Synthesis and Characterization of a Nanocomposite Based on Opuntia ficus indica for Efficient Removal of Methylene Blue Dye: Adsorption Kinetics and Optimization by Response Surface Methodology
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Cactus/Iron Oxide Adsorbent
2.2. Removal of Pollutant (MB) from Water
2.2.1. Effect of Contact Time
2.2.2. Effect of Initial Concentration of the Solution
2.2.3. Effect of Adsorbent Amount
2.2.4. Effect of Initial Solution pH
2.2.5. Effect of Temperature
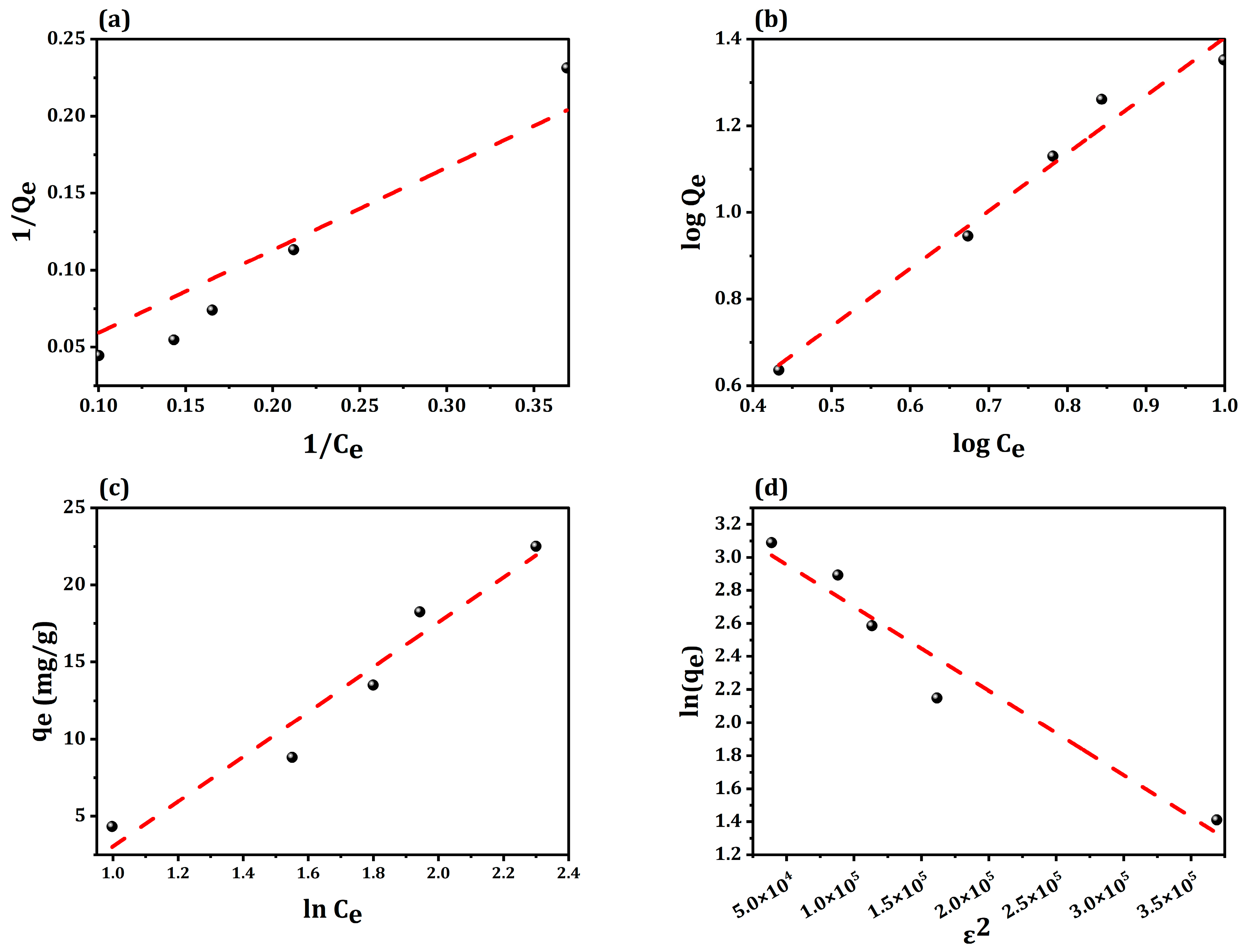
2.3. Adsorption Isotherms
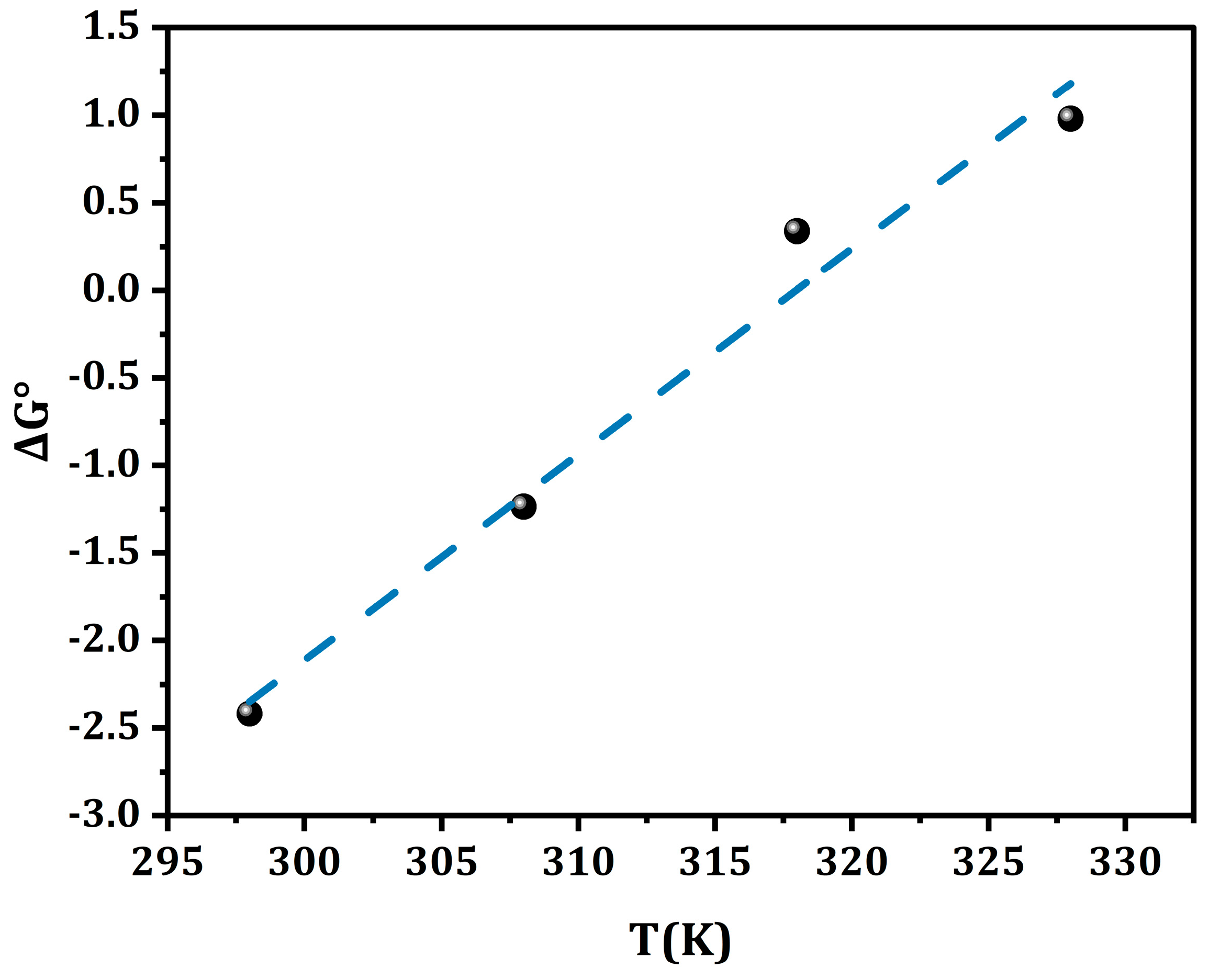
2.4. Analysis of Thermodynamic Parameters
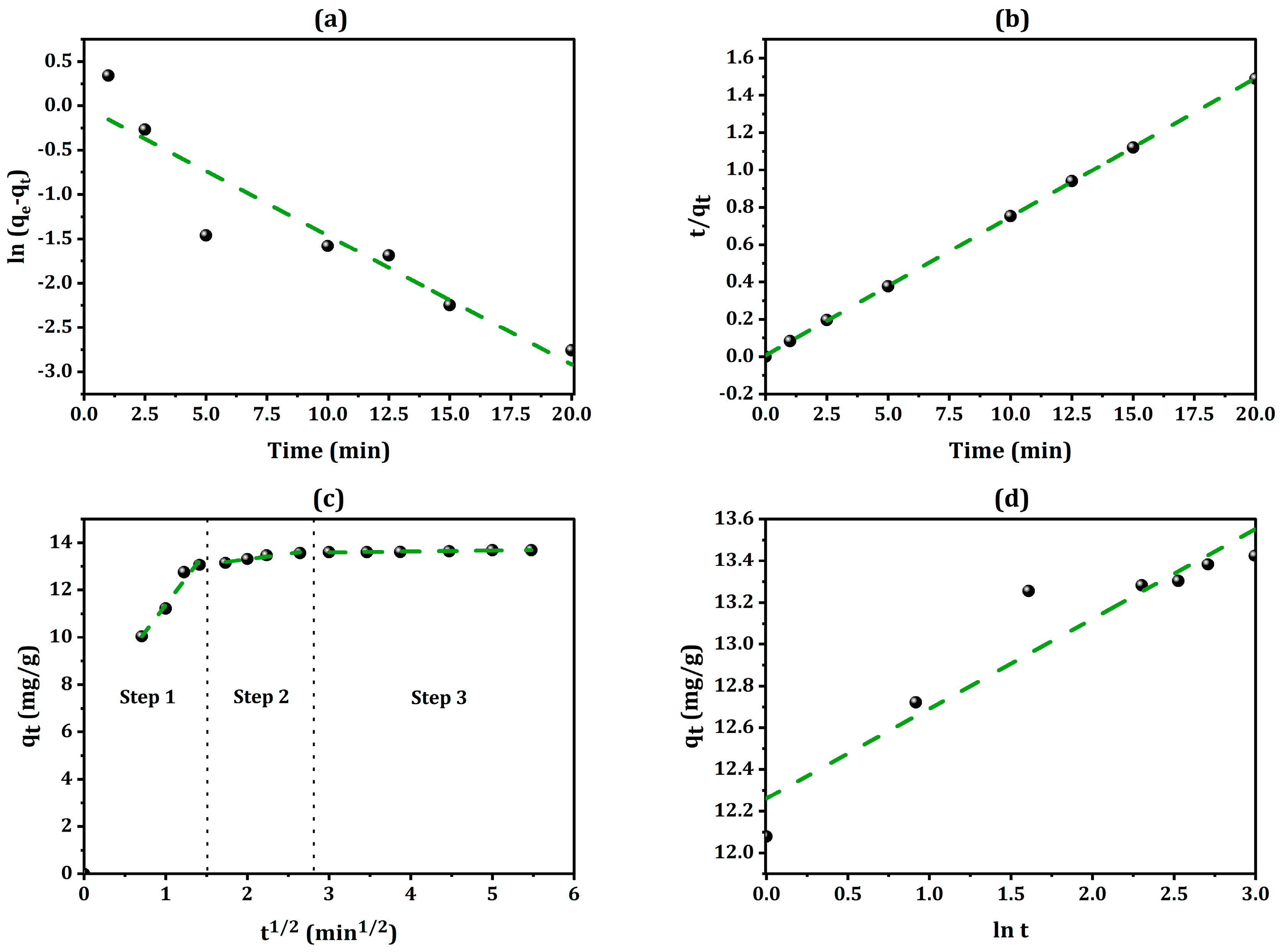
2.5. Analysis of Adsorption Kinetics
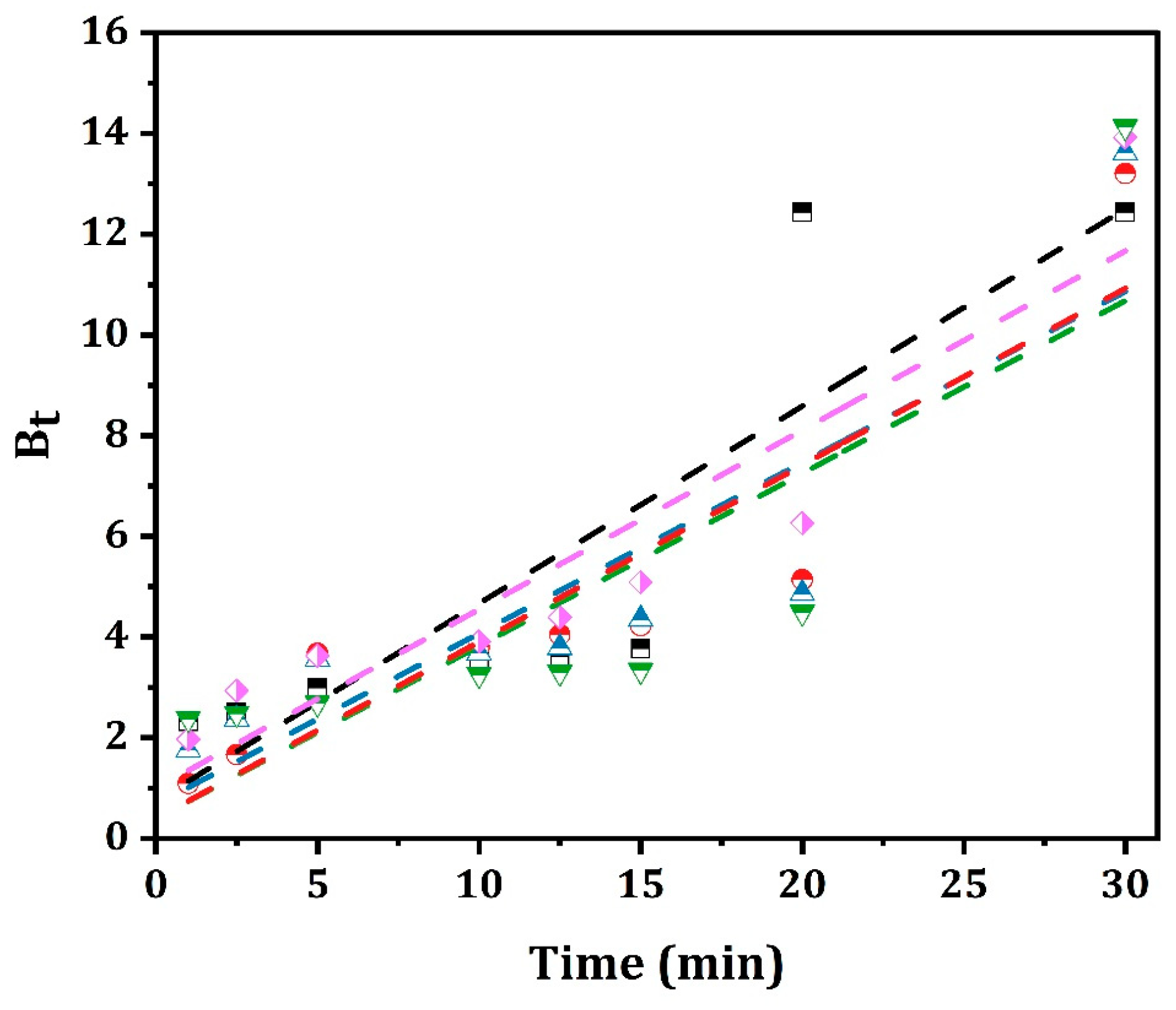
2.6. Boyd Model
2.7. Adsorption Mechanism
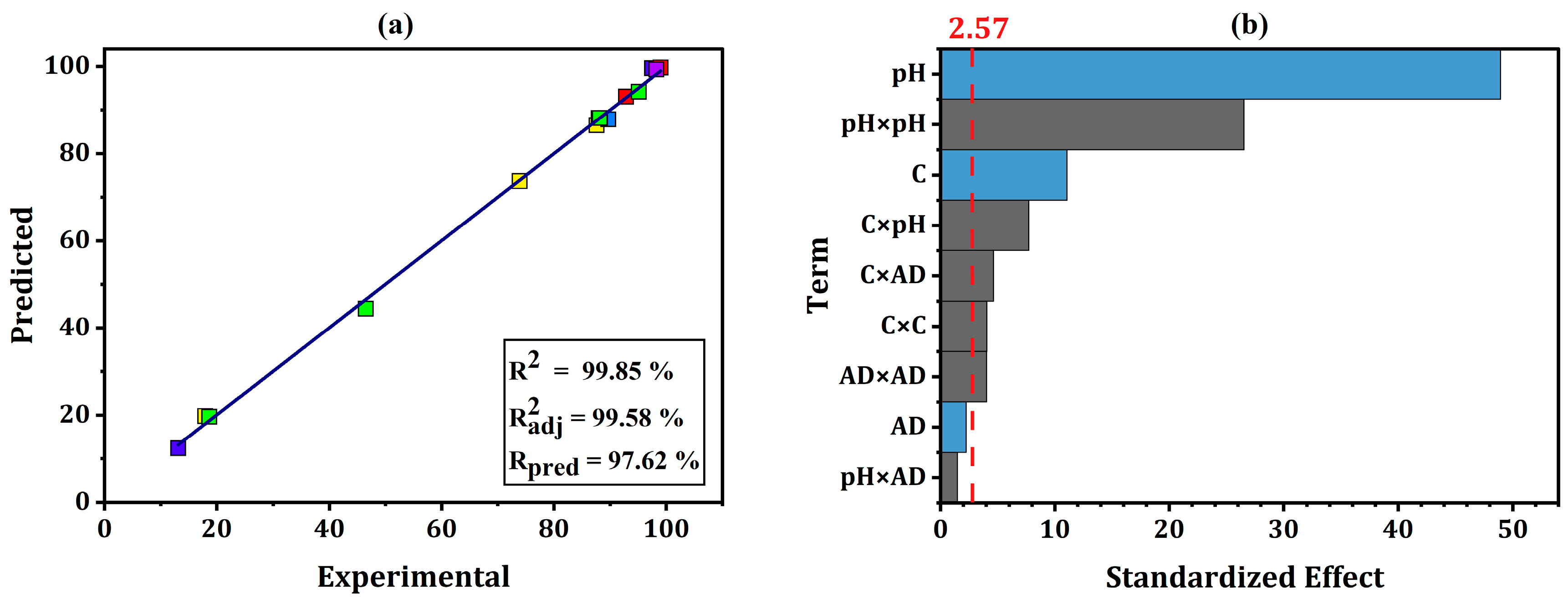
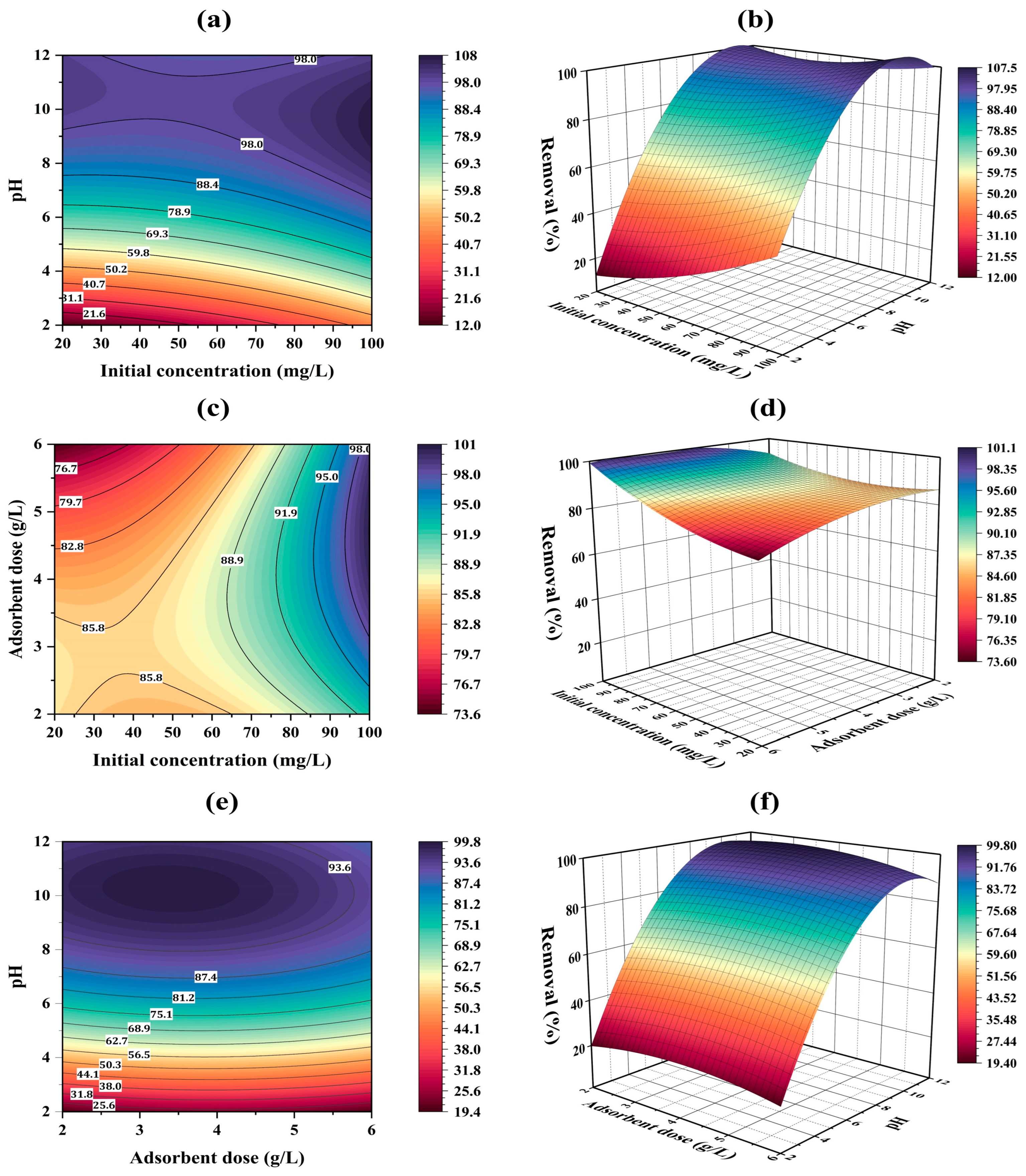
2.8. Optimization by Box Behnken Design
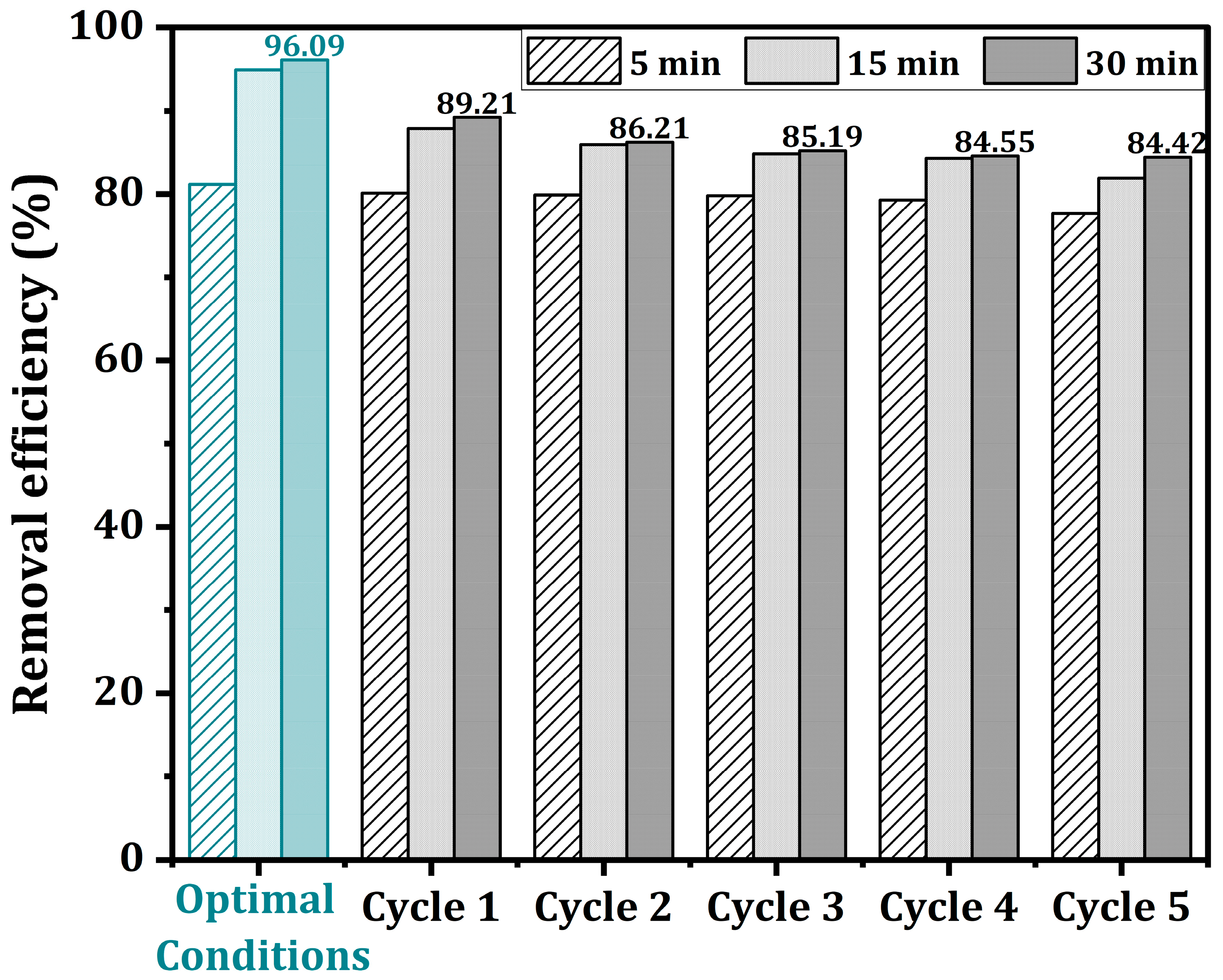
2.9. Regeneration and Reusability Potential
2.10. Comparison of the Current Material with Other Nanocomposite Materials for the Removal of Organic Pollutants
3. Materials and Methods
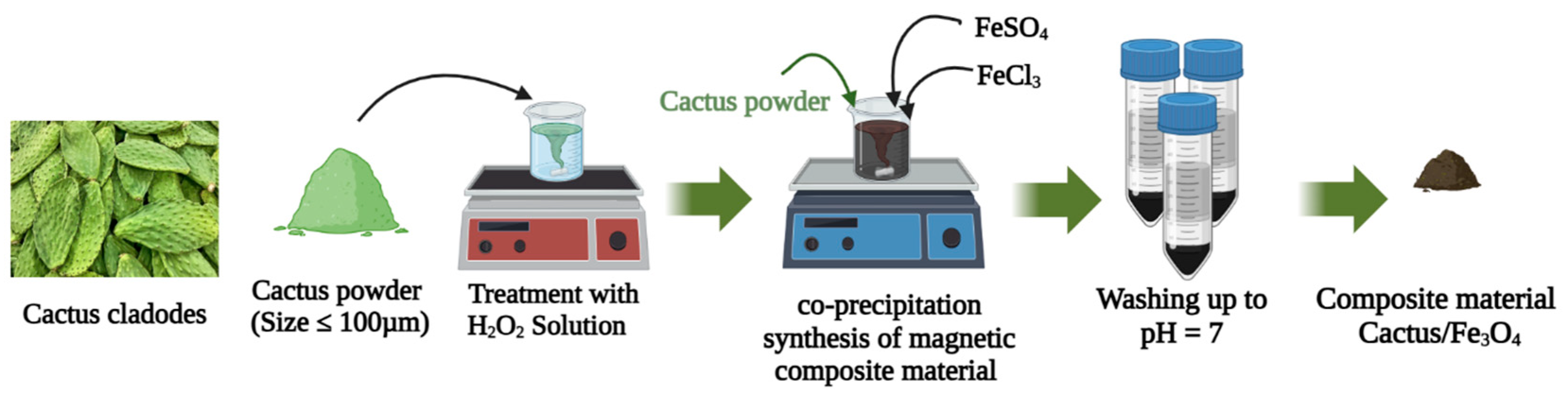
3.1. Materials
Preparation of the Magnetic Nanocomposite Material
3.2. Methods
3.2.1. Characterization
3.2.2. Batch Adsorption Experiments
3.2.3. Optimization by Response Surface Methodology
3.2.4. Regeneration Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira Fernandes, C.S.; Alves, F.; Loureiro, J. Sustainable Futures: From Causes of Environmental Degradation to Solutions. Discov. Sustain. 2024, 5, 63. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Salehi, G.; Rabiee, N. Rapid and Efficient Removal of Methylene Blue Dye from Aqueous Solutions Using Extract-Modified Zn–Al LDH. Chemosphere 2024, 350, 141011. [Google Scholar] [CrossRef]
- European Parliament. The Impact of Textile Production and Waste on the Environment (Infographics); European Environment Agency: Copenhagen, Denmark, 2022. [Google Scholar]
- Abbaz, A.; Arris, S.; Viscusi, G.; Ayat, A.; Aissaoui, H.; Boumezough, Y. Adsorption of Safranin O Dye by Alginate/Pomegranate Peels Beads: Kinetic, Isotherm and Thermodynamic Studies. Gels 2023, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Feng, L.; Han, Y.; Xu, Z.; Xu, L.; An, X.; Zhang, Q. Revealing the Degrading-Possibility of Methyl Red by Two Azoreductases of Anoxybacillus Sp. PDR2 Based on Molecular Docking. Chemosphere 2024, 351, 141173. [Google Scholar] [CrossRef]
