Synergistic Production of Lycopene and β-Alanine Through Engineered Redox Balancing in Escherichia coli
Abstract
1. Introduction
2. Results
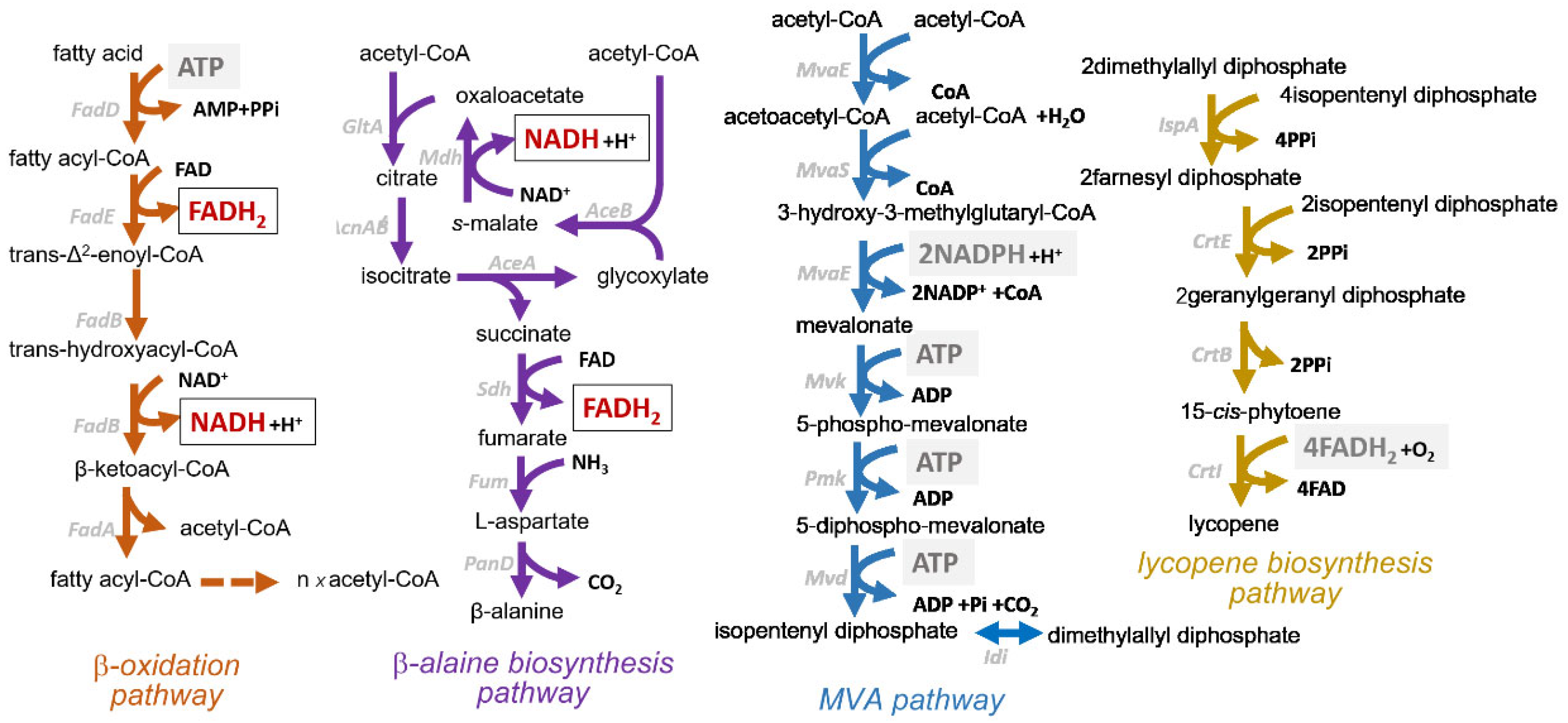
2.1. Design and Rationale of a Dual-Product Synthesis Strategy for Redox Balancing
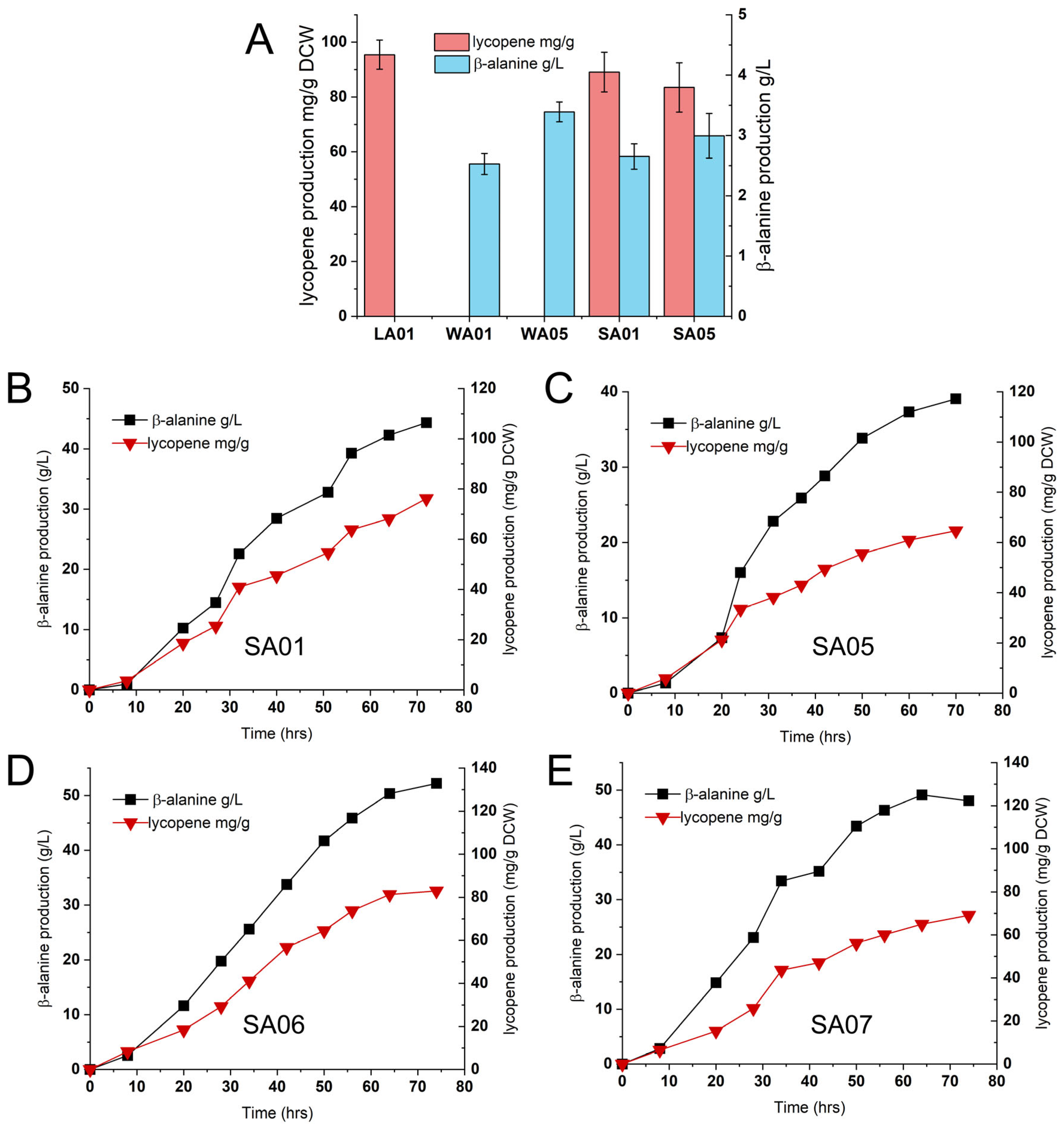
2.2. Validation and Optimization of Dual-Pathway Strains for Enhanced Co-Production
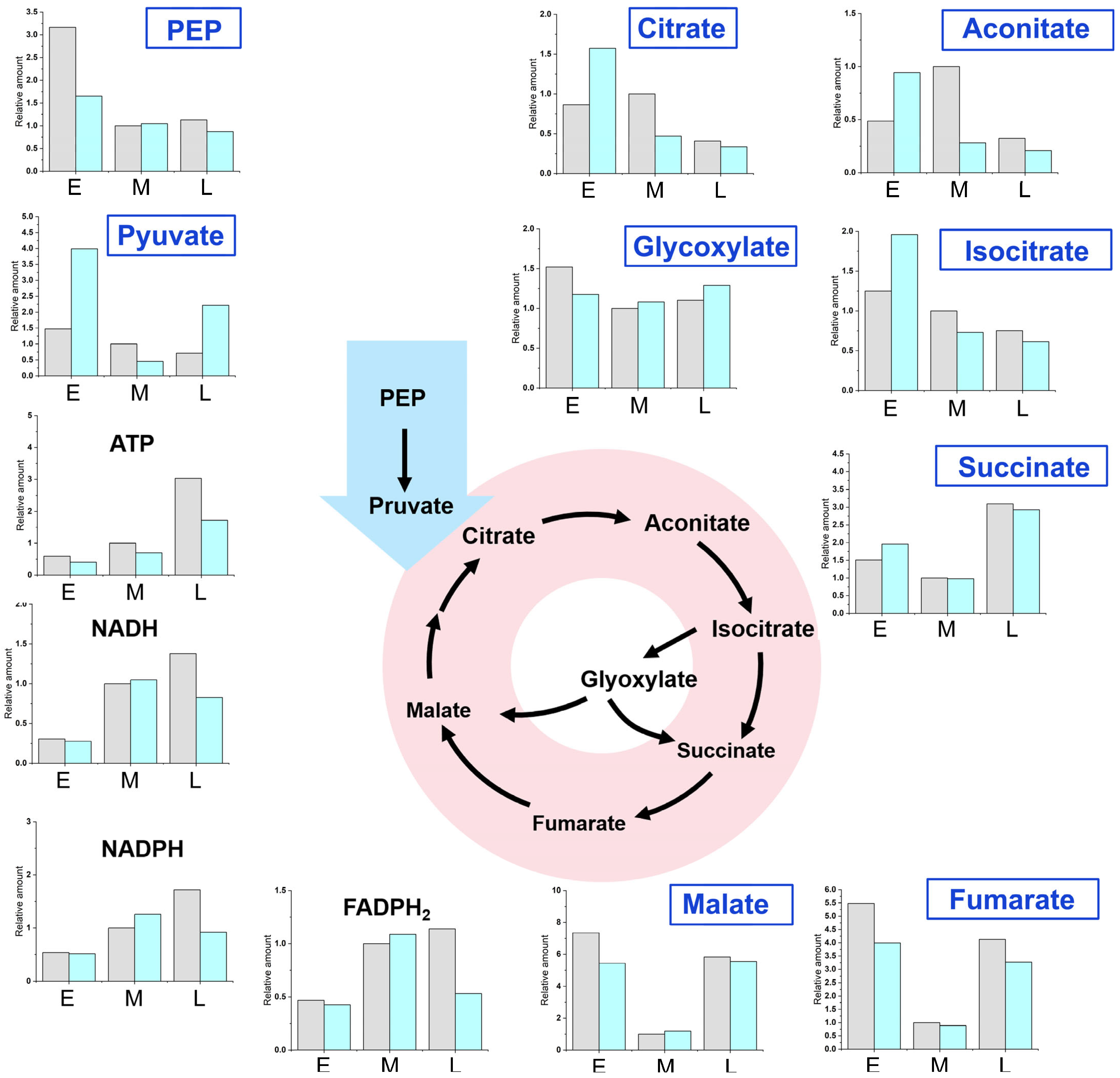
2.3. Metabolite Analysis at Different Fermentation Stages of WA01 and SA06
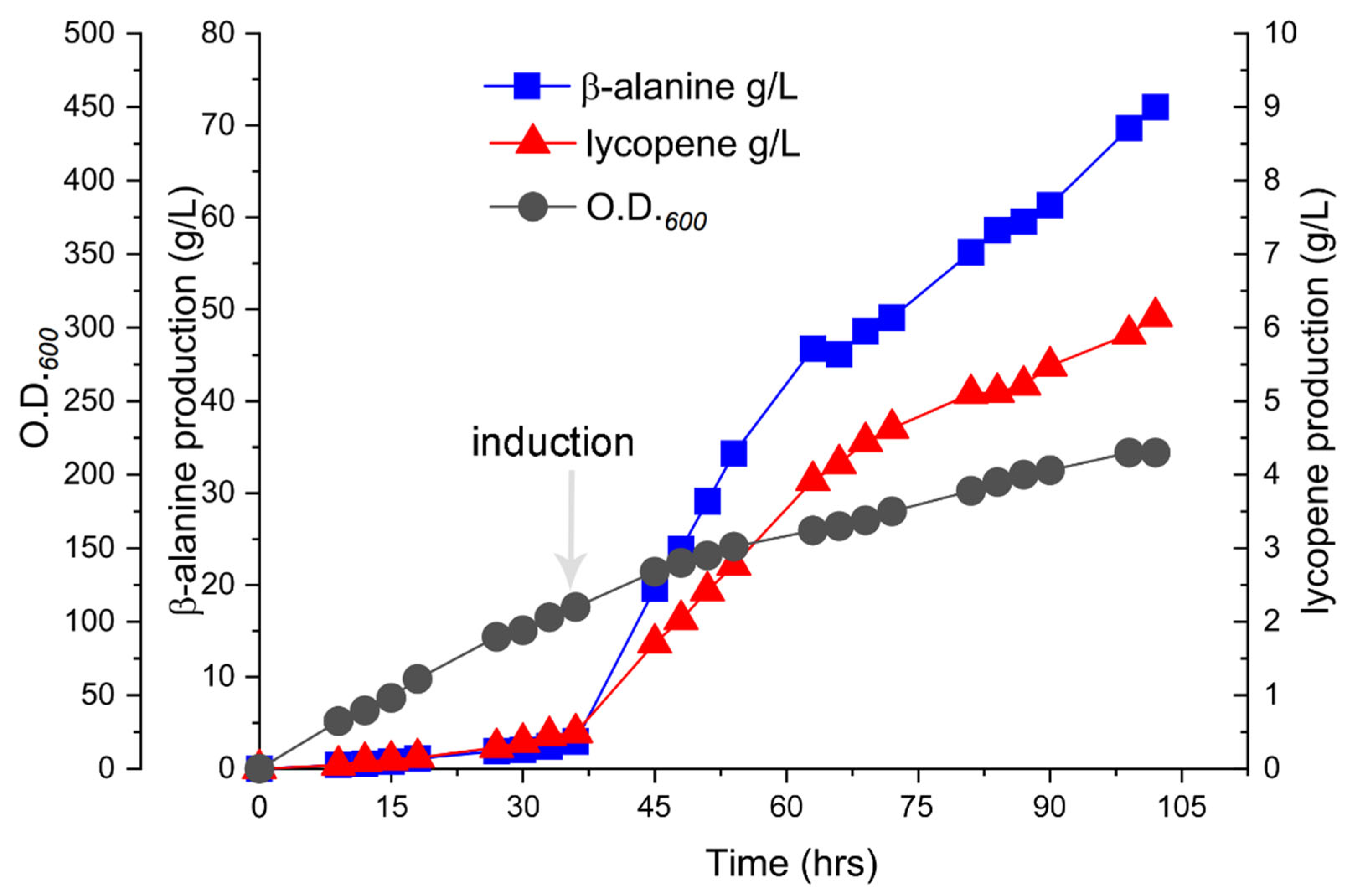
2.4. Metabolic Bottleneck and Process Optimization Strategy
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Bacterial Strains and Cultivation Conditions
4.3. Genetic Manipulations
4.4. Analytical Methods
4.5. Flask and Fed-Batch Bioconversion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cho, J.S.; Kim, G.B.; Eun, H.; Moon, C.W.; Lee, S.Y. Designing Microbial Cell Factories for the Production of Chemicals. JACS Au 2022, 2, 1781–1799. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, X.; Li, M.; Ma, M.; Zheng, Q.; Li, D.; An, T.; Wang, G. Advances in the optimization of central carbon metabolism in metabolic engineering. Microb. Cell Fact. 2023, 22, 76. [Google Scholar] [CrossRef]
- Zhang, C.; Ottenheim, C.; Weingarten, M.; Ji, L. Microbial Utilization of Next-Generation Feedstocks for the Biomanufacturing of Value-Added Chemicals and Food Ingredients. Front. Bioeng. Biotechnol. 2022, 10, 874612. [Google Scholar] [CrossRef] [PubMed]
