Strategic Advances in Targeted Delivery Carriers for Therapeutic Cancer Vaccines
Abstract
1. Introduction
2. Viral Vector Vaccines
2.1. Adenoviral (Ad) Vectors
2.2. Adeno-Associated Virus (AAV)
2.3. Poxvirus Vectors
2.4. Oncolytic Virus (OVs)
3. Non-Viral Vector Vaccines
3.1. Nanoparticle Delivery Systems
3.1.1. Lipid Nanoparticles (LNPs)
| Targeting Mechanism | Strategy | Specific Approach | Reference |
|---|---|---|---|
| Passive | 3-component formulation (3-Comp) | Cholesterol removal enhances pulmonary tropism | [160] |
| Cholesterol removal combined with miR-122/142-modified mRNA for dual organ/cell targeting | [161] | ||
| Selective Organ Targeting (SORT) | Addition of charged lipids enables organ-specific tropism | [162] | |
| Anionic lipids promote splenic accumulation | [163] | ||
| Component replacement | Bile acid substitution for cholesterol enhances splenic targeting | [164] | |
| Ionizable lipid screening | Lipid library screened for lung-specific delivery | [165] | |
| Hydrophobic tail optimization | Branched chains increase ovarian tumor selectivity | [166] | |
| Ionizable lipid + phospholipid tuning | T-cell targeting achieved via phospholipid enrichment and cholesterol reduction | [167] | |
| pH-responsive lipids | CL4H6 lipid (a synthetic ionizable lipid) enables delivery to tumor-associated macrophages | [168] | |
| Active | Antibody conjugation + chemotactic cue | Surface anti-PECAM-1 and cationic lipid chemotaxis enhance lung targeting | [169] |
| Surface peptide conjugation | D-peptide–PEG conjugates direct LNPs to PD-L1+ tumor cells | [170] | |
| Pardaxin-modified LNPs facilitate endoplasmic reticulum (ER)-specific delivery | [171] | ||
| Ganglioside insertion | CD169 targeting enabled via ganglioside incorporation | [172] | |
| Dendritic cell (DC) membrane coating | DC membrane-coated LNPs target TME | [173] |
3.1.2. Polymeric Nanoparticles (PNP)
3.1.3. Inorganic Nanoparticle Carriers
3.2. Cell-Based Delivery Platforms
3.2.1. Dendritic Cells (DCs)
3.2.2. Engineered Immune Cells
3.2.3. Stem Cells
3.3. Membrane-Derived Vesicular Carriers
3.4. Plant Virus-Derived Nanoparticles for Cancer Vaccine Delivery
3.5. Bacterial Vectors
4. Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Vergati, M.; Intrivici, C.; Huen, N.-Y.; Schlom, J.; Tsang, K.Y. Strategies for Cancer Vaccine Development. J. Biomed. Biotechnol. 2010, 2010, 596432. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; Brem, H.; Langer, R. Advancing the Field of Drug Delivery: Taking Aim at Cancer. Cancer Cell 2003, 4, 337–341. [Google Scholar] [CrossRef] [PubMed]
- The History of Cancer. First Cancer Diagnosis. Available online: https://www.cancer.org/cancer/understanding-cancer/history-of-cancer.html (accessed on 4 July 2025).
- Coley, W.B. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, K.; Singh, M.; Sharan, K.; Aggarwal, S. Coley’s Toxin to First Approved Therapeutic Vaccine—A Brief Historical Account in the Progression of Immunobiology-Based Cancer Treatment. Biomedicines 2024, 12, 2746. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Sellars, M.C.; Wu, C.J.; Fritsch, E.F. Cancer Vaccines: Building a Bridge over Troubled Waters. Cell 2022, 185, 2770–2788. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.-H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Capobianchi, M.R.; Del Porto, P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Aurisicchio, L.; Ciliberto, G. Genetic Cancer Vaccines: Current Status and Perspectives. Expert Opin. Biol. Ther. 2012, 12, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 5825401. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery Methods to Increase Cellular Uptake and Immunogenicity of DNA Vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.E.; Jansen, K. Turning the Corner on Therapeutic Cancer Vaccines. npj Vaccines 2019, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Y.; Tian, X.; Wei, X. Cancer Vaccines: Current Status and Future Directions. J. Hematol. Oncol. J Hematol. Oncol. 2025, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gao, H.; Tan, D.; Zhang, H.; Wang, J. mRNA Cancer Vaccines: Advances, Trends and Challenges. Acta Pharm. Sin. B 2022, 12, 2969–2989. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges and Prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Pounraj, S.; Chen, S.; Ma, L.; Mazzieri, R.; Dolcetti, R.; Rehm, B.H.A. Targeting Tumor Heterogeneity with Neoantigen-Based Cancer Vaccines. Cancer Res. 2024, 84, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA Vaccines: Ready for Prime Time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, Q.; Huang, X.; Koo, S.; Kong, N.; Tao, W. mRNA-Based Cancer Therapeutics. Nat. Rev. Cancer 2023, 23, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Sasso, E.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Folgori, A.; Nicosia, A. New Viral Vectors for Infectious Diseases and Cancer. Chall. Vaccinol. 2020, 50, 101430. [Google Scholar] [CrossRef] [PubMed]
- Schlom, J. Therapeutic Cancer Vaccines: Current Status and Moving Forward. JNCI J. Natl. Cancer Inst. 2012, 104, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Scarsella, L.; Ehrke-Schulz, E.; Paulussen, M.; Thal, S.C.; Ehrhardt, A.; Aydin, M. Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications. Viruses 2024, 16, 377. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, I.; Kamen, A. Production of Adenovirus Vector for Gene Therapy. Biotechnol. Adv. 2003, 20, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Sato-Dahlman, M.; LaRocca, C.J.; Yanagiba, C.; Yamamoto, M. Adenovirus and Immunotherapy: Advancing Cancer Treatment by Combination. Cancers 2020, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Majhen, D.; Calderon, H.; Chandra, N.; Fajardo, C.A.; Rajan, A.; Alemany, R.; Custers, J. Adenovirus-Based Vaccines for Fighting Infectious Diseases and Cancer: Progress in the Field. Hum. Gene Ther. 2014, 25, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Löser, P.; Cichon, G.; Arnold, W.; Both, G.W.; Strauss, M. Ovine Adenovirus Vectors Overcome Preexisting Humoral Immunity against Human Adenoviruses In Vivo. J. Virol. 1999, 73, 6930–6936. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.E.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-Chimaeric Adenovirus Serotype 5 Vectors Circumvent Pre-Existing Anti-Vector Immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of Adenovirus with Retention of Infectivity and Protection from Neutralizing Antibody in Vitro and in Vivo. Hum. Gene Ther. 1999, 10, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Dmitriev, I.P.; Brough, D.E.; Kashentseva, E.A.; Li, J.; Curiel, D.T. A New Gorilla Adenoviral Vector with Natural Lung Tropism Avoids Liver Toxicity and Is Amenable to Capsid Engineering and Vector Retargeting. J. Virol. 2020, 94, e00265-20. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nishikawaji, Y.; Kawakami, H.; Kosai, K. Adenovirus Biology, Recombinant Adenovirus, and Adenovirus Usage in Gene Therapy. Viruses 2021, 13, 2502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, W.; Wang, L.; Gao, P.; Li, Z.; Wu, J.; Zhang, H.; Wu, H.; Kong, W.; Yu, B.; et al. Enhancing the Antitumor Activity of an Engineered TRAIL-Coated Oncolytic Adenovirus for Treating Acute Myeloid Leukemia. Signal Transduct. Target. Ther. 2020, 5, 40. [Google Scholar] [CrossRef]
- Wienen, F.; Nilson, R.; Allmendinger, E.; Graumann, D.; Fiedler, E.; Bosse-Doenecke, E.; Kochanek, S.; Krutzke, L. Affilin-Based Retargeting of Adenoviral Vectors to the Epidermal Growth Factor Receptor. Biomater. Adv. 2023, 144, 213208. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.-R.; Hong, J.; Li, Y.; Shin, H.C.; Lee, H.; Kim, H.S.; Yun, C.-O. Mesenchymal Stem Cell–Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019, 79, 4503–4514. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, X.; Guo, X.; Yang, C.; Hung, T.; Lu, Z. CELO Fiber1 Knob Is a Promising Candidate to Modify the Tropism of Adenoviral Vectors. Genes 2022, 13, 2316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Huang, Y.; Wu, J.; Wang, Y.; Chen, A.; Guo, Q.; Zhang, Y.; Zhang, S.; Wang, L.; et al. An Oncolytic System Produces Oxygen Selectively in Pancreatic Tumor Cells to Alleviate Hypoxia and Improve Immune Activation. Pharmacol. Res. 2024, 199, 107053. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, J.; Ji, W.; Wang, G.; Fang, L.; Zhang, Q.; Ang, L.; Zhao, M.; Sen, Y.; Chen, L.; et al. Triple-Serotype Chimeric Oncolytic Adenovirus Exerts Multiple Synergistic Mechanisms against Solid Tumors. J. Immunother. Cancer 2022, 10, e004691. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Duffy, M.R.; Lei-Rossmann, J.; Muntzer, A.; Scott, E.M.; Hagel, J.; Campo, L.; Bryant, R.J.; Verrill, C.; Lambert, A.; et al. An Oncolytic Virus Expressing a T-Cell Engager Simultaneously Targets Cancer and Immunosuppressive Stromal Cells. Cancer Res. 2018, 78, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Møller, A.W.; Garofalo, M.; Cerullo, V.; Pesonen, S.; Alemany, R.; Jaderberg, M. Antitumor-specific T-cell Responses Induced by Oncolytic Adenovirus ONCOS-102 (AdV5/3-D24-GM-CSF) in Peritoneal Mesothelioma Mouse Model. J. Med. Virol. 2018, 90, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Zhang, J.; Chu, J.; Zhang, Z. Novel Combination Therapy Using Recombinant Oncolytic Adenovirus Silk Hydrogel and PD-L1 Inhibitor for Bladder Cancer Treatment. J. Nanobiotechnol. 2024, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Dong, T.; Phung, A.T.; Shah, J.R.; Larson, C.; Sanchez, A.B.; Blair, S.L.; Oronsky, B.; Trogler, W.C.; Reid, T.; et al. Full Remission of CAR-Deficient Tumors by DOTAP-Folate Liposome Encapsulation of Adenovirus. ACS Biomater. Sci. Eng. 2022, 8, 5199–5209. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, X.; Wang, Q.; Zhang, J.; Huang, D.; Chen, E.; Qian, H.; Zhong, Y.; Tang, Q.; Chen, W. Tumor Localization of Oncolytic Adenovirus Assisted by pH-Degradable Microgels with JQ1-Mediated Boosting Replication and PD-L1 Suppression for Enhanced Cancer Therapy. Biomater. Sci. 2020, 8, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, H.; Zhang, C.; Wang, Y.; Zhang, C.; Zhang, Y.; Zhong, A.; Zhang, D.; Liu, X. Silk-Gel Powered Adenoviral Vector Enables Robust Genome Editing of PD-L1 to Augment Immunotherapy across Multiple Tumor Models. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2206399. [Google Scholar] [CrossRef] [PubMed]
