Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment
Abstract
1. Introduction
General Considerations for Myelodysplastic Syndromes
2. Risk Stratification and Treatment Options
Prognostic Tools and Standard Treatment Approaches
3. New Treatment Approaches
Overview of Innovative Therapies Under Clinical Development
4. Oxidative Stress: Mechanisms and Disease Connections
4.1. Oxidative Stress Plays a Role in Both Physiological and Pathological Contexts
4.2. Oxidative Stress and Mitochondrial Dysfunction in Cancer Biology and Therapy Response
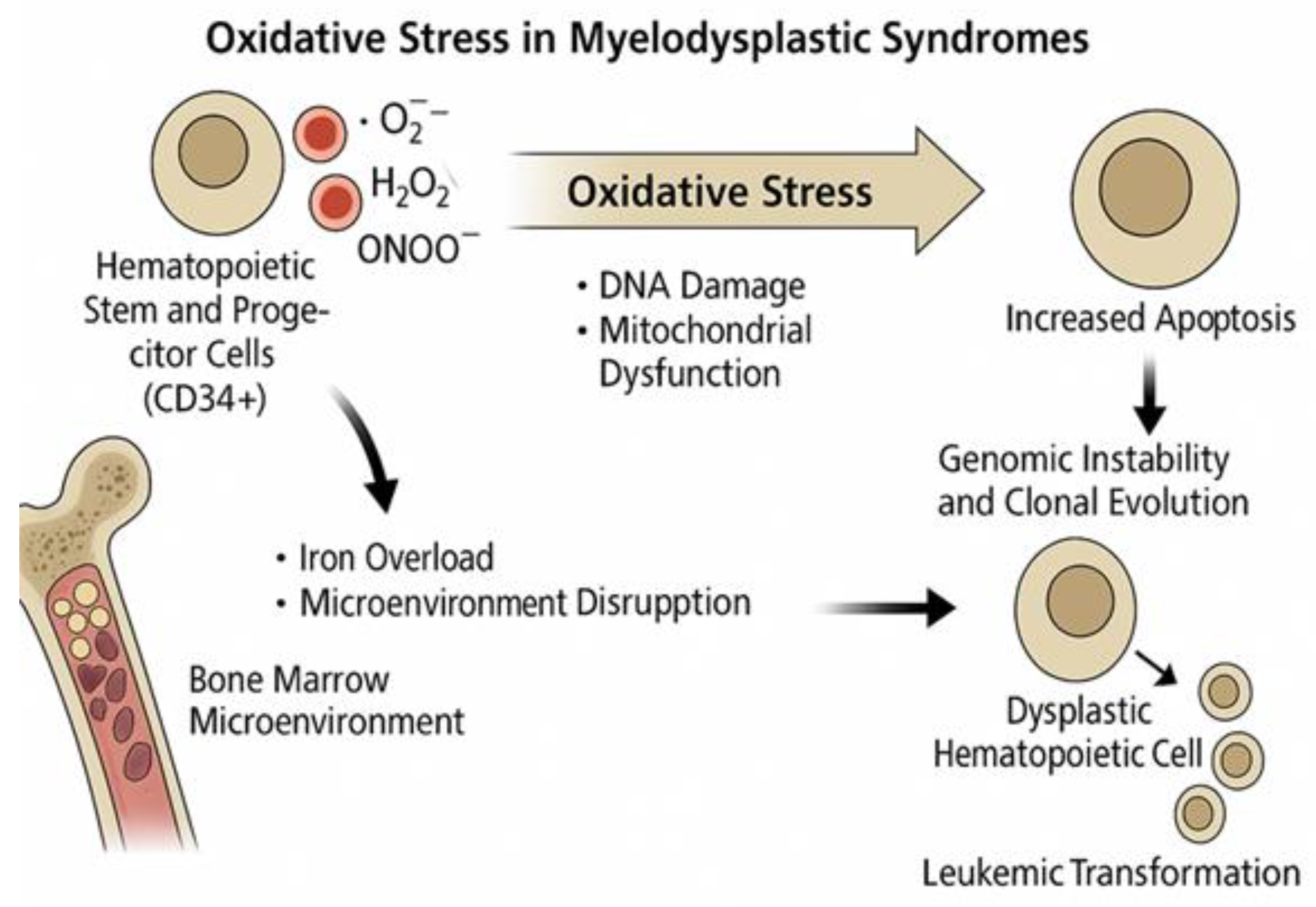
5. Oxidative Stress in Myelodysplastic Syndromes
5.1. Oxidative Mechanisms Specific to MDS Pathogenesis
5.2. The Dual Role of Reactive Oxygen Species in the Pathogenesis of Myelodysplastic Syndromes
5.3. Impacts of Iron Dysregulation and Chelation Therapy on Oxidative Stress in Myelodysplastic Syndrome
5.4. Mitochondrial Dynamics and Ferroptosis in the Metabolic Landscape of Myelodysplastic Syndromes
5.5. DNA Methylation and Epigenetic Regulation in Myelodysplastic Syndromes: Biomarkers and Functional Implications
5.6. Oxidative Stress in MDS and the Treatment Options Available
6. Future Perspectives in Oxidative Stress Research: The Role of Artificial Intelligence in Antioxidant Discovery in Myeloid Malignancies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jacobs, A. The pathogenesis and classification of the myelodysplastic syndromes. Br. J. Haematol. 1990, 75 (Suppl. 1), 7–11. [Google Scholar]
- Hofmann, W.-K.; Koeffler, H.P. Important features of myelodysplastic syndrome. Int. J. Hematol. 2002, 76 (Suppl. 2), 222–227. [Google Scholar] [CrossRef]
- Hasserjian, R.P. Myelodysplastic Syndrome Updated. Pathobiology 2019, 86, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Qin, T.; Xu, Z.; Qu, S.; Pan, L.; Li, B.; Wang, H.; Zhang, P.; Yan, X.; et al. Comparison of the revised 4th (2016) and 5th (2022) editions of the World Health Organization classification of myelodysplastic neoplasms. Leukemia 2022, 36, 2875–2882. [Google Scholar] [CrossRef]
- Tanaka, T.N.; Bejar, R. MDS overlap disorders and diagnostic boundaries. Blood 2019, 133, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Hosono, N. Genetic abnormalities and pathophysiology of MDS. Int. J. Clin. Oncol. 2019, 24, 885–892. [Google Scholar] [CrossRef]
- Pellagatti, A.; Boultwood, J. The molecular pathogenesis of the myelodysplastic syndromes. Eur. J. Haematol. 2015, 95, 3–15. [Google Scholar] [CrossRef]
- Chiereghin, C.; Travaglino, E.; Zampini, M.; Saba, E.; Saitta, C.; Riva, E.; Bersanelli, M.; Della Porta, M.G. The Genetics of Myelodysplastic Syndromes: Clinical Relevance. Genes 2021, 12, 1144. [Google Scholar] [CrossRef]
- Haferlach, T. The Molecular Pathology of Myelodysplastic Syndrome. Pathobiology 2019, 86, 24–29. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, X.; Di, T.; Tang, F.; Guo, X.; Liu, Y.; Bai, J.; Li, Y.; Li, L.; Zhang, L. The yin-yang of immunity: Immune dysregulation in myelodysplastic syndrome with different risk stratification. Front. Immunol. 2022, 13, 994053. [Google Scholar] [CrossRef]
- Glenthøj, A.; Ørskov, A.D.; Hansen, J.W.; Hadrup, S.R.; O’Connell, C.; Grønbæk, K. Immune Mechanisms in Myelodysplastic Syndrome. Int. J. Mol. Sci. 2016, 17, 944. [Google Scholar] [CrossRef]
- Giannouli, S.; Kanellopoulou, T.; Voulgarelis, M. Myelodysplasia and autoimmunity. Curr. Opin. Rheumatol. 2012, 24, 97–102. [Google Scholar] [CrossRef]
- Grignano, E.; Jachiet, V.; Fenaux, P.; Adès, L.; Fain, O.; Mekinian, A. Autoimmune manifestations associated with myelodysplastic syndromes. Ann. Hematol. 2018, 97, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Ossa, J.E.A.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef] [PubMed]
- Tobiasson, M.; Kittang, A.O. Treatment of myelodysplastic syndrome in the era of next-generation sequencing. J. Intern. Med. 2019, 286, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Adès, L. How we manage adults with myelodysplastic syndrome. Br. J. Haematol. 2020, 189, 1016–1027. [Google Scholar] [CrossRef]
