Hurdles of Sperm Success: Exploring the Role of DNases
Abstract
1. Introduction
2. Sperm Degradation Along Gametogenesis
3. Apoptotic Mechanisms in Sperm Death
3.1. DNA Cleavage in Spermatogonia
3.2. DNA Cleavage During Spermatogenesis
3.3. DNA Cleavage During Spermiogenesis
3.4. DNA Cleavage in Ejaculated Sperm
3.5. Additional Enzymes Involved in DNA Cleavage During Gametogenesis
4. Necrosis in Sperm Death
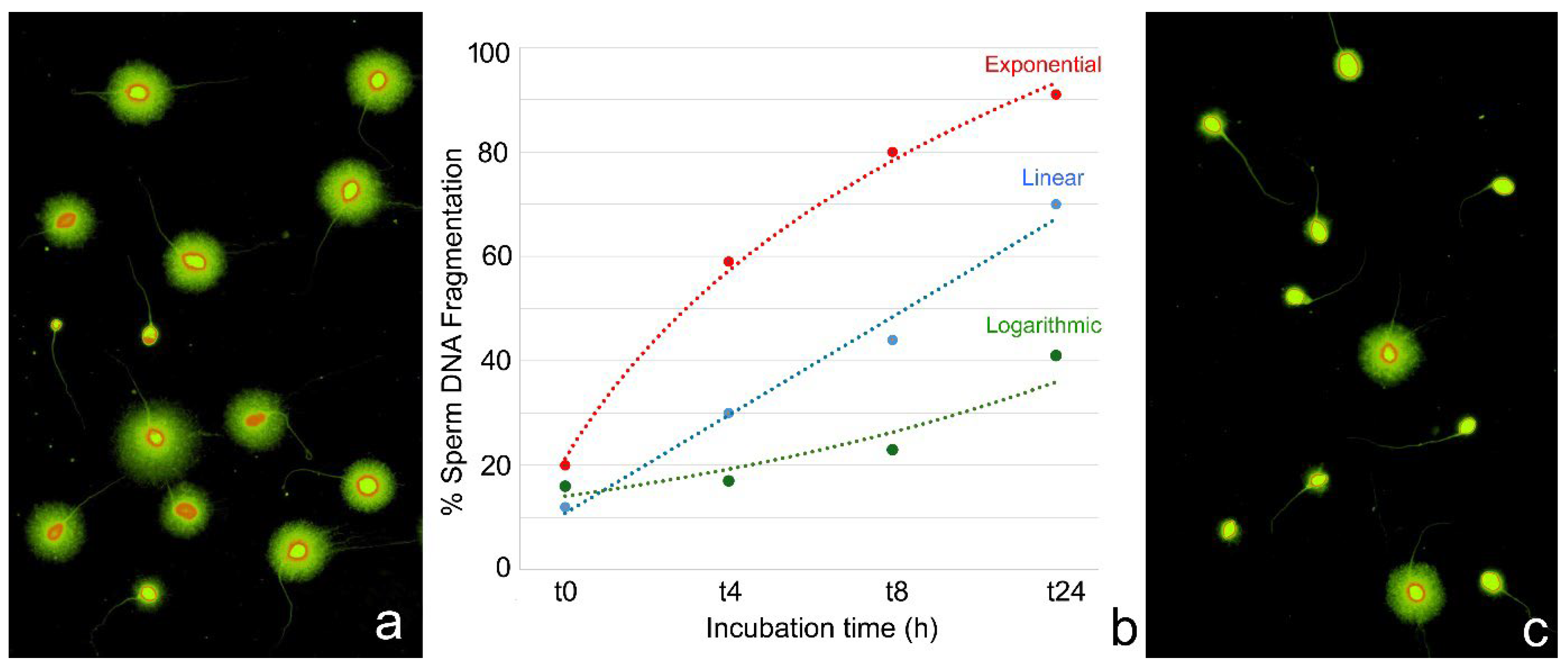
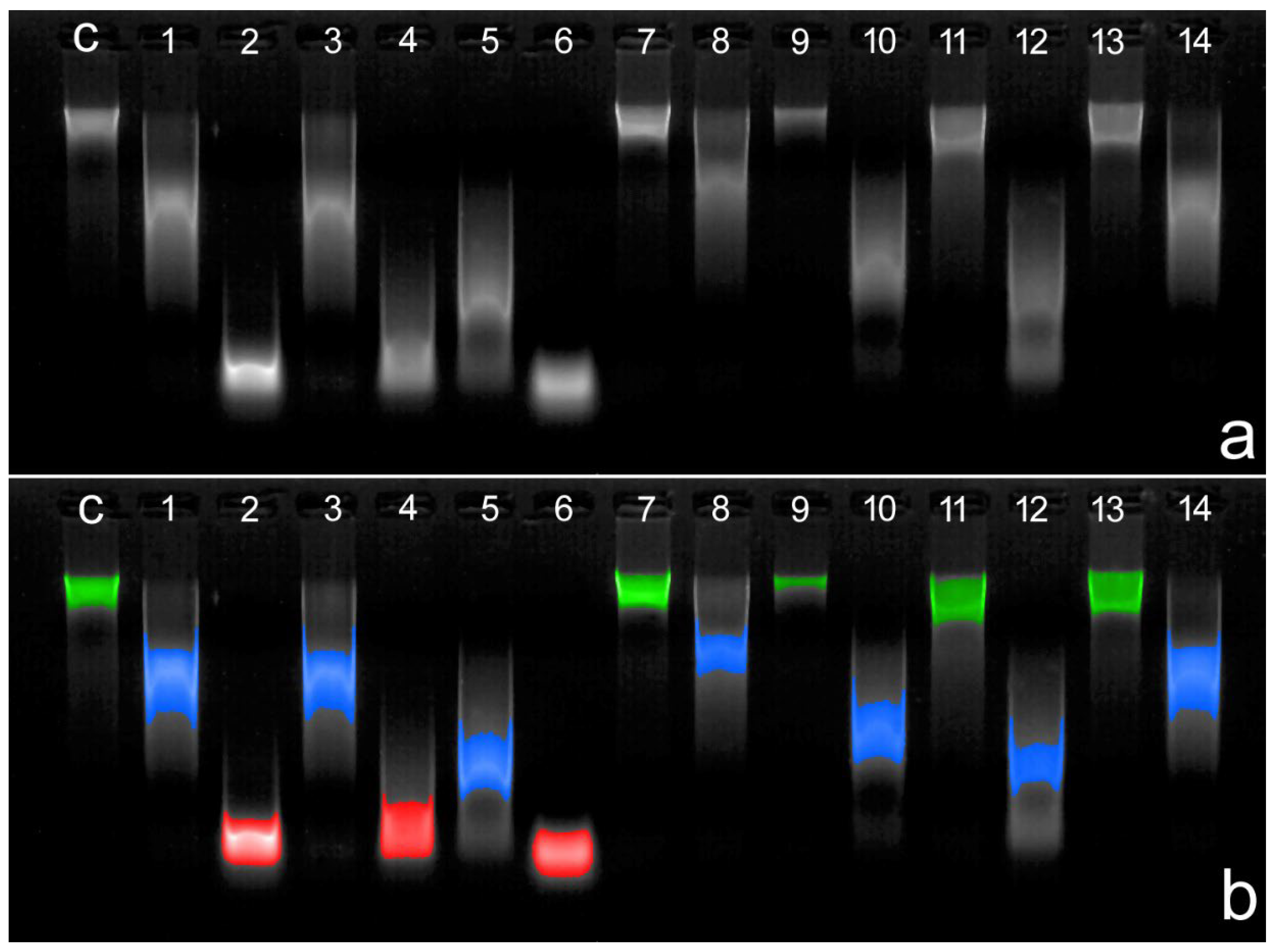
5. Sperm DNA Vulnerability During Assisted Reproductive Procedures
6. Main Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, J.L.M.; Majzoub, A.; Esteves, S.; Humaidan, P. Unraveling the impact of sperm dNA fragmentation on reproductive outcomes. Semin. Reprod. Med. 2023, 41, 241–257. [Google Scholar] [CrossRef]
- Tímermans, A.; Vázquez, R.; Otero, F.; Gosálvez, J.; Johnston, S.; Fernández, J.L. DNA fragmentation of human spermatozoa: Simple assessment of single- and double-strand DNA breaks and their respective dynamic behavioral response. Andrology 2020, 8, 1287–1303. [Google Scholar] [CrossRef]
- González-Marín, C.; Gosálvez, J.; Roy, R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int. J. Mol. Sci. 2012, 13, 14026–14052. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Segatelli, T.M.; França, L.R.; Pinheiro, P.F.; Alemida, C.C.; Martinez, M.; Martinez, F.E. Spermatogenic cycle length and spermatogenic efficiency in the gerbil (Meriones unguiculatus). J. Androl. 2004, 25, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Heller, C.G.; Clermont, Y. Spermatogenesis in man: An estimate of its duration. Science 1963, 140, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Oakberg, E.F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 1956, 99, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Gosálvez, J.; Cortés-Gutiérrez, E.; López-Fernández, C.; Fernández, J.L.; Caballero, P.; Nuñez, R. Sperm deoxyribonucleic acid fragmentation dynamics in fertile donors. Fertil. Steril. 2009, 92, 170–173. [Google Scholar] [CrossRef]
- Rougier, N.; Uriondo, H.; Papier, S.; Checa, M.A.; Sueldo, C.; Alvarez Sedó, C. Changes in DNA fragmentation during sperm preparation for intracytoplasmic sperm injection over time. Fertil. Steril. 2013, 100, 69–74. [Google Scholar] [CrossRef]
- Letkovska, K.; Babal, P.; Cierna, Z.; Schmidtova, S.; Liskova, V.; Kalavska, K.; Miskovska, V.; Horak, S.; Rejlekova, K.; Chovanec, M.; et al. Prognostic Value of Apoptosis-Inducing Factor (AIF) in Germ Cell Tumors. Cancers 2021, 13, 776. [Google Scholar] [CrossRef]
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 2002, 84, 215–222. [Google Scholar] [CrossRef]
- Rezende, N.C.; Lee, M.Y.; Monette, S.; Mark, W.; Lu, A.; Gudas, L.J. Rex1 (Zfp42) null mice show impaired testicular function, abnormal testis morphology, and aberrant gene expression. Dev. Biol. 2011, 356, 370–382. [Google Scholar] [CrossRef]
- Keeney, S.; Giroux, C.N.; Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 1997, 88, 375–384. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, M.; Li, L.; Fan, Y.; Pathre, P.; Dong, J.; Lou, D.; Wells, J.M.; Olivares-Villagómez, D.; Van Kaer, L.; et al. Endonuclease G is required for early embryogenesis and normal apoptosis in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 15782–15787. [Google Scholar] [CrossRef]
- David, K.; Sasaki, M.; Yu, S.W.; Dawson, T.M.; Dawson, V.L. EndoG is dispensable in embryogenesis and apoptosis. Cell Death Differ. 2006, 13, 1147–1155. [Google Scholar] [CrossRef]
- Wang, Z.; Meerod, T.; Cortes-Silva, N.; Chiang, A.C.; Nie, Z.; Di, Y.; Mu, P.; Verma, A.; Reid, A.J.; Ma, H. Poldip2 promotes mtDNA elimination during Drosophila spermatogenesis to ensure maternal inheritance. EMBO J. 2025, 44, 1724–1748. [Google Scholar] [CrossRef]
