microRNA-200c Mitigates Pulpitis and Promotes Dentin Regeneration
Abstract
1. Introduction
2. Results
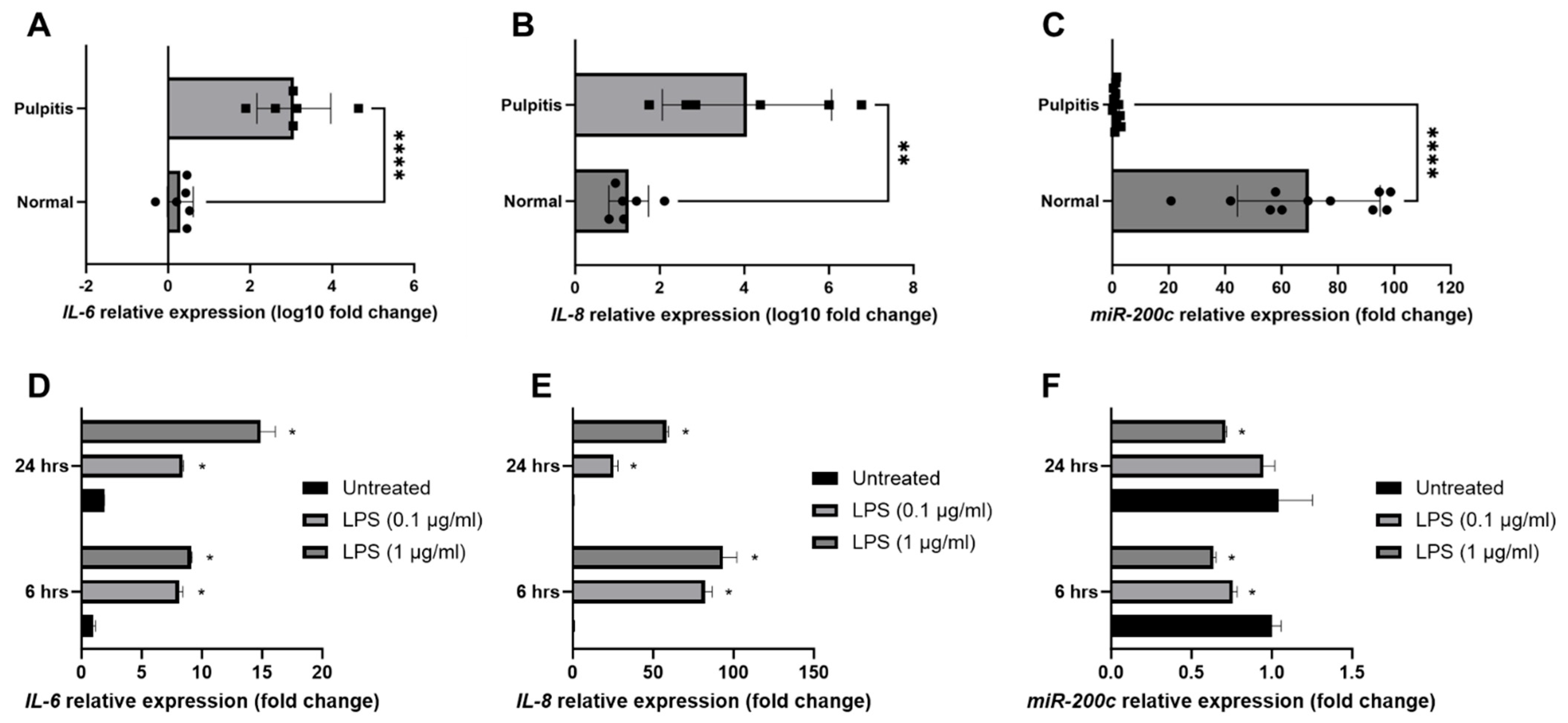
2.1. miR-200c Participates in the Pathogenesis of Pulpitis and Inflamed Human Dental Pulp Cells (hDPCs)
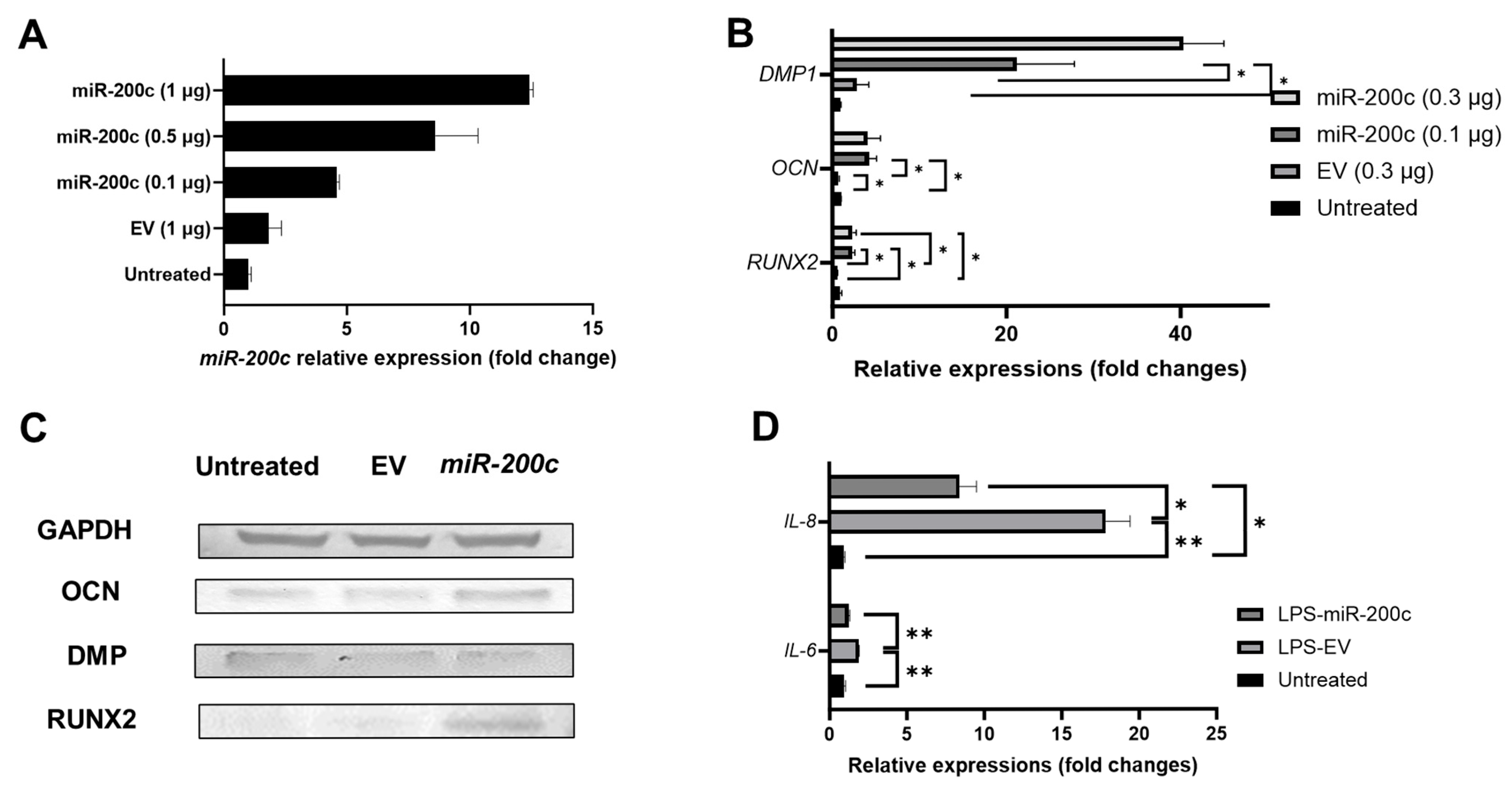
2.2. miR-200c Overexpression Promotes Odontogenic Differentiation in Human Dental Pulp Stem Cells (DPSCs) and Mitigates IL-6 and IL-8 in Inflamed Human Dental Pulp Cells (hDPCs)
2.3. Biocompatible CaCO3-Based Nanoparticles Significantly Improve the Transfection Efficiency of miR-200c in Dental Pulp Stem Cells
2.4. miR-200c Delivered by CaCO3 Promotes Odontogenic Differentiation in Human DPSCs
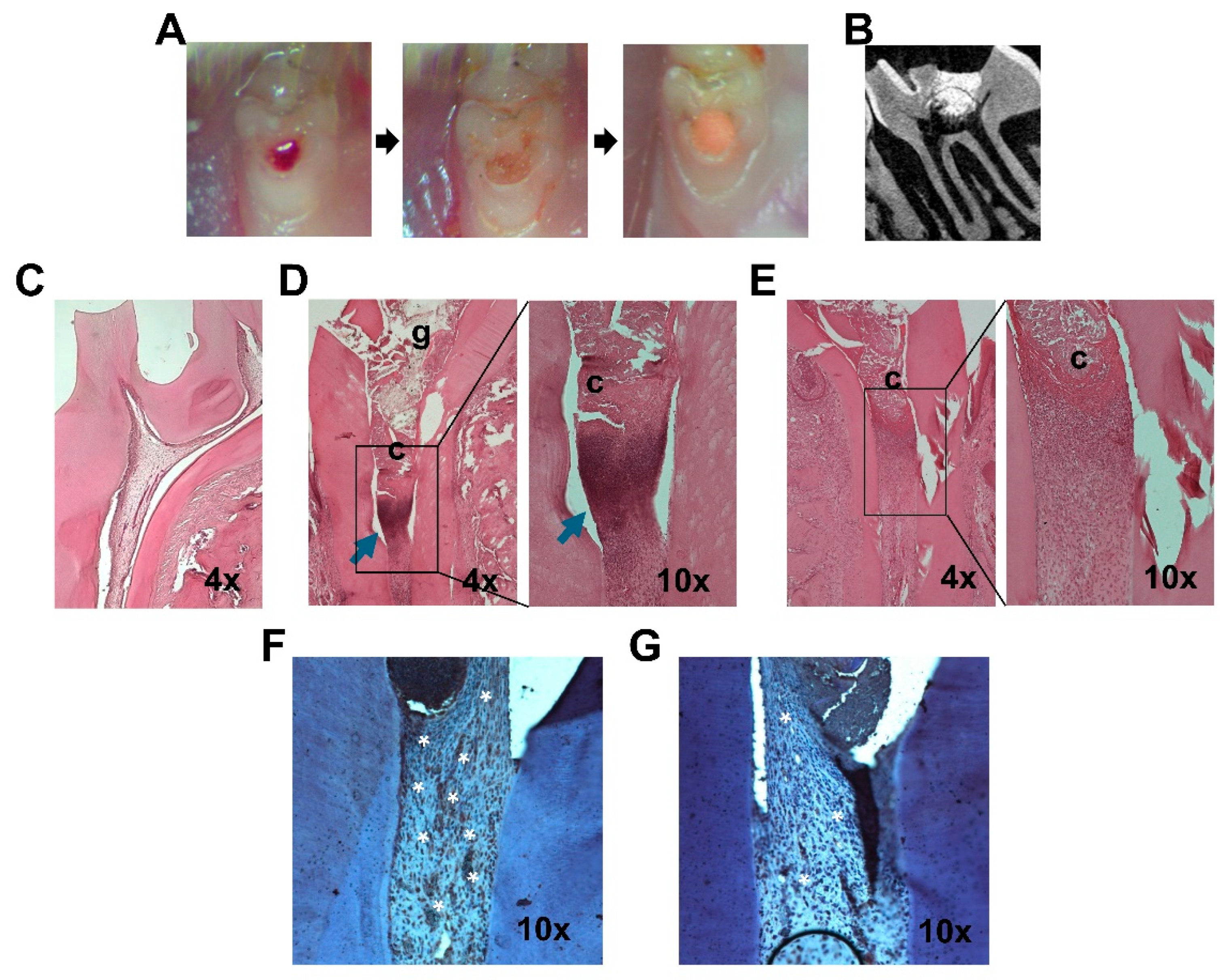
2.5. miR-200c Mitigates Pulp Inflammation and Improves Dentin Regeneration In Vivo
3. Discussion
4. Materials and Methods
4.1. Characterizing miR-200c and Proinflammatory Cytokines Within Inflamed Human Pulp Tissues and Dental Pulp Cells
4.2. Determining Odontogenic Differentiation Enhancement and Anti-Inflammation of miR-200c Overexpression in Human Dental Pulp Stem Cells and Dental Pulp Cells
4.3. Determining the Functions of pDNA miR-200c Delivered by CaCO3-Based Nanoparticles on Odontogenic Differentiation and Anti-Inflammation in Human DPSCs
4.4. Determining the Functions of pDNA Encoding miR-200c in Rat Models of Pulpitis
4.5. Quantitative Reverse Transcription PCR
4.6. Western Blot
4.7. Histological Examination
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parisay, I.; Ghoddusi, J.; Forghani, M. A review on vital pulp therapy in primary teeth. Iran. Endod. J. 2015, 10, 6–15. [Google Scholar]
- Asgary, S.; Ahmadyar, M. Vital pulp therapy using calcium-enriched mixture: An evidence-based review. J. Conserv. Dent. 2013, 16, 92–98. [Google Scholar] [CrossRef]
- Colombo, J.S.; Moore, A.N.; Hartgerink, J.D.; D’Souza, R.N. Scaffolds to control inflammation and facilitate dental pulp regeneration. J. Endod. 2014, 40 (Suppl. S4), S6–S12. [Google Scholar] [CrossRef]