- Carraway, H.E.; Saygin, C. Therapy for lower-risk MDS. Hematology 2020, 2020, 426–433. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Ciurea, S.O.; Cutler, C.; Robin, M.; Warlick, E.D.; Nakamura, R.; Brunner, A.M.; Dholaria, B.; Walker, A.R.; Kröger, N.; et al. Hematopoietic Cell Transplantation in the Management of Myelodysplastic Syndrome. Transplant. Cell. Ther. 2023, 29, 71–81. [Google Scholar] [CrossRef]
- Saygin, C.; Carraway, H.E. Current and emerging strategies for management of myelodysplastic syndromes. Blood Rev. 2021, 48, 100791. [Google Scholar] [CrossRef]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; et al. Lenalidomide in the Myelodysplastic Syndrome with Chromosome 5q Deletion. N. Engl. J. Med. 2006, 355, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Santini, V.; Spiriti, M.A.A.; Giagounidis, A.; Schlag, R.; Radinoff, A.; Gercheva-Kyuchukova, L.; Anagnostopoulos, A.; Oliva, E.N.; Symeonidis, A.; et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-α in anemic patients with low-risk MDS. Leukemia 2018, 32, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Santini, V.; Almeida, A.; Platzbecker, U.; Jonasova, A.; Silverman, L.R.; Falantes, J.; Reda, G.; Buccisano, F.; Fenaux, P.; et al. Phase III, Randomized, Placebo-Controlled Trial of CC-486 (Oral Azacitidine) in Patients With Lower-Risk Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1426–1436. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Jayade, S.; Schmier, J.; Botteman, M.; Hassan, A.; Ruiters, D.; Hill, K.; Joshi, N. Injectable Hypomethylating Agents for Management of Myelodysplastic Syndromes: Patients’ Perspectives on Treatment. Clin. Lymphoma Myeloma Leuk. 2022, 22, e185–e198. [Google Scholar] [CrossRef]
- Şenel, P.; Agar, S.; Yurtsever, M.; Gölcü, A. Voltammetric quantification, spectroscopic, and DFT studies on the binding of the antineoplastic drug Azacitidine with DNA. J. Pharm. Biomed. Anal. 2024, 237, 115746. [Google Scholar] [CrossRef]
- Teodorescu, P.; Pasca, S.; Dima, D.; Tomuleasa, C.; Ghiaur, G. Targeting the Microenvironment in MDS: The Final Frontier. Front. Pharmacol. 2020, 11, 1044. [Google Scholar] [CrossRef]
- Syed, K.; Naguib, S.; Liu, Z.-J.; Cimmino, L.; Yang, F.-C. Novel combinations to improve hematopoiesis in myelodysplastic syndrome. Stem Cell Res. Ther. 2020, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Velegraki, M.; Stiff, A.; Papadaki, H.A.; Li, Z. Myeloid-Derived Suppressor Cells: New Insights into the Pathogenesis and Therapy of MDS. J. Clin. Med. 2022, 11, 4908. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, G.; Inoue, D.; Zhang, L. Targeting low-risk myelodysplastic syndrome with novel therapeutic strategies. Trends Mol. Med. 2021, 27, 990–999. [Google Scholar] [CrossRef]
- Henry, D.H.; Glaspy, J.; Harrup, R.; Mittelman, M.; Zhou, A.; Carraway, H.E.; Bradley, C.; Saha, G.; Modelska, K.; Bartels, P.; et al. Roxadustat for the treatment of anemia in patients with LOWER-RISK myelodysplastic syndrome: Open-label, dose-selection, lead-in stage of a phase 3 study. Am. J. Hematol. 2022, 97, 174–184. [Google Scholar] [CrossRef]
- Hara, R.; Goto, N.; Furuya, D.; Kitahara, T.; Numata, H.; Watanabe, S.; Kawada, H.; Ando, K. The Effect of Roxadustat on Transfusion-Dependent Myelodysplastic Syndrome Complicated by Chronic Kidney Disease. Case Rep. Oncol. 2021, 14, 1574–1579. [Google Scholar] [CrossRef]
- Lennox, A.L.; Huang, F.; Behrs, M.K.; González-Sales, M.; Bhise, N.; Wan, Y.; Sun, L.; Berry, T.; Feller, F.; Morcos, P.N. Imetelstat, a novel, first-in-class telomerase inhibitor: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2024, 17, e70076. [Google Scholar] [CrossRef]