- Leduc, F.; Maquennehan, V.; Nkoma, G.B.; Boissonneault, G. DNA damage response during chromatin remodeling in elongating spermatids of mice. Biol. Reprod. 2008, 78, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Enari, M.; Sakahira, H.; Yokoyama, H.; Okawa, K.; Iwamatsu, A.; Nagata, S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998, 391, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Haimovici, A.; Rupp, V.; Amer, T.; Moeed, A.; Weber, A.; Häcker, G. The caspase-activated DNase promotes cellular senescence. EMBO J. 2024, 43, 3523–3544. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Arai, T.; Kijima, M.; Sato, S.; Miura, S.; Yuasa, M.; Kitamura, D.; Mizuta, R. DNase γ, DNase I and caspase-activated DNase cooperate to degrade dead cells. Genes Cells 2016, 21, 1150–1163. [Google Scholar] [CrossRef]
- Bass, B.; Tanner, E.; Mateos San Martín, D.; Blute, T.; Kinser, R.D.; Dolph, P.J.; McCall, K. Cell-autonomous requirement for DNaseII in nonapoptotic cell death. Cell Death Differ. 2009, 16, 1362–1371. [Google Scholar] [CrossRef]
- Mizuta, R.; Araki, S.; Furukawa, M.; Furukawa, Y.; Ebara, S.; Shiokawa, D.; Hayashi, K.; Tanuma, S.; Kitamura, D. DNase γ is the effector endonuclease for internucleosomal DNA fragmentation in necrosis. PLoS ONE 2013, 8, e80223. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746–754. [Google Scholar] [CrossRef]
- Bejarano, I.; Rodríguez, A.B.; Pariente, J.A. Apoptosis Is a Demanding Selective Tool During the Development of Fetal Male Germ Cells. Front. Cell Dev. Biol. 2018, 6, 65. [Google Scholar] [CrossRef]
- Dunkel, L.; Hirvonen, V.; Erkkilä, K. Clinical aspects of male germ cell apoptosis during testis development and spermatogenesis. Cell Death Differ. 1997, 4, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef]
- Hamer, G.; Novak, I.; Kouznetsova, A.; Höög, C. Disruption of pairing and synapsis of chromosomes causes stage-specific apoptosis of male meiotic cells. Theriogenology 2008, 69, 333–339. [Google Scholar] [CrossRef]
- Sakkas, D.; Mariethoz, E.; Manicardi, G.; Bizzaro, D.; Bianchi, P.G.; Bianchi, U. Origin of DNA damage in ejaculated human spermatozoa. Rev. Reprod. 1999, 4, 31–37. [Google Scholar] [CrossRef]
- Xu, Y.R.; Dong, H.S.; Yang, W.X. Regulators in the apoptotic pathway during spermatogenesis: Killers or guards? Gene 2016, 582, 97–111. [Google Scholar] [CrossRef]
- Lee, S.H.; Meng, X.W.; Flatten, K.S.; Loegering, D.A.; Kaufmann, S.H. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ. 2013, 20, 64–76. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef]
- Sakkas, D.; Seli, E.; Bizzaro, D.; Tarozzi, N.; Manicardi, G.C. Abnormal spermatozoa in the ejaculate: Abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online 2003, 7, 428–432. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L. Apoptosis during spermatogenesis: The thrill of being alive. Mol. Reprod. Dev. 2001, 58, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Ghosh, S.R.; Weil, A.C.; Zirkin, B.R. Caspase-3 and caspase-activated deoxyribonuclease are associated with testicular germ cell apoptosis resulting from reduced intratesticular testosterone. Endocrinology 2001, 142, 3809–3816. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Sato, K.; Ikeda, Y.; Imai, H.; Nakagawa, Y.; Ohba, Y.; Fujii, J. An abortive apoptotic pathway induced by singlet oxygen is due to the suppression of caspase activation. Biochem. J. 2005, 389, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Giampietri, C.; Petrungaro, S.; Coluccia, P.; D’Alessio, A.; Starace, D.; Riccioli, A.; Padula, F.; Palombi, F.; Ziparo, E.; Filippini, A.; et al. Germ cell apoptosis control during spermatogenesis. Contraception 2005, 72, 298–302. [Google Scholar] [CrossRef]
- Chen, K.Q.; Wei, B.H.; Hao, S.L.; Yang, W.X. The PI3K/AKT signaling pathway: How does it regulate development of Sertoli cells and spermatogenic cells? Histol. Histopathol. 2022, 37, 621–636. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; de la Casa, M.; Serrano, M.; Gosálvez, J.; Roy Barcelona, R. Impact of polymorphism in DNA repair genes OGG1 and XRCC1 on seminal parameters and human male infertility. Andrologia 2018, 50, e13115. [Google Scholar] [CrossRef]
