Lipidome Complexity in Physiological and Pathological Skin Pigmentation
Abstract
1. Introduction
2. Lipidomics Principles
3. Lipids in the Skin
4. Lipid Metabolism and Analysis in Skin Pigmentation
4.1. Intrinsic/Extrinsic Factors in Skin Pigmentation
4.2. Fatty Acids and Phospholipids
4.3. Cholesterol
4.4. Bioactive Lipids
4.4.1. Eicosanoids
4.4.2. Sphingolipids
4.4.3. Endocannabinoids
4.4.4. Lipidomics for Studying Melanogenesis
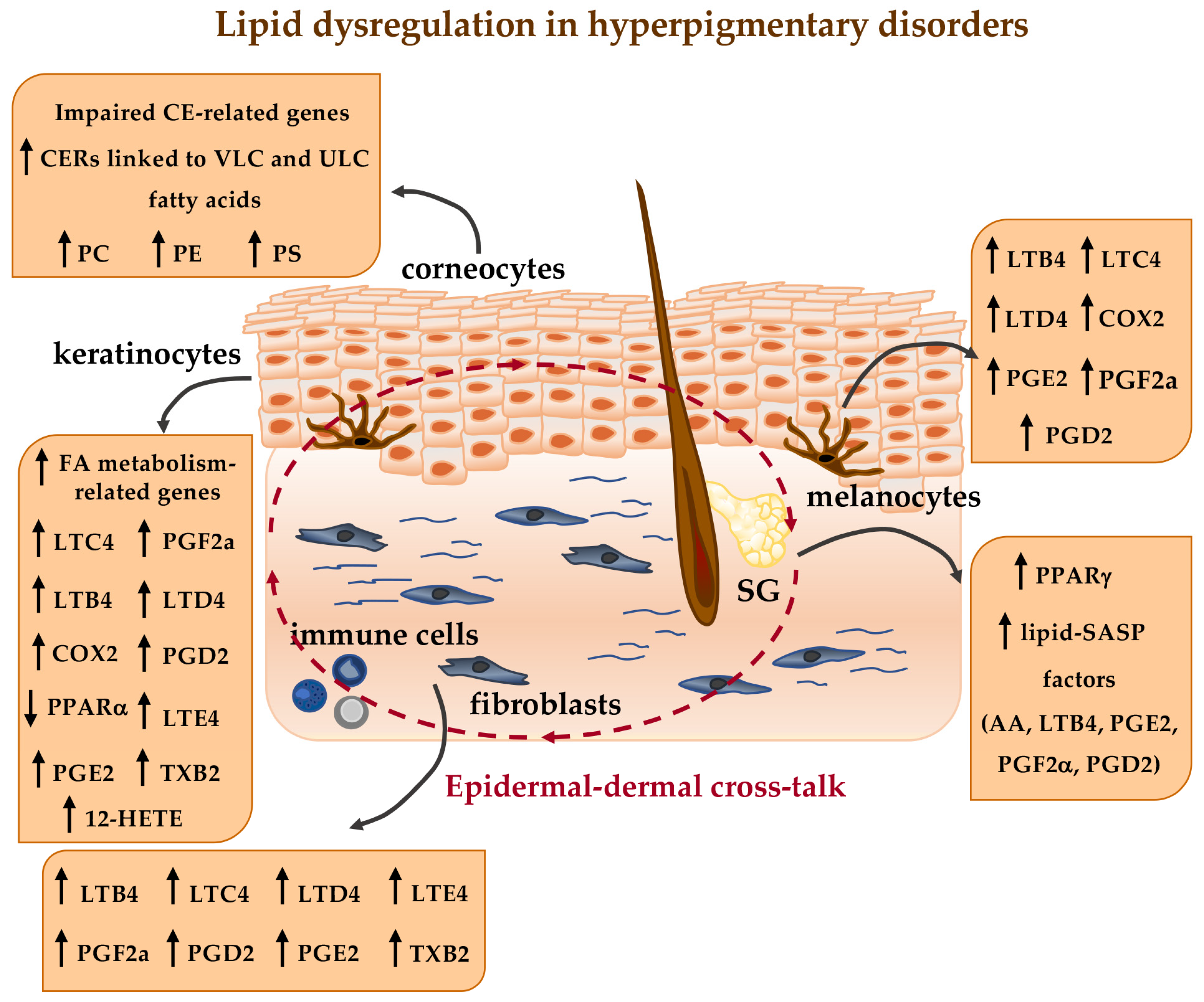
5. Lipid Metabolism and Analysis in Hyperpigmentary Disorders
6. Lipid Metabolism and Analysis in Hypopigmentary Disorders
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell. Proteomics 2023, 22, 100561. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, E.H.; Ismail, R.; Mabrouk, M.S.; Amin, E. Multi-omics data integration and analysis pipeline for precision medicine: Systematic review. Comput. Biol. Chem. 2024, 113, 108254. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Siwach, A.; Sachdeva, S.N. Metabolomics and cellular altered pathways in cancer biology: A review. J. Biochem. Mol. Toxicol. 2024, 38, e23807. [Google Scholar] [CrossRef]
- Gutierrez Reyes, C.D.; Alejo-Jacuinde, G.; Perez Sanchez, B.; Chavez Reyes, J.; Onigbinde, S.; Mogut, D.; Hernández-Jasso, I.; Calderón-Vallejo, D.; Quintanar, J.L.; Mechref, Y. Multi Omics Applications in Biological Systems. Curr. Issues Mol. Biol. 2024, 46, 5777–5793. [Google Scholar] [CrossRef]
- Guo, T.; Steen, J.A.; Mann, M. Mass-spectrometry-based proteomics: From single cells to clinical applications. Nature 2025, 638, 901–911. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Géhin, C.; Fowler, S.J.; Trivedi, D.K. Chewing the fat: How lipidomics is changing our understanding of human health and disease in 2022. Anal. Sci. Adv. 2023, 4, 104–131. [Google Scholar] [CrossRef]
- Anh, N.K.; Thu, N.Q.; Tien, N.T.N.; Long, N.P.; Nguyen, H.T. Advancements in Mass Spectrometry-Based Targeted Metabolomics and Lipidomics: Implications for Clinical Research. Molecules 2024, 29, 5934. [Google Scholar] [CrossRef]
- Dakal, T.C.; Xiao, F.; Bhusal, C.K.; Sabapathy, P.C.; Segal, R.; Chen, J.; Bai, X. Lipids dysregulation in diseases: Core concepts, targets and treatment strategies. Lipids Health Dis. 2025, 24, 61. [Google Scholar] [CrossRef]
- Kendall, A.C.; Koszyczarek, M.M.; Jones, E.A.; Hart, P.J.; Towers, M.; Griffiths, C.E.M.; Morris, M.; Nicolaou, A. Lipidomics for translational skin research: A primer for the uninitiated. Exp. Dermatol. 2018, 27, 721–728. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Narzt, M.-S. The impact of recent advances in lipidomics and redox lipidomics on dermatological research. Free Radic. Biol. Med. 2019, 144, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kiezel-Tsugunova, M.; Kendall, A.C.; Nicolaou, A. Fatty acids and related lipid mediators in the regulation of cutaneous inflammation. Biochem. Soc. Trans. 2018, 46, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, P.T.; Milne, S.B.; Myers, D.S.; Brown, H.A. Lipidomics: A mass spectrometry based systems level analysis of cellular lipids. Curr. Opin. Chem. Biol. 2009, 13, 526–531. [Google Scholar] [CrossRef]
- Murphy, R.C.; Merrill, A.H. Lipidomics. In Encyclopedia of Cell Biology, 2nd ed.; Bradshaw, R.A., Hart, G.W., Stahl, P.D., Eds.; Academic Press: Oxford, UK, 2023; pp. 202–218. ISBN 978-0-12-821624-8. [Google Scholar]
- Lagarde, M.; Géloën, A.; Record, M.; Vance, D.; Spener, F. Lipidomics is emerging. Biochim. Biophys. Acta 2003, 1634, 61. [Google Scholar] [CrossRef]
- Watson, A.D. Thematic review series: Systems Biology Approaches to Metabolic and Cardiovascular Disorders. Lipidomics: A global approach to lipid analysis in biological systems. J. Lipid Res. 2006, 47, 2101–2111. [Google Scholar] [CrossRef]
- Manni, M.M.; Sot, J.; Arretxe, E.; Gil-Redondo, R.; Falcón-Pérez, J.M.; Balgoma, D.; Alonso, C.; Goñi, F.M.; Alonso, A. The fatty acids of sphingomyelins and ceramides in mammalian tissues and cultured cells: Biophysical and physiological implications. Chem. Phys. Lipids 2018, 217, 29–34. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Addepalli, R.V.; Mullangi, R. A concise review on lipidomics analysis in biological samples. ADMET DMPK 2020, 9, 1–22. [Google Scholar] [CrossRef]
- Li, S.; Ganguli-Indra, G.; Indra, A.K. Lipidomic analysis of epidermal lipids: A tool to predict progression of inflammatory skin disease in humans. Expert Rev. Proteomics 2016, 13, 451–456. [Google Scholar] [CrossRef]
- Hancock, S.E.; Poad, B.L.J.; Batarseh, A.; Abbott, S.K.; Mitchell, T.W. Advances and unresolved challenges in the structural characterization of isomeric lipids. Anal. Biochem. 2017, 524, 45–55. [Google Scholar] [CrossRef]
- Khoury, S.; Canlet, C.; Lacroix, M.Z.; Berdeaux, O.; Jouhet, J.; Bertrand-Michel, J. Quantification of Lipids: Model, Reality, and Compromise. Biomolecules 2018, 8, 174. [Google Scholar] [CrossRef]
- Köfeler, H.C.; Ahrends, R.; Baker, E.S.; Ekroos, K.; Han, X.; Hoffmann, N.; Holčapek, M.; Wenk, M.R.; Liebisch, G. Recommendations for good practice in MS-based lipidomics. J. Lipid Res. 2021, 62, 100138. [Google Scholar] [CrossRef]
- Gerhardtova, I.; Jankech, T.; Majerova, P.; Piestansky, J.; Olesova, D.; Kovac, A.; Jampilek, J. Recent Analytical Methodologies in Lipid Analysis. Int. J. Mol. Sci. 2024, 25, 2249. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Lee, H.-C.; Yokomizo, T. Applications of mass spectrometry-based targeted and non-targeted lipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, Y.; Zhang, W. Structural Lipidomics Enabled by Isomer-Resolved Tandem Mass Spectrometry. Anal. Chem. 2025, 97, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.K.; Naushin, S.; Ray, A.; Singh, P.; Raj, A.; Pradhan, S.; Adlakha, K.; Siddiqua, T.J.; Malakar, D.; Dash, D.; et al. A High Throughput Lipidomics Method Using Scheduled Multiple Reaction Monitoring. Biomolecules 2022, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Codreanu, S.G.; Goyal, S.; Wages, P.A.; Gorti, S.K.K.; Pearson, M.J.; Uribe, I.; Sherrod, S.D.; McLean, J.A.; Porter, N.A.; et al. Evaluating a targeted multiple reaction monitoring approach to global untargeted lipidomic analyses of human plasma. Rapid Commun. Mass Spectrom. 2020, 34, e8911. [Google Scholar] [CrossRef] [PubMed]
- Dushianthan, A.; Cusack, R.; Koster, G.; Grocott, M.P.W.; Postle, A.D. Insight into erythrocyte phospholipid molecular flux in healthy humans and in patients with acute respiratory distress syndrome. PLoS ONE 2019, 14, e0221595. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ji, S.; Burla, B.; Wenk, M.R.; Torta, F.; Cazenave-Gassiot, A. LICAR: An Application for Isotopic Correction of Targeted Lipidomic Data Acquired with Class-Based Chromatographic Separations Using Multiple Reaction Monitoring. Anal. Chem. 2021, 93, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Han, X. Tutorial on lipidomics. Anal. Chim. Acta 2019, 1061, 28–41. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Zhu, Z.-J. The Use of LipidIMMS Analyzer for Lipid Identification in Ion Mobility-Mass Spectrometry-Based Untargeted Lipidomics. Methods Mol. Biol. 2020, 2084, 269–282. [Google Scholar] [CrossRef]
- Züllig, T.; Trötzmüller, M.; Köfeler, H.C. Global Lipidomics Profiling by a High Resolution LC-MS Platform. Methods Mol. Biol. 2021, 2306, 39–51. [Google Scholar] [CrossRef]
- Fahy, E.; Alvarez-Jarreta, J.; Brasher, C.J.; Nguyen, A.; Hawksworth, J.I.; Rodrigues, P.; Meckelmann, S.; Allen, S.M.; O’Donnell, V.B. LipidFinder on LIPID MAPS: Peak filtering, MS searching and statistical analysis for lipidomics. Bioinformatics 2019, 35, 685–687. [Google Scholar] [CrossRef]
