Exploring Recent Developments in the Manifestation, Diagnosis, and Treatment of Patients with Smith–Lemli–Opitz Syndrome: From Molecular Pathways to Clinical Innovations
Abstract
1. Introduction
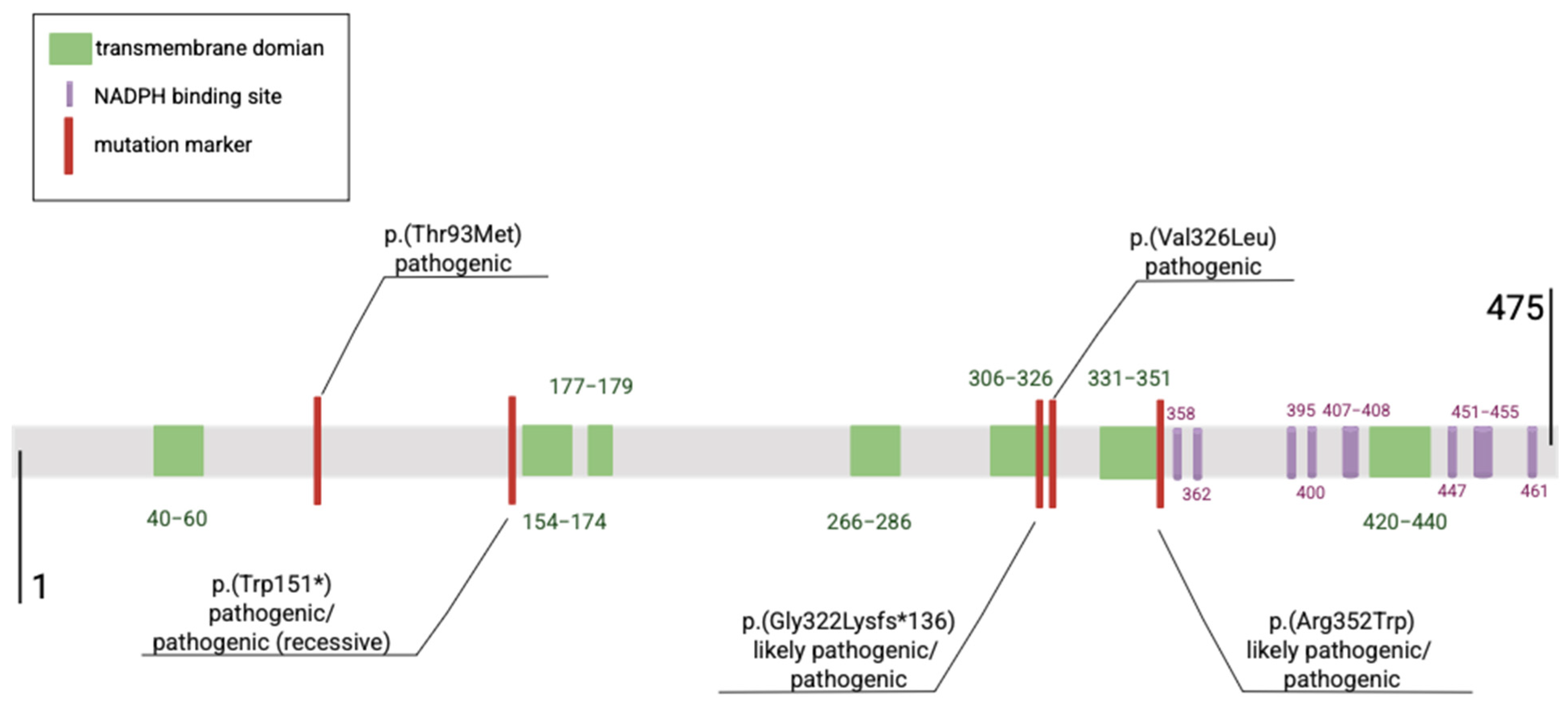
1.1. DHCR7 Gene Pathogenic Variants
| Reported | DNA Change (cDNA) | Protein | rsID | Clinical Classification | Reference |
|---|---|---|---|---|---|
| 246 | c.964-1G>G | p.(Gly322Lysfs*136) | - | likely pathogenic (recessive), pathogenic | [12,13] |
| 117 | c.452G>A | p.(Trp151*), p.(Trp151Ter) | rs80338854 | Pathogenic, pathogenic (recessive) | [14] |
| 96 | c.278C>T | p.(Thr93Met) | rs80338853 | pathogenic | [8,15,16] |
| 50 | c.976G>T | p.(Val326Leu) | rs80338859 | pathogenic | [15] |
| 31 | c.1054C>T | p.(Arg352Trp) | rs80338860 | likely pathogenic, pathogenic | [15] |
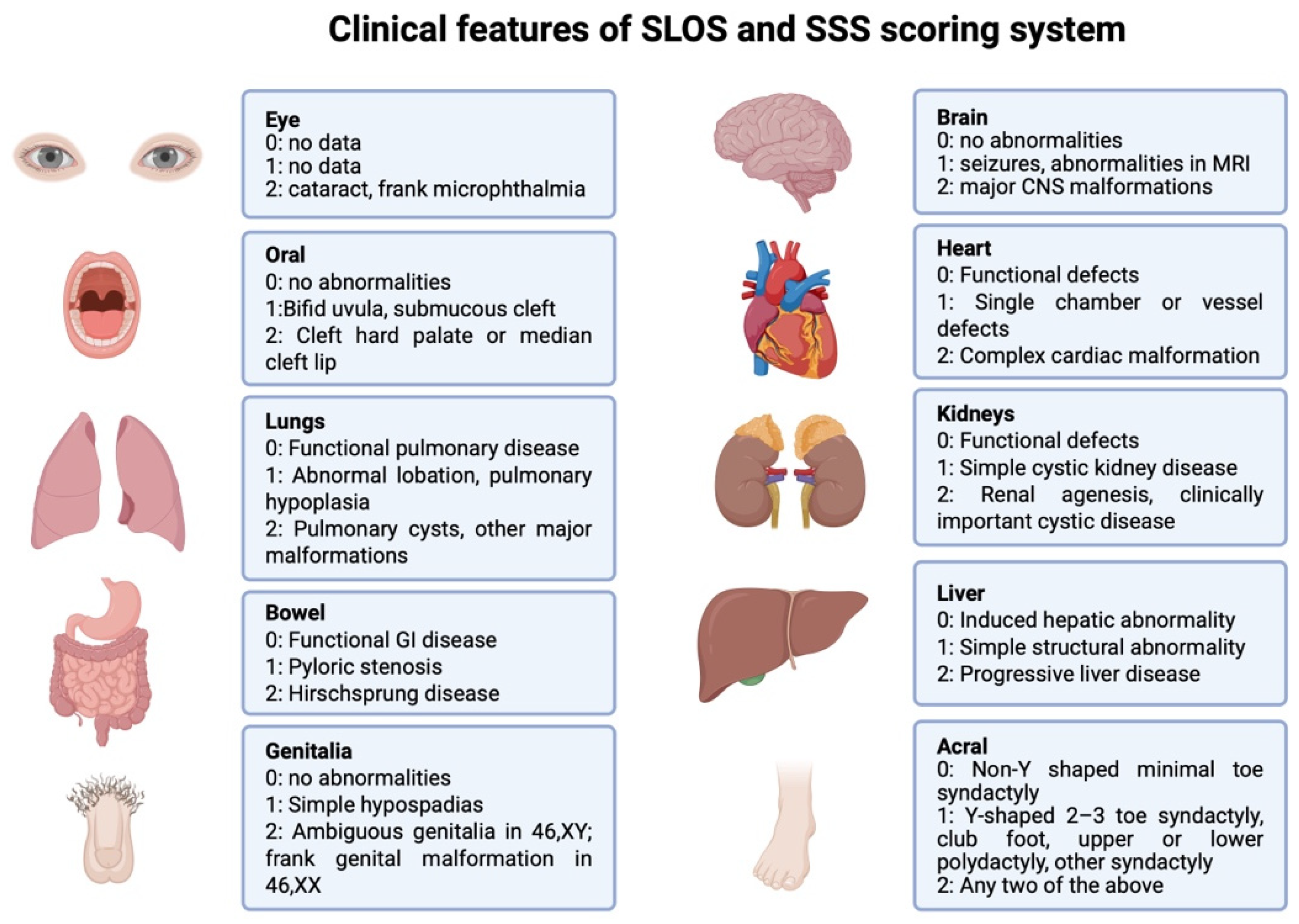
1.2. SLOS Severity Score (SSS)
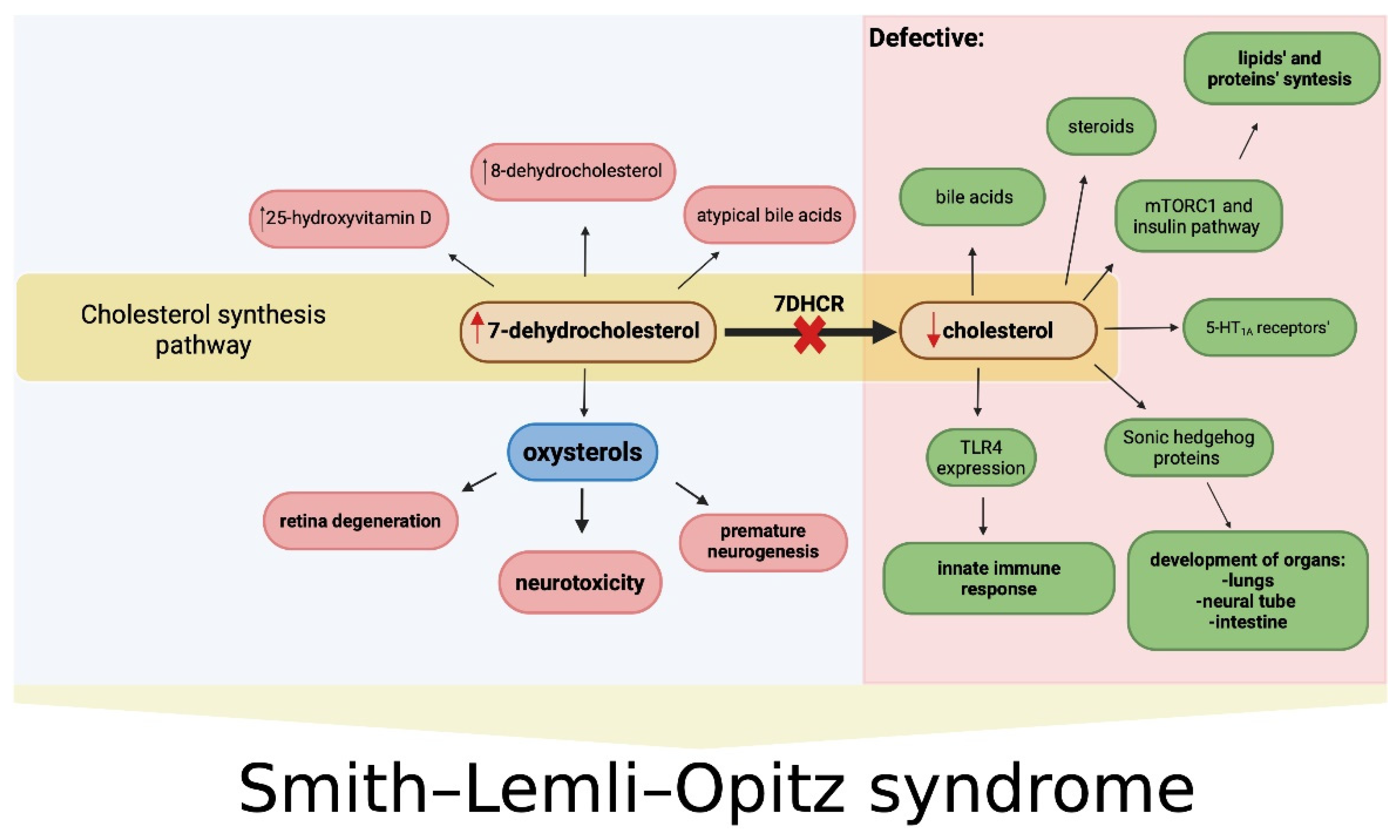
1.3. Cholesterol Deficiency and Oxysterols in the Pathogenesis of SLOS
1.3.1. Cholesterol
1.3.2. Oxysterols
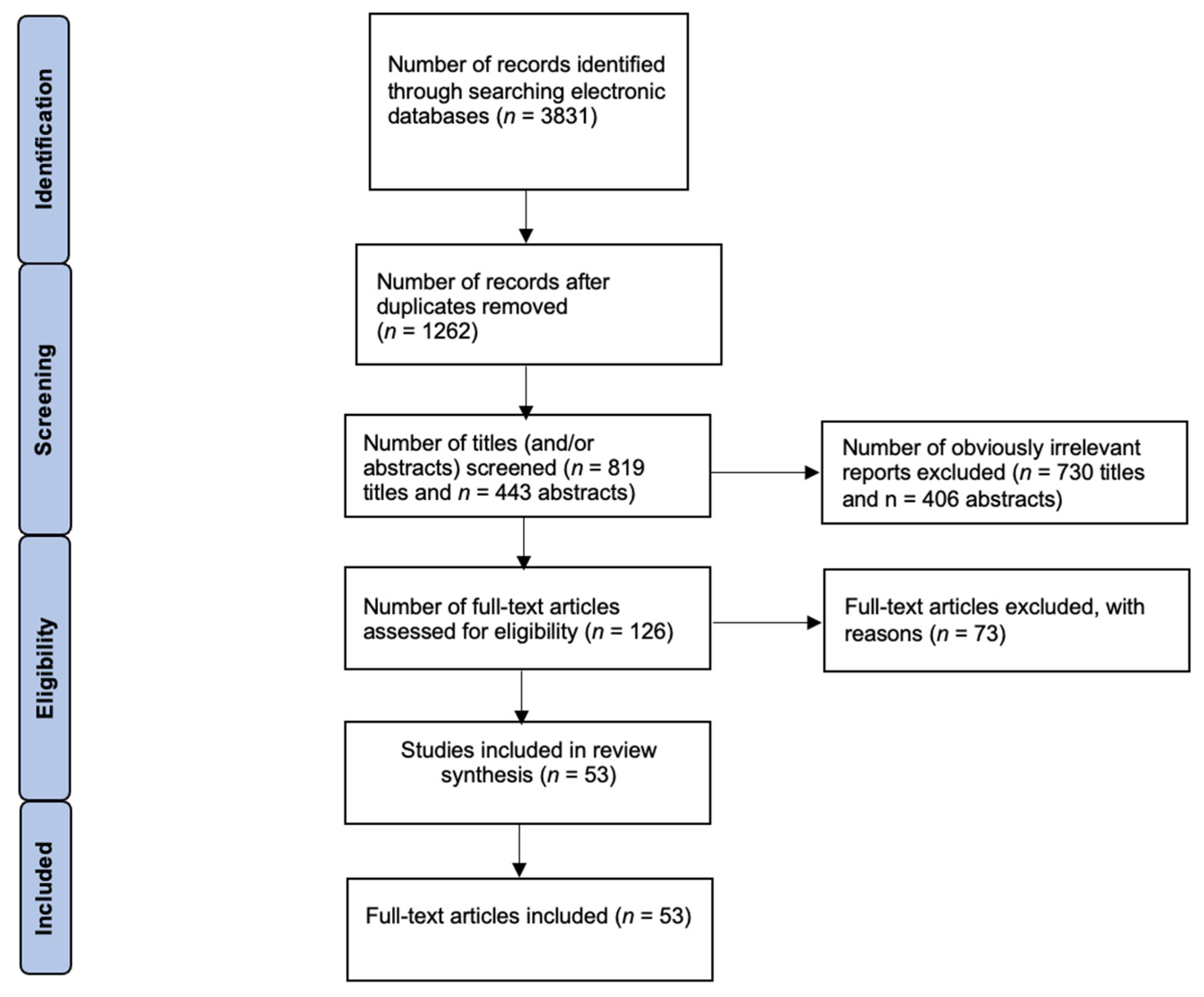
2. Methods
Eligibility Criteria and Study Selection
3. Update on Clinical Manifestation and Underlying Molecular Mechanisms in SLOS
3.1. General Features and Congenital Malformations
3.2. Neurological Disorders
Molecular Aspects
3.3. The Visual System
Molecular Aspects
3.4. Other Systemic Molecular Implications
4. Update on SLOS Diagnostics and Disease Progression Monitoring
4.1. Basic Diagnostics
4.2. Glial Fibrillary Acidic Protein (GFAP)
4.3. Neuroimaging Techniques
4.4. Prenatal and Perinatal Diagnostics
5. Treatment
5.1. Exogenous Cholesterol Supplementation
5.2. Statin Therapy
5.3. Cholic Acid Supplementation
5.4. Antioxidants
5.5. Liver Transplantation
5.6. Gene Therapy
6. Discussion
6.1. Diagnostics
6.2. Treatment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-hydroxyvitamin D |
| 4HDHC | 4β-hydroxy-7-dehydrocholesterol |
| 4-OH-DHC | 4α- and 4β-hydroxy-7-DHC |
| 5-HT1A | Serotonin 1A receptors |
| 7-DHC | 7-dehydrocholesterol |
| 7-DHCR | 7-dehydrocholesterol reductase |
| 7-kChol | 7-ketocholesterol |
| 7-kDHC | 7-keto-cholesta-5,8-dien-3β-ol |
| 7-PT | 7-dehydropregnanetriol |
| 8-DHC | 8-dehydrocholesterol |
| 8-PT | 8-dehydropregnanetriol |
| ASCVD | Atherosclerotic cardiovascular disease |
| ASD | Autism spectrum disorder |
| C/EBP | CCAAT/enhancer binding protein |
| CNS | Central nervous system |
| CRALBP | Cellular retinaldehyde-binding protein |
| CSF | Cerebrospinal fluid |