- Aguilar, P.; Linsuwanont, P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: A systematic review. J. Endod. 2011, 37, 581–587. [Google Scholar] [CrossRef]
- Coll, J.; Seale, N.S.; Vargas, K.; Chi, D.L.; Marghalani, A.A.; Graham, L. Protocol for a Systematic Review and Meta-analysis of Vital Pulp Therapy for Children with Deep Caries in the Primary Dentition. Pediatr. Dent. 2015, 37, 418–421. [Google Scholar]
- Ripa, L.W. Review. Pulp therapy for the primary dentition. I. The treatment of teeth with vital pulps. J. Conn. State Dent. Assoc. 1970, 44, 193–199. [Google Scholar]
- Marques, M.S.; Wesselink, P.R.; Shemesh, H. Outcome of Direct Pulp Capping with Mineral Trioxide Aggregate: A Prospective Study. J. Endod. 2015, 41, 1026–1031. [Google Scholar] [CrossRef]
- Bjørndal, L.; Demant, S.; Dabelsteen, S. Depth and activity of carious lesions as indicators for the regenerative potential of dental pulp after intervention. J. Endod. 2014, 40 (Suppl. S4), S76–S81. [Google Scholar] [CrossRef]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Chua, P.; Elamin, A.D.; Clarke, M.; El-Karim, I.A. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 556–571. [Google Scholar] [CrossRef]
- Xavier, M.T.; Costa, A.L.; Ramos, J.C.; Caramês, J.; Marques, D.; Martins, J.N.R. Calcium Silicate-Based Cements in Restorative Dentistry: Vital Pulp Therapy Clinical, Radiographic, and Histological Outcomes on Deciduous and Permanent Dentition-A Systematic Review and Meta-Analysis. Materials 2024, 17, 4264. [Google Scholar] [CrossRef]
- Sangwan, P.; Sangwan, A.; Duhan, J.; Rohilla, A. Tertiary dentinogenesis with calcium hydroxide: A review of proposed mechanisms. Int. Endod. J. 2013, 46, 3–19. [Google Scholar] [CrossRef]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef]
- Tomson, P.L.; Grover, L.M.; Lumley, P.J.; Sloan, A.J.; Smith, A.J.; Cooper, P.R. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J. Dent. 2007, 35, 636–642. [Google Scholar] [CrossRef]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J. Endod. 2014, 40 (Suppl. S4), S46–S51. [Google Scholar] [CrossRef]