- Conroy, M.J.; Andrews, R.M.; Andrews, S.; Cockayne, L.; Dennis, E.A.; Fahy, E.; Gaud, C.; Griffiths, W.J.; Jukes, G.; Kolchin, M.; et al. LIPID MAPS: Update to databases and tools for the lipidomics community. Nucleic Acids Res. 2024, 52, D1677–D1682. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. LC-MS-Based Lipidomics and Automated Identification of Lipids Using the LipidBlast In-Silico MS/MS Library. Methods Mol. Biol. 2017, 1609, 149–170. [Google Scholar] [CrossRef]
- Alvarez-Jarreta, J.; Rodrigues, P.R.S.; Fahy, E.; O’Connor, A.; Price, A.; Gaud, C.; Andrews, S.; Benton, P.; Siuzdak, G.; Hawksworth, J.I.; et al. LipidFinder 2.0: Advanced informatics pipeline for lipidomics discovery applications. Bioinformatics. 2021, 37, 1478–1479. [Google Scholar] [CrossRef]
- Schwudke, D.; Shevchenko, A.; Hoffmann, N.; Ahrends, R. Lipidomics informatics for life-science. J. Biotechnol. 2017, 261, 131–136. [Google Scholar] [CrossRef]
- Hoffmann, N.; Mayer, G.; Has, C.; Kopczynski, D.; Al Machot, F.; Schwudke, D.; Ahrends, R.; Marcus, K.; Eisenacher, M.; Turewicz, M. A Current Encyclopedia of Bioinformatics Tools, Data Formats and Resources for Mass Spectrometry Lipidomics. Metabolites 2022, 12, 584. [Google Scholar] [CrossRef]
- Meitei, N.S.; Shulaev, V. Bioinformatics in Lipidomics: Automating Large-Scale LC-MS-Based Untargeted Lipidomics Profiling with SimLipid Software. Methods Mol. Biol. 2022, 2396, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. The outer frontier: The importance of lipid metabolism in the skin. J. Lipid Res. 2009, 50, S417–S422. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five Functional Aspects of the Epidermal Barrier. Int. J. Mol. Sci. 2021, 22, 11676. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Berdyshev, E. Skin Lipid Barrier: Structure, Function and Metabolism. Allergy Asthma Immunol. Res. 2024, 16, 445–461. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Nădăban, A.; Bras, W.; MCabe, C.; Bunge, A.; Gooris, G.S. The skin barrier: An extraordinary interface with an exceptional lipid organization. Prog. Lipid Res. 2023, 92, 101252. [Google Scholar] [CrossRef]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef]
- Van Smeden, J.; Boiten, W.A.; Hankemeier, T.; Rissmann, R.; Bouwstra, J.A.; Vreeken, R.J. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim. Biophys. Acta 2014, 1841, 70–79. [Google Scholar] [CrossRef]
- Weerheim, A.; Ponec, M. Determination of stratum corneum lipid profile by tape stripping in combination with high-performance thin-layer chromatography. Arch. Dermatol. Res. 2001, 293, 191–199. [Google Scholar] [CrossRef]
- Wertz, P.W. Lipid Metabolic Events Underlying the Formation of the Corneocyte Lipid Envelope. Skin Pharmacol. Physiol. 2021, 34, 38–50. [Google Scholar] [CrossRef]
- Feingold, K.R.; Elias, P.M. The role of ceramides in the disruption of the cutaneous permeability barrier, a common manifestation of skin disorders. J. Lipid Res. 2024, 65, 100593. [Google Scholar] [CrossRef]
- Ponec, M.; Weerheim, A.; Kempenaar, J.; Mommaas, A.M.; Nugteren, D.H. Lipid composition of cultured human keratinocytes in relation to their differentiation. J. Lipid Res. 1988, 29, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Houben, E.; De Paepe, K.; Rogiers, V. A Keratinocyte’s Course of Life. Skin Pharmacol. Physiol. 2006, 20, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Amen, N.; Mathow, D.; Rabionet, M.; Sandhoff, R.; Langbein, L.; Gretz, N.; Jäckel, C.; Gröne, H.-J.; Jennemann, R. Differentiation of epidermal keratinocytes is dependent on glucosylceramide:ceramide processing. Hum. Mol. Genet. 2013, 22, 4164–4179. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. The how, why and clinical importance of stratum corneum acidification. Exp. Dermatol. 2017, 26, 999–1003. [Google Scholar] [CrossRef]
- Pappas, A. Epidermal surface lipids. Derm.-Endocrinol 2009, 1, 72–76. [Google Scholar] [CrossRef]
- Wertz, P.W. Lipids and the Permeability and Antimicrobial Barriers of the Skin. J. Lipids 2018, 2018, 5954034. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Q.; Zhong, Q.; Duan, C.; Krutmann, J.; Wang, J.; Xia, J. Skin Microbiome, Metabolome and Skin Phenome, from the Perspectives of Skin as an Ecosystem. Phenomics 2022, 2, 363–382. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Isom, M.; Desaire, H. Skin Surface Sebum Analysis by ESI-MS. Biomolecules 2024, 14, 790. [Google Scholar] [CrossRef]
- Kendall, A.C.; Pilkington, S.M.; Massey, K.A.; Sassano, G.; Rhodes, L.E.; Nicolaou, A. Distribution of bioactive lipid mediators in human skin. J. Investig. Dermatol. 2015, 135, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Kiezel-Tsugunova, M.; Brownbridge, L.C.; Harwood, J.L.; Nicolaou, A. Lipid functions in skin: Differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides, in a human skin organ culture model. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.; O’Boyle, N.M. Skin lipids in health and disease: A review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Jiang, Y.J. The mechanisms by which lipids coordinately regulate the formation of the protein and lipid domains of the stratum corneum. Derm.-Endocrinol. 2011, 3, 113–118. [Google Scholar] [CrossRef][Green Version]
- Casati, S.; Giannasi, C.; Niada, S.; Bergamaschi, R.F.; Orioli, M.; Brini, A.T. Bioactive Lipids in MSCs Biology: State of the Art and Role in Inflammation. Int. J. Mol. Sci. 2021, 22, 1481. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Wang, Y.; Chen, Y.; Hu, Y.; Guo, C.; Yu, Z.; Xu, P.; Ding, Y.; Mi, Q.-S.; et al. Abnormal lipid metabolism in epidermal Langerhans cells mediates psoriasis-like dermatitis. JCI Insight 2022, 7, e150223. [Google Scholar] [CrossRef]
- Garcia, C.; Andersen, C.J.; Blesso, C.N. The Role of Lipids in the Regulation of Immune Responses. Nutrients 2023, 15, 3899. [Google Scholar] [CrossRef]
- Honda, T.; Kabashima, K.; Kunisawa, J. Exploring the roles of prostanoids, leukotriens, and dietary fatty acids in cutaneous inflammatory diseases: Insights from pharmacological and genetic approaches. Immunol. Rev. 2023, 317, 95–112. [Google Scholar] [CrossRef]
- Yoshida, M.; Shin, K.-O.; Muraoka, S.; Choi, Y.; Park, J.-H.; Park, S.-H.; Hwang, J.-T.; Park, K.; Uchida, Y. The Epidermal Environment’s Influence on the Dermal Environment in Response to External Stress. Skin Pharmacol. Physiol. 2023, 36, 149–159. [Google Scholar] [CrossRef]
- Nicolaou, A.; Kendall, A.C. Bioactive lipids in the skin barrier mediate its functionality in health and disease. Pharmacol. Ther. 2024, 260, 108681. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, J.; Narita, H.; Kondo, N.; Hotta, M.; Takagi, Y.; Masukawa, Y.; Kitahara, T.; Takema, Y.; Koyano, S.; Yamazaki, S.; et al. Changes in the ceramide profile of atopic dermatitis patients. J. Investig. Dermatol. 2010, 130, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.-M.; Hwang, J.-H.; Bae, S.; Nahm, D.-H.; Park, H.-S.; Ye, Y.-M.; Lim, K.-M. Relationship of ceramide-, and free fatty acid-cholesterol ratios in the stratum corneum with skin barrier function of normal, atopic dermatitis lesional and non-lesional skins. J. Dermatol. Sci. 2015, 77, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef]
- Boiten, W.; van Smeden, J.; Bouwstra, J. The Cornified Envelope-Bound Ceramide Fraction Is Altered in Patients with Atopic Dermatitis. J. Investig. Dermatol. 2020, 140, 1097–1100.e4. [Google Scholar] [CrossRef]
- Uchino, T.; Kamiya, D.; Yagi, H.; Fujino-Shimaya, H.; Hatta, I.; Fujimori, S.; Miyazaki, Y.; Kirishita, Y.; Sano, Y.; Mizuno, H.; et al. Comparative analysis of intercellular lipid organization and composition between psoriatic and healthy stratum corneum. Chem. Phys. Lipids 2023, 254, 105305. [Google Scholar] [CrossRef]
- Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. The heterogeneity and complexity of skin surface lipids in human skin health and disease. Prog. Lipid Res. 2024, 93, 101264. [Google Scholar] [CrossRef]
- Rischke, S.; Schäfer, S.M.G.; König, A.; Ickelsheimer, T.; Köhm, M.; Hahnefeld, L.; Zaliani, A.; Scholich, K.; Pinter, A.; Geisslinger, G.; et al. Metabolomic and lipidomic fingerprints in inflammatory skin diseases—Systemic illumination of atopic dermatitis, hidradenitis suppurativa and plaque psoriasis. Clin. Immunol. 2024, 265, 110305. [Google Scholar] [CrossRef]
- Sanabria-de la Torre, R.; Montero-Vílchez, T.; García-Gavín, J.; Arias-Santiago, S. Current Insights on Lipidomics in Dermatology: A Systematic Review. J. Investig. Dermatol. 2025, 145, 1105–1116.e6. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Jetten, A.M.; Slominski, A.T. Neuro-Immuno-Endocrinology of the Skin: How Environment Regulates Body Homeostasis. Nat. Rev. Endocrinol. 2025, 21, 495–509. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-Neuro-Immuno-Endocrinology: How the Ultraviolet Radiation Regulates the Body, Brain, and Immune System. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef]
- Jablonski, N.G. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021, 34, 707–729. [Google Scholar] [CrossRef] [PubMed]
- Snyman, M.; Walsdorf, R.E.; Wix, S.N.; Gill, J.G. The metabolism of melanin synthesis—From melanocytes to melanoma. Pigment Cell Melanoma Res. 2024, 37, 438–452. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Potterf, S.B.; Hearing, V.J. Tyrosine transport into melanosomes is increased following stimulation of melanocyte differentiation. Biochem. Biophys. Res. Commun. 1998, 248, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Gaudel, C.; Soysouvanh, F.; Leclerc, J.; Bille, K.; Husser, C.; Montcriol, F.; Bertolotto, C.; Ballotti, R. Regulation of Melanogenesis by the Amino Acid Transporter SLC7A5. J. Investig. Dermatol. 2020, 140, 2253–2259.e4. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Becker, S.W.; Lerner, A.B.; Montgomery, H. Tyrosinase in Human Skin: Demonstration of Its Presence and of Its Role in Human Melanin Formation. Science 1950, 112, 223–225. [Google Scholar] [CrossRef]
- Körner, A.; Pawelek, J. Mammalian Tyrosinase Catalyzes Three Reactions in the Biosynthesis of Melanin. Science 1982, 217, 1163–1165. [Google Scholar] [CrossRef]
- Halaban, R.; Pomerantz, S.H.; Marshall, S.; Lambert, D.T.; Lerner, A.B. Regulation of Tyrosinase in Human Melanocytes Grown in Culture. J. Cell. Biol. 1983, 97, 480–488. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wazir, U.; Kothari, S.; Gibbons, N.C.J.; Moore, J.; Wood, J.M. Human phenylalanine hydroxylase is activated by H2O2: A novel mechanism for increasing the L-tyrosine supply for melanogenesis in melanocytes. Biochem. Biophys. Res. Commun. 2004, 322, 88–92. [Google Scholar] [CrossRef]
- Kim, H.-E.; Lee, S.-G. Induction of ATP synthase β by H2O2 induces melanogenesis by activating PAH and cAMP/CREB/MITF signaling in melanoma cells. Int. J. Biochem. Cell Biol. 2013, 45, 1217–1222. [Google Scholar] [CrossRef]
- Jimbow, K.; Park, J.S.; Kato, F.; Hirosaki, K.; Toyofuku, K.; Hua, C.; Yamashita, T. Assembly, target-signaling and intracellular transport of tyrosinase gene family proteins in the initial stage of melanosome biogenesis. Pigment Cell Res. 2000, 13, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Land, E.J.; Ito, S.; Wakamatsu, K.; Riley, P.A. Rate constants for the first two chemical steps of eumelanogenesis. Pigment Cell Res. 2003, 16, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Ishiguro, I. Chemical characterization of pheomelanogenesis starting from dihydroxyphenylalanine or tyrosine and cysteine: Effects of tyrosinase and cysteine concentrations and reaction time. Biochim. Biophys. Acta 1997, 1336, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.; Li, W.; Lamoreux, M.L.; Ito, S.; Wakamatsu, K.; Sviderskaya, E.V.; Bennett, D.C.; Park, Y.-M.; Gahl, W.A.; Huizing, M.; et al. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc. Natl. Acad. Sci. USA 2005, 102, 10964–10969. [Google Scholar] [CrossRef]
- Adelmann, C.H.; Traunbauer, A.K.; Chen, B.; Condon, K.J.; Chan, S.H.; Kunchok, T.; Lewis, C.A.; Sabatini, D.M. MFSD12 mediates the import of cysteine into melanosomes and lysosomes. Nature 2020, 588, 699–704. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Nagao, A.; Watanabe, M.; Nakao, K.; Ito, S. Pheomelanogenesis is promoted at a weakly acidic pH. Pigment Cell Melanoma Res. 2017, 30, 372–377. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-Tyrosine and L-Dihydroxyphenylalanine as Hormone-like Regulators of Melanocyte Functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes--vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, L.; Gao, H.; Chang, L.; Yu, X.; Zhu, Z.; He, X.; Geng, J.; Dong, Y.; Li, H.; et al. Exosomal miRNA derived from keratinocytes regulates pigmentation in melanocytes. J. Dermatol. Sci. 2019, 93, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Poy, M.N.; Stoffel, M.; Fuchs, E. A skin microRNA promotes differentiation by repressing “stemness”. Nature 2008, 452, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Dynoodt, P.; Mestdagh, P.; Van Peer, G.; Vandesompele, J.; Goossens, K.; Peelman, L.J.; Geusens, B.; Speeckaert, R.M.; Lambert, J.L.W.; Van Gele, M.J.L. Identification of miR-145 as a key regulator of the pigmentary process. J. Investig. Dermatol. 2013, 133, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin Releasing Hormone and Proopiomelanocortin Involvement in the Cutaneous Response to Stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key Role of CRF in the Skin Stress Response System. Endocr Rev 2013, 34, 827–884. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [CrossRef]
- Berson, J.F.; Harper, D.C.; Tenza, D.; Raposo, G.; Marks, M.S. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Biol. Cell 2001, 12, 3451–3464. [Google Scholar] [CrossRef]
- Goding, C.R.; Arnheiter, H. MITF-the first 25 years. Genes Dev. 2019, 33, 983–1007. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Funasaka, Y.; Slominski, A.; Ermak, G.; Hwang, J.; Pawelek, J.M.; Ichihashi, M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: Regulation by ultraviolet B. Biochim. Biophys. Acta 1996, 1313, 130–138. [Google Scholar] [CrossRef]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.P.; Ballotti, R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef]
- Rousseau, K.; Kauser, S.; Pritchard, L.E.; Warhurst, A.; Oliver, R.L.; Slominski, A.; Wei, E.T.; Thody, A.J.; Tobin, D.J.; White, A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007, 21, 1844–1856. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.; Suzuki, I.; Tada, A.; Im, S.; Akcali, C. The melanocortin-1 receptor and human pigmentation. Ann. N. Y. Acad. Sci. 1999, 885, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, E.; Wakamatsu, K.; Ito, S.; Wolber, R.; Hearing, V.J. Regulation of eumelanin/pheomelanin synthesis and visible pigmentation in melanocytes by ligands of the melanocortin 1 receptor. Pigment Cell Melanoma Res. 2008, 21, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.A.; Knittel, J.; Kadekaro, A.L.; Swope, V.B.; Starner, R. The melanocortin 1 receptor and the UV response of human melanocytes--a shift in paradigm. Photochem. Photobiol. 2008, 84, 501–508. [Google Scholar] [CrossRef]
- Dessinioti, C.; Antoniou, C.; Katsambas, A.; Stratigos, A.J. Melanocortin 1 receptor variants: Functional role and pigmentary associations. Photochem. Photobiol. 2011, 87, 978–987. [Google Scholar] [CrossRef]