| CYP27 | Sterol 27-hydroxylase |
| DEGs | Differentially expressed genes |
| DHCDO | 3β,5α-dihydroxycholesta-7,9(11)-dien-6-one |
| DHCEO | 3β,5α-dihydroxycholest-7-en-6-one |
| DHCR7 | 7-dehydrocholesterol reductase gene |
| EMT | Epithelial–mesenchymal transition |
| EPCD | 5,9-endoperoxy-cholest-7-en-3β,6α-diol |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| GC-MS | Gas chromatography–mass spectrometry |
| GFAP | Glial fibrillary acidic protein |
| GI | Gastrointestinal |
| GR | Glucocorticoid receptor |
| HDL | High-density lipoprotein |
| Hh | Hedgehog |
| hiPSCs | Human induced pluripotent stem cells |
| HMG-CoA | Hydroxymethylglutaryl-CoA |
| IF1 | Inhibitory factor 1 |
| iPSCs | Induced pluripotent stem cells |
| IUGR | Intrauterine growth restriction |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LOVD | Leiden Open Variation Database |
| LPS | Lipopolysaccharides |
| MALDI-MSI | Matrix-assisted laser desorption ionization mass spectrometry imaging |
| MAP2 | Microtubule-associated protein 2 |
| MEK | Mitogen-activated protein kinase kinase |
| MRI | Magnetic resonance imaging |
| mRPE | Monkey retinal pigment epithelium |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| PT | Pregnanetriol |
| RPE | Retinal pigment epithelial |
| RPE-65 | Retinal pigment epithelium-specific 65 kDa protein |
| RTK | Receptor tyrosine kinase |
| SHH | Sonic Hedgehog |
| SLOS | Smith–Lemli–Opitz syndrome |
| SMA | α-smooth muscle actin |
| Smad2 | Mothers against decapentaplegic homolog 2 |
| SREBPs | Sterol regulatory element-binding proteins |
| SSS | SLOS Severity Score |
| SYP | Synaptophysin |
| TGF-β | Transforming growth factor beta |
| THCEO | 3β,5α,9α-trihydroxycholest-7-en-6-one |
| TLR4 | Toll-like receptor 4 |
| TOF-SIMS | Time-of-flight secondary ion mass spectrometry |
| TUBB3 | Tubulin Beta 3 Class III |
| TβR-I | TGF-β receptor I |
| TβR-II | TGF-β receptor II |
| US | Ultrasound imaging |
| Wnt | Wingless/integrated |
| Δ8-isomerase | Steroid 8-isomerase |
| CVS | Chorionic villus sampling |
References
- Prabhu, A.V.; Luu, W.; Sharpe, L.J.; Brown, A.J. Cholesterol-Mediated Degradation of 7-Dehydrocholesterol Reductase Switches the Balance from Cholesterol to Vitamin D Synthesis. J. Biol. Chem. 2016, 291, 8363–8373. [Google Scholar] [CrossRef] [PubMed]
- DHCR7 7-Dehydrocholesterol Reductase—NIH Genetic Testing Registry (GTR)—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gtr/genes/1717/ (accessed on 16 April 2025).
