Network Pharmacology as a Tool to Investigate the Antioxidant and Anti-Inflammatory Potential of Plant Secondary Metabolites—A Review and Perspectives
Abstract
1. Introduction
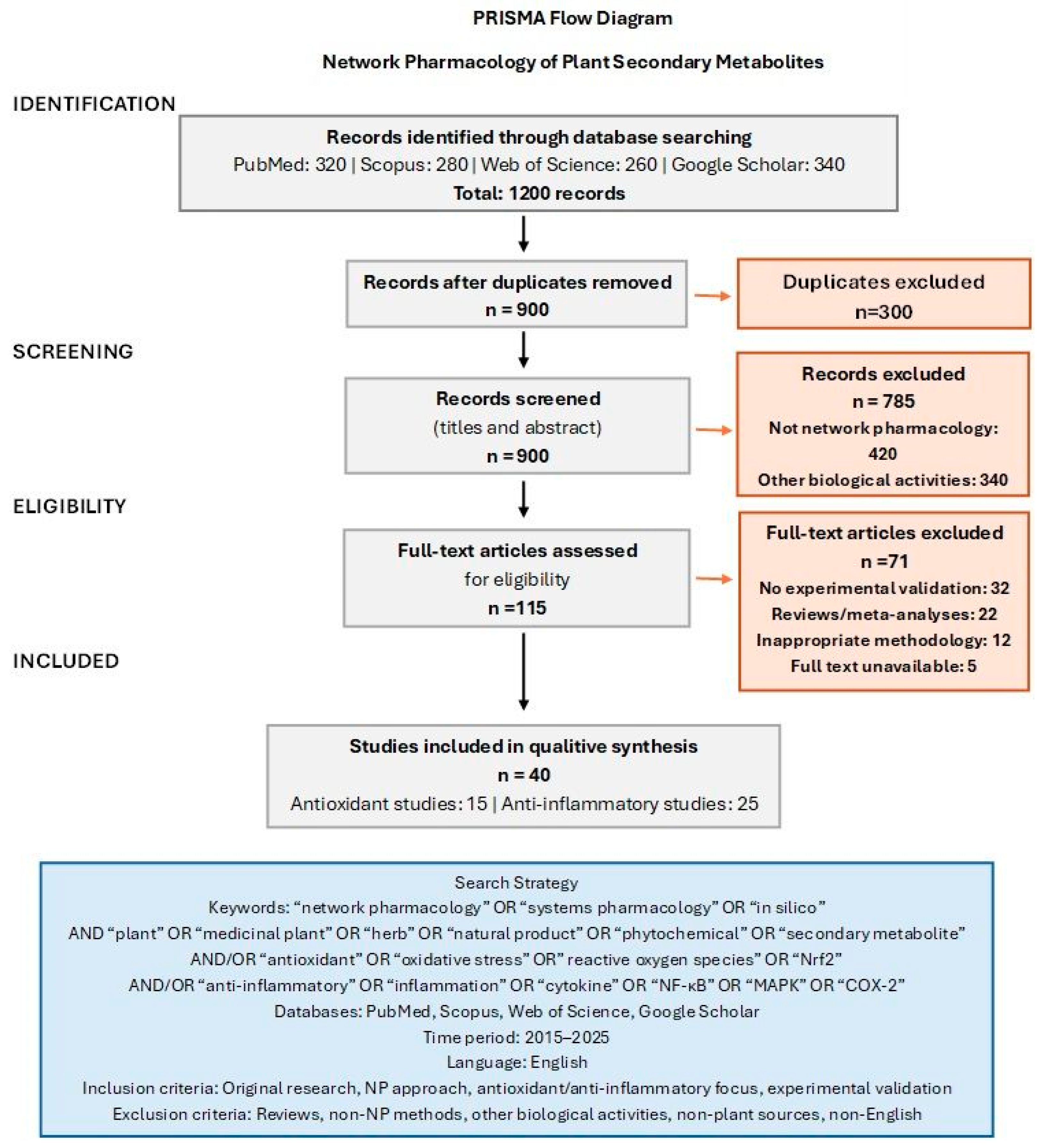
2. Review Methodology
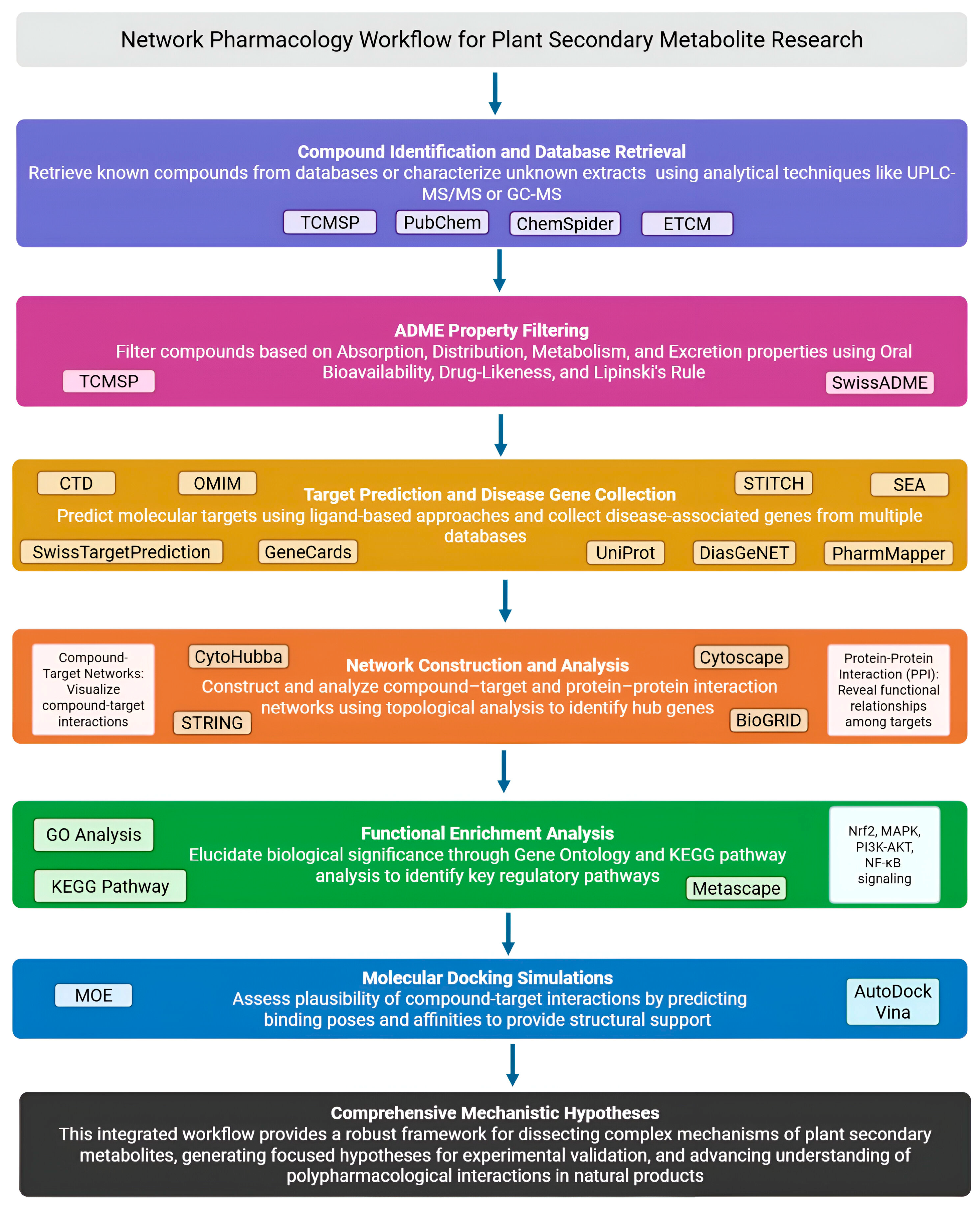
3. Typical Workflow of NP in Plant Secondary Metabolite Research
4. Applying NP to Investigate the Antioxidant Potential of Secondary Metabolites
4.1. Introduction to the Application of NP in Antioxidant Research
4.2. Main Antioxidant Mechanisms Identified Using NP—A Review of Studies
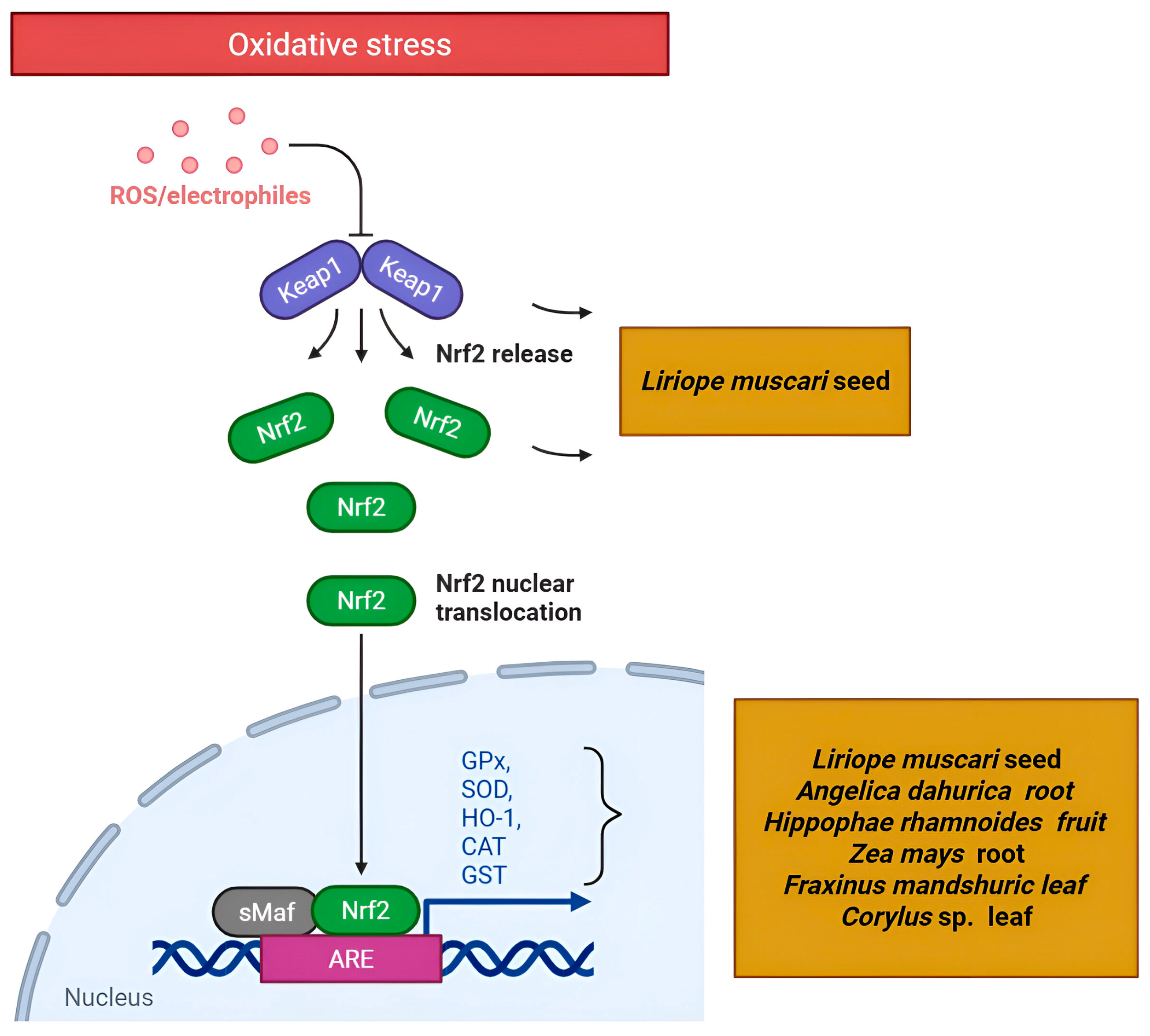
4.2.1. Targeting the Nrf2/KEAP1/ARE Pathway
4.2.2. Modulation of Oxidative Stress-Related Signaling Pathways (PI3K/AKT, MAPK, NF-κB)
4.2.3. Direct Interactions with Redox-Related Proteins and Enzymes
4.3. Key Antioxidant Compound Classes Identified via NP
4.4. Synthesis of Findings on Antioxidant Mechanisms and Comparison with Experimental Validation
4.4.1. In Vivo Validation of Predicted Antioxidant Mechanisms
4.4.2. The Role of Molecular Docking in Confirming Interactions
5. Applying NP to Investigate the Anti-Inflammatory Potential of Secondary Metabolites
5.1. Introduction to the Application of NP in Anti-Inflammatory Research
5.2. Main Anti-Inflammatory Mechanisms Identified Using NP—A Review of Studies
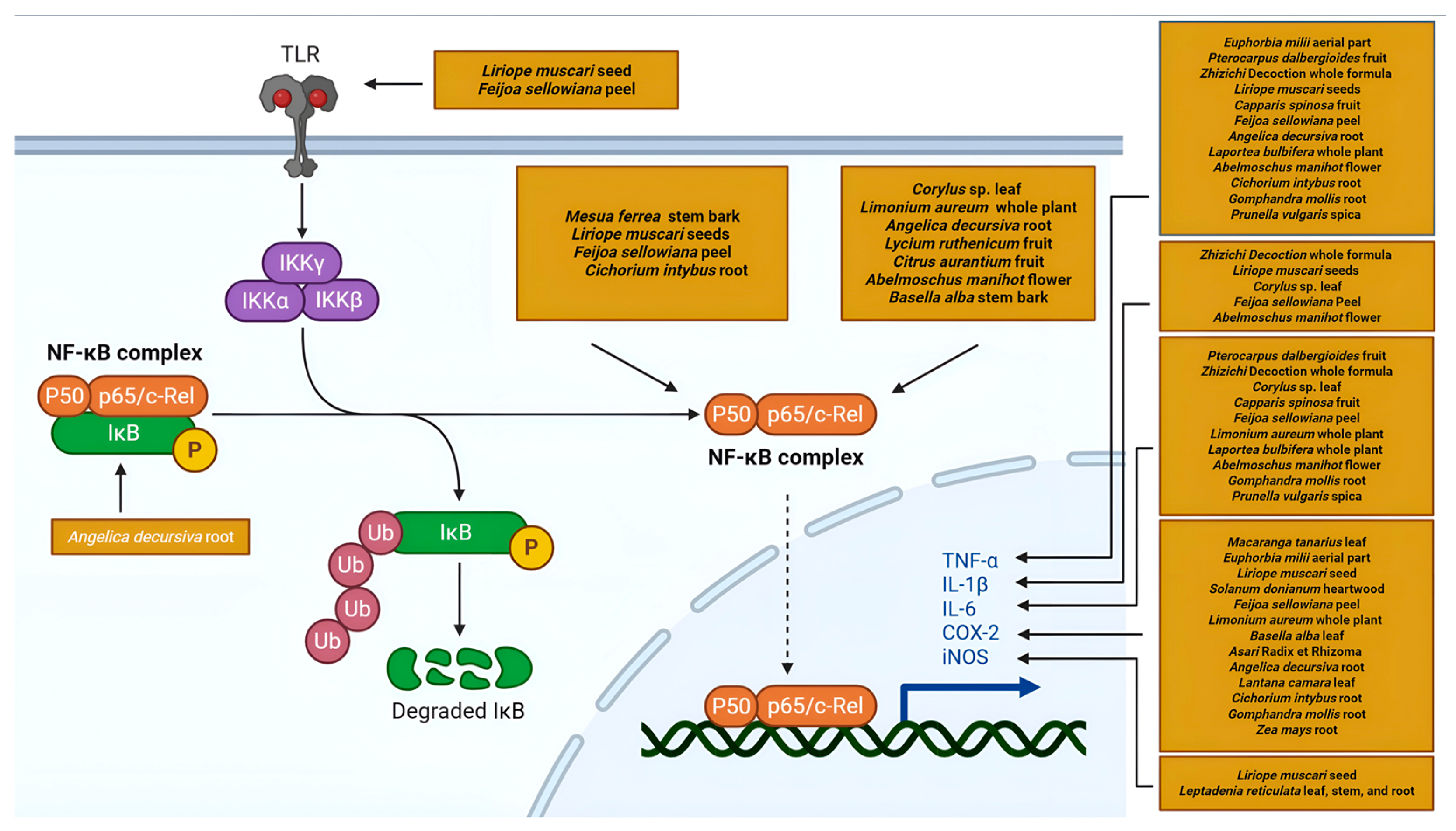
5.2.1. Modulation of the NF-κB Signaling Pathway
5.2.2. Involvement of MAPK Signaling Cascades
5.2.3. The Role of the PI3K/AKT Pathway in Inflammation
5.2.4. Targeting the JAK-STAT Pathway
5.2.5. Other Relevant Pathways
5.2.6. Direct Interactions with Inflammatory Mediators and Enzymes
5.3. Key Anti-Inflammatory Compound Classes Identified via NP
5.4. Synthesis of Findings on Anti-Inflammatory Mechanisms and Comparison with Experimental Validation
5.4.1. Concordance Between In Silico Predictions and In Vitro Anti-Inflammatory Activity
5.4.2. In Vivo Validation of Predicted Anti-Inflammatory Mechanisms
5.5. The Role of Molecular Docking in Confirming Anti-Inflammatory Interactions