- García-Borrón, J.C.; Abdel-Malek, Z.; Jiménez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef]
- Nasti, T.H.; Timares, L. Invited Review MC1R, Eumelanin and Pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, T. Role of Dermal Factors Involved in Regulating the Melanin and Melanogenesis of Mammalian Melanocytes in Normal and Abnormal Skin. Int. J. Mol. Sci. 2024, 25, 4560. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef]
- Ando, H.; Watabe, H.; Valencia, J.C.; Yasumoto, K.; Furumura, M.; Funasaka, Y.; Oka, M.; Ichihashi, M.; Hearing, V.J. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: A new aspect of ubiquitin-proteasome function. J. Biol. Chem. 2004, 279, 15427–15433. [Google Scholar] [CrossRef]
- Yamada, H.; Hakozaki, M.; Uemura, A.; Yamashita, T. Effect of fatty acids on melanogenesis and tumor cell growth in melanoma cells. J. Lipid Res. 2019, 60, 1491–1502. [Google Scholar] [CrossRef]
- Moon, K.M.; Lee, M.-K.; Park, S.-Y.; Seo, J.; Kim, A.-R.; Lee, B. Docosatrienoic Acid Inhibits Melanogenesis Partly through Suppressing the Intracellular MITF/Tyrosinase Axis. Pharmacy 2024, 17, 1198. [Google Scholar] [CrossRef] [PubMed]
- Sultan, F.; Basu, R.; Murthy, D.; Kochar, M.; Attri, K.S.; Aggarwal, A.; Kumari, P.; Dnyane, P.; Tanwar, J.; Motiani, R.K.; et al. Temporal analysis of melanogenesis identifies fatty acid metabolism as key skin pigment regulator. PLoS Biol. 2022, 20, e3001634. [Google Scholar] [CrossRef] [PubMed]
- Schuler, G.; Hönigsmann, H.; Jaschke, E.; Wolff, K. Selective accumulation of lipid within melanocytes during photochemotherapy (PUVA) of psoriasis. Br. J. Dermatol. 1982, 107, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Meira, W.V.; Heinrich, T.A.; Cadena, S.M.S.C.; Martinez, G.R. Melanogenesis inhibits respiration in B16-F10 melanoma cells whereas enhances mitochondrial cell content. Exp. Cell Res. 2017, 350, 62–72. [Google Scholar] [CrossRef]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Untargeted lipidomic analysis of primary human epidermal melanocytes acutely and chronically exposed to UV radiation. Mol. Omics 2018, 14, 170–180. [Google Scholar] [CrossRef]
- Łuczaj, W.; Dobrzyńska, I.; Skrzydlewska, E. Differences in the phospholipid profile of melanocytes and melanoma cells irradiated with UVA and treated with cannabigerol and cannabidiol. Sci. Rep. 2023, 13, 16121. [Google Scholar] [CrossRef]
- Yuri, M.; Sasahira, T.; Nakai, K.; Ishimaru, S.; Ohmori, H.; Kuniyasu, H. Reversal of expression of 15-lipoxygenase-1 to cyclooxygenase-2 is associated with development of colonic cancer. Histopathology 2007, 51, 520–527. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Robbins, G.T.; Nie, D. PPAR gamma, bioactive lipids, and cancer progression. Front. Biosci. Landmark Ed. 2012, 17, 1816–1834. [Google Scholar] [CrossRef]
- Maresca, V.; Flori, E.; Camera, E.; Bellei, B.; Aspite, N.; Ludovici, M.; Catricalà, C.; Cardinali, G.; Picardo, M. Linking αMSH with PPARγ in B16-F10 melanoma. Pigment Cell Melanoma Res. 2013, 26, 113–127. [Google Scholar] [CrossRef]
- Flori, E.; Rosati, E.; Cardinali, G.; Kovacs, D.; Bellei, B.; Picardo, M.; Maresca, V. The α-melanocyte stimulating hormone/peroxisome proliferator activated receptor-γ pathway down-regulates proliferation in melanoma cell lines. J. Exp. Clin. Cancer Res. 2017, 36, 142. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Hasse, S.; Rokos, H.; Chavan, B.; Shalbaf, M.; Spencer, J.D.; Wood, J.M. Cholesterol regulates melanogenesis in human epidermal melanocytes and melanoma cells. Exp. Dermatol. 2009, 18, 680–688. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Jung, E.; Cho, J.Y.; Park, D. Artemisinic acid inhibits melanogenesis through downregulation of C/EBP α-dependent expression of HMG-CoA reductase gene. Food Chem. Toxicol. 2013, 51, 225–230. [Google Scholar] [CrossRef]
- Hall, A.M.; Krishnamoorthy, L.; Orlow, S.J. 25-Hydroxycholesterol Acts in the Golgi Compartment to Induce Degradation of Tyrosinase. Pigment Cell Melanoma Res. 2004, 17, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Janjetovic, Z.; Slominski, R.M.; Li, W.; Jetten, A.M.; Indra, A.K.; Mason, R.S.; Tuckey, R.C. Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin. J. Investig. Dermatol. 2024, 144, 2145–2161. [Google Scholar] [CrossRef] [PubMed]
- Starner, R.J.; McClelland, L.; Abdel-Malek, Z.; Fricke, A.; Scott, G. PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Exp. Dermatol. 2010, 19, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Shin, J.Y.; Kim, M.R.; Hann, S.-K.; Oh, S.H. siRNA-mediated knock-down of COX-2 in melanocytes suppresses melanogenesis. Exp. Dermatol. 2012, 21, 420–425. [Google Scholar] [CrossRef]
- Scott, G.; Leopardi, S.; Printup, S.; Malhi, N.; Seiberg, M.; Lapoint, R. Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: Analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2alpha on melanocyte dendricity. J. Investig. Dermatol. 2004, 122, 1214–1224. [Google Scholar] [CrossRef]
- Coleman, R.A.; Eglen, R.M.; Jones, R.L.; Narumiya, S.; Shimizu, T.; Smith, W.L.; Dahlén, S.E.; Drazen, J.M.; Gardiner, P.J.; Jackson, W.T. Prostanoid and leukotriene receptors: A progress report from the IUPHAR working parties on classification and nomenclature. Adv. Prostaglandin Thromboxane Leukot. Res. 1995, 23, 283–285. [Google Scholar]
- Gledhill, K.; Rhodes, L.E.; Brownrigg, M.; Haylett, A.K.; Masoodi, M.; Thody, A.J.; Nicolaou, A.; Tobin, D.J. Prostaglandin-E2 is produced by adult human epidermal melanocytes in response to UVB in a melanogenesis-independent manner. Pigment Cell Melanoma Res. 2010, 23, 394–403. [Google Scholar] [CrossRef]
- Upadhyay, P.R.; Ho, T.; Abdel-Malek, Z.A. Participation of keratinocyte-and fibroblast-derived factors in melanocyte homeostasis, the response to UV, and pigmentary disorders. Pigment Cell Melanoma Res. 2021, 34, 762–776. [Google Scholar] [CrossRef]
- Hannun, Y.A. The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 1994, 269, 3125–3128. [Google Scholar] [CrossRef]
- Mao, C.; Obeid, L.M. Ceramidases: Regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim. Biophys. Acta 2008, 1781, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Nardo, A.D.; Collins, V.; Elias, P.M.; Holleran, W.M. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J. Investig. Dermatol. 2003, 120, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Schwudke, D.; Meurer, M.; Lehmann, B.; Shevchenko, A. Lipidome of narrow-band ultraviolet B irradiated keratinocytes shows apoptotic hallmarks. Exp. Dermatol. 2010, 19, e103–e110. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.L.; Zaman, R.; Rehman, N.; Tan, C.K. Ceramides and Skin Health: New Insights. Exp. Dermatol. 2025, 34, e70042. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.Y.; Moon, S.J.; Chung, J.H.; Kim, K.H.; Cho, K.H.; Park, K.C. Ceramide inhibits cell proliferation through Akt/PKB inactivation and decreases melanin synthesis in Mel-Ab cells. Pigment Cell Res. 2001, 14, 110–115. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, S.-Y.; Chung, J.-H.; Kim, K.-H.; Eun, H.-C.; Park, K.-C. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell. Signal. 2002, 14, 779–785. [Google Scholar] [CrossRef]
- Tokudome, Y.; Fukutomi, M. Sphingomyelin reduces melanogenesis in murine B16 melanoma cells through indirect suppression of tyrosinase. Cytotechnology 2023, 75, 93–101. [Google Scholar] [CrossRef]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta 2006, 1758, 2016–2026. [Google Scholar] [CrossRef]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, E.-H.; Lee, S.-Y.; Lee, Y.; Shin, K.-O.; Park, K.; Kang, I.-J. Morus alba L. root decreases melanin synthesis via sphingosine-1-phosphate signaling in B16F10 cells. J. Ethnopharmacol. 2023, 301, 115848. [Google Scholar] [CrossRef]