- Lee, J.N.; Bae, S.-H.; Paik, Y.-K. Structure and Alternative Splicing of the Rat 7-Dehydrocholesterol Reductase Gene. Biochim. Biophys. Acta 2002, 1576, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Witsch-Baumgartner, M.; Fitzky, B.U.; Ogorelkova, M.; Kraft, H.G.; Moebius, F.F.; Glossmann, H.; Seedorf, U.; Gillessen-Kaesbach, G.; Hoffmann, G.F.; Clayton, P.; et al. Mutational Spectrum in the Delta7-Sterol Reductase Gene and Genotype-Phenotype Correlation in 84 Patients with Smith-Lemli-Opitz Syndrome. Am. J. Hum. Genet. 2000, 66, 402–412. [Google Scholar] [CrossRef]
- Unique Variants in the DHCR7 Gene—Global Variome Shared LOVD. Available online: https://databases.lovd.nl/shared/variants/DHCR7/unique#object_id=VariantOnTranscriptUnique%2CVariantOnGenome&id=DHCR7&order=VariantOnGenome%2FClinicalClassifica-tion%2CDESC&search_transcriptid=00000101&search_VariantOnGenome/ClinicalClassification=pathogenic&page_size=100&page=1 (accessed on 16 April 2025).
- Lazarin, G.A.; Haque, I.S.; Evans, E.A.; Goldberg, J.D. Smith-Lemli-Opitz Syndrome Carrier Frequency and Estimates of in Utero Mortality Rates. Prenat. Diagn. 2017, 37, 350–355. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, T.; Ha, K.; Ki, C.-S. Carrier Frequency and Incidence Estimation of Smith-Lemli-Opitz Syndrome in East Asian Populations by Genome Aggregation Database (gnomAD) Based Analysis. Orphanet J. Rare Dis. 2021, 16, 166. [Google Scholar] [CrossRef]
- Witsch-Baumgartner, M.; Schwentner, I.; Gruber, M.; Benlian, P.; Bertranpetit, J.; Bieth, E.; Chevy, F.; Clusellas, N.; Estivill, X.; Gasparini, G.; et al. Age and Origin of Major Smith-Lemli-Opitz Syndrome (SLOS) Mutations in European Populations. J. Med. Genet. 2008, 45, 200–209. [Google Scholar] [CrossRef]
- Yılmaz, M.; Bebek, O.; Turkyilmaz, A. Smith-Lemli-Opitz Syndrome with Biallelic c.1295A>G (p.Tyr432Cys) Variant in the DHCR7 Gene in a 73-Year-Old Woman: Report of the Oldest Patient. Mol. Syndromol. 2024, 15, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Myers, R.; Zhang, W.; Alexov, E. Computational Investigation of the Missense Mutations in DHCR7 Gene Associated with Smith-Lemli-Opitz Syndrome. Int. J. Mol. Sci. 2018, 19, 141. [Google Scholar] [CrossRef]
- LOVD—An Open Source DNA Variation Database System. Available online: https://www.lovd.nl/ (accessed on 16 April 2025).
- The Deciphering Developmental Disorders Study Large-Scale Discovery of Novel Genetic Causes of Developmental Disorders. Nature 2015, 519, 223–228. [CrossRef]
- Pinz, H.; Pyle, L.C.; Li, D.; Izumi, K.; Skraban, C.; Tarpinian, J.; Braddock, S.R.; Telegrafi, A.; Monaghan, K.G.; Zackai, E.; et al. De Novo Variants in Myelin Regulatory Factor (MYRF) as Candidates of a New Syndrome of Cardiac and Urogenital Anomalies. Am. J. Med. Genet. A 2018, 176, 969–972. [Google Scholar] [CrossRef]
- Pan, X.; Tao, A.M.; Lu, S.; Ma, M.; Hannan, S.B.; Slaugh, R.; Drewes Williams, S.; O’Grady, L.; Kanca, O.; Person, R.; et al. De Novo Variants in FRYL Are Associated with Developmental Delay, Intellectual Disability, and Dysmorphic Features. Am. J. Hum. Genet. 2024, 111, 742–760. [Google Scholar] [CrossRef]
- Fitzky, B.U.; Witsch-Baumgartner, M.; Erdel, M.; Lee, J.N.; Paik, Y.-K.; Glossmann, H.; Utermann, G.; Moebius, F.F. Mutations in the Δ7-Sterol Reductase Gene in Patients with the Smith–Lemli–Opitz Syndrome. Proc. Natl. Acad. Sci. USA 1998, 95, 8181–8186. [Google Scholar] [CrossRef] [PubMed]
- Karaca, E.; Harel, T.; Pehlivan, D.; Jhangiani, S.N.; Gambin, T.; Coban Akdemir, Z.; Gonzaga-Jauregui, C.; Erdin, S.; Bayram, Y.; Campbell, I.M.; et al. Genes That Affect Brain Structure and Function Identified by Rare Variant Analyses of Mendelian Neurologic Disease. Neuron 2015, 88, 499–513. [Google Scholar] [CrossRef]
- Nowaczyk, M.J.M.; Tan, M.; Hamid, J.S.; Allanson, J.E. Smith-Lemli-Opitz Syndrome: Objective Assessment of Facial Phenotype. Am. J. Med. Genet. A 2012, 158A, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, C.; Kratz, L.E.; Moser, A.; Natowicz, M.R.; Kelley, R.I. Clinical and Biochemical Spectrum of Patients with RSH/Smith-Lemli-Opitz Syndrome and Abnormal Cholesterol Metabolism. Am. J. Med. Genet. 1997, 68, 263–269. [Google Scholar] [CrossRef]
- Ryan, A.K.; Bartlett, K.; Clayton, P.; Eaton, S.; Mills, L.; Donnai, D.; Winter, R.M.; Burn, J. Smith-Lemli-Opitz Syndrome: A Variable Clinical and Biochemical Phenotype. J. Med. Genet. 1998, 35, 558–565. [Google Scholar] [CrossRef]
- Yu, H.; Lee, M.H.; Starck, L.; Elias, E.R.; Irons, M.; Salen, G.; Patel, S.B.; Tint, G.S. Spectrum of Delta(7)-Dehydrocholesterol Reductase Mutations in Patients with the Smith-Lemli-Opitz (RSH) Syndrome. Hum. Mol. Genet. 2000, 9, 1385–1391. [Google Scholar] [CrossRef]
- Balder, J.W.; Lansberg, P.J.; Hof, M.H.; Wiegman, A.; Hutten, B.A.; Kuivenhoven, J.A. Pediatric Lipid Reference Values in the General Population: The Dutch Lifelines Cohort Study. J. Clin. Lipidol. 2018, 12, 1208–1216. [Google Scholar] [CrossRef]
- Chandar, V.; Gidvani, C.H.; Gupta, A.K.; Wilson, C.G.; Sharma, Y.V. Lipid Profile in Normal Healthy Children. Med. J. Armed Forces India 1994, 50, 101–104. [Google Scholar] [CrossRef]
- Donoghue, S.E.; Pitt, J.J.; Boneh, A.; White, S.M. Smith-Lemli-Opitz Syndrome: Clinical and Biochemical Correlates. J. Pediatr. Endocrinol. Metab. JPEM 2018, 31, 451–459. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Oh, J.; Lim, T.H.; Kang, H.; Ko, B.S.; Cho, Y.; The Korean Cardiac Arrest Research Consortium KoCARC Investigators. Association between Initial Serum Cholesterol Levels and Outcomes of Patients Hospitalized after Out-of-Hospital Cardiac Arrest: A Retrospective Multicenter Registry Study. J. Pers. Med. 2022, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; Hamilton, M.A.; Maclellan, W.R.; Fonarow, G.C. Low Serum Total Cholesterol Is Associated with Marked Increase in Mortality in Advanced Heart Failure. J. Card. Fail. 2002, 8, 216–224. [Google Scholar] [CrossRef]
- Selvaraman, A.; Rahhal, S.; Bianconi, S.; Furnary, T.; Porter, F.D. Assessing Postnatal Mortality in Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. A 2025, 197, e63875. [Google Scholar] [CrossRef]
- Jurevics, H.; Morell, P. Cholesterol for Synthesis of Myelin Is Made Locally, Not Imported into Brain. J. Neurochem. 1995, 64, 895–901. [Google Scholar] [CrossRef]
- Goritz, C.; Mauch, D.H.; Pfrieger, F.W. Multiple Mechanisms Mediate Cholesterol-Induced Synaptogenesis in a CNS Neuron. Mol. Cell. Neurosci. 2005, 29, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Anderson, A.; Allen, L.B.; Korade, Z.; Mirnics, K. Cholesterol Biosynthesis and Uptake in Developing Neurons. ACS Chem. Neurosci. 2019, 10, 3671–3681. [Google Scholar] [CrossRef]