- Dabuleanu, M. Pulpitis (reversible/irreversible). J. Can. Dent. Assoc. 2013, 79, d90. [Google Scholar]
- Rechenberg, D.K.; Galicia, J.C.; Peters, O.A. Biological Markers for Pulpal Inflammation: A Systematic Review. PLoS ONE 2016, 11, e0167289. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, X.; Zhao, Y.; Ge, L.; Wang, Y. Potential Application of Human β-Defensin 4 in Dental Pulp Repair. Front. Physiol. 2020, 11, 1077. [Google Scholar] [CrossRef]
- Cao, H.; Jheon, A.; Li, X.; Sun, Z.; Wang, J.; Florez, S.; Zhang, Z.; McManus, M.T.; Klein, O.D.; Amendt, B.A. The Pitx2:miR-200c/141:noggin pathway regulates Bmp signaling and ameloblast differentiation. Development 2013, 140, 3348–3359. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, C.; Cheng, S.; Zhao, Z.; Li, J. MicroRNA control of tooth formation and eruption. Arch. Oral. Biol. 2017, 73, 302–310. [Google Scholar] [CrossRef]
- Emfietzoglou, R.; Pachymanolis, E.; Piperi, C. Impact of Epigenetic alterations in the development of oral diseases. Curr. Med. Chem. 2020, 28, 1091–1103. [Google Scholar] [CrossRef]
- Liu, L.; Shu, S.; Cheung, G.S.; Wei, X. Effect of miR-146a/bFGF/PEG-PEI Nanoparticles on Inflammation Response and Tissue Regeneration of Human Dental Pulp Cells. Biomed. Res. Int. 2016, 2016, 3892685. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.; Yang, W.; Miao, L.; Wang, N.; Wei, S.; Ge, J.; Li, X.; Wu, J. Decreased expression of microRNA-30b promotes the development of pulpitis by upregulating the expression of interleukin-6 receptor. Exp. Ther. Med. 2019, 17, 3233–3238. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J. Downregulation of microRNA-204 increases the expression of matrix metallopeptidase 9 in pediatric patients with pulpitis and Helicobacter pylori infection in the stomach. Exp. Ther. Med. 2019, 18, 253–259. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Deng, J.; Wang, X.; Long, F.; He, J. MicroRNA-506 Is Involved in Regulation of the Occurrence of Lipopolysaccharides (LPS)-Induced Pulpitis by Sirtuin 1 (SIRT1). Med. Sci. Monit. 2019, 25, 10008–10015. [Google Scholar] [CrossRef]