- Panier, S.; Wang, S.; Schumacher, B. Genome Instability and DNA Repair in Somatic and Reproductive Aging. Annu. Rev. Pathol. 2024, 19, 261–290. [Google Scholar] [CrossRef]
- Zakariah, M.; Molele, R.A.; Mahdy, M.A.A.; Ibrahim, M.I.A.; McGaw, L.J. Regulation of spermatogenic cell apoptosis by the pro-apoptotic proteins in the testicular tissues of mammalian and avian species. Anim. Reprod. Sci. 2022, 247, 107158. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.L.; Yamashita, Y.M. Germ cell connectivity enhances cell death in response to DNA damage in the Drosophila testis. eLife 2017, 6, e27960. [Google Scholar] [CrossRef] [PubMed]
- Rübe, C.E.; Zhang, S.; Miebach, N.; Fricke, A.; Rübe, C. Protecting the heritable genome: DNA damage response mechanisms in spermatogonial stem cells. DNA Repair 2011, 10, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bergerat, A.; de Massy, B.; Gadelle, D.; Varoutas, P.C.; Nicolas, A.; Forterre, P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature 1997, 386, 414–417. [Google Scholar] [CrossRef]
- Raghavan, A.R.; Hochwagen, A. Keeping it safe: Control of meiotic chromosome breakage. Trends Genet. 2025, 41, 315–329. [Google Scholar] [CrossRef]
- Baudat, F.; Manova, K.; Yuen, J.P.; Jasin, M.; Keeney, S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell 2000, 6, 989–998. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Romanienko, P.J.; Khil, P.P.; Camerini-Otero, R.D. Gene expression profiles of Spo11-/- mouse testes with spermatocytes arrested in meiotic prophase I. Reproduction 2006, 132, 67–77. [Google Scholar] [CrossRef]
- Myers, S.; Bowden, R.; Tumian, A.; Bontrop, R.E.; Freeman, C.; MacFie, T.S.; McVean, G.; Donnelly, P. Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science 2010, 327, 876–879. [Google Scholar] [CrossRef]
- Boateng, K.A.; Bellani, M.A.; Gregoretti, I.V.; Pratto, F.; Camerini-Otero, R.D. Homologous pairing preceding SPO11-mediated double-strand breaks in mice. Dev. Cell 2013, 24, 196–205. [Google Scholar] [CrossRef]
- Tang, X.; Hu, Z.; Ding, J.; Wu, M.; Guan, P.; Song, Y.; Yin, Y.; Wu, W.; Ma, J.; Huang, Y.; et al. In vitro reconstitution of meiotic DNA double-strand-break formation. Nature 2025, 639, 800–807. [Google Scholar] [CrossRef]
- Wang, S.; Zickler, D.; Kleckner, N.; Zhang, L. Meiotic crossover patterns: Obligatory crossover, interference and homeostasis in a single process. Cell Cycle 2015, 14, 305–314. [Google Scholar] [CrossRef]
- Lange, J.; Yamada, S.; Tischfield, S.E.; Pan, J.; Kim, S.; Zhu, X.; Socci, N.D.; Jasin, M.; Keeney, S. The landscape of mouse meiotic double-strand break formation, processing, and repair. Cell 2016, 167, 695–708.e16. [Google Scholar] [CrossRef] [PubMed]
- Papanikos, F.; Clément, J.A.J.; Testa, E.; Ravindranathan, R.; Grey, C.; Dereli, I.; Bondarieva, A.; Valerio-Cabrera, S.; Stanzione, M.; Schleiffer, A.; et al. Mouse ANKRD31 regulates spatiotemporal patterning of meiotic recombination initiation and ensures recombination between X and Y sex chromosomes. Mol. Cell 2019, 74, 1069–1085.e11. [Google Scholar] [CrossRef] [PubMed]
- Romanienko, P.J.; Camerini-Otero, R.D. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 2000, 6, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.S. Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 2010, 16, 30–36. [Google Scholar] [CrossRef]
- Gaspa-Toneu, L.; Peters, A.H. Nucleosomes in mammalian sperm: Conveying paternal epigenetic inheritance or subject to reprogramming between generations? Curr. Opin. Genet. Dev. 2023, 79, 102034. [Google Scholar] [CrossRef]
- Moritz, L.; Hammoud, S.S. The art of packaging the sperm genome: Molecular and structural basis of the histone-to-protamine exchange. Front. Endocrinol. 2022, 13, 895502. [Google Scholar] [CrossRef]
- Okada, Y. Sperm chromatin structure: Insights from in vitro to in situ experiments. Curr. Opin. Cell Biol. 2022, 75, 102075. [Google Scholar] [CrossRef]
