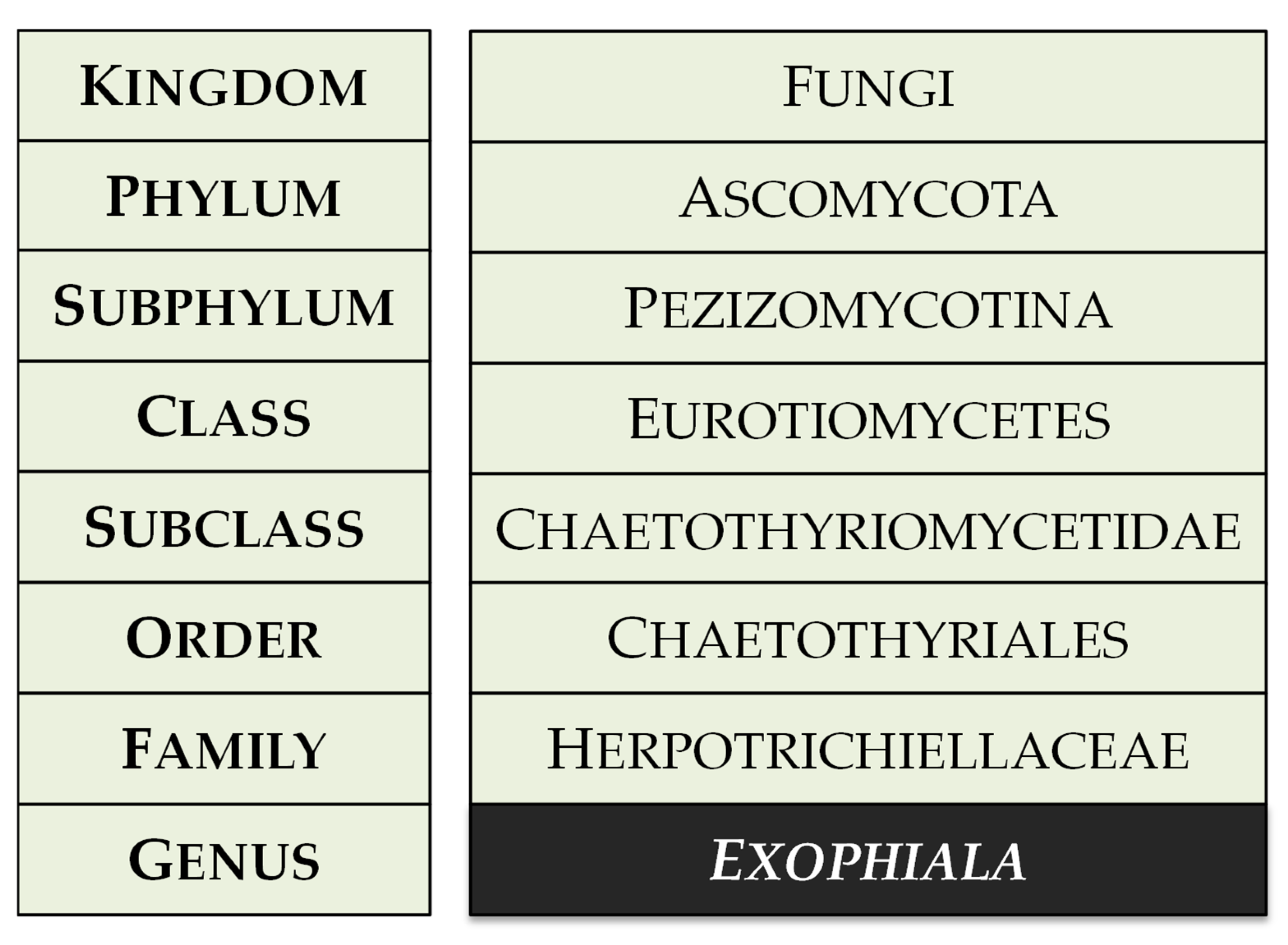
Clinical and Molecular Advances on the Black Yeast Exophiala dermatitidis
Abstract
1. Introduction
2. Study Selection
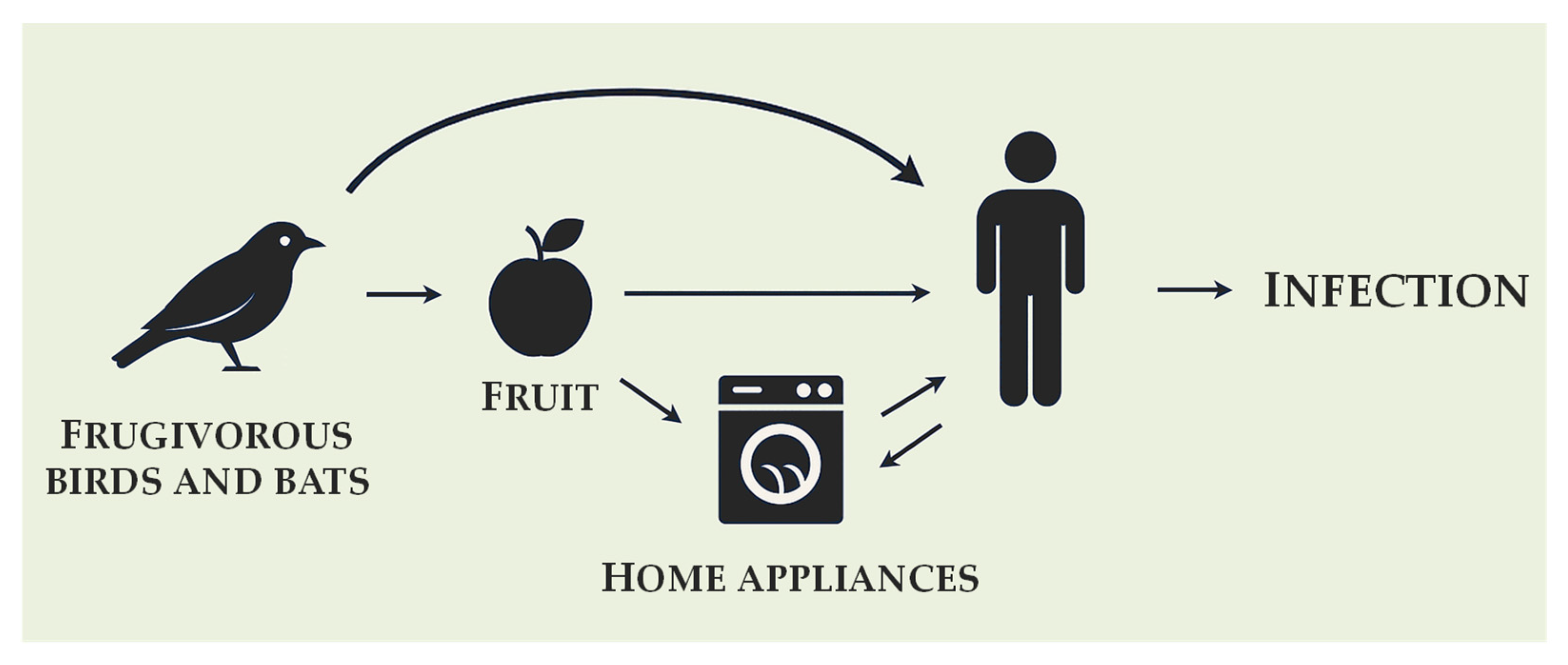
3. Ecological Background of Exophiala dermatitidis
3.1. Occurrence in Human Environment
3.2. Natural Habitat, Origin and Hypothetical Transmission Route
4. Clinical and Epidemiological Aspects of Exophiala dermatitidis
4.1. Predisposing Factors
4.1.1. Cystic Fibrosis
4.1.2. CARD9 Deficiency
4.2. Recent Clinical Outcomes
4.2.1. Pulmonary Infections Beyond Cystic Fibrosis
4.2.2. Central Nervous System Infections
4.2.3. Fungemia and Disseminated Infections
4.2.4. Association with Crohn’s Disease
4.2.5. Ocular Infections
4.2.6. Scalp Infections and Hair Loss Involving Exophiala dermatitidis
4.2.7. Exophiala dermatitidis in Polymicrobial Infections
4.2.8. Expanding Clinical Perspectives—Animal Infections
5. Updated Insights into Virulence Factors of Exophiala dermatitidis
5.1. Melanin as a Virulence Factor
5.2. Capsule Formation
5.3. Hydrolytic and Virulence-Associated Enzymes
5.3.1. Catalase
5.3.2. Urease
5.3.3. DNase
5.3.4. Protease
5.3.5. Hemolysins
5.3.6. Other Enzymes and Knowledge Gaps
5.4. Carbon Source Utilization and Metabolic Plasticity
5.5. Other Virulence-Associated Traits
6. Diagnostic and Therapeutic Considerations
6.1. Diagnostic Strategies
6.1.1. Classical Culture-Based Diagnostics
6.1.2. Morphological and Staining Techniques
6.1.3. PCR and ITS Sequencing
6.1.4. STR Genotyping and AFLP
6.1.5. MALDI-TOF Mass Spectrometry
6.1.6. Diagnostic Challenges and Misidentification
6.2. Therapeutic Management
6.2.1. Clinical Approaches
6.2.2. In Vitro Susceptibility Profiles
6.2.3. Successful Treatment Strategies
7. Conclusions and Future Research
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | amino acid |
| AFLP | amplified fragment length polymorphism |
| AIDS | acquired immunodeficiency syndrome |
| BCSA | Burkholderia cepacia selective agar |
| BYF | black yeast-like fungi |
| CARD9 | caspase recruitment domain-containing protein 9 |
| CF | cystic fibrosis |
| CFTR | cystic fibrosis transmembrane regulator |
| CD | Crohn’s disease |
| CLABSI | central line-associated bloodstream infections |
| CLRs | C-type lectin receptors |
| CNS | central nervous system |
| COPD | chronic obstructive pulmonary disease |
| COVID | coronavirus disease |
| CXCL/R | C-X-C motif chemokine ligand/receptor |
| D1/D2 | domains 1 and 2 of the large subunit ribosomal RNA gene |
| DHN | 1,8-dihydroxynaphthalene |
| DIC | differential interference contrast |
| ECA | erythritol chloramphenicol agar |
| FFPE | formalin-fixed paraffin-embedded |
| GABA | γ-aminobutyric acid |
| GI | gastrointestinal |
| HGA | homogentisic acid |
| HIV | human immunodeficiency virus |
| ID | identification |
| IL | interleukin |
| INDEL | insertion-deletion |
| ITS | internal transcribed spacer |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| KS | keto-synthase |
| MALDI-TOF | matrix-assisted laser desorption ionization time-of-flight |
| MAPK | mitogen-activated protein kinase |
| MEA | malt extract agar |
| MIC | minimal inhibitory concentration |
| mNGS | metagenomic next-generation sequencing |
| MS | mass spectrometry |
| Myb-like | myeloblastosis-like |
| NET | neutrophil extracellular trap |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NI | not investigated |
| PAMP | pathogen-associated molecular pattern |
| PCR | polymerase chain reaction |
| Pks | polyketide synthase |
| PRRs | pattern recognition receptors |
| RBC | red blood cells |
| ROS | reactive oxygen species |
| SDA | Sabouraud dextrose agar |
| SGCA | Sabouraud gentamicin chloramphenicol agar |
| SH-SY5Y | human-derived neuroblastoma cell line, subclone of SK-N-SH |
| SNP | single nucleotide polymorphism |
| SOD | superoxide dismutase |
| STR | short tandem repeat |
| TCA | tricarboxylic acid cycle |
| TH17/22 | T helper 17/22 |
| TLRs | Toll-like receptors |
| TNF-α | tumor necrosis factor α |
| YPD | yeast extract-peptone-dextrose |
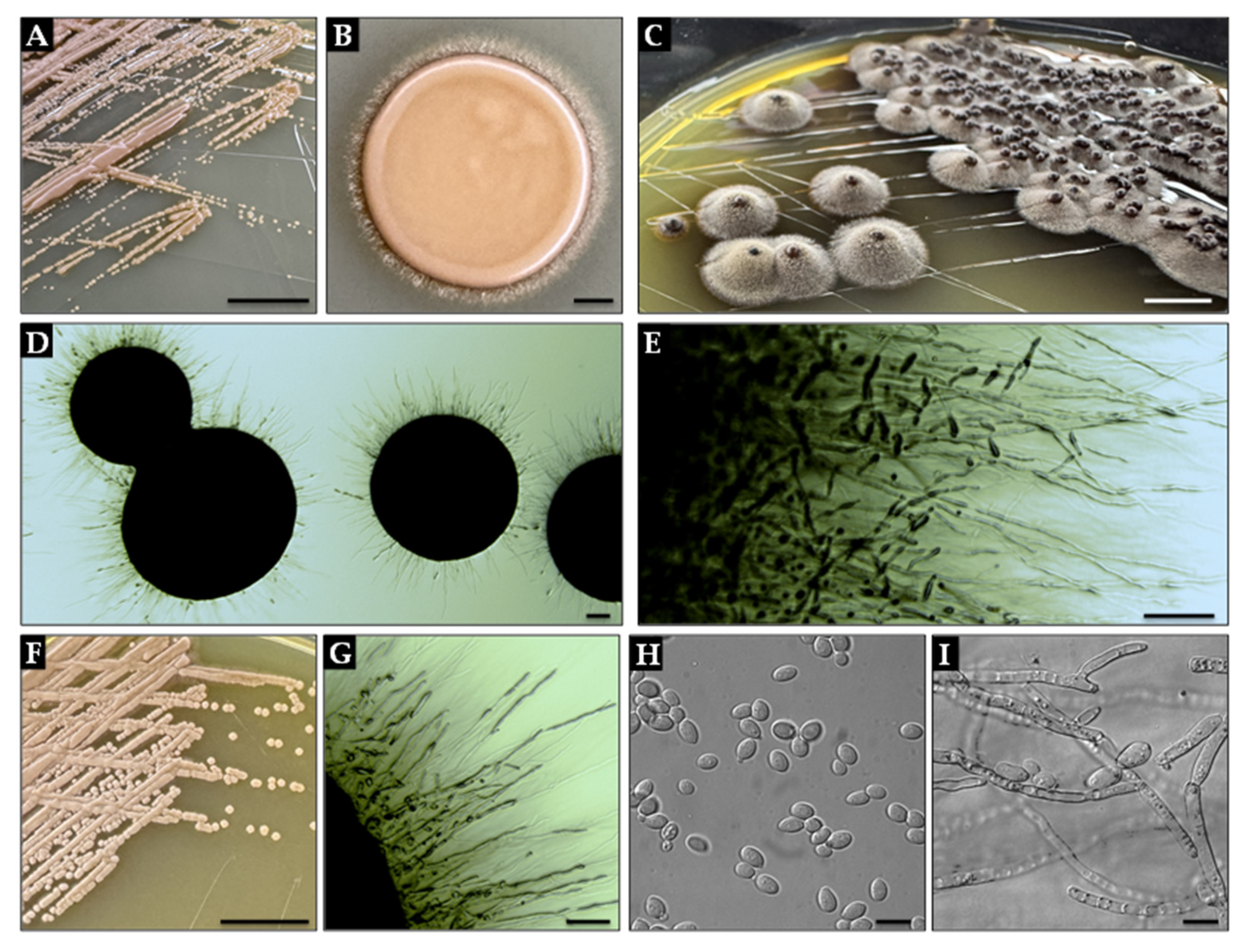
Appendix A. Extended Data on Exophiala dermatitidis
Appendix A.1. Additional Background Information
Appendix A.2. Macro- and Micromorphology of Exophiala dermatitidis
References
- Carmichael, J.W. Cerebral mycetoma of trout due to a Phialophora-like fungus. Sabouraudia 1966, 5, 120–123. [Google Scholar] [CrossRef]
- Kirchhoff, L.; Olsowski, M.; Rath, P.M.; Steinmann, J. Exophiala dermatitidis: Key issues of an opportunistic fungal pathogen. Virulence 2019, 10, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Fraser, M.; Schilling, W.; Jones, G.; Pearl, R.; Linton, C.J.; Johnson, E.M. Exophiala campbellii causing a subcutaneous palmar cyst in an otherwise healthy UK resident. Med. Mycol. Case Rep. 2020, 29, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Jabgratog, P.; Chamroensakchai, T.; Kanjanabuch, T.; Ampaipun, J.; Thongbor, N.; Hurdeal, V.G.; Hyde, K.D. Peritoneal dialysis-associated peritonitis caused by Exophiala spinifera: A case report and review of literature. Med. Mycol. Case Rep. 2022, 35, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Miyakubo, T.; Todokoro, D.; Satake, Y.; Makimura, K.; Miyakubo, S.; Akiyama, H. Exophiala lecanii-corni keratitis presenting as a serpiginous pigmented superficial lesion: A case report. Medicine 2020, 99, e22121. [Google Scholar] [CrossRef]
