Impact of SOD1 Transcript Variants on Amyotrophic Lateral Sclerosis Severity
Abstract
1. Introduction
2. Results
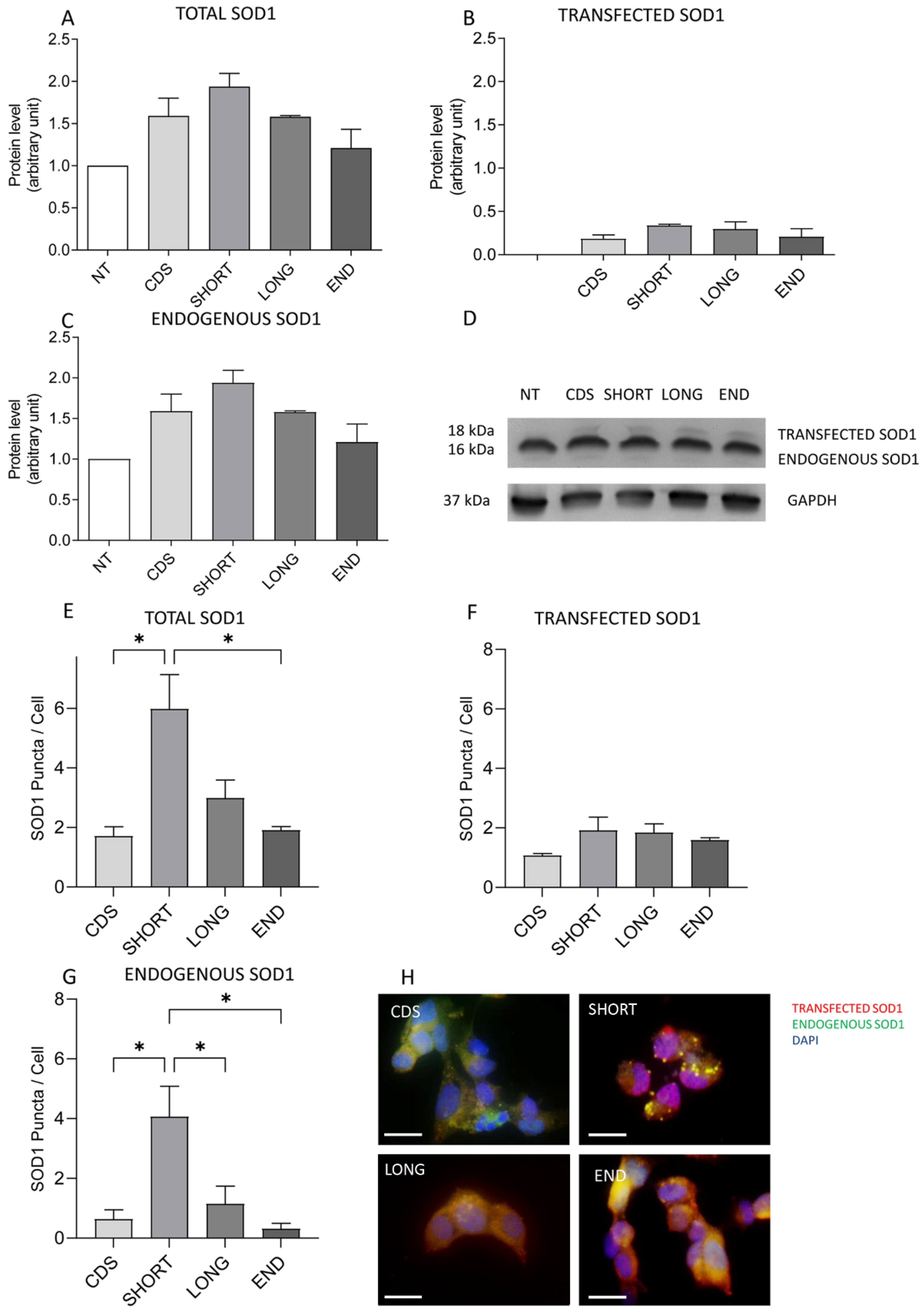
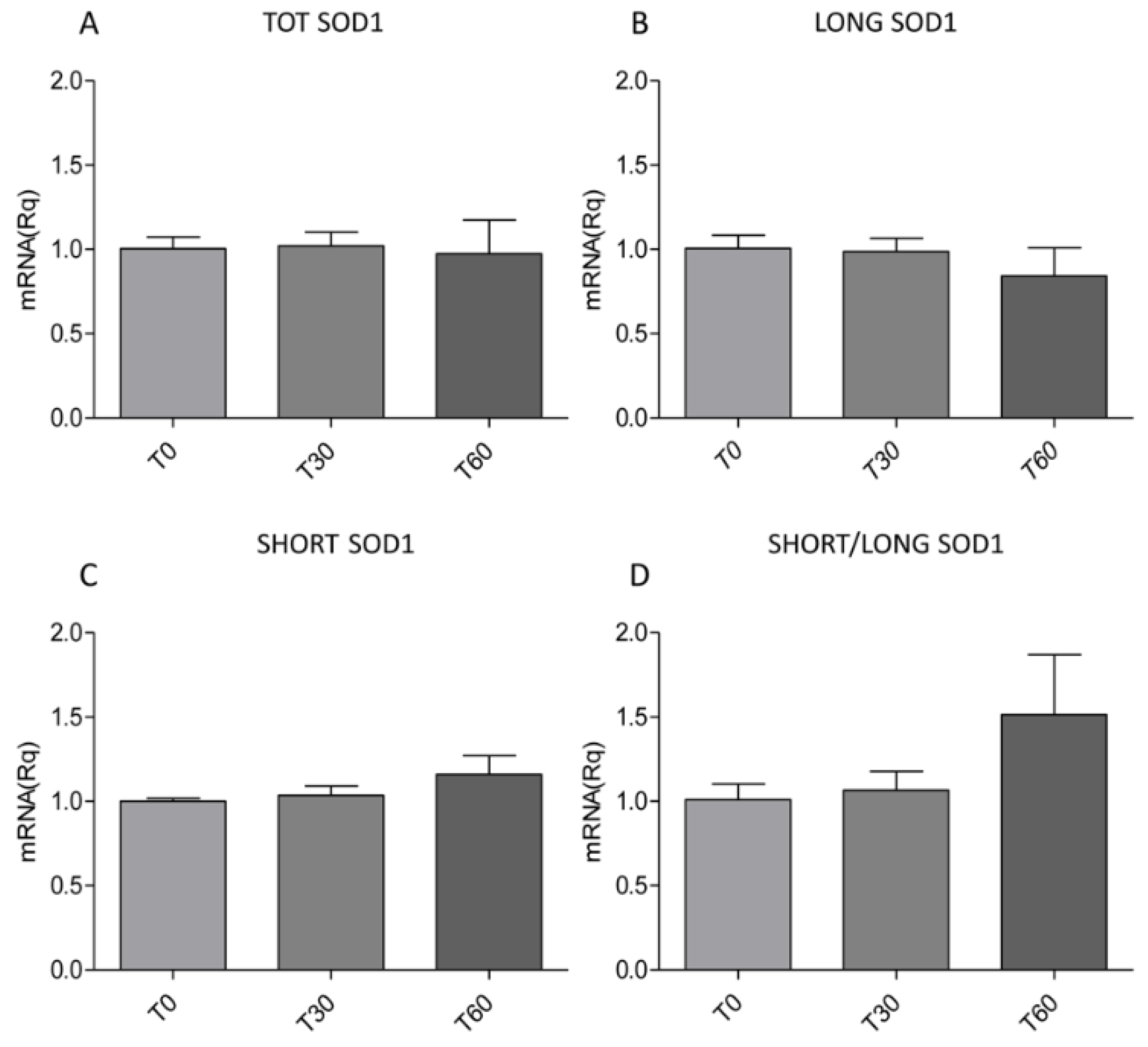
2.1. Oxidative Stress Induces the Overexpression of SHORT SOD1 Transcript Leading to the Increase and Aggregation of TOT SOD1
2.2. Peripheral Blood Mononuclear Cells of sALS Patients Present an Increased Expression of SHORT SOD1
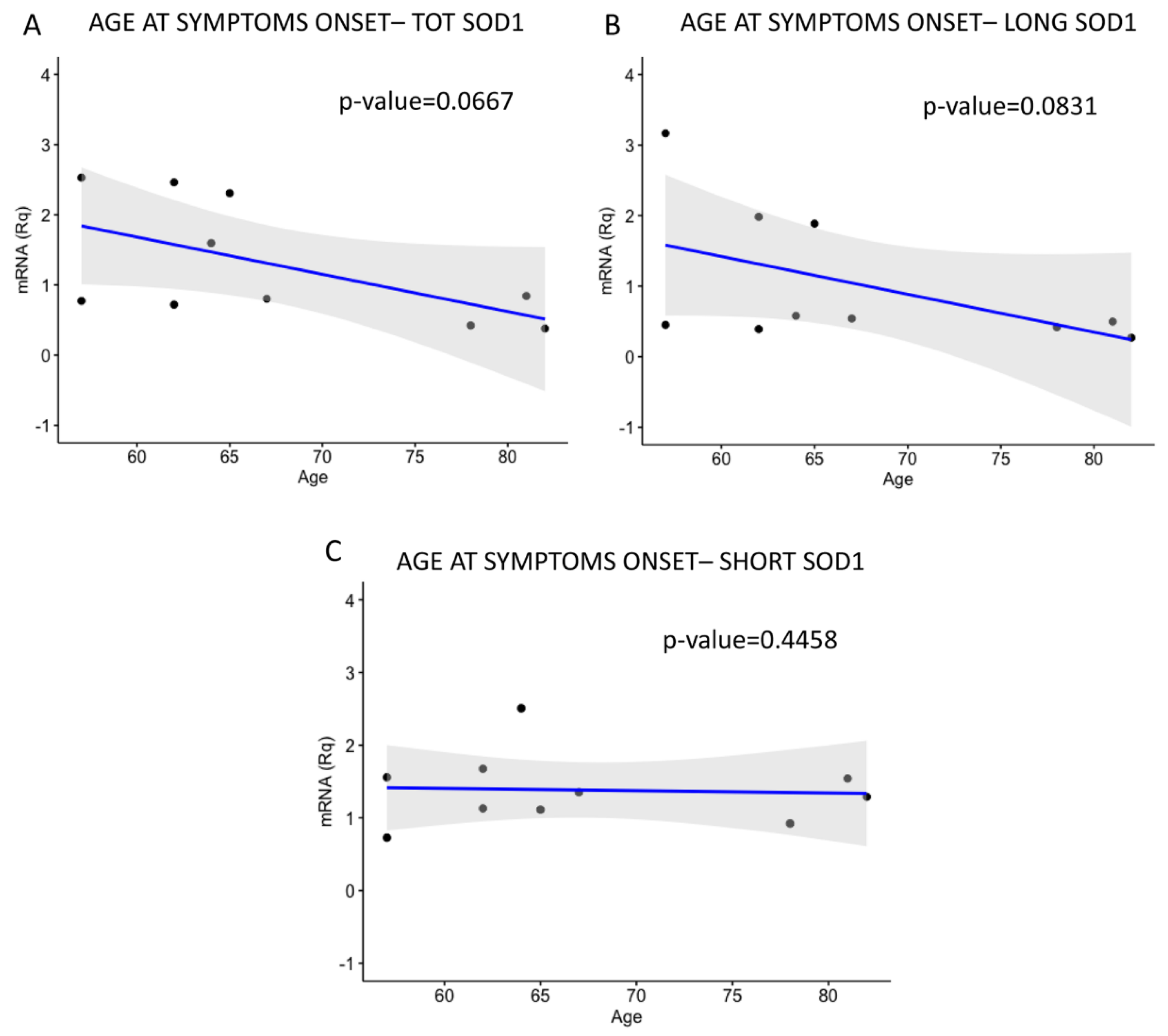
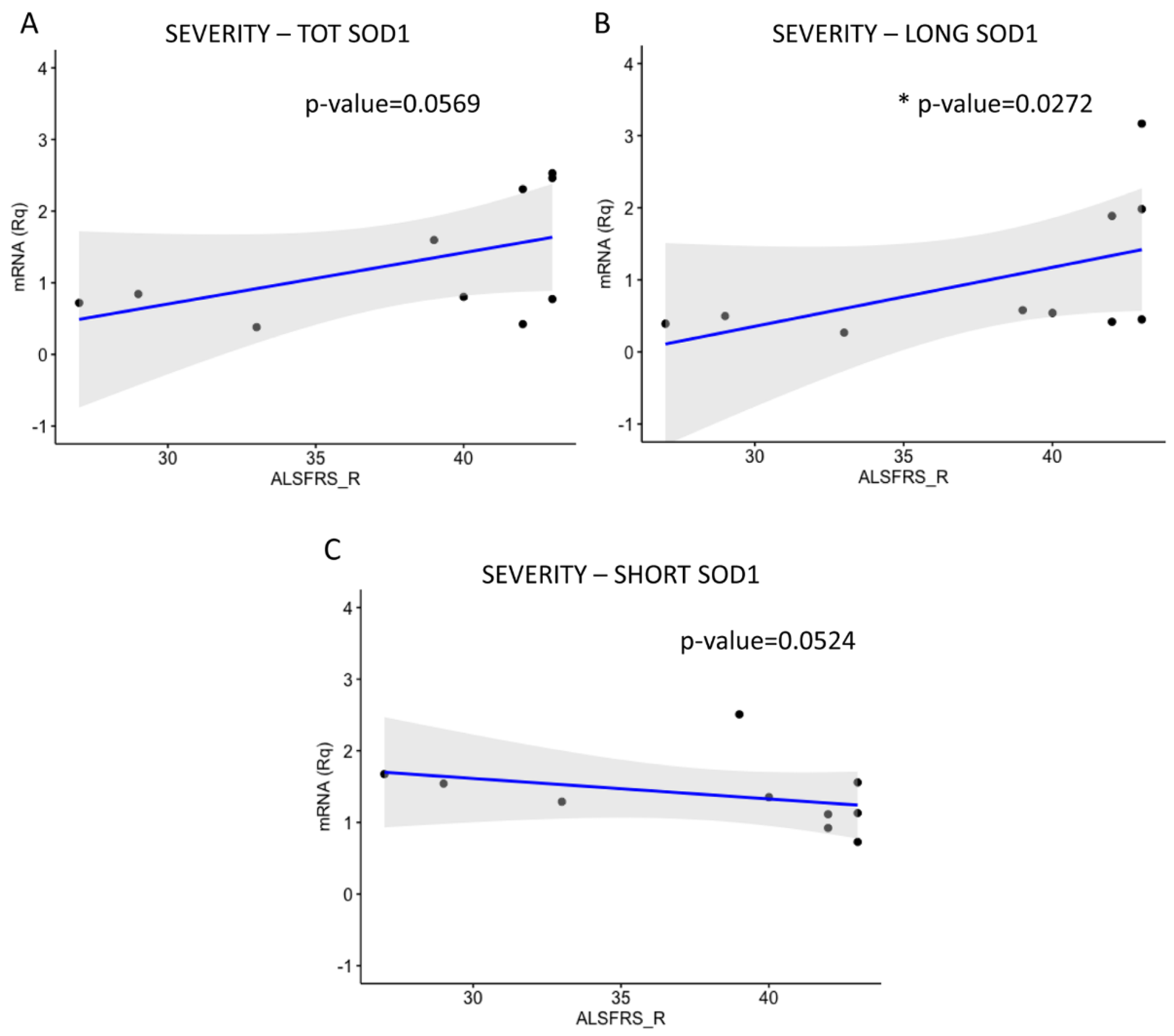
2.3. LONG SOD1 Inversely Correlates with the Severity of the Pathology
3. Discussion
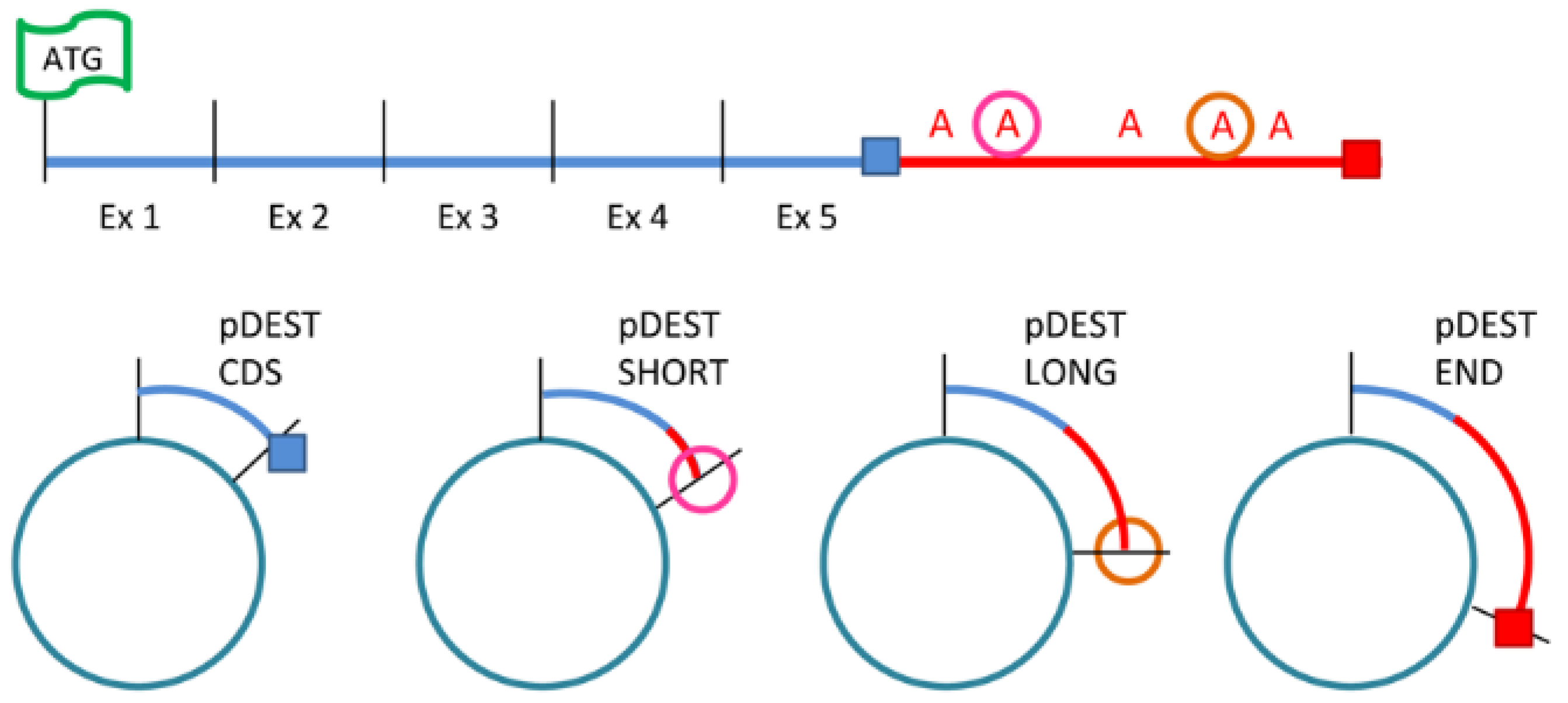
4. Materials and Methods
4.1. HeLa and SH-SY5Y Cell Culture and Treatment
4.2. Enrolment of sALS Patients and Healthy Subjects
4.3. Peripheral Blood Mononuclear Cells’ Isolation
4.4. Cell Transfection
4.5. Immunofluorescence Analysis
4.6. Western Blot
4.7. RNA Extraction
4.8. 3′ Rapid Amplification of cDNA Ends
4.9. Multiplex Reverse Transcription–Polymerase Chain Reaction
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| ALSFRS_R | ALS Functional Rating Scale_Revised |
| Ct | Cycle threshold |
| CTRL | Control |
| FLAG | N’terminal-Flag sequence |
| H2O2 | Hydrogen peroxide |
| IF | Immunofluorescence |
| LONG SOD1 | Long SOD1 transcript |
| NT | Not transfected |
| OS | Oxidative stress |
| PBMCs | Peripheral blood mononuclear cells |
