Liposomes and Extracellular Vesicles as Distinct Paths Toward Precision Glioma Treatment
Abstract
1. Introduction
2. Overview of Liposomes and EVs
2.1. Structural Features and Classification of Liposomes
2.2. Structural and Biogenetic Properties of EVs
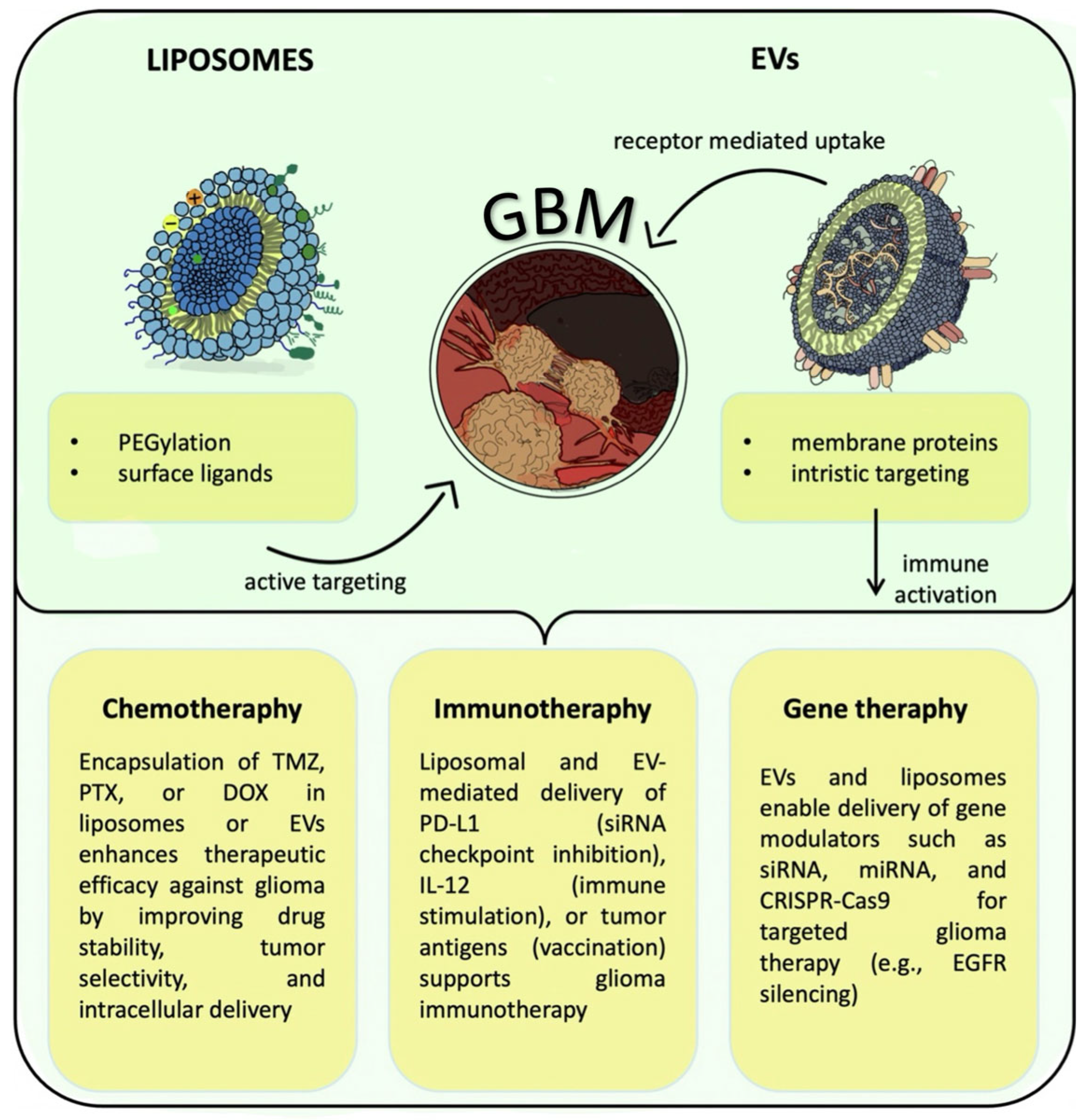
3. Applications of EVs and Liposomes in Glioma Therapy
3.1. Chemotherapy
3.2. Immunotherapy
3.3. Gene Therapy
4. Challenges and Future Directions
4.1. The Complexity of Crossing the Brain’s Defenses
4.2. Cargo Capacity vs. Cargo Compatibility
4.3. The Manufacturing Divide
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| GBM | Glioblastoma multiforme |
| BBB | Blood–Brain Barrier |
| EVs | Extracellular Vesicles |
| SUVs | Small Unilamellar Vesicles |
| LUVs | Large Unilamellar Vesicles |
| MLVs | Multilamellar Vesicles |
| MVVs | Multivesicular Vesicles |
| GUVs | Giant Unilamellar Vesicles |
| EPR | Enhanced Permeability and Retention |
| PEG | Polyethylene glycol |
| MVBs | Multivesicular bodies |
| PTX | Paclitaxel |
| NIR-II | Near-infrared II |
| mRNA | Messenger ribonucleic acid |
| miRNA | Micro-ribonucleic acid |
| gDNA | Genomic DNA |
| TMZ | Temozolomide |
| MGMT | O-6-methylguanine-DNA methyltransferase |
| CED | convection-enhanced delivery |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DOX | Doxorubicin |
| SCF | Supercritical fluid |
| TME | Tumor microenvironment |
| TAMs | Tumor-associated macrophages regulatory |
| Tregs | Regulatory T cells |
| MDSCs | Myeloid-derived suppressor cells |
| siRNA | small interfering ribonucleic acid |
| PD-L1 | Programmed death-ligand 1 |
| CpG | Cytosine–phosphate–guanine oligonucleotide |
| CTL | Cytotoxic T lymphocyte |
| Dexosomes | Dendritic cell-derived exosomes |
| MHC | Major histocompatibility complex |
| IL-2 | Interleukin-2 |
| IL-12 | Interleukin-12 |
| PD-1 | Programmed cell death protein 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| Il-6 | Interleukin-6 |
| IL-21 | Interleukin-21 |
| IL-7 | Interleukin-7 |
| LPN | Lipid nanoparticle |
| GSC | Glioma stem-like cell |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats associated protein 9 |
| EGFR | Epidermal growth factor receptor |
| RMT | Receptor-mediated transcytosis |
| AMT | Adsorptive-mediated transcytosis |
| gRNA | Guide ribonucleic acid |
| RGD | Arginine–Glycine–Aspartic motif |
| Angipoep-2 | Peptide ligand facilitating BBB transcytosis |
| ABC | Accelerated blood clearance |
| MSCs | Mesenchymal stem cells |
| RVG | Neuron-targeting peptide |
| SPAAC | Strain-promoted azide–alkyne cycloaddition |
| FKBP | FK506-binding protein |
| FRB | FKBP–rapamycin binding domain |
| GMP | Good Manufacturing Practice |
| ISEV | International Society for Extracellular Vesicles |
| TGF-β | Transforming growth factor-β |
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood–brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Perets, N.; Betzer, O.; Shapira, R.; Brenstein, S.; Angel, A.; Sadan, T.; Ashery, U.; Popovtzer, R.; Offen, D. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 2019, 23, 552–561. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Engineering extracellular vesicle–based delivery systems for enhanced therapeutic efficacy: Current status and future directions. Acta Pharmacol. Sin. B 2023, 13, 545–564. [Google Scholar] [CrossRef]