- Marcon, L.; Boissonneault, G. Transient DNA strand breaks during mouse and human spermiogenesis: New insights in stage specificity and link to chromatin remodeling. Biol. Reprod. 2004, 70, 910–918. [Google Scholar] [CrossRef]
- Rathke, C.; Baarends, W.M.; Awe, S.; Renkawitz-Pohl, R. Chromatin dynamics during spermiogenesis. Biochim. Biophys. Acta 2014, 1839, 155–168. [Google Scholar] [CrossRef]
- Gouraud, A.; Brazeau, M.A.; Grégoire, M.C.; Simard, O.; Massonneau, J.; Arguin, M.; Boissonneault, G. “Breaking news” from spermatids. Basic. Clin. Androl. 2013, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Akematsu, T.; Fukuda, Y.; Garg, J.; Fillingham, J.S.; Pearlman, R.E.; Loidl, J. Post-meiotic DNA double-strand breaks occur in Tetrahymena, and require Topoisomerase II and Spo11. eLife 2017, 6, e26176. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.A.; de Boer, P.; Philippens, M.E.; Kal, H.B.; de Rooij, D.G. Parp1-XRCC1 and the repair of DNA double strand breaks in mouse round spermatids. Mutat. Res. 2010, 683, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, B.; Collodel, G.; Piomboni, P. Apoptosis in human ejaculated sperm cells (notulae seminologicae 9). J. Submicrosc. Cytol. Pathol. 1996, 28, 587–596. [Google Scholar]
- Muratori, M.; Piomboni, P.; Baldi, E.; Filimberti, E.; Pecchioli, P.; Moretti, E.; Gambera, L.; Baccetti, B.; Biagiotti, R.; Forti, G.; et al. Functional and ultrastructural features of DNA-fragmented human sperm. J. Androl. 2000, 21, 903–912. [Google Scholar] [CrossRef]
- Lachaud, C.; Tesarik, J.; Cañadas, M.L.; Mendoza, C. Apoptosis and necrosis in human ejaculated spermatozoa. Hum. Reprod. 2004, 19, 607–610. [Google Scholar] [CrossRef]
- Grunewald, S.; Paasch, U.; Said, T.M.; Sharma, R.K.; Glander, H.J.; Agarwal, A. Caspase activation in human spermatozoa in response to physiological and pathological stimuli. Fertil. Steril. 2005, 83, 106–112. [Google Scholar] [CrossRef]
- Lu, Y.; Bhushan, S.; Tchatalbachev, S.; Marconi, M.; Bergmann, M.; Weidner, W.; Chakraborty, T.; Meinhardt, A.; Schlatt, S. Necrosis is the dominant cell death pathway in uropathogenic Escherichia coli elicited epididymo-orchitis and is responsible for damage of rat testis. PLoS ONE 2013, 8, e52919. [Google Scholar] [CrossRef]
- Fraczek, M.; Hryhorowicz, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kolanowski, T.J.; Beutin, L.; Kurpisz, M. Can apoptosis and necrosis coexist in ejaculated human spermatozoa during in vitro semen bacterial infection? J. Assist. Reprod. Genet. 2015, 32, 771–779. [Google Scholar] [CrossRef]
- Engel, K.M.; Springsguth, C.H.; Grunewald, S. What happens to the unsuccessful spermatozoa? Andrology 2018, 6, 335–344. [Google Scholar] [CrossRef]
- Kavaldzhieva, K.K.; Dimitrova-Dikanarova, D.K.; Mladenova, K.S.; Lazarov, V.V. The Death of Sperm Cells. Acta Medica Bulg. 2023, 50, 69–72. [Google Scholar] [CrossRef]
- Shihara, Y.; Shimamoto, N. Involvement of endonuclease G in nucleosomal DNA fragmentation under sustained endogenous oxidative stress. J. Biol. Chem. 2006, 281, 6726–6733. [Google Scholar] [CrossRef]
- Kitazumi, I.; Tsukahara, M. Regulation of DNA fragmentation: The role of caspases and phosphorylation. FEBS J. 2011, 278, 427–441. [Google Scholar] [CrossRef]
- Sevrioukova, I.F. Apoptosis-inducing factor: Structure, function, and redox regulation. Antioxid. Redox Signal. 2011, 14, 2545–2579. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Labilloy, A. Genetics, TREX1 Mutations; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Wei, K.; Clark, A.B.; Wong, E.; Kane, M.F.; Mazur, D.J.; Parris, T.; Kolas, N.K.; Russell, R.; Hou, H.; Kneitz, B.; et al. Inactivation of exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003, 17, 603–614. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, F.; Lee, A.; Yamine, M.; Wang, Z.H.; Zhang, G.; Combs, C.; Xu, H. Mitochondrial DNA removal is essential for sperm development and activity. EMBO J. 2025, 44, 1749–1773. [Google Scholar] [CrossRef] [PubMed]