- Kim, D.-S.; Park, S.-H.; Jeong, Y.-M.; Kwon, S.-B.; Miller, A.J.; Fisher, D.E.; Park, K.-C. Sphingosine-1-phosphate decreases melanin synthesis via microphthalmia-associated transcription factor phosphorylation through the S1P3 receptor subtype. J. Pharm. Pharmacol. 2011, 63, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.O.; Mechoulam, R. Novel natural and synthetic ligands of the endocannabinoid system. Curr. Med. Chem. 2010, 17, 1341–1359. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; De Petrocellis, L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010, 17, 1430–1449. [Google Scholar] [CrossRef]
- Labar, G.; Michaux, C. Fatty acid amide hydrolase: From characterization to therapeutics. Chem. Biodivers. 2007, 4, 1882–1902. [Google Scholar] [CrossRef]
- Fezza, F.; De Simone, C.; Amadio, D.; Maccarrone, M. Fatty acid amide hydrolase: A gate-keeper of the endocannabinoid system. Subcell. Biochem. 2008, 49, 101–132. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 1–24. [Google Scholar] [CrossRef]
- Petrosino, S.; Ligresti, A.; Di Marzo, V. Endocannabinoid chemical biology: A tool for the development of novel therapies. Curr. Opin. Chem. Biol. 2009, 13, 309–320. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Haskó, G.; Paus, R.; Pacher, P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agrò, A.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef]
- Garate, J.; Lage, S.; Fernández, R.; Velasco, V.; Abad, B.; Asumendi, A.; Gardeazabal, J.; Arroyo-Berdugo, Y.; Rodríguez, M.Á.; Artola, J.L.; et al. Imaging Mass Spectrometry-Based Lipidomic Approach to Classification of Architectural Features in Nevi. J. Investig. Dermatol. 2019, 139, 2055–2058.e7. [Google Scholar] [CrossRef]
- Qi, K.; Lv, Y.; Ren, Y.; Wang, X.; Wu, L.; Wang, J.; Zhang, X.; He, Y.; Zhang, C.; Liu, C.; et al. Cholesterol was identified as a biomarker in human melanocytic nevi using DESI and DESI/PI mass spectrometry imaging. Talanta 2021, 231, 122380. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin Pigmentation Types, Causes and Treatment—A Review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef] [PubMed]
- Chaowattanapanit, S.; Silpa-Archa, N.; Kohli, I.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J. Am. Acad. Dermatol. 2017, 77, 607–621. [Google Scholar] [CrossRef]
- Choi, W.; Yin, L.; Smuda, C.; Batzer, J.; Hearing, V.J.; Kolbe, L. Molecular and histological characterization of age spots. Exp. Dermatol. 2017, 26, 242–248. [Google Scholar] [CrossRef]
- Baxter, L.L.; Pavan, W.J. The etiology and molecular genetics of human pigmentation disorders. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 379–392. [Google Scholar] [CrossRef]
- Plensdorf, S.; Livieratos, M.; Dada, N. Pigmentation Disorders: Diagnosis and Management. Am. Fam. Physician 2017, 96, 797–804. [Google Scholar]
- Meunier, M.; Bracq, M.; Chapuis, E.; Lapierre, L.; Humeau, A.; Bernard, S.; Lambert, C.; Paulus, C.; Auriol, P.; Lemagnen, P.; et al. Targeting SDF-1 as an efficient strategy to resolve skin hyperpigmentation issues with Himanthalia elongata extract. J. Cosmet. Dermatol. 2023, 22, 383–394. [Google Scholar] [CrossRef]
- Vashi, N.A.; de Castro Maymone, M.B.; Kundu, R.V. Aging Differences in Ethnic Skin. J. Clin. Aesthetic Dermatol. 2016, 9, 31–38. [Google Scholar]
- Markiewicz, E.; Karaman-Jurukovska, N.; Mammone, T.; Idowu, O.C. Post-Inflammatory Hyperpigmentation in Dark Skin: Molecular Mechanism and Skincare Implications. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2555–2565. [Google Scholar] [CrossRef]
- Kaufman, B.P.; Aman, T.; Alexis, A.F. Postinflammatory Hyperpigmentation: Epidemiology, Clinical Presentation, Pathogenesis and Treatment. Am. J. Clin. Dermatol. 2018, 19, 489–503. [Google Scholar] [CrossRef]
- Kashetsky, N.; Feschuk, A.; Pratt, M.E. Post-inflammatory hyperpigmentation: A systematic review of treatment outcomes. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 470–479. [Google Scholar] [CrossRef]
- Tomita, Y.; Maeda, K.; Tagami, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment Cell Res. 1992, 5, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Park, T.J.; Kang, H.Y. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, T.J.; Kweon, Y.Y.; Baek, D.J.; Lee, J.W.; Kang, H.Y. Age-Dependent Sequential Increase of Senescent Cells in the Skin. J. Investig. Dermatol. 2022, 142, 2521–2523.e1. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. BioFactors 2009, 35, 193–199. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef]
- Murakami, M.; Kambe-Ohkura, T.; Kudo, I. Functional coupling between phospholipase A2S and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. Adv. Exp. Med. Biol. 2002, 507, 15–19. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, H.; Iraha, R.; Toriyama, M. Down-regulation of tyrosinase expression by acetylsalicylic acid in murine B16 melanoma. Biol. Pharm. Bull. 2008, 31, 33–37. [Google Scholar] [CrossRef]
- Gravitz, L. Chemoprevention: First line of defence. Nature 2011, 471, S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Roumie, C.L.; Choma, N.N.; Kaltenbach, L.; Mitchel, E.F.; Arbogast, P.G.; Griffin, M.R. Non-aspirin NSAIDs, cyclooxygenase-2 inhibitors and risk for cardiovascular events-stroke, acute myocardial infarction, and death from coronary heart disease. Pharmacoepidemiol. Drug Saf. 2009, 18, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Van Gele, M.; Geusens, B.; Speeckaert, R.; Dynoodt, P.; Vanhoecke, B.; Van Den Bossche, K.; Lambert, J. Development of a 3D pigmented skin model to evaluate RNAi-induced depigmentation. Exp. Dermatol. 2011, 20, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Fogh, K.; Herlin, T.; Kragballe, K. Eicosanoids in skin of patients with atopic dermatitis: Prostaglandin E2 and leukotriene B4 are present in biologically active concentrations. J. Allergy Clin. Immunol. 1989, 83, 450–455. [Google Scholar] [CrossRef]
- Brain, S.; Camp, R.; Dowd, P.; Black, A.K.; Greaves, M. The release of leukotriene B4-like material in biologically active amounts from the lesional skin of patients with psoriasis. J. Investig. Dermatol. 1984, 83, 70–73. [Google Scholar] [CrossRef]
- Hammarström, S.; Hamberg, M.; Samuelsson, B.; Duell, E.A.; Stawiski, M.; Voorhees, J.J. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc. Natl. Acad. Sci. USA 1975, 72, 5130–5134. [Google Scholar] [CrossRef]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jastrząb, A.; Dobrzyńska, M.; Biernacki, M.; Skrzydlewska, E. Exogenous Antioxidants Impact on UV-Induced Changes in Membrane Phospholipids and the Effectiveness of the Endocannabinoid System in Human Skin Cells. Antioxidants 2021, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-F.; Ma, K.-H.; Jhap, T.-Y.; Liu, P.-S.; Chueh, S.-H. Ultraviolet B irradiation induced Nrf2 degradation occurs via activation of TRPV1 channels in human dermal fibroblasts. Free Radic. Biol. Med. 2019, 141, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.-W.; Kim, Y.; Kang, J.-H.; Kang, S.-S.; Ahn, Y.K.; Park, C.-S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Yonei, N.; Kaminaka, C.; Kimura, A.; Furukawa, F.; Yamamoto, Y. Two patterns of solar lentigines: A histopathological analysis of 40 Japanese women. J. Dermatol. 2012, 39, 829–832. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.H.; Chung, B.Y.; Kim, J.E.; Shin, J.H.; Oh, S.H.; Choi, M.; Lee, S.H.; Kim, Y.C.; Ryu, H.J. Can we predict postinflammatory hyperpigmentation after laser treatment based on dermoscopic findings of solar lentigo? Lasers Med. Sci. 2023, 38, 130. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, W.; Kim, Y.-A.; Lee, Y.-J.; Kim, S.; Shin, J.; Choi, Y.; Kim, J.; Lee, Y.; Kim, M.S.; et al. Multi-System-Level Analysis Reveals Differential Expression of Stress Response-Associated Genes in Inflammatory Solar Lentigo. Int. J. Mol. Sci. 2024, 25, 3973. [Google Scholar] [CrossRef]