| PRB | Basal progression rate |
| PRL | Late progression rate |
| RACE | 3′ rapid amplification of cDNA ends |
| RT-PCR | Reverse transcription–polymerase chain reaction |
| S.E.M. | Standard error of mean |
| SDS | Sodium dodecyl sulphate |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| SHOR SOD1 | Short SOD1 transcript |
| SOD1 | Superoxide dismutase 1 |
| TOT SOD1 | Total SOD1 |
| WB | Western blot |
References
- Logroscino, G.; Urso, D.; Tortelli, R. The challenge of amyotrophic lateral sclerosis descriptive epidemiology: To estimate low incidence rates across complex phenotypes in different geographic areas. Curr. Opin. Neurol. 2022, 35, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, S.; Murn, M.; Choi, P.J. Respiratory Failure in Amyotrophic Lateral Sclerosis. Chest 2019, 155, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, S.; Dhasmana, A.; Narula, A.S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. The panoramic view of amyotrophic lateral sclerosis: A fatal intricate neurological disorder. Life Sci. 2022, 288, 120156. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.Y.; Zhou, Z.R.; Che, C.H.; Liu, C.Y.; He, R.L.; Huang, H.P. Genetic epidemiology of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, M.; Müschen, L.H.; Brakemeier, S.; Machetanz, G.; Naumann, M.; Castro-Gomez, S. Current State and Future Directions in the