- Christofferson, D.E.; Yuan, J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 2010, 22, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis—Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Yuan, W.; Sun, Z.; Ji, G.; Hu, H. Emerging roles of ferroptosis in male reproductive diseases. Cell Death Discov. 2023, 9, 358. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Krautwald, S.; Kroemer, G.; Linkermann, A. Molecular mechanisms of regulated necrosis. Semin. Cell Dev. Biol. 2014, 35, 24–32. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Eisenbach, M. Why are sperm cells phagocytosed by leukocytes in the female genital tract? Med. Hypotheses 2003, 60, 590–592. [Google Scholar] [CrossRef]
- Jewanraj, J.; Ngcapu, S.; Osman, F.; Mtshali, A.; Singh, R.; Mansoor, L.E.; Abdool Karim, S.S.; Abdool Karim, Q.; Passmore, J.S.; Liebenberg, L.J.P. The impact of semen exposure on the immune and microbial environments of the female genital tract. Front. Reprod. Health 2020, 9, 566559. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. The Female Response to Seminal Fluid. Physiol. Rev. 2020, 100, 1077–1117. [Google Scholar] [CrossRef]
- Napoletano, F.; Gibert, B.; Yacobi-Sharon, K.; Vincent, S.; Favrot, C.; Mehlen, P.; Girard, V.; Teil, M.; Chatelain, G.; Walter, L.; et al. p53-dependent programmed necrosis controls germ cell homeostasis during spermatogenesis. PLoS Genet. 2017, 13, e1007024. [Google Scholar] [CrossRef] [PubMed]
- Beumer, T.L.; Roepers-Gajadien, H.L.; Gademan, I.S.; van Buul, P.P.; Gil-Gomez, G.; Rutgers, D.H.; de Rooij, D.G. The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ. 1998, 5, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Ody, C.; Araki, K.; Garcia, I.; Vassalli, P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997, 16, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Lauková, L.; Konečná, B.; Janovičová, Ľ.; Vlková, B.; Celec, P. Deoxyribonucleases and Their Applications in Biomedicine. Biomolecules 2020, 11, 1036. [Google Scholar] [CrossRef]
- Rubartelli, A.; Lotze, M.T. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007, 28, 429–436. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef]
- Sosnin, D.Y.; Gal’kovich, K.R.; Krivtsov, A.V.; Gil’manov, A.Z. Tumor necrosis factor in the ejaculate as an indicator of reduced fertility. Urologiia 2024, 1, 80–85. [Google Scholar] [CrossRef]
- Siddighi, S.; Chan, C.A.; Patton, W.C.; Jacobson, J.D.; Chan, P.J. Male age and sperm necrosis in assisted reproductive technologies. Urol. Int. 2007, 79, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Takeuchi, T.; Yoshida, A. Preparation and incubation conditions affect the DNA integrity of ejaculated human spermatozoa. Asian J. Androl. 2010, 12, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Yelumalai, S.; Kashir, J.; Jone, C.; Bagheri, H.; Oo, S.L.; McLaren, L.; Coward, K. Clinician-induced (iatrogenic) damage incurred during human infertility treatment: Detrimental effects of sperm selection methods and cryopreservation upon the viability, DNA integrity, and function of human sperm. Asian Pac. J. Reprod. 2012, 1, 69–75. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef]
- Habibi, P.; Ostad, S.N.; Heydari, A.; Aliebrahimi, S.; Montazeri, V.; Foroushani, A.R.; Monazzam, M.R.; Ghazi-Khansari, M.; Golbabaei, F. Effect of heat stress on DNA damage: A systematic literature review. Int. J. Biometeorol. 2022, 66, 2147–2158. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, T.V.; Nguyen, T.A.T.; Nguyen, Q.H.V.; Cao, T.N. Does conventional freezing affect sperm DNA fragmentation? Clin. Exp. Reprod. Med. 2019, 46, 67–75. [Google Scholar] [CrossRef]
- Hebles, M.; Dorado, M.; Gallardo, M.; González-Martínez, M.; Sánchez-Martín, P. Seminal quality in the first fraction of ejaculate. Syst. Biol. Reprod. Med. 2015, 61, 113–116. [Google Scholar] [CrossRef]
