In Vivo and In Vitro Experimental Study Comparing the Effect of a Combination of Sodium Dichloroacetate and Valproic Acid with That of Temozolomide on Adult Glioblastoma
Abstract
1. Introduction
2. Results
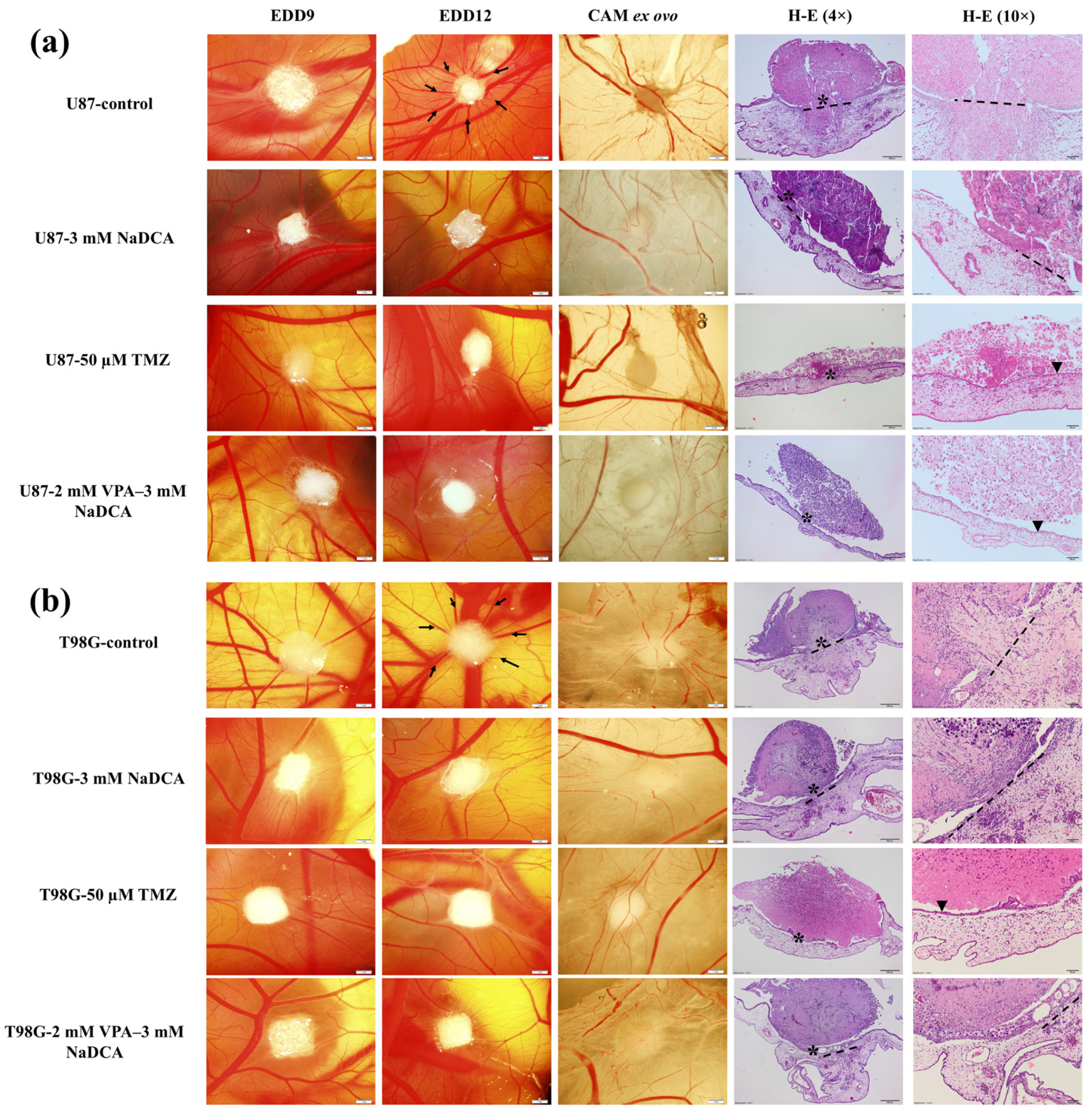
2.1. Stereomicroscopic and Histologic Images of U87 and T98G Tumors In Vivo on the CAM, the Tumor Ex Ovo, and H-E Histological Images
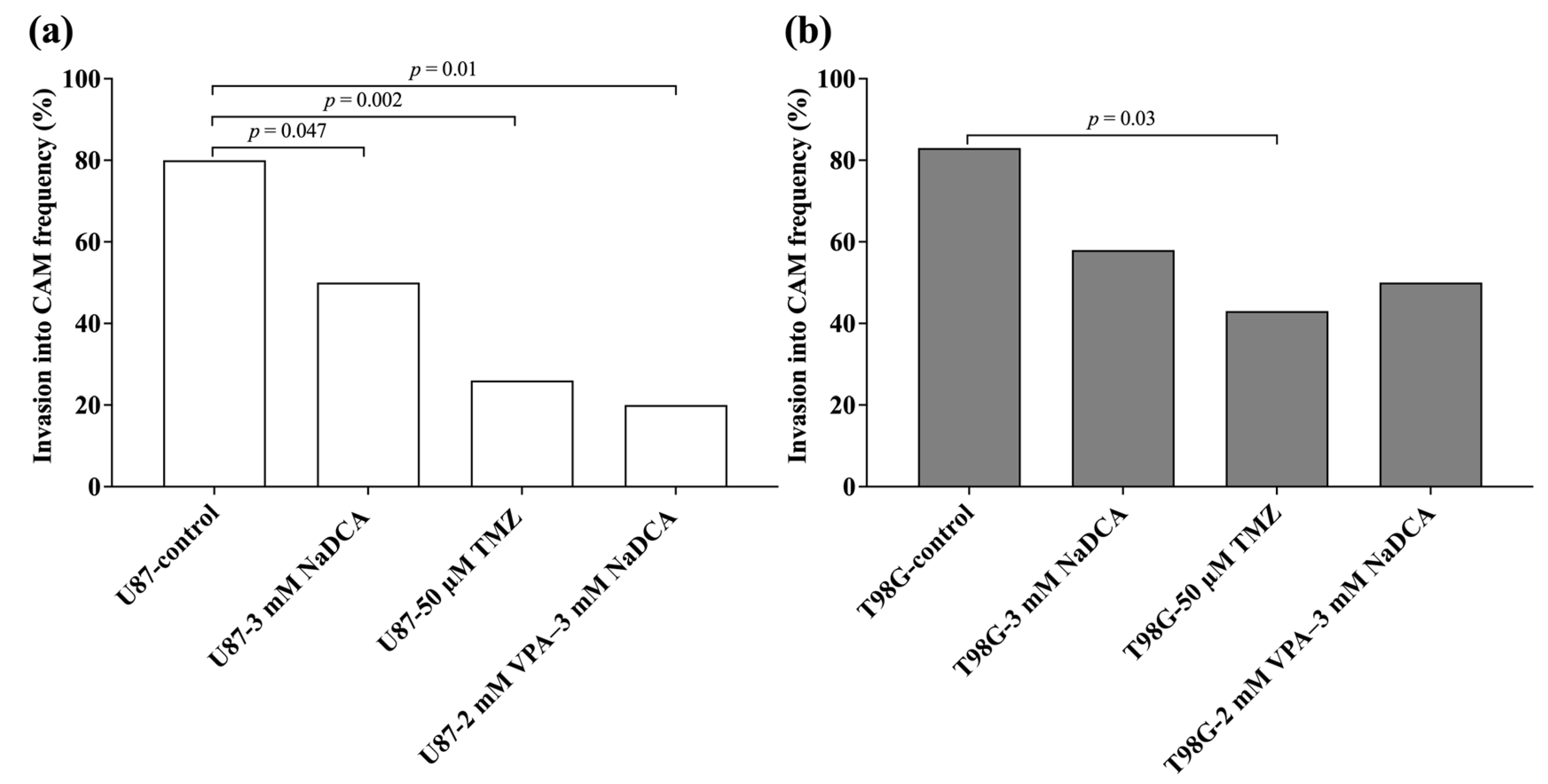
2.2. U87 and T98G Tumor Growth, Rate of Tumor Invasion into the CAM
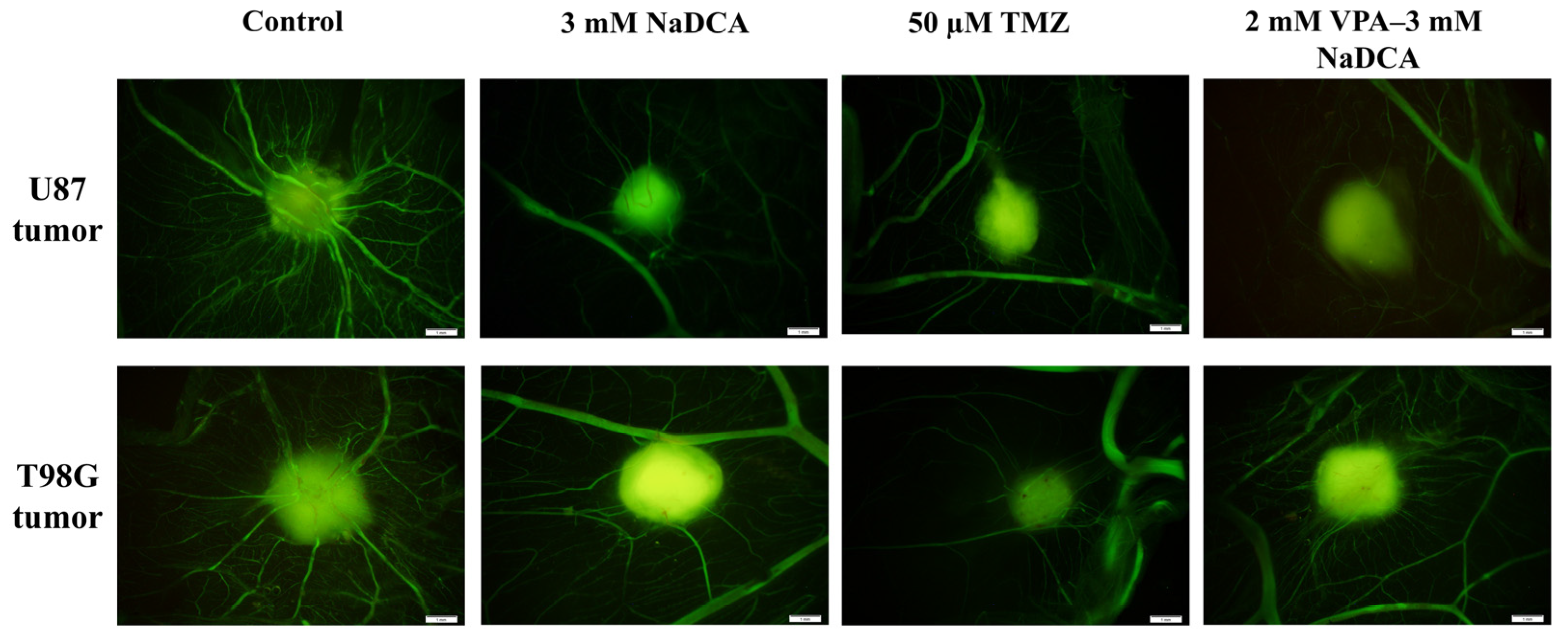
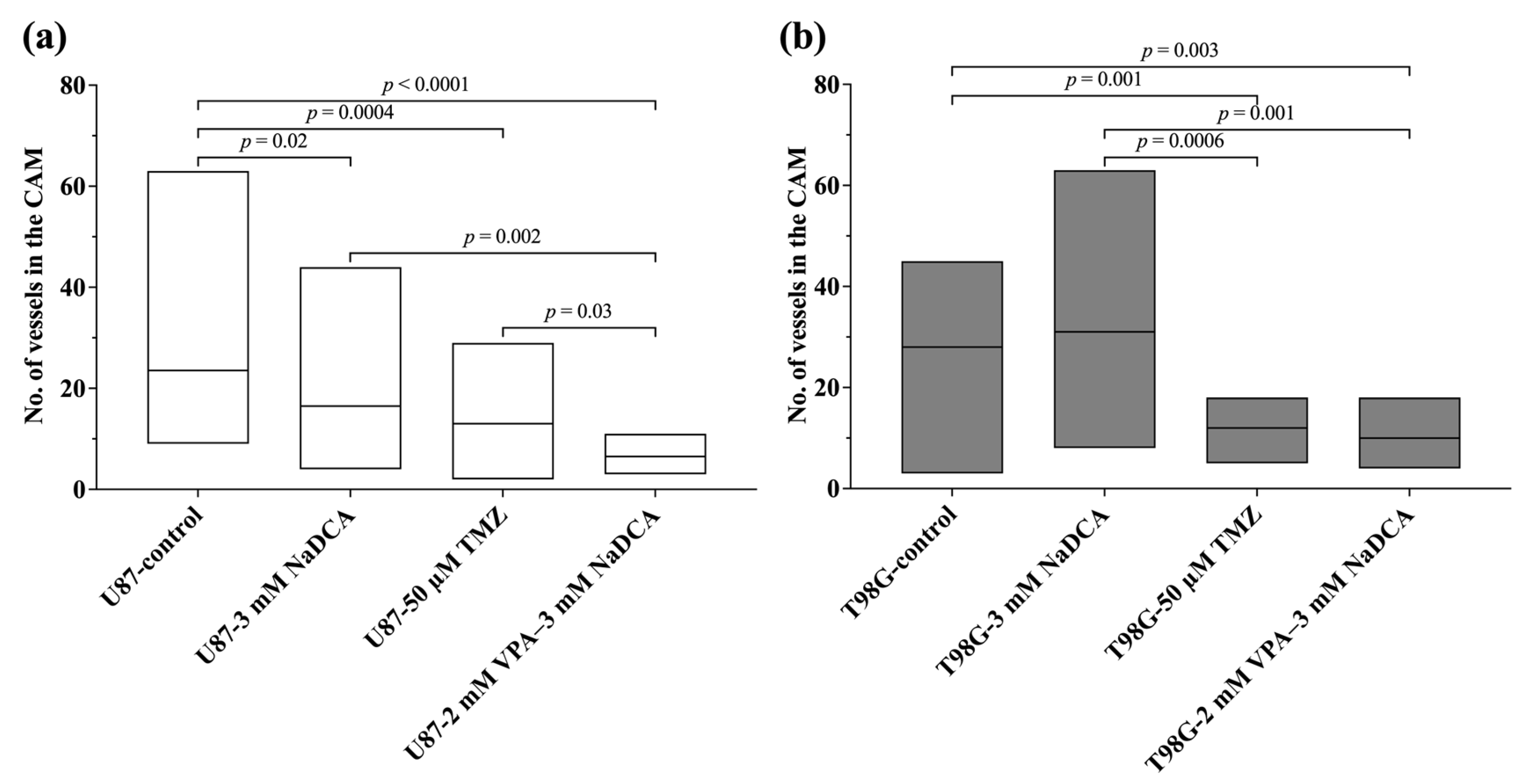
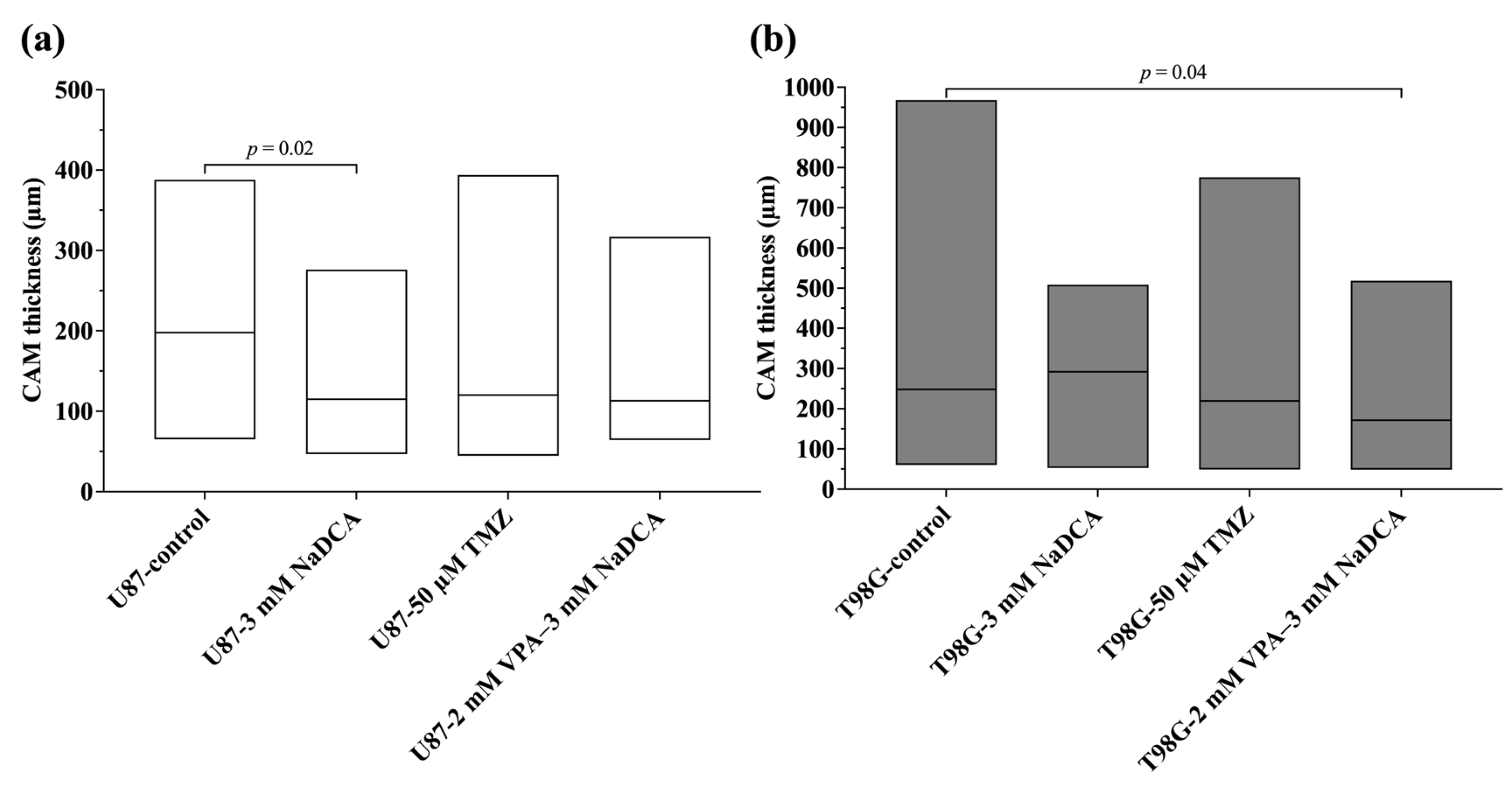
2.3. The IMP Impact on Neo-Angiogenesis in the CAM and on CAM Thickness Under the Tumor
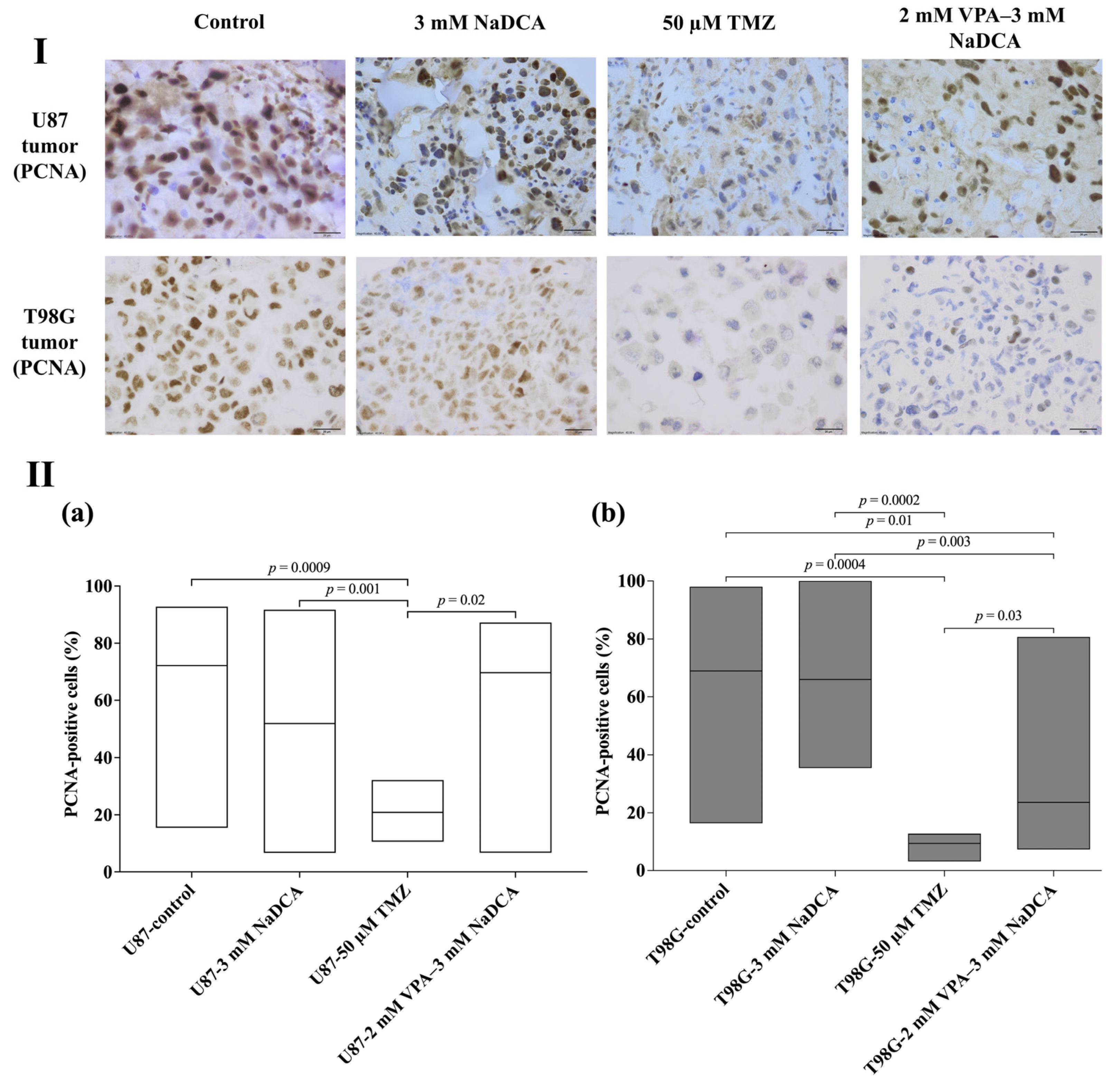
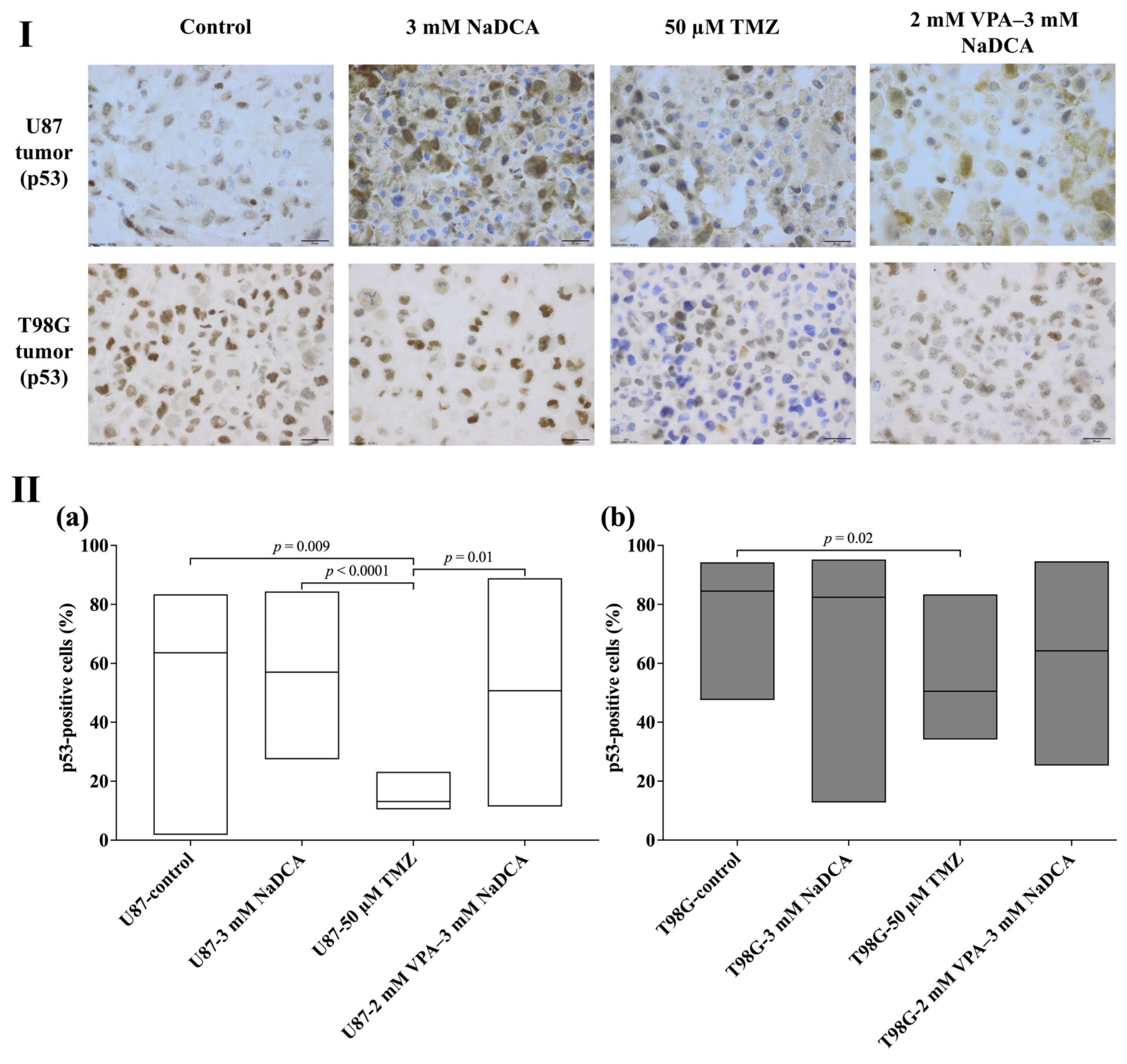
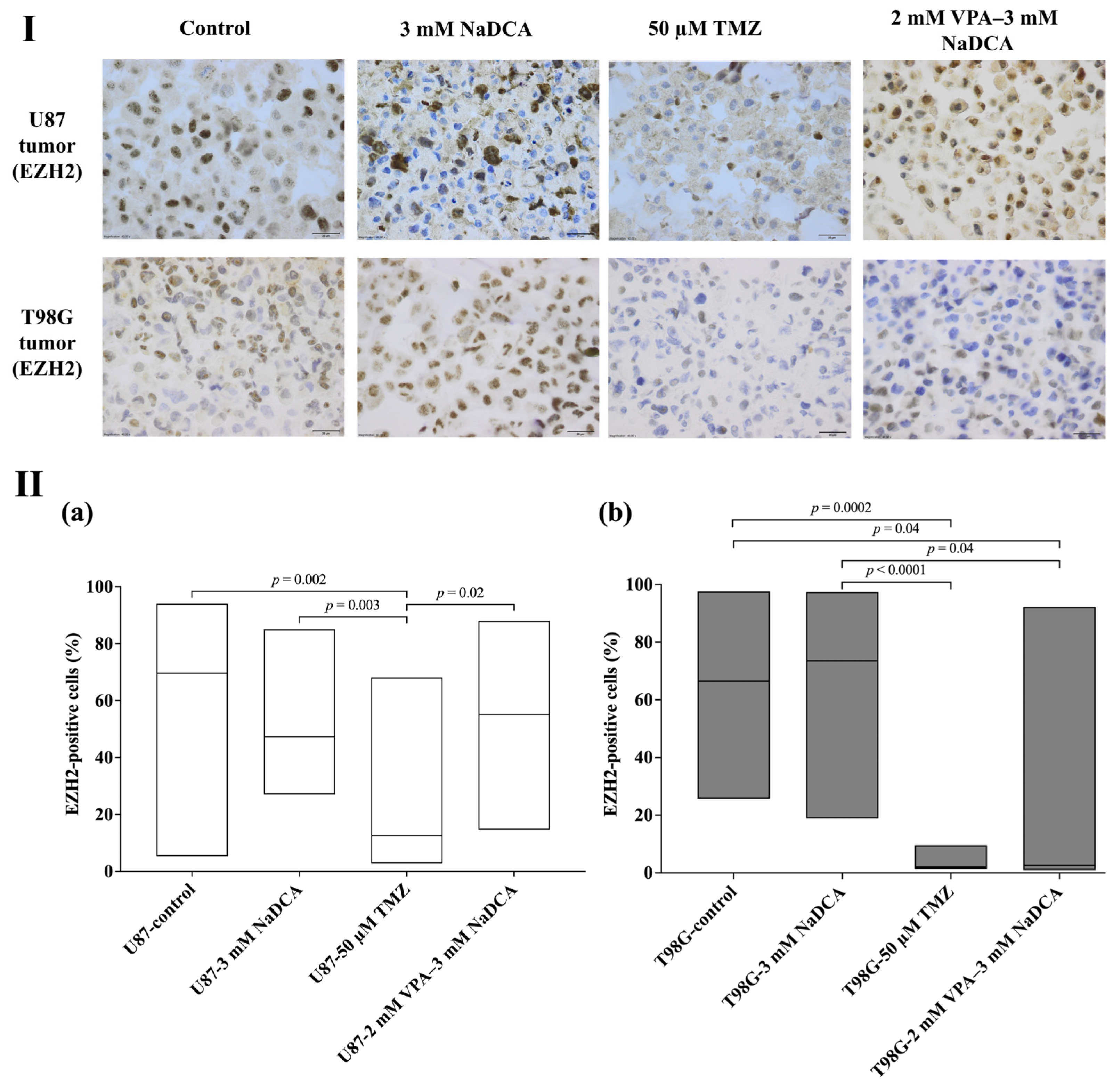
2.4. The Data of Immunohistochemical Examination of PCNA, p53, and EZH2 Marker Expression in the Tumors
2.4.1. The PCNA Expression of U87 and T98G in Control and Treated Tumors
2.4.2. The p53 Expression of U87 and T98G in Control and Treated Tumors
2.4.3. The EZH2 Expression in Studied U87 and T98G Tumors
2.5. The Data of SLC12A2, SLC12A5, SLC5A8, CHD1, and CHD2 Expression in Tested U87 and T98G Cell Groups
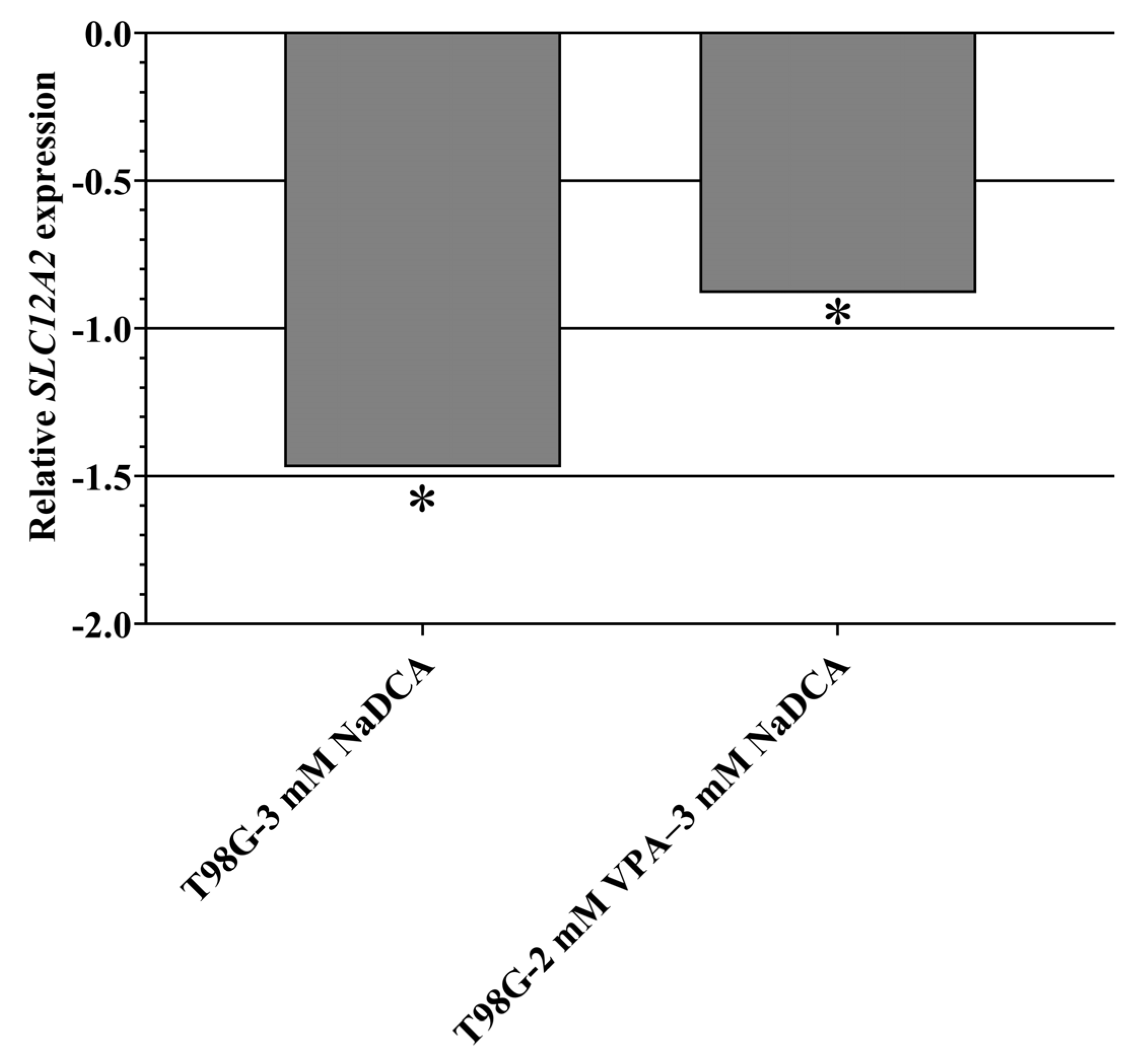
2.5.1. SLC12A2 Expression in U87 and T98G Cells
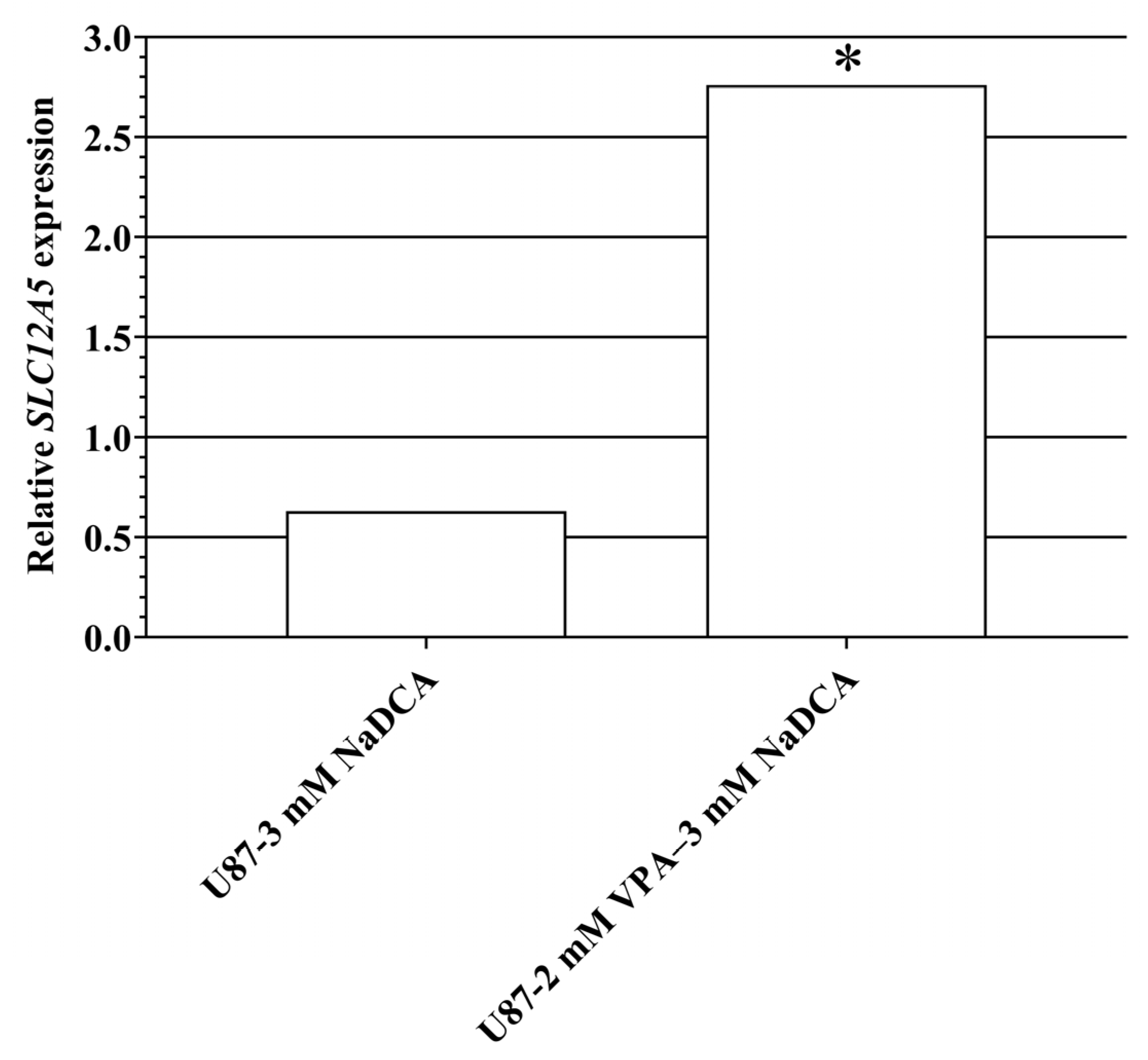
2.5.2. SLC12A5 Expression in U87 and T98G Cells
2.5.3. SLC5A8 Expression in U87 and T98G Cells
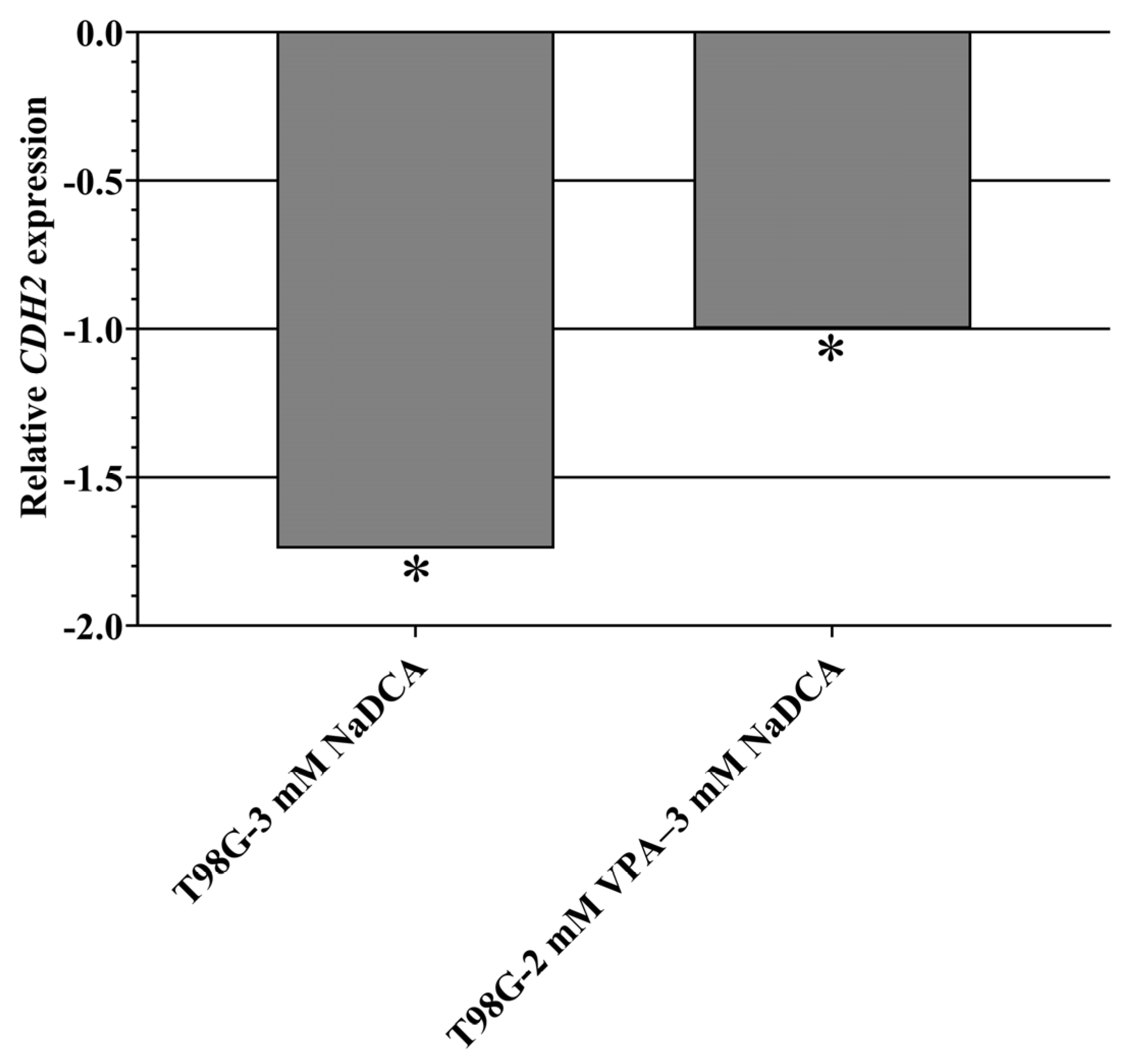
2.5.4. CDH1 and CDH2 Expression in U87 and T98G Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. U87 and T98G Tumors in the CAM Study Groups
4.3. The CAM Model
4.4. Investigational Medicinal Products
4.5. Histological and Immunohistochemical Examination of the Tumor
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMEM | Advanced Minimum Essential Medium for cell culture |
| CAM | Chick embryo chorioallantoic membrane |
| CDH1 | Gene encoding the E-cadherin protein |
| CDH2 | Gene encoding the N-cadherin protein |
| CT | Threshold cycle |
| DMEM | Dulbecco’s Modified Eagle Medium for cell culture |
| DMSO | Dimethyl sulfoxide |
| EDD | Day of embryo development |
| EZH2 | Polycomb inhibitory complex catalytic subunit 2 |
| FBS | Fetal bovine serum |
| GABA | Gamma-aminobutyric acid |
| GAPDH | Gene encoding glyceraldehyde-3-phosphate dehydrogenase protein |
| GBM | Glioblastoma |
| HDAC | Histone deacetylase |
| H-E | Hematoxylin and eosin |
| IHC | Immunohistochemistry |
| MGMT | O-6-methylguanine-DNA methyltransferase |
| NaDCA | Sodium dichloroacetate |
| OS | Overall survival |
| PCNA | Proliferating cell nuclear antigen |
| PDH | Pyruvate dehydrogenase |
| PDK | Pyruvate dehydrogenase kinase |
| p53 | TP53 gene-encoded p53 protein |
| P/S | Penicillin and streptomycin |
| SLC12A2 | Gene encoding Na+-K+-2Cl− (NKCC1) co-transporter |
| SLC12A5 | Gene encoding K+-Cl− (KCC2) co-transporter |
| SLC5A8 | Gene encoding Na+-dependent monocarboxylate transporter |
| TMZ | Temozolomide |
| T98G | Male glioblastoma cell line |
| U87 | U87-MG female glioblastoma cell line |
| VPA | Valproic acid |
| VPA–NaDCA | Combination of valproic acid and dichloroacetate |
Appendix A
| Study Group | n | Invasion Frequency (%) |
|---|---|---|
| U87-control | 20 | 80.00 |
| U87-3 mM NaDCA | 20 | 50.00 a |
| U87-50 µM TMZ | 15 | 26.67 b |
| U87-2 mM VPA–3 mM NaDCA | 10 | 20.00 c |
| T98G-control | 12 | 83.33 |
| T98G-3 mM NaDCA | 12 | 58.33 |
| T98G-50 µM TMZ | 16 | 43.75 d |
| T98G-2 mM VPA–3 mM NaDCA | 12 | 50.00 |
| Study Group | n | No. of Vessels, Median (Range) |
|---|---|---|
| U87-control | 20 | 23.5 (9–63) a |
| U87-3 mM NaDCA | 20 | 16.5 (4–44) b,c |
| U87-50 µM TMZ | 15 | 13 (2–29) d |
| U87-2 mM VPA–3 mM NaDCA | 10 |
6.5 (3–11) e |
| T98G-control | 12 | 28 (3–45) f |
| T98G-3 mM NaDCA | 12 | 31 (8–63) g,h |
| T98G-50 µM TMZ | 16 | 12 (5–18) |
| T98G-2 mM VPA–3 mM NaDCA | 12 | 10 (4–18) i |
| Study Group | n | CAM Thickness (μm), Median (Range) |
|---|---|---|
| U87-control | 20 | 197.80 (65.21–387.90) |
| U87-3 mM NaDCA | 20 | 115.00 (46.61–276.30) a |
| U87-50 µM TMZ | 15 | 120.20 (44.47–393.90) |
| U87-2 mM VPA–3 mM NaDCA | 10 | 113.10 (64.25–317.10) |
| T98G-control | 12 | 248.10 (60.11–968.20) |
| T98G-3 mM NaDCA | 12 | 292.00 (52.46–508.60) |
| T98G-50 µM TMZ | 16 | 219.60 (48.73–776.00) |
| T98G-2 mM VPA–3 mM NaDCA | 12 | 171.70 (48.26–518.70) b |
| Study Group | n | PCNA-Positive Cells (%), Median (Range) |
|---|---|---|
| U87-control | 13 | 72.12 (15.44–92.73) |
| U87-3 mM NaDCA | 18 | 51.95 (6.67–91.67) |
| U87-50 µM TMZ | 8 | 20.89 (10.59–32.13) a,b |
| U87-2 mM VPA–3 mM NaDCA | 10 | 69.71 (6.73–87.18) c |
| T98G-control | 9 | 68.95 (16.41–98.04) |
| T98G-3 mM NaDCA | 10 | 66.03 (35.43–100.0) |
| T98G-50 µM TMZ | 6 | 9.43 (3.17–12.77) d,e,f |
| T98G-2 mM VPA–3 mM NaDCA | 8 | 23.63 (7.34–80.75) g,h |