- Valenza, M.; Chen, J.Y.; Di Paolo, E.; Ruozi, B.; Belletti, D.; Ferrari Bardile, C.; Leoni, V.; Caccia, C.; Brilli, E.; Di Donato, S.; et al. Cholesterol-loaded Nanoparticles Ameliorate Synaptic and Cognitive Function in H Untington’s Disease Mice. EMBO Mol. Med. 2015, 7, 1547–1564. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Ferriero, D.M.; Jiang, X. Cholesterol in Brain Development and Perinatal Brain Injury: More than a Building Block. Curr. Neuropharmacol. 2022, 20, 1400–1412. [Google Scholar] [CrossRef]
- Pang, K.; Liu, C.; Tong, J.; Ouyang, W.; Hu, S.; Tang, Y. Higher Total Cholesterol Concentration May Be Associated with Better Cognitive Performance among Elderly Females. Nutrients 2022, 14, 4198. [Google Scholar] [CrossRef]
- Mistry, H.; Richardson, C.D.; Higginbottom, A.; Ashford, B.; Ahamed, S.U.; Moore, Z.; Matthews, F.E.; Brayne, C.; Simpson, J.E.; Wharton, S.B. Relationships of Brain Cholesterol and Cholesterol Biosynthetic Enzymes to Alzheimer’s Pathology and Dementia in the CFAS Population-Derived Neuropathology Cohort. Neurosci. Res. 2024, 204, 22–33. [Google Scholar] [CrossRef]
- Pörn, M.I.; Tenhunen, J.; Slotte, J.P. Increased Steroid Hormone Secretion in Mouse Leydig Tumor Cells after Induction of Cholesterol Translocation by Sphingomyelin Degradation. Biochim. Biophys. Acta 1991, 1093, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Harding, S.V.; Thandapilly, S.J.; Tosh, S.M.; Jones, P.J.H.; Ames, N.P. Barley β-Glucan Reduces Blood Cholesterol Levels via Interrupting Bile Acid Metabolism. Br. J. Nutr. 2017, 118, 822–829. [Google Scholar] [CrossRef]
- Chatelaine, H.; Dey, P.; Mo, X.; Mah, E.; Bruno, R.S.; Kopec, R.E. Vitamin A and D Absorption in Adults with Metabolic Syndrome versus Healthy Controls: A Pilot Study Utilizing Targeted and Untargeted LC-MS Lipidomics. Mol. Nutr. Food Res. 2021, 65, e2000413. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, B.; Holzach, P. Sub-Achilles bursitis--a biomechanical analysis and clinical study. Z. Orthop. Ihre Grenzgeb. 1992, 130, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp Genes Are Coexpressed at Many Diverse Sites of Cell-Cell Interaction in the Mouse Embryo. Dev. Biol. 1995, 172, 126–138. [Google Scholar] [CrossRef]
- Xu, S.; Tang, C. Cholesterol and Hedgehog Signaling: Mutual Regulation and Beyond. Front. Cell Dev. Biol. 2022, 10, 774291. [Google Scholar] [CrossRef]
- Koide, T.; Hayata, T.; Cho, K.W.Y. Negative Regulation of Hedgehog Signaling by the Cholesterogenic Enzyme 7-Dehydrocholesterol Reductase. Dev. Camb. Engl. 2006, 133, 2395–2405. [Google Scholar] [CrossRef]
- Honda, A.; Salen, G.; Shefer, S.; Batta, A.K.; Honda, M.; Xu, G.; Tint, G.S.; Matsuzaki, Y.; Shoda, J.; Tanaka, N. Bile Acid Synthesis in the Smith-Lemli-Opitz Syndrome: Effects of Dehydrocholesterols on Cholesterol 7alpha-Hydroxylase and 27-Hydroxylase Activities in Rat Liver. J. Lipid Res. 1999, 40, 1520–1528. [Google Scholar] [CrossRef]
- Bianconi, S.E.; Conley, S.K.; Keil, M.F.; Sinaii, N.; Rother, K.I.; Porter, F.D.; Stratakis, C.A. Adrenal Function in Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. A 2011, 155A, 2732–2738. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A. Feedback Regulation of Cholesterol Synthesis: Sterol-Accelerated Ubiquitination and Degradation of HMG CoA Reductase. Cell Res. 2008, 18, 609–621. [Google Scholar] [CrossRef]
- Shimano, H. Sterol Regulatory Element-Binding Proteins (SREBPs): Transcriptional Regulators of Lipid Synthetic Genes. Prog. Lipid Res. 2001, 40, 439–452. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Sharpe, L.J.; Brown, A.J. The Sterol-Based Transcriptional Control of Human 7-Dehydrocholesterol Reductase (DHCR7): Evidence of a Cooperative Regulatory Program in Cholesterol Synthesis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2014, 1841, 1431–1439. [Google Scholar] [CrossRef]
- Paik, Y.K.; Billheimer, J.T.; Magolda, R.L.; Gaylor, J.L. Microsomal Enzymes of Cholesterol Biosynthesis from Lanosterol. Solubilization and Purification of Steroid 8-Isomerase. J. Biol. Chem. 1986, 261, 6470–6477. [Google Scholar] [CrossRef]
- Elias, E.R.; Orth, L.E.; Li, A.; Xu, L.; Jones, S.M.; Rizzo, W.B. Cholic Acid Increases Plasma Cholesterol in Smith-Lemli-Opitz Syndrome: A Pilot Study. Mol. Genet. Metab. Rep. 2024, 38, 101030. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Korade, Z.; Rosado, D.A.; Mirnics, K.; Porter, N.A. Metabolism of Oxysterols Derived from Nonenzymatic Oxidation of 7-Dehydrocholesterol in Cells. J. Lipid Res. 2013, 54, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Korade, Z.; Porter, N.A. Oxysterols from Free Radical Chain Oxidation of 7-Dehydrocholesterol: Product and Mechanistic Studies. J. Am. Chem. Soc. 2010, 132, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, W.; Sheflin, L.G.; Fliesler, S.J.; Porter, N.A. Novel Oxysterols Observed in Tissues and Fluids of AY9944-Treated Rats: A Model for Smith-Lemli-Opitz Syndrome. J. Lipid Res. 2011, 52, 1810–1820. [Google Scholar] [CrossRef]
- Xu, L.; Korade, Z.; Rosado, J.D.A.; Liu, W.; Lamberson, C.R.; Porter, N.A. An Oxysterol Biomarker for 7-Dehydrocholesterol Oxidation in Cell/Mouse Models for Smith-Lemli-Opitz Syndrome. J. Lipid Res. 2011, 52, 1222–1233. [Google Scholar] [CrossRef]
- Xu, L.; Sheflin, L.G.; Porter, N.A.; Fliesler, S.J. 7-Dehydrocholesterol-Derived Oxysterols and Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome. Biochim. Biophys. Acta 2012, 1821, 877–883. [Google Scholar] [CrossRef]
- Shinkyo, R.; Xu, L.; Tallman, K.A.; Cheng, Q.; Porter, N.A.; Guengerich, F.P. Conversion of 7-Dehydrocholesterol to 7-Ketocholesterol Is Catalyzed by Human Cytochrome P450 7A1 and Occurs by Direct Oxidation without an Epoxide Intermediate. J. Biol. Chem. 2011, 286, 33021–33028. [Google Scholar] [CrossRef]
- Huang, S.S.; Liu, I.-H.; Chen, C.-L.; Chang, J.-M.; Johnson, F.E.; Huang, J.S. 7-Dehydrocholesterol (7-DHC), But Not Cholesterol, Causes Suppression of Canonical TGF-β Signaling and Is Likely Involved in the Development of Atherosclerotic Cardiovascular Disease (ASCVD). J. Cell. Biochem. 2017, 118, 1387–1400. [Google Scholar] [CrossRef]
- Movassaghi, M.; Bianconi, S.; Feinn, R.; Wassif, C.A.; Porter, F.D. Vitamin D Levels in Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. A 2017, 173, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Fitzky, B.U.; Moebius, F.F.; Asaoka, H.; Waage-Baudet, H.; Xu, L.; Xu, G.; Maeda, N.; Kluckman, K.; Hiller, S.; Yu, H.; et al. 7-Dehydrocholesterol-Dependent Proteolysis of HMG-CoA Reductase Suppresses Sterol Biosynthesis in a Mouse Model of Smith-Lemli-Opitz/RSH Syndrome. J. Clin. Investig. 2001, 108, 905–915. [Google Scholar] [CrossRef]