- Tvrdá, E.; Arroyo, F.; Gosálvez, J. Dynamic assessment of human sperm DNA damage I: The effect of seminal plasma-sperm co-incubation after ejaculation. Int. Urol. Nephrol. 2018, 50, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Arroyo, F.; Ďuračka, M.; López-Fernández, C.; Gosálvez, J. Dynamic assessment of human sperm DNA damage II: The effect of sperm concentration adjustment during processing. J. Assist. Reprod. Genet. 2019, 36, 799–807. [Google Scholar] [CrossRef]
- López-Fernández, C.; Crespo, F.; Arroyo, F.; Fernández, J.L.; Arana, P.; Johnston, S.D.; Gosálvez, J. Dynamics of sperm DNA fragmentation in domestic animals II. The stallion. Theriogenology 2007, 68, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Nabi, A.; Khalili, M.A.; Halvaei, I.; Roodbari, F. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia 2014, 46, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Gosálvez, J.; López-Fernández, C.; Fernández, J.L.; Gouraud, A.; Holt, W.V. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev. 2011, 78, 951–961. [Google Scholar] [CrossRef]
- Luño, V.; Martínez, F.; Muñoz, A.; Gil, L. Effect of season on the dynamics of cat sperm DNA fragmentation. BMC Vet. Res. 2023, 19, 113. [Google Scholar] [CrossRef]
- Gosálvez, J.; de la Torre, J.; López-Fernández, C.; Pérez-Gutiérrez, L.; Ortega, L.; Caballero, P.; Nuñez, R. DNA fragmentation dynamics in fresh versus frozen thawed plus gradient-isolated human spermatozoa. Syst. Biol. Reprod. Med. 2010, 56, 27–36. [Google Scholar] [CrossRef]
- Gosálvez, J.; López-Fernández, C.; Fernández, J.L. Sperm Chromatin Dispersion (SCD) test: Technical aspects and clinical applications. In Sperm DNA Damage: Biological and Clinical Applications in Male Infertility and Assisted Reproduction; Zini, A., Agarwal, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 151–170. [Google Scholar]
- Gosálvez, J.; Sánchez Gutierrez, R.; Alvarez, J. Should intracytoplasmic sperm injection always be recommended when using cryopreserved sperm? Fertil. Steril. 2019. [Google Scholar] [CrossRef]
- Imrat, P.; Mahasawangkul, S.; Gosálvez, J.; Suthanmapinanth, P.; Sombutputorn, P.; Jansittiwate, S.; Thongtip, N.; Pinyopummin, A.; Colenbrander, B.; Holt, W.V.; et al. Effect of cooled storage on quality and DNA integrity of Asian elephant (Elephas maximus) spermatozoa. Reprod. Fertil. Dev. 2012, 24, 1105–1116. [Google Scholar] [CrossRef]
- Simon, L.; Castillo, J.; Oliva, R.; Lewis, S.E. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod. Biomed. Online 2011, 23, 724–734. [Google Scholar] [CrossRef]
- Bibi, R.; Jahan, S.; Razak, S.; Hammadeh, M.E.; Almajwal, A.; Amor, H. Protamines and DNA integrity as biomarkers of sperm quality and assisted conception outcome. Andrologia 2022, 54, e14418. [Google Scholar] [CrossRef]
- García-Peiró, A.; Martínez-Heredia, J.; Oliver-Bonet, M.; Abad, C.; Amengual, M.J.; Navarro, J.; Jones, C.; Coward, K.; Gosálvez, J.; Benet, J. Protamine 1 to protamine 2 ratio correlates with dynamic aspects of DNA fragmentation in human sperm. Fertil. Steril. 2011, 95, 105–109. [Google Scholar] [CrossRef]
- Maione, B.; Pittoggi, C.; Achene, L.; Lorenzini, R.; Spadafora, C. Activation of endogenous nucleases in mature sperm cells upon interaction with exogenous DNA. DNA Cell Biol. 1997, 16, 1087–1097. [Google Scholar] [CrossRef]
- Feitosa, W.B.; Mendes, C.M.; Milazzotto, M.P.; Rocha, A.M.; Martins, L.F.; Simões, R.; Paula-Lopes, F.F.; Visintin, J.A.; Assumpção, M.E. Exogenous DNA uptake by bovine spermatozoa does not induce DNA fragmentation. Theriogenology 2010, 74, 563–568. [Google Scholar] [CrossRef]
- Bartolomé-Nebreda, J.; Vargas-Baquero, E.; López-Fernández, C.; Fernández, J.L.; Johnston, S.; Gosálvez, J. Free circulating DNA and DNase activity in the ejaculates of men with spinal cord injury. Spinal Cord 2021, 59, 167–174. [Google Scholar] [CrossRef]