Diagnosis of Amyotrophic Lateral Sclerosis. Cells 2023, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.P.; Likanje, M.N.; Andrews, J.A. Amyotrophic lateral sclerosis: Update on clinical management. Curr. Opin. Neurol. 2020, 33, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; P O, J.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Oh, K.W.; Nordin, A.; Panthi, S.; Kim, S.H.; Nordin, F.; Freischmidt, A.; Ludolph, A.C.; Ki, C.S.; Forsberg, K.; et al. De novo mutations in SOD1 are a cause of ALS. J. Neurol. Neurosurg. Psychiatry 2022, 93, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Vidal-Taboada, J.M.; Syriani, E.; Salvado, M.; Morales, M.; Gamez, J. Haplotype Analysis of the First A4V-SOD1 Spanish Family: Two Separate Founders or a Single Common Founder? Front. Genet. 2019, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown RHJr Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Je, G.; Pacut, P.; Keyhanian, K.; Gao, J.; Ghasemi, M. Gene Therapy in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, X.; Li, X.; Mou, J.; Liu, H.; Chen, J.; Wu, J. The G41D mutation in SOD1-related amyotrophic lateral sclerosis exhibits phenotypic heterogeneity among individuals: A case report and literature review. Medicine 2022, 101, e28771. [Google Scholar] [CrossRef] [PubMed]