- Orfali, D.M.; Meramo, S.; Sukumara, S. Ranking economic and environmental performance of feedstocks used in bio-based production systems. Curr. Res. Biotechnol. 2025, 9, 100275. [Google Scholar] [CrossRef]
- Li, S.; Ye, Z.; Moreb, E.A.; Hennigan, J.N.; Castellanos, D.B.; Yang, T.; Lynch, M.D. Dynamic control over feedback regulatory mechanisms improves NADPH flux and xylitol biosynthesis in engineered E. coli. Metab. Eng. 2021, 64, 26–40. [Google Scholar] [CrossRef]
- Liu, B.; Xiang, S.; Zhao, G.; Wang, B.; Ma, Y.; Liu, W.; Tao, Y. Efficient production of 3-hydroxypropionate from fatty acids feedstock in Escherichia coli. Metab. Eng. 2019, 51, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, R.; Vasmara, C.; Bertin, L.; Fiume, F. Conversion of waste cooking oil into biogas: Perspectives and limits. Appl. Microbiol. Biotechnol. 2020, 104, 2833–2856. [Google Scholar] [CrossRef] [PubMed]
- Nurfarahin, A.H.; Mohamed, M.S.; Phang, L.Y. Development of Palm Fatty Acid Distillate-Containing Medium for Biosurfactant Production by Pseudomonas sp. LM19. Molecules 2019, 24, 2613. [Google Scholar] [CrossRef]
- Diaz, L.; Borges, M.E. Low-quality vegetable oils as feedstock for biodiesel production using K-pumice as solid catalyst. Tolerance of water and free fatty acids contents. J. Agric. Food Chem. 2012, 60, 7928–7933. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, J.; Wang, X.; Liu, B.; Liu, W.; Tao, Y. Fatty acid feedstocks enable a highly efficient glyoxylate-TCA cycle for high-yield production of beta-alanine. mLife 2022, 1, 171–182. [Google Scholar] [CrossRef]
- Wang, L.; Mao, Y.; Wang, Z.; Ma, H.; Chen, T. Advances in biotechnological production of beta-alanine. World J. Microbiol. Biotechnol. 2021, 37, 79. [Google Scholar] [CrossRef] [PubMed]
- De Brandt, J.; Derave, W.; Vandenabeele, F.; Pomiès, P.; Blancquaert, L.; Keytsman, C.; Barusso-Grüninger, M.S.; de Lima, F.F.; Hayot, M.; Spruit, M.A.; et al. Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: A double-blind, randomized, placebo-controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Kopach, O.; Rusakov, D.A.; Sylantyev, S. Multi-target action of beta-alanine protects cerebellar tissue from ischemic damage. Cell Death Dis. 2022, 13, 747. [Google Scholar] [CrossRef]
- Zhang, Y.; Yun, J.; Zabed, H.M.; Dou, Y.; Zhang, G.; Zhao, M.; Taherzadeh, M.J.; Ragauskas, A.; Qi, X. High-level co-production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol: Metabolic engineering and process optimization. Bioresour. Technol. 2023, 369, 128438. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, S.; Qi, Q. Expression of active recombinant human tissue-type plasminogen activator by using in vivo polyhydroxybutyrate granule display. Appl. Environ. Microbiol. 2010, 76, 7226–7230. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnological production of lycopene by microorganisms. Appl. Microbiol. Biotechnol. 2020, 104, 10307–10324. [Google Scholar] [CrossRef]