- Ma, L.; Liu, W.; Liu, B.; Tang, Y. Removal of Methylene Blue by Acrylic Polymer Adsorbents Loaded with Magnetic Iron Manganese Oxides: Synthesis, Characterization, and Adsorption Mechanisms. Chemosphere 2024, 346, 140588. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, X.; Hou, J.; Li, Z. Adsorption Performance of Mineral-Carbon Adsorbents Derived from Coal Gasification Fine Ash: Prepared via Low-Temperature Alkali Fusion Method. Environ. Res. 2024, 248, 118311. [Google Scholar] [CrossRef]
- Wang, H.; Yi, L.; Huang, F.; Huang, Q.; Zhou, T. Facile Synthesis of Graphene Nanosheets on Wastewater Sediments for High Efficient Adsorption of Methylene Blue. Sep. Purif. Technol. 2024, 337, 126366. [Google Scholar] [CrossRef]
- Kurniawan, T.; Saepurahman; Azis, M.A.; Jayanudin. Simultaneous Impregnation-Dealumination to Produce SnO2-Hierarchical Zeolite for Methylene Blue Elimination via Adsorption-Photodegradation. Case Stud. Chem. Environ. Eng. 2024, 9, 100613. [Google Scholar] [CrossRef]
- Kaewtrakulchai, N.; Chanpee, S.; Pasee, W.; Putta, A.; Chutipaijit, S.; Kaewpanha, M.; Suriwong, T.; Puengjinda, P.; Panomsuwan, G.; Fuji, M.; et al. Valorization of Horse Manure Conversion to Magnetic Carbon Nanofiber for Dye Adsorption by Hydrothermal Treatment Coupled with Carbonization. Case Stud. Chem. Environ. Eng. 2024, 9, 100563. [Google Scholar] [CrossRef]
- Mohamed, S.M.I.; Yılmaz, M.; Güner, E.K.; El Nemr, A. Synthesis and Characterization of Iron Oxide-Commercial Activated Carbon Nanocomposite for Removal of Hexavalent Chromium (Cr6+) Ions and Mordant Violet 40 (MV40) Dye. Sci. Rep. 2024, 14, 1241. [Google Scholar] [CrossRef]
- Khan, Z.A.; Elwakeel, K.Z.; Mashabi, R.A.; Elgarahy, A.M. Adsorption of Anionic Dyes onto 1,5-Diphenylcarbazide Functionalized Magnetic Hybrid Polymer: Impact of Water Salinity and Surfactants on Adsorption Isotherms. J. Ind. Eng. Chem. 2024, 131, 569–584. [Google Scholar] [CrossRef]
- Fei, J.; Li, J. Metal Oxide Nanomaterials for Water Treatment. In Nanotechnologies for the Life Sciences; Kumar, C.S.S.R., Ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-3-527-31301-3. [Google Scholar]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent Advances on Sustainable Adsorbents for the Remediation of Noxious Pollutants from Water and Wastewater: A Critical Review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Dwivedi, S.; Dey, S. Review on Biochar as an Adsorbent Material for Removal of Dyes from Waterbodies. Int. J. Environ. Sci. Technol. 2023, 20, 9335–9350. [Google Scholar] [CrossRef]