6. Assessment of the Potential and Limitations of NP in Secondary Metabolite Research
6.1. The Emerging Prominence of NP
6.2. Therapeutic Insights and Strategic Advantages
6.3. Inherent Limitations and Contemporary Challenges
6.3.1. Database Dependency and Algorithmic Variability
6.3.2. Static Models and Biological Complexity
6.3.3. Standardization and Methodological Transparency
6.4. The Critical Role of Experimental Validation
6.5. Concluding Assessment
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Sezer, F.; Deniz, S.; Sevim, D.; Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxid. Med. Cell Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1. [Google Scholar] [CrossRef]
- Press, N.J.; Joly, E.; Ertl, P. Natural Product Drug Delivery: A Special Challenge? Prog. Med. Chem. 2019, 58, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Simoben, C.V.; Babiaka, S.B.; Moumbock, A.F.A.; Namba-Nzanguim, C.T.; Eni, D.B.; Medina-Franco, J.L.; Günther, S.; Ntie-Kang, F.; Sippl, W. Challenges in Natural Product-Based Drug Discovery Assisted with in Silico-Based Methods. RSC Adv. 2023, 13, 31578–31594. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.D.; Tatonetti, N.P. Informatics and Computational Methods in Natural Product Drug Discovery: A Review and Perspectives. Front. Genet. 2019, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Tessema, F.B.; Asfaw, T.B.; Tadesse, M.G.; Gonfa, Y.H.; Bachheti, R.K. In Silico Studies as Support for Natural Products Research. Medinformatics 2025, 2, 11–21. [Google Scholar] [CrossRef]
- Medina-Franco, J.L. Computational Approaches for the Discovery and Development of Pharmacologically Active Natural Products. Biomolecules 2021, 11, 630. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Liu, K.; Hu, X.; Gu, X. Advances in Drug Discovery Based on Network Pharmacology and Omics Technology. Curr. Pharm. Anal. 2024, 21, 33–43. [Google Scholar] [CrossRef]
