Sex-Dependent Effects of Aging and Insulin Resistance on Skeletal Muscle Function and Structure in Rats
Abstract
1. Introduction
2. Results
2.1. The Impact of Aging and Insulin Resistance on Hormonal Levels and Muscle Mass in Male and Female Rats
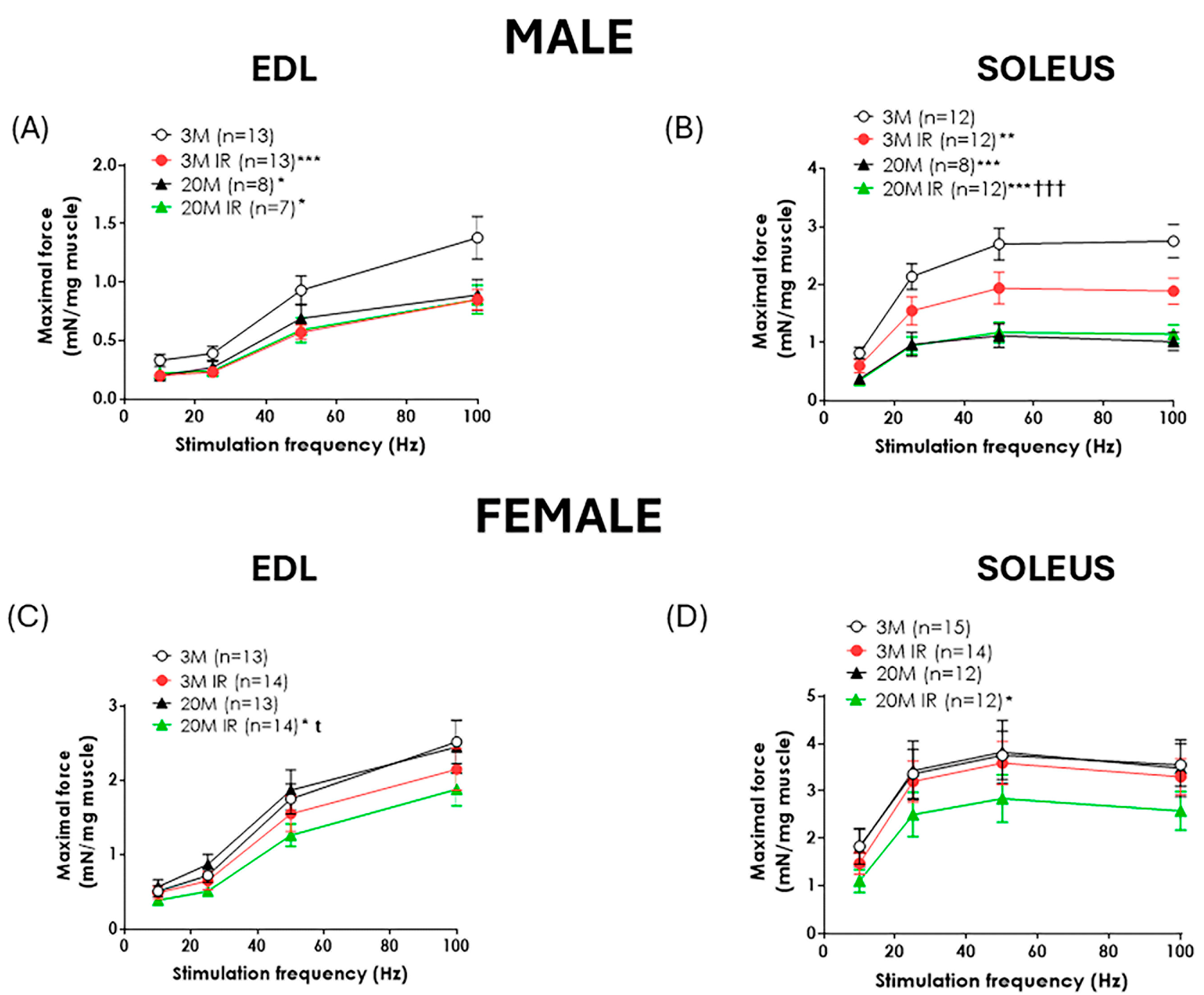
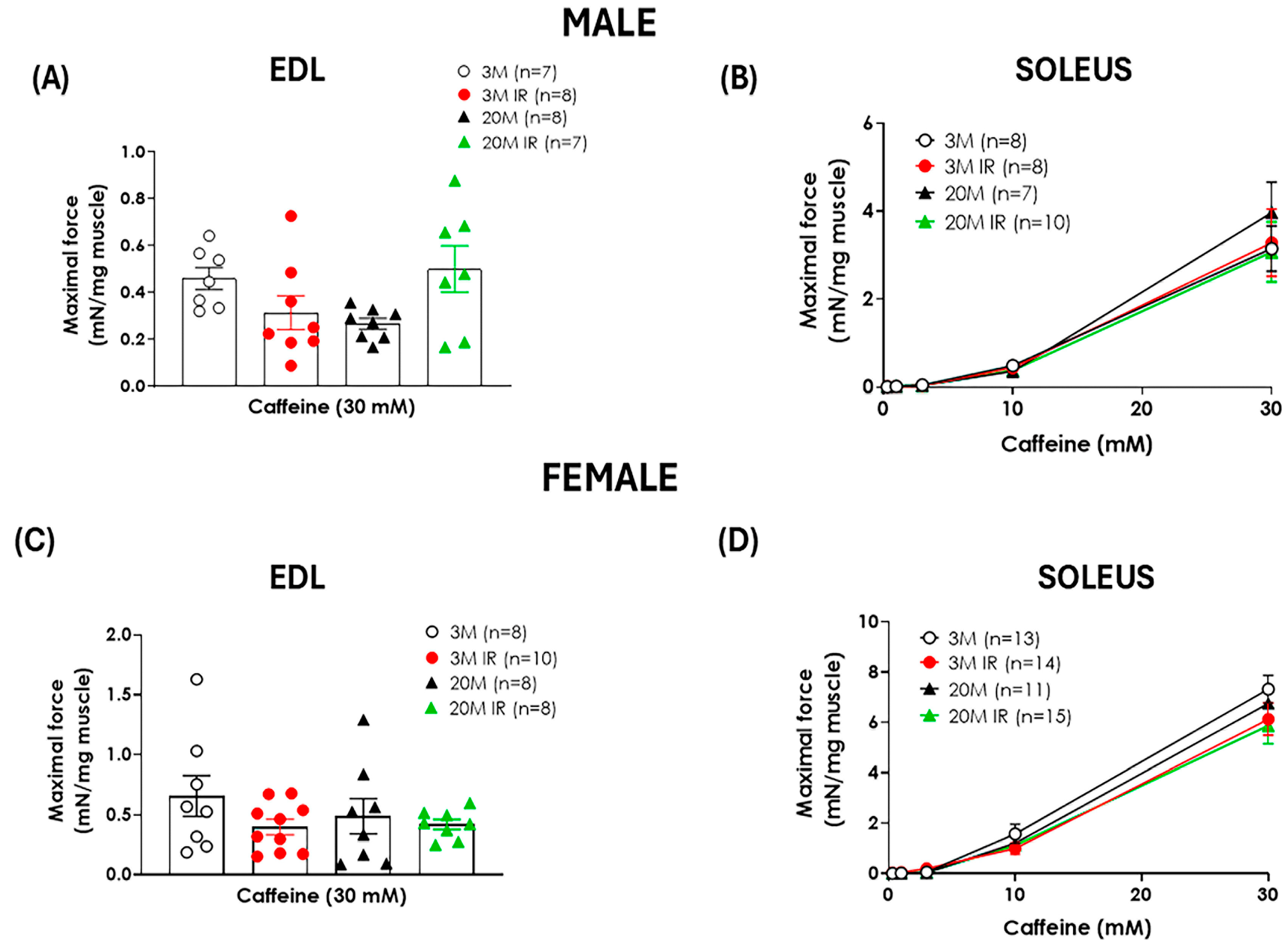
2.2. Sex Influences the Impact of Aging and Insulin Resistance on Muscle Function
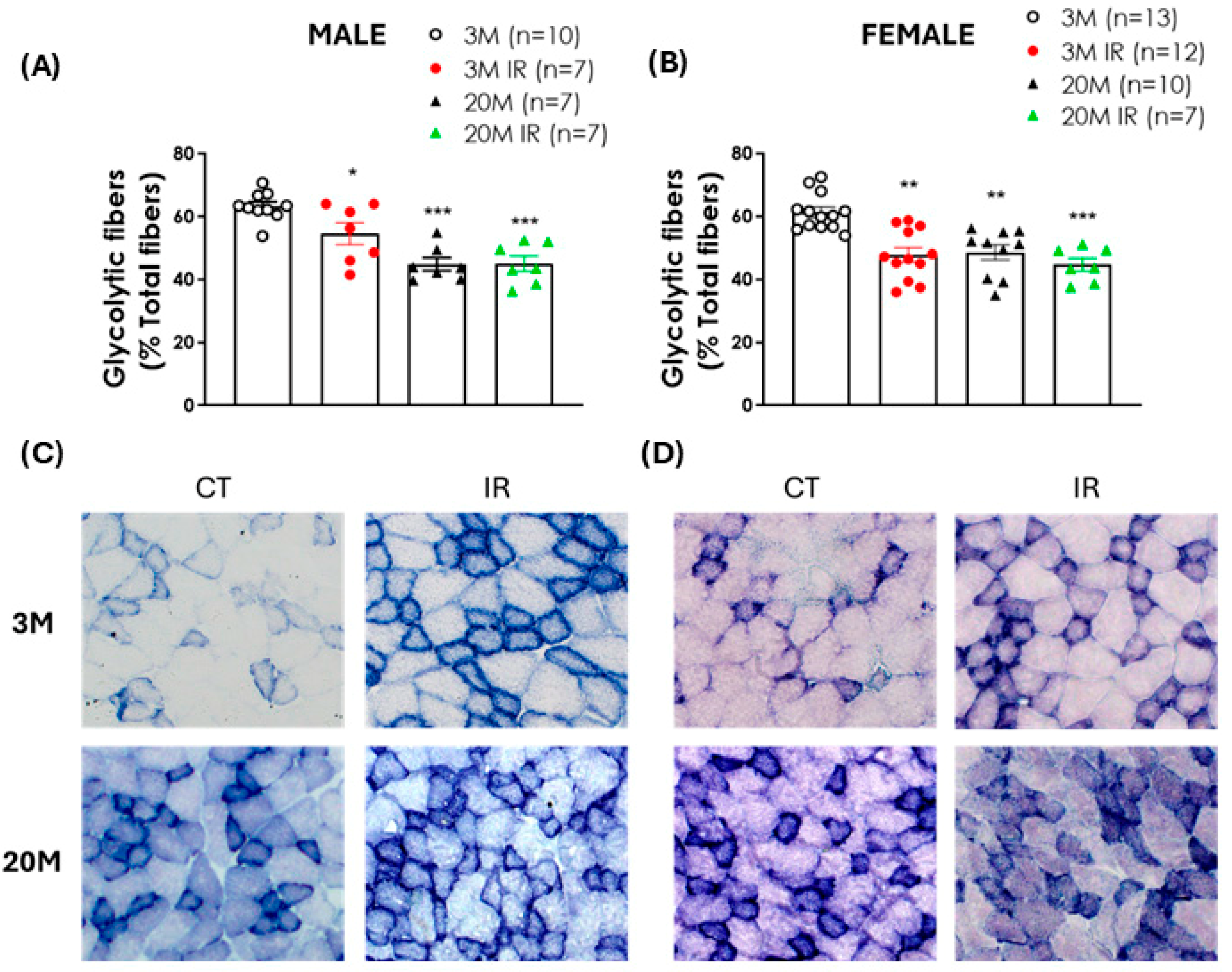
2.3. Impact of Aging and IR on Glycolytic Fiber Content in EDL Muscle
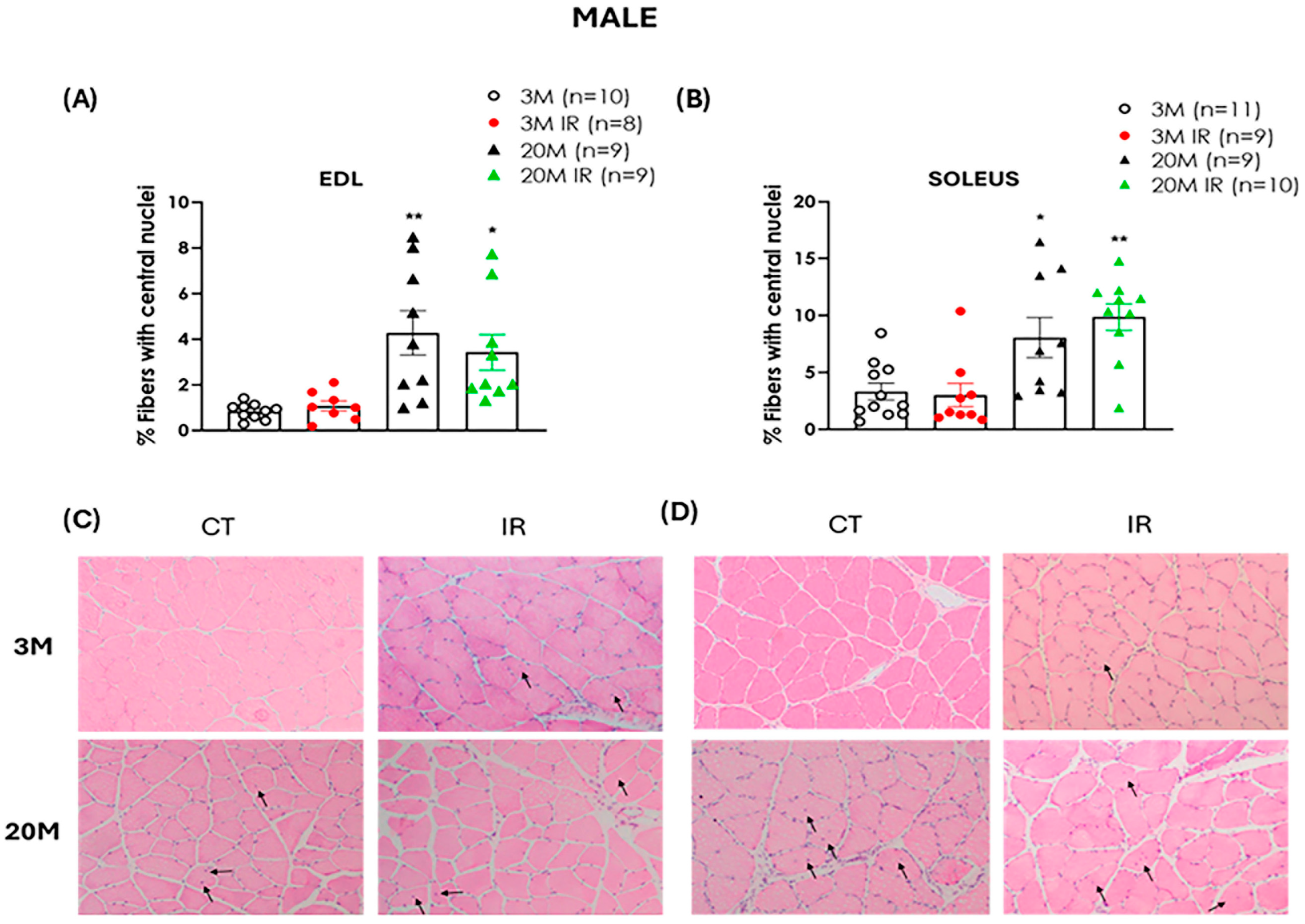
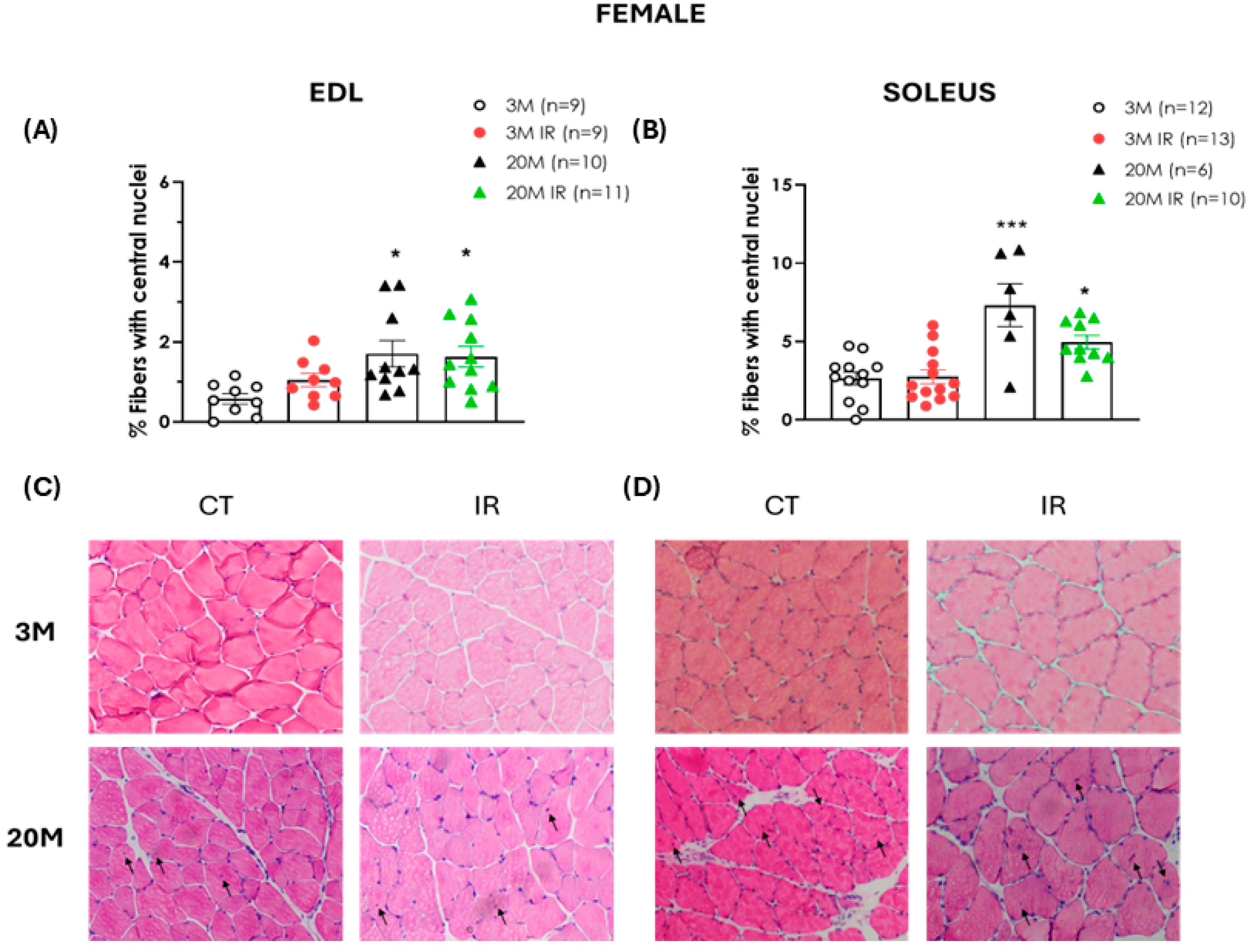
2.4. Aging Is a Determinant Factor in Altering Muscle Regenerative Capacity
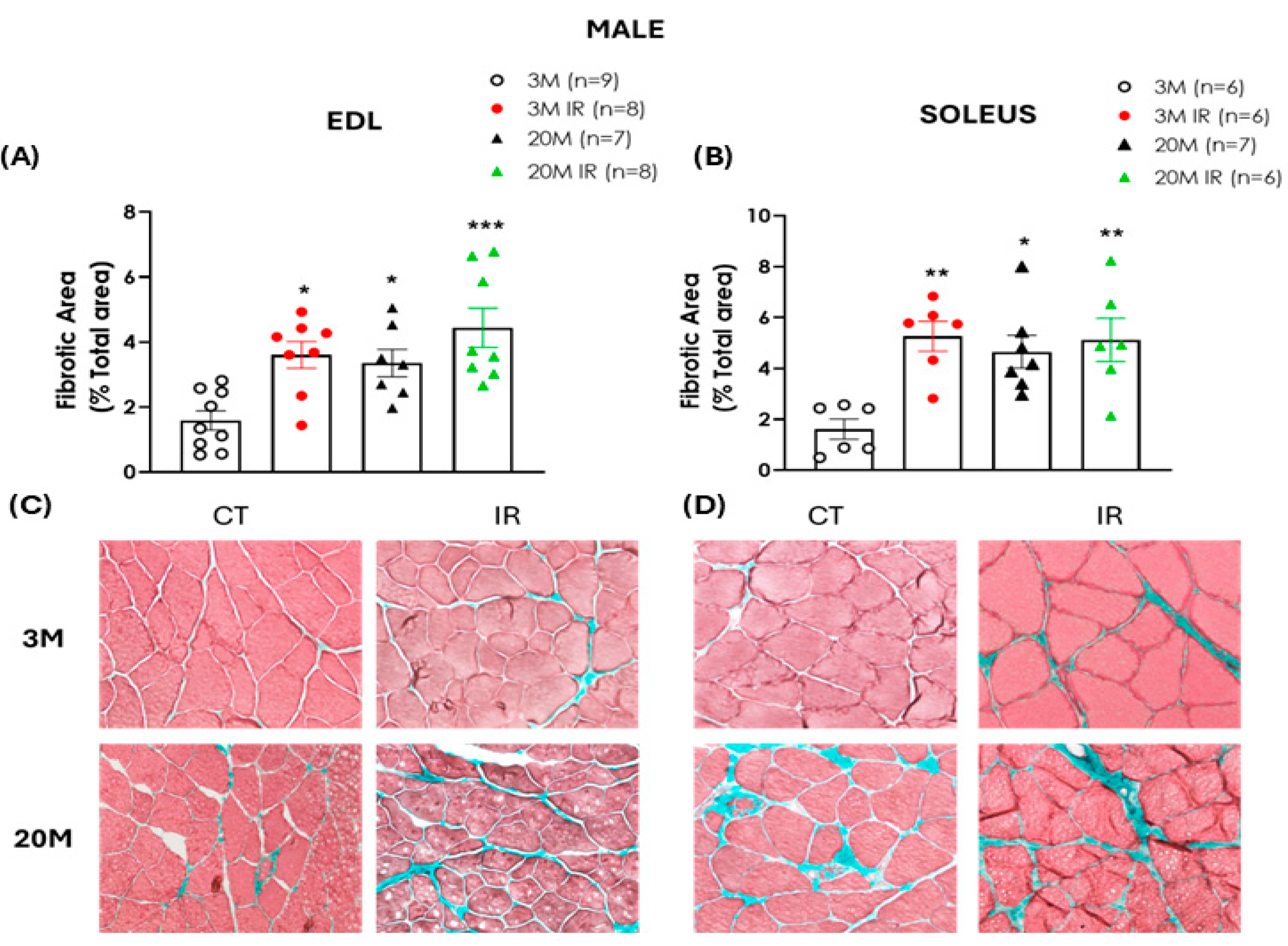
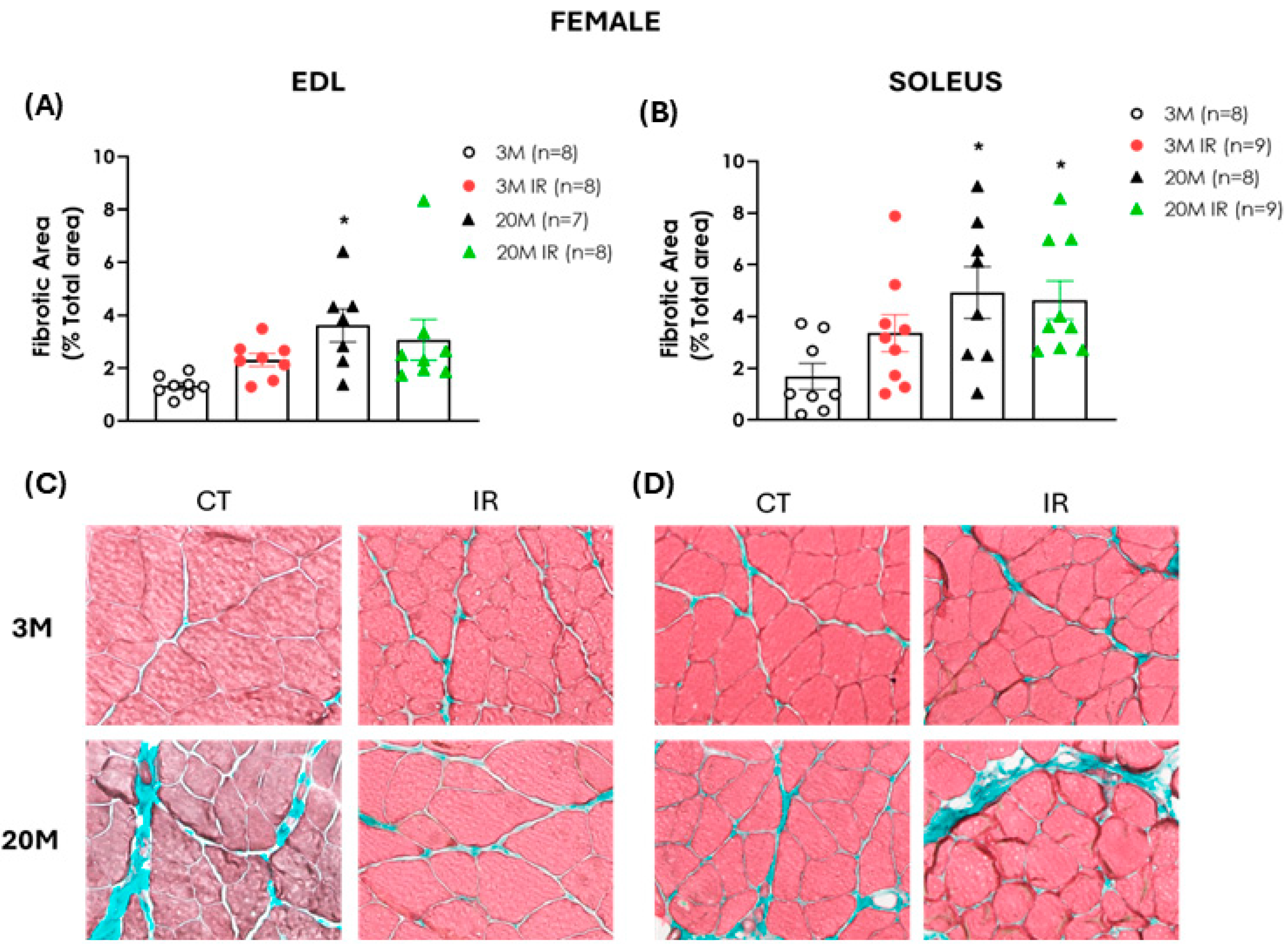
2.5. The Impact of Aging and Insulin Resistance on Muscle Fibrosis in Male and Female Rats
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Biochemical Determinations
4.3. Determination of Contractile Responses of EDL and Soleus Muscles to Electrical Stimulation
4.4. Histological Staining
4.4.1. Hematoxylin and Eosin (H&E) Staining
4.4.2. Succinate Dehydrogenase Assay (SDH)
4.4.3. Masson’s Trichrome Staining
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Warner, M.E.; Zhang, X.; Guillemot, J. Demographic Ageing: An Opportunity to Rethink Economy, Society and Regions. Cambridge J. Reg. Econ. Soc. 2024, 18, 79–92. [Google Scholar] [CrossRef]
- Olshansky, S.J. From Lifespan to Healthspan. JAMA—J. Am. Med. Assoc. 2018, 320, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mañas, L.; Rodríguez-Artalejo, F.; Sinclair, A.J. The Third Transition: The Clinical Evolution Oriented to the Contemporary Older Patient. J. Am. Med. Dir. Assoc. 2017, 18, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud. Acción Multisectorial Para Un Envejecimiento Saludable Basado En El Ciclo de Vida: Proyecto de Estrategia y Plan de Acción Mundiales Sobre El Envejecimiento y La Salud. Organ. Mund. Salud 2016, A69, 1–43. [Google Scholar]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) Approach to Healthy Ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Brocca, L.; McPhee, J.S.; Longa, E.; Canepari, M.; Seynnes, O.; Vito, G.D.; Pellegrino, M.A.; Narici, M.; Bottinelli, R. Structure and Function of Human Muscle Fibres and Muscle Proteome in Physically Active Older Men. J. Physiol. 2017, 595, 4823–4844. [Google Scholar] [CrossRef] [PubMed]