- Scarian, E.; Bordoni, M.; Fantini, V.; Jacchetti, E.; Raimondi, M.T.; Diamanti, L.; Carelli, S.; Cereda, C.; Pansarasa, O. Patients’ Stem Cells Differentiation in a 3D Environment as a Promising Experimental Tool for the Study of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 5344. [Google Scholar] [CrossRef] [PubMed]
- Pansarasa, O.; Bordoni, M.; Diamanti, L.; Sproviero, D.; Gagliardi, S.; Cereda, C. SOD1 in Amyotrophic Lateral Sclerosis: “Ambivalent” Behavior Connected to the Disease. Int. J. Mol. Sci. 2018, 19, 1345. [Google Scholar] [CrossRef] [PubMed]
- Pansarasa, O.; Garofalo, M.; Scarian, E.; Dragoni, F.; Garau, J.; Di Gerlando, R.; Diamanti, L.; Bordoni, M.; Gagliardi, S. Biomarkers in Human Peripheral Blood Mononuclear Cells: The State of the Art in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 2580. [Google Scholar] [CrossRef] [PubMed]
- Grad, L.I.; Yerbury, J.J.; Turner, B.J.; Guest, W.C.; Pokrishevsky, E.; O’Neill, M.A.; Yanai, A.; Silverman, J.M.; Zeineddine, R.; Corcoran, L.; et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 3620–3625. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.I.; Fromholt, S.E.; O’Neal, V.M.; Diamond, J.H.; Borchelt, D.R. Prion-like propagation of mutant SOD1 misfolding and motor neuron disease spread along neuroanatomical pathways. Acta Neuropathol. 2016, 131, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Christy, D.; Shyu, C.C.; Moon, K.