- Heath, C.P.; Sharma, P.C.; Sontakke, S.; Smith, D.J.; Jhaveri, T.A. The brief case: Hidden in plain sight—Exophiala jeanselmei subcutaneous phaeohyphomycosis of hand masquerading as a hematoma. J. Clin. Microbiol. 2024, 62, e0106824. [Google Scholar] [CrossRef]
- Anandabhavan, A.M.; Anoop, T.; Narayanan, D.P.; Payyappilly, R.J. Subcutaneous phaeohyphomycoses caused by Exophiala xenobiotica in an immunocompromised patient: A case report. Indian J. Dermatol. Venereol. Leprol. 2024, 90, 266. [Google Scholar] [CrossRef]
- AlAgha, R.; Chew, K.L.; Tay, W.J.; Lum, L. Answer to photo quiz: Phaeohyphomycosis caused by Exophiala oligosperma. J. Clin. Microbiol. 2021, 59, e0219520. [Google Scholar] [CrossRef]
- Carlos, D.S.; Isa-pimentel, M.; Arenas, R. Phaeohyphomycosis: A review. Microbiol. Res. 2023, 14, 1751–1763. [Google Scholar] [CrossRef]
- de León, L.R.; Moreno-Perlín, T.; Castillo-Marenco, T.; del Rayo Sánchez-Carbente, M.; Gostinčar, C.; Ramírez-Durán, N.; Ocaña, A.M.F.; Sánchez, N.C.; Dávila-Ramos, S.; Gunde-Cimerman, N.; et al. Polyextremotolerant, opportunistic, and melanin-driven resilient black yeast Exophiala dermatitidis in environmental and clinical contexts. Sci. Rep. 2025, 15, 6472. [Google Scholar] [CrossRef]
- Lebecque, P.; Leonard, A.; Huang, D.; Reychler, G.; Boeras, A.; Leal, T.; Symoens, F. Exophiala (Wangiella) dermatitidis and cystic fibrosis–prevalence and risk factors. Med. Mycol. 2010, 48 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- de Jong, C.C.M.; Slabbers, L.; Engel, T.G.P.; Yntema, J.B.; van Westreenen, M.; Croughs, P.D.; Roeleveld, N.; Brimicombe, R.; Verweij, P.E.; Meis, J.F.; et al. Clinical relevance of Scedosporium spp. and Exophiala dermatitidis in patients with cystic fibrosis: A nationwide study. Med. Mycol. 2020, 58, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Diemert, D.; Kunimoto, D.; Sand, C.; Rennie, R. Sputum isolation of Wangiella dermatitidis in patients with cystic fibrosis. Scand. J. Infect. Dis. 2001, 33, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.; Rautemaa-Richardson, R.; Wilkinson, S.; Patel, L.; Maitra, A.; Horsley, A. Impact of airway Exophiala spp. on children with cystic fibrosis. J. Cyst. Fibros. 2021, 20, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Kondori, N.; Gilljam, M.; Lindblad, A.; Jönsson, B.; Moore, E.R.B.; Wennerås, C. High rate of Exophiala dermatitidis recovery in the airways of patients with cystic fibrosis is associated with pancreatic insufficiency. J. Clin. Microbiol. 2011, 49, 1004–1009. [Google Scholar] [CrossRef]
- Tanuskova, D.; Horakova, J.; Buzassyova, D.; Poczova, M.; Bodova, I.; Svec, P.; Chocholova, A.; Adamcakova, J.; Sykora, T.; Pozdechova, M.; et al. A case of Exophiala dermatitidis infection in a child after allogeneic stem cell transplantation: Case report and literature review of paediatric cases. JMM Case Rep. 2017, 4, e005102. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakamura, A.; Fujieda, A.; Nakase, K.; Katayama, N. Pulmonary infection caused by Exophiala dermatitidis in a patient with multiple myeloma: A case report and a review of the literature. Med. Mycol. Case Rep. 2012, 1, 95–98. [Google Scholar] [CrossRef]
- Babič, M.N.; Zupančič, J.; Gunde-Cimerman, N.; de Hoog, S.; Zalar, P. Ecology of the human opportunistic black yeast Exophiala dermatitidis indicates preference for human-made habitats. Mycopathologia 2018, 183, 201–212. [Google Scholar] [CrossRef]
- Shoff, C.J.; Perfect, J.R. Uncommon yeasts and molds causing human disease. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 813–834. ISBN 9780323851800. [Google Scholar]
- Sudhadham, M.; Prakitsin, S.; Sivichai, S.; Chaiyarat, R.; Dorrestein, G.M.; Menken, S.B.J.; de Hoog, G.S. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 2008, 61, 145–155. [Google Scholar] [CrossRef]
- Blasi, B.; Tafer, H.; Tesei, D.; Sterflinger, K. From glacier to sauna: RNA-seq of the human pathogen black fungus Exophiala dermatitidis under varying temperature conditions exhibits common and novel fungal response. PLoS ONE 2015, 10, e0127103. [Google Scholar] [CrossRef]
- Gümral, R.; Özhak-Baysan, B.; Tümgör, A.; Saraçlı, M.A.; Yıldıran, Ş.T.; Ilkit, M.; Zupančič, J.; Novak-Babič, M.; Gunde-Cimerman, N.; Zalar, P.; et al. Dishwashers provide a selective extreme environment for human-opportunistic yeast-like fungi. Fungal Divers. 2016, 76, 1–9. [Google Scholar] [CrossRef]
- Zupančič, J.; Babič, M.N.; Zalar, P.; Gunde-Cimerman, N. The black yeast Exophiala dermatitidis and other selected opportunistic human fungal pathogens spread from dishwashers to kitchens. PLoS ONE 2016, 11, e0148166. [Google Scholar] [CrossRef] [PubMed]
- Toberna, C.P.; Kram, J.J.F.; Beck, E.T.; Ray, S.; Gavinski, T.; Sterkel, A.K.; Baumgardner, D.J. Attempted isolation of Cryptococcus species and incidental isolation of Exophiala dermatitidis from human oral cavities. Mycopathologia 2020, 185, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Voidaleski, M.F.; Costa, F.d.F.; de Hoog, G.S.; Gomes, R.R.; Vicente, V.A. Metagenomics reveals an abundance of black yeast-like fungi in the skin microbiome. Mycoses 2023, 66, 488–496. [Google Scholar] [CrossRef]