- Song, J.; Wang, D.; Chen, H.; Huang, X.; Zhong, Y.; Jiang, N.; Chen, C.; Xia, M. Association of Plasma 7-Ketocholesterol With Cardiovascular Outcomes and Total Mortality in Patients With Coronary Artery Disease. Circ. Res. 2017, 120, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.; Wolf, C.; Chevy, F.; Benachi, A.; Dumez, Y.; Munnich, A.; Cormier-Daire, V. Antenatal Manifestations of Smith-Lemli-Opitz (RSH) Syndrome: A Retrospective Survey of 30 Cases. Am. J. Med. Genet. A 2004, 124A, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Różdżyńska-Świątkowska, A.; Ciara, E.; Halat-Wolska, P.; Krajewska-Walasek, M.; Jezela-Stanek, A. Anthropometric Characteristics of 65 Polish Smith-Lemli-Opitz Patients. J. Appl. Genet. 2021, 62, 469–475. [Google Scholar] [CrossRef]
- Gabor, K.; Mesev, E.V.; Madenspacher, J.; Meacham, J.; Rai, P.; Moon, S.; Wassif, C.A.; Shaikh, S.R.; Tucker, C.J.; Karmaus, P.; et al. Sterol Biosynthesis Regulates TLR Signaling and the Innate Immune Response in a Smith-Lemli-Opitz Syndrome Model. J. Clin. Investig. 2024, 134, e167633. [Google Scholar] [CrossRef]
- Schoner, K.; Witsch-Baumgartner, M.; Behunova, J.; Petrovic, R.; Bald, R.; Kircher, S.G.; Ramaswamy, A.; Kluge, B.; Meyer-Wittkopf, M.; Schmitz, R.; et al. Smith-Lemli-Opitz Syndrome—Fetal Phenotypes with Special Reference to the Syndrome-specific Internal Malformation Pattern. Birth Defects Res. 2020, 112, 175–185. [Google Scholar] [CrossRef]
- Eren, E.E.; Bilgin, N.; Urganci, N.; Kose, G. A Case of Smith-Lemli-Opitz Syndrome Diagnosed with Hypertrophic Pyloric Stenosis. Sisli Etfal Hastan. Tip Bul. 2021, 55, 268–271. [Google Scholar] [CrossRef]
- The Lancet Neurology. Rare Diseases: Maintaining Momentum. Lancet Neurol. 2022, 21, 203. [Google Scholar] [CrossRef]
- Thurm, A.; Tierney, E.; Farmer, C.; Albert, P.; Joseph, L.; Swedo, S.; Bianconi, S.; Bukelis, I.; Wheeler, C.; Sarphare, G.; et al. Development, Behavior, and Biomarker Characterization of Smith-Lemli-Opitz Syndrome: An Update. J. Neurodev. Disord. 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Sikora, D.M.; Pettit-Kekel, K.; Penfield, J.; Merkens, L.S.; Steiner, R.D. The near Universal Presence of Autism Spectrum Disorders in Children with Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. A 2006, 140, 1511–1518. [Google Scholar] [CrossRef]
- Kaub, P.A.; Sharp, P.C.; Ranieri, E.; Fletcher, J.M. Isolated Autism Is Not an Indication for Smith-Lemli-Opitz Syndrome Biochemical Testing. J. Paediatr. Child Health 2022, 58, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Lalovic, A.; Merkens, L.; Russell, L.; Arsenault-Lapierre, G.; Nowaczyk, M.J.M.; Porter, F.D.; Steiner, R.D.; Turecki, G. Cholesterol Metabolism and Suicidality in Smith-Lemli-Opitz Syndrome Carriers. Am. J. Psychiatry 2004, 161, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- Cenik, B.; Palka, J.M.; Thompson, B.M.; McDonald, J.G.; Tamminga, C.A.; Cenik, C.; Brown, E.S. Desmosterol and 7-Dehydrocholesterol Concentrations in Post Mortem Brains of Depressed People: The Role of Trazodone. Transl. Psychiatry 2022, 12, 139. [Google Scholar] [CrossRef]
- Zalewski, C.K.; Sydlowski, S.A.; King, K.A.; Bianconi, S.; Dang Do, A.; Porter, F.D.; Brewer, C.C. Auditory Phenotype of Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. A 2021, 185, 1131–1141. [Google Scholar] [CrossRef]
- Miyazaki, S.; Shimizu, N.; Miyahara, H.; Teranishi, H.; Umeda, R.; Yano, S.; Shimada, T.; Shiraishi, H.; Komiya, K.; Katoh, A.; et al. DHCR7 Links Cholesterol Synthesis with Neuronal Development and Axonal Integrity. Biochem. Biophys. Res. Commun. 2024, 712–713, 149932. [Google Scholar] [CrossRef]
- Seger, R.; Ahn, N.G.; Posada, J.; Munar, E.S.; Jensen, A.M.; Cooper, J.A.; Cobb, M.H.; Krebs, E.G. Purification and Characterization of Mitogen-Activated Protein Kinase Activator(s) from Epidermal Growth Factor-Stimulated A431 Cells. J. Biol. Chem. 1992, 267, 14373–14381. [Google Scholar] [CrossRef]
- Yuan, J.; Ng, W.H.; Tian, Z.; Yap, J.; Baccarini, M.; Chen, Z.; Hu, J. Activating Mutations in MEK1 Enhance Homodimerization and Promote Tumorigenesis. Sci. Signal. 2018, 11, eaar6795. [Google Scholar] [CrossRef]
- Tomita, H.; Hines, K.M.; Herron, J.M.; Li, A.; Baggett, D.W.; Xu, L. 7-Dehydrocholesterol-Derived Oxysterols Cause Neurogenic Defects in Smith-Lemli-Opitz Syndrome. eLife 2022, 11, e67141. [Google Scholar] [CrossRef]
- Murphy, A.R.; Haynes, J.M.; Laslett, A.L.; Cameron, N.R.; O’Brien, C.M. Three-Dimensional Differentiation of Human Pluripotent Stem Cell-Derived Neural Precursor Cells Using Tailored Porous Polymer Scaffolds. Acta Biomater. 2020, 101, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Dreser, N.; Madjar, K.; Holzer, A.-K.; Kapitza, M.; Scholz, C.; Kranaster, P.; Gutbier, S.; Klima, S.; Kolb, D.; Dietz, C.; et al. Development of a Neural Rosette Formation Assay (RoFA) to Identify Neurodevelopmental Toxicants and to Characterize Their Transcriptome Disturbances. Arch. Toxicol. 2020, 94, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, H.; Guan, X.; Fiesler, V.; Bhuiyan, M.I.H.; Liu, R.; Jalali, S.; Hasan, M.N.; Tai, A.K.; Chattopadhyay, A.; et al. Activation of Endothelial Wnt/β-Catenin Signaling by Protective Astrocytes Repairs BBB Damage in Ischemic Stroke. Prog. Neurobiol. 2021, 199, 101963. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Jha, B. Imaging Findings of Congenital Glaucoma in Opitz Syndrome. AJNR Am. J. Neuroradiol. 2008, 29, 1003–1005. [Google Scholar] [CrossRef][Green Version]
- Goodwin, H.; Brooks, B.P.; Porter, F.D. Acute Postnatal Cataract Formation in Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. A 2008, 146A, 208–211. [Google Scholar] [CrossRef]
- Rossin, E.J.; Tsui, I.; Wong, S.C.; Hou, K.K.; Prakhunhungsit, S.; Blair, M.P.; Shapiro, M.J.; Leishman, L.; Nagiel, A.; Lifton, J.A.; et al. Traumatic Retinal Detachment in Patients with Self-Injurious Behavior: An International Multicenter Study. Ophthalmol. Retina 2021, 5, 805–814. [Google Scholar] [CrossRef] [PubMed]
- López-Cañizares, A.; Al-Khersan, H.; Fernandez, M.P.; Lin, B.R.; Goduni, L.; Berrocal, A.M. Smith-Lemli-Optiz Syndrome: Importance of Ophthalmology Referral and Follow-Up. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2023, 27, 100–102. [Google Scholar] [CrossRef]
- Pfeffer, B.A.; Xu, L.; Porter, N.A.; Rao, S.R.; Fliesler, S.J. Differential Cytotoxic Effects of 7-Dehydrocholesterol-Derived Oxysterols on Cultured Retina-Derived Cells: Dependence on Sterol Structure, Cell Type, and Density. Exp. Eye Res. 2016, 145, 297–316. [Google Scholar] [CrossRef]
- Pfeffer, B.A.; Xu, L.; Fliesler, S.J. Transcriptomic Changes Associated with Loss of Cell Viability Induced by Oxysterol Treatment of a Retinal Photoreceptor-Derived Cell Line: An In Vitro Model of Smith-Lemli-Opitz Syndrome. Int. J. Mol. Sci. 2021, 22, 2339. [Google Scholar] [CrossRef]
- Farkas, M.H.; Skelton, L.A.; Ramachandra-Rao, S.; Au, E.; Fliesler, S.J. Morphological, Biochemical, and Transcriptomic Characterization of iPSC-Derived Human RPE Cells from Normal and Smith-Lemli-Opitz Syndrome Patients. Mol. Vis. 2022, 28, 394–411. [Google Scholar]
