Pulmonary Embolism in Acute Ischaemic Stroke: Evolving Evidence, Diagnostic Challenges, and a Novel Thromboinflammatory Axis Hypothesis
Abstract
1. Introduction
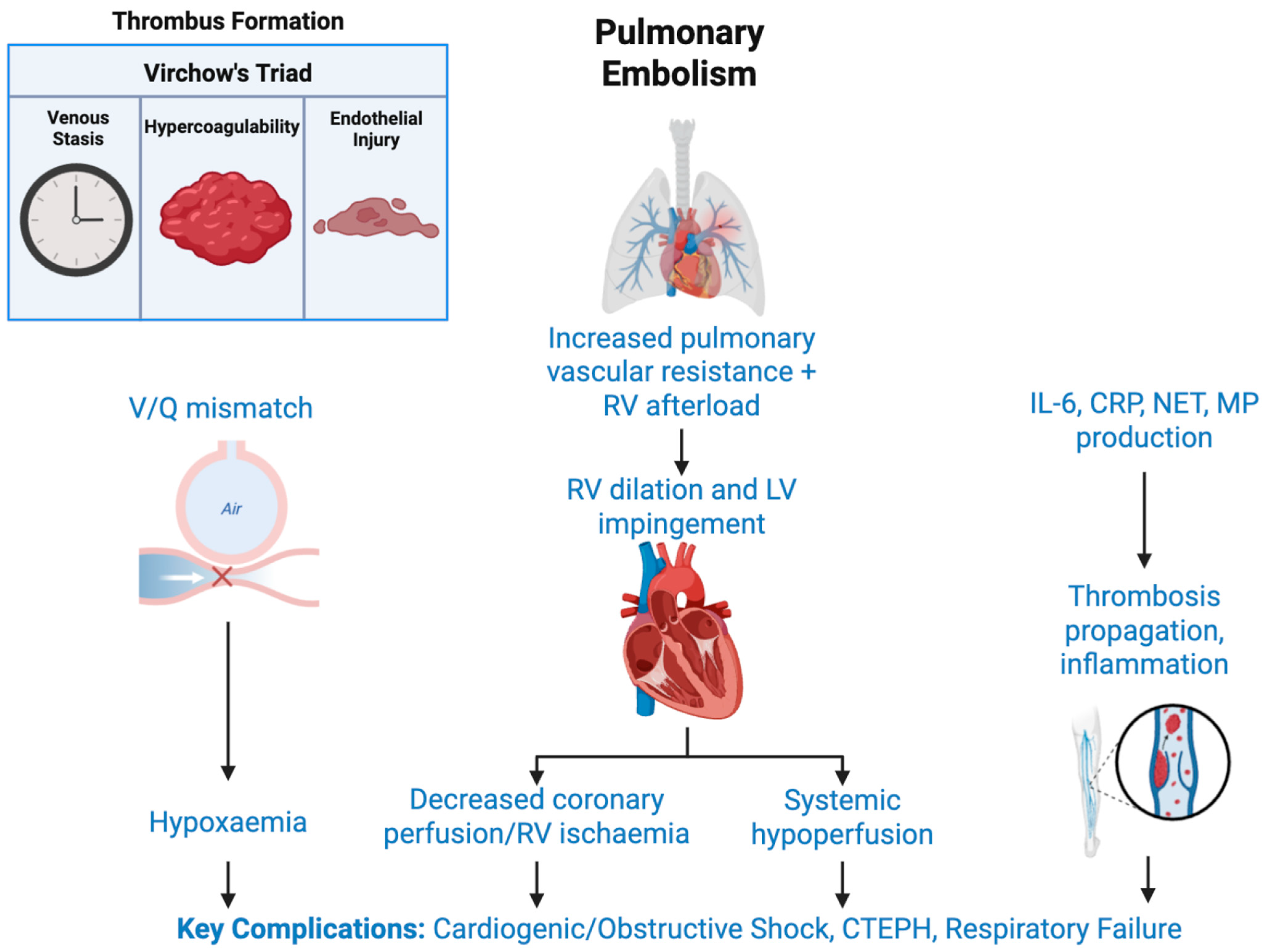
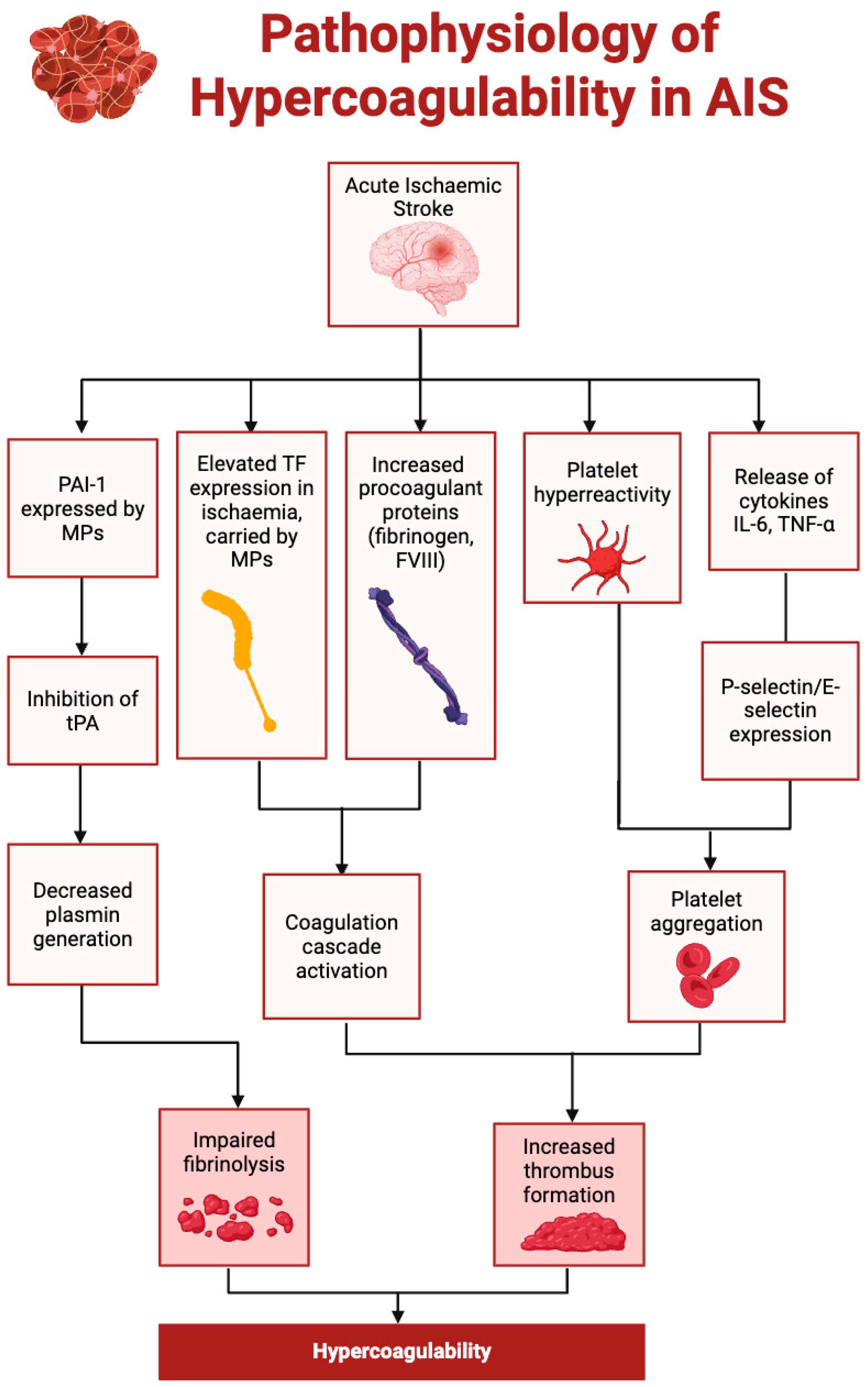
2. Risk Factors and Pathophysiology
2.1. Pathophysiology of Pulmonary Embolism
2.2. Risk Factors
2.2.1. Immobility
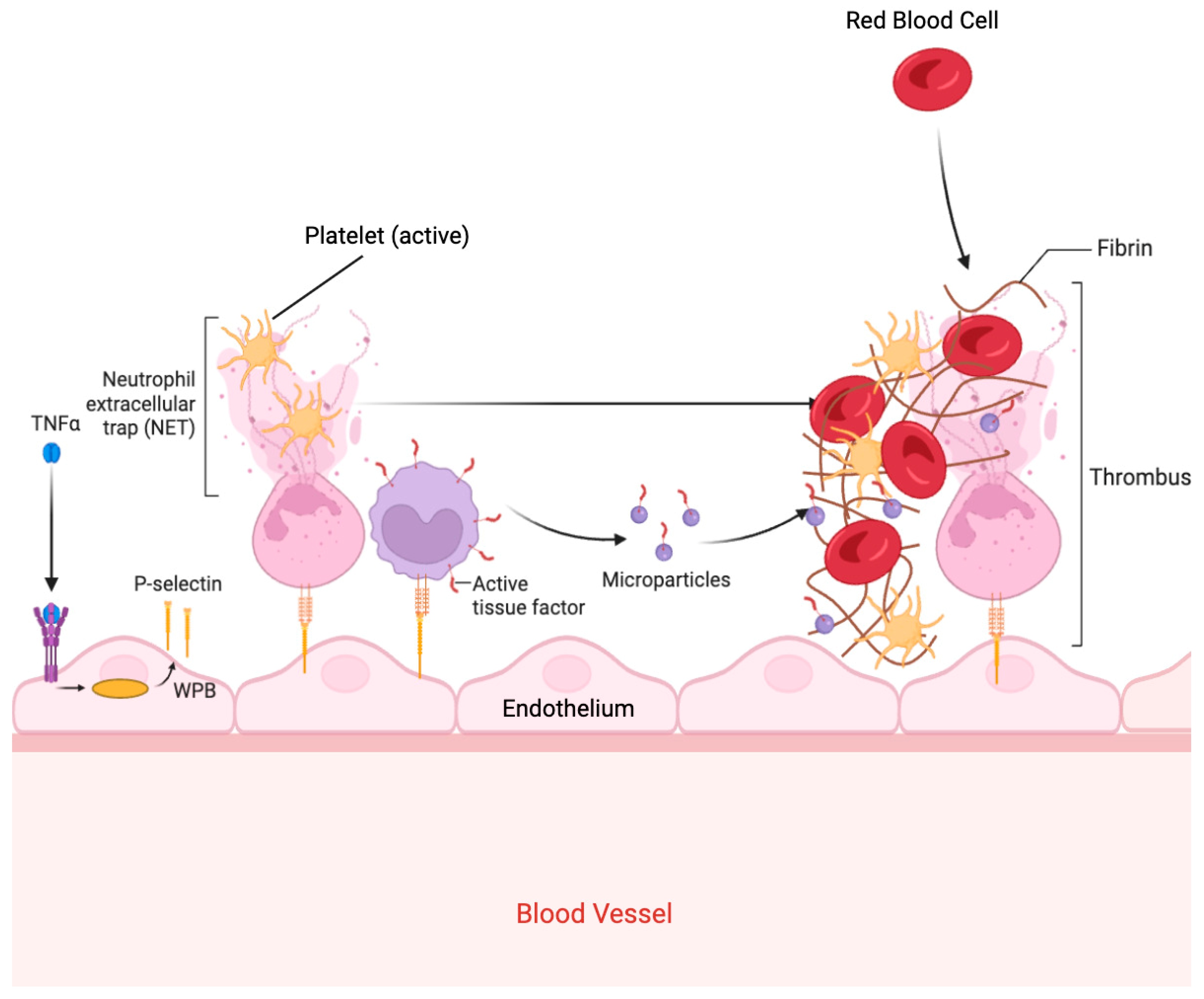
2.2.2. Hypercoagulability
2.2.3. Patent Foramen Ovale
2.2.4. Atrial Fibrillation
2.2.5. Malignancy
2.2.6. Obesity and Metabolic Syndrome
2.2.7. Sex-Specific Risk
2.2.8. Inherited and Acquired Thrombophilia
2.3. Additional Consideration in Young Stroke and Emerging Risk Populations
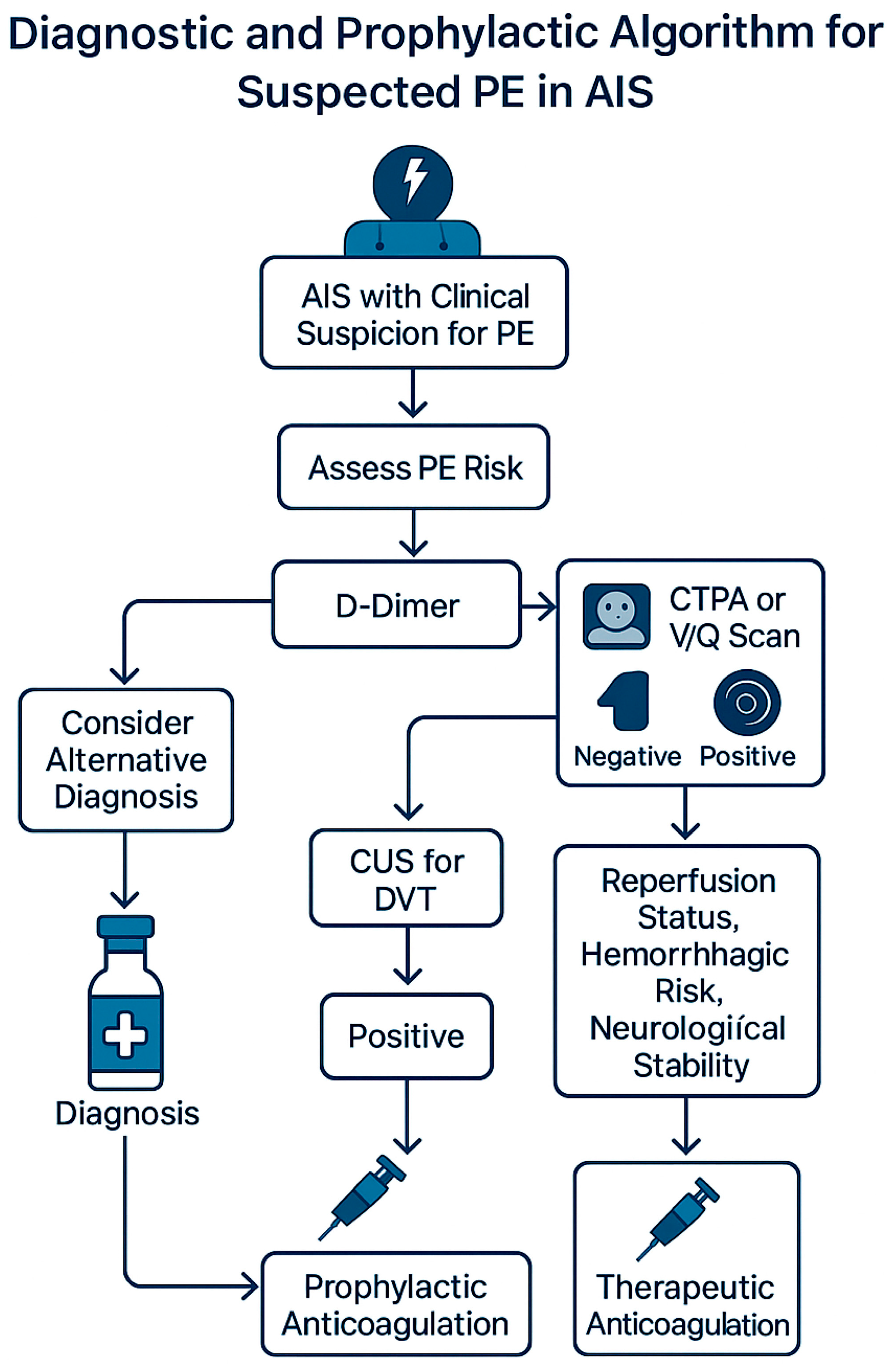
3. Diagnostics
3.1. Diagnostic and Management Algorithm
3.2. D-Dimer
3.3. CT Pulmonary Angiography
3.4. Ventilation Perfusion (V/Q) Scan
3.5. Compression Ultrasonography
3.6. Echocardiography
4. Management
4.1. Risk Stratification and Diagnosis
4.2. Prophylactic Management
4.2.1. Pharmacological Prophylaxis
4.2.2. Mechanical Prophylaxis
4.2.3. Prophylaxis in the Context of Reperfusion Therapy
- Sequential management—reperfusion first, followed by delayed anticoagulation—is preferred over simultaneous thrombolysis for PE [10].
4.3. Acute Management
4.4. Long-Term Management
4.4.1. Anticoagulation Strategies
4.4.2. Cognitive Sequelae of Subclinical PE
4.4.3. Secondary Prevention and Rehabilitation
4.4.4. Integrated Neuro-Pulmonary Rehabilitation
5. Discussion
5.1. The Brain–Lung Thromboinflammatory Axis Hypothesis
5.2. Pathophysiological Considerations
5.3. Diagnostic and Management Challenges
5.4. Future Directions
- I.