- Chinvattanachot, G.; Rivas, D.; Duque, G. Mechanisms of Muscle Cells Alterations and Regeneration Decline during Aging. Ageing Res. Rev. 2024, 102, 102589. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Behnke, B.J.; Arjmandi, B.; Ghosh, P.; Chen, B.; Brooks, R.; Maraj, J.J.; Elam, M.L.; Maher, P.; Kurien, D.; et al. Daily Muscle Stretching Enhances Blood Flow, Endothelial Function, Capillarity, Vascular Volume and Connectivity in Aged Skeletal Muscle. J. Physiol. 2018, 596, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, Y.; Miyachi, R.; Higuchi, T.; Sato, H. Effects of Aging on Collagen in the Skeletal Muscle of Mice. Int. J. Mol. Sci. 2023, 24, 13121. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-K.; Lin, L.-Y.; Yu, Y.-H.; Wu, K.-H.; Kuo, H.-K. Inverse Association between Insulin Resistance and Gait Speed in Nondiabetic Older Men: Results from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2002. BMC Geriatr. 2009, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, J.I.; Cotsonis, G.A.; Walston, J.; Schwartz, A.V.; Satterfield, S.; Miljkovic, I.; Harris, T.B. Insulin Resistance Is Associated with Decreased Quadriceps Muscle Strength in Nondiabetic Adults Aged ≥70 Years. Diabetes Care 2009, 32, 736–738. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Carnicero, J.A.; Angulo, J.; Cámara-Hernández, V.; García-García, F.J.; Rodríguez-Mañas, L. Fat Mass Accounts for Insulin Resistance Impact on Functional Decline and Mortality in Nondiabetic Older Adults. J. Am. Med. Dir. Assoc. 2024, 25, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and Skeletal Muscle Mass: The Role of IGF Signalling, the Sirtuins, Dietary Restriction and Protein Intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Templeman, N.M.; Flibotte, S.; Chik, J.H.L.; Sinha, S.; Lim, G.E.; Foster, L.J.; Nislow, C.; Johnson, J.D. Reduced Circulating Insulin Enhances Insulin Sensitivity in Old Mice and Extends Lifespan. Cell Rep. 2017, 20, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gatineau, E.; Savary-Auzeloux, I.; Migné, C.; Polakof, S.; Dardevet, D.; Mosoni, L. Chronic Intake of Sucrose Accelerates Sarcopenia in Older Male Rats through Alterations in Insulin Sensitivity and Muscle Protein Synthesis. J. Nutr. 2015, 145, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Choi, J.W.; Park, J.H.; Kim, D.S.; Ahn, Y.; Kim, Y.; Kong, S.H.; Oh, C.M. Association of Appendicular Skeletal Muscle Mass Index and Insulin Resistance With Mortality in Multi-Nationwide Cohorts. J. Cachexia Sarcopenia Muscle 2025, 16, e13811. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Rizzo, M. Age-Related Changes in Insulin Resistance and Muscle Mass: Clinical Implications in Obese Older Adults. Medicina 2024, 60, 1648. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Nkembo, A.; Tipparaju, S.M.; Ashraf, M.; Wanling, X. Sarcopenia: Recent Advances for Detection, Progression and Metabolic Alterations along with Therapeutic Targets. Can. J. Physiol. Pharmacol. 2024, 102, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Knaub, L.A.; Hull, S.E.; Pott, G.B.; Peelor, R.; Miller, B.F.; Shankar, K.; Rudolph, M.C.; Reusch, J.E.B.; Scalzo, R.L. Sex Differences in the Skeletal Muscle Response to a High Fat, High Sucrose Diet in Rats. Nutrients 2023, 15, 4438. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Jensen, M.D. Metabolic Changes in Aging Humans: Current Evidence and Therapeutic Strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, S.; Grillone, G.; Michele, R.D.; Cortesi, M. A Comparison between Male and Female Athletes in Relative Strength and Power Performances. J. Funct. Morphol. Kinesiol. 2021, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Carnicero, J.A.; Molina-Baena, B.; García-García, F.J.; Sosa, P.; Rodríguez-Mañas, L. Gender-Specific Capacity of Insulin Resistance Proxies to Predict Functional Decline in Older Adults. J. Nutr. Health Aging 2024, 28, 100376. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Santos-Ruiz, M.; Moreno, P.; Novials, A.; Villanueva-Peñacarrillo, M.L.; Rodríguez-Mañas, L. Differential Effect of Amylin on Endothelial-Dependent Vasodilation in Mesenteric Arteries from Control and Insulin Resistant Rats. PLoS ONE 2015, 10, e0120479. [Google Scholar] [CrossRef] [PubMed]