- Charvériat, M.; Lafon, V.; Mouthon, F.; Zimmer, L. Innovative Approaches in CNS Drug Discovery. Therapies 2021, 76, 101–109. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network Pharmacology: The next Paradigm in Drug Discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Yadav, D.K. Metabolomics and Network Pharmacology in the Exploration of the Multi-Targeted Therapeutic Approach of Traditional Medicinal Plants. Plants 2022, 11, 3243. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Mäkelä, S.; Aittokallio, T. Network Pharmacology Applications to Map the Unexplored Target Space and Therapeutic Potential of Natural Products. Nat. Prod. Rep. 2015, 32, 1249–1266. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2010, 12, 122–132. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese Medicine Network Pharmacology: Theory, Methodology and Application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.I.; Iyengar, R. Network Analyses in Systems Pharmacology. Bioinformatics 2009, 25, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H.H.W. Network Pharmacology: Curing Causal Mechanisms Instead of Treating Symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM Network Pharmacology: A New Trend towards Combining Computational, Experimental and Clinical Approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, Y.Q.; Liu, Z.M.; Chen, T.; Lv, C.Y.; Tang, S.H.; Zhang, X.B.; Zhang, W.; Li, Z.Y.; Zhou, R.R.; et al. ETCM: An Encyclopaedia of Traditional Chinese Medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current Approaches and Challenges for the Metabolite Profiling of Complex Natural Extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W3664. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating Protein Pharmacology by Ligand Chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 Update: A Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting Protein-Chemical Interaction Networks with Tissue and Affinity Data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards Direct Deposition of Bioassay Data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 2016, 1–33. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.Org: Online Mendelian Inheritance in Man (OMIM®), an Online Catalog of Human Genes and Genetic Disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2021. Nucleic Acids Res. 2021, 49, D1138–D1143. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID Database: A Comprehensive Biomedical Resource of Curated Protein, Genetic, and Chemical Interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Carbon, S.; Douglass, E.; Good, B.M.; Unni, D.R.; Harris, N.L.; Mungall, C.J.; Basu, S.; Chisholm, R.L.; Dodson, R.J.; Hartline, E.; et al. The Gene Ontology Resource: Enriching a GOld Mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software News and Updates AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox. Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox. Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of Hypoxia-Inducible Factor-1a by Reactive Oxygen Species: New Developments in an Old Debate. J. Cell Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive Oxygen Species in the Activation of MAP Kinases. Methods Enzym. 2013, 528, 27–48. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of Reactive Oxygen Species and NF-ΚB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 Regulatory Network Provides an Interface between Redox and Intermediary Metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell Mol. Life Sci. 2016, 73, 3221. [Google Scholar] [CrossRef]
- Truong, V.L.; Bae, Y.J.; Rarison, R.H.G.; Bang, J.H.; Park, S.Y.; Jeong, W.S. Anti-Inflammatory and Antioxidant Activities of Lipophilic Fraction from Liriope platyphylla Seeds Using Network Pharmacology, Molecular Docking, and In Vitro Experiments. Int. J. Mol. Sci. 2023, 24, 14958. [Google Scholar] [CrossRef]
- Dan, Z.; Xiujun, W.; Yanbei, Z.; Zihang, L.; Xiaotian, Q.; Li, Q. Comprehensive Metabolomics and Network Pharmacology Reveal the Mechanism of Antioxidant Activities of Chimonanthus Praecox Chemical Components. Available online: https://ssrn.com/abstract=4924486 (accessed on 15 May 2025).