- Sultan Karakoyun, A.; Ünal, N.; Metin, B.; Yıldırım Servi, E.; Spruijtenburg, B.; Alper Özarslan, M.; Gümral, R.; Döğen, A.; Ilkit, M.; Kandemir, H.; et al. Opportunistic yeasts on stored apples: A One Health perspective. One Health Mycol. 2023, 1, 4–13. [Google Scholar] [CrossRef]
- Sudhadham, M.; Gerrits Van Den Ende, A.H.G.; Sihanonth, P.; Sivichai, S.; Chaiyarat, R.; Menken, S.B.J.; Van Belkum, A.; De Hoog, G.S. Elucidation of distribution patterns and possible infection routes of the neurotropic black yeast Exophiala dermatitidis using AFLP. Fungal Biol. 2011, 115, 1051–1065. [Google Scholar] [CrossRef]
- Marques, G.N.; Cota, J.B.; Leal, M.O.; Silva, N.U.; Flanagan, C.A.; Crosta, L.; Tavares, L.; Oliveira, M. First documentation of Exophiala spp. isolation in Psittaciformes. Animals 2022, 12, 1699. [Google Scholar] [CrossRef]
- Seng, P.; Abat, C.; Rolain, J.M.; Colson, P.; Lagier, J.C.; Gouriet, F.; Fournier, P.E.; Drancourt, M.; La Scola, B.; Raoult, D. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: Impact of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 2182–2194. [Google Scholar] [CrossRef]
- Kang, S.J.; Choi, S.M.; Choi, J.A.; Choi, J.U.; Oh, T.H.; Kim, S.E.; Kim, U.J.; Won, E.J.; Jang, H.C.; Park, K.H.; et al. Factors affecting the clinical relevance of Corynebacterium striatum isolated from blood cultures. PLoS ONE 2018, 13, e0199454. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, Y.; Zheng, J. A review of cystic fibrosis: Basic and clinical aspects. Anim. Model. Exp. Med. 2021, 4, 220–232. [Google Scholar] [CrossRef]
- Kurbessoian, T.; Murante, D.; Crocker, A.; Hogan, D.A.; Stajich, J.E. In-host evolution of Exophiala dermatitidis in cystic fibrosis lung micro-environment. G3 Genes Genomes Genet. 2023, 13, jkad126. [Google Scholar] [CrossRef]
- Haase, G.; Skopnik, H.; Kusenbach, G. Exophiala dermatitidis infection in cystic fibrosis. Lancet 1990, 336, 188–189. [Google Scholar] [CrossRef]
- Kusenbach, G.; Skopnik, H.; Haase, G.; Friedrichs, F.; Döhmen, H. Exophiala dermatitidis pneumonia in cystic fibrosis. Eur. J. Pediatr. 1992, 151, 344–346. [Google Scholar] [CrossRef]
- Symington, L.S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 2002, 66, 630–670. [Google Scholar] [CrossRef]
- Murante, D.; Demers, E.G.; Kurbessoian, T.; Ruzic, M.; Ashare, A.; Stajich, J.E.; Hogan, D.A. Mrs4 loss of function in fungi during adaptation to the cystic fibrosis lung. mBio 2023, 14, e0117123. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, B.; Hao, H.; Liu, Z. CARD9 signaling, inflammation, and diseases. Front. Immunol. 2022, 13, 880879. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, C. Role of CARD9 in cell- and organ-specific immune responses in various infections. Int. J. Mol. Sci. 2024, 25, 2598. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Lin, Z.; Wang, X.; Li, T.; Yu, J.; Liu, W.; Tong, Z.; Xu, Y.; Zhang, J.; et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J. Allergy Clin. Immunol. 2014, 133, 905–908. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Gao, J.; Zhang, C.; Zhang, X.; Dang, E.; Li, W.; Qiao, H.; Liao, W.; Wang, G.; et al. The phytopathogenic fungus Pallidocercospora crystallina-caused localized subcutaneous phaeohyphomycosis in a patient with a homozygous missense CARD9 mutation. J. Clin. Immunol. 2019, 39, 713–725. [Google Scholar] [CrossRef]
- Glocker, E.-O.; Hennigs, A.; Nabavi, M.; Schäffer, A.A.; Woellner, C.; Salzer, U.; Pfeifer, D.; Veelken, H.; Warnatz, K.; Tahami, F.; et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009, 361, 1727–1735. [Google Scholar] [CrossRef]
- Imanaka, Y.; Taniguchi, M.; Doi, T.; Tsumura, M.; Nagaoka, R.; Shimomura, M.; Asano, T.; Kagawa, R.; Mizoguchi, Y.; Karakawa, S.; et al. Inherited CARD9 deficiency in a child with invasive disease due to Exophiala dermatitidis and two older but asymptomatic siblings. J. Clin. Immunol. 2021, 41, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhao, Y.; Tang, M.; Xia, H.; Li, D.; Lu, G. Concurrent infection of Exophiala dermatitidis and Angiostrongylus cantonensis in central nervous system of a child with inherited CARD9 deficiency: A case report and literature review. J. Med. Mycol. 2024, 34, 101455. [Google Scholar] [CrossRef] [PubMed]
- Alves de Medeiros, A.K.; Lodewick, E.; Bogaert, D.J.A.; Haerynck, F.; Van daele, S.; Lambrecht, B.; Bosma, S.; Vanderdonckt, L.; Lortholary, O.; Migaud, M.; et al. Chronic and invasive fungal infections in a family with CARD9 deficiency. J. Clin. Immunol. 2016, 36, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ju, J.; Luo, M.; Zeng, L.; Xu, H.; Li, Z.; Wang, Y. Exophiala dermatitidis aggravates colitis through the Syk-CARD9 signaling pathway. J. Crohn’s Colitis 2025, 19, i292–i293. [Google Scholar] [CrossRef]
- Vinh, D.C. The molecular immunology of human susceptibility to fungal diseases: Lessons from single gene defects of immunity. Expert Rev. Clin. Immunol. 2019, 15, 461–486. [Google Scholar] [CrossRef]
- Klasinc, R.; Riesenhuber, M.; Bacher, A.; Willinger, B. Invasive fungal infection caused by Exophiala dermatitidis in a patient after lung transplantation: Case report and literature review. Mycopathologia 2019, 184, 107–113. [Google Scholar] [CrossRef]
- Barrera, C.; Schwarz, C.; Delhaes, L.; Le Gal, S.; Ramel, S.; Gangneux, J.P.; Guitard, J.; Hoffmann, C.; Bellanger, A.P.; Bouchara, J.P.; et al. Detection of specific IgE against molds involved in allergic bronchopulmonary mycoses in patients with cystic fibrosis. Mycopathologia 2024, 189, 68. [Google Scholar] [CrossRef]