- Del Bene, M.; Osti, D.; Faletti, S.; Beznoussenko, G.V.; DiMeco, F.; Pelicci, G. Extracellular vesicles: The key for precision medicine in glioblastoma. Neuro-Oncology 2022, 24, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Harmancey, R.; McPherson, D.D.; Kim, H.; Huang, S.L. Liposome-based carriers for CRISPR genome editing. Int. J. Mol. Sci. 2023, 24, 12844. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gao, Y.; Wang, L. Challenges and innovations in nanoparticle-mediated RNA delivery. Nat. Rev. Drug Discov. 2021, 20, 1014–1035. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role in drug targeting. Adv. Drug Deliv. Rev. 2012, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ningaraj, N.S.; Rao, M.; Hashizume, K.; Asotra, K.; Black, K.L. Regulation of blood–brain tumor barrier permeability by calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2002, 301, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Kawak, P.; Sawaftah, N.M.A.; Pitt, W.G.; Husseini, G.A. Transferrin-targeted liposomes in glioblastoma therapy: A review. Int. J. Mol. Sci. 2023, 24, 13262. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.; Khan, I.; Kartal, Z.; Mahfooz, S.; Hatiboglu, M.A.H. Status quo in the liposome-based therapeutic strategies against glioblastoma: ‘targeting the tumor and tumor microenvironment’. Int. J. Mol. Sci. 2024, 25, 11271. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002, 4, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, P.; Chakraborty, D.D.; Nath, L.K. Advancements in Nanoengineered Paclitaxel Formulations: A Comprehensive Review of Blood-Brain Barrier Infiltration Strategies for Glioblastoma Therapy. Biomed. Eng. Adv. 2024, 7, 100122. [Google Scholar] [CrossRef]
- Du, L.; Wang, P.; Huang, H.; Li, M.; Roy, S.; Zhang, Y.; Guo, B. Light-activatable and hyperthermia-sensitive ‘all-in-one’ theranostics: NIR-II fluorescence imaging and chemo-photothermal therapy of subcutaneous glioblastoma by temperature-sensitive liposome-containing AIEgens and paclitaxel. Front. Bioeng. Biotechnol. 2023, 11, 1343694. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Madhankumar, A.B.; Miller, P.A.; Duck, K.A.; Hafenstein, S.; Rizk, E.; Slagle-Webb, B.; Sheehan, J.M.; Connor, J.R.; Yang, Q.X. MRI contrast agent for targeting glioma: Interleukin-13 labeled liposome encapsulating gadolinium-DTPA. Neuro-Oncology 2016, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, D.; Gao, D.; Gong, P.; Zheng, H.; Sun, M.; Sheng, Z. Engineered apoptotic bodies hitchhiking across the blood-brain barrier achieved a combined photothermal-chemotherapeutic effect against glioma. Theranostics 2023, 13, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.P.K.; Holme, M.N.; Stevens, M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liang, R.; Erel-Akbaba, G.; Saad, L.; Obeid, P.J.; Gao, J.; Chiocca, E.A.; Weissleder, R.; Tannous, B.A. Immune checkpoint inhibition in GBM primed with radiation by engineered extracellular vesicles. ACS Nano 2022, 16, 1940–1953. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Tyagi, A.; Tyagi, N. Exosomes: Mediators of neurodegeneration, neuroprotection and therapeutics. Mol. Neurobiol. 2014, 49, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.N.; Whaley, L.A.; Norton, E.S.; Zarco, N.; Guerrero-Cázares, H. Extracellular Vesicles in the Glioblastoma Microenvironment: A Diagnostic and Therapeutic Perspective. Mol. Aspects Med. 2023, 91, 101167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.Y.M.; Li, Y.; Chan, A.H.Y.; Ng, S.C.P.; Loong, H.H.F.; Chan, D.T.M.; Wong, G.K.C.; Poon, W.-S. A multifaceted review of temozolomide resistance mechanisms in glioblastoma beyond O-6-methylguanine-DNA methyltransferase. Glioma 2019, 2, 68–82. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wen, H.; Lu, W.L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.J.; Yang, T.Y.; et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release 2010, 141, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, Z.; Zhang, S.; Pang, Z.; Jiang, X. Internalization and subcellular fate of aptamer and peptide dual-functioned nanoparticles in brain endothelial cells under physiological condition. J. Drug Target. 2014, 22, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.M.; Palkar, P.; Mutalik, S.P.; Mutalik, S.; Goda, J.S.; Rao, B.S.S. Enhancing glioblastoma cytotoxicity through encapsulating O6-benzylguanine and temozolomide in PEGylated liposomal nanocarrier: An in vitro study. 3 Biotech 2024, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Swetha, K.L.; Roy, A.; Saha, R.N.; Singhvi, G. Exploring temozolomide encapsulated PEGylated liposomes and lyotropic liquid crystals for effective treatment of glioblastoma: In-vitro, cell line, and pharmacokinetic studies. Eur. J. Pharm. Biopharm. 2023, 186, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Li, R.J.; Huang, C.Y.; Wei, K.C.; Chen, P.Y. Controlled release of liposome-encapsulated temozolomide for brain tumour treatment by convection-enhanced delivery. Pharmaceutics 2018, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Nordling-David, M.M.; Yaffe, R.; Guez, D.; Meirow, H.; Last, D.; Grad, E.; Salomon, S.; Sharabi, S.; Levi-Kalisman, Y.; Golomb, G.; et al. Liposomal temozolomide drug delivery using convection enhanced delivery. J. Control. Release 2017, 261, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-y.; Liu, Y.; Zhang, L.; Guo, R.-b.; Liu, Y.; Li, X.-t.; Ma, L.; Kong, L. Menthol-modified paclitaxel multifunctional cationic liposomes cross the blood-brain barrier and target glioma stem cells for treatment of glioblastoma. J. Drug Deliv. Sci. Technol. 2024, 93, 105387. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H. Paclitaxel-loaded liposomes decorated with a multifunctional tandem peptide (R8–RGD) for glioma targeting. Biomaterials 2014, 35, 4835–4847. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shin, D.H.; Kim, J.S. Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells. J. Control. Release 2018, 269, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, M.; Desideri, M.; Paoletti, C.; Bossi, G.; D’Ambrosio, E.; Picardo, M.; Marchese, C.; Bonanno, E.; Ranelletti, F.O.; Mileo, A.M. BET protein inhibition sensitizes glioblastoma cells to temozolomide by attenuating MGMT expression. Cell Death Dis. 2022, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.-L.; Hwang, M.K.; Maffa, A.; Balk, S.P.; Floyd, S.R.; Fréchet, J.M.J.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, P.; Abdouss, M.; Farhadi, M.; Jameie, S.B.; Farhadi, M. Co-delivery of temozolomide and quercetin with folic acid-conjugated exosomes in glioblastoma treatment. Nanomedicine 2024, 19, 2271–2287. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Liu, X.; Wang, X.; Zhang, H.; Lin, X.; Li, Z.; Guo, X.; Liu, X.; Sun, Z. Apoptotic body-mediated intercellular delivery for enhanced drug delivery. Sci. Adv. 2022, 8, abg0880. [Google Scholar] [CrossRef]
- Qian, J.; Zhao, Y.; Liu, J.; Zhou, L.; Wang, P.; Wang, W. Exosomal transfer of miR-1238 contributes to temozolomide re-sistance in glioblastoma. EBioMedicine 2019, 42, 238–251. [Google Scholar] [CrossRef]