| Study Group | n | p53-Positive Cells (%), MEDIAN (Range) |
|---|---|---|
| U87-control | 15 | 63.58 (1.81–83.44) |
| U87-3 mM NaDCA | 20 | 57.04 (27.48–84.39) |
| U87-50 µM TMZ | 7 | 13.07 (10.40–23.17) a,b |
| U87-2 mM VPA–3 mM NaDCA | 10 | 50.70 (11.41–88.89) c |
| T98G-control | 9 | 84.52 (47.57–94.23) |
| T98G-3 mM NaDCA | 11 | 82.42 (12.75–95.21) |
| T98G-50 µM TMZ | 7 | 50.53 (34.09–83.38) d |
| T98G-2 mM VPA–3 mM NaDCA | 8 | 64.21 (25.29–94.59) |
| Study Group | n | EZH2-Positive Cells (%), Median (Range) |
|---|---|---|
| U87-control | 17 | 69.57 (5.34–94.06) |
| U87-3 mM NaDCA | 19 | 47.23 (26.98–85.05) |
| U87-50 µM TMZ | 8 | 12.55 (2.84–68.12) a,b |
| U87-2 mM VPA–3 mM NaDCA | 10 | 55.07 (14.62–87.96) c |
| T98G-control | 9 | 66.47 (25.74–97.65) d |
| T98G-3 mM NaDCA | 11 | 73.59 (18.90–97.35) d,e |
| T98G-50 µM TMZ | 7 | 2.02 (1.31–9.59) f |
| T98G-2 mM VPA–3 mM NaDCA | 9 | 2.60 (0.98–92.23) |
| Study Groups | n | Indicator, Mean ± SD | ||||
|---|---|---|---|---|---|---|
| CT | ∆CT | ∆∆CT | 2−∆∆CT | |||
| SLC12A2 | GAPDH | |||||
| U87-control | 6 | 22.07 ± 1.23 | 18.14 ± 1.41 | 3.93 ± 0.64 | ||
| U87-3 mM NaDCA | 6 | 23.13 ± 0.33 | 18.92 ± 1.51 | 4.21 ± 1.22 | 0.29 | 0.82 |
| U87-50 µM TMZ | 6 | 21.67 ± 0.18 | 17.21 ± 0.19 | 4.46 ± 0.30 a | 0.53 | 0.69 |
| U87-2 mM VPA–3 mM NaDCA | 6 | 22.71 ± 0.31 | 18.85 ± 0.20 | 3.86 ± 0.27 | −0.07 | 1.05 |
| T98G-control | 6 | 21.63 ± 0.60 | 19.87 ± 0.23 | 1.76 ± 0.60 b | ||
| T98G-3 mM NaDCA | 6 | 22.01 ± 0.65 | 18.78 ± 0.63 | 3.23 ± 0.15 c | 1.47 | 0.36 |
| T98G-50 µM TMZ | 6 | 22.84 ± 0.80 | 21.18 ± 1.16 | 1.66 ± 0.40 d,e | −0.10 | 1.07 |
| T98G-2 mM VPA–3 mM NaDCA | 6 | 20.99 ± 0.49 | 18.35 ± 0.24 | 2.64 ± 0.58 f | 0.88 | 0.54 |
| Study Groups | n | Indicator, Mean ± SD | ||||
|---|---|---|---|---|---|---|
| CT | ∆CT | ∆∆CT | 2−∆∆CT | |||
| SLC12A5 | GAPDH | |||||
| U87-control | 6 | 33.79 ± 1.21 | 16.25 ± 0.26 | 17.54 ± 1.07 a | ||
| U87-3 mM NaDCA | 6 | 33.93 ± 1.55 | 16.70 ± 0.66 | 17.23 ± 0.98 a | −0.31 | 1.55 |
| U87-50 µM TMZ | 6 | 32.41 ± 0.15 | 15.21 ± 0.16 | 17.20 ± 0.25 b | −0.33 | 1.28 |
| U87-2 mM VPA–3 mM NaDCA | 6 | 31.42 ± 0.22 | 16.31 ± 0.89 | 15.12 ± 0.87 | −2.42 | 6.79 |
| T98G-control | 6 | 37.31 ± 0.46 | 17.41 ± 0.22 | - | ||
| T98G-3 mM NaDCA | 6 | 37.14 ± 0.24 | 16.63 ± 0.63 | - | - | - |
| T98G-50 µM TMZ | 6 | 37.53 ± 0.05 | 19.16 ± 2.39 | - | - | - |
| T98G-2 mM VPA–3 mM NaDCA | 6 | 33.50 ± 0.36 | 16.80 ± 2.00 | 16.70 ± 2.12 | - | - |
| Study Groups | n | Indicator, Mean ± SD | ||||
|---|---|---|---|---|---|---|
| CT | ∆CT | ∆∆CT | 2−∆∆CT | |||
| CDH2 | GAPDH | |||||
| U87-control | 6 | 23.83 ± 0.72 | 18.14 ± 1.41 | 5.68 ± 1.26 a | ||
| U87-3 mM NaDCA | 6 | 23.53 ± 0.82 | 18.92 ± 1.51 | 4.62 ± 0.71 | −1.07 | 2.09 |
| U87-50 µM TMZ | 6 | 22.52 ± 0.13 | 17.21 ± 0.19 | 5.32 ± 0.28 | −0.37 | 1.29 |
| U87-2 mM VPA–3 mM NaDCA | 6 | 23.88 ± 0.28 | 18.85 ± 0.20 | 5.03 ± 0.31 | −0.66 | 1.58 |
| T98G-control | 6 | 23.64 ± 0.22 | 19.87 ± 0.23 | 3.78 ± 0.30 | ||
| T98G-3 mM NaDCA | 6 | 24.29 ± 0.59 | 18.78 ± 0.63 | 5.51 ± 0.20 a,b | 1.73 | 0.30 |
| T98G-50 µM TMZ | 6 | 24.31 ± 0.46 | 21.18 ± 1.16 | 3.13 ± 0.75 | −0.65 | 1.57 |
| T98G-2 mM VPA–3 mM NaDCA | 6 | 23.13 ± 0.45 | 18.35 ± 0.24 | 4.77 ± 0.32 a,b,c | 1.00 | 0.50 |
References
- Park, C.M.; Park, M.J.; Kwak, H.J.; Moon, S.I.; Yoo, D.H.; Lee, H.C.; Park, I.C.; Rhee, C.H.; Hong, S.I. Induction of P53-Mediated Apoptosis and Recovery of Chemosensitivity through P53 Transduction in Human Glioblastoma Cells by Cisplatin. Int. J. Oncol. 2006, 28, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Gittleman, H.; Ostrom, Q.T.; Stetson, L.C.; Waite, K.; Hodges, T.R.; Wright, C.H.; Wright, J.; Rubin, J.B.; Berens, M.E.; Lathia, J.; et al. Sex Is an Important Prognostic Factor for Glioblastoma but Not for Nonglioblastoma. Neurooncol. Pract. 2019, 6, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.A.; Hovinga, K.; Reiner, A.S.; Esquenazi, Y.; Tabar, V.; Panageas, K.S. The Relationship between Repeat Resection and Overall Survival in Patients with Glioblastoma: A Time-Dependent Analysis. J. Neurosurg. 2018, 129, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gilbert, M.R.; Chakravarti, A. Chemoradiotherapy in Malignant Glioma: Standard of Care and Future Directions. J. Clin. Oncol. 2007, 25, 4127–4136. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.C.; Pentheroudakis, G. High-Grade Glioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25, III93–III101. [Google Scholar] [CrossRef] [PubMed]