- Liu, N.; Liu, B.; Wang, G.; Soong, Y.-H.V.; Tao, Y.; Liu, W.; Xie, D. Lycopene Production from Glucose, Fatty acid and Waste Cooking Oil by Metabolically Engineered Escherichia coli. Biochem. Eng. J. 2020, 155, 107488. [Google Scholar] [CrossRef]
- Kuzuyama, T.; Seto, H. Two distinct pathways for essential metabolic precursors for isoprenoid biosynthesis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 41–52. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, S.; Shao, X.; Park, J.-B.; Jeong, S.-H.; Park, H.-J.; Kwak, W.-J.; Wei, G.; Kim, S.-W. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb. Cell Fact. 2019, 18, 55. [Google Scholar] [CrossRef]
- Huang, G.; Li, J.; Lin, J.; Duan, C.; Yan, G. Multi-modular metabolic engineering and efflux engineering for enhanced lycopene production in recombinant Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae015. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.; Misawa, N.; Linden, H.; Yamano, S.; Kobayashi, K.; Sandmann, G. Expression in Escherichia coli, purification, and reactivation of the recombinant Erwinia uredovora phytoene desaturase. J. Biol. Chem. 1992, 267, 19891–19895. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, K.; Zheng, P.; Huo, X.; Liao, F.; Zhu, L.; Hu, M.; Tao, Y. Enhanced production of D-psicose from D-fructose by a redox-driven multi-enzyme cascade system. Enzyme Microb. Technol. 2023, 163, 110172. [Google Scholar] [CrossRef]
- Dong, X.; Liu, B.; Bao, Y.; Liu, W.; Tao, Y. Metabolic engineering of Escherichia coli for high-level production of violaxanthin. Microb. Cell Fact. 2023, 22, 115. [Google Scholar]
- Mu, Q.; Zhang, S.; Mao, X.; Tao, Y.; Yu, B. Highly efficient production of L-homoserine in Escherichia coli by engineering a redox balance route. Metab. Eng. 2021, 67, 321–329. [Google Scholar] [CrossRef]
- van Hoek, M.J.; Merks, R.M. Redox balance is key to explaining full vs. partial switching to low-yield metabolism. BMC Syst. Biol. 2012, 6, 22. [Google Scholar] [CrossRef]
- Liang, Q.; Qi, Q. From a co-production design to an integrated single-cell biorefinery. Biotechnol. Adv. 2014, 32, 1328–1335. [Google Scholar] [CrossRef]
- Yadav, B.; Talan, A.; Tyagi, R.; Drogui, P. Concomitant production of value-added products with polyhydroxyalkanoate (PHA) synthesis: A review. Bioresour. Technol. 2021, 337, 125419. [Google Scholar] [CrossRef]
- Piao, X.; Wang, L.; Lin, B.; Chen, H.; Liu, W.; Tao, Y. Metabolic engineering of Escherichia coli for production of L-aspartate and its derivative beta-alanine with high stoichiometric yield. Metab. Eng. 2019, 54, 244–254. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S.; Kelly, R.M. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef]