- Tang, L.; Feng, Y.; Gao, S.; Mu, Q.; Liu, C. Nanotherapeutics overcoming the blood–brain barrier for glioblastoma treatment. Front. Pharmacol. 2021, 12, 786700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Agiba, A.M.; Arreola-Ramírez, J.L.; Carbajal, V.; Segura-Medina, P. Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies. Molecules 2024, 29, 636. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-F.; Wei, J.; Guo, Q.; Chen, J.; Deng, Y.; Liu, X.; Gong, W.; Zeng, Q. The efficacy and safety of anti-PD-1/PD-L1 in treatment of glioma: A single-arm meta-analysis. Front. Immunol. 2023, 14, 1168244. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Hatamipour, M.; Alani, B.; Nikzad, H.; Mohammadian Roshan, N.; Verdi, J.; Jaafari, M.R.; Badiee, A. Liposomal gp100 vaccine combined with CpG ODN sensitizes established B16F10 melanoma tumors to anti-PD-1 therapy. Iran. J. Basic Med. Sci. 2020, 23, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.; Wu, H.; Zhang, G.; Zhan, S.; Zhang, L.; Chen, Y. Active immunotherapy based on dendritic cells exosomes for glioma: A novel strategy for glioma treatment. Med. Hypotheses 2011, 77, 371–373. [Google Scholar] [CrossRef][Green Version]
- Himes, B.T.; Peterson, T.E.; de Mooij, T.; Cumba Garcia, L.M.; Jung, M.-Y.; Uhm, S.; Yan, D.; Tyson, J.; Jin-Lee, H.J.; Parney, D.; et al. The role of extracellular vesicles and PD-L1 in glioblastoma-mediated immunosuppressive monocyte induction. Neuro-Oncology 2020, 22, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Jackson, E.; Giamas, G. Breaking through the glioblastoma microenvironment via extracellular vesicles. Oncogene 2020, 39, 4477–4490. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.R.P.; Scheyltjens, I.; Duerinck, J.; Neyns, B.; Movahedi, K.; Van Ginderachter, J.A. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife 2020, 9, e52176. [Google Scholar] [CrossRef] [PubMed]
- Pavlyukov, M.S.; Yu, H.; Bastola, S.; Minata, M.; Shender, V.O.; Lee, Y.; Wang, J.; Komarova, S.; Wang, J.; Chin, A.; et al. Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell 2018, 34, 119–135.e10. [Google Scholar] [CrossRef] [PubMed]
- Musatova, O.E.; Rubtsov, Y.P. Effects of glioblastoma-derived extracellular vesicles on the functions of immune cells. Front. Cell Dev. Biol. 2023, 11, 1060000. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhu, G.; Zhang, Z.; Yu, Y.; Zeng, L.; Xu, Z.; Weng, J.; Xia, J.; Li, J.; Pathak, J.L. Apoptotic bodies: Bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J. Nanobiotechnol. 2023, 21, 218. [Google Scholar] [CrossRef] [PubMed]
- Broderick, L.; Yokota, S.J.; Reineke, J.; Mathiowitz, E.; Stewart, C.C.; Barcos, M.; Kelleher, R.J. Human CD4⁺ effector memory T cells persisting in the microenvironment of lung cancer xenografts are activated by local delivery of IL-12 to proliferate, produce IFN-γ, and eradicate tumor cells. J. Immunol. 2005, 174, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Chen, X.; Hu, J.; Li, Y.; Zhang, T.; Zhao, Q.; Ma, R.; Sun, W. Extracellular vesicle-based targeted RNA therapies against cancer. Sci. Transl. Med. 2024, 16, eabn0325. [Google Scholar] [CrossRef]
- Linder, B.; Weirauch, U.; Ewe, A.; Uhmann, A.; Seifert, V.; Mittelbronn, M.; Harter, P.N.; Aigner, A.; Kögel, D. Therapeutic targeting of Stat3 using lipopolyplex nanoparticle-formulated siRNA in a syngeneic orthotopic mouse glioma model. Cancers 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.E.I.; Filtjens, J.; Brabants, E.; Kancheva, D.; Debraekeleer, A.; Brughmans, J.; Jacobs, L.; Bardet, P.M.R.; Knetemann, E.; Lefesvre, P.; et al. Intratumoral delivery of lipid nano-particle-formulated mRNA encoding IL-21, IL-7, and 4-1BBL induces systemic anti-tumor immunity. Nat. Commun. 2024, 15, 10635. [Google Scholar] [CrossRef]