- Yu, H.Y.; Qu, T.T.; Yang, Q.; Hu, J.H.; Sheng, J.F. A fatal case of Exophiala dermatitidis meningoencephalitis in an immunocompetent host: A case report and literature review. J. Infect. Chemother. 2021, 27, 1520–1524. [Google Scholar] [CrossRef]
- Lavrin, T.; Konte, T.; Kostanjšek, R.; Sitar, S.; Sepcic, K.; Mihevc, S.P.; Žagar, E.; Župunski, V.; Lenassi, M.; Rogelj, B.; et al. The neurotropic black yeast Exophiala dermatitidis induces neurocytotoxicity in neuroblastoma cells and progressive cell death. Cells 2020, 9, 963. [Google Scholar] [CrossRef]
- Tzar, M.N.; Meor Jamaludin, W.H.B.; Abdul Wahab, A.; Ding, C.H. Exophiala dermatitidis, ‘the real black fungus’ fungemia in a patient with COVID-19. IDCases 2022, 27, e01428. [Google Scholar] [CrossRef]
- Yurtsever, N.; Dougherty, P.; Condon, S.; Orsini, R.; Berman, M.; Berry, G.J.; Duong, S. Exophiala dermatitidis isolated from blood in patient with steroid use. Am. J. Clin. Pathol. 2020, 154 (Suppl. S1), S154–S155. [Google Scholar] [CrossRef]
- Hagiya, H.; Maeda, T.; Kusakabe, S.; Kawasaki, K.; Hori, Y.; Kimura, K.; Ueda, A.; Yoshioka, N.; Sunada, A.; Nishi, I.; et al. A fatal case of Exophiala dermatitidis disseminated infection in an allogenic hematopoietic stem cell transplant recipient during micafungin therapy. J. Infect. Chemother. 2019, 25, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.; Goldberg, M.; Mody, R. Noninvasive colonic phaeohyphomycosis caused by Exophiala oligosperma in a patient with Crohn’s disease. Am. J. Gastroenterol. 2015, 110, 388. [Google Scholar] [CrossRef]
- Homa, M.; Manikandan, P.; Saravanan, V.; Revathi, R.; Anita, R.; Narendran, V.; Panneerselvam, K.; Shobana, C.S.; Aidarous, M.A.; Galgóczy, L.; et al. Exophiala dermatitidis endophthalmitis: Case report and literature review. Mycopathologia 2018, 183, 603–609. [Google Scholar] [CrossRef]
- Zeng, L.; Feng, Z.; Zhuo, M.; Wen, Z.; Zhu, C.; Tang, C.; Liu, L.; Wang, Y. Fecal fungal microbiota alterations associated with clinical phenotypes in Crohn’s disease in Southwest China. PeerJ 2022, 10, e14260. [Google Scholar] [CrossRef]
- Shah, S.J.; Nakhwa, C.; Shah, S.; Rai, M. Late onset endophthalmitis with rare fungus Exophiala dermatitidis. Indian J. Ophthalmol.-Case Rep. 2022, 2, 480–482. [Google Scholar] [CrossRef]
- Mormeneo Bayo, S.; Bellés Bellés, A.; Prats Sánchez, I.; López González, E.; Aramburu Arnuelos, J.; Bernet Sánchez, A.; García González, M. Fungal corneal abscess caused by Exophiala dermatitidis. Rev. Esp. Quimioter. 2023, 36, 425–426. [Google Scholar] [CrossRef]
- Beniwal, A.; Manumuraleekrishna; Das, A.K.; Ahmed, N.H.; Rathod, P.; Tandon, R. A rare case of Exophiala dermatitidis graft infection. Indian J. Ophthalmol.-Case Rep. 2023, 3, 671–672. [Google Scholar] [CrossRef]
- Atta, M.M.; Hussein, F.A. Isolation and identification of fungi that cause hair loss (alopecia). Adv. Biosci. Biotechnol. 2023, 14, 337–345. [Google Scholar] [CrossRef]
- Miyoshi, S.; Tanabe, M.; Semba, M.; Sato, C.; Aoyama, S.; Watanabe, A.; Ito, R.; Hamada, K.; Watanabe, A.; Abe, M. Exophiala dermatitidis coinfection with nontuberculous mycobacteria: A case report and literature review. Respirol. Case Rep. 2023, 11, e01221. [Google Scholar] [CrossRef]
- Ahamad, A.; Tehreem, B.; Farooqi, M.; Maramara, B. Case report and literature review: Double jeopardy–Exophiala dermatitidis and Mycobacterium canariasense central line-associated bloodstream infection in a patient. Access Microbiol. 2022, 4, 000347. [Google Scholar] [CrossRef]
- Kohli, A.; Rifai, Z.J.; Foray, N. Exploring a rare pulmonary coinfection: Cryptococcal pneumonia and Exophiala dermatitidis in an immunocompetent host. Cureus 2024, 16, e61085. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.; Veiga, F.F.; Svidzinski, T.I.E.; Negri, M. Case of mixed infection of toenail caused by Candida parapsilosis and Exophiala dermatitidis and in vitro effectiveness of propolis extract on mixed biofilm. J. Fungi 2023, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, L.; Weisner, A.K.; Schrepffer, M.; Hain, A.; Scharmann, U.; Buer, J.; Rath, P.M.; Steinmann, J. Phenotypical characteristics of the black yeast Exophiala dermatitidis are affected by Pseudomonas aeruginosa in an artificial sputum medium mimicking cystic fibrosis–like conditions. Front. Microbiol. 2020, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y. Ecology and Evolution of the Order Chaetothyriales in Search of Human Pathogenicity; Radboud Institute for Molecular Life Sciences: Nijmegen, The Netherlands, 2024. [Google Scholar]
- Hatta, J.; Anzawa, K.; Kubota, K.; Ohtani, T.; Mochizuki, T. A case of recalcitrant phaeohyphomycosis of the face caused by Exophiala lecanii-corni. Med. Mycol. J. 2021, 62, 35–39. [Google Scholar] [CrossRef]
- Ling, J.; Pu, Y.; Gan, J.; Zhou, W.; Chen, X.; Zhang, Q.; Jiang, S.; Liu, C.; Kuang, L. CARD9 deficiency in combination with invasive infection by Exophiala dermatitidis in a pediatric patient. Mycopathologia 2022, 187, 299–303. [Google Scholar] [CrossRef]
- Nakatani, R.; Ashiarai, M.; Yoshihara, H.; Yada, K.; Nozaki, T.; Ushigusa, T.; Mori, N.; Hasegawa, D. Multidisciplinary management of disseminated Exophiala dermatitidis mycosis in an infant with mixed phenotype acute leukemia: A case report. BMC Infect. Dis. 2022, 22, 797. [Google Scholar] [CrossRef]