- Chekaoui, A.; Garofalo, M.; Gad, B.; Staniszewska, M.; Chiaro, J.; Pancer, K.; Gryciuk, A.; Cerullo, V.; Salmaso, S.; Caliceti, P.; et al. Cancer Vaccines: An Update on Recent Achievements and Prospects for Cancer Therapy. Clin. Exp. Med. 2024, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, S.; Luo, Z. Oncolytic Adenovirus, a New Treatment Strategy for Prostate Cancer. Biomedicines 2022, 10, 3262. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Asokan, A.; Samulski, R.J. Adeno-Associated Virus Serotypes: Vector Toolkit for Human Gene Therapy. Mol. Ther. 2006, 14, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Electron Microscopy of Adenovirus-Associated Virus (AAV) in Cell Cultures. Virology 1966, 29, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Kay, M. From Virus Evolution to Vector Revolution: Use of Naturally Occurring Serotypes of Adeno-Associated Virus (AAV) as Novel Vectors for Human Gene Therapy. Curr. Gene Ther. 2003, 3, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Basar, E.; Mead, H.; Shum, B.; Rauter, I.; Ay, C.; Skaletz-Rorowski, A.; Brockmeyer, N.H. Biological Barriers for Drug Delivery and Development of Innovative Therapeutic Approaches in HIV, Pancreatic Cancer, and Hemophilia a/B. Pharmaceutics 2024, 16, 1207. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Samulski, R.J. Engineering Adeno-Associated Virus Vectors for Gene Therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Chang, L.S.; Shenk, T. A Recombinant Plasmid from Which an Infectious Adeno-Associated Virus Genome Can Be Excised in Vitro and Its Use to Study Viral Replication. J. Virol. 1987, 61, 3096–3101. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.M.; Flotte, T.R. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol. 2019, 6, 601–621. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D.M.; Young, S.M.; Samulski, R.J. Integration of Adeno-Associated Virus (AAV) and Recombinant AAV Vectors. Annu. Rev. Genet. 2004, 38, 819–845. [Google Scholar] [CrossRef] [PubMed]
- Dagotto, G.; Fisher, J.L.; Li, D.; Li, Z.; Jenni, S.; Li, Z.; Tartaglia, L.J.; Abbink, P.; Barouch, D.H. Identification of a Novel Neutralization Epitope in Rhesus AAVs. Mol. Ther. Methods Clin. Dev. 2024, 32, 101350. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.-Y.; Lai, C.-Y.; Hung, W.-Z.; Chang, H.-Y.; Lin, P.-C.; Chiang, S.-F.; Ke, T.-W.; Liang, J.-A.; Shiau, A.-C.; Yang, P.-C.; et al. A Novel Engineered AAV-Based Neoantigen Vaccine in Combination with Radiotherapy Eradicates Tumors. Cancer Immunol. Res. 2023, 11, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Krotova, K.; Day, A.; Aslanidi, G. An Engineered AAV6-Based Vaccine Induces High Cytolytic Anti-Tumor Activity by Directly Targeting DCs and Improves Ag Presentation. Mol. Ther. Oncolytics 2019, 15, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.-C.; Hardet, R.; Prager, L.; Bentler, M.; Demeules, M.; John-Neek, P.; Jäschke, N.M.; Ha, T.C.; Hacker, U.T.; Adriouch, S.; et al. Capsid-Modified Adeno-Associated Virus Vectors as Novel Vaccine Platform for Cancer Immunotherapy. Mol. Ther. Methods Clin. Dev. 2023, 29, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Olarewaju, O.; Held, F.; Curtis, P.; Kenny, C.H.; Maier, U.; Panavas, T.; du Plessis, F. αFAP-Specific Nanobodies Mediate a Highly Precise Retargeting of Modified AAV2 Capsids Thereby Enabling Specific Transduction of Tumor Tissues. Mol. Ther. Methods Clin. Dev. 2024, 32, 101378. [Google Scholar] [CrossRef] [PubMed]
- Strecker, M.I.; Wlotzka, K.; Strassheimer, F.; Roller, B.; Ludmirski, G.; König, S.; Röder, J.; Opitz, C.; Alekseeva, T.; Reul, J.; et al. AAV-Mediated Gene Transfer of a Checkpoint Inhibitor in Combination with HER2-Targeted CAR-NK Cells as Experimental Therapy for Glioblastoma. Oncoimmunology 2022, 11, 2127508. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.A.; Edwin C Fluck, I.I.I.; Murphy, J.; Wang, Q.; Hoff, H.; Pumroy, R.A.; Lee, C.Y.; Sims, J.J.; Roy, S.; Moiseenkova-Bell, V.Y.; et al. Context-Specific Function of the Engineered Peptide Domain of PHP.B. J. Virol. 2021, 95, e01164. [Google Scholar] [CrossRef] [PubMed]
- Krotova, K.; Kuoch (Yoshitomi), H.; Caine, C.; Aslanidi, G. Tumor Antigen-Loaded AAV Vaccine Drives Protective Immunity in a Melanoma Animal Model. Mol. Ther. Methods Clin. Dev. 2023, 28, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Mulcrone, P.L.; Herzog, R.W.; Xiao, W. Adding Recombinant AAVs to the Cancer Therapeutics Mix. Mol. Ther. Oncolytics 2022, 27, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Ortiz, J.L.; Schaffer, D.V. Adeno-Associated Virus (AAV) Vectors in Cancer Gene Therapy. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Hensel, J.A.; Khattar, V.; Ashton, R.; Ponnazhagan, S. Recombinant AAV-CEA Tumor Vaccine in Combination with an Immune Adjuvant Breaks Tolerance and Provides Protective Immunity. Mol. Ther. Oncolytics 2018, 12, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, J.C., Jr.; Singh, J.; Carlson, R.; Leong, E.; Baybutt, T.R.; Barton, J.; Caparosa, E.; Pattison, A.; Rappaport, J.A.; Roh, J.; et al. Chimeric Ad5.F35 Vector Evades Anti-Adenovirus Serotype 5 Neutralization Opposing GUCY2C-Targeted Antitumor Immunity. J. Immunother. Cancer 2020, 8, e001046. [Google Scholar] [CrossRef] [PubMed]
- Daradoumis, J.; Ragonnaud, E.; Skandorff, I.; Nielsen, K.N.; Bermejo, A.V.; Andersson, A.-M.; Schroedel, S.; Thirion, C.; Neukirch, L.; Holst, P.J. An Endogenous Retrovirus Vaccine Encoding an Envelope with a Mutated Immunosuppressive Domain in Combination with Anti-PD1 Treatment Eradicates Established Tumours in Mice. Viruses 2023, 15, 926. [Google Scholar] [CrossRef] [PubMed]
- Rosewell Shaw, A.; Porter, C.; Biegert, G.; Jatta, L.; Suzuki, M. HydrAd: A Helper-Dependent Adenovirus Targeting Multiple Immune Pathways for Cancer Immunotherapy. Cancers 2022, 14, 2769. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.-J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, Heterologous Chimpanzee Adenovirus and Self-Amplifying mRNA Neoantigen Vaccine for Advanced Metastatic Solid Tumors: Phase 1 Trial Interim Results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Tejero, M.; Duzenli, O.F.; Kuoch, H.; Caine, C.; Krotova, K.; Paulaitis, M.; Aslanidi, G. Insights in AAV-Mediated Antigen-Specific Immunity and a Strategy for AAV Vaccine Dose Reduction through AAV-Extracellular Vesicle Association. Mol. Ther. Methods Clin. Dev. 2024, 32, 101358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, C.; Wang, X.-Y.; Yang, H. “Double-Punch” Strategy for Delivery of Viral Immunotherapy with Prolonged Tumor Retention and Enhanced Transfection Efficacy. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, S.; Kuryk, L.; Prygiel, M.; Lupo, M.G.; Zasada, A.A.; Pesce, C.; Ferri, N.; Rinner, B.; Salmaso, S.; Garofalo, M. Extracellular Vesicles Powered Cancer Immunotherapy: Targeted Delivery of Adenovirus-Based Cancer Vaccine in Humanized Melanoma Model. J. Control. Release 2024, 376, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chiu, M.S.; Yan, C.W.; Man, K.; Chen, Z. Eliminating Mesothelioma by AAV-Vectored, PD1-Based Vaccination in the Tumor Microenvironment. Mol. Ther. Oncolytics 2021, 20, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Jones, S. Zoonotic Poxvirus Infections in Humans. Curr. Opin. Infect. Dis. 2004, 17, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxviridae. In Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Volz, A.; Sutter, G. Chapter Five—Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 97, pp. 187–243. [Google Scholar]
- Liu, M.A. Immunologic Basis of Vaccine Vectors. Immunity 2010, 33, 504–515. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G. Poxvirus Tropism. Nat. Rev. Microbiol. 2005, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- de Vries, C.R.; Monken, C.E.; Lattime, E.C. The Addition of Recombinant Vaccinia HER2/Neu to Oncolytic Vaccinia-GMCSF given into the Tumor Microenvironment Overcomes MDSC-Mediated Immune Escape and Systemic Anergy. Cancer Gene Ther. 2015, 22, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ylösmäki, E.; Malorzo, C.; Capasso, C.; Honkasalo, O.; Fusciello, M.; Martins, B.; Ylösmäki, L.; Louna, A.; Feola, S.; Paavilainen, H.; et al. Personalized Cancer Vaccine Platform for Clinically Relevant Oncolytic Enveloped Viruses. Mol. Ther. 2018, 26, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Grundy, M.; Bau, L.; Wallington, S.; Balkaran, J.; Ramos, V.; Fisher, K.; Seymour, L.; Coussios, C.; Carlisle, R. Polymer Stealthing and Mucin-1 Retargeting for Enhanced Pharmacokinetics of an Oncolytic Vaccinia Virus. Mol. Ther. Oncolytics 2021, 21, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Su, Q.; Liang, Y.; Hu, J.; Yuan, S. Oncolytic Vaccine Virus Harbouring the IL-24 Gene Suppresses the Growth of Lung Cancer by Inducing Apoptosis. Biochem. Biophys. Res. Commun. 2016, 476, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Yan, W.; Wang, R.; Wang, X.; Guo, Y.; Dun, H.; Huan, Z.; Xu, L.; Han, R.; Sun, X.; et al. GM-CSF and IL-21-Armed Oncolytic Vaccinia Virus Significantly Enhances Anti-Tumor Activity and Synergizes with Anti-PD1 Immunotherapy in Pancreatic Cancer. Front. Immunol. 2025, 15, 1506632. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Freistaedter, A.; Jones, G.J.B.; Zervos, E.; Roper, R.L. Development of Improved Therapeutic Mesothelin-Based Vaccines for Pancreatic Cancer. PLoS ONE 2018, 13, e0193131. [Google Scholar] [CrossRef] [PubMed]