- Brodzikowska, A.; Gondek, A.; Rak, B.; Paskal, W.; Pełka, K.; Cudnoch-Jędrzejewska, A.; Włodarski, P. Metalloproteinase 14 (MMP-14) and hsa-miR-410-3p expression in human inflamed dental pulp and odontoblasts. Histochem. Cell Biol. 2019, 152, 345–353. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, K.; Chen, Z.; Wu, B. Noncoding RNAs: New insights into the odontogenic differentiation of dental tissue-derived mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 297. [Google Scholar] [CrossRef]
- Gay, I.; Cavender, A.; Peto, D.; Sun, Z.; Speer, A.; Cao, H.; Amendt, B.A. Differentiation of human dental stem cells reveals a role for microRNA-218. J. Periodontal Res. 2014, 49, 110–120. [Google Scholar] [CrossRef]
- Hara, E.S.; Ono, M.; Eguchi, T.; Kubota, S.; Pham, H.T.; Sonoyama, W.; Tajima, S.; Takigawa, M.; Calderwood, S.K.; Kuboki, T. miRNA-720 controls stem cell phenotype, proliferation and differentiation of human dental pulp cells. PLoS ONE 2013, 8, e83545. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Dao, J.; Gan, Z.; Zeng, X. Differential expression of lncRNA/miRNA/mRNA and their related functional networks during the osteogenic/odontogenic differentiation of dental pulp stem cells. J. Cell Physiol. 2020, 235, 3350–3361. [Google Scholar] [CrossRef]
- Qiu, Z.; Lin, S.; Hu, X.; Zeng, J.; Xiao, T.; Ke, Z.; Lv, H. Involvement of miR-146a-5p/neurogenic locus notch homolog protein 1 in the proliferation and differentiation of STRO-1(+) human dental pulp stem cells. Eur. J. Oral. Sci. 2019, 127, 294–303. [Google Scholar] [CrossRef]