- Su, G.; Huang, P.; Liu, D.; Xing, G.; Guo, R.; Li, S.; Fan, S.; Cheng, L.; Yan, Q. Gut mycobiome alterations and network interactions with the bacteriome in patients with atherosclerotic cardiovascular disease. Microbiol. Spectr. 2024, 13, e0218224. [Google Scholar] [CrossRef]
- Muotoe-Okafor, F.A.; Gugnani, H.C. Isolation of Lecythophora mutabilis and Wangiella dermatitidis from the fruit-eating bat, Eidolon helvum. Mycopathologia 1993, 122, 95–100. [Google Scholar] [CrossRef]
- Kano, R.; Kusuda, M.; Nakamura, Y.; Watanabe, S.; Tsujimoto, H.; Hasegawa, A. First isolation of Exophiala dermatitidis from a dog: Identification by molecular analysis. Vet. Microbiol. 2000, 76, 201–205. [Google Scholar] [CrossRef]
- Murphy, K.F.; Malik, R.; Barnes, A.; Hotston-Moore, A.; Pearson, G.R.; Barr, F.J. Successful treatment of intra-abdominal Exophiala dermatitidis infection in a dog. Vet. Rec. 2011, 168, 217a. [Google Scholar] [CrossRef]
- Osada, H.; Nagashima-Fukui, M.; Okazawa, T.; Omura, M.; Makimura, K.; Ohmori, K. Case report: First isolation of Exophiala dermatitidis from subcutaneous phaeohyphomycosis in a cat. Front. Vet. Sci. 2023, 10, 1259115. [Google Scholar] [CrossRef]
- Irie, M.; Kita, C.; Yamagami, T.; Miyoshi, T.; Fujiki, N.; Kuriyagawa, Y.; Hanafusa, Y.; Chambers, J.K.; Uchida, K. A case of Exophiala dermatitidis-induced phaeohyphomycosis in a cat with multiple intra-abdominal masses. J. Vet. Med. Sci. 2024, 86, 550–554. [Google Scholar] [CrossRef]
- Chhoker, K.; Hausner, G.; Harris, S.D. Genetic Analysis of Pigment Production in the Fungus Exophiala dermatitidis. bioRxiv 2025. Available online: https://www.themoonlight.io/file?url=https%3A%2F%2Fwww.biorxiv.org%2Fcontent%2F10.1101%2F2025.03.19.644242.full.pdf (accessed on 7 July 2025). [CrossRef]
- Poyntner, C.; Mirastschijski, U.; Sterflinger, K.; Tafer, H. Transcriptome study of an Exophiala dermatitidis PKS1 mutant on an ex vivo skin model: Is melanin important for infection? Front. Microbiol. 2018, 9, 1457. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.E.; Schultzhaus, Z.; Frank, C.; Romsdahl, J.; Wang, Z.; Dadachova, E. Transcriptomic and genomic changes associated with radioadaptation in Exophiala dermatitidis. Comput. Struct. Biotechnol. J. 2021, 19, 196–205. [Google Scholar] [CrossRef]
- Schultzhaus, Z.; Chen, A.; Shuryak, I.; Wang, Z. The transcriptomic and phenotypic response of the melanized yeast Exophiala dermatitidis to ionizing particle exposure. Front. Microbiol. 2021, 11, 609996. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, K. Dimorphic fungi, molds, and mold-like agents of medical importance. In Microbes of Medical Importance; Prajapati, A.K., Ed.; Iterative International Publishers (IIP), Selfypage Developers Pvt Ltd.: Chikmagalur, India, 2024; pp. 354–438. ISBN 9789362520203. [Google Scholar]
- Wear, M.P.; Jacobs, E.; Wang, S.; McConnell, S.A.; Bowen, A.; Strother, C.; Cordero, R.J.B.; Crawford, C.J.; Casadevall, A. Cryptococcus neoformans capsule regrowth experiments reveal dynamics of enlargement and architecture. J. Biol. Chem. 2022, 298, 101769. [Google Scholar] [CrossRef]
- Nishimura, K.; Miyaji, M. Studies on a saprophyte of Exophiala dermatitidis isolated from a humidifier. Mycopathologia 1982, 77, 173–181. [Google Scholar] [CrossRef]
- Song, Y.; da Silva, N.M.; Weiss, V.A.; Vu, D.; Moreno, L.F.; Vicente, V.A.; Li, R.; de Hoog, G.S. Comparative genomic analysis of capsule-producing black yeasts Exophiala dermatitidis and Exophiala spinifera, potential agents of disseminated mycoses. Front. Microbiol. 2020, 11, 586. [Google Scholar] [CrossRef]
- Pradhan, A.; Herrero-de-Dios, C.; Belmonte, R.; Budge, S.; Lopez Garcia, A.; Kolmogorova, A.; Lee, K.K.; Martin, B.D.; Ribeiro, A.; Bebes, A.; et al. Elevated catalase expression in a fungal pathogen is a double-edged sword of iron. PLoS Pathog. 2017, 13, e1006405. [Google Scholar] [CrossRef]
- Song, Y.; Laureijssen-van de Sande, W.W.J.; Moreno, L.F.; van den Ende, B.G.; Li, R.; de Hoog, S. Comparative ecology of capsular Exophiala species causing disseminated infection in humans. Front. Microbiol. 2017, 8, 2514. [Google Scholar] [CrossRef]
- Sav, H.; Ozakkas, F.; Altinbas, R.; Kiraz, N.; Tümgör, A.; Gümral, R.; Döğen, A.; Ilkit, M.; de Hoog, G.S. Virulence markers of opportunistic black yeast in Exophiala. Mycoses 2016, 59, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Kondori, N.; Jaén-Luchoro, D.; Karlsson, R.; Abedzaedeh, B.; Hammarström, H.; Jönsson, B. Exophiala species in household environments and their antifungal resistance profile. Sci. Rep. 2024, 14, 17622. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, N.; Xu, L.; Deng, Z.; Limwachiranon, J.; Guo, Y.; Han, Y.; Yang, W.; Scharf, D.H. Urease of Aspergillus fumigatus is required for survival in macrophages and virulence. Microbiol. Spectr. 2023, 11, e0350822. [Google Scholar] [CrossRef] [PubMed]
- Baumgardner, D.J.; Kram, J.J.F. Ammonia tolerance of Exophiala dermatitidis: A tale of two fungi. In Proceedings of the Advocate Aurora Health Scientific Day, Milwaukee, WI, USA, 25 May 2022. [Google Scholar]
- Sánchez, M.; Colom, F. Extracellular DNase activity of Cryptococcus neoformans and Cryptococcus gattii. Rev. Iberoam. Micol. 2010, 27, 10–13. [Google Scholar] [CrossRef]