- Kochneva, G.; Sivolobova, G.; Tkacheva, A.; Grazhdantseva, A.; Troitskaya, O.; Nushtaeva, A.; Tkachenko, A.; Kuligina, E.; Richter, V.; Koval, O. Engineering of Double Recombinant Vaccinia Virus with Enhanced Oncolytic Potential for Solid Tumor Virotherapy. Oncotarget 2016, 7, 74171–74188. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Nguyen, P.; Hong, B.; DeRenzo, C.; Rainusso, N.C.; Rodriguez Cruz, T.; Wu, M.-F.; Liu, H.; Song, X.-T.; Suzuki, M.; et al. Engineering Oncolytic Vaccinia Virus to Redirect Macrophages to Tumor Cells. Adv. Cell Gene Ther. 2021, 4, e99. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia Virus-Mediated Cancer Immunotherapy: Cancer Vaccines and Oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.G.; Geoffroy, K.; Marguerie, M.; Khan, S.T.; Martin, N.T.; Kmiecik, J.; Bobbala, D.; Aitken, A.S.; de Souza, C.T.; Stephenson, K.B.; et al. Adjuvant Oncolytic Virotherapy for Personalized Anti-Cancer Vaccination. Nat. Commun. 2021, 12, 2626. [Google Scholar] [CrossRef] [PubMed]
- Ricordel, M.; Foloppe, J.; Antoine, D.; Findeli, A.; Kempf, J.; Cordier, P.; Gerbaud, A.; Grellier, B.; Lusky, M.; Quemeneur, E.; et al. Vaccinia Virus Shuffling: deVV5, a Novel Chimeric Poxvirus with Improved Oncolytic Potency. Cancers 2018, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Draganov, D.D.; Santidrian, A.F.; Minev, I.; Nguyen, D.; Kilinc, M.O.; Petrov, I.; Vyalkova, A.; Lander, E.; Berman, M.; Minev, B.; et al. Delivery of Oncolytic Vaccinia Virus by Matched Allogeneic Stem Cells Overcomes Critical Innate and Adaptive Immune Barriers. J. Transl. Med. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Mirbahari, S.N.; Silva, M.D.; Zúñiga, A.I.M.; Zamani, N.K.; St-Laurent, G.; Totonchi, M.; Azad, T. Recent Progress in Combination Therapy of Oncolytic Vaccinia Virus. Front. Immunol. 2024, 15, 1272351. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.J.; Hawkins, R.E.; Kaufman, H.L.; Thompson, J.A.; Tomczak, P.; Szczylik, C.; McDonald, M.; Eastty, S.; Shingler, W.H.; de Belin, J.; et al. Vaccination of Metastatic Renal Cancer Patients with MVA-5T4: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. Clin. Cancer Res. 2010, 16, 5539–5547. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pal, S.K.; Alex, A.; Agarwal, N. Development of PROSTVAC Immunotherapy in Prostate Cancer. Future Oncol. 2015, 11, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, R.; Miest, T.; Shashkova, E.V.; Barry, M.A. Reprogrammed Viruses as Cancer Therapeutics: Targeted, Armed and Shielded. Nat. Rev. Microbiol. 2008, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, Z.; Jing, R.; Zuo, B.; Gao, X.; Han, G.; Qi, H.; Wu, L.; Liu, Y.; Yin, H. Alarmin Augments the Antitumor Immunity of Lentiviral Vaccine in Ectopic, Orthotopic and Autochthonous Hepatocellular Carcinoma Mice. Theranostics 2019, 9, 4006–4018. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Norton, T.D.; Leibowitz, R.; Landau, N.R. Checkpoint Inhibitor-Expressing Lentiviral Vaccine Suppresses Tumor Growth in Preclinical Cancer Models. J. Immunother. Cancer 2024, 12, e008761. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, J.; Zhou, H.; Li, J.; Sun, C.; Zhu, W.; Yin, Y.; Li, X. Enhanced Anti-Tumor Response Elicited by a Novel Oncolytic HSV-1 Engineered with an Anti-PD-1 Antibody. Cancer Lett. 2021, 518, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Huang, H.; Grenier, J.M.; Perez, O.A.; Smilowitz, H.M.; Adler, B.; Khanna, K.M. Cytomegalovirus-Based Vaccine Expressing a Modified Tumor Antigen Induces Potent Tumor-Specific CD8(+) T-Cell Response and Protects Mice from Melanoma. Cancer Immunol. Res. 2015, 3, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Wu, C.; Qi, R.; Zeng, Y.; Huang, P.; Cao, J.; Chen, T.; Chen, K.; Lin, L.; Han, Q.; et al. Swine Pseudorabies Virus Attenuated Vaccine Reprograms the Kidney Cancer Tumor Microenvironment and Synergizes with PD-1 Blockade. J. Med. Virol. 2024, 96, e29568. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.F.; Verweij, M.C.; Nair, S.S.; Morrow, D.; Mansouri, M.; Chakravarty, D.; Beechwood, T.; Meyer, C.; Uebelhoer, L.; Lauron, E.J.; et al. CD8+ T Cell Targeting of Tumor Antigens Presented by HLA-E. Sci. Adv. 2024, 10, eadm7515. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, J.; An, Y.; Wang, X.; Liu, Y.; Yan, S.; Ye, X.; Qi, J.; Zhu, S.; Yu, Q.; et al. Recombinant Newcastle Disease Virus (NDV/Anh-IL-2) Expressing Human IL-2 as a Potential Candidate for Suppresses Growth of Hepatoma Therapy. J. Pharmacol. Sci. 2016, 132, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Pliquet, E.; Ruffie, C.; Escande, M.; Thalmensi, J.; Najburg, V.; Combredet, C.; Bestetti, T.; Julithe, M.; Liard, C.; Huet, T.; et al. Strong Antigen-Specific T-Cell Immunity Induced by a Recombinant Human TERT Measles Virus Vaccine and Amplified by a DNA/Viral Vector Prime Boost in IFNAR/CD46 Mice. Cancer Immunol. Immunother. CII 2019, 68, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Vannini, A.; Gatta, V.; Rambaldi, J.; Sanapo, M.; Barboni, C.; Zaghini, A.; Nanni, P.; Lollini, P.-L.; Casiraghi, C.; et al. A Fully-Virulent Retargeted Oncolytic HSV Armed with IL-12 Elicits Local Immunity and Vaccine Therapy towards Distant Tumors. PLoS Pathog. 2018, 14, e1007209. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, N.; Block, M.S.; Kim, J.W.; Shapiro, G.I.; Do, K.T.; Hwu, P.; Eder, J.P.; Jones, R.L.; Lu, H.; ter Meulen, J.H.; et al. First-in-Class, First-in-Human Study Evaluating LV305, a Dendritic-Cell Tropic Lentiviral Vector, in Sarcoma and Other Solid Tumors Expressing NY-ESO-1. Clin. Cancer Res. 2019, 25, 5808–5817. [Google Scholar] [CrossRef] [PubMed]
- Šustić, M.; Cokarić Brdovčak, M.; Lisnić, B.; Materljan, J.; Juranić Lisnić, V.; Rožmanić, C.; Indenbirken, D.; Hiršl, L.; Busch, D.H.; Brizić, I.; et al. Memory CD8 T Cells Generated by Cytomegalovirus Vaccine Vector Expressing NKG2D Ligand Have Effector-like Phenotype and Distinct Functional Features. Front. Immunol. 2021, 12, 681380. [Google Scholar] [CrossRef] [PubMed]
- Bryson, P.D.; Han, X.; Truong, N.; Wang, P. Breast Cancer Vaccines Delivered by Dendritic Cell-Targeted Lentivectors Induce Potent Antitumor Immune Responses and Protect Mice from Mammary Tumor Growth. Vaccine 2017, 35, 5842–5849. [Google Scholar] [CrossRef] [PubMed]
- Barasa, A.K.; Ye, P.; Phelps, M.; Arivudainambi, G.T.; Tison, T.; Ogembo, J.G. BALB/c Mice Immunized with a Combination of Virus-like Particles Incorporating Kaposi Sarcoma-Associated Herpesvirus (KSHV) Envelope Glycoproteins gpK8.1, gB, and gH/gL Induced Comparable Serum Neutralizing Antibody Activity to UV-Inactivated KSHV. Oncotarget 2017, 8, 34481–34497. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.O.; Ossmann, S.; Kaufmann, A.M.; Leitner, J.; Steinberger, P.; Willimsky, G.; Raftery, M.J.; Schönrich, G. Development of a Human Cytomegalovirus (HCMV)-Based Therapeutic Cancer Vaccine Uncovers a Previously Unsuspected Viral Block of MHC Class I Antigen Presentation. Front. Immunol. 2019, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, I.A.; Ramsay, A.J. The prime-boost strategy: Exciting prospects for improved vaccination. Immunol. Today 2000, 21, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Rühl, J.; Citterio, C.; Engelmann, C.; Haigh, T.; Dzionek, A.; Dreyer, J.; Khanna, R.; Taylor, G.S.; Wilson, J.B.; Leung, C.S.; et al. Heterologous Prime-Boost Vaccination Protects against EBV Antigen–Expressing Lymphomas. J. Clin. Investig. 2019, 129, 2071–2087. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Quach, T.H.T.; Tran, T.M.; Phuoc, H.N.; Nguyen, H.T.; Vo, T.K.; Vo, G.V. Reactogenicity and Immunogenicity of Heterologous Prime-Boost Immunization with COVID-19 Vaccine. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 147, 112650. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.-T.; Du, W.-L.; Liu, Y.-Y.; Lan, H.-R.; Si, J.-X.; Mou, X.-Z. Oncolytic Virotherapy in Solid Tumors: The Challenges and Achievements. Cancers 2021, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic Virotherapy: Basic Principles, Recent Advances and Future Directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.; Li, R.; Huang, X.; Liu, Q. Design of Outer Membrane Vesicles as Cancer Vaccines: A New Toolkit for Cancer Therapy. Cancers 2019, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Du, S.; Dong, Y. mRNA Delivery in Cancer Immunotherapy. Acta Pharm. Sin. B 2023, 13, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Li, G.; Fu, W.; Lei, C. Exosomes: The next Frontier in Vaccine Development and Delivery. Front. Immunol. 2024, 15, 1435426. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Peng, L.; Han, Y.; Wang, D.; He, X.; Wang, J.; Ou, C. Lipid Nanoparticle-Based mRNA Vaccines in Cancers: Current Advances and Future Prospects. Front. Immunol. 2022, 13, 922301. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Whitehead, K.A. Tools for Translation: Non-Viral Materials for Therapeutic mRNA Delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Kon, E.; Elia, U.; Peer, D. Principles for Designing an Optimal mRNA Lipid Nanoparticle Vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.-Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard Nanomaterial Clearance by the Liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Ad-El, N.; Hazan-Halevy, I.; Stotsky-Oterin, L.; Peer, D. Targeting Cancer with mRNA–Lipid Nanoparticles: Key Considerations and Future Prospects. Nat. Rev. Clin. Oncol. 2023, 20, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef] [PubMed]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin Formulated in Solid Lipid Nanoparticles Has Enhanced Efficacy in Hodgkin’s Lymphoma in Mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Bahadori, M.B.; Vandghanooni, S.; Eskandani, M.; Nakhlband, A.; Eskandani, M. Preparation, characterization and anti-proliferative effects of sclareol-loaded solid lipid nanoparticles on A549 human lung epithelial cancer cells. J. Drug Deliv. Sci. Technol. 2018, 45, 272–280. [Google Scholar] [CrossRef]
- Yoo, S.; Faisal, M.; Bae, S.-H.; Youn, K.; Park, H.-J.; Kwon, S.P.; Hwang, I.K.; Lee, J.; Kim, H.J.; Nam, J.-H.; et al. Novel Less Toxic, Lymphoid Tissue-Targeted Lipid Nanoparticles Containing a Vitamin B5-Derived Ionizable Lipid for mRNA Vaccine Delivery. Adv. Healthc. Mater. 2024, 14, 2403366. [Google Scholar] [CrossRef] [PubMed]
- Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupzak, B.; Dahlman, J.E. Constrained Nanoparticles Deliver siRNA and sgRNA to T Cells In Vivo without Targeting Ligands. Adv. Mater. 2019, 31, e1902251. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid Nanoparticle-Mediated Lymph Node–Targeting Delivery of mRNA Cancer Vaccine Elicits Robust CD8+T Cell Response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, D.; Wang, Z.; Huang, Q.; Huang, F.; Ye, Z.; Wich, D.; Chen, M.; Khirallah, J.; Gao, S.; et al. Antitumour Vaccination via the Targeted Proteolysis of Antigens Isolated from Tumour Lysates. Nat. Biomed. Eng. 2025, 9, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhao, G.; Gong, N.; Han, X.; Shepherd, S.J.; Xiong, X.; Xiao, Z.; Palanki, R.; Xu, J.; Swingle, K.L.; et al. Combinatorial Design of Siloxane-Incorporated Lipid Nanoparticles Augments Intracellular Processing for Tissue-Specific mRNA Therapeutic Delivery. Nat. Nanotechnol. 2025, 20, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, J.; Shen, R.; Lin, J.; Li, S.; Lu, X.; Stelzel, J.L.; Kong, J.; Cheng, L.; Vuong, I.; et al. Screening for Lipid Nanoparticles That Modulate the Immune Activity of Helper T Cells towards Enhanced Antitumour Activity. Nat. Biomed. Eng. 2024, 8, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Naidu, G.S.; Karpov, O.; Goldsmith, M.; Sharma, P.; Ezra, A.; Stotsky, L.; Breier, D.; Peer, D. Lipid Nanoparticles with Fine-Tuned Composition Show Enhanced Colon Targeting as a Platform for mRNA Therapeutics. Adv. Sci. 2025, 12, 2408744. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Hamilton, A.G.; Zhao, G.; Xiao, Z.; El-Mayta, R.; Han, X.; Gong, N.; Xiong, X.; Xu, J.; Figueroa-Espada, C.G.; et al. High-Throughput Barcoding of Nanoparticles Identifies Cationic, Degradable Lipid-like Materials for mRNA Delivery to the Lungs in Female Preclinical Models. Nat. Commun. 2024, 15, 1884. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Y.; Zhou, M.; Xu, S.; Varley, A.J.; Golubovic, A.; Lu, R.X.Z.; Wang, K.C.; Yeganeh, M.; Vosoughi, D.; et al. Combinatorial Design of Ionizable Lipid Nanoparticles for Muscle-Selective mRNA Delivery with Minimized off-Target Effects. Proc. Natl. Acad. Sci. USA 2023, 120, e2309472120. [Google Scholar] [CrossRef] [PubMed]
- Bevers, S.; Kooijmans, S.A.A.; Van De Velde, E.; Evers, M.J.W.; Seghers, S.; Gitz-Francois, J.J.J.M.; Van Kronenburg, N.C.H.; Fens, M.H.A.M.; Mastrobattista, E.; Hassler, L.; et al. mRNA-LNP Vaccines Tuned for Systemic Immunization Induce Strong Antitumor Immunity by Engaging Splenic Immune Cells. Mol. Ther. 2022, 30, 3078–3094. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, B.; Yang, Y.; Jiang, Y.; Wang, R.; Wei, Q.; Pan, Y.; Chen, Y.; Wang, H.; Fan, J.; et al. Low-Dose Mildronate-Derived Lipidoids for Efficient mRNA Vaccine Delivery with Minimal Inflammation Side Effects. ACS Nano 2024, 18, 23289–23300. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Yu, Z.; Wang, J.; Li, N.; Wang, A.; Xue, T.; Wang, Q.; Shi, Y.; Han, L.; Qin, W.; et al. Discovery of Ketal-Ester Ionizable Lipid Nanoparticle with Reduced Hepatotoxicity, Enhanced Spleen Tropism for mRNA Vaccine Delivery. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2024, 11, e2404684. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Xu, Y.; Qiu, H.; Cao, F.; Xiao, Z.-X.; Zhang, C.; Zha, G.-F. Personalized membrane protein vaccine based on a lipid nanoparticle delivery system prevents postoperative recurrence in colorectal cancer models. Acta Biomater. 2025, 192, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, J.; Sui, D.; Yang, Q.; Wang, T.; Xu, Z.; Li, X.; Gao, X.; Yan, X.; Liu, X.; et al. Simultaneous dendritic cells targeting and effective endosomal escape enhance sialic acid-modified mRNA vaccine efficacy and reduce side effects. J. Control. Release 2023, 364, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, M.M.; Gong, N.; Mukalel, A.J.; Thatte, A.S.; El-Mayta, R.; Patel, S.K.; Metzloff, A.E.; Swingle, K.L.; Han, X.; Xue, L.; et al. In Vivo mRNA CAR T Cell Engineering via Targeted Ionizable Lipid Nanoparticles with Extrahepatic Tropism. Small 2024, 20, 2304378. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Qi, S.; Yu, X.; Gao, X.; Yang, K.; Zhang, X.; Cheng, M.; Bai, B.; Feng, Y.; Lu, M.; et al. Development of Mannosylated Lipid Nanoparticles for mRNA Cancer Vaccine with High Antigen Presentation Efficiency and Immunomodulatory Capability. Angew. Chem. Int. Ed. 2024, 63, e202318515. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Shuvaev, V.V.; Pardi, N.; Khoshnejad, M.; Kiseleva, R.Y.; Brenner, J.S.; Uhler, T.; Tuyishime, S.; Mui, B.L.; Tam, Y.K.; et al. PECAM-1 Directed Re-Targeting of Exogenous mRNA Providing Two Orders of Magnitude Enhancement of Vascular Delivery and Expression in Lungs Independent of Apolipoprotein E-Mediated Uptake. J. Control. Release Off. J. Control. Release Soc. 2018, 291, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, I.; Laczkó, D.; Shahnawaz, H.; Muramatsu, H.; Natesan, A.; Yadegari, A.; Papp, T.E.; Alameh, M.-G.; Shuvaev, V.; Mui, B.L.; et al. Highly Efficient CD4+ T Cell Targeting and Genetic Recombination Using Engineered CD4+ Cell-Homing mRNA-LNPs. Mol. Ther. 2021, 29, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Méndez Fernández, P.O.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T Cells Produced In Vivo to Treat Cardiac Injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Choi, J.; Hwang, J.; Kim, S.; Kim, Y.; Shim, M.K.; Park, W.; Yu, S.; Jung, S.; Yang, Y.; et al. Apolipoprotein Fusion Enables Spontaneous Functionalization of mRNA Lipid Nanoparticles with Antibody for Targeted Cancer Therapy. ACS Nano 2025, 19, 6412–6425. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Feng, Y.; Lu, Y.; Huang, R.; Zhang, Y.; Zhao, Y.; Mo, R. Lymph-Targeted High-Density Lipoprotein-Mimetic Nanovaccine for Multi-Antigenic Personalized Cancer Immunotherapy. Sci. Adv. 2024, 10, eadk2444. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, K.; Yang, K.; Ma, W.; Qi, S.; Yu, X.; He, J.; Lin, X.; Yu, G. Circular RNA Cancer Vaccines Drive Immunity in Hard-to-Treat Malignancies. Theranostics 2022, 12, 6422–6436. [Google Scholar] [CrossRef] [PubMed]
- Ramos da Silva, J.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.F.M.M.; Moreno, A.C.R.; Aps, L.R.M.M.; Venceslau-Carvalho, A.A.; et al. Single Immunizations of Self-Amplifying or Non-Replicating mRNA-LNP Vaccines Control HPV-Associated Tumors in Mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Liu, M.; Zhu, F.; Zhao, D.; Liu, G.; Han, T.; Fei, C.; Zeng, W.; Chen, S.; Wu, Q.; et al. FcRn-Guided Antigen Trafficking Enhances Cancer Vaccine Efficacy. Cancer Immunol. Immunother. CII 2025, 74, 54. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Mészáros, T.; Vashegyi, I.; Fülöp, T.; Örfi, E.; Dézsi, L.; Rosivall, L.; Bavli, Y.; Urbanics, R.; Mollnes, T.E.; et al. Pseudo-Anaphylaxis to Polyethylene Glycol (PEG)-Coated Liposomes: Roles of Anti-PEG IgM and Complement Activation in a Porcine Model of Human Infusion Reactions. ACS Nano 2019, 13, 9315–9324. [Google Scholar] [CrossRef] [PubMed]
- 157Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal Escape: A Bottleneck for LNP-Mediated Therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef] [PubMed]
- Jacob, E.M.; Huang, J.; Chen, M. Lipid Nanoparticle-Based mRNA Vaccines: A New Frontier in Precision Oncology. Precis. Clin. Med. 2024, 7, pbae017. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Materials 2023, 35, 2303261. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Shi, L.; Sheng, T.; Yan, X.; Lin, L.; Meng, C.; Wu, S.; Chen, Y.; Zhang, Y.; Wang, C.; et al. Reformulating Lipid Nanoparticles for Organ-Targeted mRNA Accumulation and Translation. Nat. Commun. 2024, 15, 5659. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Yu, X.; Liu, P.; Ren, H.; Wei, T.; Cheng, Q. Simplified Lipid Nanoparticles for Tissue- and Cell-Targeted mRNA Delivery Facilitate Precision Tumor Therapy in a Lung Metastasis Mouse Model. Adv. Mater. 2024, 36, 2409812. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective ORgan Targeting (SORT) Nanoparticles for Tissue Specific mRNA Delivery and CRISPR/Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lin, Y.; Meng, Y.; Li, M.; Ren, H.; Shi, H.; Cheng, Q.; Wei, T. Spleen-Targeted mRNA Vaccine Doped with Manganese Adjuvant for Robust Anticancer Immunity In Vivo. ACS Nano 2024, 18, 30701–30715. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Billingsley, M.M.; Mukalel, A.J.; Thatte, A.S.; Hamilton, A.G.; Gong, N.; El-Mayta, R.; Safford, H.C.; Merolle, M.; Mitchell, M.J. Bile Acid-Containing Lipid Nanoparticles Enhance Extrahepatic mRNA Delivery. Theranostics 2024, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, Y.; Solek, N.C.; Chen, J.; Gong, F.; Varley, A.J.; Golubovic, A.; Pan, A.; Dong, S.; Zheng, G.; et al. Tumor-Tailored Ionizable Lipid Nanoparticles Facilitate IL-12 Circular RNA Delivery for Enhanced Lung Cancer Immunotherapy. Adv. Mater. 2024, 36, 2400307. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, X.; Wang, L.; Wu, C.; Shuai, Q.; Zhang, Y.; Liu, S. Branched Hydrophobic Tails in Lipid Nanoparticles Enhance mRNA Delivery for Cancer Immunotherapy. Biomaterials 2023, 301, 122279. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, M.M.; Hamilton, A.G.; Mai, D.; Patel, S.K.; Swingle, K.L.; Sheppard, N.C.; June, C.H.; Mitchell, M.J. Orthogonal Design of Experiments for Optimization of Lipid Nanoparticles for mRNA Engineering of CAR T Cells. Nano Lett. 2022, 22, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the Function of Tumor-Associated Macrophages by siRNA-Loaded Lipid Nanoparticles for Cancer Immunotherapy. J. Control. Release 2020, 325, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.E.; Omo-Lamai, S.; Patel, M.N.; Wu, J.; Arguiri, E.; Muzykantov, V.R.; Myerson, J.W.; Marcos-Contreras, O.A.; Brenner, J.S. Combination of Physicochemical Tropism and Affinity Moiety Targeting of Lipid Nanoparticles Enhances Organ Targeting. Nano Lett. 2024, 24, 4774–4784. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J.; Kim, E.H.; Park, W.; Jang, H.; Jang, Y.; Chi, S.-G.; Kweon, D.-H.; Lee, K.; Kim, S.H.; et al. Design of PD-L1-Targeted Lipid Nanoparticles to Turn on PTEN for Efficient Cancer Therapy. Adv. Sci. 2024, 11, 2309917. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Li, X.; Huang, J.; Guo, X.; Zhang, J.; Luo, Z.; Shi, Y.; Jiang, M.; Qin, B.; et al. ER-Targeting PDT Converts Tumors into In Situ Therapeutic Tumor Vaccines. ACS Nano 2022, 16, 9240–9253. [Google Scholar] [CrossRef] [PubMed]