| E. coli Strains | Genotypes | Reference |
|---|---|---|
| BW25113 | F− DE(araD-araB)567 lacZ4787(del)::rrnB-3 LAM− rph-1 DE(rhaD-rhaB)568 hsdR514 | CGSC # |
| DH5α | F− φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK−, mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | CGSC # |
| WA01 | BW25113, ΔfadR, PCPA1-fadD, P119-fadL, ΔiclR::P119-BspanD, ΔaspC::P119-glcB-RBS-aceA, P119-btuE, P119-gor, PCPA1-aspA, pXB1k-TcpanD (p15A ori, KanR) * [10] | This study |
| WA0X | WA01, pSB1s-Bsnox (pSC101 ori, StrR) * [23] | This study |
| WA02 | WA01, GTG-icd | This study |
| WA03 | WA01, ATGAGG-icd | This study |
| WA04 | WA01, ATG(AGG)2-icd | This study |
| WA05 | WA01, ATG(AGG)3-icd | This study |
| WA06 | WA01, Δicd | This study |
| LA01 | BW25113, ΔlpxM::ParaBAD-mvaS-mvaE-mvk-ParaBAD-pmk-mvd-idi, pSB1s-crtEBI (pSC101 ori, StrR) * [18] | This study |
| SA01 | WA01, ΔlpxM::ParaBAD-mvaS-mvaE-mvk-ParaBAD-pmk-mvd-idi, pSB1s-crtEBI | This study |
| SA05 | WA05, ΔlpxM::ParaBAD-mvaS-mvaE-mvk-ParaBAD-pmk-mvd-idi, pSB1s-crtEBI | This study |
| SA06 | SA01, ΔsthA, P119-ptnAB | This study |
| SA07 | SA06, pSB1s-crtEBI > pMB1s-crtEBI (MBI ori, StrR) * [24] | This study |
| Strains | Lycopene Production (72 h) | β-Alanine Production | O.D.600 | ||
|---|---|---|---|---|---|
| mg/g DCW | g/L | 50–51 h | 70–72 h | ||
