Abstract
Retinoids are used in cosmetics as anti-aging ingredients, along with other substances. However, due to limitations in use (such as photodegradation), it seems necessary to look for retinoid alternatives to be applied in cosmetic products. Bakuchiol, a natural alternative of retinoids, isolated from Psolarea corylifolia, is one such compound. It has great cosmetic potential and its mechanism of action is not yet fully explored. From the point of view of the bioactive compound, it is also essential to develop a method for rapid quality control of cosmetic preparations containing bakuchiol. The aim of this study was to apply and compare methods for the quantification of bakuchiol in cosmetic products using UV-Vis, 1H qNMR, and HPLC. The results show the possibility of using the 1H NMR method in the routine quality control of cosmetics with bakuchiol because of its comparable results with HPLC analysis and significantly shorter analysis time.
1. Introduction
Aging is a natural and inevitable process which is largely influenced by genetic factors. Among other symptoms, it includes various changes in the skin. Skin aging results from intrinsic processes like telomerase activity as well as external factors such as UV radiation due to sun exposure and pollution, known as photo-aging [1]. Cosmeceuticals, bridging cosmetics and pharmaceuticals, target those factors to rejuvenate the skin, with vitamin A derivatives (retinoids) being particularly effective due to the activation of RAR (retinoic acid receptor) [2,3]. Retinoids, while beneficial for anti-aging, can cause adverse reactions like skin irritation and increased sensitivity to light.
Bakuchiol (C22H34O2, Figure 1) is believed to be an efficient agent in the prevention of skin aging as it exhibits antioxidant activity [4] and stimulates the expression of the same genes as natural retinoids [5]. Bakuchiol was first isolated from the seeds of Psolarea corylifolia (Fabaceae) in 1966 [6]. In the same year, its complete structural configuration was established [7]. Bakuchiol is an optically active substance and the naturally occurring stereoisomer is S-(+)-bakuchiol. In 1973, the first successful biochemical synthesis of bakuchiol was carried out [8]. A natural source from which bakuchiol can be obtained is the fruit of Psoralea corylifolia (syn. Cullen corylifolium) [9]. The concentration of bakuchiol in those fruits ranges from 1% to 7% by dry weight [10]. This compound has also been isolated from roots, seeds, dry leaves, and fruits of other plant species belonging to various families [11,12,13,14,15,16,17].
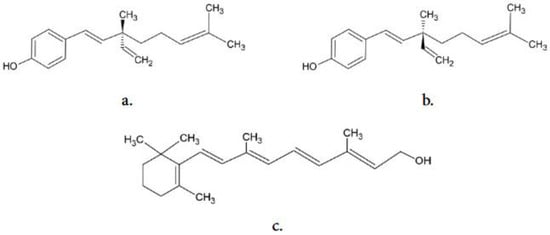
Figure 1.
The structure of: (a) (S)-bakuchiol, (b) (R)-bakuchiol, and (c) all-trans retinol.
The mechanism of action and all of the possible molecular targets for bakuchiol are yet to be studied [18]; however, based on the observed positive effects on the skin [19], it is frequently applied in products available on the market. Thus, methods of quality control are urgently needed. This is why, in our study, spectroscopic and chromatographic methods of qualitative and quantitative analysis were applied to preselect the most promising ones in terms of the simplicity, cost, and time of analysis.
2. Results
Six commercially available cosmetic formulations described as ‘face serum’ were obtained from the Polish market. The samples varied both in composition (the majority of them were oil solutions, two of them were emulsions) and in price (ranging from drug stores to ‘eco’ stores). The major ingredients are listed in Table 1.

Table 1.
Declared composition of the studied cosmetic products.
The chief aim of this research was to develop a method for the rapid and simple identification of the active ingredient bakuchiol in cosmetic products in a direct manner. The samples were obtained from drugstores. The selection criteria comprised the popularity ranking among consumers as well as the price of the product. It was assumed that the price of the product would depend on the quality of the active substance and the form of the preparation, and hence its effectiveness, as well as the packaging. The ranking encompassed both high-end and lower-priced preparations. Six cosmetic preparations (in the form of sera) were selected for analysis.