- Resnier, P.; Motard, C.; Pittet, J.-F.; Benoit, J.-P.; Passirani, C. EGFR siRNA lipid nanocapsules efficiently transfect glioma cells in vitro. Int. J. Pharm. 2013, 454, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Natsume, A.; Toda, H.; Iwamizu, H.; Sugita, T.; Hachisu, R.; Watanabe, R.; Yuki, K.; Motomura, K.; Bankiewicz, K.; et al. Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxicity of temozolomide in GBM-initiating cells. Gene Ther. 2010, 17, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.; Devi, S.N.; Bhardwaj, A.K.; Yashavarddhan, M.H.; Bohra, D.; Ganguly, N.K. Exosomes as nature’s nano carriers: Promising drug delivery tools and targeted therapy for glioma. Biomed. Pharmacother. 2025, 182, 117754. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, C.; Li, L.; Ma, W. Classification and translational potential of RNA-based therapies in cancer treatment. Trends Pharmacol. Sci. 2020, 41, 979–993. [Google Scholar] [CrossRef]
- Fierro, J.; DiPasquale, J.; Perez, J.; Chin, B.; Chokpapone, Y.; Tran, A.M.; Holden, A.; Factoriza, C.; Sivagnanakumar, N.; Aguilar, R.; et al. Dual-sgRNA CRISPR/Cas9 knockout of PD-L1 in human U87 glioblastoma tumor cells inhibits proliferation, invasion, and tumor-associated macrophage polarization. Sci. Rep. 2022, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Zhang, N.; Niu, W.; Mu, M.; Zhang, X.; Hu, S.; Niu, C. Exosomal miR-155-5p derived from glioma stem-like cells promotes mesenchymal transition via targeting ACOT12. Cell Death Dis. 2022, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, J.; Yu, D.; Liu, Y.; Song, D.; Tian, K.; Chen, H.; Ye, Q.; Wang, X.; Xu, T.; et al. Polymer-locking fusogenic liposomes for glioblastoma-targeted delivery of siRNA and CRISPR–Cas9 RNP. Nat. Nanotechnol. 2024, 19, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, Z.; Wang, W.; Zou, C.; Wang, Y.; Pan, L.; Jia, B.; Zhang, K.; Zhang, W.; Li, W.; et al. Engineered extracellular vesicles delivering CRISPR–Cas9 RNP for radiosensitization of glioblastoma. ACS Nano 2023, 17, 16432–16447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, T.; Zhu, Y.; Jaffar Ali, D.; Hu, F.; Qi, Y.; Sun, B.; Xiao, Z. Exosomes transfer among different species cells and mediating miRNAs delivery. J. Cell. Biochem. 2017, 118, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jaffar Ali, D.; Xu, H.; Kumaravel, S.; Si, K.; Li, Y.; Sun, B.; Ma, J.; Xiao, Z. Epithelial cell-derived microvesicles: A safe delivery platform of CRISPR/Cas9 conferring synergistic anti-tumor effect with sorafenib. Exp. Cell Res. 2020, 392, 112040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Zhang, C.; Wang, Y.; Wang, F.; Jia, Y.; Li, Z.; Zhao, Y.; Cheng, Y.; Wang, K. Exosome versus liposome delivery of siRNA in a murine glioma model: Evaluation of efficacy and biodistribution. J. Cancer Res. Clin. Oncol. 2022, 148, 3311–3324. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sui, B.; Zhang, X.; Liu, J.; Hao, X.; Zheng, L. miR-92a-1-5p enriched prostate cancer extracellular vesicles regulate oste-oclast function via MAPK1 and FoxO1. J. Exp. Clin. Cancer Res. 2023, 42, 109. [Google Scholar] [CrossRef] [PubMed]
- Whitford, W.; Guterstam, P. Exosome manufacturing status. Future Med. Chem. 2019, 11, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Kabilova, T.O.; Shmendel, E.V.; Gladkikh, D.V.; Chernolovskaya, E.L.; Markov, O.V.; Morozova, N.G.; Maslov, M.A.; Zenkova, M.A. Targeted delivery of nucleic acids into xenograft tumors mediated by novel folate-equipped liposomes. Eur. J. Pharm. Biopharm. 2018, 123, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Cabrera-Pastor, A. Emerging role of extracellular vesicles as biomarkers in neurodegenerative diseases and their clinical and therapeutic potential in central nervous system pathologies. Int. J. Mol. Sci. 2024, 25, 10068. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Pajecki, D.; Vykhodtseva, N.; Park, J.; Xia, L.; McDannold, N.; Han, Y. Delivery of liposomes with different sizes to mice brain after BBB disruption by focused ultrasound with microbubbles. Ultrasound Med. Biol. 2016, 42, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Bhimreddy, M.; Weber-Levine, C.; Jiang, K.; Alomari, S.; Theodore, N.; Manbachi, A.; Tyler, B.M. Applications of focused ultrasound for the treatment of glioblastoma: A new frontier. Cancers 2022, 14, 4920. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the treatment of brain cancer—A review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef] [PubMed]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and extracellular vesicles as drug delivery systems: A comparison of composition, pharmacokinetics, and functionalization. Adv. Healthc. Mater. 2022, 11, e2100639. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Xu, J.; Wang, D.; Zhang, T.; Ji, Y.; Jiang, W.; Li, C. Targeted co-delivery of TGF-β inhibitor and temozolomide via a liposomal system for enhanced glioma immunochemotherapy. Acta Pharmacol. Sin. B 2022, 12, 1726–1739. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Chen, K.T.J.; Ryan, G.M.; Anantha, M.; Wretham, N.; Nosrati, Z.; Heroux, D.; Wang, L.; Chow, N.; Dai, Z.; et al. DMPC/Chol liposomal copper CX5461 is therapeutically superior to a DSPC/Chol formulation. J. Control. Release 2022, 345, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood–brain barrier. Expert Opin. Drug Deliv. 2020, 17, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Andresen, T.L.; Simonsen, J.B. Extracellular vesicle-mediated delivery of RNA therapeutics: Current advances and future challenges. Adv. Drug Deliv. Rev. 2023, 191, 114658. [Google Scholar] [CrossRef]
- Ribovski, L.; Joshi, B.; Gao, J.; Zuhorn, I. Breaking free: Endocytosis and endosomal escape of extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S.E. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Song, B.; Yuan, L.; Yang, G. Multiplexed strategies toward clinical translation of extracellular vesicles. Theranostics 2022, 12, 6740–6761. [Google Scholar] [CrossRef] [PubMed]
- Matarredona, E.R.; Pastor, A.M. Extracellular vesicle-mediated communication between the glioblastoma and its microenvironment. Cells 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.A.; El Andaloussi, S.; et al. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [PubMed]
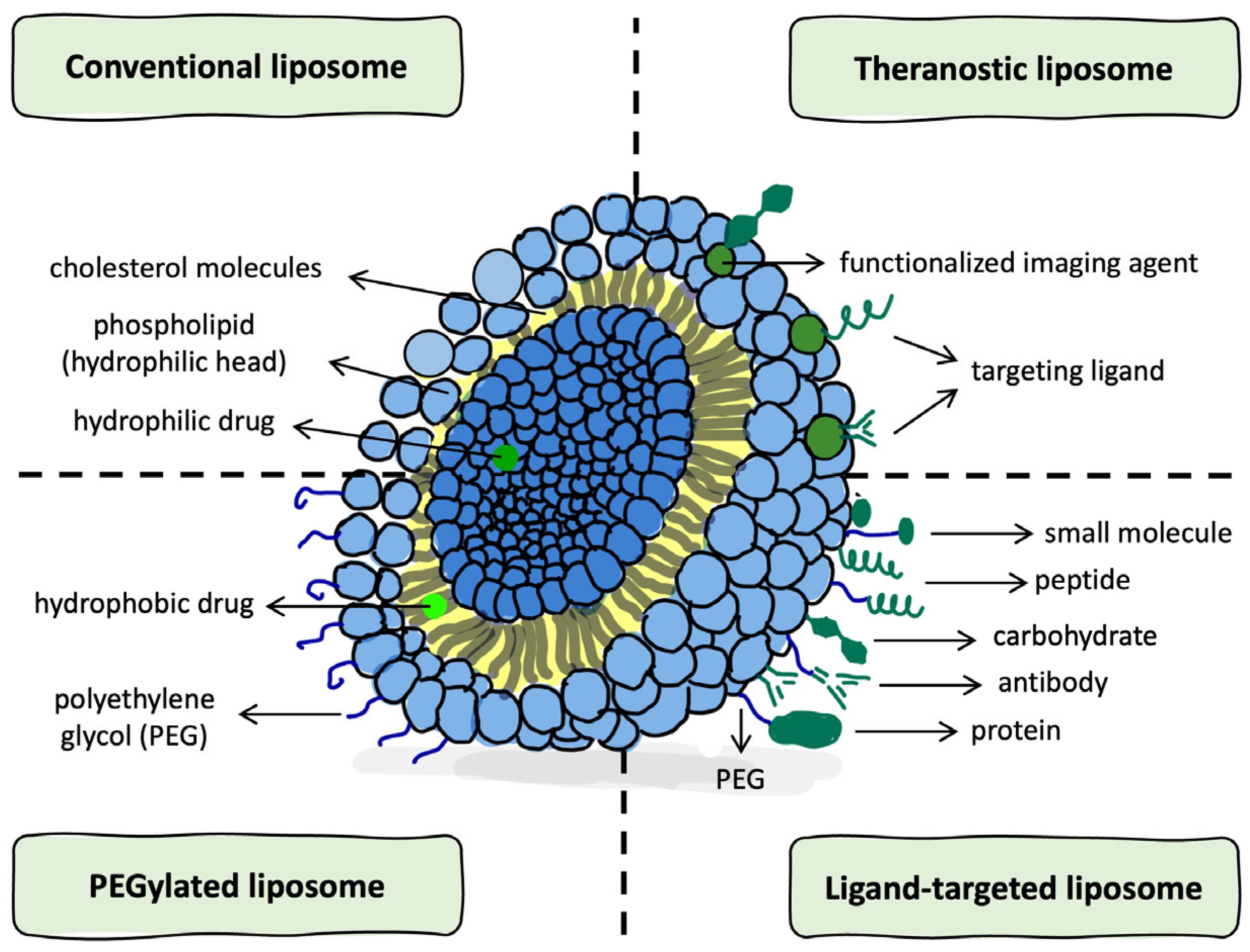
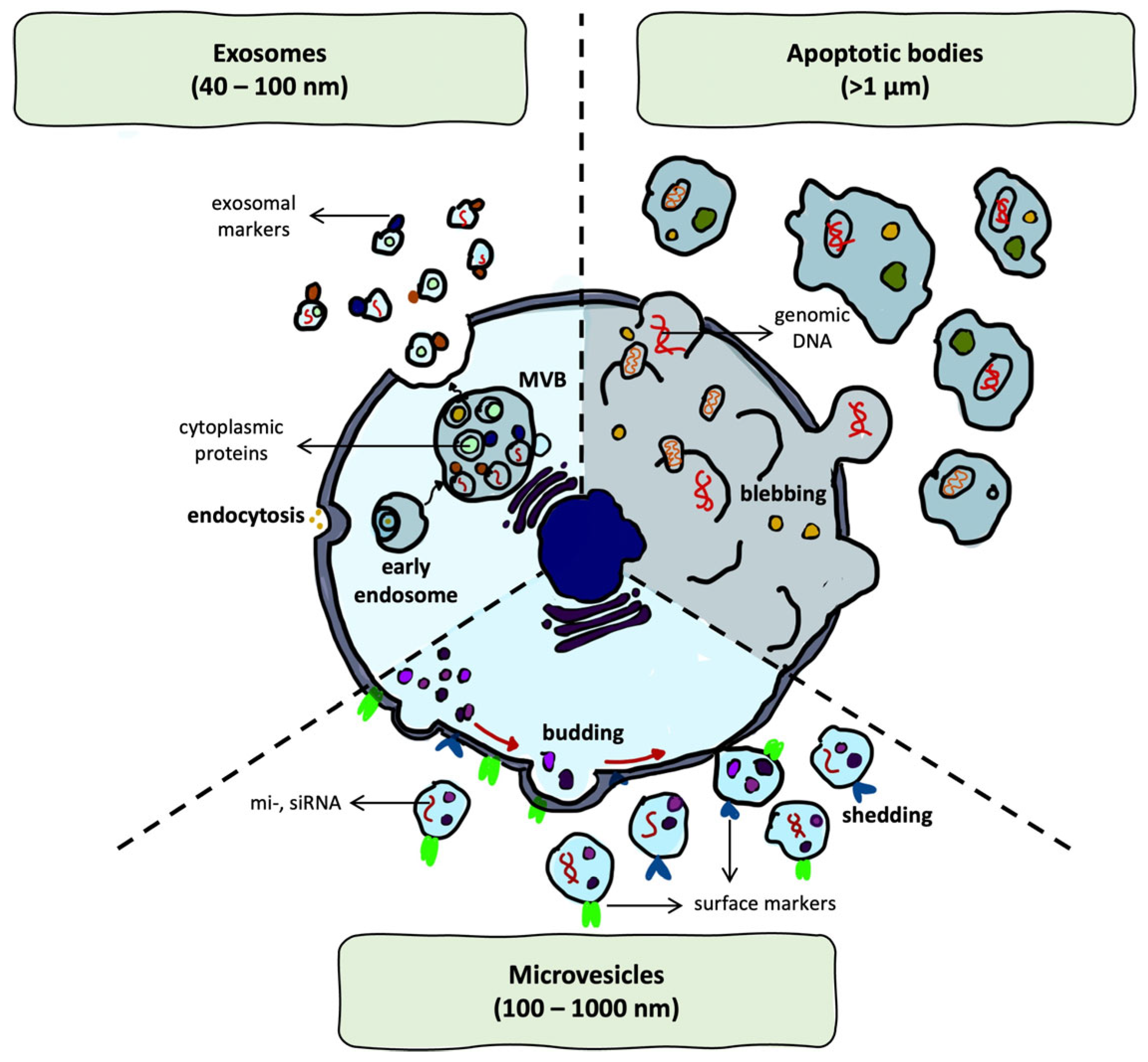
| Type | Size Range | Lamellarity | Encapsulation Capacity | Biological Analogy | References |
|---|---|---|---|---|---|
| Small Unilamellar Vesicle (SUV) | 20–100 nm | Single bilayer | Low (limited aqueous volume) | Structurally and dimensionally comparable to exosomes | [16,23] |
| Large Unilamellar Vesicle (LUV) | 100–1000 nm | Single bilayer | Moderate to high | Overlap in size with larger exosomes and early endosomes | [13,17,23] |
| Multilamellar Vesicle (MLV) | >500 nm | Multiple concentric bilayers | Low (limited aqueous space) | May resemble multilamellar EVs or complex apoptotic bodies | [16,23] |
| Multivesicular Vesicle (MVV) | >500 nm | Outer bilayer enclosing internal vesicles | High (dual aqueous compartments) | Comparable to multivesicular bodies or large EV subtypes | [17,23] |
| Giant Unilamellar Vesicle (GUV) | >1 µm | Single bilayer | Variable (suitable for large macromolecules) | Used as membrane models; less common in vivo | [23] |