- Platzbecker, U.; Santini, V.; Fenaux, P.; Sekeres, M.A.; Savona, M.R.; Madanat, Y.F.; Díez-Campelo, M.; Valcárcel, D.; Illmer, T.; Jonášová, A.; et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): A multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 403, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Abaza, Y.; Zeidan, A.M. Immune Checkpoint Inhibition in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Cells 2022, 11, 2249. [Google Scholar] [CrossRef]
- Sallman, D.A.; Al Malki, M.M.; Asch, A.S.; Wang, E.S.; Jurcic, J.G.; Bradley, T.J.; Flinn, I.W.; Pollyea, D.A.; Kambhampati, S.; Tanaka, T.N.; et al. Magrolimab in Combination With Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study. J. Clin. Oncol. 2023, 41, 2815–2826. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Simon, F.; Trollet, C.; Santibañez, J.F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies Oxid. Oxidative Med. Cell. Longev. 2017, 2017, 4310469. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, Q.; Wu, R.; Tuo, Z.; Wang, J.; Ye, L.; Shao, F.; Chaipanichkul, P.; Yoo, K.H.; Wei, W.; et al. Interactions between oxidative stress and senescence in cancer: Mechanisms, therapeutic implications, and future perspectives. Redox Biol. 2024, 73, 103208. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, B.; Cheng, Y.; Song, Y.; Li, Q.; Luo, C.; Birla, H. Targeting Molecular Mediators of Ferroptosis and Oxidative Stress for Neurological Disorders. Oxidative Med. Cell. Longev. 2022, 2022, 3999083. [Google Scholar] [CrossRef]
- Zeng, W.; Long, X.; Liu, P.; Xie, X. Interplay of oncogenic signaling, oxidative stress, and ferroptosis in cancer. Int. J. Cancer 2023, 153, 918–931. [Google Scholar] [CrossRef]
- Jourdain, A.A.; Begg, B.E.; Mick, E.; Shah, H.; Calvo, S.E.; Skinner, O.S.; Sharma, R.; Blue, S.M.; Yeo, G.W.; Burge, C.B.; et al. Loss of LUC7L2 and U1 snRNP subunits shifts energy metabolism from glycolysis to OXPHOS. Mol. Cell 2021, 81, 1905–1919.e12. [Google Scholar] [CrossRef]
- Danieli, M.G.; Antonelli, E.; Piga, M.A.; Cozzi, M.F.; Allegra, A.; Gangemi, S. Oxidative stress, mitochondrial dysfunction, and respiratory chain enzyme defects in inflammatory myopathies. Autoimmun. Rev. 2023, 22, 103308. [Google Scholar] [CrossRef]
- Canton, M.; Menazza, S.; Di Lisa, F. Oxidative stress in muscular dystrophy: From generic evidence to specific sources and targets. J. Muscle Res. Cell Motil. 2014, 35, 23–36. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Devkar, R.V.; Nammi, S. Oxidative Stress in Liver Diseases: Pathogenesis, Prevention, and Therapeutics. Oxidative Med. Cell. Longev. 2017, 2017, 8341286. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Li, J.; Hu, J.; Yang, K.; Tao, L. Oxidative stress: A common pathological state in a high-risk population for osteoporosis. Biomed. Pharmacother. 2023, 163, 114834. [Google Scholar] [CrossRef]
- De Martinis, M.; Allegra, A.; Sirufo, M.M.; Tonacci, A.; Pioggia, G.; Raggiunti, M.; Ginaldi, L.; Gangemi, S. Vitamin D Deficiency, Osteoporosis and Effect on Autoimmune Diseases and Hematopoiesis: A Review. Int. J. Mol. Sci. 2021, 22, 8855. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Takano, Y.; Satrialdi; Abe, J.; Hibino, M.; Harashima, H. Therapeutic strategies for regulating mitochondrial oxidative stress. Biomolecules 2020, 10, 83. [Google Scholar] [CrossRef]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Allegra, A.; Caserta, S.; Genovese, S.; Pioggia, G.; Gangemi, S. Gender differences in oxidative stress in relation to cancer susceptibility and survival. Antioxidants 2023, 12, 1255. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef]
- Soundararajan, L.; Warrier, S.; Dharmarajan, A.; Bhaskaran, N. Predominant factors influencing reactive oxygen species in cancer stem cells. J. Cell. Biochem. 2024, 125, 3–21. [Google Scholar] [CrossRef]