- Makhado, E.; Motshabi, B.R.; Allouss, D.; Ramohlola, K.E.; Modibane, K.D.; Hato, M.J.; Jugade, R.M.; Shaik, F.; Pandey, S. Development of a Ghatti Gum/Poly (Acrylic Acid)/TiO2 Hydrogel Nanocomposite for Malachite Green Adsorption from Aqueous Media: Statistical Optimization Using Response Surface Methodology. Chemosphere 2022, 306, 135524. [Google Scholar] [CrossRef]
- Deb, A.; Debnath, A.; Bhowmik, K.; Rudra Paul, S.; Saha, B. Application of Polyaniline Impregnated Mixed Phase Fe2O3, MnFe2 O4 and ZrO2 Nanocomposite for Rapid Abatement of Binary Dyes from Aqua Matrix: Response Surface Optimisation. Int. J. Environ. Anal. Chem. 2023, 103, 5938–5956. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Alijani, N.; Alizadeh, A.H. Preparation of Chitosan-Based Ternary Nanocomposite Hydrogel Film by Loading Graphene Oxide Nanosheets as Adsorbent for Enhanced Methylene Blue Dye Removal. Int. J. Biol. Macromol. 2023, 253, 126585. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Aamer, N.; Abdallah, H.M.; López-Maldonado, E.A.; Mohamed, Y.M.A.; El Nazer, H.A.; Mohamed, R.R. Novel High-Efficient Adsorbent Based on Modified Gelatin/Montmorillonite Nanocomposite for Removal of Malachite Green Dye. Sci. Rep. 2024, 14, 1228. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guivar, J.A.; Flores-Cano, D.A.; Caetano Passamani, E. Differentiating Nanomaghemite and Nanomagnetite and Discussing Their Importance in Arsenic and Lead Removal from Contaminated Effluents: A Critical Review. Nanomaterials 2021, 11, 2310. [Google Scholar] [CrossRef]
- Qasim, H.M.; Abudi, Z.N.; Alzubaidi, L.A. Cobalt Ion Removal Using Magnetic Biochar Obtained from Conocarpus Erectus Leaves. Biomass Conv. Bioref. 2023, 13, 16865–16875. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, R.; Bao, B.; Liu, S.; Hu, C.; Ding, W.; Zheng, H. Selective Removal of Phosphate by Magnetic NaCe(CO3)2/Fe3O4 Nanocomposites: Performance and Mechanism. Sep. Purif. Technol. 2023, 325, 124741. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, Q.; Jin, T.; Ren, G.; Liu, Z.; Qian, Y. Effective Adsorption of Thorium Ion by Novel Self-Crosslinking Polyamide Acid-Grafted Magnetic Nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132279. [Google Scholar] [CrossRef]
- Kassim, M.A.B.M.; Kaus, N.H.M.; Imam, S.S.; Sagadevan, S.; Salaeh, S. Rapid and Facile Chemical Synthesis of Fe3O4/Biochar Nanocomposite for the Adsorptive Removal of Fluoroquinolones from Aqueous Solution. Inorg. Chem. Commun. 2023, 156, 111156. [Google Scholar] [CrossRef]