- Vargas-Baquero, E.; Johnston, S.; Sánchez-Ramos, A.; Arévalo-Martín, A.; Wilson, R.; Gosálvez, J. The incidence and etiology of sperm DNA fragmentation in the ejaculates of males with spinal cord injuries. Spinal Cord 2020, 58, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Gosálvez, J.; Vargas-Baquero, E.; López-Fernández, C.; Bartolomé-Nebreda, J.; Fernández, J.L.; Johnston, S. Sperm DNA damage in men with spinal cord injury: The relative incidence of single- and double-strand DNA breaks. Andrology 2022, 10, 1292–1301. [Google Scholar] [CrossRef]
- Szczygiel, M.A.; Ward, W.S. Combination of dithiothreitol and detergent treatment of spermatozoa causes paternal chromosomal damage. Biol. Reprod. 2002, 67, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Sotolongo, B.; Lino, E.; Ward, W.S. Ability of hamster spermatozoa to digest their own DNA. Biol. Reprod. 2003, 69, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Nguyen, H.; Wu, H.; Ward, W.S. Functional aspects of sperm chromatin organization. Results Probl. Cell Differ. 2022, 70, 295–311. [Google Scholar] [CrossRef]
- Sotolongo, B.; Huang, T.T.; Isenberger, E.; Ward, W.S. An endogenous nuclease in hamster, mouse, and human spermatozoa cleaves DNA into loop-sized fragments. J. Androl. 2005, 26, 272–280. [Google Scholar] [CrossRef]
- Shastina, V.V.; Menzorova, N.I.; Sibirtsev, Y.T.; Rasskazov, V.A. Purification and characteristics of Ca2+, Mg2+- and Ca2+,Mn2+-dependent and acid DNases from spermatozoa of the sea urchin Strongylocentrotus intermedius. Biochemistry 2003, 68, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Sibirtsev, J.T.; Shastina, V.V.; Menzorova, N.I.; Makarieva, T.N.; Rasskazov, V.A. Ca2+, Mg2+-dependent DNase involvement in apoptotic effects in spermatozoa of sea urchin Strongylocentrotus intermedius induced by two-headed sphingolipid rhizochalin. Mar. Biotechnol. 2011, 13, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Shaman, J.A.; Prisztoka, R.; Ward, W.S. Topoisomerase IIB and an extracellular nuclease interact to digest sperm DNA in an apoptotic-like manner. Biol. Reprod. 2006, 75, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Ajduk, A.; Riel, J.M.; Ward, M.A. Ejaculated and epididymal mouse spermatozoa are different in their susceptibility to nuclease-dependent DNA damage and in their nuclease activity. Biol. Reprod. 2007, 77, 636–647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singer, R.; Sagiv, M.; Allalouf, D.; Levinsky, H.; Servadio, C. Deoxyribonuclease activity in human seminal fluid. Arch. Androl. 1983, 10, 169–172. [Google Scholar] [CrossRef]
- Yasuda, T.; Nadano, D.; Awazu, S.; Kishi, K. Human urine deoxyribonuclease II (DNase II) isoenzymes: A novel immunoaffinity purification, biochemical multiplicity, genetic heterogeneity and broad distribution among tissues and body fluids. Biochim. Biophys. Acta 1992, 1119, 185–193. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; De La Vega, C.G.; Bartolomé-Nebreda, J.; Gosálvez, J. Characterization of DNA cleavage produced by seminal plasma using leukocytes as a cell target. Syst. Biol. Reprod. Med. 2019, 65, 420–429. [Google Scholar] [CrossRef]
- Fernández-Encinas, A.; García-Peiró, A.; Ribas-Maynou, J.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Characterization of nuclease activity in human seminal plasma and its relationship to semen parameters, sperm DNA fragmentation and male infertility. J. Urol. 2016, 195, 213–219. [Google Scholar] [CrossRef]
- Bartolomé, J.; Romeo, S.C.; Dorado-Silva, M.; García de la Vega, C.; López, C.; Sánchez-Martín, P.; Johnston, S.; Gosálvez, J. DNase activity in human seminal plasma and follicular fluid and its inhibition by follicular fluid chelating agents. Reprod. Biomed. Online 2021, 43, 1079–1086. [Google Scholar] [CrossRef]
- Sato, F.; Soh, T.; Hattori, M.A.; Fujihara, N. Evaluation of deoxyribonuclease activity in seminal plasma of ejaculated chicken semen. Asian J. Androl. 2003, 5, 213–216. [Google Scholar]