- Xu, X.; Chen, X.; Man, Q.; Li, W.; Wang, L.; Liu, X.; Chen, J.; Cui, J. Multi-Omics, Network Pharmacology, and Molecular Docking Provide Insights into the Genetic Basis, Bioactive, and Potential Antioxidant Mechanisms in Potato (Solanum tuberosum L.) Flesh. Sci. Hortic. 2025, 345, 114148. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Zhang, Z.; Tong, L.; Yu, S.; Liu, Y.; Yang, F. Flavonoid Compounds in Hippophae rhamnoides L. Protect Endothelial Cells from Oxidative Damage Through the PI3K/AKT-ENOS Pathway. Chem. Biodivers 2024, 21, e202400300. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Wang, Y.; Lv, G.; Lin, H.; Lin, Z. UPLC-MS/MS Profiling, Antioxidant and Anti-Inflammatory Activities, and Potential Health Benefits Prediction of Phenolic Compounds in Hazel Leaf. Front. Nutr. 2023, 10, 1092071. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, H.; Song, Y.; Xiao, M.; Hu, H.; Yu, S.; Xie, F. Metabolic Profiles and Potential Antioxidant Mechanisms of Hawk Tea. Sci. Rep. 2025, 15, 3600. [Google Scholar] [CrossRef]
- Wei, X.; Jiao, P.; Jiang, Z.; Wang, C.; Liu, Q.; Li, Y.; Liu, S.; Guan, S.; Ma, Y. Study on Differential Metabolite Active Ingredients in Maize Roots Based on Network Pharmacology and Metabolomics Analysis. J. Agric. Food Chem. 2025, 73, 6646–6658. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Xiang, Y.; Wei, Z.; Zheng, W.; Shen, P.; Huang, L. Network Pharmacology of Dracaena sp. in Guangxi and Its Related Species Leaf Secondary Metabolites Possess Antioxidant Properties. Arab. J. Chem. 2024, 17, 105812. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Chen, Y.; Wang, M.; Yu, J.; Bai, W.; Hong, L. Combined Metabolomics and Network Pharmacology Analysis Reveal the Effect of Rootstocks on Anthocyanins, Lipids, and Potential Pharmacological Ingredients of Tarroco Blood Orange (Citrus sinensis L. Osbeck). Plants 2024, 13, 2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, Q. Simultaneous Extraction and Analysis of Seven Major Saikosaponins from Bupleuri Radix and the Exploration of Antioxidant Activity and Its Mechanism. Molecules 2023, 28, 5872. [Google Scholar] [CrossRef]
- Guo, J.; Ho, C.-T.; Bai, N. New Utilization of Fraxinus mandshurica Leaves: As a Safe and Promising Natural Antioxidant. J. Agric. Food Chem. 2024, 72, 20470–20482. [Google Scholar] [CrossRef]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Li, Q.; Wang, T.; Wang, C.; Ding, X. Ultrasonic-Assisted DES Extraction Optimization, Characterization, Antioxidant and in Silico Study of Polysaccharides from Angelica dahurica Radix. Arab. J. Chem. 2024, 17, 106044. [Google Scholar] [CrossRef]
- Polvani, S.; Tarocchi, M.; Galli, A. PPARγ and Oxidative Stress: Con(β) Catenating NRF2 and FOXO. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef]
- Wonggo, D.; Anwar, C.; Dotulong, V.; Reo, A.; Taher, N.; Syahputra, R.A.; Nurkolis, F.; Tallei, T.E.; Kim, B.; Tsopmo, A. Subcritical Water Extraction of Mangrove Fruit Extract (Sonneratia alba) and Its Antioxidant Activity, Network Pharmacology, and Molecular Connectivity Studies. J. Agric. Food Res. 2024, 18, 101334. [Google Scholar] [CrossRef]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef][Green Version]
- Nam, D.G.; Kim, M.; Choi, A.J.; Choe, J.S. Health Benefits of Antioxidant Bioactive Compounds in Ginger (Zingiber officinale) Leaves by Network Pharmacology Analysis Combined with Experimental Validation. Antioxidants 2024, 13, 652. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, T.; Jiang, S.; Xiao, Z.; Shan, Y.; Li, T.; Pan, Z.; Li, Q.; Fu, F. Antioxidant Effects and Potential Mechanisms of Citrus Reticulata ‘Chachi’ Components: An Integrated Approach of Network Pharmacology and Metabolomics. Foods 2024, 13, 4018. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and Scoring in Virtual Screening for Drug Discovery: Methods and Applications. Nat. Rev. Drug. Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-Activated Protein Kinases in Innate Immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. TNF and MAP Kinase Signalling Pathways. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Hawkins, P.T.; Stephens, L.R. PI3K Signalling in Inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 882–897. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Chai, C.; Jin, B.; Bi, J.; Cui, Y.; Cui, X.; Shan, C.; Yu, S.; Wen, H. Exploring of Antidepressant Components and Mechanisms of Zhizichi Decoction: Integration of Serum Pharmacochemistry, Network Pharmacology and Anti-Inflammatory Analysis Verification. Anal. Sci. Adv. 2025, 6, e70002. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Wang, M.; He, D.; Liu, L.; Shu, Y.; Song, Z.; Li, H.; Liu, Y.; Lu, A. Anti-Inflammatory Effects of Zhishi and Zhiqiao Revealed by Network Pharmacology Integrated with Molecular Mechanism and Metabolomics Studies. Phytomedicine 2018, 50, 61–72. [Google Scholar] [CrossRef]