- Krex, D.; Klink, B.; Hartmann, C.; Von Deimling, A.; Pietsch, T.; Simon, M.; Sabel, M.; Steinbach, J.P.; Heese, O.; Reifenberger, G.; et al. Long-Term Survival with Glioblastoma Multiforme. Brain 2007, 130, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma Survival in the United States before and during the Temozolomide Era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Singh, S.; Singh, P.; Chauhan, P.; Zaidi, M. Tumor Markers: A Diagnostic Tool. Natl. J. Maxillofac. Surg. 2016, 7, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, C.M.; Storevik, S.; Guyon, J.; Pagano Zottola, A.C.; Bouchez, C.L.; Derieppe, M.A.; Tan, T.Z.; Miletic, H.; Lorens, J.; Tronstad, K.J.; et al. Refining the Role of Pyruvate Dehydrogenase Kinases in Glioblastoma Development. Cancers 2022, 14, 3769. [Google Scholar] [CrossRef] [PubMed]
- Sutendra, G.; Michelakis, E.D. Pyruvate Dehydrogenase Kinase as a Novel Therapeutic Target in Oncology. Front. Oncol. 2013, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Chinopoulos, C.; Seyfried, T.N. Mitochondrial Substrate-Level Phosphorylation as Energy Source for Glioblastoma: Review and Hypothesis. ASN Neuro. 2018, 10, 1759091418818261. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yu, M.; Tsoli, M.; Chang, C.; Joshi, S.; Liu, J.; Ryall, S.; Chornenkyy, Y.; Siddaway, R.; Hawkins, C.; et al. Targeting Reduced Mitochondrial DNA Quantity as a Therapeutic Approach in Pediatric High-Grade Gliomas. Neuro Oncol. 2020, 22, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.S.; Sousa, M.; Morais, C.M.; Oancea-Castillo, L.R.; Régnier-Vigouroux, A.; Rebelo, O.; Tão, H.; Barbosa, M.; Pedroso, M.C.D.L.; Jurado, A.S. MiR-144 Overexpression as a Promising Therapeutic Strategy to Overcome Glioblastoma Cell Invasiveness and Resistance to Chemotherapy. Hum. Mol. Genet. 2019, 28, 2738–2751. [Google Scholar] [CrossRef] [PubMed]
- Wicks, R.T.; Azadi, J.; Mangraviti, A.; Zhang, I.; Hwang, L.; Joshi, A.; Bow, H.; Hutt-Cabezas, M.; Martin, K.L.; Rudek, M.A.; et al. Local Delivery of Cancer-Cell Glycolytic Inhibitors in High-Grade Glioma. Neuro Oncol. 2015, 17, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Meng, G.; Su, L.; Chen, A.; Xia, M.; Xu, C.; Yu, D.; Jiang, A.; Wei, J. Dichloroacetate Blocks Aerobic Glycolytic Adaptation to Attenuated Measles Virus and Promotes Viral Replication Leading to Enhanced Oncolysis in Glioblastoma. Oncotarget 2015, 6, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Hau, E.; Joshi, S.; Dilda, P.J.; McDonald, K.L. Sensitization of Glioblastoma Cells to Irradiation by Modulating the Glucose Metabolism. Mol. Cancer Ther. 2015, 14, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Wigfield, S.; Gee, H.E.; Devlin, C.M.; Singleton, D.; Li, J.L.; Buffa, F.; Huffman, M.; Sinn, A.L.; Silver, J.; et al. Dichloroacetate Reverses the Hypoxic Adaptation to Bevacizumab and Enhances Its Antitumor Effects in Mouse Xenografts. J. Mol. Med. 2013, 91, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. Oxid. Med. Cell Longev. 2019, 2019, 8201079. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Ahmed, E.; Kader, G.A.; Abdel-mottaleb, Y.; ElSayed, M.H.; Youssef, A.M.; Zaitone, S.A. Inhibitory Effect of Valproate Sodium on Pain Behavior in Diabetic Mice Involves Suppression of Spinal Histone Deacetylase 1 and Inflammatory Mediators. Int. Immunopharmacol. 2019, 70, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Wadia, R.J.; Stolar, M.; Grens, C.; Ehrlich, B.E.; Chao, H.H. The Prevention of Chemotherapy Induced Peripheral Neuropathy by Concurrent Treatment with Drugs Used for Bipolar Disease: A Retrospective Chart Analysis in Human Cancer Patients. Oncotarget 2018, 9, 7322–7331. [Google Scholar] [CrossRef] [PubMed]
- Um, J.H.; Yun, J. Emerging Role of Mitophagy in Human Diseases and Physiology. BMB Rep. 2017, 50, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gaffke, L.; Firyn, N.; Rintz, E.; Pierzynowska, K.; Piotrowska, E.; Mazur–Marzec, H.; Węgrzyn, G. Therapeutic Potential of Lithium Chloride and Valproic Acid against Neuronopathic Types of Mucopolysaccharidoses through Induction of the Autophagy Process. Arch. Biochem. Biophys. 2023, 747, 109754. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Botrugno, O.A.; Dal Zuffo, R.; Pallavicini, I.; Matthews, G.M.; Cluse, L.; Barozzi, I.; Senese, S.; Fornasari, L.; Moretti, S.; et al. A Dual Role for Hdac1: Oncosuppressor in Tumorigenesis, Oncogene in Tumor Maintenance. Blood 2013, 121, 3459–3468. [Google Scholar] [CrossRef] [PubMed]
- Stakišaitis, D.; Kapočius, L.; Valančiūte, A.; Balnyte, I.; Tamošuitis, T.; Vaitkevičiūs, A.; Sužiedelis, K.; Urboniene, D.; Tatarūnas, V.; Kilimaite, E.; et al. SARS-CoV-2 Infection, Sex-Related Differences, and a Possible Personalized Treatment Approach with Valproic Acid: A Review. Biomedicines 2022, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Bezecny, P. Histone Deacetylase Inhibitors in Glioblastoma: Pre-Clinical and Clinical Experience. Med. Oncol. 2014, 31, 985. [Google Scholar] [CrossRef] [PubMed]