- Satala, D.; Bras, G.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. More than just protein degradation: The regulatory roles and moonlighting functions of extracellular proteases produced by fungi pathogenic for humans. J. Fungi 2023, 9, 121. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Fong, P.H.L.; Wong, S.S.W.; Lee, V.H.F. Antifungal susceptibility and phenotypic characterization of oral isolates of a black fungus from a nasopharyngeal carcinoma patient under radiotherapy. BMC Oral Health 2015, 15, 39. [Google Scholar] [CrossRef]
- Angiolella, L. Virulence regulation and drug-resistance mechanism of fungal infection. Microorganisms 2022, 10, 409. [Google Scholar] [CrossRef]
- Liu, Z.; Basso, P.; Hossain, S.; Liston, S.D.; Robbins, N.; Whitesell, L.; Noble, S.M.; Cowen, L.E. Multifactor transcriptional control of alternative oxidase induction integrates diverse environmental inputs to enable fungal virulence. Nat. Commun. 2023, 14, 4528. [Google Scholar] [CrossRef]
- Shankar, S.; Mahadevan, A.; Sundaram, C.; Sarkar, C.; Chacko, G.; Lanjewar, D.; Santosh, V.; Yasha, T.; Radhakrishnan, V. Pathobiology of fungal infections of the central nervous system with special reference to the Indian scenario. Neurol. India 2007, 55, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, H.; Piccinini, A.M. A Complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 2018, 155, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Ma, Q.; de Assis, L.J.; Leaves, I.; Larcombe, D.E.; Rodriguez Rondon, A.V.; Nev, O.A.; Brown, A.J.P. Anticipatory stress responses and immune evasion in fungal pathogens. Trends Microbiol. 2021, 29, 416–427. [Google Scholar] [CrossRef]
- Ismail, Y.M.; Fayed, S.M.; Wehedy, A.H. Virulence factors of Pseudomonas aeruginosa isolated from ICU. Egypt. J. Med. Microbiol. 2021, 30, 9–14. [Google Scholar] [CrossRef]
- Shivaperumal, N.; Knight, D.R.; Imwattana, K.; Androga, G.O.; Chang, B.J.; Riley, T.V. Esculin hydrolysis negative and TcdA-only producing strains of Clostridium (Clostridioides) difficile from the environment in Western Australia. J. Appl. Microbiol. 2022, 133, 1183–1196. [Google Scholar] [CrossRef]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. Lactate like fluconazole reduces ergosterol content in the plasma membrane and synergistically kills Candida albicans. Int. J. Mol. Sci. 2021, 22, 5219. [Google Scholar] [CrossRef]
- Suchodolski, J.; Krasowska, A. Fructose induces fluconazole resistance in Candida albicans through activation of Mdr1 and Cdr1 transporters. Int. J. Mol. Sci. 2021, 22, 2127. [Google Scholar] [CrossRef]
- Lok, B.; Adam, M.A.A.; Kamal, L.Z.M.; Chukwudi, N.A.; Sandai, R.; Sandai, D. The assimilation of different carbon sources in Candida albicans: Fitness and pathogenicity. Med. Mycol. 2021, 59, 115–125. [Google Scholar] [CrossRef]
- Ene, I.V.; Adya, A.K.; Wehmeier, S.; Brand, A.C.; Maccallum, D.M.; Gow, N.A.R.; Brown, A.J.P. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell. Microbiol. 2012, 14, 1319–1335. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Haase, G. Nutritional physiology and selective isolation of Exophiala dermatitidis. Antonie Van Leeuwenhoek 1993, 64, 17–26. [Google Scholar] [CrossRef]
- Cuesta-Zedeno, L.F.; Batista-García, R.A.; Gunde-Cimerman, N.; Amabilis-Sosa, L.E.; Ramirez-Pereda, B. Utilizing black yeast for sustainable solutions: Pioneering clean energy production and wastewater treatment with Exophiala dermatitidis. Process Biochem. 2024, 147, 630–643. [Google Scholar] [CrossRef]
- Kindler, B.L.J.; Krämer, H.J.; Nies, S.; Gradicsky, P.; Haase, G.; Mayser, P.; Spiteller, M.; Spiteller, P. Generation of indole alkaloids in the human-pathogenic fungus Exophiala dermatitidis. Eur. J. Org. Chem. 2010, 2010, 2084–2090. [Google Scholar] [CrossRef]
- Wang, C.; Xing, H.; Jiang, X.; Zeng, J.; Liu, Z.; Chen, J.; Wu, Y. Cerebral phaeohyphomycosis caused by Exophiala dermatitidis in a Chinese CARD9-deficient patient: A case report and literature review. Front. Neurol. 2019, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Maraki, S.; Katzilakis, N.; Neonakis, I.; Stafylaki, D.; Meletiadis, J.; Hamilos, G.; Stiakaki, E. Exophiala dermatitidis central line-associated bloodstream infection in a child with Ewing’s sarcoma: Case report and literature review on paediatric infections. Mycopathologia 2022, 187, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.L.; Cognialli, R.C.R.; Barros, R.C.; Pinto, T.d.A.; Cunha, M.F.M.; Tahan, T.T.; Voidaleski, M.F.; Gomes, R.R.; Becker, G.N.; Andrade, L.V.; et al. Peritonitis by Exophiala dermatitidis in a pediatric patient. Med. Mycol. Case Rep. 2019, 24, 18–22. [Google Scholar] [CrossRef]
- Zoqi, H.; Schmidt, D.; Sedlacek, L.; Rath, P.M.; Steinmann, J.; Kirchhoff, L. Establishment of a novel short tandem repeat typing method for Exophiala dermatitidis. Mycopathologia 2024, 189, 5. [Google Scholar] [CrossRef]
- Engel, T.G.P.; Slabbers, L.; de Jong, C.; Melchers, W.J.G.; Hagen, F.; Verweij, P.E.; Merkus, P.; Meis, J.F. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients—A Dutch, multicentre study. J. Cyst. Fibros. 2019, 18, 221–226. [Google Scholar] [CrossRef]
- Futatsuya, T.; Mura, T.; Anzawa, K.; Mochizuki, T.; Shimizu, A.; Iinuma, Y. MALDI-TOF MS identification of Exophiala species isolated in Japan: Library enrichment and faster sample preparation. J. Dermatol. 2023, 50, 1313–1320. [Google Scholar] [CrossRef]