- Dkhissi, O.; El Hakmaoui, A.; Chatoui, M.; Bouyakhsass, R.; Bakraouy, H.; Kurniawan, T.A.; Anouzla, A.; Jada, A.; Souabi, S. Vegetable Oil Refinery Wastewater Treatment by Using the Cactus as a Bio-Flocculant in the Coagulation-Flocculation Process. Water Air Soil Pollut 2023, 234, 322. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Cactus Stems (Opuntia spp.): A Review on Their Chemistry, Technology, and Uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Ayat, A.; Arris, S.; Abbaz, A.; Bencheikh-Lehocine, M.; Meniai, A.H. Application of Response Surface Methodology For Modeling and Optimization of A Bio Coagulation Process (Sewage Wastewater Treatment Plant). Environ. Manag. 2021, 67, 489–497. [Google Scholar] [CrossRef]
- Barbera, M.; Indelicato, S.; Bongiorno, D.; Censi, V.; Saiano, F.; Piazzese, D. Untreated Opuntia ficus indica for the Efficient Adsorption of Ni(II), Pb(II), Cu(II) and Cd(II) Ions from Water. Molecules 2023, 28, 3953. [Google Scholar] [CrossRef] [PubMed]
- Buhani; Dewi, J.S.; Fajriyah, N.S.; Rilyanti, M.; Suharso; Sumadi; Elwakeel, K.Z. Modification of Non-Activated Carbon from Rubber Fruit Shells with 3-(Aminopropyl)-Triethoxysilane and Its Adsorption Study on Coomassie Brilliant Blue and Methylene Blue in Solution. Water Air Soil Pollut 2023, 234, 578. [Google Scholar] [CrossRef]
- Essekri, A.; Laabd, M.; Albourine, A. Efficient Adsorption of Crystal Violet Dye Using Functionalized Argan Shell: Experiments and Statistical Optimization Modeling. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133401. [Google Scholar] [CrossRef]
- Akkari, I.; Graba, Z.; Bezzi, N.; Merzeg, F.A.; Bait, N.; Ferhati, A.; Kaci, M.M. Biosorption of Basic Red 46 Using Raw Cactus Fruit Peels: Equilibrium, Kinetic and Thermodynamic Studies. Biomass Conv. Bioref. 2024, 14, 1825–1836. [Google Scholar] [CrossRef]
- Dogari, H.; Salehi, M.M.; Hassanzadeh-Afruzi, F.; Saeidirad, M.; Maleki, A. Magnetic Polyacrylonitrile-Melamine Nanoadsorbent (PAN-Mel@Fe3O4) for Effective Adsorption of Cd (II) and Pb (II) from Aquatic Area. Mater. Sci. Eng. B 2023, 298, 116871. [Google Scholar] [CrossRef]
- Benhamou, A.; Boussetta, A.; Grimi, N.; Idrissi, M.E.; Nadifiyine, M.; Barba, F.J.; Moubarik, A. Characteristics of Cellulose Fibers from Opuntia ficus indica Cladodes and Its Use as Reinforcement for PET Based Composites. J. Nat. Fibers 2022, 19, 6148–6164. [Google Scholar] [CrossRef]
- Reddy, S.S.; Bhaduri, S.K.; Sen, S.K. Infrared Spectra of Alkali Treated Jute Stick. J Appl. Polym. Sci 1990, 41, 329–336. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Galluzzi, A.; Polichetti, M.; Gorrasi, G. Fabrication of Novel Hybrid Materials Based on Iron-Aluminum Modified Hemp Fibers: Comparison between Two Proposed Methodologies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128683. [Google Scholar] [CrossRef]