- Yelton, C.J.; Ray, S.K. Histone Deacetylase Enzymes and Selective Histone Deacetylase Inhibitors for Antitumor Effects and Enhancement of Antitumor Immunity in Glioblastoma. Neuroimmunol. Neuroinflamm. 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Babu, E.; Ramachandran, S.; Coothankandaswamy, V.; Elangovan, S.; Prasad, P.D.; Ganapathy, V.; Thangaraju, M. Role of SLC5A8, a Plasma Membrane Transporter and a Tumor Suppressor, in the Antitumor Activity of Dichloroacetate. Oncogene 2011, 30, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Veronezi, G.M.B.; Felisbino, M.B.; Gatti, M.S.V.; Mello, M.L.S.; De Vidal, B.C. DNA Methylation Changes in Valproic Acid-Treated HeLa Cells as Assessed by Image Analysis, Immunofluorescence and Vibrational Microspectroscopy. PLoS ONE 2017, 12, e0170740. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of Temozolomide Resistance in Glioblastoma—A Comprehensive Review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Guan, W. Valproic Acid: A Promising Therapeutic Agent in Glioma Treatment. Front. Oncol. 2021, 11, 687362. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Li, J.R.; Wu, C.C.; Ou, Y.C.; Chen, W.Y.; Kuan, Y.H.; Wang, W.Y.; Chen, C.J. Valproic Acid Sensitizes Human Glioma Cells to Gefitinib-Induced Autophagy. IUBMB Life 2015, 67, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Wang, J.; Hong, M.; Zheng, L.; Tong, Q. Valproic Acid Suppresses Warburg Effect and Tumor Progression in Neuroblastoma. Biochem. Biophys. Res. Commun. 2019, 508, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, A.H.L.; Pimentel, R.S.; Prado, A.P.; Garcia, J.; Frozza, R.L.; Bernardi, A. Neuroinflammation in Glioblastoma: The Role of the Microenvironment in Tumour Progression. Curr. Cancer Drug Targets 2024, 24, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Tsai, C.H.; Pucino, V.; Ho, P.C.; Mauro, C. Lactate Modulation of Immune Responses in Inflammatory versus Tumour Microenvironments. Nat. Rev. Immunol. 2021, 21, 151–161. [Google Scholar] [CrossRef] [PubMed]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, E.A.N.; Mahmoud, H.M.; Azouz, A.A. Management of Ulcerative Colitis by Dichloroacetate: Impact on NFATC1/NLRP3/IL1B Signaling Based on Bioinformatics Analysis Combined with in Vivo Experimental Verification. Inflammopharmacology 2024, 32, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Amirzargar, M.A.; Yaghubi, F.; Hosseinipanah, M.; Jafari, M.; Pourjafar, M.; Rezaeepoor, M.; Rezaei, H.; Roshanaei, G.; Hajilooi, M.; Solgi, G. Anti-Inflammatory Effects of Valproic Acid in a Rat Model of Renal Ischemia/Reperfusion Injury: Alteration in Cytokine Profile. Inflammation 2017, 40, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.B.; Aires, C.C.P.; Luis, P.B.M.; Ruiter, J.P.N.; IJlst, L.; Duran, M.; Wanders, R.J.A.; Tavares de Almeida, I. Valproic Acid Metabolism and Its Effects on Mitochondrial Fatty Acid Oxidation: A Review. J. Inherit. Metab. Dis. 2008, 31, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Stakišaitis, D.; Kapočius, L.; Kilimaitė, E.; Gečys, D.; Šlekienė, L.; Balnytė, I.; Palubinskienė, J.; Lesauskaitė, V. Preclinical Study in Mouse Thymus and Thymocytes: Effects of Treatment with a Combination of Sodium Dichloroacetate and Sodium Valproate on Infectious Inflammation Pathways. Pharmaceutics 2023, 15, 2715. [Google Scholar] [CrossRef] [PubMed]
- Gaca-Tabaszewska, M.; Bogusiewicz, J.; Bojko, B. Metabolomic and Lipidomic Profiling of Gliomas—A New Direction in Personalized Therapies. Cancers 2022, 14, 5041. [Google Scholar] [CrossRef] [PubMed]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A Review of Glioblastoma Immunotherapy. J. Neurooncol. 2021, 151, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide Resistance in Glioblastoma Multiforme. Genes. Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Somasundaram, K. Glioblastoma vs. Temozolomide: Can the Red Queen Race Be Won? Cancer Biol. Ther. 2019, 20, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Kohl, C.; Aung, T.; Haerteis, S.; Ignatov, A.; Ortmann, O.; Papathemelis, T. The 3D in Vivo Chorioallantoic Membrane Model and Its Role in Breast Cancer Research. J. Cancer Res. Clin. Oncol. 2022, 148, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.-C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The Chick Chorioallantoic Membrane (CAM) as a Versatile Patient-Derived Xenograft (PDX) Platform for Precision Medicine and Preclinical Research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar] [PubMed]
- Wick, W.; Platten, M. Understanding and Targeting Alkylator Resistance in Glioblastoma. Cancer Discov. 2014, 4, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J. Natl. Cancer. Inst. 2017, 109, djx071. [Google Scholar] [CrossRef] [PubMed]