- Sundaram, J.K.; Hanumanthappa, M.; Kandagalla, S.; Shekarappa, S.B.; Gollapalli, P.; Hani, U. Evaluating the Anti-Inflammatory Potential of Mesua ferrea Linn. Stem Bark through Network Pharmacology Approach. J. Appl. Biol. Biotechnol. 2023, 11, 174–197. [Google Scholar] [CrossRef]
- Yang, Z.; Man, J.; Liu, Y.; Zhang, H.; Wu, D.; Shao, D.; Hao, B.; Wang, S. Study on the Alleviating Effect and Potential Mechanism of Ethanolic Extract of Limonium aureum (L.) Hill. on Lipopolysaccharide-Induced Inflammatory Responses in Macrophages. Int. J. Mol. Sci. 2023, 24, 16272. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Q. Study on the Anti-Inflammatory Mechanism of Coumarins in Peucedanum decursivum Based on Spatial Metabolomics Combined with Network Pharmacology. Molecules 2024, 29, 3346. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zheng, Y.; Pei, J.; Huang, L. Potential Mechanism of Laportea bulbifera on Treating Inflammation and Tumor via Metabolomics, Network Pharmacology and Molecular Docking. J. Biomol. Struct. Dyn. 2024, 1–17. [Google Scholar] [CrossRef]
- Tran Huynh, Q.D.; Phan, T.T.T.; Chu, M.H.; Nguyen, T.V.; Duong, T.L.T.; Hsu, S.J.; Wang, Y.H.; Pham, N.T.; Bui, B.T.N.; Nguyen, D.K.; et al. Anti-Inflammatory Gomphandranosides I–VIII from the Roots of Gomphandra Molis Merr. Available online: https://ssrn.com/abstract=5153553 (accessed on 15 May 2025).
- Gao, Y.; Liang, Z.; Lv, N.; Shan, J.; Zhou, H.; Zhang, J.; Shi, L. Exploring the Total Flavones of Abelmoschus manihot against IAV-Induced Lung Inflammation by Network Pharmacology. BMC Complement Med. Ther. 2022, 22, 36. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, Q.; Lin, Y.; Xia, B.; Li, Y.; Xie, J.; Wu, P.; Liao, D.; Lin, L. The Dynamics of Bioactive Ingredients with Anti-Inflammatory and Anti-Breast Cancer Activity During Prunellae spica Development. Nat. Prod. Commun. 2024, 19, e202401788. [Google Scholar] [CrossRef]
- Remorosa, A.G.B.; Tsai, P.W.; De Castro-Cruz, K.A.; Hsueh, C.C.; Chen, R.Y.; Chen, B.Y. Deciphering Characteristics of Macaranga tanarius Leaves Extract with Electron Shuttle-Associated Anti-Inflammatory Activity via Microbial Fuel Cells, Molecular Docking, and Network Pharmacology. Biochem. Eng. J. 2024, 208, 109345. [Google Scholar] [CrossRef]
- Dong, W.M.; Zhang, Y.T.; Wang, H.M.; Deng, M.X.; Chen, Z.X.; He, H.P.; Dong, F.W. Anti-Inflammatory Activities of New Compounds Isolated from the Fruits of Foeniculum vulgare Mill. Chem. Biodivers 2025, 22, e202401788. [Google Scholar] [CrossRef]
- Zhao, J.N.; Yu, S.F.; Wu, Z.H.; Chen, L.; Fu, R.; Li, Z.; Qu, Y.L.; Huang, J.; Wang, L.B.; Piao, X.M.; et al. Chemical Constituents from the Heartwood of Solanum verbascifolium L. and Their Anti-Inflammatory Activities Combined Network Pharmacology. Chem. Biodivers 2024, 21, e202302111. [Google Scholar] [CrossRef]
- Li, S.; Ou, P.; Zhang, X.; Jiao, G.; Chen, Y.; Pan, Y.; Wang, Y.; Liu, Q.; Wang, W. Anti-Inflammatory Mechanisms of Acca sellowiana Peel in RAW264.7 Cells: Involvement of the JAK-STAT Signaling Pathway. ACS Food Sci. Technol. 2025, 5, 1601–1613. [Google Scholar] [CrossRef]
- Liu, X.; Aimaier, A.; Wang, W.; Dong, Y.; Han, P.; He, J.; Mu, L.; Wang, X.; Li, J. Quality Variation and Biosynthesis of Anti-Inflammatory Compounds for Capparis spinosa Based on the Metabolome and Transcriptome Analysis. Front. Plant. Sci. 2023, 14, 1224073. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.H.; Bueno, P.R.P.; Garcia, P.J.B.; Lee, M.J.; De Castro-Cruz, K.A.; Leron, R.B.; Tsai, P.W. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of Osmanthus fragrans (Thunb.) Lour. Flower Extr. 2023, 12, 3168. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Wang, D.; Du, G. Analysis of Bioactive Components in the Fruit, Roots, and Leaves of Alpinia oxyphylla by UPLC-MS/MS. Evid.-Based Complement. Altern. Med. 2021, 2021, 5592518. [Google Scholar] [CrossRef]