| Feature | Liposomes | Extracellular Vesicles | References |
|---|---|---|---|
| Drug Encapsulation Efficiency | High encapsulation efficiency is possible, but highly dependent on the preparation method, lipid composition, and vesicle size | Moderate and less tunable; limited by parent cell type and passive loading approaches, size, etc. | Liposomes: [13,48,51] EVs: [58,61] |
| Stability in Circulation | Inherently unstable; improved by PEGylation, but remains a formulation challenge | Stable in biofluids, but stability can vary depending on isolation methods and donor cell variability | Liposomes: [5,13,16,51] EVs: [8,9,54] |
| Tumor Targeting | Requires the addition of targeting ligands (e.g., transferrin, angiopep) | Naturally enriched surface proteins aid glioma cell uptake | Liposomes: [26,27,54] EVs: [10,35,62,63] |
| Therapeutic Efficacy | Effective tumor delivery with proper formulation; relies on EPR effect and surface design | Often show superior penetration and retention in glioma models, especially in dense tumor tissue | Liposomes: [5,12,48,52,54,64] EVs: [10,56,57,58] |
| Immunogenicity | Potentially immunogenic, especially upon repeated administration | Low immunogenicity; naturally derived, generally well tolerated | Liposomes: [13,17] EVs: [10,57,63] |
| Scalability and Manufacturing | Industrial processes exist, but standard methods are not fully scalable; SCF is emerging | Lacks scalable methods; isolation and reproducibility remain major hurdles | Liposomes: [3,16] EVs: [65,66] |
| Strategy | Liposomes | Extracellular Vesicles | References |
|---|---|---|---|
| Checkpoint Inhibition | Encapsulated PD-L1 siRNA or anti-PD-1 antibodies (engineered) | Engineered EVs loaded with PD-L1 siRNA | Liposomes: [8,27] EVs: [1,2,7,19,38,72] |
| Cancer Vaccines | Tumor-associated peptides + CpG co-loaded liposomes | Dexosomes enriched with MHC I/II and glioma-associated antigens | Liposomes: [70] EVs: [37,72] |
| Cytokine Delivery | IL-12-loaded liposomes improve T cell activation and anti-tumor immunity | EVs carrying IL-12 mRNA promote localized cytokine expression and immune activation in GBM | Liposomes: [79] EVs: [74,80] |
| Macrophage Reprogramming | Experimental delivery of immunomodulators (rarely used) | Glioma-derived EVs carrying IL-6/miR-155-3p promote M2 polarization via STAT3 activation | Liposomes: [27] EVs: [61,72,77] |
| Antigen Presentation/Priming | Requires synthetic adjuvants | Apoptotic bodies and Dexosomes present native tumor antigens | Liposomes: [70] EVs: [38,72,77] |
| Therapeutic Tool | Immunotherapy Use | Gene Therapy Use | Reference |
|---|---|---|---|
| siRNA (e.g., PD-L1) | Restores T cell activation by silencing immune checkpoints | Silences immune-suppressive genes, enhancing anti-tumor response | [39,87] |
| miRNA (e.g., miR-155) | Polarizes macrophages toward the M1 phenotype and boosts cytotoxic immune activity | Reprograms tumor-associated macrophages and modifies the glioma-supportive stroma | [61,88] |
| mRNA (e.g., IL-12) | Enables localized expression of immune-stimulatory cytokines | Introduces tumor-suppressing cytokines and therapeutic proteins through transient expression | [63,75,82] |
| CRISPR-Cas9 | - | Enables genome editing of oncogenes and resistance-related genes like MGMT | [84,87,89] |
| Feature | Liposomes | Extracellular Vesicles | References |
|---|---|---|---|
| siRNA Delivery | Liposomal formulations carrying siRNA (e.g., PD-L1) inhibit glioma; require PEGylation or targeting moieties | EVs naturally internalized by glioma cells deliver siRNA via fusion or endocytosis | Liposomes: [83,84,89] EVs: [93,94] |
