, 26(14), 6883. https://doi.org/10.3390/ijms26146883