- Akkouch, A.; Eliason, S.; Sweat, M.E.; Romero-Bustillos, M.; Zhu, M.; Qian, F.; Amendt, B.A.; Hong, L. Enhancement of MicroRNA-200c on Osteogenic Differentiation and Bone Regeneration by Targeting Sox2-Mediated Wnt Signaling and Klf4. Hum. Gene Ther. 2019, 30, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Gu, R.; Zhang, W.; Shao, L.; Li, F.; Wu, C.; Sun, Y. MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/β-Catenin signaling pathway via downregulating Myd88. J. Cell Physiol. 2019, 234, 22675–22686. [Google Scholar] [CrossRef]
- Wendlandt, E.B.; Graff, J.W.; Gioannini, T.L.; McCaffrey, A.P.; Wilson, M.E. The role of MicroRNAs miR-200b and miR-200c in TLR4 signaling and NF-kappa B activation. Innate Immun. 2012, 18, 846–855. [Google Scholar] [CrossRef]
- Chuang, T.D.; Khorram, O. miR-200c Regulates IL8 Expression by Targeting IKBKB: A Potential Mediator of Inflammation in Leiomyoma Pathogenesis. PLoS ONE 2014, 9, e95370. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Sharp, T.; Khorsand, B.; Fischer, C.; Eliason, S.; Salem, A.; Akkouch, A.; Brogden, K.; Amendt, B.A. MicroRNA-200c Represses IL-6, IL-8, and CCL-5 Expression and Enhances Osteogenic Differentiation. PLoS ONE 2016, 11, e0160915. [Google Scholar] [CrossRef] [PubMed]
- Krongbaramee, T.; Zhu, M.; Qian, Q.; Zhang, Z.; Eliason, S.; Shu, Y.; Qian, F.; Akkouch, A.; Su, D.; Amendt, B.A.; et al. Plasmid encoding microRNA-200c ameliorates periodontitis and systemic inflammation in obese mice. Mol. Ther. Nucleic Acids 2021, 23, 1204–1216. [Google Scholar] [CrossRef]
- Akkouch, A.; Zhu, M.; Romero-Bustillos, M.; Eliason, S.; Qian, F.; Salem, A.K.; Amendt, B.A.; Hong, L. MicroRNA-200c Attenuates Periodontitis by Modulating Proinflammatory and Osteoclastogenic Mediators. Stem Cells Dev. 2019, 28, 1026–1036. [Google Scholar] [CrossRef]
- Chen, C.; Han, H.; Yang, W.; Ren, X.; Kong, X. Polyethyleneimine-modified calcium carbonate nanoparticles for p53 gene delivery. Regen. Biomater. 2016, 3, 57–63. [Google Scholar] [CrossRef]