- The development of AIS-specific VTE risk calculators that integrate the NIHSS, infarct subtype, immobility, and biomarkers to guide prophylaxis.
- II.
- Randomised trials evaluating the safety and efficacy of DOACs in early post-stroke prophylaxis, particularly after IVT or EVT.
- III.
- Biomarker-guided anticoagulation protocols, using serial measurements of IL-6, D-dimer, and potentially NET-related markers, to personalise therapy and determine the timing of anticoagulation.
- IV.
- Emerging therapeutic avenues include targeting NETs and endothelial activation [186]. Preclinical studies have shown that PAD4 inhibitors and DNase I can reduce NET-mediated thrombosis and improve outcomes in thromboinflammatory conditions. Similarly, agents that stabilise endothelial function, such as recombinant thrombomodulin, statins, and sphingosine-1-phosphate analogues, may mitigate endothelial injury and hyperpermeability in the pulmonary vasculature [190]. Anti-cytokine therapies (e.g., IL-6 or TNF-α inhibitors) may also hold promise in modulating the systemic inflammatory response post-stroke [111]. These strategies warrant an investigation in AIS populations, particularly in those at high risk for in situ pulmonary thrombosis.
- V.
- VI.
- The validation of stroke–PE rehabilitation models and remote monitoring platforms for the early detection of anticoagulation-related complications and functional recovery.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Rinde, L.B.; Smabrekke, B.; Mathiesen, E.B.; Lochen, M.L.; Njolstad, I.; Hald, E.M.; Wilsgaard, T.; Braekkan, S.K.; Hansen, J.B. Ischemic Stroke and Risk of Venous Thromboembolism in the General Population: The Tromso Study. J. Am. Heart Assoc. 2016, 5, e004311. [Google Scholar] [CrossRef]
- Sherman, D.G.; Albers, G.W.; Bladin, C.; Fieschi, C.; Gabbai, A.A.; Kase, C.S.; O’Riordan, W.; Pineo, G.F.; PREVAIL Investigators. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): An open-label randomised comparison. Lancet 2007, 369, 1347–1355. [Google Scholar] [CrossRef]
- Amin, A.N.; Lin, J.; Thompson, S.; Wiederkehr, D. Rate of deep-vein thrombosis and pulmonary embolism during the care continuum in patients with acute ischemic stroke in the United States. BMC Neurol. 2013, 13, 17. [Google Scholar] [CrossRef]
- Kelly, J.; Rudd, A.; Lewis, R.; Hunt, B.J. Venous thromboembolism after acute stroke. Stroke 2001, 32, 262–267. [Google Scholar] [CrossRef]
- Smith, S.B.; Geske, J.B.; Maguire, J.M.; Zane, N.A.; Carter, R.E.; Morgenthaler, T.I. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest 2010, 137, 1382–1390. [Google Scholar] [CrossRef]
- Nyquist, P.; Bautista, C.; Jichici, D.; Burns, J.; Chhangani, S.; DeFilippis, M.; Goldenberg, F.D.; Kim, K.; Liu-DeRyke, X.; Mack, W.; et al. Prophylaxis of Venous Thrombosis in Neurocritical Care Patients: An Evidence-Based Guideline: A Statement for Healthcare Professionals from the Neurocritical Care Society. Neurocrit. Care 2016, 24, 47–60. [Google Scholar] [CrossRef]
- Pongmoragot, J.; Rabinstein, A.A.; Nilanont, Y.; Swartz, R.H.; Zhou, L.; Saposnik, G.; Investigators of Registry of Canadian Stroke Network (RCSN) and University of Toronto Stroke Program for Stroke Outcomes Research Canada (SORCan [www.sorcan.ca]) Working Group. Pulmonary embolism in ischemic stroke: Clinical presentation, risk factors, and outcome. J. Am. Heart Assoc. 2013, 2, e000372. [Google Scholar] [CrossRef]
- Saleh Velez, F.G.; Ortiz Garcia, J.G. Management dilemmas in acute ischemic stroke and concomitant acute pulmonary embolism: Case series and literature review. eNeurologicalSci 2021, 23, 100341. [Google Scholar] [CrossRef]
- Han, L.; Yang, J.M.; Qian, W.Y.; Xu, X.P.; Tung, T.H.; Liu, Y.; Wang, F. Risk factors for lower extremity deep vein thrombosis in acute stroke patients following endovascular thrombectomy: A retrospective cohort study. Front. Neurol. 2023, 14, 1249365. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Guo, Z.N.; Jin, H.; Sun, T.; Ni, C.; Yan, X. Incidence and Risk Factors of Lower-Extremity Deep Vein Thrombosis After Thrombolysis Among Patients with Acute Ischemic Stroke. Pharmgenomics Pers. Med. 2021, 14, 1107–1114. [Google Scholar] [CrossRef]
- Esmon, C.T. Inflammation and thrombosis. J. Thromb. Haemost. 2003, 1, 1343–1348. [Google Scholar] [CrossRef]
- Lio, K.U.; Jimenez, D.; Moores, L.; Rali, P. Clinical conundrum: Concomitant high-risk pulmonary embolism and acute ischemic stroke. Emerg. Radiol. 2020, 27, 433–439. [Google Scholar] [CrossRef]
- Shah, I.K.; Merfeld, J.M.; Chun, J.; Tak, T. Pathophysiology and Management of Pulmonary Embolism. Int. J. Angiol. 2022, 31, 143–149. [Google Scholar] [CrossRef]
- Stanimirovic, D.; Satoh, K. Inflammatory mediators of cerebral endothelium: A role in ischemic brain inflammation. Brain Pathol. 2000, 10, 113–126. [Google Scholar] [CrossRef]
- Goldhaber, S.Z. Pulmonary embolism. Lancet 2004, 363, 1295–1305. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Mackman, N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Trenor, C.C., 3rd; Furie, B.C.; Furie, B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 728–733. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Z.; Long, Q.; Huang, J.; Hong, T.; Liu, W.; Lin, J. Insights Into Immunothrombosis: The Interplay Among Neutrophil Extracellular Trap, von Willebrand Factor, and ADAMTS13. Front. Immunol. 2020, 11, 610696. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, W.; Shen, F.; Du, W.; Xu, Z.; Liu, Z. The Emerging Role of Neutrophil Extracellular Traps in Arterial, Venous and Cancer-Associated Thrombosis. Front. Cardiovasc. Med. 2021, 8, 786387. [Google Scholar] [CrossRef]
- Simonneau, G.; Torbicki, A.; Dorfmuller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Navi Babak, B.; Reiner Anne, S.; Kamel, H.; Iadecola, C.; Okin Peter, M.; Elkind Mitchell, S.V.; Panageas Katherine, S.; DeAngelis Lisa, M. Risk of Arterial Thromboembolism in Patients With Cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef]
- Tondel, B.G.; Morelli, V.M.; Hansen, J.B.; Braekkan, S.K. Risk factors and predictors for venous thromboembolism in people with ischemic stroke: A systematic review. J. Thromb. Haemost. 2022, 20, 2173–2186. [Google Scholar] [CrossRef]
- Harvey, R.L.; Roth, E.J.; Yarnold, P.R.; Durham, J.R.; Green, D. Deep vein thrombosis in stroke. The use of plasma D-dimer level as a screening test in the rehabilitation setting. Stroke 1996, 27, 1516–1520. [Google Scholar] [CrossRef]
- Dennis, M.; Caso, V.; Kappelle, L.J.; Pavlovic, A.; Sandercock, P.; European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur. Stroke J. 2016, 1, 6–19. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Zhou, L.; Yin, X.; Dong, Q. Stratification of venous thromboembolism risk in stroke patients by Caprini score. Ann. Palliat. Med. 2020, 9, 631–636. [Google Scholar] [CrossRef]
- Poletto, S.R.; Rebello, L.C.; Valenca, M.J.; Rossato, D.; Almeida, A.G.; Brondani, R.; Chaves, M.L.; Nasi, L.A.; Martins, S.C. Early mobilization in ischemic stroke: A pilot randomized trial of safety and feasibility in a public hospital in Brazil. Cerebrovasc. Dis. Extra 2015, 5, 31–40. [Google Scholar] [CrossRef]