- Rawat, S.; Ahammed, M.M. Clay-Moringa Seedcake Composite for Removal of Cationic and Anionic Dyes. Chemosphere 2024, 350, 141083. [Google Scholar] [CrossRef]
- Hegde, V.; Uthappa, U.T.; Mane, P.V.; Ji, S.M.; Suneetha, M.; Wang, B.; Altalhi, T.; Subrahmanya, T.M.; Kurkuri, M.D. Design of Low-Cost Natural Casein Biopolymer Based Adsorbent for Efficient Adsorption of Multiple Anionic Dyes and Diclofenac Sodium from Aqueous Solutions. Chemosphere 2024, 353, 141571. [Google Scholar] [CrossRef]
- Farch, S.; Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Kebir, M.; Zamouche, M.; Amrane, A.; Zhang, J.; Hadadi, A.; et al. Application of Walnut Shell Biowaste as an Inexpensive Adsorbent for Methylene Blue Dye: Isotherms, Kinetics, Thermodynamics, and Modeling. Separations 2023, 10, 60. [Google Scholar] [CrossRef]
- Khan, S.A.; Riaz-ur-Rehman; Khan, M.A. Adsorption of Chromium (III), Chromium (VI) and Silver (I) on Bentonite. Waste Manag. 1995, 15, 271–282. [Google Scholar] [CrossRef]
- Fatima, B.; Siddiqui, S.I.; Nirala, R.K.; Vikrant, K.; Kim, K.-H.; Ahmad, R.; Chaudhry, S.A. Facile Green Synthesis of ZnO–CdWO4 Nanoparticles and Their Potential as Adsorbents to Remove Organic Dye. Environ. Pollut. 2021, 271, 116401. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. ISBN 978-0-12-818489-9. [Google Scholar]
- Hameed, B.H.; El-Khaiary, M.I. Equilibrium, Kinetics and Mechanism of Malachite Green Adsorption on Activated Carbon Prepared from Bamboo by K2CO3 Activation and Subsequent Gasification with CO2. J. Hazard. Mater. 2008, 157, 344–351. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Design of Sodium Alginate/Soybean Extract Beads Loaded with Hemp Hurd and Halloysite as Novel and Sustainable Systems for Methylene Blue Adsorption. Polym. Eng. Sci 2022, 62, 129–144. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Jawad, A.H.; Kashi, E.; Radzun, K.A.; ALOthman, Z.A.; Wilson, L.D. Insight into Adsorption Mechanism, Modeling, and Desirability Function of Crystal Violet and Methylene Blue Dyes by Microalgae: Box-Behnken Design Application. Algal Res. 2022, 67, 102864. [Google Scholar] [CrossRef]
- Joshi, S.; Garg, V.K.; Kataria, N.; Kadirvelu, K. Applications of Fe3O4@AC Nanoparticles for Dye Removal from Simulated Wastewater. Chemosphere 2019, 236, 124280. [Google Scholar] [CrossRef]
- Sharma, A.; Mangla, D.; Shehnaz; Chaudhry, S.A. Recent Advances in Magnetic Composites as Adsorbents for Wastewater Remediation. J. Environ. Manag. 2022, 306, 114483. [Google Scholar] [CrossRef] [PubMed]