Bakuchiol is insoluble in water but soluble in alcohol, DMSO, plant oils, triglyceride, silicone oils, and a wide-range of hydrophobic emollients. Hence, an oil-in-water type emulsion can be implemented to increase the bioavailability of bakuchiol via the skin. This type of emulsion (containing water) was used in cosmetic samples 5 and 6. The other products were squalene/oil/oil mixture solutions.
2.1. UV-Vis Analysis
All the examined samples and the standard were subjected to UV analysis in ethanol.
The shape of the spectra in samples 1, 3, and 4 was similar to the standard (Figure 2a and Figure S1). Based on the UV-Vis spectrum, the wavelength of 262 nm was chosen for the quantitative determination of bakuchiol. The analysis of the UV-Vis spectra of sample 2 shows no presence of bakuchiol (Figure 2a). The content of bakuchiol was determined using a standard curve (see Section 4).
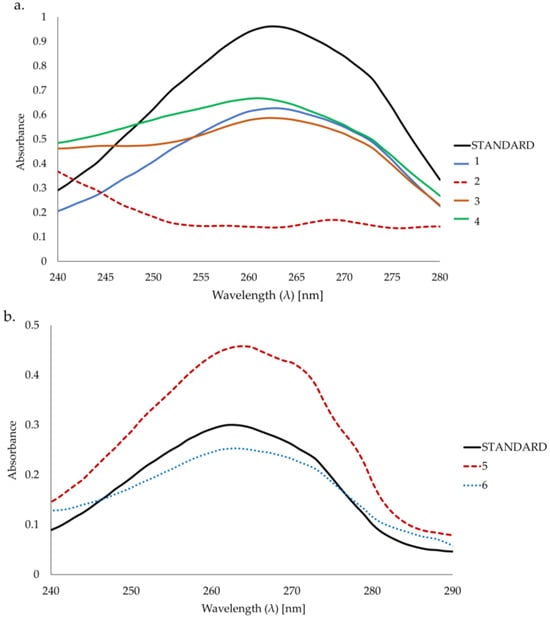
Figure 2.
(a). UV-Vis spectra of bakuchiol (standard) (15 μg/mL) in samples 1, 2, 3, and 4. (b). UV-Vis spectra of bakuchiol (standard) (5 μg/mL) in samples 5 and 6.
Samples 5 and 6 could not be dissolved completely and bakuchiol could not be properly extracted because of the oil-in-water type of emulsion used; however, in the UV spectra of the solutions obtained from partial dissolution (Figure 2b and Figure S2), a maximum at λ = 262 nm is present and the shape of the spectra allows one to conclude that bakuchiol is probably present in the sample but could not be quantified.
2.2. HPLC Analysis
Bakuchiol content in cosmetic products was analyzed using the HPLC-DAD method. The components are well-separated using a reverse-phase column (endcapped C18) and an isocratic elution with acetonitrile containing 1% formic acid. In accordance with the UV spectra the detection wavelength was set at λ = 260 nm. The peak at RT 31.8 min was assigned to bakuchiol. The limit of detection (LOD) and limit of quantification (LOQ) were determined with the formulas LOD = 3.3 × σ/S and LOQ = 10 × σ/S, where σ is the standard deviation of the y-intercept and S is the slope of the calibration curve. The relative standard deviation (% RSD) for intraday variation remained below 2.5%.
Representative chromatograms of samples 1 and 4 are presented in Figure 3 (all obtained chromatograms are shown in Figure S3). The late-eluting bakuchiol content could be determined because no peak interference with the other ingredients was observed.