- Talec, P.; Gaujoux, S.; Samama, C.M. Early ambulation and prevention of post-operative thrombo-embolic risk. J. Visc. Surg. 2016, 153, S11–S14. [Google Scholar] [CrossRef]
- AVERT Trial Collaboration Group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet 2015, 386, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Sadaghianloo, N.; Dardik, A. The efficacy of intermittent pneumatic compression in the prevention of lower extremity deep venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Costantini, T.W.; Kornblith, L.Z.; Pritts, T.; Coimbra, R. The intersection of coagulation activation and inflammation after injury: What you need to know. J. Trauma. Acute Care Surg. 2024, 96, 347–356. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhang, S.; Nanda, A.; Li, G. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl. Res. 2013, 6, 834–851. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; di Sciacca, R.; Pinto, A.; Licata, G. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 2008, 14, 3574–3589. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, T.W.; Myers, D.D.; Henke, P.K. Mechanisms of venous thrombosis and resolution. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 387–391. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Lindgren, A.; Lindoff, C.; Norrving, B.; Astedt, B.; Johansson, B.B. Tissue plasminogen activator and plasminogen activator inhibitor-1 in stroke patients. Stroke 1996, 27, 1066–1071. [Google Scholar] [CrossRef]
- Falcione, S.; Munsterman, D.; Joy, T.; Kamtchum-Tatuene, J.; Sykes, G.; Jickling, G. Association of Thrombin Generation with Leukocyte Inflammatory Profile in Patients with Acute Ischemic Stroke. Neurology 2022, 99, e1356–e1363. [Google Scholar] [CrossRef]
- Sfredel, M.D.; Burada, E.; Catalin, B.; Dinescu, V.; Tartea, G.; Iancau, M.; Osiac, E. Blood Coagulation Following an Acute Ischemic Stroke. Curr. Health Sci. J. 2018, 44, 118–121. [Google Scholar] [CrossRef]
- Butenas, S.; Orfeo, T.; Mann, K.G. Tissue factor in coagulation: Which? Where? When? Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1989–1996. [Google Scholar] [CrossRef]
- Caine, G.J.; Stonelake, P.S.; Lip, G.Y.; Kehoe, S.T. The hypercoagulable state of malignancy: Pathogenesis and current debate. Neoplasia 2002, 4, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Abu Zaanona, M.I.; Mantha, S. Cancer-Associated Thrombosis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Heo, J.H.; Yun, J.; Kim, K.H.; Jung, J.W.; Yoo, J.; Kim, Y.D.; Nam, H.S. Cancer-Associated Stroke: Thrombosis Mechanism, Diagnosis, Outcome, and Therapeutic Strategies. J. Stroke 2024, 26, 164–178. [Google Scholar] [CrossRef]
- Buyuksirin, M.; Anar, C.; Polat, G.; Karadeniz, G. Can the Level of CRP in Acute Pulmonary Embolism Determine Early Mortality? Turk. Thorac. J. 2021, 22, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, H.; Zhang, J.; Xu, X. Research progress on biomarkers of pulmonary embolism. Clin. Respir. J. 2021, 15, 1046–1055. [Google Scholar] [CrossRef]
- Zhou, P.; Waresi, M.; Zhao, Y.; Lin, H.C.; Wu, B.; Xiong, N.; Li, H.; Huang, Q.; Luo, X.; Li, J. Increased serum interleukin-6 level as a predictive biomarker for atrial fibrillation: A systematic review and meta-analysis. Rev. Port. Cardiol. (Engl. Ed.) 2020, 39, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Bacila, C.I.; Vladoiu, M.G.; Valeanu, M.; Moga, D.F.; Pumnea, P.M. The Role of IL-6 and TNF-Alpha Biomarkers in Predicting Disability Outcomes in Acute Ischemic Stroke Patients. Life 2025, 15, 47. [Google Scholar] [CrossRef]
- Braadt, L.; Naumann, M.; Freuer, D.; Schmitz, T.; Linseisen, J.; Ertl, M. Novel inflammatory biomarkers associated with stroke severity: Results from a cross-sectional stroke cohort study. Neurol. Res. Pract. 2023, 5, 31. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Zaman, M.O.; Elgendy, I.Y.; Mahmoud, A.N.; Patel, N.K.; Agarwal, N.; Tobis, J.M.; Meier, B. Cryptogenic Stroke and Patent Foramen Ovale. J. Am. Coll. Cardiol. 2018, 71, 1035–1043. [Google Scholar] [CrossRef]
- Maron, B.A.; Shekar, P.S.; Goldhaber, S.Z. Paradoxical embolism. Circulation 2010, 122, 1968–1972. [Google Scholar] [CrossRef]
- Windecker, S.; Stortecky, S.; Meier, B. Paradoxical embolism. J. Am. Coll. Cardiol. 2014, 64, 403–415. [Google Scholar] [CrossRef]
- Guo, S.; Roberts, I.; Missri, J. Paradoxical embolism, deep vein thrombosis, pulmonary embolism in a patient with patent foramen ovale: A case report. J. Med. Case Rep. 2007, 1, 104. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Lee, J.-Y.; Brochet, E.; Kim Jong, S.; Song, J.-K.; Mas, J.-L.; CLOSE and DEFENSE-PFO Trial Investigators. Atrial Septal Aneurysm, Shunt Size, and Recurrent Stroke Risk in Patients With Patent Foramen Ovale. J. Am. Coll. Cardiol. 2020, 75, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Guideline Updates Needed: Closure Devices to Prevent Recurrent Stroke in Patients With Patent Foramen Ovale. J. Am. Heart Assoc. 2024, 13, e035937. [Google Scholar] [CrossRef] [PubMed]
- Sposato, L.A.; Albin, C.S.W.; Elkind, M.S.V.; Kamel, H.; Saver, J.L.; Goldstein, L.B.; Das, A.S.; Gokcal, E.; Merino, J.G.; Broderick, J.; et al. Patent Foramen Ovale Management for Secondary Stroke Prevention: State-of-the-Art Appraisal of Current Evidence. Stroke 2024, 55, 236–247. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.D.; Dong, Y.; Dong, Q. Patent foramen ovale closure for patients with cryptogenic stroke: A systematic review and comprehensive meta-analysis of 5 randomized controlled trials and 14 observational studies. CNS Neurosci. Ther. 2018, 24, 853–862. [Google Scholar] [CrossRef]
- Eltelbany, M.; Gattani, R.; Ofosu-Somuah, A.; Damluji, A.; Epps, K.C.; Batchelor, W.B. Transcatheter PFO closure for cryptogenic stroke: Current approaches and future considerations. Front. Cardiovasc. Med. 2024, 11, 1391886. [Google Scholar] [CrossRef]
- Robinson, A.A.; Ikuta, K.; Soverow, J. Anticoagulation for the acute management of ischemic stroke. Yale J. Biol. Med. 2014, 87, 199–206. [Google Scholar]
- Kasner, S.E.; Lattanzi, S.; Fonseca, A.C.; Elgendy, A.Y. Uncertainties and Controversies in the Management of Ischemic Stroke and Transient Ischemic Attack Patients With Patent Foramen Ovale. Stroke 2021, 52, e806–e819. [Google Scholar] [CrossRef]
- Patel, J.; Bhaskar, S.M.M. Diagnosis and Management of Atrial Fibrillation in Acute Ischemic Stroke in the Setting of Reperfusion Therapy: Insights and Strategies for Optimized Care. J. Cardiovasc. Dev. Dis. 2023, 10, 458. [Google Scholar] [CrossRef]
- Hald, E.M.; Rinde, L.B.; Lochen, M.L.; Mathiesen, E.B.; Wilsgaard, T.; Njolstad, I.; Braekkan, S.K.; Hansen, J.B. Atrial Fibrillation and Cause-Specific Risks of Pulmonary Embolism and Ischemic Stroke. J. Am. Heart Assoc. 2018, 7, e006502. [Google Scholar] [CrossRef] [PubMed]