- Khairan, K.; Maulydia, N.B.; Faddillah, V.; Tallei, T.E.; Fauzi, F.M.; Idroes, R. Uncovering Anti-Inflammatory Potential of Lantana camara Linn: Network Pharmacology and In Vitro Studies. Narra J. 2024, 4, e894. [Google Scholar] [CrossRef]
- Mallepura Adinarayanaswamy, Y.; Padmanabhan, D.; Natarajan, P.; Palanisamy, S. Metabolomic Profiling of Leptadenia reticulata: Unveiling Therapeutic Potential for Inflammatory Diseases through Network Pharmacology and Docking Studies. Pharmaceuticals 2024, 17, 423. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Liang, Y.; Liu, R.; Lv, X.; Zhang, Q.; Xu, H.; Bi, K.; Li, Z.; Li, Q. A Systematic Strategy for Uncovering Quality Marker of Asari Radix et Rhizoma on Alleviating Inflammation Based Chemometrics Analysis of Components. J. Chromatogr. A 2021, 1642, 461960. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Arellano, J.I.; Gómez-Verjan, J.C.; Rojano-Vilchis, N.A.; Mendoza-Cruz, M.; Jiménez-Estrada, M.; López-Valdés, H.E.; Martínez-Coria, H.; Gutiérrez-Juárez, R.; González-Espinosa, C.; Reyes-Chilpa, R.; et al. Chemoinformatic Analysis of Selected Cacalolides from Psacalium decompositum (A. Gray) H. Rob. & Brettell and Psacalium peltatum (Kunth) Cass. and Their Effects on FcεRI-Dependent Degranulation in Mast Cells. Molecules 2018, 23, 3367. [Google Scholar] [CrossRef]
- Alnusaire, T.S.; Sabouni, I.L.; Khojah, H.; Qasim, S.; Al-Sanea, M.M.; Siddique, S.; Mokhtar, F.A.; Ahmed, S.R. Integrating Chemical Profiling, In Vivo Study, and Network Pharmacology to Explore the Anti-Inflammatory Effect of Pterocarpus dalbergioides Fruits and Its Correlation with the Major Phytoconstituents. ACS Omega 2023, 8, 32544–32554. [Google Scholar] [CrossRef]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction Networks of Chemicals and Proteins. Nucleic Acids Res. 2008, 36, D684–D688. [Google Scholar] [CrossRef]
- Halayal, R.Y.; Bagewadi, Z.K.; Khan, T.M.Y.; Shamsudeen, S.M. Investigating Compounds from Basella Alba for Their Antioxidant, Anti-Inflammatory, and Anticancer Properties through in Vitro and Network Pharmacology, Molecular Simulation Approach. Green Chem. Lett. Rev. 2025, 18, 2481945. [Google Scholar] [CrossRef]
- Negm, W.A.; Elekhnawy, E.; Mokhtar, F.A.; Binsuwaidan, R.; Attallah, N.G.M.; Mostafa, S.A.; Moglad, E.; Ibrahim, S.; Al-Fakhrany, O.M.; Eliwa, D. Phytochemical Inspection and Anti-Inflammatory Potential of Euphorbia milii Des Moul. Integrated with Network Pharmacology Approach. Arab. J. Chem. 2024, 17, 105568. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, L.; Zhang, F.; Chen, X.; Wang, H.; Zhou, J.; Chai, K.; Liu, J.; Lei, H.; Lu, P.; et al. Network Pharmacology: A Crucial Approach in Traditional Chinese Medicine Research. Chin. Med. 2025, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.P.; Baldi, A.; Kumar, N.; Pradhan, J. Harnessing Network Pharmacology in Drug Discovery: An Integrated Approach. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 398, 4689–4703. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A Web Server for Target Prediction of Bioactive Small Molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper Server: A Web Server for Potential Drug Target Identification Using Pharmacophore Mapping Approach. Nucleic Acids Res. 2010, 38, W609. [Google Scholar] [CrossRef]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A Large-Scale Bioactivity Database for Drug Discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef]
- Zdrazil, B.; Felix, E.; Hunter, F.; Manners, E.J.; Blackshaw, J.; Corbett, S.; de Veij, M.; Ioannidis, H.; Lopez, D.M.; Mosquera, J.F.; et al. The ChEMBL Database in 2023: A Drug Discovery Platform Spanning Multiple Bioactivity Data Types and Time Periods. Nucleic Acids Res. 2024, 52, D1180–D1192. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Rodríguez-Pérez, J.R.; Cortés-Hernández, H.F.; López-López, E. Rethinking the “best Method” Paradigm: The Effectiveness of Hybrid and Multidisciplinary Approaches in Chemoinformatics. Artif. Intell. Life Sci. 2024, 6, 100117. [Google Scholar] [CrossRef]
| Feature | TCMSP | SwissTargetPrediction | PharmMapper | STITCH | ChEMBL |
|---|---|---|---|---|---|
| Access Type | Web-based | Web-based | Web-based | Web-based | Web-based, API |
| Primary Data Source (Compounds) | Proprietary database (mainly TCM), PubChem, ChEMBL | ChEMBL | ZINC, DrugBank, ChEMBL, TCMSP, and others | PubChem, ChEMBL | Own curated database (bioactive drug-like molecules) |