- Setoguchi, D.; Iwanaga, N.; Ito, Y.; Ashizawa, N.; Hirayama, T.; Takeda, K.; Ide, S.; Takemoto, S.; Tashiro, M.; Hosogaya, N.; et al. Pulmonary phaeohyphomycosis due to Exophiala dermatitidis in a patient with pulmonary non-tuberculous mycobacterial infection. J. Infect. Chemother. 2023, 29, 615–619. [Google Scholar] [CrossRef]
- Li, Z.; Tang, J.; Zhu, J.; Xie, M.; Huang, S.; Li, S.; Zhan, Y.; Zeng, W.; Xu, T.; Ye, F. The convoluted process of diagnosing pulmonary mycosis caused by Exophiala dermatitidis: A case report. BMC Infect. Dis. 2022, 22, 433. [Google Scholar] [CrossRef]
- Kumar, A.; Nandakumar, A.; Nair, S.; Singh, A.; Shashindran, N.; Thulasidharan, S.; Subhash, K.; Ramachandran, A.; Chowdhary, A. Exophiala dermatitidis as a cause of central line associated bloodstream infection in an infant: Case report and literature review. Rev. Iberoam. Micol. 2021, 38, 12–15. [Google Scholar] [CrossRef]
- Setoguchi, D.; Iwanaga, N.; Ito, Y.; Hirayama, T.; Yoshida, M.; Takeda, K.; Ide, S.; Takemoto, S.; Tashiro, M.; Hosogaya, N.; et al. Neglected pulmonary infection caused by Exophiala dermatitidis misidentified as Rhodotorula spp. Mycoses 2024, 67, e13804. [Google Scholar] [CrossRef]
- Setoguchi, D.; Iwanaga, N.; Hirayama, T.; Yoshida, M.; Takeda, K.; Ide, S.; Tashiro, M.; Takazono, T.; Kosai, K.; Izumikawa, K.; et al. Clinical review of respiratory tract infections caused by Exophiala dermatitidis misidentified as genus Rhodotorula: Case series. Open Forum Infect. Dis. 2025, 12, ofae631.2295. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshinouchi, T.; Okumura, M.; Yokoyama, T. Diverse antifungal potency of terbinafine as a therapeutic agent against Exophiala dermatitidis in vitro. Sci. Rep. 2024, 14, 27500. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rudramurthy, S.M.; Padhye, A.A.; Hemashetter, B.M.; Iyer, R.; Hallur, V.; Sharma, A.; Agnihotri, S.; Gupta, S.; Ghosh, A.; et al. Clinical spectrum, molecular characterization, antifungal susceptibility testing of Exophiala spp. from India and description of a novel Exophiala species, E. arunalokei sp. nov. Front. Cell. Infect. Microbiol. 2021, 11, 686120. [Google Scholar] [CrossRef]
- Yoshinouchi, T.; Yamamoto, K.; Migita, M.; Yokoyama, T.; Nakamura, T.; Matsuoka, M. Diagnosis and clinical management of Exophiala dermatitidis pneumonia in a patient with anorexia nervosa: A case report. Med. Mycol. Case Rep. 2023, 42, 100617. [Google Scholar] [CrossRef]
- Haase, G.; Sonntag, L.; van de Peer, Y.; Uijthof, J.M.J.; Podbielski, A.; Melzer-Krick, B. Phylogenetic analysis of ten black yeast species using nuclear small subunit rRNA gene sequences. Antonie Van Leeuwenhoek 1995, 68, 19–33. [Google Scholar] [CrossRef]
- Suh, M.K.; Lee, H.C.; Kim, D.M.; Ha, G.Y. Molecular phylogenetics of Exophiala species isolated from Korea. Ann. Dermatol. 2012, 24, 287–294. [Google Scholar] [CrossRef]
- Céspedes, L.D.; Marta, Y.; Fonseca, C.; González, Y.O.; Leyva, L. Dematiaceous fungi causing human mycoses. General considerations. Sisla Med. J. Microbiol. 2024, 1, 33–38. [Google Scholar]
| Clinical Manifestation | Immunocompetence | Predisposing Factors | Outcome | Refs. |
|---|---|---|---|---|
| Pulmonary (non-CF) | No | Transplant, chemotherapy | Often fatal | [47,48] |
| CNS | Often yes | CARD9 1, East Asia | High mortality | [49,50] |
| Fungemia | No | Catheter, malignancy | Poor if delayed | [51,52,53] |
| Crohn’s disease | Possibly | Immune dysregulation | Variable | [45,54,55,56] |
| Ocular | No | Surgery, steroids | Graft failure | [57,58,59] |
| Hair loss/scalp | Possibly | Possibly dysbiosis | Not confirmed | [60] |
| Polymicrobial (lung, blood) | Both | Biofilm, devices | Complex | [43,61,62,63,64,65,66,67] |
| Melanin Type | Biosynthetic Pathway | Environmental Triggers | Functions | Refs. |
|---|---|---|---|---|
| DHN–melanin | Polyketide pathway (via Pks1p) | Constitutively produced | Invasion, resistance to ROS, structural reinforcement | [77,78,79] |
| L-DOPA–melanin | From L-DOPA via laccase/tyrosinase | Host-like conditions (e.g., CNS) | Possible neurotropism mimicry | [76] |
| Pyomelanin | Tyrosine degradation (via HGA) | Oxidative stress | Iron scavenging, stress resistance | [76] |
| Enzyme | Function | Detection Frequency | Comments | Refs. |
|---|---|---|---|---|
| Catalase | Detoxifies H2O2; oxidative stress protection | 100% of strains | Core survival factor; bifunctional catalase/peroxidase also predicted | [84,85,86,87] |
| Urease | pH neutralization; evasion of macrophages | Variable (5–100%) | Correlates with ammonia tolerance; possibly host-induced | [85,86,87,88,89] |
| DNase | Degrades NETs/DNA traps | Rare (0–2%) in vitro; present in ex vivo RNA-seq | Likely conditionally expressed | [77,86,87,90] |
| Protease | Host tissue degradation; immune evasion | Rare in vitro | Serine protease expression upregulated in host context | [77,85,86,91] |
| Hemolysin | Iron acquisition via RBC lysis | Variable (0–93%) | Only α-hemolysis reported; strain-dependent | [10,85,92] |
| Phospholipase | Membrane degradation | Not detected | No activity observed under current methods | [86,87,93,94] |
| Oxidases | ROS metabolism | Not detected | No activity observed under current methods | [95,96,97] |