- Affandi, A.J.; Grabowska, J.; Olesek, K.; Lopez Venegas, M.; Barbaria, A.; Rodríguez, E.; Mulder, P.P.G.; Pijffers, H.J.; Ambrosini, M.; Kalay, H.; et al. Selective Tumor Antigen Vaccine Delivery to Human CD169+ Antigen-Presenting Cells Using Ganglioside-Liposomes. Proc. Natl. Acad. Sci. USA 2020, 117, 27528–27539. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Choi, J.; Jin, D.; Xu, E.; Lee, J.; Byun, J.; Oh, Y.-K. Hybrid Lipid Nanoparticles with Tumor Antigen-Primed Dendritic Cell Membranes for Post-Surgical Tumor Immunotherapy. J. Control. Release 2025, 379, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Biodegradable, Polymeric Nanoparticle Delivery Systems for Cancer Therapy. Nanomed 2007, 2, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Prakash, G.; Ozturk, A.; Saghazadeh, S.; Sohail, M.F.; Seo, J.; Dockmeci, M.; Zhang, Y.S.; Khademhosseini, A. Evolution and Clinical Translation of Drug Delivery Nanomaterials. Nano Today 2017, 15, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Folkman, J. Polymers for the Sustained Release of Proteins and Other Macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, R.; Li, D.; Xiang, J.; Zhang, W.; Shi, X.; Xu, H.; Yao, S.; Liu, J.; Shao, S.; et al. Guanidine-Modified Nanoparticles as Robust BTZ Delivery Carriers and Activators of Immune Responses. J. Control. Release 2023, 357, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wilhelm, J.; Li, W.; Li, S.; Wang, Z.; Huang, G.; Wang, J.; Tang, H.; Khorsandi, S.; Sun, Z.; et al. Polycarbonate-Based Ultra-pH Sensitive Nanoparticles Improve Therapeutic Window. Nat. Commun. 2020, 11, 5828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Li, X.; Yu, Y.; Zhang, L.; Zhang, H.; Chen, C.; Chen, D.; Wang, M.; Xing, N.; et al. Targeting Hypoxia and Autophagy Inhibition via Delivering Sonodynamic Nanoparticles with HIF-2α Inhibitor for Enhancing Immunotherapy in Renal Cell Carcinoma. Adv. Healthc. Mater. 2024, 13, 2402973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cheng, F.; Zhang, Y.; Su, T.; Zhu, G. Engineering and Delivery of cGAS-STING Immunomodulators for the Immunotherapy of Cancer and Autoimmune Diseases. Acc. Chem. Res. 2023, 56, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Moses, A.S.; Demessie, A.A.; Singh, P.; Lee, H.; Korzun, T.; Taratula, O.R.; Alani, A.G.; Taratula, O. Poly(Aspartic Acid)-Based Polymeric Nanoparticle for Local and Systemic mRNA Delivery. Mol. Pharm. 2022, 19, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, J.; Li, R.; Tang, D.; Cao, Z.; Xu, C.; Xiao, H. Activating CD8+ T Cells by Pt(IV) Prodrug-Based Nanomedicine and aPD-L1 Antibody for Enhanced Cancer Immunotherapy. Adv. Mater. 2024, 36, 2311640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yue, L.; Cao, L.; Liu, K.; Yang, S.; Liang, S.; Liu, L.; Zhao, C.; Wu, D.; Wang, Z.; et al. Tumor Microenvironment-Responsive Macrophage-Mediated Immunotherapeutic Drug Delivery. Acta Biomater. 2024, 186, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.-T.N.; Duwa, R.; Lee, S.; Kwon, T.K.; Chang, J.-H.; Jeong, J.-H.; Yook, S. Targeting Tumor-Associated Macrophages with Mannosylated Nanotherapeutics Delivering TLR7/8 Agonist Enhances Cancer Immunotherapy. J. Control. Release 2024, 372, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Ferreira, E.; Miranda, A.; Ferreira, D.; Relvas-Santos, M.; Castro, F.; Santos, B.; Gonçalves, M.; Quintas, S.; Peixoto, A.; et al. Targeted and Self-Adjuvated Nanoglycovaccine Candidate for Cancer Immunotherapy. ACS Nano 2024, 18, 10088–10103. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Stephan, S.B.; Koehne, A.L.; Nelson, P.S.; Stephan, M.T. In Vitro-Transcribed Antigen Receptor mRNA Nanocarriers for Transient Expression in Circulating T Cells in Vivo. Nat. Commun. 2020, 11, 6080. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Nie, W.; Lyu, L.; Zhang, X.; Wang, W.; Zhang, Y.; He, S.; Guo, A.; Liu, F.; Wang, B.; et al. Tumor-Microenvironment-Activatable Nanoparticle Mediating Immunogene Therapy and M2 Macrophage-Targeted Inhibitor for Synergistic Cancer Immunotherapy. ACS Nano 2024, 18, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Weichselbaum, R.R.; Lin, W. Mitochondria-targeted Multifunctional Nanoparticles Combine Cuproptosis and Programmed Cell Death-1 Downregulation for Cancer Immunotherapy. Adv. Sci. 2024, 11, 2403520. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yang, J.; Peng, A.; Qian, Y.; Liu, Y.; Pan, P.; Liu, Q. Lysosome Targeted Nanoparticle Aggregation Reverses Immunosuppressive Tumor Microenvironment for Cancer Immunotherapy. Adv. Mater. 2024, 36, 2412730. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, Y.; Huang, X.; Shen, Y.; Zou, Q.; Yang, G.; Fu, L.; Liu, Q.; Luo, D. Photosensitive and Dual-Targeted Chromium Nanoparticle Delivering Small Interfering RNA YTHDF1 for Molecular-Targeted Immunotherapy in Liver Cancer. J. Nanobiotechnology 2024, 22, 348. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Zhang, M.; Ma, R.; Wang, Y.; Weng, X.; Zhang, J.; Zhang, Z.; Chen, X.; Yang, W. Polymeric Nanocarrier via Metabolism Regulation Mediates Immunogenic Cell Death with Spatiotemporal Orchestration for Cancer Immunotherapy. Nat. Commun. 2024, 15, 8586. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wang, H.; Yan, J.; Li, Y.; Dong, X.; Tian, S.; Sun, Y.; Luo, K.; He, B.; Liang, Y. Tailor-Made Autophagy Cascade Amplification Polymeric Nanoparticles for Enhanced Tumor Immunotherapy. Small 2023, 19, 2207898. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-Y.; Lin, S.-Y.; Wu, Y.-N.; Shen, C.-Y.; Sheu, M.-T.; Ho, H.-O. Glycosylation of OVA Antigen-Loaded PLGA Nanoparticles Enhances DC-Targeting for Cancer Vaccination. J. Control. Release 2022, 351, 970–988. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, M.; Pang, L.; Wang, S.; Kong, Y.; Zhu, X.; Zhou, X.; Wang, X.; Chen, C.; Ning, H.; et al. Identification of a Novel DEC-205 Binding Peptide to Develop Dendritic Cell-Targeting Nanovaccine for Cancer Immunotherapy. J. Control. Release 2024, 373, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Hao, Y.-Y.; Huang, L.-L.; Li, X.; Zou, J.; Zhang, S.-Y.; Yang, X.-Y.; Chen, H.-F.; Guo, Y.-X.; et al. Tumor-Selective Nano-Dispatcher Enforced Cancer Immunotherapeutic Effects via Regulating Lactate Metabolism and Activating Toll-like Receptors. Small Weinh. Bergstr. Ger. 2025, 21, e2406870. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fu, J.; Peng, H.; He, Y.; Chang, A.; Zhang, H.; Hao, Y.; Xu, X.; Li, S.; Zhao, J.; et al. Co-Delivery of Polyphyllin II and IR780 PLGA Nanoparticles Induced Pyroptosis Combined with Photothermal to Enhance Hepatocellular Carcinoma Immunotherapy. J. Nanobiotechnology 2024, 22, 647. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Padmanaban, S.; Chahal, S.; Mohapatra, A.; Sundaram, A.; Cho, C.-S.; Park, I.-K. Targeted Nanoparticle Delivery Unleashes Synergistic Photothermal and Immunotherapeutic Effects against Hepatocellular Carcinoma. J. Nanobiotechnology 2024, 22, 778. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, L.; He, W.; Teng, C.; Sun, S.; Lu, H.; Li, S.; Lv, L.; Cao, X.; Yin, H.; et al. In Situ Targeting Nanoparticles-Hydrogel Hybrid System for Combined Chemo-Immunotherapy of Glioma. J. Control. Release 2022, 345, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.-N.; Zhu, L.; Liang, J.; Zhao, D.-K.; Tian, T.-Y.; Fan, Y.-N.; Ye, S.-Y.; Liu, H.; Huang, X.-Y.; Cao, Z.-T.; et al. Orchestrating NK and T Cells via Tri-Specific Nano-Antibodies for Synergistic Antitumor Immunity. Nat. Commun. 2024, 15, 6211. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhao, C.; Pan, Y.; Guo, Y.; Liu, L.; Wu, J.; Zhang, Y.; Rao, L.; Li, Q. Genetically Engineered Cellular Nanoparticles Loaded with Curcuminoids for Cancer Immunotherapy. Theranostics 2024, 14, 6409–6425. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Agrawal, P.; Singh, S.K.; Chhonker, Y.S.; Sun, J.; Murry, D.J. Polymer-Based Drug Delivery Systems for Cancer Therapeutics. Polymers 2024, 16, 843. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Ocaña, A.; Castro-Osma, J.A.; Bravo, I.; Alonso-Moreno, C. Polyester Polymeric Nanoparticles as Platforms in the Development of Novel Nanomedicines for Cancer Treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ghassemi, A.H.; Hennink, W.E.; Schwendeman, S.P. The Microclimate pH in Poly(D,L-Lactide-Co-Hydroxymethyl Glycolide) Microspheres during Biodegradation. Biomaterials 2012, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 880337. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 1977. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhu, H.; Qin, Y.; Zhang, R.; Wang, L.; Zhang, E.; Zhou, X.; Meng, R. GP60 and SPARC as Albumin Receptors: Key Targeted Sites for the Delivery of Antitumor Drugs. Front. Pharmacol. 2024, 15, 1329636. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Li, Y.H.; Gao, Y.Q.; Wei, N.; Zhao, X.; Wang, C.X.; Li, Y.F.; Xiu, X.; Cui, J.X. Direct Comparison of Two Albumin-Based Paclitaxel-Loaded Nanoparticle Formulations: Is the Crosslinked Version More Advantageous? Int. J. Pharm. 2014, 468, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Brayden, D.J.; Cheung, D.L.; Liew, A.; Fitzgerald, M.; Pandit, A. Albumin-Based Delivery Systems: Recent Advances, Challenges, and Opportunities. J. Control. Release 2025, 380, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Hesemans, E.; Saffarzadeh, N.; Maksoudian, C.; Izci, M.; Chu, T.; Rios Luci, C.; Wang, Y.; Naatz, H.; Thieme, S.; Richter, C.; et al. Cu-Doped TiO2 Nanoparticles Improve Local Antitumor Immune Activation and Optimize Dendritic Cell Vaccine Strategies. J. Nanobiotechnology 2023, 21, 87. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Yuan, H.; Tian, M.; Zhang, X.; Xia, P.; Shi, G.; Hou, R.; Li, J.; Jiang, H.; Yang, Z.; et al. Precise Photodynamic Therapy by Midkine Nanobody-Engineered Nanoparticles Remodels the Microenvironment of Pancreatic Ductal Adenocarcinoma and Potentiates the Immunotherapy. ACS Nano 2024, 18, 4019–4037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, Z.; Cui, D.; He, S.; Jiang, Y.; Li, J.; Huang, J.; Pu, K. Semiconducting Polymer Nano-PROTACs for Activatable Photo-Immunometabolic Cancer Therapy. Nat. Commun. 2021, 12, 2934. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Niu, R.; Zhang, X.; Zhang, B.; Chen, X.; Guo, J.; Song, S.; Wang, Y.; Zhang, H.; Zhao, Y. Metal-Organic Framework-Based Nanovaccine for Relieving Immunosuppressive Tumors via Hindering Efferocytosis of Macrophages and Promoting Pyroptosis and Cuproptosis of Cancer Cells. ACS Nano 2024, 18, 12386–12400. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, M.K.; Nguyen, T.L.; Kim, J. Hollow Mesoporous Silica Nanoparticles with Extra-Large Mesopores for Enhanced Cancer Vaccine. ACS Appl. Mater. Interfaces 2020, 12, 34658–34666. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ming, H.; Li, B.; Liu, S.; Chen, L.; Zhang, T.; Gao, Y.; He, T.; Huang, C.; Du, Z. A pH and Glutathione-Responsive Carbon Monoxide-Driven Nano-Herb Delivery System for Enhanced Immunotherapy in Colorectal Cancer. J. Control. Release 2024, 376, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, F.; Zhang, L.; Liu, W.; Wang, X.; Zhu, R.; Qiao, Z.-A.; Yu, B.; Yu, X. TRAIL-Modified, Doxorubicin-Embedded Periodic Mesoporous Organosilica Nanoparticles for Targeted Drug Delivery and Efficient Antitumor Immunotherapy. Acta Biomater. 2022, 143, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Liao, Y.; Zuo, Q.; Liu, N.; Liu, Z. MnO2 Nanoparticles as a Minimalist Multimode Vaccine Adjuvant/Delivery System to Regulate Antigen Presenting Cells for Tumor Immunotherapy. J. Mater. Chem. B 2022, 10, 3474–3490. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Zou, F.; Gu, J.; Deng, S.; Cao, Y.; Cai, K. The Role of Inorganic Nanomaterials in Overcoming Challenges in Colorectal Cancer Diagnosis and Therapy. Pharmaceutics 2025, 17, 409. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Han, Y.; Ling, Z.; Meng, X.; Zhang, B.; Dong, W.; Zhang, D.; Chen, K. Nanomaterials: Breaking the Bottleneck of Breast Cancer Drug Resistance. Front. Immunol. 2024, 15, 1492546. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A Review of Mesoporous Silica Nanoparticle Delivery Systems in Chemo-Based Combination Cancer Therapies. Front. Chem. 2020, 8, 598722. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, S.; Sun, M.; Wei, G.; Chen, C. Silica Nanoparticles as Versatile Carriers for Nanofertilizers and Nanopesticides: Design and Applications. J. Agric. Food Chem. 2025, 73, 14742–14759. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Katiyar, P.; Kushwaha, K. Recent Advances in Mesoporous Silica Nanoparticle: Synthesis, Drug Loading, Release Mechanisms, and Diverse Applications. Front. Nanotechnol. 2025, 7, 1564188. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Multifunctional Iron Oxide Nanoparticles as Promising Magnetic Biomaterials in Drug Delivery: A Review. J. Funct. Biomater. 2024, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.F.; Bharadwaj, P.; Leblond Chain, J.; Roullin, V.G. Purification Processes of Polymeric Nanoparticles: How to Improve Their Clinical Translation? J. Control. Release 2023, 360, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wu, Y.; Xie, Y.; Yu, M.; Chen, Y. Advanced Polymeric Nanoparticles for Cancer Immunotherapy: Materials Engineering, Immunotherapeutic Mechanism and Clinical Translation. Adv. Mater. 2025, 37, 2413603. [Google Scholar] [CrossRef] [PubMed]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. Unlocking the Promise of mRNA Therapeutics. Nat. Biotechnol. 2022, 40, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C. Dendritic Cell Subsets in T Cell Programming: Location Dictates Function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Okamura, K.; Riding, R.L.; Fan, X.; Afshari, K.; Haddadi, N.-S.; McCauley, S.M.; Guney, M.H.; Luban, J.; Funakoshi, T.; et al. AIM2 Regulates Anti-Tumor Immunity and Is a Viable Therapeutic Target for Melanoma. J. Exp. Med. 2021, 218, e20200962. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, Z.; Heng, X.; Niu, X.; Wang, Y.; Yao, L.; Sun, L.; Liu, Z.; Chen, H. Click-Chemistry-Mediated Cell Membrane Glycopolymer Engineering to Potentiate Dendritic Cell Vaccines. Angew. Chem. 2024, 136, e202315782. [Google Scholar] [CrossRef]
- Lim, R.J.; Salehi-Rad, R.; Tran, L.M.; Oh, M.S.; Dumitras, C.; Crosson, W.P.; Li, R.; Patel, T.S.; Man, S.; Yean, C.E.; et al. CXCL9/10-Engineered Dendritic Cells Promote T Cell Activation and Enhance Immune Checkpoint Blockade for Lung Cancer. Cell Rep. Med. 2024, 5, 101479. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Martinez-Usatorre, A.; Li, L.; Hicham, M.; Guichard, A.; Marcone, R.; Fournier, N.; Torchia, B.; Martinez Bedoya, D.; Davanture, S.; et al. Cytokine-Armed Dendritic Cell Progenitors for Antigen-Agnostic Cancer Immunotherapy. Nat. Cancer 2024, 5, 240–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xing, F.; Dai, Y.; Zhang, Z.; Zhou, G.; Yang, S.; Liu, Y.-C.; Yuan, Z.; Luo, K.Q.; Ying, T.; et al. Navigating Chimeric Antigen Receptor-Engineered Natural Killer Cells as Drug Carriers via Three-Dimensional Mapping of the Tumor Microenvironment. J. Control. Release 2023, 362, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Du, F.; Wang, H.; Gong, X.; Xia, Y.; Zhang, X.; Deng, H.; Zhang, R.; Wang, Z. Genetically engineered macrophages as living cell drug carriers for targeted cancer therapy. J. Control. Release 2024, 367, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, R.; Liang, T.; Ren, H.; Park, C.; Tai, C.-H.; Ni, W.; Zhou, J.; Mackay, S.; Edmondson, E.; et al. Camel Nanobody-Based B7-H3 CAR-T Cells Show High Efficacy against Large Solid Tumours. Nat. Commun. 2023, 14, 5920. [Google Scholar] [CrossRef] [PubMed]
- Röder, J.; Alekseeva, T.; Kiefer, A.; Kühnel, I.; Prüfer, M.; Zhang, C.; Bodden, M.; Rosigkeit, S.; Waldmann, A.; Tonn, T.; et al. ErbB2/HER2-Targeted CAR-NK Cells Eliminate Breast Cancer Cells in an Organoid Model That Recapitulates Tumor Progression. Mol. Ther. 2025, 33, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Du, Z.; Li, L.; Qiao, L.; Zhang, S.; Yin, X.; Chang, X.; Li, C.; Hua, Z. Camouflaging Attenuated Salmonella by Cryo-Shocked Macrophages for Tumor-Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Milton, P.; Sittplangkoon, C.; Liu, X.; Sui, Z.; Boyce, B.F.; Yao, Z. Chimeric Antigen Receptor Dendritic Cells Targeted Delivery of a Single Tumoricidal Factor for Cancer Immunotherapy. Cancer Immunol. Immunother. CII 2024, 73, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Ma, T.; Zhu, D.; Liu, T.; Lv, F. Tumor Targeted Combination Therapy Mediated by Functional Macrophages under Fluorescence Imaging Guidance. J. Control. Release 2020, 328, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Y.; Xie, X.; Song, T.; Yang, G.; Su, Q.; Li, T.; Li, S.; Wu, C.; You, F.; et al. Engineered Mesenchymal Stem Cells as a Biotherapy Platform for Targeted Photodynamic Immunotherapy of Breast Cancer. Adv. Healthc. Mater. 2022, 11, 2101375. [Google Scholar] [CrossRef] [PubMed]
- Costa-Garcia, M.; Moya-Borrego, L.; Alemany Bonastre, R.; Moreno Olié, R. Optimized Protocol for Culturing Menstrual Blood-Derived MSCs for Combination with Oncolytic Adenoviruses in Cancer Treatment. Mol. Ther. Oncol. 2024, 32, 200907. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal Stem/Stromal Cells in Cancer Therapy. J. Hematol. Oncol. 2021, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kosuke, K.; Nishikawa, M. Mesenchymal Stem/Stromal Cells as next-Generation Drug Delivery Vehicles for Cancer Therapeutics. Expert Opin. Drug Deliv. 2021, 18, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Kamenšek, U.; Božič, T.; Čemažar, M.; Švajger, U. Antitumor Efficacy of Interleukin 12-Transfected Mesenchymal Stem Cells in B16-F10 Mouse Melanoma Tumor Model. Pharmaceutics 2025, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Adamus, T.; Hung, C.-Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K.; et al. Glioma-Targeted Delivery of Exosome-Encapsulated Antisense Oligonucleotides Using Neural Stem Cells. Mol. Ther. Nucleic Acids 2021, 27, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Kitadani, J.; Ojima, T.; Iwamoto, H.; Tabata, H.; Nakamori, M.; Nakamura, M.; Hayata, K.; Katsuda, M.; Miyajima, M.; Yamaue, H. Cancer Vaccine Therapy Using Carcinoembryonic Antigen—Expressing Dendritic Cells Generated from Induced Pluripotent Stem Cells. Sci. Rep. 2018, 8, 4569. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 Targeted and Cargo-Loaded Exosomes Facilitate Simultaneous Imaging and Therapy of Glioma in Vitro and in Vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, E.; Atkin, S.L.; Sahebkar, A. Use of Stem Cells as Carriers of Oncolytic Viruses for Cancer Treatment. J. Cell. Physiol. 2019, 234, 14906–14913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Duan, X.; Liu, Y.; Xu, J.; Al-bashari, A.A.G.; Ye, P.; Ye, Q.; He, Y. The Application of Mesenchymal Stem Cells in Future Vaccine Synthesis. Vaccines 2023, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Mirkazemi, K.; Kamalinejad, A.; Doroudian, M. Exosome-Based Advances in Pancreatic Cancer: The Potential of Mesenchymal Stem Cells. Crit. Rev. Oncol. Hematol. 2025, 207, 104594. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, E.; Hesami, S.; Movahed, E.; Keshel, S.H.; Doroudian, M. Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Strategy in the Brain Tumors. Stem Cell Res. Ther. 2022, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huang, W.; Liu, M.; Chen, J.; Xiao, D.; Peng, Z.; He, H.; Shen, H.; Jin, Q.; Chen, L.; et al. Progress of Mesenchymal Stem Cell-Derived Exosomes in Targeted Delivery of Antitumor Drugs. Cancer Cell Int. 2025, 25, 169. [Google Scholar] [CrossRef] [PubMed]