- Niewisch, M.R.; Kuçi, Z.; Wolburg, H.; Sautter, M.; Krampen, L.; Deubzer, B.; Handgretinger, R.; Bruchelt, G. Influence of Dichloroacetate (DCA) on Lactate Production and Oxygen Consumption in Neuroblastoma Cells: Is DCA a Suitable Drug for Neuroblastoma Therapy? Cell. Physiol. Biochem. 2012, 29, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Sutendra, G.; Dromparis, P.; Webster, L.; Haromy, A.; Niven, E.; Maguire, C.; Gammer, T.L.; Mackey, J.R.; Fulton, D.; et al. Metabolic Modulation of Glioblastoma with Dichloroacetate. Sci. Transl. Med. 2010, 2, 31ra34. [Google Scholar] [CrossRef] [PubMed]
- Velpula, K.K.; Tsung, A.J. PDK1: A New Therapeutic Target for Glioblastoma? CNS Oncol. 2014, 3, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Velpula, K.K.; Guda, M.R.; Sahu, K.; Tuszynski, J.; Asuthkar, S.; Bach, S.E.; Lathia, J.D.; Tsung, A.J. Metabolic Targeting of EGFRvIII/PDK1 Axis in Temozolomide Resistant Glioblastoma. Oncotarget 2017, 8, 35639–35655. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.L.; Licht, J.D. Targeting Epigenetics in Cancer. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Thotala, D.; Karvas, R.M.; Engelbach, J.A.; Garbow, J.R.; Hallahan, A.N.; DeWees, T.A.; Laszlo, A.; Hallahan, D.E. Valproic Acid Enhances the Efficacy of Radiation Therapy by Protecting Normal Hippocampal Neurons and Sensitizing Malignant Glioblastoma Cells. Oncotarget 2015, 6, 35004–35022. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Texakalidis, P.; McDonald, K.L.; Mekary, R.A.; Smith, T.R. The Survival Effect of Valproic Acid in Glioblastoma and Its Current Trend: A Systematic Review and Meta-Analysis. Clin. Neurol. Neurosurg. 2018, 174, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Kresbach, C.; Bronsema, A.; Guerreiro, H.; Rutkowski, S.; Schüller, U.; Winkler, B. Long-Term Survival of an Adolescent Glioblastoma Patient under Treatment with Vinblastine and Valproic Acid Illustrates Importance of Methylation Profiling. Child’s Nerv. Syst. 2022, 38, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Wang, H.; Niu, J.; Hou, H.; Jiang, Y. Histone Deacetylase Inhibitor, Valproic Acid, Radiosensitizes the C6 Glioma Cell Line in Vitro. Oncol. Lett. 2014, 7, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhang, J.; Yi, Y.; Huang, Y.; Wang, Y.; Wang, Y.; Zhang, W. Proliferating Cell Nuclear Antigen Has an Association with Prognosis and Risks Factors of Cancer Patients: A Systematic Review. Mol. Neurobiol. 2016, 53, 6209–6217. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, M.; Gu, M.; Cai, Z.; Wu, Q.; Yu, J.; Tang, M.; He, C.; Wang, Y.; Sun, P.; et al. Design and Synthesis of 7-Oxabicyclo [2.2.1] Heptane-2,3-Dicarboxylic Acid Derivatives as PP5 Inhibitors To Reverse Temozolomide Resistance in Glioblastoma Multiforme. J. Med. Chem. 2024, 67, 15691–15710. [Google Scholar] [CrossRef] [PubMed]
- Damanskienė, E.; Balnytė, I.; Valančiūtė, A.; Lesauskaitė, V.; Alonso, M.M.; Stakišaitis, D. The Comparative Experimental Study of Sodium and Magnesium Dichloroacetate Effects on Pediatric PBT24 and SF8628 Cell Glioblastoma Tumors Using a Chicken Embryo Chorioallantoic Membrane Model and on Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 10455. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Ko, H.J.; Chiou, S.J.; Lai, Y.L.; Hou, C.C.; Javaria, T.; Huang, Z.Y.; Cheng, T.S.; Hsu, T.I.; Chuang, J.Y.; et al. Nbm-Bmx, an Hdac8 Inhibitor, Overcomes Temozolomide Resistance in Glioblastoma Multiforme by Downregulating the β-Catenin/c-Myc/Sox2 Pathway and Upregulating P53-Mediated Mgmt Inhibition. Int. J. Mol. Sci. 2021, 22, 5907. [Google Scholar] [CrossRef] [PubMed]
- AghaAmiri, S.; Ghosh, S.C.; Hernandez Vargas, S.; Halperin, D.M.; Azhdarinia, A. Somatostatin Receptor Subtype-2 Targeting System for Specific Delivery of Temozolomide. J. Med. Chem. 2024, 67, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular Targeted Therapy of Glioblastoma. Cancer Treat Rev 2019, 80. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Takagi, Y.; Toriya, Y.; Nakagawa, T.; Aruga, T.; Iida, S.; Uetake, H.; Sugihara, K. Inverse Correlation between the Expression of O6-Methylguanine-DNA Methyl Transferase (MGMT) and P53 in Breast Cancer. Jpn. J. Clin. Oncol. 2005, 35, 121–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamagishi, M.; Uchimaru, K. Targeting EZH2 in Cancer Therapy. Curr. Opin. Oncol. 2017, 29, 375–381. [Google Scholar] [CrossRef] [PubMed]