- Lanes, C.F.; Sampaio, L.A.; Marins, L.F. Evaluation of DNase activity in seminal plasma and uptake of exogenous DNA by spermatozoa of the Brazilian flounder Paralichthys orbignyanus. Theriogenology 2009, 71, 525–533. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Funnell, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, D.N. Comparative studies on bull and stallion seminal DNase activity and interaction with semen extender and spermatozoa. Anim. Reprod. Sci. 2010, 121, 249–258. [Google Scholar] [CrossRef]
- Wang, Y.; Du, C.; Zhang, Y.; Zhu, L. Composition and Function of Neutrophil Extracellular Traps. Biomolecules 2024, 29, 416. [Google Scholar] [CrossRef]
- Wrenzycki, C. Interaction of sperm cells with the female reproductive tract in cattle: Focus on neutrophil extracellular trap formation. Anim. Reprod. Sci. 2022, 246, 107056. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, D.N. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol. Reprod. 2005, 73, 1174–1181. [Google Scholar] [CrossRef]
- Dorado-Silva, M.; Bartolomé-Nebreda, J.; Sánchez-Martín, P.; Johnston, S.; Gosálvez, J. Co-incubation of spermatozoa with human follicular fluid reduces sperm DNA fragmentation by mitigating DNase activity in the seminal plasma. J. Assist. Reprod. Genet. 2020, 37, 63–69. [Google Scholar] [CrossRef] [PubMed]
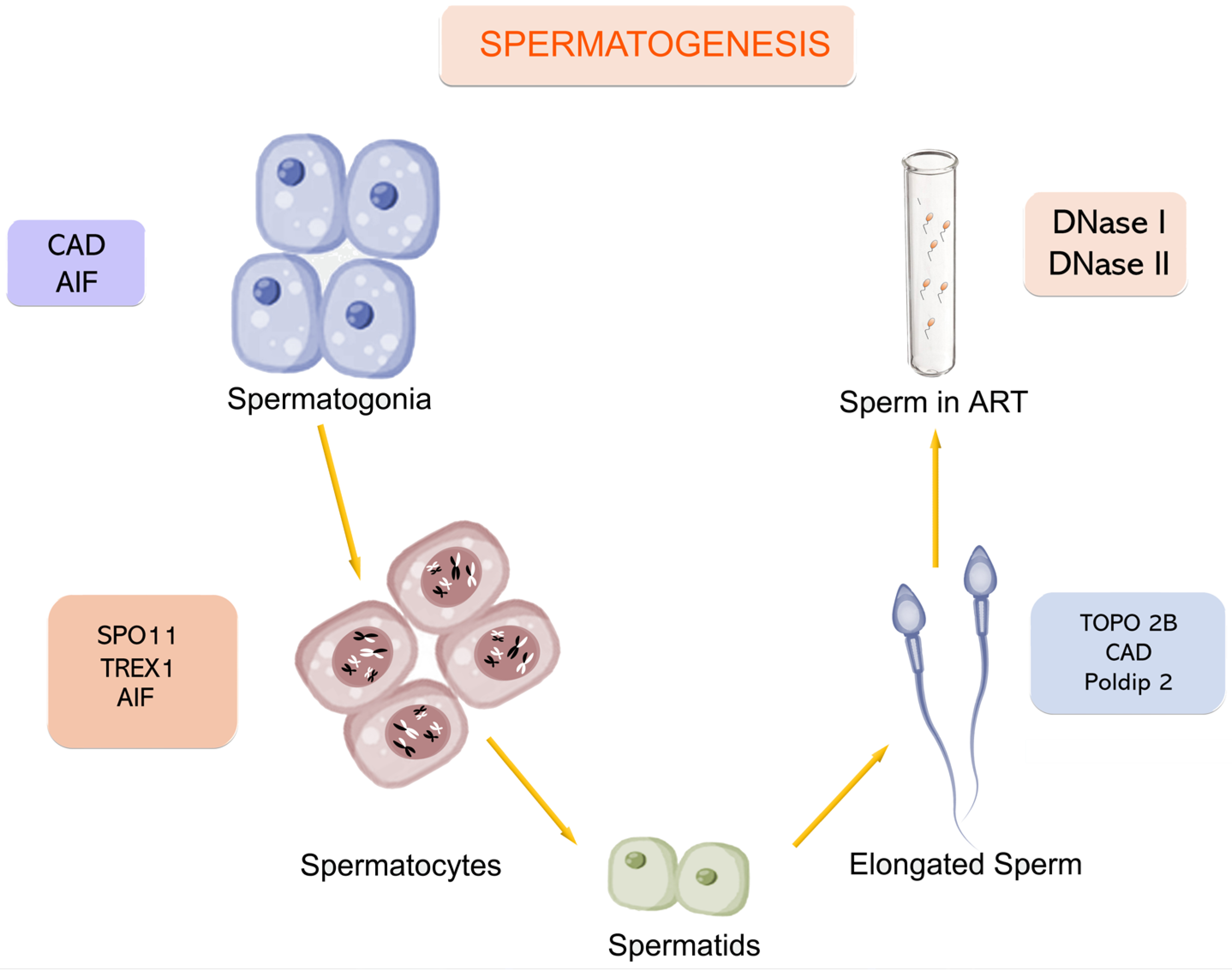
| Enzyme | Stage | Localization | Function/Role | Notes * |
|---|---|---|---|---|
| AIF | Spermatogonia and primary spermatocytes | Mitochondria → Nucleus | Caspase-independent apoptosis, large-scale DNA fragmentation (50 kb–1 Mb) | Low expression in mature sperm [10,11] |
| TREX1 | Early spermatogenesis | Cytosol/Nucleus of Sertoli cells | Degrading DNA fragments post-apoptosis. Preventing inflammation | Clearing DNA debris from CAD activity [12] |
| SPO11 | Early meiosis I | Nucleus | Introducing programmed DSBs to initiate meiotic recombination | Required for synapsis [13] |
| EndoG | Late spermatogenesis (post-meiotic) | Mitochondria → Nucleus | Cleaving nuclear and mitochondrial DNA during apoptosis | Role in sperm is debated [14,15] |
| Poldip2 | Spermiogenesis | Mitochondria | Clearing mitochondrial DNA in elongating spermatids | Loss leading to genome fragmentation [16] |
| TOPO2B | Spermiogenesis | Nucleus | Inducing DSBs during histone-to-protamine exchange | Working with torsional stress [17] |
| CAD | Early spermatogenesis and spermiogenesis | Nucleus | Cleaving chromosomal DNA into nucleosomal fragments during apoptosis | Activated by Caspase-3/-7 [18,19] |
| DNase I | Ejaculated sperm | Extracellular and lysosomal | Random DNA cleavage in necrotic cells | Ca2+ and Mg2+ required [20] |
| DNase II | Ejaculated sperm | Lysosomes | Digesting apoptotic/necrotic DNA under acidic pH in the female tract | Independent of divalent cations [21] |
| DNase γ | Possibly across all stages | Unclear-multi-organ | Fragmenting internucleosomal DNA. Cooperating with DNase I especially during necrosis | Suggested but not confirmed in sperm [20,22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosálvez, J.; López-Fernández, C.; Bartolomé-Nebreda, J.; García de la Vega, C. Hurdles of Sperm Success: Exploring the Role of DNases. Int. J. Mol. Sci. 2025, 26, 6789. https://doi.org/10.3390/ijms26146789
Gosálvez J, López-Fernández C, Bartolomé-Nebreda J, García de la Vega C. Hurdles of Sperm Success: Exploring the Role of DNases. International Journal of Molecular Sciences. 2025; 26(14):6789. https://doi.org/10.3390/ijms26146789
Chicago/Turabian StyleGosálvez, Jaime, Carmen López-Fernández, Javier Bartolomé-Nebreda, and Carlos García de la Vega. 2025. "Hurdles of Sperm Success: Exploring the Role of DNases" International Journal of Molecular Sciences 26, no. 14: 6789. https://doi.org/10.3390/ijms26146789
APA StyleGosálvez, J., López-Fernández, C., Bartolomé-Nebreda, J., & García de la Vega, C. (2025). Hurdles of Sperm Success: Exploring the Role of DNases. International Journal of Molecular Sciences, 26(14), 6789. https://doi.org/10.3390/ijms26146789