- Hole, P.S.; Darley, R.L.; Tonks, A. Do reactive oxygen species play a role in myeloid leukemias? Blood 2011, 117, 5816–5826. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G.; et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.; Cristani, M.; Saija, A.; Stelitano, C.; Callea, V.; Tomaino, A.; Minciullo, P.L.; Gangemi, S. Lipid peroxidation and protein oxidation in patients affected by Hodgkin’s lymphoma. Mediat. Inflamm. 2004, 13, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-B.; Huang, S.-J.; Yang, T.-B.; Wang, W.; Chen, B.-Y.; Guo, L. Comparison of machine learning methods for Predicting 3-Year survival in elderly esophageal squamous cancer patients based on oxidative stress. BMC Cancer 2024, 24, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-F.; Chen, S.; Tseng, L.-M.; Lee, H.-C. Role of the mitochondrial stress response in human cancer progression. Exp. Biol. Med. 2020, 245, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Yadav, T.; Rutter, J. Stressed to death: Mitochondrial stress responses connect respiration and apoptosis in cancer. Mol. Cell 2022, 82, 3321–3332. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, J.; Lu, W. The significance of mitochondrial dysfunction in cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Testa, U.; Labbaye, C.; Castelli, G.; Pelosi, E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp. Hematol. 2016, 44, 540–560. [Google Scholar] [CrossRef]
- Kocabas, F.; Xie, L.; Xie, J.; Yu, Z.; DeBerardinis, R.J.; Kimura, W.; Thet, S.; Elshamy, A.F.; Abouellail, H.; Muralidhar, S.; et al. Hypoxic metabolism in human hematopoietic stem cells. Cell Biosci. 2015, 5, 39. [Google Scholar] [CrossRef]
- Gonzalez-Menendez, P.; Romano, M.; Yan, H.; Deshmukh, R.; Papoin, J.; Oburoglu, L.; Daumur, M.; Dumé, A.-S.; Phadke, I.; Mongellaz, C.; et al. An IDH1-vitamin C crosstalk drives human erythroid development by inhibiting pro-oxidant mitochondrial metabolism. Cell Rep. 2021, 34, 108723. [Google Scholar] [CrossRef]
- Ivanov, S.; Nano, O.; Hana, C.; Bonano-Rios, A.; Hussein, A. Molecular Targeting of the Isocitrate Dehydrogenase Pathway and the Implications for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 7337. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Kriegsheim, A.V.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Deynoux, M.; Sunter, N.; Hérault, O.; Mazurier, F. Hypoxia and hypoxia-inducible factors in leukemias. Front. Oncol. 2016, 6, 41. [Google Scholar] [CrossRef]
- Magliulo, D.; Bernardi, R. Hypoxic stress and hypoxia-inducible factors in leukemias. Front. Oncol. 2022, 12, 973978. [Google Scholar] [CrossRef]
- Stergiou, I.E.; Kambas, K.; Poulaki, A.; Giannouli, S.; Katsila, T.; Dimitrakopoulou, A.; Vidali, V.; Mouchtouris, V.; Kloukina, I.; Xingi, E.; et al. Exploiting the role of hypoxia-inducible factor 1 and pseudohypoxia in the myelodysplastic syndrome pathophysiology. Int. J. Mol. Sci. 2021, 22, 4099. [Google Scholar] [CrossRef]
- Benito, J.; Zeng, Z.; Konopleva, M.; Wilson, W.R. Targeting hypoxia in the leukemia microenvironment. Int. J. Hematol. Oncol. 2013, 2, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Steidl, U. Inhibition of HIF1α signaling: A grand slam for MDS therapy? Cancer Discov. 2018, 8, 1355–1357. [Google Scholar] [CrossRef]
- Saigo, K.; Takenokuchi, M.; Hiramatsu, Y.; Tada, H.; Hishita, T.; Takata, M.; Misawa, M.; Imoto, S.; Imashuku, S. Oxidative Stress Levels in Myelodysplastic Syndrome Patients: Their Relationship to Serum Ferritin and Haemoglobin Values. J. Int. Med. Res. 2011, 39, 1941–1945. [Google Scholar] [CrossRef]
- Farquhar, M.J.; Bowen, D.T. Oxidative Stress and the Myelodysplastic Syndromes. Int. J. Hematol. 2003, 77, 342–350. [Google Scholar] [CrossRef]
- Chung, Y.J.; Robert, C.; Gough, S.M.; Rassool, F.V.; Aplan, P.D. Oxidative stress leads to increased mutation frequency in a murine model of myelodysplastic syndrome. Leuk. Res. 2014, 38, 95–102. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Lobão, M.J.; Pereira, A.; Costa, J.M.N.; et al. Oxidative stress and mitochondrial dysfunction play a role in myelodysplastic syndrome development, diagnosis, and prognosis: A pilot study. Free. Radic. Res. 2015, 49, 1081–1094. [Google Scholar] [CrossRef]