-M.; Fernando, S.; Gidden, Z.; Cowan, C.M.; Ban, Y.; Stacey, R.G.; Grad, L.I.; et al. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)G93A ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J. Biol. Chem. 2019, 294, 3744–3759. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Bigio, E.H.; Ince, P.G.; Geser, F.; Neumann, M.; Cairns, N.J.; Kwong, L.K.; Forman, M.S.; Ravits, J.; Stewart, H.; et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 2007, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Benson, B.C.; Shaw, P.J.; Azzouz, M.; Highley, J.R.; Hautbergue, G.M. Proteinopathies as Hallmarks of Impaired Gene Expression, Proteostasis and Mitochondrial Function in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2021, 15, 783624. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.; Levanon, D.; Lieman-Hurwitz, J.; Dafni, N.; Groner, Y. Human Cu/Zn superoxide dismutase gene: Molecular characterization of its two mRNA species. Nucleic Acids Res. 1984, 12, 9349–9365. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Amadio, M.; Laforenza, U.; Dell, M.; Diamanti, L.; Sardone, V.; Gagliardi, S.; Govoni, S.; Ceroni, M.; Pascale, A.; et al. Posttranscriptional regulation of SOD1 gene expression under oxidative stress: Potential role of ELAV proteins in sporadic ALS. Neurobiol. Dis. 2013, 60, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orco, M.; Milani, P.; Arrigoni, L.; Pansarasa, O.; Sardone, V.; Maffioli, E.; Polveraccio, F.; Bordoni, M.; Diamanti, L.; Ceroni, M.; et al. Hydrogen peroxide-mediated induction of SOD1 gene transcription is independent from Nrf2 in a cellular model of neurodegeneration. Biochim. Biophys. Acta 2016, 1859, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Pansarasa, O.; Dell’Orco, M.; Crippa, V.; Gagliardi, S.; Sproviero, D.; Bernuzzi, S.; Diamanti, L.; Ceroni, M.; Tedeschi, G.; et al. Nuclear Phospho-SOD1 Protects DNA from Oxidative Stress Damage in Amyotrophic Lateral Sclerosis. J. Clin. Med. 2019, 8, 729. [Google Scholar] [CrossRef] [PubMed]
- Meiering, E.M. The threat of instability: Neurodegeneration predicted by protein destabilization and aggregation propensity. PLoS Biol. 2008, 6, e193. [Google Scholar] [CrossRef] [PubMed]
- McAlary, L.; Plotkin, S.S.; Yerbury, J.J.; Cashman, N.R. Prion-Like Propagation of Protein Misfolding and Aggregation in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Pesaresi, M.G.; Gerbino, V.; Grosskreutz, J.; Carrì, M.T. Amyotrophic lateral sclerosis: New insights into underlying molecular mechanisms and opportunities for therapeutic intervention. Antioxid. Redox Signal. 2012, 17, 1277–1330. [Google Scholar] [CrossRef] [PubMed]