- Galland, S.; Stamenkovic, I. Mesenchymal Stromal Cells in Cancer: A Review of Their Immunomodulatory Functions and Dual Effects on Tumor Progression. J. Pathol. 2020, 250, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular Vesicles in Immunomodulation and Tumor Progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Yang, Z.; Li, H.; Wei, M.; Yang, G.; Xing, H.; Li, Q. Smart Exosomes with Lymph Node Homing and Immune-Amplifying Capacities for Enhanced Immunotherapy of Metastatic Breast Cancer. Mol. Ther. Nucleic Acids 2021, 26, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Phung, C.D.; Pham, T.T.; Nguyen, H.T.; Nguyen, T.T.; Ou, W.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Anti-CTLA-4 Antibody-Functionalized Dendritic Cell-Derived Exosomes Targeting Tumor-Draining Lymph Nodes for Effective Induction of Antitumor T-Cell Responses. Acta Biomater. 2020, 115, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yao, Z.; Ke, X.; Hu, M.; Ren, H.; Gao, S.; Zhang, H. Extracellular Vesicles-Based Vaccines: Emerging Immunotherapies against Cancer. J. Control. Release 2025, 378, 438–459. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Nguyen, T.M.; Jayasinghe, M.K.; Gao, C.; Pham, T.T.; Vu, L.T.; Yeo, E.Y.M.; Yap, G.; Wang, L.; Goh, B.C.; et al. Robust Delivery of RIG-I Agonists Using Extracellular Vesicles for Anti-cancer Immunotherapy. J. Extracell. Vesicles 2022, 11, e12187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Zhuo, Y.; Zhang, Z.; Chen, R.; Liang, L.; Jiang, X.; Nie, D.; Liu, C.; Zou, Z.; et al. Endoplasmic Reticulum-Targeted Delivery of Celastrol and PD-L1 siRNA for Reinforcing Immunogenic Cell Death and Potentiating Cancer Immunotherapy. Acta Pharm. Sin. B 2024, 14, 3643–3660. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-Based Immunotherapy: A Promising Approach for Cancer Treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Fu, T.; Qian, Y.; Bian, X.; Zhang, X.; Zhang, J. Extracellular Vesicle-Based Drug Delivery Systems in Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhang, H.; Zhang, X.; Yu, X.; Wang, Y.; Meng, Q.-F.; Yang, K.; Bai, B.; Tian, R.; Zhu, S.; et al. Supramolecular Engineering of Cell Membrane Vesicles for Cancer Immunotherapy. Sci. Bull. 2022, 67, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Byun, J.; Kim, D.; Wu, Y.; Lee, J.; Oh, Y.-K. Cell Membrane-Coated mRNA Nanoparticles for Enhanced Delivery to Dendritic Cells and Immunotherapy. Asian J. Pharm. Sci. 2024, 19, 100968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miao, F.; Gu, L.; Zhang, R.; Ma, Y.; Li, Y.; Zheng, J.; Lin, Z.; Gao, Y.; Huang, L.; et al. Stimulator of Interferon Genes-Activated Biomimetic Dendritic Cell Nanovaccine as a Chemotherapeutic Booster to Enhance Systemic Fibrosarcoma Treatment. ACS Nano 2024, 18, 24219–24235. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.T.; Zhou, J.; Kroll, A.V.; Fang, R.H.; Yan, M.; Xiao, C.; Chen, X.; Kline, J.; Zhang, L.; Zhang, D.-E. Acute Myeloid Leukemia Cell Membrane-Coated Nanoparticles for Cancer Vaccination Immunotherapy. Leukemia 2021, 36, 994. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Wang, J.; Xu, X.; Zhang, A.; Li, Y.; Zhang, Z. Macrophage Membrane-Coated Nano-Gemcitabine Promotes Lymphocyte Infiltration and Synergizes AntiPD-L1 to Restore the Tumoricidal Function. ACS Nano 2023, 17, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Zhang, J.; Raza, F.; Pan, X.; Hu, Z.; Feng, H.; Shen, Q. Biomimetic Gold Nanocages Incorporating Copper-Human Serum Albumin for Tumor Immunotherapy via Cuproptosis-Lactate Regulation. J. Control. Release 2024, 372, 446–466. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Wang, Y.; Luan, Z.; Zhao, C.; Yang, K. Tumor-Associated Macrophage Membrane-Camouflaged pH-Responsive Polymeric Micelles for Combined Cancer Chemotherapy-Sensitized Immunotherapy. Int. J. Pharm. 2022, 624, 121911. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, S.; Zhang, S.; Wang, L.; Yuan, H.; Hu, F. Cell Membrane Coated-Nanoparticles for Cancer Immunotherapy. Acta Pharm. Sin. B 2022, 12, 3233–3254. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Y.; Liu, J.; Zhang, W.; He, Y.; Chen, F.; Xie, X.; Tang, J.; Guan, S.; Shao, D.; et al. Leveraging Senescent Cancer Cell Membrane to Potentiate Cancer Immunotherapy through Biomimetic Nanovaccine. Adv. Sci. 2024, 11, 2400630. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chang, R.; Liang, B.; Wang, Y.; Zhu, Y.; Jia, Z.; Fan, J.; Zhang, Z.; Du, B.; Kong, D. Overcoming Drug Resistance through Extracellular Vesicle-Based Drug Delivery System in Cancer Treatment. Cancer Drug Resist. 2024, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Pumpens, P.; Pushko, P. Virus-Like Particles: A Comprehensive Guide; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-000-56987-2. [Google Scholar]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yui, M.; Deo, V.K.; Park, E.Y. Development of Rous Sarcoma Virus-like Particles Displaying hCC49 scFv for Specific Targeted Drug Delivery to Human Colon Carcinoma Cells. Pharm. Res. 2015, 32, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Dewangan, H.K. Nanoparticles as Adjuvants in Vaccine Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 2. [Google Scholar] [CrossRef] [PubMed]
- Ruzzi, F.; Semprini, M.S.; Scalambra, L.; Palladini, A.; Angelicola, S.; Cappello, C.; Pittino, O.M.; Nanni, P.; Lollini, P.-L. Virus-like Particle (VLP) Vaccines for Cancer Immunotherapy. Int. J. Mol. Sci. 2023, 24, 12963. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Hefferon, K. Application of Plant Viruses in Biotechnology, Medicine, and Human Health. Viruses 2021, 13, 1697. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Cuevas, E.; Garcia-Atutxa, I.; Huerta-Saquero, A.; Villanueva-Flores, F. The Role of Plant Virus-like Particles in Advanced Drug Delivery and Vaccine Development: Structural Attributes and Application Potential. Viruses 2025, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ledezma, D.K.; Affonso de Oliveira, J.F.; Omole, A.O.; Steinmetz, N.F. A Cowpea Mosaic Virus Adjuvant Conjugated to Liposomes Loaded with Tumor Cell Lysates as an Ovarian Cancer Vaccine. Nat. Commun. 2025, 16, 5047. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9733. [Google Scholar] [CrossRef] [PubMed]
- Shahgolzari, M.; Venkataraman, S.; Osano, A.; Akpa, P.A.; Hefferon, K. Plant Virus Nanoparticles Combat Cancer. Vaccines 2023, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Park, J.; Cai, H.; Steinmetz, N.F. S100A9-targeted Cowpea Mosaic Virus as a Prophylactic and Therapeutic Immunotherapy against Metastatic Breast Cancer and Melanoma. Adv. Sci. 2021, 8, 2101796. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.H.; Lewis, J.D. Cowpea Mosaic Virus Nanoparticles for Cancer Imaging and Therapy. Adv. Drug Deliv. Rev. 2019, 145, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Shahgolzari, M.; Pazhouhandeh, M.; Milani, M.; Yari Khosroushahi, A.; Fiering, S. Plant Viral Nanoparticles for Packaging and in Vivo Delivery of Bioactive Cargos. WIREs Nanomed. Nanobiotechnology 2020, 12, e1629. [Google Scholar] [CrossRef] [PubMed]
- Shahgolzari, M.; Dianat-Moghadam, H.; Yavari, A.; Fiering, S.N.; Hefferon, K. Multifunctional Plant Virus Nanoparticles for Targeting Breast Cancer Tumors. Vaccines 2022, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Komane, M.D.; Kayoka-Kabongo, P.N.; Rutkowska, D.A. The Use of Plant Viral Nanoparticles in Cancer Biotherapy—A Review. Viruses 2025, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Shahgolzari, M.; Fiering, S. Emerging Potential of Plant Virus Nanoparticles (PVNPs) in Anticancer Immunotherapies. J. Cancer Immunol. 2022, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Affonso de Oliveira, J.F.; Gatus, J.; Edmondson, E.; Neun, B.W.; Dobrovolskaia, M.A.; Steinmetz, N.F. Preclinical SC and IV Repeat-Dose Toxicology of a Cowpea Mosaic Virus—A Cancer Immunotherapy Candidate. Toxicol. Rep. 2025, 14, 102022. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Steinmetz, N.F. The Pharmacology of Plant Virus Nanoparticles. Virology 2021, 556, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.C.; Jaroentomeechai, T.; Moeller, T.D.; Hershewe, J.M.; Warfel, K.F.; Moricz, B.S.; Martini, A.M.; Dubner, R.S.; Hsu, K.J.; Stevenson, T.C.; et al. On-Demand Biomanufacturing of Protective Conjugate Vaccines. Sci. Adv. 2021, 7, eabe9444. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-Cancer Interactions: Bacteria-Based Cancer Therapy. Exp. Mol. Med. 2019, 51, 152. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.F. The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Kalaora, S.; Nagler, A.; Nejman, D.; Alon, M.; Barbolin, C.; Barnea, E.; Ketelaars, S.L.C.; Cheng, K.; Vervier, K.; Shental, N.; et al. Identification of Bacteria-Derived HLA-Bound Peptides in Melanoma. Nature 2021, 592, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Naghavian, R.; Faigle, W.; Oldrati, P.; Wang, J.; Toussaint, N.C.; Qiu, Y.; Medici, G.; Wacker, M.; Freudenmann, L.K.; Bonté, P.-E.; et al. Microbial Peptides Activate Tumour-Infiltrating Lymphocytes in Glioblastoma. Nature 2023, 617, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-Targeted Salmonella as a Novel Anticancer Vector. Cancer Res. 1997, 57, 4537–4544. [Google Scholar] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Redenti, A.; Im, J.; Redenti, B.; Li, F.; Rouanne, M.; Sheng, Z.; Sun, W.; Gurbatri, C.R.; Huang, S.; Komaranchath, M.; et al. Probiotic Neoantigen Delivery Vectors for Precision Cancer Immunotherapy. Nature 2024, 635, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.-H.; You, S.-H.; Ngo, H.T.-T.; Van Nguyen, K.; Tran, K.V.; Chu, T.-H.; Kim, S.; Ha, S.-J.; Hong, Y.; Min, J.-J. Reprogramming the Tumor Immune Microenvironment Using Engineered Dual-Drug Loaded Salmonella. Nat. Commun. 2024, 15, 6680. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Madaan, T.; Kamble, N.S.; Siddiqui, N.A.; Pauletti, G.M.; Kotagiri, N. Engineered Bacteria Enhance Immunotherapy and Targeted Therapy through Stromal Remodeling of Tumors. Adv. Healthc. Mater. 2022, 11, e2101487. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Kitagawa, K.; Kato, M.; Yanase, S.; Okamura, Y.; Bando, Y.; Hara, T.; Terakawa, T.; Furukawa, J.; Nakano, Y.; et al. An Oral Cancer Vaccine Using Bifidobacterium Vector Augments Combination of Anti-PD-1 and Anti-CTLA-4 Antibodies in Mouse Renal Cell Carcinoma Model. Sci. Rep. 2023, 13, 9994. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Tatsumi, M.; Kato, M.; Komai, S.; Doi, H.; Hashii, Y.; Katayama, T.; Fujisawa, M.; Shirakawa, T. An Oral Cancer Vaccine Using a Bifidobacterium Vector Suppresses Tumor Growth in a Syngeneic Mouse Bladder Cancer Model. Mol. Ther. Oncolytics 2021, 22, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, Y.; Ou, Q.; Chen, M.; Tian, S.; Tang, J.; Li, R.; Liang, Q.; Chen, Z.; Wang, C. Listeria-Vectored Cervical Cancer Vaccine Candidate Strains Reduce MDSCs via the JAK-STAT Signaling Pathway. BMC Biol. 2024, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, M.; Liu, X.; Chen, Y.; Xun, Z.; Sun, Y.; Tan, W.; He, J.; Zheng, J.H. Photodynamic Therapy-Improved Oncolytic Bacterial Immunotherapy with FAP-Encoding S. Typhimurium. J. Control. Release 2022, 351, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, F.; Ma, Y.; Zhao, Y.; Zhang, W.; Zhang, Z.; Liu, H.; Yang, X.; Zhang, C.; Zhang, J.; et al. Redirecting Antigens by Engineered Photosynthetic Bacteria and Derived Outer Membrane Vesicles for Enhanced Cancer Immunotherapy. ACS Nano 2023, 17, 18716–18731. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Feng, Y.; Li, L.; Kong, F.; Gao, J.; Kong, X. Engineered Bacterial Outer Membrane Vesicles: A Versatile Bacteria-Based Weapon against Gastrointestinal Tumors. Theranostics 2024, 14, 761–787. [Google Scholar] [CrossRef] [PubMed]
- Qing, S.; Lyu, C.; Zhu, L.; Pan, C.; Wang, S.; Li, F.; Wang, J.; Yue, H.; Gao, X.; Jia, R.; et al. Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy. Adv. Mater. 2020, 32, 2002085. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Svennerholm, K.; Crescitelli, R.; Lässer, C.; Gribonika, I.; Lötvall, J. Synthetic Bacterial Vesicles Combined with Tumour Extracellular Vesicles as Cancer Immunotherapy. J. Extracell. Vesicles 2021, 10, e12120. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, L.P.; Fuhrmann, G. Bacterial Extracellular Vesicles: Understanding Biology Promotes Applications as Nanopharmaceuticals. Adv. Drug Deliv. Rev. 2021, 173, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial Extracellular Vesicles and Their Novel Therapeutic Applications in Health and Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 962216. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-R.; Nguyen, D.-H.; Yoo, S.W.; Min, J.-J. Bacteria and Bacterial Derivatives as Delivery Carriers for Immunotherapy. Adv. Drug Deliv. Rev. 2022, 181, 114085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tang, Y.; Xu, W.; Hao, X.; Li, Y.; Huang, S.; Xiang, D.; Wu, J. Bacteria-Based Immunotherapy for Cancer: A Systematic Review of Preclinical Studies. Front. Immunol. 2023, 14, 1140463. [Google Scholar] [CrossRef] [PubMed]
- Dailey, K.M.; Small, J.M.; Pullan, J.E.; Winfree, S.; Vance, K.E.; Orr, M.; Mallik, S.; Bayles, K.W.; Hollingsworth, M.A.; Brooks, A.E. An Intravenous Pancreatic Cancer Therapeutic: Characterization of CRISPR/Cas9n-Modified Clostridium Novyi-Non Toxic. PLoS ONE 2023, 18, e0289183. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Yin, S.; Zhang, Z.; Yu, Y.; Fang, H.; Liang, Z.; Zhu, R.; Zhou, H.; Li, J.; Cao, K.; et al. Multimodal Targeting Chimeras Enable Integrated Immunotherapy Leveraging Tumor-Immune Microenvironment. Cell 2024, 187, 7470–7491. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, S.; Dai, P.; Liu, F.; Shang, Y.; Yang, Q.; Qin, J.; Yuchi, Z.; Wang, Z.; Zhao, Y. Tailored Trojan Horse Nanocarriers for Enhanced Redox-Responsive Drug Delivery. J. Control. Release 2022, 342, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guan, T.; Ke, Y.; Lin, Y.; Tai, R.; Ye, J.; Deng, Z.; Deng, S.; Ou, C. Biologically Logic-Gated Trojan-Horse Strategy for Personalized Triple-Negative Breast Cancer Precise Therapy by Selective Ferroptosis and STING Pathway Provoking. Biomaterials 2025, 315, 122905. [Google Scholar] [CrossRef] [PubMed]
- Yasothamani, V.; Karthikeyan, L.; Sarathy, N.P.; Vivek, R. Targeted Designing of Multimodal Tumor-Seeking Nanomedicine for Breast Cancer-Specific Triple-Therapeutic Effects. ACS Appl. Bio Mater. 2021, 4, 6575–6588. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zeng, Z.; Tang, Y.; Tian, J.; Hao, X.; Sun, P.; Peng, Y.; Tian, T.; Xiang, D.; Wang, R.; et al. Spatiotemporal-Controllable ROS-Responsive Camptothecin Nano-Bomb for Chemo/Photo/Immunotherapy in Triple-Negative Breast Cancer. J. Nanobiotechnol. 2024, 22, 798. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, S.; Srinivasan, K.; M, D.; Vincent, P.M.D.R. Personalized Cancer Vaccine Design Using AI-Powered Technologies. Front. Immunol. 2024, 15, 1357217. [Google Scholar] [CrossRef] [PubMed]
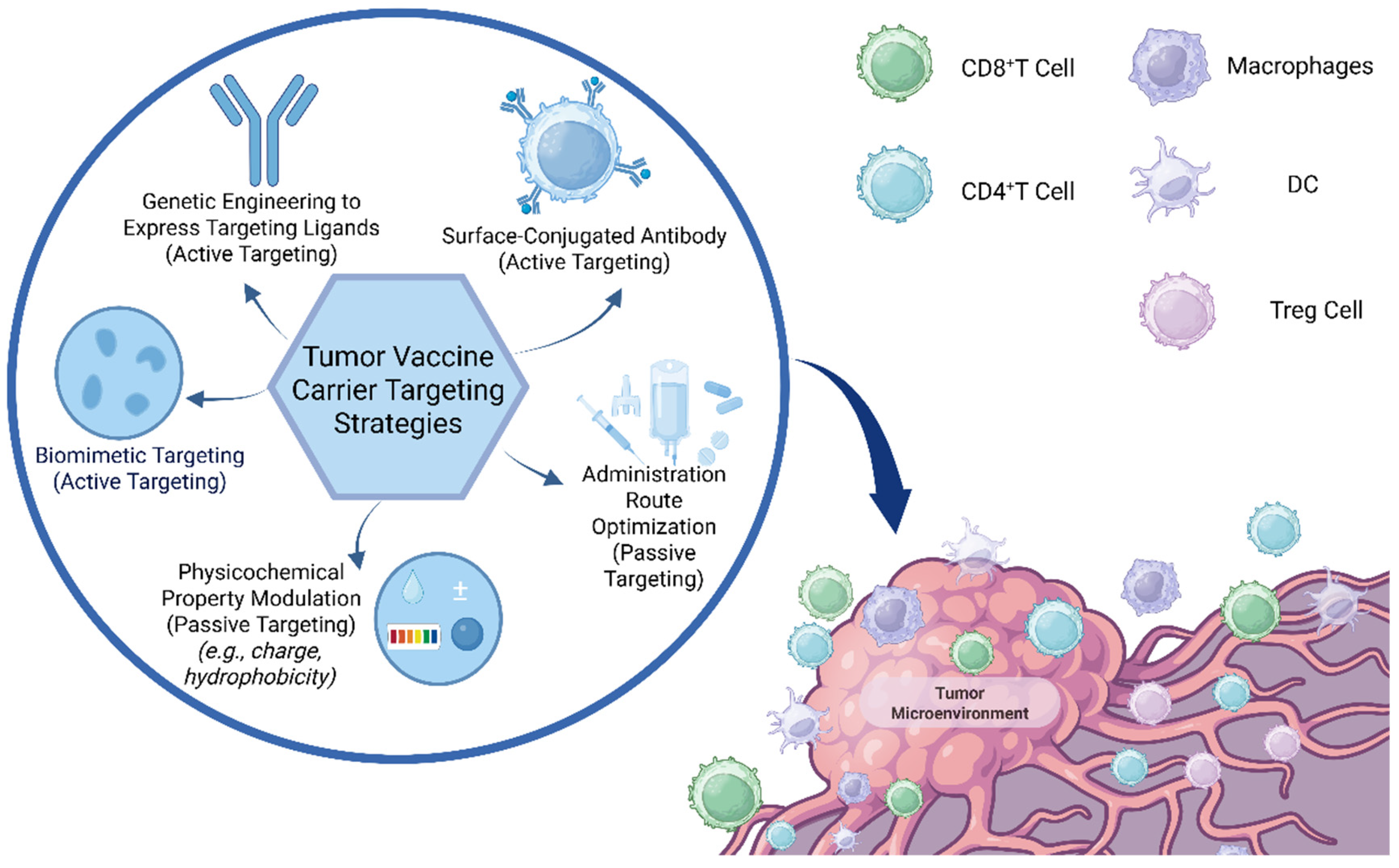

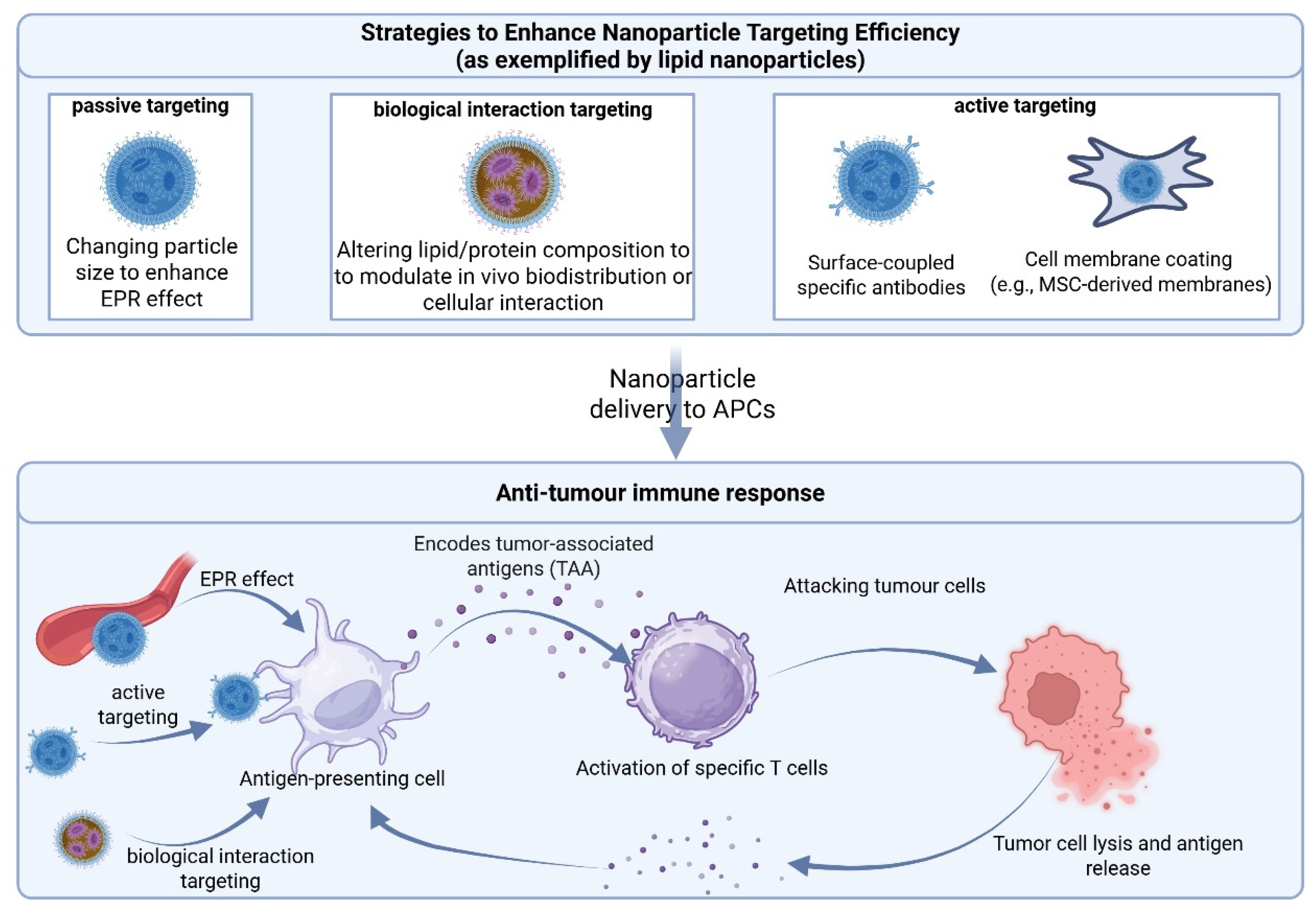
| Strategy Type | Modification | Target | Reference |
|---|---|---|---|
| Ad fiber engineering | Ad35 fiber replacement to evade neutralization and improve uptake | Guanylyl cyclase C+ gastrointestinal tumors | [69] |
| Ad5 genetic reprogramming | MelARV expression with ISD domain mutation | Tumors expressing endogenous retroviral antigens | [70] |
| OAd + HDAd vector combination | CAdVEC platform for complex TME adaptation | Solid tumors | [71] |
| Heterologous Ad-based prime–boost | ChAd68 priming with VEE-samRNA boost | Tumors bearing personalized neoantigens | [72] |
| AAV capsid S663 mutation + EV delivery | EV-mediated delivery to bypass immune memory and receptor binding | Melanoma | [73] |
| Ad–PAMAM + hydrogel formulation | Localized release via hydrogel for enhanced regional accumulation | [74] | |
| Oncolytic Ad + tumor-derived EVs | Trojan horse delivery using tumor membrane camouflage | [75] | |
| Multi-serotype AAV vector | Co-delivery of PD-1 and tumor antigens to dendritic cells | Mesothelioma | [76] |
| Polymer Type | Advantage | Disadvantage | Common Applications | Reference |
|---|---|---|---|---|
| PLGA | Biodegradable; good biocompatibility; FDA-approved; controlled release | Acidic degradation products acidify microenvironment; rapid clearance without modification | Laser-activated controlled drug delivery at targeted sites | [204,205,206] |
| PEG | Reduces protein adsorption/opsonization; verified safety; improves nanoparticle stability and circulation | Non-biodegradable, accumulates; antibody induction upon repeated use; cannot form nanoparticles alone | Copolymer to enhance carrier circulation and stability | [206,207] |
| Chitosan (natural polycation) | Biodegradable; mucosal absorption; strongly binds negatively charged drugs | Batch-to-batch variability; high solubility only under acidic conditions, limiting systemic administration | Mucosal vaccine delivery; systemic vaccine adjuvant | [204,208,209] |
| Polycaprolactone (PCL) | Biodegradable; good biocompatibility; FDA-approved; efficiently encapsulates hydrophobic drugs | Slow degradation; poor hydrophilic drug encapsulation; forms semi-crystalline matrices, delaying release | Targeted drug delivery (active/passive) | [204,210] |
| Albumin (protein polymer nanoparticles) | Biocompatible; biodegradable; clinically established; low immunogenicity; tumor-targeting capability | Instability (easy dissociation); biological sourcing complicates purification; limited control over size/drug loading | Delivery of chemotherapeutics (e.g., paclitaxel) | [211,212,213,214] |
| Nanoparticle Type | Composition, Size, Morphology | Key Applications | Challenges | Reference |
|---|---|---|---|---|
| Gold Nanoparticles (AuNPs) | Gold core stabilized by surface modifications; spherical (5–100 nm) or tunable morphologies (rods, shells) with adjustable optical properties | Photothermal therapy, drug/gene delivery, imaging | Non-degradable; immune activation; high production cost | [225,226] |
| Mesoporous Silica Nanoparticles (MSNs) | Amorphous silica with ordered nanopores; spherical (~50–200 nm), pore diameter 2–6 nm; usually surface-modified | Sustained-release drug delivery, combination therapy | Residual additives; liver accumulation; poor biodegradability; mechanical brittleness | [227,228,229] |
| Iron Oxide Nanoparticles (Magnetic NPs) | Crystalline iron oxide stabilized by coatings; spherical (5–50 nm); ≤20 nm particles show superparamagnetism (no residual magnetization) | Magnetic targeting, hyperthermia, MRI imaging contrast agents | Coating-dependent stability; limited targeting depth; dose-related toxicity | [230,231] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Liang, J.; Zhang, Y.; Dong, C.; Tan, D.; Wang, H.; Zheng, Y.; He, Q. Strategic Advances in Targeted Delivery Carriers for Therapeutic Cancer Vaccines. Int. J. Mol. Sci. 2025, 26, 6879. https://doi.org/10.3390/ijms26146879
Wu J, Liang J, Zhang Y, Dong C, Tan D, Wang H, Zheng Y, He Q. Strategic Advances in Targeted Delivery Carriers for Therapeutic Cancer Vaccines. International Journal of Molecular Sciences. 2025; 26(14):6879. https://doi.org/10.3390/ijms26146879
Chicago/Turabian StyleWu, Junxi, Jinghui Liang, Yuan Zhang, Chunyan Dong, Dejiang Tan, Hongyu Wang, Yiyang Zheng, and Qing He. 2025. "Strategic Advances in Targeted Delivery Carriers for Therapeutic Cancer Vaccines" International Journal of Molecular Sciences 26, no. 14: 6879. https://doi.org/10.3390/ijms26146879
APA StyleWu, J., Liang, J., Zhang, Y., Dong, C., Tan, D., Wang, H., Zheng, Y., & He, Q. (2025). Strategic Advances in Targeted Delivery Carriers for Therapeutic Cancer Vaccines. International Journal of Molecular Sciences, 26(14), 6879. https://doi.org/10.3390/ijms26146879