- Ding, Q.J.; Remy, M.T.; Upara, C.; Hu, J.; Mora Mata, A.V.; Haes, A.J.; Lanzel, E.; Sun, H.; Buchakjian, M.R.; Hong, L. CaCO3 Nanoparticles Delivering MicroRNA-200c Suppress Oral Squamous Cell Carcinoma. J. Dent. Res. 2023, 103, 220345231216110. [Google Scholar] [CrossRef]
- Remy, M.T.; Ding, Q.; Krongbaramee, T.; Hu, J.; Mora Mata, A.V.; Haes, A.J.; Amendt, B.A.; Sun, H.; Buchakjian, M.R.; Hong, L. Plasmid encoding miRNA-200c delivered by CaCO3-based nanoparticles enhances rat alveolar bone formation. Nanomedicine 2022, 17, 1339–1354. [Google Scholar] [CrossRef]
- Hong, H.; Chen, X.; Li, K.; Wang, N.; Li, M.; Yang, B.; Yu, X.; Wei, X. Dental follicle stem cells rescue the regenerative capacity of inflamed rat dental pulp through a paracrine pathway. Stem Cell Res. Ther. 2020, 11, 333. [Google Scholar] [CrossRef]
- Costa, C.A.; Giro, E.M.; do Nascimento, A.B.; Teixeira, H.M.; Hebling, J. Short-term evaluation of the pulpo-dentin complex response to a resin-modified glass-ionomer cement and a bonding agent applied in deep cavities. Dent. Mater. 2003, 19, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Y.; Gonzalez-Martin, A.; Miletic, A.V.; Lai, M.; Knight, S.; Sabouri-Ghomi, M.; Head, S.R.; Macauley, M.S.; Rickert, R.C.; Xiao, C. Transfection of microRNA Mimics Should Be Used with Caution. Front. Genet. 2015, 6, 340. [Google Scholar] [CrossRef]
- Hong, L.; Sun, H.; Amendt, B.A. MicroRNA function in craniofacial bone formation, regeneration and repair. Bone 2021, 144, 115789. [Google Scholar] [CrossRef]
- Su, D.; Krongbaramee, T.; Sun, H.; Hong, L.; Amendt, B.A. Exploring craniofacial and dental development with microRNAs. Biochem. Soc. Trans. 2022, 50, 1897–1909. [Google Scholar] [CrossRef]
- Alsadi, N.; Yasavoli-Sharahi, H.; Mueller, R.; Cuenin, C.; Chung, F.; Herceg, Z.; Matar, C. Protective Mechanisms of Polyphenol-Enriched Blueberry Preparation in Preventing Inflammation in the Skin against UVB-Induced Damage in an Animal Model. Antioxidants 2023, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, X.; Zhang, L.; Zhang, W.; Zhou, Q.; Tian, Z.; Zheng, Y.; Liao, Q.; Wang, H.; Li, G.; et al. MiR-200c is a cMyc-activated miRNA that promotes nasopharyngeal carcinoma by downregulating PTEN. Oncotarget 2017, 8, 5206–5218. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Watson, K.; Bruce, A.; Nersesian, S.; Kitz, J.; Moorehead, R. Re-expression of miR-200c suppresses proliferation, colony formation and in vivo tumor growth of murine claudin-low mammary tumor cells. Oncotarget 2017, 8, 23727–23749. [Google Scholar] [CrossRef]
- Qi, S.; Qian, J.; Chen, F.; Zhou, P.; Yue, J.; Tang, F.; Zhang, Y.; Gong, S.; Shang, G.; Cui, C.; et al. Expression of autophagy-associated proteins in rat dental irreversible pulpitis. Mol. Med. Rep. 2019, 19, 2749–2757. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krongbaramee, T.; Upara, C.; Remy, M.T.; Jiang, L.; Hu, J.; Tikkhanarak, K.; Cavalcanti, B.; Sun, H.; Teixeira, F.B.; Hong, L. microRNA-200c Mitigates Pulpitis and Promotes Dentin Regeneration. Int. J. Mol. Sci. 2025, 26, 6734. https://doi.org/10.3390/ijms26146734
Krongbaramee T, Upara C, Remy MT, Jiang L, Hu J, Tikkhanarak K, Cavalcanti B, Sun H, Teixeira FB, Hong L. microRNA-200c Mitigates Pulpitis and Promotes Dentin Regeneration. International Journal of Molecular Sciences. 2025; 26(14):6734. https://doi.org/10.3390/ijms26146734
Chicago/Turabian StyleKrongbaramee, Tadkamol, Chawin Upara, Matthew T. Remy, Long Jiang, Jue Hu, Kittiphoj Tikkhanarak, Bruno Cavalcanti, Hongli Sun, Fabricio B. Teixeira, and Liu Hong. 2025. "microRNA-200c Mitigates Pulpitis and Promotes Dentin Regeneration" International Journal of Molecular Sciences 26, no. 14: 6734. https://doi.org/10.3390/ijms26146734
APA StyleKrongbaramee, T., Upara, C., Remy, M. T., Jiang, L., Hu, J., Tikkhanarak, K., Cavalcanti, B., Sun, H., Teixeira, F. B., & Hong, L. (2025). microRNA-200c Mitigates Pulpitis and Promotes Dentin Regeneration. International Journal of Molecular Sciences, 26(14), 6734. https://doi.org/10.3390/ijms26146734