- Motataianu, A.; Serban, G.; Barcutean, L.; Balasa, R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tiwari, A.; Sharma, K.; Sharma, S.K. Does conserved domain SOD1 mutation has any role in ALS severity and therapeutic outcome? BMC Neurosci. 2020, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Couratier, P.; Lautrette, G.; Luna, J.A.; Corcia, P. Phenotypic variability in amyotrophic lateral sclerosis. Rev. Neurol. 2021, 177, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.; Drory, V.; Hardiman, O.; Nakano, I.; Ravits, J.; Robberecht, W.; Shefner, J.; WFN Research Group On ALS/MND. A revision of the El Escorial criteria—2015. Amyotroph. Lateral Scler. Front. Degener 2015, 16, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Filosto, M.; Piccinelli, S.C.; Palmieri, I.; Necchini, N.; Valente, M.; Zanella, I.; Biasiotto, G.; Di Lorenzo, D.; Cereda, C.; Padovani, A. A Novel Mutation in the Stalk Domain of KIF5A Causes a Slowly Progressive Atypical Motor Syndrome. J. Clin. Med. 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.H.; Miller, R.G.; Moore, D.H. ALSFRS-R. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5 (Suppl. S1), 90–93. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S.G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxid. Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef] [PubMed]
- Kjældgaard, A.L.; Pilely, K.; Olsen, K.S.; Lauritsen, A.Ø.; Pedersen, S.W.; Svenstrup, K.; Karlsborg, M.; Thagesen, H.; Blaabjerg, M.; Theódórsdóttir, Á.; et al. Complement Profiles in Patients with Amyotrophic Lateral Sclerosis: A Prospective Observational Cohort Study. J. Inflamm. Res. 2021, 14, 1043–1053. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, M.; Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Pansarasa, O. Impact of SOD1 Transcript Variants on Amyotrophic Lateral Sclerosis Severity. Int. J. Mol. Sci. 2025, 26, 6788. https://doi.org/10.3390/ijms26146788
Bordoni M, Scarian E, Viola C, Dragoni F, Di Gerlando R, Rizzo B, Diamanti L, Gagliardi S, Pansarasa O. Impact of SOD1 Transcript Variants on Amyotrophic Lateral Sclerosis Severity. International Journal of Molecular Sciences. 2025; 26(14):6788. https://doi.org/10.3390/ijms26146788
Chicago/Turabian StyleBordoni, Matteo, Eveljn Scarian, Camilla Viola, Francesca Dragoni, Rosalinda Di Gerlando, Bartolo Rizzo, Luca Diamanti, Stella Gagliardi, and Orietta Pansarasa. 2025. "Impact of SOD1 Transcript Variants on Amyotrophic Lateral Sclerosis Severity" International Journal of Molecular Sciences 26, no. 14: 6788. https://doi.org/10.3390/ijms26146788
APA StyleBordoni, M., Scarian, E., Viola, C., Dragoni, F., Di Gerlando, R., Rizzo, B., Diamanti, L., Gagliardi, S., & Pansarasa, O. (2025). Impact of SOD1 Transcript Variants on Amyotrophic Lateral Sclerosis Severity. International Journal of Molecular Sciences, 26(14), 6788. https://doi.org/10.3390/ijms26146788