- Lin, C.B.; Hu, Y.; Rossetti, D.; Chen, N.; David, C.; Slominski, A.; Seiberg, M. Immuno-histochemical evaluation of solar lentigines: The association of KGF/KGFR and other factors with lesion development. J. Dermatol. Sci. 2010, 59, 91–97. [Google Scholar] [CrossRef]
- Aoki, H.; Moro, O.; Tagami, H.; Kishimoto, J. Gene expression profiling analysis of solar lentigo in relation to immunohistochemical characteristics. Br. J. Dermatol. 2007, 156, 1214–1223. [Google Scholar] [CrossRef]
- Shin, J.; Park, J.-Y.; Kim, S.J.; Kang, H.Y. Characteristics of keratinocytes in facial solar lentigo with flattened rete ridges: Comparison with melasma. Clin. Exp. Dermatol. 2015, 40, 489–494. [Google Scholar] [CrossRef]
- Lee, A.-Y. Skin Pigmentation Abnormalities and Their Possible Relationship with Skin Aging. Int. J. Mol. Sci. 2021, 22, 3727. [Google Scholar] [CrossRef]
- Yoon, J.E.; Kim, Y.; Kwon, S.; Kim, M.; Kim, Y.H.; Kim, J.-H.; Park, T.J.; Kang, H.Y. Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics 2018, 8, 4620–4632. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, B.; Kim, J.C.; Park, T.J.; Kang, H.Y. Senescent Fibroblast-Derived GDF15 Induces Skin Pigmentation. J. Investig. Dermatol. 2020, 140, 2478–2486.e4. [Google Scholar] [CrossRef]
- Aoki, H.; Moro, O. Upregulation of the IFN-gamma-stimulated genes in the development of delayed pigmented spots on the dorsal skin of F1 mice of HR-1 x HR/De. J. Investig. Dermatol. 2005, 124, 1053–1061. [Google Scholar] [CrossRef]
- Fronza, M.; Caetano, G.F.; Leite, M.N.; Bitencourt, C.S.; Paula-Silva, F.W.G.; Andrade, T.A.M.; Frade, M.A.C.; Merfort, I.; Faccioli, L.H. Hyaluronidase modulates inflammatory response and accelerates the cutaneous wound healing. PLoS ONE 2014, 9, e112297. [Google Scholar] [CrossRef] [PubMed]
- Cario, M. How hormones may modulate human skin pigmentation in melasma: An in vitro perspective. Exp. Dermatol. 2019, 28, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.C.; Lima, P.B.; Tonolli, V.M.; Miot, L.D.B.; Miot, H.A. Risk factors for facial melasma in women: A case-control study. Br. J. Dermatol. 2014, 171, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Na, J.-I.; Choi, J.-Y.; Park, K.-C. Melasma: Updates and perspectives. Exp. Dermatol. 2019, 28, 704–708. [Google Scholar] [CrossRef]
- Passeron, T.; Picardo, M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018, 31, 461–465. [Google Scholar] [CrossRef]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma-Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Hwang, Y.-J.; Lee, S.-K.; Park, K.-C. Heterogeneous Pathology of Melasma and Its Clinical Implications. Int. J. Mol. Sci. 2016, 17, 824. [Google Scholar] [CrossRef]
- Hernández-Barrera, R.; Torres-Alvarez, B.; Castanedo-Cazares, J.P.; Oros-Ovalle, C.; Moncada, B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin. Exp. Dermatol. 2008, 33, 305–308. [Google Scholar] [CrossRef]
- Torres-Álvarez, B.; Mesa-Garza, I.G.; Castanedo-Cázares, J.P.; Fuentes-Ahumada, C.; Oros-Ovalle, C.; Navarrete-Solis, J.; Moncada, B. Histochemical and immunohistochemical study in melasma: Evidence of damage in the basal membrane. Am. J. Dermatopathol. 2011, 33, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Boukari, F.; Jourdan, E.; Fontas, E.; Montaudié, H.; Castela, E.; Lacour, J.-P.; Passeron, T. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: A prospective randomized comparative trial. J. Am. Acad. Dermatol. 2015, 72, 189–190.e1. [Google Scholar] [CrossRef] [PubMed]
- Duteil, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.-P.; Passeron, T. Differences in visible light-induced pigmentation according to wavelengths: A clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, J.; Lu, J. Mechanisms of ultraviolet-induced melasma formation: A review. J. Dermatol. 2022, 49, 1201–1210. [Google Scholar] [CrossRef]
- Byun, J.W.; Park, I.S.; Choi, G.S.; Shin, J. Role of fibroblast-derived factors in the pathogenesis of melasma. Clin. Exp. Dermatol. 2016, 41, 601–609. [Google Scholar] [CrossRef]
- Hasegawa, K.; Fujiwara, R.; Sato, K.; Shin, J.; Kim, S.J.; Kim, M.; Kang, H.Y. Possible Involvement of Keratinocyte Growth Factor in the Persistence of Hyperpigmentation in both Human Facial Solar Lentigines and Melasma. Ann. Dermatol. 2015, 27, 626–629. [Google Scholar] [CrossRef]
- Kapoor, R.; Dhatwalia, S.K.; Kumar, R.; Rani, S.; Parsad, D. Emerging role of dermal compartment in skin pigmentation: Comprehensive review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2757–2765. [Google Scholar] [CrossRef]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.-Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Dermatol. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.-P. Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J. Investig. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef]
- Rodríguez-Arámbula, A.; Torres-Álvarez, B.; Cortés-García, D.; Fuentes-Ahumada, C.; Castanedo-Cázares, J.P. CD4, IL-17, and COX-2 Are Associated With Subclinical Inflammation in Malar Melasma. Am. J. Dermatopathol. 2015, 37, 761–766. [Google Scholar] [CrossRef]
- Chung, B.Y.; Noh, T.K.; Yang, S.H.; Kim, I.H.; Lee, M.W.; Yoon, T.J.; Chang, S.E. Gene expression profiling in melasma in Korean women. Dermatology 2014, 229, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, H.; Luo, H.; Hu, Y.; Chen, Y.; Xie, B.; Du, X.; Hua, Y.; Song, X. Tape stripping and lipidomics reveal skin surface lipid abnormity in female melasma. Pigment Cell Melanoma Res. 2021, 34, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Janssens, M.; van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, J.; Song, X.; Xiang, W. Comparative study of melasma in patients before and after treatment based on lipomics. Lipids Health Dis. 2024, 23, 138. [Google Scholar] [CrossRef]
- Clayton, R.W.; Langan, E.A.; Ansell, D.M.; de Vos, I.J.H.M.; Göbel, K.; Schneider, M.R.; Picardo, M.; Lim, X.; van Steensel, M.A.M.; Paus, R. Neuroendocrinology and neurobiology of sebaceous glands. Biol. Rev. Camb. Philos. Soc. 2020, 95, 592–624. [Google Scholar] [CrossRef]
- Picardo, M.; Mastrofrancesco, A.; Bíró, T. Sebaceous gland-a major player in skin homoeostasis. Exp. Dermatol. 2015, 24, 485–486. [Google Scholar] [CrossRef]
- Szöllősi, A.G.; Oláh, A.; Bíró, T.; Tóth, B.I. Recent advances in the endocrinology of the sebaceous gland. Derm.-Endocrinol. 2017, 9, e1361576. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Bolognia, J.L.; Pawelek, J.M. Biology of hypopigmentation. J. Am. Acad. Dermatol. 1988, 19, 217–255. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naser, M.B.; Seltmann, H.; Zouboulis, C.C. SZ95 sebocytes induce epidermal melanocyte dendricity and proliferation in vitro. Exp. Dermatol. 2012, 21, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Zompetta, C.; De Luca, C.; Cirone, M.; Faggioni, A.; Nazzaro-Porro, M.; Passi, S.; Prota, G. Role of skin surface lipids in UV-induced epidermal cell changes. Arch. Dermatol. Res. 1991, 283, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Foolad, N.; Shi, V.Y.; Prakash, N.; Kamangar, F.; Sivamani, R.K. The association of the sebum excretion rate with melasma, erythematotelangiectatic rosacea, and rhytides. Dermatol. Online J. 2015, 21, 2. [Google Scholar] [CrossRef]