- Assar, M.E.; Sánchez-Puelles, J.M.; Royo, I.; López-Hernández, E.; Sánchez-Ferrer, A.; Aceña, J.L.; Rodríguez-Mañas, L.; Angulo, J. FM19G11 Reverses Endothelial Dysfunction in Rat and Human Arteries through Stimulation of the PI3K/Akt/ENOS Pathway, Independently of MTOR/HIF-1α Activation. Br. J. Pharmacol. 2015, 172, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, Epidemiology, and Pathophysiology. J. Bone Metab. 2013, 20, 1090254. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Muñoz-Aguirre, M.; Kim-Hellmuth, S.; Wucher, V.; Gewirtz, A.D.H.; Cotter, D.J.; Parsana, P.; Kasela, S.; Balliu, B.; Viñuela, A.; et al. The Impact of Sex on Gene Expression across Human Tissues. Science 2020, 369, eaba3066. [Google Scholar] [CrossRef] [PubMed]
- Cully, T.R.; Edwards, J.N.; Murphy, R.M.; Launikonis, B.S. A Quantitative Description of Tubular System Ca2+ Handling in Fast- and Slow-Twitch Muscle Fibres. J. Physiol. 2016, 594, 2795–2810. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.L.; Warren, G.L.; Lowe, D.A. Soleus and EDL Muscle Contractility across the Lifespan of Female C57BL/6 Mice. Exp. Gerontol. 2005, 40, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Edwén, C.E.; Thorlund, J.B.; Magnusson, S.P.; Slinde, F.; Svantesson, U.; Hulthén, L.; Aagaard, P. Stretch-Shortening Cycle Muscle Power in Women and Men Aged 18–81 Years: Influence of Age and Gender. Scand. J. Med. Sci. Sports 2014, 24, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; James, R.S.; Cox, V.M.; Seebacher, F.; Tallis, J. Age-Related Changes in Isolated Mouse Skeletal Muscle Function Are Dependent on Sex, Muscle, and Contractility Mode. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2020, 319, R296–R314. [Google Scholar] [CrossRef] [PubMed]