- Sharma, A.; Kumar, G.A.; Chattopadhyay, A. Late Endosomal/Lysosomal Accumulation of a Neurotransmitter Receptor in a Cellular Model of Smith-Lemli-Opitz Syndrome. Traffic 2021, 22, 332–344. [Google Scholar] [CrossRef]
- Navyasree, K.V.; Ramesh, S.T.; Umasankar, P.K. Cholesterol Regulates Insulin-Induced mTORC1 Signaling. J. Cell Sci. 2023, 136, jcs261402. [Google Scholar] [CrossRef]
- Genoux, A.; Pons, V.; Radojkovic, C.; Roux-Dalvai, F.; Combes, G.; Rolland, C.; Malet, N.; Monsarrat, B.; Lopez, F.; Ruidavets, J.-B.; et al. Mitochondrial Inhibitory Factor 1 (IF1) Is Present in Human Serum and Is Positively Correlated with HDL-Cholesterol. PLoS ONE 2011, 6, e23949. [Google Scholar] [CrossRef] [PubMed]
- Pires Da Silva, J.; Wargny, M.; Raffin, J.; Croyal, M.; Duparc, T.; Combes, G.; Genoux, A.; Perret, B.; Vellas, B.; Guyonnet, S.; et al. Plasma Level of ATPase Inhibitory Factor 1 (IF1) Is Associated with Type 2 Diabetes Risk in Humans: A Prospective Cohort Study. Diabetes Metab. 2023, 49, 101391. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; Rapone, B.; Simonetti, S.; Fecarotta, S.; De Carlo, G.; Favoino, E.; Loverro, M.T.; Romano, A.M.I.; Taurino, F.; Di Naro, E.; et al. Dietary Cholesterol Supplementation and Inhibitory Factor 1 Serum Levels in Two Dizygotic Smith-Lemli-Opitz Syndrome Twins: A Case Report. Ital. J. Pediatr. 2020, 46, 161. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Chu, V.; Zhao, J.; Gohs, F.; Thornhill, M.H.; Pihlstrom, B.; Mougeot, F.B.; Rose, G.A.; Sun, Y.-P.; Napenas, J.; et al. Oral Hygiene and Infective Endocarditis: A Case Control Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, 333–342. [Google Scholar] [CrossRef]
- Piekoszewska-Ziętek, P.; Witt-Porczyk, A.; Turska-Szybka, A.; Olczak-Kowalczyk, D. Hygienic Behaviors and Use of Dental Care in Patients with Genetic Syndromes. Sci. Rep. 2024, 14, 30756. [Google Scholar] [CrossRef]
- Kelley, R.I. Diagnosis of Smith-Lemli-Opitz Syndrome by Gas Chromatography/Mass Spectrometry of 7-Dehydrocholesterol in Plasma, Amniotic Fluid and Cultured Skin Fibroblasts. Clin. Chim. Acta Int. J. Clin. Chem. 1995, 236, 45–58. [Google Scholar] [CrossRef]
- Honda, A.; Batta, A.K.; Salen, G.; Tint, G.S.; Chen, T.S.; Shefer, S. Screening for Abnormal Cholesterol Biosynthesis in the Smith-Lemli-Opitz Syndrome: Rapid Determination of Plasma 7-Dehydrocholesterol by Ultraviolet Spectrometry. Am. J. Med. Genet. 1997, 68, 288–293. [Google Scholar] [CrossRef]
- Zimmerman, P.A.; Hercules, D.M.; Naylor, E.W. Direct Analysis of Filter Paper Blood Specimens for Identification of Smith-Lemli-Opitz Syndrome Using Time-of-Flight Secondary Ion Mass Spectrometry. Am. J. Med. Genet. 1997, 68, 300–304. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Z.; Zeng, Y.; Zhang, Y.; Luan, Y.; Ma, L.; Chen, L.; Zou, L.; Yang, J.; Huang, Z.; et al. A Reliable Tool for Detecting 7-Dehydrocholesterol and Cholesterol in Human Plasma and Its Use in Diagnosis of Smith-Lemli-Opitz Syndrome. J. Sep. Sci. 2022, 45, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Westbye, A.B.; Dizdarevic, L.L.; Dahl, S.R.; Asprusten, E.A.; Bliksrud, Y.T.; Sandblom, A.L.; Diczfalusy, U.; Thorsby, P.M.; Retterstøl, K. A Sterol Panel for Rare Lipid Disorders: Sitosterolemia, Cerebrotendinous Xanthomatosis and Smith-Lemli-Opitz Syndrome. J. Lipid Res. 2025, 66, 100698. [Google Scholar] [CrossRef]
- Becker, S.; Röhnike, S.; Empting, S.; Haas, D.; Mohnike, K.; Beblo, S.; Mütze, U.; Husain, R.A.; Thiery, J.; Ceglarek, U. LC-MS/MS-Based Quantification of Cholesterol and Related Metabolites in Dried Blood for the Screening of Inborn Errors of Sterol Metabolism. Anal. Bioanal. Chem. 2015, 407, 5227–5233. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, X.; Wang, A.; Yang, J.; Zheng, Y.; Dong, H.; Tian, Y.; Zhang, Z.; Wang, M.; Song, R. LC-MS/MS Profiling of Colon Oxysterols and Cholesterol Precursors in Mouse Model of Ulcerative Colitis. J. Chromatogr. A 2024, 1722, 464865. [Google Scholar] [CrossRef] [PubMed]
- Roumain, M.; Muccioli, G.G. Development and Application of an LC-MS/MS Method for the Combined Quantification of Oxysterols and Bile Acids. J. Lipid Res. 2025, 66, 100697. [Google Scholar] [CrossRef]
- Yasuda, Y.; Tateishi, N.; Shimoda, T.; Satoh, S.; Ogitani, E.; Fujita, S. Relationship between S100beta and GFAP Expression in Astrocytes during Infarction and Glial Scar Formation after Mild Transient Ischemia. Brain Res. 2004, 1021, 20–31. [Google Scholar] [CrossRef]
- Esgul, N.; Orhan Varoglu, A.; Baysal, B. Association of Gray and White Matter Volumes, Clinical Features, Neurofilament Light Chain, and Glial Fibrillary Acidic Protein in Relapsing-Remitting Multiple Sclerosis. Acta Radiol. 2025, 66, 470–476. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an Emerging Biomarker in Brain and Spinal Cord Disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Biberthaler, P.; Musaelyan, K.; Krieg, S.; Meyer, B.; Stimmer, H.; Zapf, J.; Von Matthey, F.; Chandran, R.; Marino, J.A.; Beligere, G.; et al. Evaluation of Acute Glial Fibrillary Acidic Protein and Ubiquitin C-Terminal Hydrolase-L1 Plasma Levels in Traumatic Brain Injury Patients with and without Intracranial Lesions. Neurotrauma Rep. 2021, 2, 617–625. [Google Scholar] [CrossRef]
- Fukuyama, R.; Izumoto, T.; Fushiki, S. The Cerebrospinal Fluid Level of Glial Fibrillary Acidic Protein Is Increased in Cerebrospinal Fluid from Alzheimer’s Disease Patients and Correlates with Severity of Dementia. Eur. Neurol. 2001, 46, 35–38. [Google Scholar] [CrossRef]
- Zhu, N.; Santos-Santos, M.; Illán-Gala, I.; Montal, V.; Estellés, T.; Barroeta, I.; Altuna, M.; Arranz, J.; Muñoz, L.; Belbin, O.; et al. Plasma Glial Fibrillary Acidic Protein and Neurofilament Light Chain for the Diagnostic and Prognostic Evaluation of Frontotemporal Dementia. Transl. Neurodegener. 2021, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Norgren, N.; Sundström, P.; Svenningsson, A.; Rosengren, L.; Stigbrand, T.; Gunnarsson, M. Neurofilament and Glial Fibrillary Acidic Protein in Multiple Sclerosis. Neurology 2004, 63, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Freel, B.A.; Kelvington, B.A.; Sengupta, S.; Mukherjee, M.; Francis, K.R. Sterol Dysregulation in Smith-Lemli-Opitz Syndrome Causes Astrocyte Immune Reactivity through Microglia Crosstalk. Dis. Model. Mech. 2022, 15, dmm049843. [Google Scholar] [CrossRef] [PubMed]
- Tcw, J.; Qian, L.; Pipalia, N.H.; Chao, M.J.; Liang, S.A.; Shi, Y.; Jain, B.R.; Bertelsen, S.E.; Kapoor, M.; Marcora, E.; et al. Cholesterol and Matrisome Pathways Dysregulated in Astrocytes and Microglia. Cell 2022, 185, 2213–2233.e25. [Google Scholar] [CrossRef]