- Henninger, N.; Goddeau, R.P., Jr.; Karmarkar, A.; Helenius, J.; McManus, D.D. Atrial Fibrillation Is Associated With a Worse 90-Day Outcome Than Other Cardioembolic Stroke Subtypes. Stroke 2016, 47, 1486–1492. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nyaga, U.F.; Middeldorp, M.E.; Fitzgerald, J.L.; Ariyaratnam, J.P.; Thomas, G.; Sanders, P. Frequency and prognostic significance of atrial fibrillation in acute pulmonary embolism: A pooled analysis. Respir. Med. 2022, 199, 106862. [Google Scholar] [CrossRef] [PubMed]
- White, C.W.; Kerber, R.E.; Weiss, H.R.; Marcus, M.L. The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ. Res. 1982, 51, 205–215. [Google Scholar] [CrossRef]
- Koizume, S.; Miyagi, Y. Tissue factor in cancer-associated thromboembolism: Possible mechanisms and clinical applications. Br. J. Cancer 2022, 127, 2099–2107. [Google Scholar] [CrossRef]
- Dardiotis, E.; Aloizou, A.M.; Markoula, S.; Siokas, V.; Tsarouhas, K.; Tzanakakis, G.; Libra, M.; Kyritsis, A.P.; Brotis, A.G.; Aschner, M.; et al. Cancer-associated stroke: Pathophysiology, detection and management (Review). Int. J. Oncol. 2019, 54, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Dunkler, D.; Pirker, R.; Thaler, J.; Quehenberger, P.; Wagner, O.; Zielinski, C.; Pabinger, I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012, 97, 1158–1164. [Google Scholar] [CrossRef]
- Yoo, J.; Kwon, I.; Kim, S.; Kim, H.M.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Coagulation Factor Expression and Composition of Arterial Thrombi in Cancer-Associated Stroke. Stroke 2023, 54, 2981–2989. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Oyakawa, T.; Muraoka, N.; Iida, K.; Kusuhara, M.; Mori, K. Use of direct oral anti-coagulants for the treatment of venous thromboembolism in patients with advanced cancer: A prospective observational study. Int. J. Clin. Oncol. 2019, 24, 876–881. [Google Scholar] [CrossRef]
- Ogino, Y.; Ishigami, T.; Minamimoto, Y.; Kimura, Y.; Akiyama, E.; Okada, K.; Matsuzawa, Y.; Maejima, N.; Iwahashi, N.; Hibi, K.; et al. Direct Oral Anticoagulant Therapy for Cancer-Associated Venous Thromboembolism in Routine Clinical Practice. Circ. J. 2020, 84, 1330–1338. [Google Scholar] [CrossRef]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef]
- Navi, B.B.; Zhang, C.; Sherman, C.P.; Genova, R.; LeMoss, N.M.; Kamel, H.; Tagawa, S.T.; Saxena, A.; Ocean, A.J.; Kasner, S.E.; et al. Ischemic stroke with cancer: Hematologic and embolic biomarkers and clinical outcomes. J. Thromb. Haemost. 2022, 20, 2046–2057. [Google Scholar] [CrossRef]
- van der Wall, S.J.; Klok, F.A.; den Exter, P.L.; Barrios, D.; Morillo, R.; Cannegieter, S.C.; Jimenez, D.; Huisman, M.V. Continuation of low-molecular-weight heparin treatment for cancer-related venous thromboembolism: A prospective cohort study in daily clinical practice. J. Thromb. Haemost. 2017, 15, 74–79. [Google Scholar] [CrossRef]
- Soff, G.A. Use of Direct Oral Anticoagulants for Treating Venous Thromboembolism in Patients With Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Stecker, E.; Bruce, A.W. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef] [PubMed]
- Ihaddadene, R.; Corsi, D.J.; Lazo-Langner, A.; Shivakumar, S.; Zarychanski, R.; Tagalakis, V.; Solymoss, S.; Routhier, N.; Douketis, J.; Le Gal, G.; et al. Risk factors predictive of occult cancer detection in patients with unprovoked venous thromboembolism. Blood 2016, 127, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef]
- Connolly, G.C.; Khorana, A.A. Risk stratification for cancer-associated venous thromboembolism. Best. Pract. Res. Clin. Haematol. 2009, 22, 35–47. [Google Scholar] [CrossRef]
- Hotoleanu, C. Association between obesity and venous thromboembolism. Med. Pharm. Rep. 2020, 93, 162–168. [Google Scholar] [CrossRef]
- Landin, K.; Stigendal, L.; Eriksson, E.; Krotkiewski, M.; Risberg, B.; Tengborn, L.; Smith, U. Abdominal obesity is associated with an impaired fibrinolytic activity and elevated plasminogen activator inhibitor-1. Metabolism 1990, 39, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Adipose Tissue-Derived Plasminogen Activator Inhibitor-1 Function and Regulation. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2025; pp. 1873–1896. [Google Scholar]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Russo, I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int. J. Mol. Sci. 2020, 21, 623. [Google Scholar] [CrossRef]
- Kim, P.-J.; Kim, C.; Lee, S.-S.; Shon, J.-H.; Kwon, Y.; Kim, D.-K.; Yu, H.; Ahn, H.-J.; Jeon, J.-P.; Kim, Y.; et al. Another Look at Obesity Paradox in Acute Ischemic Stroke: Association Rule Mining. J. Pers. Med. 2021, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Saleemi, A.; Asfaw, M.; Aldaoud, N.; Chalasani, P.; Lavu, V.K.; Bhui, P.; Nagar, T.; Agarwal, A.; Yesilyaprak, A.; et al. The outcomes of the obesity paradox in pulmonary embolism: A study of the national inpatient sample database from 2016 to 2020. Ann. Hematol. 2025, 104, 1187–1193. [Google Scholar] [CrossRef]
- Freeman, A.L.; Pendleton, R.C.; Rondina, M.T. Prevention of venous thromboembolism in obesity. Expert Rev. Cardiovasc. Ther. 2010, 8, 1711–1721. [Google Scholar] [CrossRef]
- Wang, T.F.; Milligan, P.E.; Wong, C.A.; Deal, E.N.; Thoelke, M.S.; Gage, B.F. Efficacy and safety of high-dose thromboprophylaxis in morbidly obese inpatients. Thromb. Haemost. 2014, 111, 88–93. [Google Scholar] [CrossRef]
- Delis, K.T.; Nicolaides, A.N. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: A prospective randomized controlled study with 1-year follow-up. Ann. Surg. 2005, 241, 431–441. [Google Scholar] [CrossRef]
- Kakkos, S.; Kirkilesis, G.; Caprini, J.A.; Geroulakos, G.; Nicolaides, A.; Stansby, G.; Reddy, D.J. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst. Rev. 2022, 1, Cd005258. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Y.; Luo, J.; Zhang, J.; Yan, Q. Sex-Based Differences in the Presentation and Outcomes of Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Tex. Heart Inst. J. 2023, 50, e238113. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.; McCullough, L.D. Sex differences in stroke: The contribution of coagulation. Exp. Neurol. 2014, 259, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Beule, J.; Balzer, J.O.; Dippold, W. D-Dimer and thrombus burden in acute pulmonary embolism. Am. J. Emerg. Med. 2018, 36, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Leyva, H.; Muriel, A.; Lin, Z.; Piazza, G.; Khairani, C.D.; Rosovsky, R.P.; Mehdipour, G.; O’Donoghue, M.L.; Madridano, O.; et al. Sex differences in treatment strategies for pulmonary embolism in older adults: The SERIOUS-PE study of RIETE participants and US Medicare beneficiaries. Vasc. Med. 2025, 30, 58–66. [Google Scholar] [CrossRef]
- Newman, J.; Bruno, E.; Allen, S.; Moore, J.; Zilinyi, R.; Khaliq, A.; Alkhafan, F.; Vitarello, C.; Lookstein, R.; Keeling, B.; et al. The influence of patient sex on pulmonary embolism evaluation, treatment modality, and outcomes. Vasc. Med. 2025, 30, 67–75. [Google Scholar] [CrossRef]
- van Mens, T.E.; van der Pol, L.M.; van Es, N.; Bistervels, I.M.; Mairuhu, A.T.A.; van der Hulle, T.; Klok, F.A.; Huisman, M.V.; Middeldorp, S. Sex-specific performance of pre-imaging diagnostic algorithms for pulmonary embolism. J. Thromb. Haemost. 2018, 16, 858–865. [Google Scholar] [CrossRef]