| LA10 | 56.4 | 1.76 | 0 | 0 | 97.5 |
| WA01 | 0 | 0 | 38.13 | 36.87 | 86.3 |
| WA0X | 0 | 0 | 16.76 | 19.43 | 68.0 |
| WA05 | 0 | 0 | 26.38 | 22.53 | 64.1 |
| WA06 | 0 | 0 | 11.22 | 7.49 | 46.2 |
| SA01 | 76.20 | 3.07 | 36.79 | 44.34 | 125.6 |
| SA05 | 64.70 | 2.17 | 33.83 | 39.06 | 104.5 |
| SA06 | 83.01 | 3.61 | 41.72 | 52.21 | 135.9 |
| SA07 | 69.08 | 2.92 | 43.41 | 48.05 | 131.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Miao, Y.; Liu, W.; Tao, Y. Synergistic Production of Lycopene and β-Alanine Through Engineered Redox Balancing in Escherichia coli. Int. J. Mol. Sci. 2025, 26, 6727. https://doi.org/10.3390/ijms26146727
Wang X, Miao Y, Liu W, Tao Y. Synergistic Production of Lycopene and β-Alanine Through Engineered Redox Balancing in Escherichia coli. International Journal of Molecular Sciences. 2025; 26(14):6727. https://doi.org/10.3390/ijms26146727
Chicago/Turabian StyleWang, Xuanlin, Yingchun Miao, Weifeng Liu, and Yong Tao. 2025. "Synergistic Production of Lycopene and β-Alanine Through Engineered Redox Balancing in Escherichia coli" International Journal of Molecular Sciences 26, no. 14: 6727. https://doi.org/10.3390/ijms26146727
APA StyleWang, X., Miao, Y., Liu, W., & Tao, Y. (2025). Synergistic Production of Lycopene and β-Alanine Through Engineered Redox Balancing in Escherichia coli. International Journal of Molecular Sciences, 26(14), 6727. https://doi.org/10.3390/ijms26146727