- El Baba, R.; Pasquereau, S.; Haidar Ahmad, S.; Monnien, F.; Abad, M.; Bibeau, F.; Herbein, G. EZH2-Myc Driven Glioblastoma Elicited by Cytomegalovirus Infection of Human Astrocytes. Oncogene 2023, 42, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Roberts, C.W.M. Targeting EZH2 in Cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Helin, K.; Dhanak, D. Chromatin Proteins and Modifications as Drug Targets. Nature 2013, 502, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskaite, D.; Stakišaitis, D.; Martinkute, J.; Šlekiene, L.; Kazlauskas, A.; Balnyte, I.; Lesauskaite, V.; Valančiute, A. The Effect of Sodium Valproate on the Glioblastoma U87 Cell Line Tumor Development on the Chicken Embryo Chorioallantoic Membrane and on EZH2 and P53 Expression. Biomed. Res. Int. 2017, 2017, 6326053. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Muenzner, J.K.; Caliskan, A.; Ndreshkjana, B.; Erlenbach-Wünsch, K.; Merkel, S.; Croner, R.; Rau, T.T.; Geppert, C.I.; Hartmann, A.; et al. Loss of Enhancer of Zeste Homologue 2 (EZH2) at Tumor Invasion Front Is Correlated with Higher Aggressiveness in Colorectal Cancer Cells. J. Cancer Res. Clin. Oncol. 2019, 145, 2227–2240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, X.; Chen, L.; Zhang, Z.; Feng, S. EZH2 Overexpression Is Associated with Poor Prognosis in Patients with Glioma. Oncotarget 2017, 8, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Mehrabi, A.; Gholami, M.H.; Zabolian, A.; Ranjbar, E.; Saleki, H.; Ranjbar, A.; Hashemi, M.; Ertas, Y.N.; Hushmandi, K.; et al. EZH2 as a New Therapeutic Target in Brain Tumors: Molecular Landscape, Therapeutic Targeting and Future Prospects. Biomed. Pharmacother. 2022, 146, 112532. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Cao, E. Structure of the Human Cation–Chloride Cotransporter NKCC1 Determined by Single-Particle Electron Cryo-Microscopy. Nat. Commun. 2020, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wu, M.; Shen, J.; Tang, J.; Li, J.; Xu, J.; Dang, B.; Chen, G. Inhibition of the NKCC1/NF-ΚB Signaling Pathway Decreases Inflammation and Improves Brain Edema and Nerve Cell Apoptosis in an SBI Rat Model. Front. Mol. Neurosci. 2021, 14, 641993. [Google Scholar] [CrossRef] [PubMed]
- Cong, D.; Zhu, W.; Kuo, J.S.; Hu, S.; Sun, D. Ion Transporters in Brain Tumors. Curr. Med. Chem. 2015, 22, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Long, S.; Wu, B.; Liu, J.; Li, G. NKCC1 Involvement in the Epithelial-to-Mesenchymal Transition Is a Prognostic Biomarker in Gliomas. PeerJ 2020, 8, e8787. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.L.; Jin, Y.; Park, S.Y.; Kim, H.; Park, E.; Jheon, S.; Kim, K.; Lee, C.T.; Chung, J.H. Expression of Na+-K+-2Cl− Cotransporter Isoform 1 (NKCC1) Predicts Poor Prognosis in Lung Adenocarcinoma and EGFR-Mutated Adenocarcinoma Patients. QJM Int. J. Med. 2016, 109, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Miyazaki, H.; Niisato, N.; Iwasaki, Y.; Kawauchi, A.; Miki, T.; Marunaka, Y. Chloride Ion Modulates Cell Proliferation of Human Androgen-Independent Prostatic Cancer Cell. Cell. Physiol. Biochem. 2010, 25, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miyazaki, H.; Shiozaki, A.; Ichikawa, D.; Otsuji, E.; Marunaka, Y. Cytosolic Cl–Affects the Anticancer Activity of Paclitaxel in the Gastric Cancer Cell Line, MKN28 Cell. Cell. Physiol. Biochem. 2017, 42, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Damanskienė, E.; Balnytė, I.; Valančiūtė, A.; Alonso, M.M.; Preikšaitis, A.; Stakišaitis, D. The Different Temozolomide Effects on Tumorigenesis Mechanisms of Pediatric Glioblastoma PBT24 and SF8628 Cell Tumor in CAM Model and on Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 2001. [Google Scholar] [CrossRef] [PubMed]
- East, M.P.; Laitinen, T.; Asquith, C.R.M. WNK Kinases: An Untapped Opportunity to Modulate Ion Transport. Nat. Rev. Drug Discov. 2020, 19, 828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Begum, G.; Pointer, K.; Clark, P.A.; Yang, S.S.; Lin, S.H.; Kahle, K.T.; Kuo, J.S.; Sun, D. WNK1-OSR1 Kinase-Mediated Phospho-Activation of Na+-K+-2Cl− Cotransporter Facilitates Glioma Migration. Mol. Cancer 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.R.; Sontheimer, H. Inhibition of the Sodium-Potassium-Chloride Cotransporter Isoform-1 Reduces Glioma Invasion. Cancer Res. 2010, 70, 5597–5606. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, D.; Chini, B. Quest for Pharmacological Regulators of KCC2. In Neuronal Chloride Transporters in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2020; pp. 709–727. [Google Scholar] [CrossRef]
- McMoneagle, E.; Zhou, J.; Zhang, S.; Huang, W.; Josiah, S.S.; Ding, K.; Wang, Y.; Zhang, J. Neuronal K+-Cl− Cotransporter KCC2 as a Promising Drug Target for Epilepsy Treatment. Acta Pharmacol. Sin. 2024, 45, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Dai, Y.B.; Li, X.; Cao, K.; Xie, D.; Tong, Z.T.; Long, Z.; Xiao, H.; Chen, M.K.; Ye, Y.L.; et al. Solute Carrier Family 12 Member 5 Promotes Tumor Invasion/Metastasis of Bladder Urothelial Carcinoma by Enhancing NF-ΚB/MMP-7 Signaling Pathway. Cell Death Dis. 2017, 8, e2691. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Deng, C.; Fei, M. SLC12A5 as a Novel Potential Biomarker of Glioblastoma Multiforme. Mol. Biol. Rep. 2023, 50, 4285–4299. [Google Scholar] [CrossRef] [PubMed]