| Primary Data Source (Targets/Interactions) | DrugBank, HIT, TTD, PharmGKB, UniProt | ChEMBL (known interactions) | PDB (protein structures), DrugBank, UniProt | Experimental, predicted, database-derived, text-mined data | Literature-derived data, bioactivity data deposition |
| Core Target Prediction Method | ADME-based compound filtering (e.g., OB, DL) combined with structural similarity and pharmacokinetic properties | 2D and 3D similarity (shape) to known ligands | Pharmacophore-based reverse docking | Combination of experimental, predicted (e.g., structural similarity), text mining, and homology transfer | Stores known interactions; not a primary prediction tool |
| Required User Input | Compound/TCM ingredient name, structure (SMILES) | 2D/3D structure (SMILES, draw interface) | 3D compound structure (mol2 format) | Compound name, SMILES, InChIKey | Compound name, structure, database ID |
| Key Outputs | Potential targets, ADME parameters, related diseases, network analysis | List of potential targets with probability scores | Ranking of potential targets (proteins) with pharmacophore fit scores | Compound–protein and protein–protein interaction networks, interaction confidence scores | Bioactivity data, targets, physicochemical properties |
| Pathway/GO Analysis Integration | Yes (e.g., KEGG, GO via linked targets) | Limited directly, targets can be exported | Limited directly, targets can be exported | Yes (e.g., KEGG, GO for associated proteins) | Data can be exported to external tools |
| Special Features/Strengths | Focus on TCM, ADME integration, user-friendly for TCM researchers | Rapid prediction for a wide range of compounds, clear interface | Identifies targets for novel compounds based on binding pocket fit | Broad scope of interactions (not just direct), confidence scoring, network visualization | Comprehensive, curated bioactivity database, standard for chemical and biological data |
| Limitations/Challenges | Limited database coverage beyond TCM, “black box” nature of some ADME parameters | Dependency on ChEMBL data quality, may favor well-studied targets | Requires 3D structure, results depend on pharmacophore model quality and coverage | Output can be very extensive, distinguishing direct vs. indirect interactions | Primarily a database rather than a prediction tool; requires API knowledge or external analytical tools for target prediction |
| Typical Use in Natural-Product NP | Identifying active ingredients and mechanisms from TCM extracts | Quick prediction of potential targets for single compounds or small libraries | Predicting targets for compounds with known 3D structures, novel target fishing | Building interaction networks, identifying key proteins in compound-related networks | Source of known activities and targets for compounds, validating predictions |
| References | [19,26] | [34,118] | [36,119] | [113] | [120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merecz-Sadowska, A.; Sadowski, A.; Zielińska-Bliźniewska, H.; Zajdel, K.; Zajdel, R. Network Pharmacology as a Tool to Investigate the Antioxidant and Anti-Inflammatory Potential of Plant Secondary Metabolites—A Review and Perspectives. Int. J. Mol. Sci. 2025, 26, 6678. https://doi.org/10.3390/ijms26146678
Merecz-Sadowska A, Sadowski A, Zielińska-Bliźniewska H, Zajdel K, Zajdel R. Network Pharmacology as a Tool to Investigate the Antioxidant and Anti-Inflammatory Potential of Plant Secondary Metabolites—A Review and Perspectives. International Journal of Molecular Sciences. 2025; 26(14):6678. https://doi.org/10.3390/ijms26146678
Chicago/Turabian StyleMerecz-Sadowska, Anna, Arkadiusz Sadowski, Hanna Zielińska-Bliźniewska, Karolina Zajdel, and Radosław Zajdel. 2025. "Network Pharmacology as a Tool to Investigate the Antioxidant and Anti-Inflammatory Potential of Plant Secondary Metabolites—A Review and Perspectives" International Journal of Molecular Sciences 26, no. 14: 6678. https://doi.org/10.3390/ijms26146678
APA StyleMerecz-Sadowska, A., Sadowski, A., Zielińska-Bliźniewska, H., Zajdel, K., & Zajdel, R. (2025). Network Pharmacology as a Tool to Investigate the Antioxidant and Anti-Inflammatory Potential of Plant Secondary Metabolites—A Review and Perspectives. International Journal of Molecular Sciences, 26(14), 6678. https://doi.org/10.3390/ijms26146678