- Luke, R.A.; Cawley, N.X.; Rahhal, S.; Selvaraman, A.; Thurm, A.; Wassif, C.A.; Porter, F.D. Elevated Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels in Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab. 2024, 143, 108570. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M.; Bezukladova, S.; Matilainen, M.; Sucksdorff, M.; Kuhle, J.; Leppert, D.; Airas, L. Increased Serum Glial Fibrillary Acidic Protein Associates with Microstructural White Matter Damage in Multiple Sclerosis: GFAP and DTI. Mult. Scler. Relat. Disord. 2021, 50, 102810. [Google Scholar] [CrossRef]
- Aladia, A.H.; Hamdan, S.; Alkheder, A. First Documented Case of Smith-Lemli-Opitz Syndrome in Syria: Clinical Presentation, Diagnosis, and Experimental Management with Simvastatin. Oxf. Med. Case Rep. 2024, 2024, omae129. [Google Scholar] [CrossRef]
- Lee, R.W.Y.; Conley, S.K.; Gropman, A.; Porter, F.D.; Baker, E.H. Brain Magnetic Resonance Imaging Findings in Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. A 2013, 161A, 2407–2419. [Google Scholar] [CrossRef]
- Li, A.; Xu, L. MALDI-IM-MS Imaging of Brain Sterols and Lipids in a Mouse Model of Smith-Lemli-Opitz Syndrome. BioRxiv Prepr. Serv. Biol. 2023. [Google Scholar] [CrossRef]
- Haas, D.; Haege, G.; Hoffmann, G.F.; Burgard, P. Prenatal Presentation and Diagnostic Evaluation of Suspected Smith-Lemli-Opitz (RSH) Syndrome. Am. J. Med. Genet. A 2013, 161A, 1008–1011. [Google Scholar] [CrossRef]
- Waye, J.S.; Eng, B.; Nowaczyk, M.J.M. Prenatal Diagnosis of Smith-Lemli-Opitz Syndrome (SLOS) by DHCR7 Mutation Analysis. Prenat. Diagn. 2007, 27, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Mikaelian, I.; Gonzalo, P. Amniotic Fluid Glial Fibrillary Acidic Protein (AF-GFAP), a Biomarker of Open Neural Tube Defects. Prenat. Diagn. 2013, 33, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, C.H.L.; Marcos, J.; Palomaki, G.E.; Craig, W.Y.; Kelley, R.I.; Kratz, L.E.; Haddow, J.E. Dehydrosteroid Measurements in Maternal Urine or Serum for the Prenatal Diagnosis of Smith-Lemli-Opitz Syndrome (SLOS). Am. J. Med. Genet. A 2007, 143A, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Rohanizadegan, M.; Sacharow, S. Desmosterolosis Presenting with Multiple Congenital Anomalies. Eur. J. Med. Genet. 2018, 61, 152–156. [Google Scholar] [CrossRef]
- Kroes, I.; Janssens, S.; Defoort, P. Ultrasound Features in Trisomy 13 (Patau Syndrome) and Trisomy 18 (Edwards Syndrome) in a Consecutive Series of 47 Cases. Facts Views Vis. ObGyn 2014, 6, 245–249. [Google Scholar]
- Luo, Y.; Zhang, C.; Ma, L.; Zhang, Y.; Liu, Z.; Chen, L.; Wang, R.; Luan, Y.; Rao, Y. Measurement of 7-Dehydrocholesterol and Cholesterol in Hair Can Be Used in the Diagnosis of Smith-Lemli-Opitz Syndrome. J. Lipid Res. 2022, 63, 100228. [Google Scholar] [CrossRef]
- Pappu, A.S.; Steiner, R.D.; Connor, S.L.; Flavell, D.P.; Lin, D.S.; Hatcher, L.; Illingworth, D.R.; Connor, W.E. Feedback Inhibition of the Cholesterol Biosynthetic Pathway in Patients with Smith-Lemli-Opitz Syndrome as Demonstrated by Urinary Mevalonate Excretion. J. Lipid Res. 2002, 43, 1661–1669. [Google Scholar] [CrossRef]
- Wassif, C.A.; Kratz, L.; Sparks, S.E.; Wheeler, C.; Bianconi, S.; Gropman, A.; Calis, K.A.; Kelley, R.I.; Tierney, E.; Porter, F.D. A Placebo-Controlled Trial of Simvastatin Therapy in Smith-Lemli-Opitz Syndrome. Genet. Med. 2017, 19, 297–305. [Google Scholar] [CrossRef]
- Björkhem, I. Crossing the Barrier: Oxysterols as Cholesterol Transporters and Metabolic Modulators in the Brain. J. Intern. Med. 2006, 260, 493–508. [Google Scholar] [CrossRef]
- Gosselet, F.; Saint-Pol, J.; Fenart, L. Effects of Oxysterols on the Blood-Brain Barrier: Implications for Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2014, 446, 687–691. [Google Scholar] [CrossRef]
- Gao, Y.; Ye, S.; Tang, Y.; Tong, W.; Sun, S. Brain Cholesterol Homeostasis and Its Association with Neurodegenerative Diseases. Neurochem. Int. 2023, 171, 105635. [Google Scholar] [CrossRef] [PubMed]
- Sikora, D.M.; Ruggiero, M.; Petit-Kekel, K.; Merkens, L.S.; Connor, W.E.; Steiner, R.D. Cholesterol Supplementation Does Not Improve Developmental Progress in Smith-Lemli-Opitz Syndrome. J. Pediatr. 2004, 144, 783–791. [Google Scholar] [CrossRef]
- Maciejak, A.; Leszczynska, A.; Warchol, I.; Gora, M.; Kaminska, J.; Plochocka, D.; Wysocka-Kapcinska, M.; Tulacz, D.; Siedlecka, J.; Swiezewska, E.; et al. The Effects of Statins on the Mevalonic Acid Pathway in Recombinant Yeast Strains Expressing Human HMG-CoA Reductase. BMC Biotechnol. 2013, 13, 68. [Google Scholar] [CrossRef]
- Ballout, R.A.; Livinski, A.; Fu, Y.-P.; Steiner, R.D.; Remaley, A.T. Statins for Smith-Lemli-Opitz Syndrome. Cochrane Database Syst. Rev. 2022, 11, CD013521. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, E.; Matarazzo, L.; Franchi-Abella, S.; Dabadie, A.; Cohen, J.; Habes, D.; Hillaire, S.; Guettier, C.; Taburet, A.-M.; Myara, A.; et al. Cholic Acid for Primary Bile Acid Synthesis Defects: A Life-Saving Therapy Allowing a Favorable Outcome in Adulthood. Orphanet J. Rare Dis. 2018, 13, 190. [Google Scholar] [CrossRef]
- Korade, Z.; Xu, L.; Harrison, F.E.; Ahsen, R.; Hart, S.E.; Folkes, O.M.; Mirnics, K.; Porter, N.A. Antioxidant Supplementation Ameliorates Molecular Deficits in Smith-Lemli-Opitz Syndrome. Biol. Psychiatry 2014, 75, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Koczok, K.; Horváth, L.; Korade, Z.; Mezei, Z.A.; Szabó, G.P.; Porter, N.A.; Kovács, E.; Mirnics, K.; Balogh, I. Biochemical and Clinical Effects of Vitamin E Supplementation in Hungarian Smith-Lemli-Opitz Syndrome Patients. Biomolecules 2021, 11, 1228. [Google Scholar] [CrossRef]
- Ertugrul, G.; Yankol, Y.; Mecit, N.; Kirimlioglu, H.; Kanmaz, T.; Acarli, K.; Kalayoglu, M. Liver Transplant and Improvements in Cholesterol Biosynthesis Defects: A Case Report of Smith-Lemli-Opitz Syndrome. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2022, 20, 104–107. [Google Scholar] [CrossRef]
- Pasta, S.; Akhile, O.; Tabron, D.; Ting, F.; Shackleton, C.; Watson, G. Delivery of the 7-Dehydrocholesterol Reductase Gene to the Central Nervous System Using Adeno-Associated Virus Vector in a Mouse Model of Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab. Rep. 2015, 4, 92–98. [Google Scholar] [CrossRef]
- Phillips, C.; Parkinson, A.; Namsrai, T.; Chalmers, A.; Dews, C.; Gregory, D.; Kelly, E.; Lowe, C.; Desborough, J. Time to Diagnosis for a Rare Disease: Managing Medical Uncertainty. A Qualitative Study. Orphanet J. Rare Dis. 2024, 19, 297. [Google Scholar] [CrossRef]
- Fisch, G.S. Whither the Genotype-phenotype Relationship? An Historical and Methodological Appraisal. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 343–353. [Google Scholar] [CrossRef]