- Rossatto, D.L.; Netto, M.S.; Jahn, S.L.; Mallmann, E.S.; Dotto, G.L.; Foletto, E.L. Highly Efficient Adsorption Performance of a Novel Magnetic Geopolymer/Fe3O4 Composite towards Removal of Aqueous Acid Green 16 Dye. J. Environ. Chem. Eng. 2020, 8, 103804. [Google Scholar] [CrossRef]
- Ayad, M.; Salahuddin, N.; Fayed, A.; Bastakoti, B.P.; Suzuki, N.; Yamauchi, Y. Chemical Design of a Smart Chitosan–Polypyrrole–Magnetite Nanocomposite toward Efficient Water Treatment. Phys. Chem. Chem. Phys. 2014, 16, 21812–21819. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Magnetic Hydrochar Grafted-Chitosan for Enhanced Efficient Adsorption of Malachite Green Dye from Aqueous Solutions: Modeling, Adsorption Behavior, and Mechanism Analysis. Int. J. Biol. Macromol. 2024, 254, 127767. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Jiang, R.; Xiao, L.; Li, W. A Novel Magnetically Separable γ-Fe2O3/Crosslinked Chitosan Adsorbent: Preparation, Characterization and Adsorption Application for Removal of Hazardous Azo Dye. J. Hazard. Mater. 2010, 179, 251–257. [Google Scholar] [CrossRef]
- Sun, P.; Hui, C.; Azim Khan, R.; Du, J.; Zhang, Q.; Zhao, Y.-H. Efficient Removal of Crystal Violet Using Fe3O4-Coated Biochar: The Role of the Fe3O4 Nanoparticles and Modeling Study Their Adsorption Behavior. Sci. Rep. 2015, 5, 12638. [Google Scholar] [CrossRef]
- Qiao, J.; Gao, S.; Yao, J.; Zhang, L.; Li, N. A Rapid and Green Method for the Removal of Anionic Dyes from Aqueous Solution Using Sulfobetaine-Modified Magnetic Nanoparticles. AIP Adv. 2019, 9, 065308. [Google Scholar] [CrossRef]
- Cai, N.; Larese-Casanova, P. Facile Synthesis and Reuse of Magnetic Black Carbon Magnetite (BC-Mag) for Fast Carbamazepine Removal from Water. Nanomaterials 2020, 10, 213. [Google Scholar] [CrossRef]
- Agilandeswari, P.; Venkateshbabu, S.; Sarojini, G.; Rajasimman, M. Sustainable Development and Analysis of a Novel Bio-Derived (Biochar) Nanocomposite for the Remediation of Carbamazepine from Aqueous Solution. Chemosphere 2024, 347, 140696. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.M.; Elgarahy, A.M.; Zoromba, M.S.; Elwakeel, K.Z. A Microwave-Regenerable Multi-Walled Carbon Nanotube/Polyaniline/Fe3O4 Ternary Nanocomposite for Quantifiable Sorption of Cationic and Anionic Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134438. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Lebepe, T.C.; Oluwafemi, O.S.; Osibote, O.A. Prediction and Optimizing of Methylene Blue Sequestration to Activated Charcoal/Magnetic Nanocomposites Using Artificial Neutral Network and Response Surface Methodology. Chemosphere 2024, 355, 141751. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, R.; Huang, Z.; Liu, X.; Wang, T.; Chen, K. Preparation and Characterization of Carboxymethylcellulose Based Citric Acid Cross-Linked Magnetic Aerogel as an Efficient Dye Adsorbent. Int. J. Biol. Macromol. 2021, 181, 1030–1038. [Google Scholar] [CrossRef]

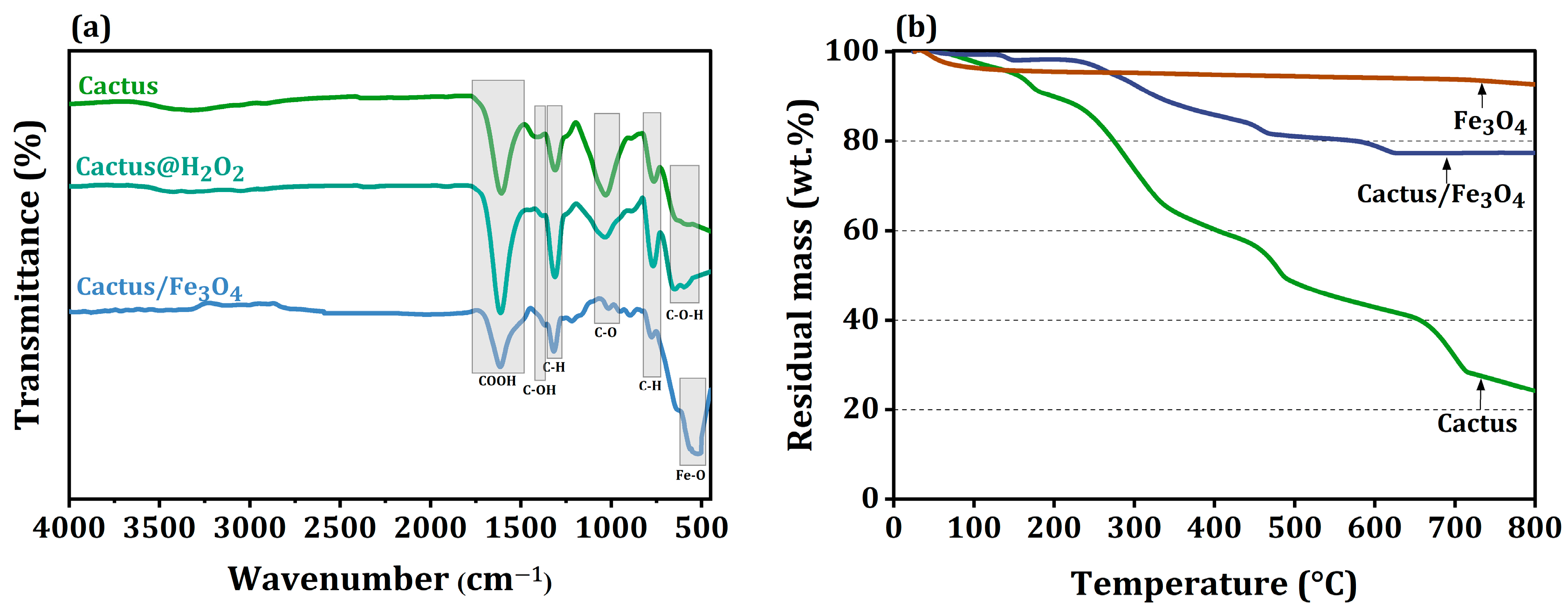


| Sample | Specific Surface Area (m2/g) | Mean Dimension (nm) | Zeta Potential (mV) |
|---|---|---|---|
| Cactus | 3.3 | 11,000 | −21 ± 0.21 |
| Cactus/iron oxide | 39.3 | 57.7 | −27 ± 0.33 |
| Isotherm Model | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg/g) | 174.82 |
| KL (L/mg) | 0.011 | |
| RL | 1 | |
| R2 | 0.971 | |
| Freundlich | Kf (L/mg) | 1.18 |
| 1/n | 1.33 | |
| R2 | 0.975 | |
| Temkin | Bj | 14.53 |
| KT (L/mg) | 0.45 | |
| R2 | 0.948 | |
| Dubinin–Radushkevich | qm (mg/g) | 24.8098 |
| ß (mol2/kJ2) | 5.09 × 10−6 | |
| E (kJ/mol) | 3,133,035 | |
| R2 | 0.9505 |
| Temperature (K) | ΔG° (kJ/mol) | ΔS° (kJ/mol K) | ΔH° (kJ/mol) |
|---|---|---|---|
| 298 | −2.42 | −0.118 | −37.61 |
| 308 | −1.24 | ||
| 318 | 0.34 | ||
| 328 | 0.97 |
| Kinetic Model | Parameters | Values |
|---|---|---|
| Pseudo-first-order | K1 (min−1) | −0.007 |
| qe (mg·g−1) | 0.991 | |
| R2 | 0.8796 | |
| Pseudo-second-order | K2 ((g/mg)min) | 0.815 |
| qe, cal(mg/g−1) | 13.454 | |
| R2 | 0.9999 | |
| Intraparticle diffusion Step 1 | Kid ((mg/g)min−0.5) | 4.539 |
| C (mg·g−1) | 6.836 | |
| R2 | 0.9683 | |
| Intraparticle diffusion Step 2 | Kid ((mg/g)min−0.5) | 0.465 |
| C (mg·g−1) | 12.375 | |
| R2 | 0.9478 | |
| Intraparticle diffusion Step 3 | Kid ((mg/g)min−0.5) | 0.040 |
| C (mg·g−1) | 13.473 | |
| R2 | 0.9448 | |
| Elovich | α | 2.322 |
| β | 99.17 × 1010 | |
| R2 | 0.898 |
| Adsorbent | Pollutant | Conditions | Qmax (mg·g−1) | References |
|---|---|---|---|---|
| Geopolymer/Fe3O4 | Acid green 16 | Equilibrium time 30 min pH 2.3 Adsorbent dosage 0.75 g·L−1 | 400 | [47] |
| Chitosan-polypyrrole-Fe3O4 | Acid green | Equilibrium time 60 min pH 5.4 Adsorbent dosage 1 g·L−1 | 26.2 | [48] |
| Magnetic hydrochar/chitosan | Malachite green | Equilibrium time 420 min pH 7.5 Adsorbent dosage 0.4 g·L−1 | 420.02 | [49] |
| Mag-γ-Fe2O3-cross linked-chitosan composite | Methyl orange | Equilibrium time 100–270 min pH 4 Adsorbent dosage 1 g·L−1 | 29.50 | [50] |
| Fe3O4/corn stalks biochar | Crystal violet | Equilibrium time 5 min pH 6 Adsorbent dosage 0.25 g·L−1 | 349.4 | [51] |