- Keller, K.; Rappold, L.; Gerhold-Ay, A.; Hobohm, L.; Hasenfuß, G.; Konstantinides, S.V.; Dellas, C.; Lankeit, M. Sex-specific differences in pulmonary embolism. Thromb. Res. 2019, 178, 173–181. [Google Scholar] [CrossRef]
- Bauer, K.A. The thrombophilias: Well-defined risk factors with uncertain therapeutic implications. Ann. Intern. Med. 2001, 135, 367–373. [Google Scholar] [CrossRef]
- Murin, S.; Marelich, G.P.; Arroliga, A.C.; Matthay, R.A. Hereditary Thrombophilia and Venous Thromboembolism. Am. J. Respir. Crit. Care Med. 1998, 158, 1369–1373. [Google Scholar] [CrossRef]
- Dhabhar, J.; Mehta, V.; Desai, N.; Dawoodi, S.; Zaman, S.B. Case Report: Unprovoked venous thromboembolism in a young adult male. F1000Research 2019, 8, 182. [Google Scholar] [CrossRef]
- Kassis, J.; Neville, C.; Rauch, J.; Busque, L.; Chang, E.R.; Joseph, L.; Le Comte, M.; Subang, R.; Fortin, P.R. Antiphospholipid antibodies and thrombosis: Association with acquired activated protein C resistance in venous thrombosis and with hyperhomocysteinemia in arterial thrombosis. Thromb. Haemost. 2004, 92, 1312–1319. [Google Scholar] [CrossRef]
- Patriarcheas, V.; Tsamos, G.; Vasdeki, D.; Kotteas, E.; Kollias, A.; Nikas, D.; Kaiafa, G.; Dimakakos, E. Antiphospholipid Syndrome: A Comprehensive Clinical Review. J. Clin. Med. 2025, 14, 733. [Google Scholar] [CrossRef]
- Sayar, Z.; Moll, R.; Isenberg, D.; Cohen, H. Thrombotic antiphospholipid syndrome: A practical guide to diagnosis and management. Thromb. Res. 2021, 198, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Celia, A.I.; Vescovo, G.M.; Sarto, G.; Alessandri, C.; Iaconelli, A.; D’Amario, D.; Frati, G.; Conti, F.; Sciarretta, S.; Angiolillo, D.J.; et al. Direct oral anticoagulants versus Vitamin K antagonists in antiphospholipid syndrome: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2025, 73, 152741. [Google Scholar] [CrossRef] [PubMed]
- Salehi Omran, S.; Hartman, A.; Zakai, N.A.; Navi, B.B. Thrombophilia Testing After Ischemic Stroke. Stroke 2021, 52, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Putaala, J. Ischemic stroke in the young: Current perspectives on incidence, risk factors, and cardiovascular prognosis. Eur. Stroke J. 2016, 1, 28–40. [Google Scholar] [CrossRef]
- Barber, M.R.W.; Clarke, A.E.; Adams, C.D.; Skeith, L. Severe thrombotic complications secondary to antiphospholipid syndrome and undiagnosed systemic lupus erythematosus. Can. Med. Assoc. J. 2022, 194, E1243–E1247. [Google Scholar] [CrossRef]
- Anderson, M.; Belmont, H.M. Severe thrombotic events associated with pre-procedural interruption of anticoagulation in systemic lupus erythematosus with secondary antiphospholipid syndrome: Cases and literature review. Lupus 2022, 31, 261–267. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sinha, A.; Banach, M.; Mittoo, S.; Weissert, R.; Kass, J.S.; Rajagopal, S.; Pai, A.R.; Kutty, S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front. Immunol. 2020, 11, 1648. [Google Scholar] [CrossRef]
- El Husseini, N.; Katzan, I.L.; Rost, N.S.; Blake, M.L.; Byun, E.; Pendlebury, S.T.; Aparicio, H.J.; Marquine, M.J.; Gottesman, R.F.; Smith, E.E.; et al. Cognitive Impairment After Ischemic and Hemorrhagic Stroke: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke 2023, 54, e272–e291. [Google Scholar] [CrossRef]
- Murray, H.W.; Ellis, G.C.; Blumenthal, D.S.; Sos, T.A. Fever and pulmonary thromboembolism. Am. J. Med. 1979, 67, 232–235. [Google Scholar] [CrossRef]
- Sabapathy, C.; Amid, A.; Kumar, R.; Avila, L.; van Ommen, H. Diagnostic Approach to Thrombosis and Thrombophilia. In SickKids Handbook of Pediatric Thrombosis and Hemostasis; Blanchette, V.S., Brandão, L.R., Carcao, M.D., Kumar, R., Rand, M.L., Revel-Vilk, S., Eds.; S.Karger AG: Basel, Switzerland, 2024; pp. 233–255. [Google Scholar]
- Innocenti, F.; Lazzari, C.; Ricci, F.; Paolucci, E.; Agishev, I.; Pini, R. D-Dimer Tests in the Emergency Department: Current Insights. Open Access Emerg. Med. 2021, 13, 465–479. [Google Scholar] [CrossRef]
- Righini, M.; Perrier, A.; De Moerloose, P.; Bounameaux, H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J. Thromb. Haemost. 2008, 6, 1059–1071. [Google Scholar] [CrossRef]
- Kabrhel, C.; Mark Courtney, D.; Camargo, C.A., Jr.; Moore, C.L.; Richman, P.B.; Plewa, M.C.; Nordenholtz, K.E.; Smithline, H.A.; Beam, D.M.; Brown, M.D.; et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad. Emerg. Med. 2009, 16, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tapson, V.F. Acute pulmonary embolism. N. Engl. J. Med. 2008, 358, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Mirza, H.; Hashmi, M.F. Lung Ventilation Perfusion Scan (VQ Scan). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Waxman, A.D.; Bajc, M.; Brown, M.; Fahey, F.H.; Freeman, L.M.; Haramati, L.B.; Julien, P.; Le Gal, G.; Neilly, B.; Rabin, J.; et al. Appropriate Use Criteria for Ventilation-Perfusion Imaging in Pulmonary Embolism: Summary and Excerpts. J. Nucl. Med. 2017, 58, 13n–15n. [Google Scholar]
- Yuriditsky, E.; Horowitz, J.M.; Taslakian, B.; Saric, M. Saddle Pulmonary Embolism Detected by Transthoracic Echocardiography in a Patient With Suspected Myocardial Infarction. Cardiovasc. Imaging Case Rep. 2024, 8, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Bhaskar, S.M.M. Diagnostic Utility of N-Terminal Pro-B-Type Natriuretic Peptide in Identifying Atrial Fibrillation Post-Cryptogenic Stroke: A Systematic Review and Meta-Analysis. Pathophysiology 2024, 31, 331–349. [Google Scholar] [CrossRef]
- Oh, J.K.; Park, J.H. Role of echocardiography in acute pulmonary embolism. Korean J. Intern. Med. 2023, 38, 456–470. [Google Scholar] [CrossRef]
- Er, C.; Cohen, A.T. Limitations of venous thromboembolism risk assessment models in medical patients: Response to “Unmet definitions in thromboprophylaxis for hospitalized medical patients: An appraisal for the need of recommendation”. Res. Pract. Thromb. Haemost. 2023, 7, 100027. [Google Scholar] [CrossRef]
- Girardi, A.M.; Bettiol, R.S.; Garcia, T.S.; Ribeiro, G.L.H.; Rodrigues, E.M.; Gazzana, M.B.; Rech, T.H. Wells and Geneva Scores Are Not Reliable Predictors of Pulmonary Embolism in Critically Ill Patients: A Retrospective Study. J. Intensive Care Med. 2020, 35, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.; Bhaskar, S.M.M. The role of artificial intelligence in optimizing management of atrial fibrillation in acute ischemic stroke. Ann. N. Y. Acad. Sci. 2024, 1541, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Wiener, R.S.; Schwartz, L.M.; Woloshin, S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch. Intern. Med. 2011, 171, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Heart and Stroke Foundation of Canada. Inpatient Prevention and Management of Complications Following Stroke. Available online: https://www.strokebestpractices.ca/recommendations/acute-stroke-management/inpatient-prevention-and-management-of-complications-following-stroke (accessed on 10 April 2025).