- Irion, G.L.; Vasthare, U.S.; Tuma, R.F. Age-Related Change in Skeletal Muscle Blood Flow in the Rat. J. Gerontol. 1987, 42, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Irion, G.L.; Vasthare, U.S.; Tuma, R.F. Preservation of Skeletal Muscle Hyperemic Response to Contraction with Aging in Female Rats. Exp. Gerontol. 1988, 23, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pérez, Y.; Gianotti, M.; Proenza, A.M.; Lladó, I. Age-Related Decline of Skeletal Muscle Insulin Sensitivity in Rats: Effect of Sex and Muscle Type. Rejuvenation Res. 2011, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gado, M.; Tsaousidou, E.; Bornstein, S.R.; Perakakis, N. Sex-Based Differences in Insulin Resistance. J. Endocrinol. 2024, 261, e230245. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, M.; Khathi, A. Investigating the Effects of Diet-Induced Prediabetes on Skeletal Muscle Strength in Male Sprague Dawley Rats. Int. J. Mol. Sci. 2024, 25, 4076. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.E.; Lou, P.-H.; Lucchinetti, E.; Zhang, L.; Clanachan, A.S.; Affolter, A.; Hersberger, M.; Zaugg, M.; Lemieux, H. Early Mitochondrial Dysfunction in Glycolytic Muscle, but Not Oxidative Muscle, of the Fructose-Fed Insulin-Resistant Rat. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E658–E667. [Google Scholar] [CrossRef] [PubMed]
- Chaves, D.F.S.; Carvalho, P.C.; Lima, D.B.; Nicastro, H.; Lorenzeti, F.M.; Siqueira-Filho, M.; Hirabara, S.M.; Alves, P.H.M.; Moresco, J.J.; Yates, J.R.; et al. Comparative Proteomic Analysis of the Aging Soleus and Extensor Digitorum Longus Rat Muscles Using TMT Labeling and Mass Spectrometry. J. Proteome Res. 2013, 12, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle Metabolism and Atrophy: Let’s Talk about Sex. Biol. Sex Differ. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Critchlow, A.J.; Hiam, D.; Williams, R.; Scott, D.; Lamon, S. The Role of Estrogen in Female Skeletal Muscle Aging: A Systematic Review. Maturitas 2023, 178, 107844. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Alencar, A.; Lin, M.; Sun, X.; Sudo, R.T.; Zapata-Sudo, G.; Lowe, D.A.; Groban, L. Activation of GPR30 Improves Exercise Capacity and Skeletal Muscle Strength in Senescent Female Fischer344 × Brown Norway Rats. Biochem. Biophys. Res. Commun. 2016, 475, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Fernández, R.; Moreno, M.; Ordóñez, P.; González-Pardo, H.; Conejo, N.M.; Díaz, F.; González, C. Positive Effects of 17β-Estradiol on Insulin Sensitivity in Aged Ovariectomized Female Rats. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2006, 61, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sarbjit-Singh, S.S.; Matthews, H.R.; Huang, C.L.H. Ryanodine Receptor Modulation by Caffeine Challenge Modifies Na+ Current Properties in Intact Murine Skeletal Muscle Fibres. Sci. Rep. 2020, 10, 2199. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Shah, S.B.; Lovering, R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021, 22, 8058. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial Degeneration Precedes the Development of Muscle Atrophy in Progression of Cancer Cachexia in Tumour-Bearing Mice. J. Cachexia. Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for Fiber-Type Specificity of Skeletal Muscle Atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial Dysfunction and Sarcopenia of Aging: From Signaling Pathways to Clinical Trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramírez-Pardo, I.; Segalés, J.; Hernansanz-Agustín, P.; Curtabbi, A.; Deryagin, O.; Pollán, A.; et al. Mitochondrial Dynamics Maintain Muscle Stem Cell Regenerative Competence throughout Adult Life by Regulating Metabolism and Mitophagy. Cell Stem Cell 2022, 29, 1298–1314.e10. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Muñoz-Cánoves, P. Regenerative Decline of Stem Cells in Sarcopenia. Mol. Aspects Med. 2016, 50, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Bennett, B.T.; Wilson, J.C.; Edens, N.K.; Pereira, S.L. Epigallocatechin-3-Gallate Improves Plantaris Muscle Recovery after Disuse in Aged Rats. Exp. Gerontol. 2014, 50, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Folker, E.S.; Baylies, M.K. Nuclear Positioning in Muscle Development and Disease. Front. Physiol. 2013, 4, 363. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, R.; Matsuoka, T.; Nakanishi, K.; Tani, A.; Kakimoto, S.; Kato, Y.; Kawatani, T.; Nakagawa, S.; Baba, Y.; Kobayashi, M.; et al. Effects of Green Tea Catechins and Exercise on Age-Related Muscle Atrophy and Satellite Cell Functions in a Mouse Model of Sarcopenia. Exp. Gerontol. 2025, 202, 112720. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.A.; Fry, C.S.; Kirby, T.J.; Jackson, J.R.; Lee, J.D.; White, S.H.; Dupont-Versteegden, E.E.; McCarthy, J.J.; Peterson, C.A. Starring or Supporting Role? Satellite Cells and Skeletal Muscle Fiber Size Regulation. Physiology 2018, 33, 26. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, G.; Fernandes, K.; Williams, P.E.; Wells, D.J. Age-Related Changes in Collagen Gene Expression in the Muscles of Mdx Dystrophic and Normal Mice. Neuromuscul. Disord. 1994, 4, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Jiang, Y.; Barnes, R.H.; Tokunaga, M.; Martinez-Santibañez, G.; Geletka, L.; Lumeng, C.N.; Buchner, D.A.; Chun, T.H. Thrombospondin 1 Mediates High-Fat Diet-Induced Muscle Fibrosis and Insulin Resistance in Male Mice. Endocrinology 2013, 154, 4548–4559. [Google Scholar] [CrossRef] [PubMed]
- Lacraz, G.; Rouleau, A.-J.; Couture, V.; Söllrald, T.; Drouin, G.; Veillette, N.; Grandbois, M.; Grenier, G. Increased Stiffness in Aged Skeletal Muscle Impairs Muscle Progenitor Cell Proliferative Activity. PLoS ONE 2015, 10, e0136217. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and Adipogenesis Originate from a Common Mesenchymal Progenitor in Skeletal Muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, H.; Lee, I.H.; Modi, S.; Wang, X.; Du, J.; Mitch, W.E. PTEN Inhibition Improves Muscle Regeneration in Mice Fed a High-Fat Diet. Diabetes 2010, 59, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Shavlakadze, T.; Xiong, K.; Mishra, S.; McEwen, C.; Gadi, A.; Wakai, M.; Salmon, H.; Stec, M.J.; Negron, N.; Ni, M.; et al. Related Gene Expression Signatures from Limb Skeletal Muscles and the Diaphragm in Mice and Rats Reveal Common and Species-Specific Changes. Skelet. Muscle 2023, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Fl-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
| 3 M | 3 M IR | 20 M | 20 M IR | |
|---|---|---|---|---|
| MALES | ||||
| Body weight (g) | 488.7 ± 26.06 (n = 20) | 512.9 ± 30.33 (n = 15) | 674.3 ± 36.02 (n = 11) ** † | 661.5 ± 47.99 (n = 15) ** † |
| Serum FT (pg/mL) | 5.419 ± 0.77 (n = 13) | 6.49 ± 1.24 (n = 14) | 1.64 ± 0.36 (n = 10) * †† | 1.90 ± 0.48 (n = 12) * †† |