| Sulfobetaine-modified magnetic nanoparticles | Methyl blue | Equilibrium time 15 min pH 2–2.5 Adsorbent dosage 10 mg | 127.06 | [52] |
| Magnetic black carbon | Carbamazepine | Equilibrium time 3 min pH 2–10 Adsorbent dosage 0.5 g·L−1 | 31.8 | [53] |
| Polypyrrole/GO/biochar | Carbamazepine | Equilibrium time 90 min pH 7.8 Adsorbent dosage 1.4 g·L−1 | 45.04 | [54] |
| Carbon nanotube/polyaniline/magnetite | Methylene blue | Equilibrium time 180 min pH 2.94–10.03 Adsorbent dosage 1.5 g·L−1 | 166.66 | [55] |
| Magnetic activated charcoal | Methylene blue | Equilibrium time 60 min pH 9.6 Adsorbent dosage 1.46 g·L−1 | 455 | [56] |
| Magnetic aerogel | Methylene blue | Equilibrium time 6 h Natural pH | 83.6 | [57] |
| Cactus/iron oxide nanocomposite | Methylene blue | Equilibrium time 10 min pH 10.98 Adsorbent dosage 2.24 g·L−1 | 174.82 | This work |
| Factors | Symbol | Coded and Real Values | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Initial concentration (mg/L) | X1 | 20 | 60 | 100 |
| pH | X2 | 2 | 7 | 12 |
| Adsorbent dose (g/L) | X3 | 2 | 4 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boumezough, Y.; Viscusi, G.; Arris, S.; Gorrasi, G.; Carabineiro, S.A.C. Synthesis and Characterization of a Nanocomposite Based on Opuntia ficus indica for Efficient Removal of Methylene Blue Dye: Adsorption Kinetics and Optimization by Response Surface Methodology. Int. J. Mol. Sci. 2025, 26, 6717. https://doi.org/10.3390/ijms26146717
Boumezough Y, Viscusi G, Arris S, Gorrasi G, Carabineiro SAC. Synthesis and Characterization of a Nanocomposite Based on Opuntia ficus indica for Efficient Removal of Methylene Blue Dye: Adsorption Kinetics and Optimization by Response Surface Methodology. International Journal of Molecular Sciences. 2025; 26(14):6717. https://doi.org/10.3390/ijms26146717
Chicago/Turabian StyleBoumezough, Yasser, Gianluca Viscusi, Sihem Arris, Giuliana Gorrasi, and Sónia A. C. Carabineiro. 2025. "Synthesis and Characterization of a Nanocomposite Based on Opuntia ficus indica for Efficient Removal of Methylene Blue Dye: Adsorption Kinetics and Optimization by Response Surface Methodology" International Journal of Molecular Sciences 26, no. 14: 6717. https://doi.org/10.3390/ijms26146717
APA StyleBoumezough, Y., Viscusi, G., Arris, S., Gorrasi, G., & Carabineiro, S. A. C. (2025). Synthesis and Characterization of a Nanocomposite Based on Opuntia ficus indica for Efficient Removal of Methylene Blue Dye: Adsorption Kinetics and Optimization by Response Surface Methodology. International Journal of Molecular Sciences, 26(14), 6717. https://doi.org/10.3390/ijms26146717