- Flori, E.; Mastrofrancesco, A.; Mosca, S.; Ottaviani, M.; Briganti, S.; Cardinali, G.; Filoni, A.; Cameli, N.; Zaccarini, M.; Zouboulis, C.C.; et al. Sebocytes contribute to melasma onset. iScience 2022, 25, 103871. [Google Scholar] [CrossRef]
- Dozsa, A.; Dezso, B.; Toth, B.I.; Bacsi, A.; Poliska, S.; Camera, E.; Picardo, M.; Zouboulis, C.C.; Bíró, T.; Schmitz, G.; et al. PPARγ-mediated and arachidonic acid-dependent signaling is involved in differentiation and lipid production of human sebocytes. J. Investig. Dermatol. 2014, 134, 910–920. [Google Scholar] [CrossRef]
- Gruber, F.; Marchetti-Deschmann, M.; Kremslehner, C.; Schosserer, M. The Skin Epilipidome in Stress, Aging, and Inflammation. Front. Endocrinol. 2020, 11, 607076. [Google Scholar] [CrossRef]
- Kohli, J.; Wang, B.; Brandenburg, S.M.; Basisty, N.; Evangelou, K.; Varela-Eirin, M.; Campisi, J.; Schilling, B.; Gorgoulis, V.; Demaria, M. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat. Protoc. 2021, 16, 2471–2498. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Narzt, M.-S.; Pils, V.; Kremslehner, C.; Nagelreiter, I.-M.; Schosserer, M.; Bessonova, E.; Bayer, A.; Reifschneider, R.; Terlecki-Zaniewicz, L.; Waidhofer-Söllner, P.; et al. Epilipidomics of Senescent Dermal Fibroblasts Identify Lysophosphatidylcholines as Pleiotropic Senescence-Associated Secretory Phenotype (SASP) Factors. J. Investig. Dermatol. 2021, 141, 993–1006.e15. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Xia, Y. New Mechanistic Insights of Melasma. Clin. Cosmet. Investig. Dermatol. 2023, 16, 429–442. [Google Scholar] [CrossRef]
- Gu, D.; Pan, R.; Meng, X.; Liu, T.; Zhong, H.; Chen, N.; Xu, Y. What lies behind melasma: A review of the related skin microenvironment. Int. J. Dermatol. 2025, 64, 256–265. [Google Scholar] [CrossRef]
- Gauthier, Y.; Cario, M.; Pain, C.; Lepreux, S.; Benzekri, L.; Taieb, A. Oestrogen associated with ultraviolet B irradiation recapitulates the specific melanosome distribution observed in caucasoid melasma. Br. J. Dermatol. 2019, 180, 951–953. [Google Scholar] [CrossRef]
- Kendall, A.C.; Pilkington, S.M.; Wray, J.R.; Newton, V.L.; Griffiths, C.E.M.; Bell, M.; Watson, R.E.B.; Nicolaou, A. Menopause induces changes to the stratum corneum ceramide profile, which are prevented by hormone replacement therapy. Sci. Rep. 2022, 12, 21715. [Google Scholar] [CrossRef]
- Speeckaert, R.; Caelenberg, E.V.; Belpaire, A.; Speeckaert, M.M.; Geel, N. van Vitiligo: From Pathogenesis to Treatment. J. Clin. Med. 2024, 13, 5225. [Google Scholar] [CrossRef]
- Ezzedine, K.; Tannous, R.; Pearson, T.F.; Harris, J.E. Recent clinical and mechanistic insights into vitiligo offer new treatment options for cell-specific autoimmunity. J. Clin. Investig. 2025, 135, e185785. [Google Scholar] [CrossRef]
- Bastonini, E.; Bellei, B.; Filoni, A.; Kovacs, D.; Iacovelli, P.; Picardo, M. Involvement of non-melanocytic skin cells in vitiligo. Exp. Dermatol. 2019, 28, 667–673. [Google Scholar] [CrossRef]
- Tulic, M.K.; Kovacs, D.; Bastonini, E.; Briganti, S.; Passeron, T.; Picardo, M. Focusing on the Dark Side of the Moon: Involvement of the Nonlesional Skin in Vitiligo. J. Investig. Dermatol. 2024, 145, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.L.; Ottaviani, M.; Albanesi, V.; Vidolin, A.P.; Leone, G.; Ferraro, C.; Cossarizza, A.; Rossi, L.; Picardo, M. Membrane lipid alterations as a possible basis for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2007, 127, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.L.; Ottaviani, M.; Bellei, B.; Albanesi, V.; Cossarizza, A.; Rossi, L.; Picardo, M. Membrane lipid defects are responsible for the generation of reactive oxygen species in peripheral blood mononuclear cells from vitiligo patients. J. Cell. Physiol. 2010, 223, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Lee, B.; Boniface, K.; Seneschal, J.; Sahoo, S.K.; Seki, T.; Wang, C.; Das, S.; Han, X.; Steppie, M.; et al. MicroRNA-211 Regulates Oxidative Phosphorylation and Energy Metabolism in Human Vitiligo. J. Investig. Dermatol. 2017, 137, 1965–1974. [Google Scholar] [CrossRef]
- Kovacs, D.; Bastonini, E.; Ottaviani, M.; Cota, C.; Migliano, E.; Dell’Anna, M.L.; Picardo, M. Vitiligo Skin: Exploring the Dermal Compartment. J. Investig. Dermatol. 2018, 138, 394–404. [Google Scholar] [CrossRef]
- Liang, L.; Li, Y.; Tian, X.; Zhou, J.; Zhong, L. Comprehensive lipidomic, metabolomic and proteomic profiling reveals the role of immune system in vitiligo. Clin. Exp. Dermatol. 2019, 44, e216–e223. [Google Scholar] [CrossRef]
- Kovacs, D.; Bastonini, E.; Briganti, S.; Ottaviani, M.; D’Arino, A.; Truglio, M.; Sciuto, L.; Zaccarini, M.; Pacifico, A.; Cota, C.; et al. Altered epidermal proliferation, differentiation, and lipid composition: Novel key elements in the vitiligo puzzle. Sci. Adv. 2022, 8, eabn9299. [Google Scholar] [CrossRef]
- Ni, Q.; Ye, Z.; Wang, Y.; Chen, J.; Zhang, W.; Ma, C.; Li, K.; Liu, Y.; Liu, L.; Han, Z.; et al. Gut Microbial Dysbiosis and Plasma Metabolic Profile in Individuals With Vitiligo. Front. Microbiol. 2020, 11, 592248. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Chen, J.; Du, P.; Ni, Q.; Li, B.; Zhang, Z.; Wang, Q.; Cui, T.; Yi, X.; Li, C.; et al. Metabolomics Signature and Potential Application of Serum Polyunsaturated Fatty Acids Metabolism in Patients With Vitiligo. Front. Immunol. 2022, 13, 839167. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, M.; Vasiljevic, D.; Vucic, V.; Arsic, A.; Petrovic, S.; Tomic-Lucic, A.; Savic, M.; Zivanovic, S.; Stojic, V.; Jakovljevic, V. Clinical Benefits of n-3 PUFA and ɤ-Linolenic Acid in Patients with Rheumatoid Arthritis. Nutrients 2017, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Arriens, C.; Hynan, L.S.; Lerman, R.H.; Karp, D.R.; Mohan, C. Placebo-controlled randomized clinical trial of fish oil’s impact on fatigue, quality of life, and disease activity in Systemic Lupus Erythematosus. Nutr. J. 2015, 14, 82. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Zhou, F.; Song, D.; Chang, L.; Liu, W.; Yan, G.; Zhang, G. From actinic keratosis to cutaneous squamous cell carcinoma: The key pathogenesis and treatments. Front. Immunol. 2025, 16, 1518633. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, Y.; Wang, Z.; Guo, S.; Qiu, S.; Zhang, A. Understanding the physiological mechanisms and therapeutic targets of diseases: Lipidomics strategies. Life Sci. 2025, 363, 123411. [Google Scholar] [CrossRef]
- Morin, S.; Simard, M.; Rioux, G.; Julien, P.; Pouliot, R. Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model. Cells 2022, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Ottaviani, M.; Truglio, M.; D’Arino, A.; Caputo, S.; Pacifico, A.; Iacovelli, P.; Di Nardo, A.; Picardo, M.; Bellei, B. Markers of Metabolic Abnormalities in Vitiligo Patients. Int. J. Mol. Sci. 2024, 25, 10201. [Google Scholar] [CrossRef] [PubMed]
- D’Arino, A.; Picardo, M.; Truglio, M.; Pacifico, A.; Iacovelli, P. Metabolic Comorbidities in Vitiligo: A Brief Review and Report of New Data from a Single-Center Experience. Int. J. Mol. Sci. 2021, 22, 8820. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Guo, F.; Zhang, M.; Wang, C.; Lin, N.; Liu, L.; Chen, Y.; Liu, F.; Du, Y.; Li, L.; et al. Risk factors for cardiovascular diseases in patients with vitiligo: An analysis of current evidence. Ann. Med. 2024, 56, 2326297. [Google Scholar] [CrossRef]
- Tanacan, E.; Atakan, N. Higher incidence of metabolic syndrome components in vitiligo patients: A prospective cross-sectional study. An. Bras. Dermatol. 2020, 95, 165–172. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, H.J.; Seo, J.M.; Almurayshid, A.; Kim, G.M.; Ezzedine, K.; Bae, J.M. Comorbidities in Patients with Vitiligo: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2023, 143, 777–789.e6. [Google Scholar] [CrossRef]