- Bajenaru, O.; Antochi, F.; Balasa, R.; Buraga, I.; Patrichi, S.; Simu, M.; Szabolcs, S.; Tiu, C.; Zaharia, C.; VTE-NEURO Study Group. Assessment of Venous Thromboembolism Prophylaxis in Neurological Patients with Restricted Mobility–VTE-NEURO Study. Maedica 2014, 9, 6–14. [Google Scholar]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Guo, J.; Guan, T.; Fan, S.; Chao, B.; Wang, L.; Liu, Y. Underuse of Oral Anticoagulants in Patients With Ischemic Stroke and Atrial Fibrillation in China. Am. J. Cardiol. 2018, 122, 2055–2061. [Google Scholar] [CrossRef]
- Seiffge, D.J.; Werring, D.J.; Paciaroni, M.; Dawson, J.; Warach, S.; Milling, T.J.; Engelter, S.T.; Fischer, U.; Norrving, B. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019, 18, 117–126. [Google Scholar] [CrossRef]
- Macha, K.; Volbers, B.; Bobinger, T.; Kurka, N.; Breuer, L.; Huttner, H.B.; Schwab, S.; Kohrmann, M. Early Initiation of Anticoagulation with Direct Oral Anticoagulants in Patients after Transient Ischemic Attack or Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2317–2321. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Tanaka, K.; Toyoda, K.; Yoshimura, S.; Itabashi, R.; Takagi, M.; Todo, K.; Shiozawa, M.; Yagita, Y.; Yoshimoto, T.; et al. Early Initiation of Direct Oral Anticoagulants After Onset of Stroke and Short- and Long-Term Outcomes of Patients With Nonvalvular Atrial Fibrillation. Stroke 2020, 51, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, M.; Guloglu, R.; Ertekin, C.; Taviloglu, K.; Alimoglu, O. Intermittent pneumatic compression in the prevention of venous thromboembolism in high-risk trauma and surgical ICU patients. Ulus. Travma Acil Cerrahi Derg. 2005, 11, 38–42. [Google Scholar] [PubMed]
- CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration; Dennis, M.; Sandercock, P.; Reid, J.; Graham, C.; Forbes, J.; Murray, G. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): A multicentre randomised controlled trial. Lancet 2013, 382, 516–524. [Google Scholar] [CrossRef]
- Kearon, C.; O’Donnell, M. Should patients with stroke wear compression stockings to prevent venous thromboembolism? Ann. Intern. Med. 2010, 153, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Partsch, H.; Morrison, N.; Meissner, M.H.; Mosti, G.; Lattimer, C.R.; Carpentier, P.H.; Gaillard, S.; Junger, M.; Urbanek, T.; et al. Risks and contraindications of medical compression treatment–A critical reappraisal. An international consensus statement. Phlebology 2020, 35, 447–460. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Wang, Q.; Gao, W. Effect of neuromuscular electrical stimulation in critically ill adults with mechanical ventilation: A systematic review and network meta- analysis. BMC Pulm. Med. 2024, 24, 56. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Gao, Y.; Deng, B.; Liu, K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst. Rev. 2021, 3, Cd004434. [Google Scholar] [CrossRef]
- Yaghi, S.; Willey, J.Z.; Cucchiara, B.; Goldstein, J.N.; Gonzales, N.R.; Khatri, P.; Kim, L.J.; Mayer, S.A.; Sheth, K.N.; Schwamm, L.H.; et al. Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e343–e361. [Google Scholar] [CrossRef]
- Safouris, A.; Psychogios, K.; Palaiodimou, L.; Orosz, P.; Magoufis, G.; Kargiotis, O.; Theodorou, A.; Karapanayiotides, T.; Spiliopoulos, S.; Nardai, S.; et al. Update of Anticoagulation Use in Cardioembolic Stroke With a Special Reference to Endovascular Treatment. J. Stroke 2024, 26, 13–25. [Google Scholar] [CrossRef]
- Goel, N.; Jain, D.; Haddad, D.B.; Shanbhogue, D. Anticoagulation in Patients with End-Stage Renal Disease and Atrial Fibrillation: Confusion, Concerns and Consequences. J. Stroke 2020, 22, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, G.; Dey, R.C.; Kutikuppala, L.V.S.; Maheshwari, A.R.; Josey, E.; Chintala, J.S.; Abdullah, M.; Godugu, S. Mechanical thrombectomy for AIS from large vessel occlusion—Current trends and future perspectives. Ann. Med. Surg. 2023, 85, 6021–6028. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.J.; Gibson, C.M.; Giri, J.; Khandhar, S.; Jaber, W.; Toma, C.; Mina, B.; Bowers, T.; Greenspon, L.; Kado, H.; et al. Outcomes in High-Risk Pulmonary Embolism Patients Undergoing FlowTriever Mechanical Thrombectomy or Other Contemporary Therapies: Results From the FLAME Study. Circ. Cardiovasc. Interv. 2023, 16, e013406. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.M.; Jaber, W.A.; Stegman, B.; Rosenberg, M.; Fanola, C.; Bhat, A.P.; Gondi, S.; Castle, J.; Ahmed, M.; Brown, M.A.; et al. Mechanical Thrombectomy for High-Risk Pulmonary Embolism: Insights From the US Cohort of the FLASH Registry. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101124. [Google Scholar] [CrossRef]
- Panahi, L.; Udeani, G.; Horseman, M.; Weston, J.; Samuel, N.; Joseph, M.; Mora, A.; Bazan, D. Review of Medical Therapies for the Management of Pulmonary Embolism. Medicina 2021, 57, 110. [Google Scholar] [CrossRef] [PubMed]
- Naoum, J.J. Anticoagulation Management Post Pulmonary Embolism. Methodist Debakey Cardiovasc. J. 2024, 20, 27–35. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014, 123, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Malicke, D.T.; Schramski, J.T. Stroke Anticoagulation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hirano, T.; Kaneko, H.; Mishina, S.; Wang, F.; Morita, S. Suboptimal Anticoagulant Management in Japanese Patients with Nonvalvular Atrial Fibrillation Receiving Warfarin for Stroke Prevention. J. Stroke Cerebrovasc. Dis. 2017, 26, 2102–2110. [Google Scholar] [CrossRef]
- Desai, P.V.; Krepostman, N.; Collins, M.; De Sirkar, S.; Hinkleman, A.; Walsh, K.; Fareed, J.; Darki, A. Neurological Complications of Pulmonary Embolism: A Literature Review. Curr. Neurol. Neurosci. Rep. 2021, 21, 59. [Google Scholar] [CrossRef]
- Sadigh, Y.; Volovici, V. Understanding Cognitive Decline After Stroke in the Acute Setting. JAMA Netw. Open 2024, 7, e2437145. [Google Scholar] [CrossRef]
- del Ser, T.; Barba, R.; Morin, M.M.; Domingo, J.; Cemillan, C.; Pondal, M.; Vivancos, J. Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke 2005, 36, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Mouradian, M.S.; Majumdar, S.R.; Senthilselvan, A.; Khan, K.; Shuaib, A. How well are hypertension, hyperlipidemia, diabetes, and smoking managed after a stroke or transient ischemic attack? Stroke 2002, 33, 1656–1659. [Google Scholar] [CrossRef]
- Yu, A.; Ding, W.; Lin, W.; Cai, J.; Huang, W. Application of pulmonary rehabilitation in patients with pulmonary embolism (Review). Exp. Ther. Med. 2022, 23, 96. [Google Scholar] [CrossRef]
- Harver, A.; Mahler, D.A.; Daubenspeck, J.A. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Ann. Intern. Med. 1989, 111, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Martin-Suarez, S.; Loforte, A.; Cavalli, G.G.; Gliozzi, G.; Botta, L.; Mariani, C.; Orioli, V.; Votano, D.; Costantino, A.; Santamaria, V.; et al. Therapeutic alternatives in chronic thromboembolic pulmonary hypertension: From pulmonary endarterectomy to balloon pulmonary angioplasty to medical therapy. State of the art from a multidisciplinary team. Ann. Cardiothorac. Surg. 2022, 11, 120–127. [Google Scholar] [CrossRef]
- Dong, J.; Li, Z.; Luo, L.; Xie, H. Efficacy of pulmonary rehabilitation in improving the quality of life for patients with chronic obstructive pulmonary disease: Evidence based on nineteen randomized controlled trials. Int. J. Surg. 2020, 73, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Jervan, O.; Haukeland-Parker, S.; Gleditsch, J.; Tavoly, M.; Klok, F.A.; Steine, K.; Johannessen, H.H.; Spruit, M.A.; Atar, D.; Holst, R.; et al. The Effects of Exercise Training in Patients With Persistent Dyspnea Following Pulmonary Embolism: A Randomized Controlled Trial. Chest 2023, 164, 981–991. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Paul, G.A.; Strange, J.W.; Tunariu, N.; Gin-Sing, W.; Banya, W.A.; Westwood, M.A.; Stefanidis, A.; Ng, L.L.; Pennell, D.J.; et al. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am. J. Respir. Crit. Care Med. 2005, 171, 1292–1297. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Lane, D.A.; Lenarczyk, R.; Boriani, G.; Doehner, W.; Benjamin, L.A.; Fisher, M.; Lowe, D.; Sacco, R.L.; Schnabel, R.; et al. Integrated care for optimizing the management of stroke and associated heart disease: A position paper of the European Society of Cardiology Council on Stroke. Eur. Heart J. 2022, 43, 2442–2460. [Google Scholar] [CrossRef] [PubMed]