- Novotna, B.; Bagryantseva, Y.; Siskova, M.; Neuwirtova, R. Oxidative DNA damage in bone marrow cells of patients with low-risk myelodysplastic syndrome. Leuk. Res. 2009, 33, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Ghoti, H.; Amer, J.; Winder, A.; Rachmilewitz, E.; Fibach, E. Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur. J. Haematol. 2007, 79, 463–467. [Google Scholar] [CrossRef]
- Jing, Q.; Zhou, C.; Zhang, J.; Zhang, P.; Wu, Y.; Zhou, J.; Tong, X.; Li, Y.; Du, J.; Wang, Y. Role of reactive oxygen species in myelodysplastic syndromes. Cell. Mol. Biol. Lett. 2024, 29, 53. [Google Scholar] [CrossRef]
- McCrann, D.J.; Eliades, A.; Makitalo, M.; Matsuno, K.; Ravid, K. Differential expression of NADPH oxidases in megakaryocytes and their role in polyploidy. Blood 2009, 114, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Anadón, A.; Wang, X.; et al. Cancer Metabolism: The Role of ROS in DNA Damage and Induction of Apoptosis in Cancer Cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef]
- Boura-Theodorou, A.; Psatha, K.; Maniatsi, S.; Kourti, A.; Kaiafa, G.; Aivaliotis, M.; Makedou, K. Oxidative Stress and Its Role in the Emergence and Progression of Myelodysplastic Syndromes: Insights from Proteomic Analysis and Other Methodologies. Proteomes 2025, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017, 547, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Hole, P.S.; Zabkiewicz, J.; Munje, C.; Newton, Z.; Pearn, L.; White, P.; Marquez, N.; Hills, R.K.; Burnett, A.K.; Tonks, A.; et al. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood 2013, 122, 3322–3330. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef]
- Słomka, A.; Pokrzywa, A.; Strzała, D.; Kubiaczyk, M.; Wesolowska, O.; Denkiewicz, K.; Styczyński, J. The Role of Hepcidin in Myelodysplastic Syndromes (MDS): A Systematic Review of Observational Studies. Cancers 2024, 16, 332. [Google Scholar] [CrossRef]
- Fibach, E.; Rachmilewitz, E.A. Iron overload in hematological disorders. Presse Méd. 2017, 46, e296–e305. [Google Scholar] [CrossRef]
- Gattermann, N.; Rachmilewitz, E.A. Iron overload in MDS—Pathophysiology, diagnosis, and complications. Ann. Hematol. 2011, 90, 1–10. [Google Scholar] [CrossRef]
- Bruzzese, A.; Martino, E.A.; Mendicino, F.; Lucia, E.; Olivito, V.; Bova, C.; Filippelli, G.; Capodanno, I.; Neri, A.; Morabito, F.; et al. Iron chelation therapy. Eur. J. Haematol. 2023, 110, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Leitch, H.A.; Buckstein, R.; Zhu, N.; Nevill, T.J.; Yee, K.W.L.; Leber, B.; Keating, M.-M.; Hilaire, E.S.; Kumar, R.; Delage, R.; et al. Iron overload in myelodysplastic syndromes: Evidence based guidelines from the Canadian consortium on MDS. Leuk. Res. 2018, 74, 21–41. [Google Scholar] [CrossRef]
- Gattermann, N. Iron overload in acquired sideroblastic anemias and MDS: Pathophysiology and role of chelation and luspatercept. Hematology 2024, 2024, 443–449. [Google Scholar] [CrossRef]
- Liu, H.; Yang, N.; Meng, S.; Zhang, Y.; Zhang, H.; Zhang, W. Iron chelation therapy for myelodysplastic syndrome: A systematic review and meta-analysis. Clin. Exp. Med. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, A.; Morales, M.L.; Garrido-García, V.; García-Baquero, I.; Leivas, A.; Carreño-Tarragona, G.; Sánchez, R.; Arenas, A.; Cedena, T.; Ayala, R.M.; et al. Protein Carbonylation in Patients with Myelodysplastic Syndrome: An Opportunity for Deferasirox Therapy. Antioxidants 2019, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Gattermann, N. When is iron overload deleterious, and when and how should iron chelation therapy be administered in myelodysplastic syndromes? Best Pract. Res. Clin. Haematol. 2013, 26, 431–444. [Google Scholar] [CrossRef]
- Kim, C.H.; Leitch, H.A. Iron overload-induced oxidative stress in myelodysplastic syndromes and its cellular sequelae. Crit. Rev. Oncol. Hematol. 2021, 163, 103367. [Google Scholar] [CrossRef] [PubMed]