| Esterase | Various (e.g., invasion, ROS defense) | Unknown or unexplored | Identified in other fungi; not studied in E. dermatitidis | [95,96,97] |
| SOD | ||||
| Hyaluronidase | ||||
| Elastase |
| Carbon Source | Physiological Context in the Human Host | Refs. |
|---|---|---|
| Common sugars and derivatives | ||
| Glucose | Abundant in blood and tissues; primary carbon source efficiently assimilated. | [50,86,104] |
| Fructose | Present in diet and bloodstream at low levels; assimilation likely via sorbitol intermediates. | [10,86,105] |
| Sucrose | Dietary disaccharide not naturally present in tissues; transiently available in GI tract. | [10,86,104] |
| Inulin | Dietary fructan polysaccharide not digested by humans; fermented by gut microbiota. | [10,104] |
| Galactose | Released from mucins and glycoproteins during tissue degradation. | [10,86,104] |
| N-acetyl-D-glucosamine | Component of microbial and fungal cell walls present in human niches. | [10,86] |
| Polyols and sugar alcohols | ||
| Sorbitol | Accumulates in diabetic tissues as polyol pathway intermediate. | [10,86] |
| Glycerol | Lipid metabolite, available in tissues and blood. | [10,86,104] |
| Organic acids and metabolites | ||
| 2-keto-D-gluconate | Intermediate metabolite in different metabolic pathways. | [86,104] |
| Succinate | Common TCA cycle intermediate found in host cells and tissues. | [10,104] |
| Glucuronate | Involved in detoxification pathways; present in extracellular matrix. | [10,104] |
| Lactate | Present in inflamed tissues and vaginal environments. | [104] |
| Neurotransmitters and AAs | ||
| GABA | Major neurotransmitter in CNS. | [50] |
| Dopamine | Neurotransmitter and melanin precursor. | [10,50] |
| Serotonin | CNS and GI tract neurotransmitter. | [10] |
| Norepinephrine/epinephrine | Neurotransmitters and hormones, present in blood and various tissues. | [10] |
| Tryptophan | Aromatic amino acid with catabolites linked to virulence. | [10,106] |
| Glutamate | Major excitatory neurotransmitter in the CNS and a key amino acid in tissue metabolism. | [10,50] |
| Aromatic and environmental compounds | ||
| Phenol | Environmental and host-derived toxin. | [10] |
| Catechol | Oxidized metabolite and melanin precursor. | [10] |
| Method | Time | Specificity | Advantages | Limitations | Refs. |
|---|---|---|---|---|---|
| Culture | 2–7 days | Low–Moderate | Widely available, supports further testing | Slow growth, risk of contamination | [2,57,59] |
| Microscopy | <1 day | Low | Simple, quick | Low discriminatory power | [57] |
| ITS Sequencing | 1–3 days | High | Species-level ID, phylogenetic value | Requires sequencing facility | [48,55,107,108,109] |
| STR Genotyping | 2–3 days | High | Strain typing, outbreak tracing | Requires multiplex PCR and interpretation | [110] |
| AFLP | 2–4 days | High | High genotypic resolution | Technically demanding | [111] |
| MALDI-TOF MS | <1 day | Moderate–High | Rapid, cost-effective | Database-dependent, variable reliability | [58,62,112,113] |
| mNGS | 1–3 days | High | Unbiased detection of rare/novel pathogens; works on FFPE | Expensive; requires bioinformatics infrastructure | [114] |
| Agent | MIC Range (µg/L) | Median In Vitro Activity | Biofilm Efficacy | Clinical Notes | Refs. |
|---|---|---|---|---|---|
| Voriconazole | 0.002–8 | High | Reduced | First-line therapy in many case reports. | [26,61,87,115,118,119] |
| Fluconazole | 0.5–256 | Poor | Poor | Largely ineffective. | [10,26,61,87,115,118,119] |
| Itraconazole | 0.03–2 | High | Reduced | Effective in CF and localized infections. | [10,61,64,87,115,118,119] |
| Posaconazole | 0.002–0.5 | High | Reduced | Alternative to voriconazole. | [61,87,115,118,119] |
| Miconazole | 0.12–5 | Moderate | NI | Rarely used. | [61,118] |
| Amphotericin B | 0.064–2 | Moderate | Reduced | Used in severe and disseminated cases. | [26,61,87,115,118] |
| 5-fluorocytosine | 1–128 | Poor | Poor | Largely ineffective. | [61,87,115] |
| Micafungin | 0.125–16 | Poor | Poor | Echinocandin class; limited activity. | [115,118] |
| Anidulafungin | 0.008–32 | Poor | Poor | Similar limitations as micafungin. | [26,87,115,119] |
| Caspofungin | 0.008–32 | Poor | Poor | Not recommended due to resistance. | [87,115,118,119] |
| Terbinafine | 0.06–0.13 | High | High | Rarely used. | [10,115,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchodolski, J.; Parol, M.; Pawlak, K.; Piecuch, A.; Ogórek, R. Clinical and Molecular Advances on the Black Yeast Exophiala dermatitidis. Int. J. Mol. Sci. 2025, 26, 6804. https://doi.org/10.3390/ijms26146804
Suchodolski J, Parol M, Pawlak K, Piecuch A, Ogórek R. Clinical and Molecular Advances on the Black Yeast Exophiala dermatitidis. International Journal of Molecular Sciences. 2025; 26(14):6804. https://doi.org/10.3390/ijms26146804
Chicago/Turabian StyleSuchodolski, Jakub, Mateusz Parol, Karolina Pawlak, Agata Piecuch, and Rafał Ogórek. 2025. "Clinical and Molecular Advances on the Black Yeast Exophiala dermatitidis" International Journal of Molecular Sciences 26, no. 14: 6804. https://doi.org/10.3390/ijms26146804
APA StyleSuchodolski, J., Parol, M., Pawlak, K., Piecuch, A., & Ogórek, R. (2025). Clinical and Molecular Advances on the Black Yeast Exophiala dermatitidis. International Journal of Molecular Sciences, 26(14), 6804. https://doi.org/10.3390/ijms26146804