- Juknevičienė, M.; Balnytė, I.; Valančiūtė, A.; Alonso, M.M.; Preikšaitis, A.; Sužiedėlis, K.; Stakišaitis, D. Differential Impact of Valproic Acid on SLC5A8, SLC12A2, SLC12A5, CDH1, and CDH2 Expression in Adult Glioblastoma Cells. Biomedicines 2024, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Boer, K.; Redeker, S.; Spliet, W.G.M.; van Rijen, P.C.; Troost, D.; Gorter, J.A. Differential Expression Patterns of Chloride Transporters, Na+-K+-2Cl—-Cotransporter and K+-Cl−-Cotransporter, in Epilepsy-Associated Malformations of Cortical Development. Neuroscience 2007, 145, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Di Cristo, G.; Awad, P.N.; Hamidi, S.; Avoli, M. KCC2, Epileptiform Synchronization, and Epileptic Disorders. Prog. Neurobiol. 2018, 162, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Palma, E.; Roseti, C.; Lauro, C.; Cipriani, R.; De Groot, M.; Aronica, E.; Limatola, C. Anomalous Levels of Cl- Transporters Cause a Decrease of GABAergic Inhibition in Human Peritumoral Epileptic Cortex. Epilepsia 2011, 52, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, G.; O’Toole, K.K.; Maguire, J. Compromised GABAergic Inhibition Contributes to Tumor-Associated Epilepsy. Epilepsy Res. 2016, 126, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gyimesi, G.; Pujol-Giménez, J.; Kanai, Y.; Hediger, M.A. Sodium-Coupled Glucose Transport, the SLC5 Family, and Therapeutically Relevant Inhibitors: From Molecular Discovery to Clinical Application. Pflug. Arch. 2020, 472, 1177–1206. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Maunakea, A.; Jun, P.; Bollen, A.W.; Hodgson, J.G.; Goldenberg, D.D.; Weiss, W.A.; Costello, J.F. Shared Epigenetic Mechanisms in Human and Mouse Gliomas Inactivate Expression of the Growth Suppressor SLC5A8. Cancer Res. 2005, 65, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Kaji, I.; Iwanaga, T.; Watanabe, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D.; Akiba, Y. SCFA Transport in Rat Duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G188–G197. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, M.; Ananth, S.; Martin, P.M.; Roon, P.; Smith, S.B.; Sterneck, E.; Prasad, P.D.; Ganapathy, V. C/Ebpdelta Null Mouse as a Model for the Double Knock-out of Slc5a8 and Slc5a12 in Kidney. J. Biol. Chem. 2006, 281, 26769–26773. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.; Gröger, N.; Diener, M.; Becker, C.; Braun, T.; Boettger, T. Lactaturia and Loss of Sodium-Dependent Lactate Uptake in the Colon of SLC5A8-Deficient Mice. J. Biol. Chem. 2008, 283, 24729–24737. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Shiraishi, K.; Koga, T.; Motooka, Y.; Fujino, K.; Shibata, H.; Mori, T.; Suzuki, M. Prognostic Significance of Aberrant Methylation of Solute Carrier Gene Family 5A8 in Lung Adenocarcinoma. Ann. Thorac. Surg. 2015, 99, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, S.; Detich, N.; Szyf, M. Valproate Induces Widespread Epigenetic Reprogramming Which Involves Demethylation of Specific Genes. Carcinogenesis 2007, 28, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.; Ribeiro, A.S.; Taipa, R.; Castro, D.S.; Reis, J.; Faria, C.; Paredes, J. Cadherin Expression and Emt: A Focus on Gliomas. Biomedicines 2021, 9, 1328. [Google Scholar] [CrossRef] [PubMed]
- Förster, A.; Brand, F.; Banan, R.; Hüneburg, R.; Weber, C.A.M.; Ewert, W.; Kronenberg, J.; Previti, C.; Elyan, N.; Beyer, U.; et al. Rare Germline Variants in the E-Cadherin Gene CDH1 Are Associated with the Risk of Brain Tumors of Neuroepithelial and Epithelial Origin. Acta Neuropathol. 2021, 142, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Tuffin, L.J.; Rodriguez, F.; Giannini, C.; Scheithauer, B.; Necela, B.M.; Sarkaria, J.N.; Anastasiadis, P.Z. Misregulated E-Cadherin Expression Associated with an Aggressive Brain Tumor Phenotype. PLoS ONE 2010, 5, e13665. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.J.; Scheithauer, B.W.; Giannini, C.; Bryant, S.C.; Jenkins, R.B. Epithelial and Pseudoepithelial Differentiation in Glioblastoma and Gliosarcoma: A Comparative Morphologic and Molecular Genetic Study. Cancer 2008, 113, 2779–2789. [Google Scholar] [CrossRef] [PubMed]
- Belut, D.R.; Lima, E.D.O.; Zanini, M.A.; Galvani, A.F.; Furtado, F.B.; Ferrasi, A.C. CDH1 Hypermethylation: A Potential Molecular Pathway for Invasiveness in Glioblastoma. Eur. J. Cancer Prev. 2024, 33, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Chen, S.; Guo, Y.; Li, W.; Qi, X.; Yang, H.; Xiao, S.; Fang, G.; Hu, J.; Wen, C.; et al. Establishment and Evaluation of Four Different Types of Patient-Derived Xenograft Models. Cancer Cell Int. 2017, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.; Harper, K.; Brochu-Gaudreau, K.; Perreault, A.; Roy, L.O.; Lucien, F.; Tian, S.; Fortin, D.; Dubois, C.M. The Development of a Rapid Patient-Derived Xenograft Model to Predict Chemotherapeutic Drug Sensitivity/Resistance in Malignant Glial Tumors. Neuro Oncol. 