- Simats, A.; Liesz, A. Systemic inflammation after stroke: Implications for post-stroke comorbidities. EMBO Mol. Med. 2022, 14, e16269. [Google Scholar] [CrossRef]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D.; et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132, e154225. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Wienkamp, A.K.; Erpenbeck, L.; Rossaint, J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022, 13, 953129. [Google Scholar] [CrossRef] [PubMed]
- Rayes, J.; Bourne, J.H.; Brill, A.; Watson, S.P. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res. Pract. Thromb. Haemost. 2020, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Nie, J.; Zhou, L.; Tian, W.; Liu, X.; Yang, L.; Yang, X.; Zhang, Y.; Wei, S.; Wang, D.W.; Wei, J. Deep insight into cytokine storm: From pathogenesis to treatment. Signal Transduct. Target. Ther. 2025, 10, 112. [Google Scholar] [CrossRef]
- Schulze, J.; Vogelgesang, A.; Dressel, A. Catecholamines, steroids and immune alterations in ischemic stroke and other acute diseases. Aging Dis. 2014, 5, 327–339. [Google Scholar] [CrossRef]
- Won, T.; Wood, M.K.; Hughes, D.M.; Talor, M.V.; Ma, Z.; Schneider, J.; Skinner, J.T.; Asady, B.; Goerlich, E.; Halushka, M.K.; et al. Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. EBioMedicine 2022, 75, 103812. [Google Scholar] [CrossRef]
- Colling, M.E.; Tourdot, B.E.; Kanthi, Y. Inflammation, Infection and Venous Thromboembolism. Circ. Res. 2021, 128, 2017–2036. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Spring, K.J.; Bhaskar, S.M.M. Neutrophil-lymphocyte ratio in acute ischemic stroke: Immunopathology, management, and prognosis. Acta Neurol. Scand. 2021, 144, 486–499. [Google Scholar] [CrossRef]
- Huang, J.C.; Bhaskar, S.M.M. Clot Morphology in Acute Ischemic Stroke Decision Making. Int. J. Mol. Sci. 2022, 23, 12373. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Cancer cell-derived tissue factor-positive extracellular vesicles: Biomarkers of thrombosis and survival. Curr. Opin. Hematol. 2019, 26, 349–356. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Wang, T.; Xiao, R.; Zhu, L.; Ruiz, M.; Dupuis, J.; Hu, Q. Extracellular vesicles in venous thromboembolism and pulmonary hypertension. J. Nanobiotechnology 2023, 21, 461. [Google Scholar] [CrossRef]
- Mallard, C.; Ferriero, D.M.; Vexler, Z.S. Immune-Neurovascular Interactions in Experimental Perinatal and Childhood Arterial Ischemic Stroke. Stroke 2024, 55, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Artner, T.; Sharma, S.; Lang, I.M. Nucleic acid liquid biopsies in cardiovascular disease: Cell-free DNA liquid biopsies in cardiovascular disease. Atherosclerosis 2024, 398, 118583. [Google Scholar] [CrossRef]
- Fang, H.; Bo, Y.; Hao, Z.; Mang, G.; Jin, J.; Wang, H. A promising frontier: Targeting NETs for stroke treatment breakthroughs. Cell Commun. Signal 2024, 22, 238. [Google Scholar] [CrossRef]
- Li, H.; Shan, W.; Zhao, X.; Sun, W. Neutrophils: Linking Inflammation to Thrombosis and Unlocking New Treatment Horizons. Int. J. Mol. Sci. 2025, 26, 1965. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Sista, A.K. Catheter-Directed Thrombolysis for Pulmonary Embolism: The State of Practice. Tech. Vasc. Interv. Radiol. 2018, 21, 78–84. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Zhang, F.; Wu, X.; Dai, T.; Kuang, J.; Cheng, Z.; Chen, W.; Yin, M.; Guo, T.; et al. Anticoagulation therapy after reperfusion treatment for acute ischemic stroke with non-valvular atrial fibrillation: A multicenter retrospective study. Sci. Rep. 2025, 15, 9619. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.T.; Wang, T.; Garcia, J.G.N. Acute Lung Injury: The Injured Lung Endothelium, Therapeutic Strategies for Barrier Protection, and Vascular Biomarkers. Textb. Pulm. Vasc. Dis. 2010, 28, 197–222. [Google Scholar]
- Hansell, L.; Delaney, A.; Milross, M.; Henderson, E. Reducing unnecessary use of intermittent pneumatic compression in intensive care: A before-and-after pilot study with environmental perspective. Aust. Crit. Care 2025, 38, 101125. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.A.; Sista, A.K.; Addison, D.; Bikdeli, B.; Bishay, V.L.; Gu, S.; Hood, M.N.; Litmanovich, D.; Misra, S.; Reddy, G.; et al. Disparities in Current Pulmonary Embolism Management and Outcomes: A Scientific Statement From the American Heart Association. Circulation 2025, 151, e944–e955. [Google Scholar] [CrossRef]
- Lauw, M.; Gangaraju, R.; Carrier, M. Treatment of Venous Thromboembolism (VTE) in Patients with Cancer [Pocket Guide]. American Society of Hematology. Available online: https://www.hematology.org/education/clinicians/guidelines-and-quality-care/pocket-guides (accessed on 10 July 2025).
- Biswas, R.; Wijeratne, T.; Zelenak, K.; Huasen, B.B.; Iacobucci, M.; Killingsworth, M.C.; Beran, R.G.; Gebreyohanns, M.; Sekhar, A.; Khurana, D.; et al. Disparities in Access to Reperfusion Therapy for Acute Ischemic Stroke (DARTS): A Comprehensive Meta-Analysis of Ethnicity, Socioeconomic Status, and Geographical Factors. CNS Drugs 2025, 39, 417–442. [Google Scholar] [CrossRef]
- Kumar, S.; Chou, S.H.Y.; Smith, C.J.; Nallaparaju, A.; Laurido-Soto, O.J.; Leonard, A.D.; Singla, A.K.; Leonhardt-Caprio, A.; Stein, D.J.; American Heart Association Stroke Council; et al. Addressing Systemic Complications of Acute Stroke: A Scientific Statement From the American Heart Association. Stroke 2025, 56, e15–e29. [Google Scholar] [CrossRef]
| Modality | Utility | Limitations in AIS patients |
|---|---|---|
| D-dimer [51,52] |
|
|
| CTPA [50] |
|
|
| V/Q scan [19] |
|
|
| Compression Ultrasonography (CUS) [14] |
|
|
| Echocardiography [14] |
|
|
| Guidelines | Pharmacological Prophylaxis | Mechanical Prophylaxis |
|---|---|---|
| American Health Association (2019) [128] | Aspirin + hydration for VTE prophylaxis in immobile stroke patients. UFH/LMWH evidence is limited, and with increased bleeding risks noted. | IPC effective (CLOTS 3 trial). GCS contraindicated. |
| European Stroke Organisation (2016) [27] | LMWH/UFH can be used if the VTE risk outweighs the bleeding risk. LMWH is preferred over UFH (better DVT reduction, more convenient). | Strongest evidence for IPC. GCS contraindicated. Neuromuscular electrical stimulation (NMES) needs more evidence as prophylaxis. |
| Neurocritical Care Society (2016) [7] | PREVAIL trial: LMWH superior to UFH for DVT prevention in AIS. Pharmacological and mechanical prophylaxis may act synergistically. | CLOTS 3 trial: IPC = absolute risk reduction of 3.6% of VTE when started within 0–3 days post-stroke. GCS may dislodge VTE and cause skin breakdown in immobile patients. |
| European Society of Cardiology (2021) [129] | Limited specific recommendations on PE prophylaxis in AIS patients. Early anticoagulation (<48 h) after AIS can increase the risk of intracranial haemorrhage. | No clear recommendation. |
| Canadian Stroke Best Practice (2022) [130] | LMWH/UFH on admission if no contraindications (e.g., haemorrhage). | IPC recommended. GCS not recommended. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Bhaskar, S.M.M. Pulmonary Embolism in Acute Ischaemic Stroke: Evolving Evidence, Diagnostic Challenges, and a Novel Thromboinflammatory Axis Hypothesis. Int. J. Mol. Sci. 2025, 26, 6733. https://doi.org/10.3390/ijms26146733
Chen D, Bhaskar SMM. Pulmonary Embolism in Acute Ischaemic Stroke: Evolving Evidence, Diagnostic Challenges, and a Novel Thromboinflammatory Axis Hypothesis. International Journal of Molecular Sciences. 2025; 26(14):6733. https://doi.org/10.3390/ijms26146733
Chicago/Turabian StyleChen, Darryl, and Sonu M. M. Bhaskar. 2025. "Pulmonary Embolism in Acute Ischaemic Stroke: Evolving Evidence, Diagnostic Challenges, and a Novel Thromboinflammatory Axis Hypothesis" International Journal of Molecular Sciences 26, no. 14: 6733. https://doi.org/10.3390/ijms26146733
APA StyleChen, D., & Bhaskar, S. M. M. (2025). Pulmonary Embolism in Acute Ischaemic Stroke: Evolving Evidence, Diagnostic Challenges, and a Novel Thromboinflammatory Axis Hypothesis. International Journal of Molecular Sciences, 26(14), 6733. https://doi.org/10.3390/ijms26146733