| miRNA Delivery | Liposomal miR-124 or miR-7 modulate STAT3/MGMT pathways, sensitizing glioma to chemotherapy | EVs loaded with miR-155 polarize TAMs (M1); miR-124 EVs inhibit glioma progression | Liposomes: [55,84] EVs: [61,88,95] |
| mRNA Delivery | Liposomes encapsulating IL-12 mRNA or mRNA vaccines trigger local immune activation | Engineered EVs deliver immune-modulating mRNA, enhancing T cell response in GBM | Liposomes: [82] EVs: [39,80] |
| Gene Editing | Liposomal CRISPR–Cas9 systems enable efficient knock-out of PD-L1 or MGMT | EVs co-delivering Cas9 protein demonstrate enhanced uptake and editing efficiency in glioma cells | Liposomes: [11,89] EVs: [90,92] |
| Feature | Liposomes | Extracellular Vesicles | References |
|---|---|---|---|
| Drug Encapsulation | High encapsulation efficiency; tunable by lipid composition, vesicle size, and preparation method | Moderate efficiency; dependent on parental cell type and passive loading techniques | Liposomes: [13,16,31,53] EVs: [20,57,58,78] |
| BBB Penetration | Requires surface modification (e.g., Angiopep-2) to cross the BBB | Crosses BBB via endogenous receptor-mediated transcytosis mechanisms | Liposomes: [5,16,26,27,66] EVs: [9,20,60,94,103] |
| Tumor Targeting | Achieved by attaching ligands (e.g., transferrin, folate, antibodies) to the surface | Inherent targeting potential due to membrane proteins and cell-of-origin tropism | Liposomes: [26,27,28,54] EVs: [9,35,36,94,106] |
| Endosomal Escape | Limited; many liposomes are degraded in endosomes unless modified with pH-sensitive components | EVs demonstrate improved cytoplasmic delivery due to their natural membrane composition | Liposomes: [16,84,106] EVs: [88,104,109,110,111] |
| Gene Delivery | Liposomes deliver siRNA, mRNA, and CRISPR-Cas9 components via encapsulation and surface engineering | EVs naturally carry and transfer miRNAs, siRNAs, and CRISPR-Cas9 machinery | Liposomes: [11,82,83,84,86,89] EVs: [18,61,90,94] |
| Clinical Scalability | Scalable using established pharmaceutical manufacturing processes; proven in other cancer types | Scalability remains challenging due to heterogeneity and a lack of standardized production methods | Liposomes: [5,16,17,64] EVs: [65,112,113,114] |
| Innovative Hybrids | Liposome–EV hybrid vesicles under development; aim to improve delivery and targeting | Hybrid EV mimetic platforms show enhanced uptake and controlled drug release | Liposomes: [12,64] EVs: [12,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraczek, W.; Szmidt, M.; Kregielewski, K.; Grodzik, M. Liposomes and Extracellular Vesicles as Distinct Paths Toward Precision Glioma Treatment. Int. J. Mol. Sci. 2025, 26, 6775. https://doi.org/10.3390/ijms26146775
Fraczek W, Szmidt M, Kregielewski K, Grodzik M. Liposomes and Extracellular Vesicles as Distinct Paths Toward Precision Glioma Treatment. International Journal of Molecular Sciences. 2025; 26(14):6775. https://doi.org/10.3390/ijms26146775
Chicago/Turabian StyleFraczek, Wiktoria, Maciej Szmidt, Kacper Kregielewski, and Marta Grodzik. 2025. "Liposomes and Extracellular Vesicles as Distinct Paths Toward Precision Glioma Treatment" International Journal of Molecular Sciences 26, no. 14: 6775. https://doi.org/10.3390/ijms26146775
APA StyleFraczek, W., Szmidt, M., Kregielewski, K., & Grodzik, M. (2025). Liposomes and Extracellular Vesicles as Distinct Paths Toward Precision Glioma Treatment. International Journal of Molecular Sciences, 26(14), 6775. https://doi.org/10.3390/ijms26146775