- Merkel, D.; Soffer, S.; Filanovsky, K.; Braester, A.; Fibach, E.; Dana, M.; Ofran, Y.; Greenbaum, U.; Nagler, A.; Amitai, I.; et al. The Effect of Oral Iron Chelator Deferiprone on Iron Overload and Oxidative Stress in Patients with Myelodysplastic Syndromes: A Study by the Israeli MDS Working Group. Acta Haematol. 2024, 147, 427–434. [Google Scholar] [CrossRef]
- Cappellini, A.; Mongiorgi, S.; Finelli, C.; Fazio, A.; Ratti, S.; Marvi, M.V.; Curti, A.; Salvestrini, V.; Pellagatti, A.; Billi, A.M.; et al. Phospholipase C beta1 (PI-PLCβ1)/Cyclin D3/protein kinase C (PKC) alpha signaling modulation during iron-induced oxidative stress in myelodysplastic syndromes (MDS). FASEB J. 2020, 34, 15400–15416. [Google Scholar] [CrossRef]
- Pilo, F.; Angelucci, E. A storm in the niche: Iron, oxidative stress and haemopoiesis. Blood Rev. 2018, 32, 29–35. [Google Scholar] [CrossRef]
- Ivars, D.; Orero, M.T.; Javier, K.; Díaz-Vico, L.; García-Giménez, J.L.; Mena, S.; Tormos, C.; Egea, M.; Pérez, P.L.; Arrizabalaga, B.; et al. Oxidative imbalance in low/intermediate-1-risk myelodysplastic syndrome patients: The influence of iron overload. Clin. Biochem. 2017, 50, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Li, Q.; Xu, A.; Hu, Y.; Sun, C. Ferroptosis in hematological malignancies and its potential network with abnormal tumor metabolism. Biomed. Pharmacother. 2022, 148, 112747. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, Y.; Liu, D.; Wang, M.; Wu, Y.; Tang, D.; Liu, X. Mitochondria as multifaceted regulators of ferroptosis. Life Metab. 2022, 1, 134–148. [Google Scholar] [CrossRef]
- Hayashi, Y.; Harada, H. Mitochondrial dynamics as a pathobiological mediator of clonal myeloid disorders. Cancer Sci. 2023, 114, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Alberghina, L. The Warburg Effect Explained: Integration of Enhanced Glycolysis with Heterogeneous Mitochondria to Promote Cancer Cell Proliferation. Int. J. Mol. Sci. 2023, 24, 15787. [Google Scholar] [CrossRef]
- Morganti, C.; Cabezas-Wallscheid, N.; Ito, K. Metabolic Regulation of Hematopoietic Stem Cells. HemaSphere 2022, 6, e740. [Google Scholar] [CrossRef]
- Liao, Y.; Octaviani, S.; Tian, Z.; Wang, S.R.; Huang, C.; Huang, J. Mitochondrial quality control in hematopoietic stem cells: Mechanisms, implications, and therapeutic opportunities. Stem Cell Res. Ther. 2025, 16, 180. [Google Scholar] [CrossRef]
- Guo, X.; Can, C.; Liu, W.; Wei, Y.; Yang, X.; Liu, J.; Jia, H.; Jia, W.; Wu, H.; Ma, D. Mitochondrial transfer in hematological malignancies. Biomark. Res. 2023, 11, 29. [Google Scholar] [CrossRef]
- Dexheimer, G.M.; Alves, J.; Reckziegel, L.; Lazzaretti, G.; Abujamra, A.L. DNA methylation events as markers for diagnosis and management of acute myeloid leukemia and myelodysplastic syndrome. Dis. Markers 2017, 2017, 5472893. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Alves, R.; Baldeiras, I.; Marques, B.; Oliveiros, B.; Pereira, A.; Costa, J.M.N.; Cortesão, E.; Vieira, L.M.; Ribeiro, A.B.S. DNA Methylation Is Correlated with Oxidative Stress in Myelodysplastic Syndrome—Relevance as Complementary Prognostic Biomarkers. Cancers 2021, 13, 3138. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Pereira, S.; Pereira, A.; Costa, J.M.N.; et al. Oxidative stress levels are correlated with P15 and P16 gene promoter methylation in myelodysplastic syndrome patients. Clin. Exp. Med. 2016, 16, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Pulikkan, J.A.; Tenen, D.G.; Behre, G. C/EBPα deregulation as a paradigm for leukemogenesis. Leukemia 2017, 31, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-M.; Hu, J.-B.; Yang, J.; Qian, W.; Yao, D.-M.; Deng, Z.-Q.; Zhang, Y.-Y.; Zhu, X.-W.; Guo, H.; Lin, J.; et al. CEBPA methylation and mutation in myelodysplastic syndrome. Med. Oncol. 2015, 32, 192. [Google Scholar] [CrossRef]
- Petzer, V.; Theurl, I.; Weiss, G.; Wolf, D. EnvIRONmental Aspects in Myelodysplastic Syndrome. Int. J. Mol. Sci. 2021, 22, 5202. [Google Scholar] [CrossRef]