- Bathina, N.; Ganni, S.; Kolalapudi, S.A.; Konala, S.; Dharavath, K.; Reddy, M.T.K. Prevalence of Metabolic Syndrome in Vitiligo Patients and its Relation to Vitiligo Severity—A Cross-Sectional Study. Indian Dermatol. Online J. 2024, 15, 612–615. [Google Scholar] [CrossRef]
- Denisenko, Y.K.; Kytikova, O.Y.; Novgorodtseva, T.P.; Antonyuk, M.V.; Gvozdenko, T.A.; Kantur, T.A. Lipid-Induced Mechanisms of Metabolic Syndrome. J. Obes. 2020, 2020, 5762395. [Google Scholar] [CrossRef]
- An, L.; Kim, D.; Donahue, L.R.; Mejooli, M.A.; Eom, C.-Y.; Nishimura, N.; White, A.C. Sexual dimorphism in melanocyte stem cell behavior reveals combinational therapeutic strategies for cutaneous repigmentation. Nat. Commun. 2024, 15, 796. [Google Scholar] [CrossRef]
- Ismail, I.B.; Bhat, Y.J.; Ul Islam, M.S. Treatment Advances in Vitiligo: An Updated Review. Dermatol. Pract. Concept. 2025, 15, 4600. [Google Scholar] [CrossRef]
- Pourriyahi, H.; Hosseini, N.-S.; Nooshabadi, M.P.; Pourriahi, H.; Baradaran, H.R.; Abtahi-Naeini, B.; Goodarzi, A. Utility of prostaglandin analogues and phosphodiesterase inhibitors as promising last resorts for the treatment of vitiligo: A systematic review, from mechanisms of action to mono-, combination and comparative therapies. J. Cosmet. Dermatol. 2024, 23, 3466–3487. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guo, S.-P. Efficacy of local prostaglandin analogues for vitiligo treatment: A systematic review and meta-analysis. Expert. Rev. Clin. Pharmacol. 2022, 15, 341–349. [Google Scholar] [CrossRef]
- Anbar, T.S.; El-Ammawi, T.S.; Abdel-Rahman, A.T.; Hanna, M.R. The effect of latanoprost on vitiligo: A preliminary comparative study. Int. J. Dermatol. 2015, 54, 587–593. [Google Scholar] [CrossRef]
- Grimes, P.E. Bimatoprost 0.03% Solution for the Treatment of Nonfacial Vitiligo. J. Drugs Dermatol. 2016, 15, 703–710. [Google Scholar]
- Chang, Y.; Li, S.; Guo, W.; Yang, Y.; Zhang, W.; Zhang, Q.; He, Y.; Yi, X.; Cui, T.; An, Y.; et al. Simvastatin Protects Human Melanocytes from H2O2-Induced Oxidative Stress by Activating Nrf2. J. Investig. Dermatol. 2017, 137, 1286–1296. [Google Scholar] [CrossRef]
- Agarwal, P.; Rashighi, M.; Essien, K.I.; Richmond, J.M.; Randall, L.; Pazoki-Toroudi, H.; Hunter, C.A.; Harris, J.E. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J. Investig. Dermatol. 2015, 135, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Noël, M.; Gagné, C.; Bergeron, J.; Jobin, J.; Poirier, P. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zdravković, T.P.; Wang, T.; Liu, Y.; Jin, H. Efficacy and safety of oral simvastatin in the treatment of patients with vitiligo. J. Investig. Med. 2021, 69, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, A.; Winnicki, A.; Krysiński, J.; Niezgoda, P.; Nowowiejska, L.; Czajkowski, R. Topical application of simvastatin acid sodium salt and atorvastatin calcium salt in vitiligo patients. Results of the randomized, double-blind EVRAAS pilot study. Sci. Rep. 2024, 14, 14612. [Google Scholar] [CrossRef]
- Shaker, E.S.E.; Allam, S.H.; Mabrouk, M.M.; Elgharbawy, N.M.; Salaam, S.F.A. Simvastatin and non-segmental vitiligo: A new potential treatment option? Dermatol. Ther. 2022, 35, e15969. [Google Scholar] [CrossRef]
- Paganelli, A.; Papaccio, F.; Picardo, M.; Bellei, B. Metabolic anomalies in vitiligo: A new frontier for drug repurposing strategies. Front. Pharmacol. 2025, 16, 1546836. [Google Scholar] [CrossRef]
- Białczyk, A.; Kamińska, B.; Czajkowski, R. The association between metabolic syndrome and vitiligo: A systematic review. Postepy Dermatol. Alergol. 2025, 42, 134–142. [Google Scholar] [CrossRef]

| Lipid Category | Structure | Class and Subclasses | Physiological Effect on Skin Pigmentation | References |
|---|---|---|---|---|
| Fatty Acyls | 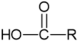 | R = Alkyl chain of 4 to 28 carbons (usually even), with varying numbers of double bonds: 0: Saturated fatty acid 1: Monounsaturated fatty acid (MUFA) 2: Polyunsaturated fatty acid (PUFA) | Unsaturated fatty acids -inhibition of melanin synthesis -regulation of tyrosinase degradation Saturated fatty acids -tyrosinase stabilization -promotion of melanosome maturation | [129,130,131,132] [130] |
| Glycerophospholipids | 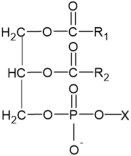 | X= Choline: Phosphatidylcholine (PC) Ethanolamine: Phosphatidylethanolamine (PE) Inositol: Phosphatidylinositol (PI) Serine: Phosphatidylserine (PS) | -involvement in metabolic changes associated with pigmentation | [136,137,138] |
| Glycerolipids | 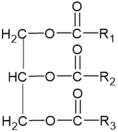 | N of esterified acids= 1: Monoacylglycerol (MG) 2: Diacylglycerol (DG) 3: Triacylglycerol (TG) | -involvement in metabolic changes associated with pigmentation | [132,133,134,135] |
| Sphingolipids | 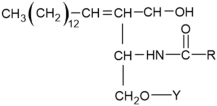 Sphingosine 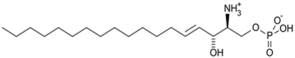 Sphingosine-1-P | Y= H: Ceramides Fatty acid: Sphingomyelin (SM) | -inhibition of pro-melanogenesis related signaling pathway -inhibition of tyrosinase activity | [158,159,160,161,162,163] |
| Sterols | 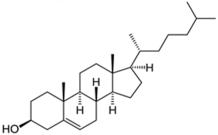 Cholesterol 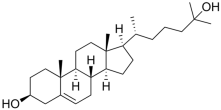 25-hydroxycholesterol | -induction of pro-melanogenesis related signaling pathway -inhibition of tyrosinase proteosomal degradation -induction of tyrosinase proteosomal degradation | [143,144] [145] | |
| Prostaglandins |  PGD2 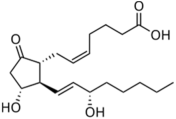 PGE2  PGF2α | -induction of pro-melanogenesis related signaling pathway -induction of melanin synthesis -promotion of dendricity | [147,148,149] | |
| Leukotrienes | 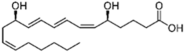 LTB4 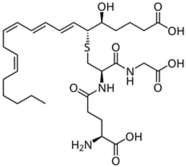 LTC4 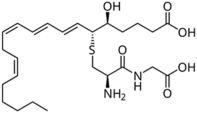 LTD4 | -induction of tyrosinase expression -promotion of dendricity | [152] | |
| Tromboxanes | 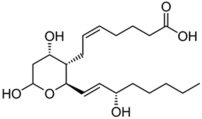 TBXB2 | -induction of tyrosinase expression -promotion of dendricity | [152] | |
| Endocannabinoids | 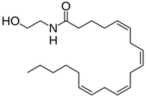 Anandamide or N-arachidonoylethanolamine (AEA) 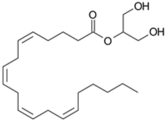 2-Arachidonoylglycerol (2-AG) | -induction of melanin synthesis -induction of pro-melanogenesis related signaling pathway | [172,173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastonini, E.; Kovacs, D.; Maresca, V.; Ottaviani, M.; Di Nardo, A.; Flori, E.; Cardinali, G.; Briganti, S. Lipidome Complexity in Physiological and Pathological Skin Pigmentation. Int. J. Mol. Sci. 2025, 26, 6785. https://doi.org/10.3390/ijms26146785
Bastonini E, Kovacs D, Maresca V, Ottaviani M, Di Nardo A, Flori E, Cardinali G, Briganti S. Lipidome Complexity in Physiological and Pathological Skin Pigmentation. International Journal of Molecular Sciences. 2025; 26(14):6785. https://doi.org/10.3390/ijms26146785
Chicago/Turabian StyleBastonini, Emanuela, Daniela Kovacs, Vittoria Maresca, Monica Ottaviani, Anna Di Nardo, Enrica Flori, Giorgia Cardinali, and Stefania Briganti. 2025. "Lipidome Complexity in Physiological and Pathological Skin Pigmentation" International Journal of Molecular Sciences 26, no. 14: 6785. https://doi.org/10.3390/ijms26146785
APA StyleBastonini, E., Kovacs, D., Maresca, V., Ottaviani, M., Di Nardo, A., Flori, E., Cardinali, G., & Briganti, S. (2025). Lipidome Complexity in Physiological and Pathological Skin Pigmentation. International Journal of Molecular Sciences, 26(14), 6785. https://doi.org/10.3390/ijms26146785