2023, 25, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Tang, Z.; Li, Y.; Yin, G.; Liu, Z.; Gao, J.; Chen, Y.; Chen, F. Patient-Derived Xenografts of Different Grade Gliomas Retain the Heterogeneous Histological and Genetic Features of Human Gliomas. Cancer Cell Int. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.M.; Kim, J.; Jin, J.; Kim, M.; Seol, H.J.; Muradov, J.; Yang, H.; Choi, Y.L.; Park, W.Y.; Kong, D.S.; et al. Patient-Specific Orthotopic Glioblastoma Xenograft Models Recapitulate the Histopathology and Biology of Human Glioblastomas In Situ. Cell Rep. 2013, 3, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Piaskowski, S.; Bienkowski, M.; Stoczynska-Fidelus, E.; Stawski, R.; Sieruta, M.; Szybka, M.; Papierz, W.; Wolanczyk, M.; Jaskolski, D.J.; Liberski, P.P.; et al. Glioma Cells Showing IDH1 Mutation Cannot Be Propagated in Standard Cell Culture Conditions. Br. J. Cancer 2011, 104, 968–970. [Google Scholar] [CrossRef] [PubMed]
- William, D.; Mullins, C.S.; Schneider, B.; Orthmann, A.; Lamp, N.; Krohn, M.; Hoffmann, A.; Classen, C.F.; Linnebacher, M. Optimized Creation of Glioblastoma Patient Derived Xenografts for Use in Preclinical Studies. J. Transl. Med. 2017, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Stakišaitis, D.; Damanskienė, E.; Curkūnavičiūtė, R.; Juknevičienė, M.; Alonso, M.M.; Valančiūtė, A.; Ročka, S.; Balnytė, I. The Effectiveness of Dichloroacetate on Human Glioblastoma Xenograft Growth Depends on Na+ and Mg2+ Cations. Dose-Response 2021, 19, 1559325821990166. [Google Scholar] [CrossRef] [PubMed]
- ATCC: T98G [T98-G] CRL-1690TM Product Information. Available online: https://www.atcc.org/products/crl-1690 (accessed on 12 July 2025).
- Ribatti, D. Chapter 5 Chick Embryo Chorioallantoic Membrane as a Useful Tool to Study Angiogenesis. Int. Rev. Cell Mol. Biol. 2008, 270, 181–224. [Google Scholar] [CrossRef] [PubMed]
- Stakišaitis, D.; Valančiūtė, A.; Balnytė, I.; Lesauskaitė, V.; Juknevičienė, J.; Stanevičiūtė, J.; Jasaitis, D.J. Valpro Rūgšties ir Dichloroacetato Druskų Derinio Taikymas Vėžio Gydymui. LT6874B, 25 October 2021. [Google Scholar]
- European Patent Application. Derivative of Valproic Acid and Dichloroacetate Used for the Treatment of Cancer. European Patent Application No. 21168796.7, 16 April 2021. Available online: https://register.epo.org/application?number=EP21168796 (accessed on 6 November 2024).
- Šlekienė, L.; Stakišaitis, D.; Balnytė, I.; Valančiūtė, A. Sodium Valproate Inhibits Small Cell Lung Cancer Tumor Growth on the Chicken Embryo Chorioallantoic Membrane and Reduces the P53 and EZH2 Expression. Dose-Response 2018, 16, 1559325818772486. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Perazzoli, G.; Cabeza, L.; Jiménez-Luna, C.; Luque, R.; Prados, J.; Melguizo, C. Temozolomide: An Updated Overview of Resistance Mechanisms, Nanotechnology Advances and Clinical Applications. Curr. Neuropharmacol. 2021, 19, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Control and Treated Study Group | Invasion, No. of Vessels, CAM Thickness | PCNA | p53 | EZH2 | ||||
|---|---|---|---|---|---|---|---|---|
| n | ||||||||
| U87 | T98G | U87 | T98G | U87 | T98G | U87 | T98G | |
| Control | 20 | 12 | 13 | 9 | 15 | 9 | 17 | 9 |
| 3 mM NaDCA | 20 | 12 | 18 | 10 | 20 | 11 | 19 | 11 |
| 50 µM TMZ | 15 | 16 | 8 | 6 | 7 | 7 | 8 | 7 |
| 2 mM VPA–3 mM NaDCA | 10 | 12 | 10 | 8 | 10 | 8 | 10 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skredėnienė, R.; Stakišaitis, D.; Valančiūtė, A.; Balnytė, I. In Vivo and In Vitro Experimental Study Comparing the Effect of a Combination of Sodium Dichloroacetate and Valproic Acid with That of Temozolomide on Adult Glioblastoma. Int. J. Mol. Sci. 2025, 26, 6784. https://doi.org/10.3390/ijms26146784
Skredėnienė R, Stakišaitis D, Valančiūtė A, Balnytė I. In Vivo and In Vitro Experimental Study Comparing the Effect of a Combination of Sodium Dichloroacetate and Valproic Acid with That of Temozolomide on Adult Glioblastoma. International Journal of Molecular Sciences. 2025; 26(14):6784. https://doi.org/10.3390/ijms26146784
Chicago/Turabian StyleSkredėnienė, Rūta, Donatas Stakišaitis, Angelija Valančiūtė, and Ingrida Balnytė. 2025. "In Vivo and In Vitro Experimental Study Comparing the Effect of a Combination of Sodium Dichloroacetate and Valproic Acid with That of Temozolomide on Adult Glioblastoma" International Journal of Molecular Sciences 26, no. 14: 6784. https://doi.org/10.3390/ijms26146784
APA StyleSkredėnienė, R., Stakišaitis, D., Valančiūtė, A., & Balnytė, I. (2025). In Vivo and In Vitro Experimental Study Comparing the Effect of a Combination of Sodium Dichloroacetate and Valproic Acid with That of Temozolomide on Adult Glioblastoma. International Journal of Molecular Sciences, 26(14), 6784. https://doi.org/10.3390/ijms26146784