- Boy, M.; Bisio, V.; Zhao, L.-P.; Guidez, F.; Schell, B.; Lereclus, E.; Henry, G.; Villemonteix, J.; Rodrigues-Lima, F.; Gagne, K.; et al. Myelodysplastic syndrome associated TET2 mutations affect NK cell function and genome methylation. Nat. Commun. 2023, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Singh, D.K.; Verma, M.; Fahl, S.P.; Rhodes, M.; Sprinkle, S.R.; Wang, M.; Zhang, Y.; Perrigoue, J.; Kessel, R.; et al. Ribosomal protein control of hematopoietic stem cell transformation through direct, non-canonical regulation of metabolism. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gonzalez-Menendez, P.; Phadke, I.; Olive, M.E.; Joly, A.; Papoin, J.; Yan, H.; Galtier, J.; Platon, J.; Kang, S.W.S.; McGraw, K.L.; et al. Arginine metabolism regulates human erythroid differentiation through hypusination of eIF5A. Blood 2023, 141, 2520–2536. [Google Scholar] [CrossRef]
- Fandy, T.E.; Jiemjit, A.; Thakar, M.; Rhoden, P.; Suarez, L.; Gore, S.D. Decitabine Induces Delayed Reactive Oxygen Species (ROS) Accumulation in Leukemia Cells and Induces the Expression of ROS Generating Enzymes. Clin. Cancer Res. 2014, 20, 1249–1258. [Google Scholar] [CrossRef]
- Lübbert, M.; Rüter, B.H.; Claus, R.; Schmoor, C.; Schmid, M.; Germing, U.; Kuendgen, A.; Rethwisch, V.; Ganser, A.; Platzbecker, U.; et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica 2012, 97, 393–401. [Google Scholar] [CrossRef]
- Montes, P.; Guerra-Librero, A.; García, P.; Cornejo-Calvo, M.E.; López, M.d.S.; de Haro, T.; Martínez-Ruiz, L.; Escames, G.; Acuña-Castroviejo, D. Effect of 5-Azacitidine Treatment on Redox Status and Inflammatory Condition in MDS Patients. Antioxidants 2022, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Mpakou, V.; Spathis, A.; Bouchla, A.; Tsakiraki, Z.; Kontsioti, F.; Papageorgiou, S.; Bazani, E.; Gkontopoulos, K.; Thomopoulos, T.; Glezou, I.; et al. Upregulated hypoxia inducible factor 1α sig-naling pathway in high risk myelodysplastic syndrome and acute myeloid leukemia patients is associated with better response to 5-azacytidine—Data from the Hellenic myelodysplastic syndrome study group. Hematol. Oncol. 2021, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, L.; Ørskov, A.D.; Liu, M.; Harsløf, L.B.; Jones, P.A.; Grønbæk, K. Vitamin C—A new player in regulation of the cancer epigenome. Semin. Cancer Biol. 2018, 51, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ottone, T.; Faraoni, I.; Fucci, G.; Divona, M.; Travaglini, S.; De Bellis, E.; Marchesi, F.; Angelini, D.F.; Palmieri, R.; Gurnari, C.; et al. Vitamin C Deficiency in Patients With Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 890344. [Google Scholar] [CrossRef]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017, 170, 1079–1095.e20. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Alves, V.; Silva, T.; Carvalho, C.; de Oliveira, C.R.; Sarmento-Ribeiro, A.B. Oxidative stress mediates apoptotic effects of ascorbate and dehydroascorbate in human Myelodysplasia cells in vitro. Toxicol. Vitr. 2013, 27, 1542–1549. [Google Scholar] [CrossRef]
- Kumar, S.; Mathew, S.O.; Aharwal, R.P.; Tulli, H.S.; Mohan, C.D.; Sethi, G.; Ahn, K.-S.; Webber, K.; Sandhu, S.S.; Bishayee, A. Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal. Pharmaceuticals 2023, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Oben, K.Z.; Alhakeem, S.S.; McKenna, M.K.; Brandon, J.A.; Mani, R.; Noothi, S.K.; Jinpeng, L.; Akunuru, S.; Dhar, S.K.; Singh, I.P.; et al. Oxidative stress-induced JNK/AP-1 signaling is a major pathway involved in selective apoptosis of myelodysplastic syndrome cells by Withaferin-A. Oncotarget 2017, 8, 77436–77452. [Google Scholar] [CrossRef]
- Okamoto, S.; Tsujioka, T.; Suemori, S.; Kida, J.; Kondo, T.; Tohyama, Y.; Tohyama, K. Withaferin A suppresses the growth of myelodysplasia and leukemia cell lines by inhibiting cell cycle progression. Cancer Sci. 2016, 107, 1302–1314. [Google Scholar] [CrossRef]
- Vannini, N.; Campos, V.; Girotra, M.; Trachsel, V.; Rojas-Sutterlin, S.; Tratwal, J.; Ragusa, S.; Stefanidis, E.; Ryu, D.; Rainer, P.Y.; et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 2019, 24, 405–418.e7. [Google Scholar] [CrossRef]