- Ciara, E.; Nowaczyk, M.; Witsch-Baumgartner, M.; Malunowicz, E.; Popowska, E.; Jezela-Stanek, A.; Piotrowicz, M.; Waye, J.; Utermann, G.; Krajewska-Walasek, M. DHCR7 Mutations and Genotype–Phenotype Correlation in 37 Polish Patients with Smith–Lemli–Opitz Syndrome. Clin. Genet. 2004, 66, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Santiago, E.; Jabato, F.M.; Rojano, E.; Seoane, P.; Pazos, F.; Perkins, J.R.; Ranea, J.A.G. Phenotype-Genotype Comorbidity Analysis of Patients with Rare Disorders Provides Insight into Their Pathological and Molecular Bases. PLoS Genet. 2020, 16, e1009054. [Google Scholar] [CrossRef]
- Trujillano, D.; Oprea, G.; Schmitz, Y.; Bertoli-Avella, A.M.; Abou Jamra, R.; Rolfs, A. A Comprehensive Global Genotype–Phenotype Database for Rare Diseases. Mol. Genet. Genom. Med. 2017, 5, 66–75. [Google Scholar] [CrossRef]
- Schmidt, A.; Danyel, M.; Grundmann, K.; Brunet, T.; Klinkhammer, H.; Hsieh, T.-C.; Engels, H.; Peters, S.; Knaus, A.; Moosa, S.; et al. Next-Generation Phenotyping Integrated in a National Framework for Patients with Ultrarare Disorders Improves Genetic Diagnostics and Yields New Molecular Findings. Nat. Genet. 2024, 56, 1644–1653. [Google Scholar] [CrossRef]
- Rillig, F.; Grüters, A.; Schramm, C.; Krude, H. The Interdisciplinary Diagnosis of Rare Diseases—Results of the Translate-NAMSE Project. Dtsch. Ärztebl. Int. 2022, 119, 469. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; De Rezende, V.L.; De Aguiar Da Costa, M.; De Oliveira, J.; Gonçalves, C.L. Cholesterol Metabolism Pathway in Autism Spectrum Disorder: From Animal Models to Clinical Observations. Pharmacol. Biochem. Behav. 2023, 223, 173522. [Google Scholar] [CrossRef]
- Abedalthagafi, M.; Bawazeer, S.; Fawaz, R.I.; Heritage, A.M.; Alajaji, N.M.; Faqeih, E. Non-Invasive Prenatal Testing: A Revolutionary Journey in Prenatal Testing. Front. Med. 2023, 10, 1265090. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.; Escobar, R.; Zarante, I. Craniofacial Anomalies Associated with Hypospadias. Description of a Hospital Based Population in South America. Int. Braz J Urol Off. J. Braz. Soc. Urol. 2016, 42, 793–797. [Google Scholar] [CrossRef]
- Joss, S.K.; Paterson, W.; Donaldson, M.D.C.; Tolmie, J.L. Cleft Palate, Hypotelorism, and Hypospadias: Schilbach-Rott Syndrome. Am. J. Med. Genet. 2002, 113, 105–107. [Google Scholar] [CrossRef]
- Wadman, E.; Fernandes, E.; Muss, C.; Powell-Hamilton, N.; Wojcik, M.H.; Madden, J.A.; Carreon, C.K.; Clark, R.D.; Stenftenagel, A.; Chikalard, K.; et al. A Novel Syndrome Associated with Prenatal Fentanyl Exposure. Genet. Med. Open 2023, 1, 100834. [Google Scholar] [CrossRef] [PubMed]
- Elias, E.R.; Irons, M.B.; Hurley, A.D.; Tint, G.S.; Salen, G. Clinical Effects of Cholesterol Supplementation in Six Patients with the Smith-Lemli-Opitz Syndrome (SLOS). Am. J. Med. Genet. 1997, 68, 305–310. [Google Scholar] [CrossRef]
- Starck, L.; Lövgren-Sandblom, A.; Björkhem, I. Simvastatin Treatment in the SLO Syndrome: A Safe Approach? Am. J. Med. Genet. 2002, 113, 183–189. [Google Scholar] [CrossRef]
- Calvo, P.L.; Brunati, A.; Spada, M.; Romagnoli, R.; Corso, G.; Parenti, G.; Rossi, M.; Baldi, M.; Carbonaro, G.; David, E.; et al. Liver Transplantation in Defects of Cholesterol Biosynthesis: The Case of Lathosterolosis. Am. J. Transplant. 2014, 14, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.; Prütz, F. Rare Diseases: A Challenge for Medicine and Public Health. J. Health Monit. 2023, 8, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Depping, M.K.; Uhlenbusch, N.; von Kodolitsch, Y.; Klose, H.F.E.; Mautner, V.-F.; Löwe, B. Supportive Care Needs of Patients with Rare Chronic Diseases: Multi-Method, Cross-Sectional Study. Orphanet J. Rare Dis. 2021, 16, 44. [Google Scholar] [CrossRef]
- Gutenbrunner, C.; Schiller, J.; Goedecke, V.; Lemhoefer, C.; Boekel, A. Screening of Patient Impairments in an Outpatient Clinic for Suspected Rare Diseases-A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 8874. [Google Scholar] [CrossRef]
- Begic, N.; Begic, Z.; Begic, E. Smith-Lemli-Opitz Syndrome: Bosnian and Herzegovinian Experience. Balk. J. Med. Genet. BJMG 2021, 24, 99–102. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, K.; Chen, L. Molecular Biomarkers, Network Biomarkers, and Dynamic Network Biomarkers for Diagnosis and Prediction of Rare Diseases. Fundam. Res. 2022, 2, 894–902. [Google Scholar] [CrossRef]
- Bai, J.P.F.; Barrett, J.S.; Burckart, G.J.; Meibohm, B.; Sachs, H.C.; Yao, L. Strategic Biomarkers for Drug Development in Treating Rare Diseases and Diseases in Neonates and Infants. AAPS J. 2013, 15, 447–454. [Google Scholar] [CrossRef]
- Ayoglu, B.; Chaouch, A.; Lochmüller, H.; Politano, L.; Bertini, E.; Spitali, P.; Hiller, M.; Niks, E.H.; Gualandi, F.; Pontén, F.; et al. Affinity Proteomics within Rare Diseases: A BIO-NMD Study for Blood Biomarkers of Muscular Dystrophies. EMBO Mol. Med. 2014, 6, 918–936. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.P.; Wootton, S.H.; Tyson, J.E. N-of-1 Trials: The Epitome of Personalized Medicine? J. Clin. Transl. Sci. 2023, 7, e161. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Matabosch, X.; Serra, M.; Watson, B.; Shackleton, C.; Watson, G. Biochemical and Physiological Improvement in a Mouse Model of Smith–Lemli–Opitz Syndrome (SLOS) Following Gene Transfer with AAV Vectors. Mol. Genet. Metab. Rep. 2014, 1, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Horgan, C.; Watts, K.; Ram, D.; Rust, S.; Hutton, R.; Jones, S.; Wynn, R. A Retrospective Cohort Study of Libmeldy (Atidarsagene Autotemcel) for MLD: What We Have Accomplished and What Opportunities Lie Ahead. JIMD Rep. 2023, 64, 346–352. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żukowska, A.; Król, M.; Kupnicka, P.; Bąk, K.; Janawa, K.; Chlubek, D. Exploring Recent Developments in the Manifestation, Diagnosis, and Treatment of Patients with Smith–Lemli–Opitz Syndrome: From Molecular Pathways to Clinical Innovations. Int. J. Mol. Sci. 2025, 26, 6672. https://doi.org/10.3390/ijms26146672
Żukowska A, Król M, Kupnicka P, Bąk K, Janawa K, Chlubek D. Exploring Recent Developments in the Manifestation, Diagnosis, and Treatment of Patients with Smith–Lemli–Opitz Syndrome: From Molecular Pathways to Clinical Innovations. International Journal of Molecular Sciences. 2025; 26(14):6672. https://doi.org/10.3390/ijms26146672
Chicago/Turabian StyleŻukowska, Aleksandra, Małgorzata Król, Patrycja Kupnicka, Katarzyna Bąk, Kamil Janawa, and Dariusz Chlubek. 2025. "Exploring Recent Developments in the Manifestation, Diagnosis, and Treatment of Patients with Smith–Lemli–Opitz Syndrome: From Molecular Pathways to Clinical Innovations" International Journal of Molecular Sciences 26, no. 14: 6672. https://doi.org/10.3390/ijms26146672
APA StyleŻukowska, A., Król, M., Kupnicka, P., Bąk, K., Janawa, K., & Chlubek, D. (2025). Exploring Recent Developments in the Manifestation, Diagnosis, and Treatment of Patients with Smith–Lemli–Opitz Syndrome: From Molecular Pathways to Clinical Innovations. International Journal of Molecular Sciences, 26(14), 6672. https://doi.org/10.3390/ijms26146672