| % EDL mass (muscle/body weight) | 0.041 ± 0.001 (n = 10) | 0.042 ± 0.003 (n = 8) | 0.032 ± 0.001 (n = 10) ** †† | 0.030 ± 0.001 (n = 10) ** †† |
| % Soleus mass (muscle/body weight) | 0.048 ± 0.001 (n = 11) | 0.045 ± 0.002 (n = 8) | 0.037 ± 0.001 (n = 8) ** | 0.040 ± 0.002 (n = 9) * |
| FEMALES | ||||
| Body weight (g) | 286.2 ± 9.89 (n = 30) | 313.1 ± 9.94(n = 22) | 389.1 ± 11.74 (n = 28) *** †† | 440 ± 26.37 (n = 19) *** ††† |
| Serum estradiol (pg/mL) | 27.26 ± 2.87 (n = 9) | 24.23 ± 2.37 (n = 10) | 21.46 ± 2.53 (n = 10) | 15.68 ± 1.27 (n = 9) ** |
| % EDL mass (muscle/body weight) | 0.043 ± 0.002 (n = 10) | 0.042 ± 0.001 (n = 10) | 0.033 ± 0.002 (n = 9) ** †† | 0.026 ± 0.002 (n = 15) *** ††† t |
| % Soleus mass (muscle/body weight) | 0.057 ± 0.003 (n = 9) | 0.055 ± 0.001 (n = 10) | 0.042 ± 0.004 (n = 9) * † | 0.029 ± 0.002 (n = 12) *** ††† t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa, P.; Angulo, J.; Sánchez-Ferrer, A.; Gómez-Cabrera, M.C.; Fernández, A.; Rodríguez-Mañas, L.; El Assar, M. Sex-Dependent Effects of Aging and Insulin Resistance on Skeletal Muscle Function and Structure in Rats. Int. J. Mol. Sci. 2025, 26, 6783. https://doi.org/10.3390/ijms26146783
Sosa P, Angulo J, Sánchez-Ferrer A, Gómez-Cabrera MC, Fernández A, Rodríguez-Mañas L, El Assar M. Sex-Dependent Effects of Aging and Insulin Resistance on Skeletal Muscle Function and Structure in Rats. International Journal of Molecular Sciences. 2025; 26(14):6783. https://doi.org/10.3390/ijms26146783
Chicago/Turabian StyleSosa, Patricia, Javier Angulo, Alberto Sánchez-Ferrer, Maria Carmen Gómez-Cabrera, Argentina Fernández, Leocadio Rodríguez-Mañas, and Mariam El Assar. 2025. "Sex-Dependent Effects of Aging and Insulin Resistance on Skeletal Muscle Function and Structure in Rats" International Journal of Molecular Sciences 26, no. 14: 6783. https://doi.org/10.3390/ijms26146783
APA StyleSosa, P., Angulo, J., Sánchez-Ferrer, A., Gómez-Cabrera, M. C., Fernández, A., Rodríguez-Mañas, L., & El Assar, M. (2025). Sex-Dependent Effects of Aging and Insulin Resistance on Skeletal Muscle Function and Structure in Rats. International Journal of Molecular Sciences, 26(14), 6783. https://doi.org/10.3390/ijms26146783