- Torello, C.O.; Alvarez, M.C.; Saad, S.T.O. Polyphenolic flavonoid compound quercetin effects in the treatment of acute my-eloid leukemia and myelodysplastic syndromes. Molecules 2021, 26, 5781. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.; Hwang, J.; Van Den Broek, T.J.; Oh, B.; Kim, J.Y.; Wopereis, S.; Bouwman, J.; Kwon, O. A Machine Learning Algorithm for Quantitatively Diag-nosing Oxidative Stress Risks in Healthy Adult Individuals Based on Health Space Methodology: A Proof-of-Concept Study Using Korean Cross-Sectional Cohort Data. Antioxidants 2021, 10, 1132. [Google Scholar] [CrossRef]
- Idowu, S.O.; Fatokun, A.A. Artificial Intelligence (AI) to the Rescue: Deploying Machine Learning to Bridge the Biorelevance Gap in Antioxidant Assays. SLAS Technol. Transl. Life Sci. Innov. 2021, 26, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Moon, J.-O.; Ahn, S.I.; Lee, H. Predicting antioxidant activity of compounds based on chemical structure using machine learning methods. Korean J. Physiol. Pharmacol. 2024, 28, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Su, W.; Zhong, L.; Yang, Z.; Li, T.; Liang, Y.; Ruan, T.; Jiang, G. Comprehensive Characterization of Oxidative Stress-Modulating Chemicals Using GPT-Based Text Mining. Environ. Sci. Technol. 2024, 58, 20540–20552. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recom-mendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Tanoli, Z.; Vähä-Koskela, M.; Aittokallio, T. Artificial intelligence, machine learning, and drug repurposing in cancer. Expert Opin. Drug Discov. 2021, 16, 977–989. [Google Scholar] [CrossRef]
- Issa, N.T.; Stathias, V.; Schürer, S.; Dakshanamurthy, S. Machine and deep learning approaches for cancer drug repurposing. Semin. Cancer Biol. 2021, 68, 132–142. [Google Scholar] [CrossRef]
- Ambreen, S.; Umar, M.; Noor, A.; Jain, H.; Ali, R. Advanced AI and ML frameworks for transforming drug discovery and opti-mization: With innovative insights in polypharmacology, drug repurposing, combination therapy and nanomedicine. Eur. J. Med. Chem. 2025, 284, 117164. [Google Scholar] [CrossRef]
| Mechanism | Molecular Drivers | Cellular Impact | Therapeutic Strategies |
|---|---|---|---|
| DNA Damage | ROS, RNS | Genomic instability, mutations, apoptosis | Antioxidants, DNA repair enhancers |
| Mitochondrial Dysfunction | Iron overload, mtDNA mutations | Altered energy metabolism, increased ROS | Iron chelators (deferasirox), mitochondrial-targeted therapies |
| Epigenetic Alterations | TET2, DNMT3A, CEBPA methylation | Aberrant gene expression, impaired differentiation | HMAs, vitamin C (TET cofactor) |
| Redox Imbalance | Low GSH, high NOX activity | Sustained oxidative stress, ineffective hematopoiesis | GSH donors, NOX inhibitors |
| Immune Dysfunction | NK cells, inflammatory cytokines | Impaired immune surveillance | Immunomodulators, azacitidine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierro, F.; Fazio, M.; Murdaca, G.; Stagno, F.; Gangemi, S.; Allegra, A. Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment. Int. J. Mol. Sci. 2025, 26, 6415. https://doi.org/10.3390/ijms26136415
Pierro F, Fazio M, Murdaca G, Stagno F, Gangemi S, Allegra A. Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment. International Journal of Molecular Sciences. 2025; 26(13):6415. https://doi.org/10.3390/ijms26136415
Chicago/Turabian StylePierro, Federico, Manlio Fazio, Giuseppe Murdaca, Fabio Stagno, Sebastiano Gangemi, and Alessandro Allegra. 2025. "Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment" International Journal of Molecular Sciences 26, no. 13: 6415. https://doi.org/10.3390/ijms26136415
APA StylePierro, F., Fazio, M., Murdaca, G., Stagno, F., Gangemi, S., & Allegra, A. (2025). Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment. International Journal of Molecular Sciences, 26(13), 6415. https://doi.org/10.3390/ijms26136415