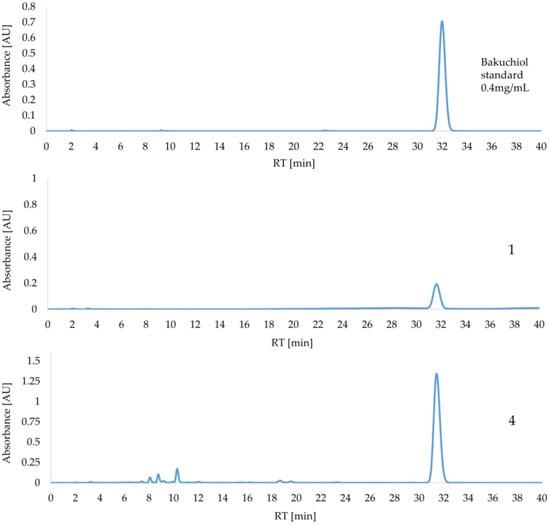
Figure 3.
The chromatograms of bakuchiol standard (0.4 mg/mL) and samples 1 and 4 at λ = 260 nm.
The HPLC findings correspond to the results of the UV/VIS method. Sample 4 had the highest bakuchiol content at 3.6%. Sample 3 matched the amount stated on the product label at 1%, whereas sample 1 contained only 50% of the declared content, namely 0.51%. No bakuchiol was detected in sample 2.
2.3. NMR Analysis
Based on the recorded sets of spectra (1H, 13C, DEPT, COSY, HSQC, and HMBC) of bakuchiol (standard) (Figure 4 and Figures S4–S12 in Supplementary Materials), the structure was confirmed by assigning 1H and 13C signals. The 1H and 13C chemical shifts are summarized in Table 2 and the assignment is presented in Figure 4.
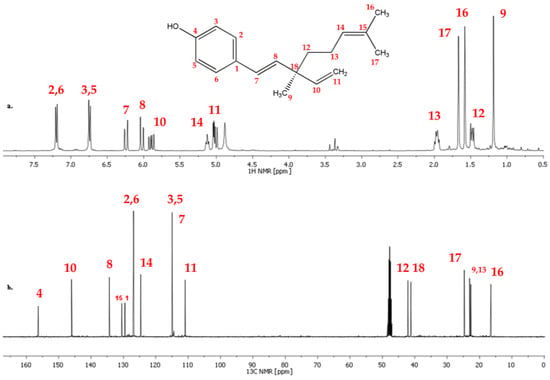
Figure 4.
(a) 1H and (b) 13C NMR spectra of bakuchiol (standard) in CDCl3 (300 MHz) with atom assignment (red numbers).

Table 2.
Assignment of signals in 1H and 13C NMR spectra of bakuchiol (standard).
The 1H spectrum of bakuchiol measured in CDCl3 consists of characteristic signals of a para-substituted aromatic at δ = 7.25–7.20 ppm and δ = 6.80–6.70 ppm; the signals at δ = 6.30–6.20 ppm; δ = 6.10–6.00 ppm; δ = 5.95–5.85 ppm are typical of hydrogens attached to a double bond (Figure 4).
Since the cosmetic products contain multiple other components (cf. Table 1), the signals of bakuchiol are slightly shifted due to possible interactions. The spectra of the six samples are presented in Figure 5. The additional signals can be easily identified. They occur primarily in the aliphatic range (0–5.0 ppm; cf. also Figure S13 in Supplementary Materials). The hydrogen signals of the CH2 and CH3 groups present in unsaturated fatty acids (δ range 0.89–0.91 ppm) are observed for samples 1–5, and proton signals of the CH2 and CH3 groups of the squalene are present in samples 1–4 [20]. The signals at δ 1.5 ppm are protons from acyl chains (-OCO-CH2-CH2-) in oils. For instance, in samples 2–4, a multiplet at δ 2.3 ppm signifies the presence of the -OCO-CH2- groups derived from acyl chains in unsaturated fatty acids, whereas the multiplets at approximately δ 4.4 ppm indicate the presence of triacylglycerols in the product. Sample 6 shows a different spectrum in the analyzed range. The multiplet at approximately δ 4.7 ppm shows the presence of glycerin, a finding that is corroborated by the composition of the preparation (Table 1). In three of the six analyzed samples (1, 5, and 6), a singlet appears in the spectrum at approximately δ 2.0 ppm; its chemical shift value indicates that it is a signal from the hydroxyl proton of the alcohol.
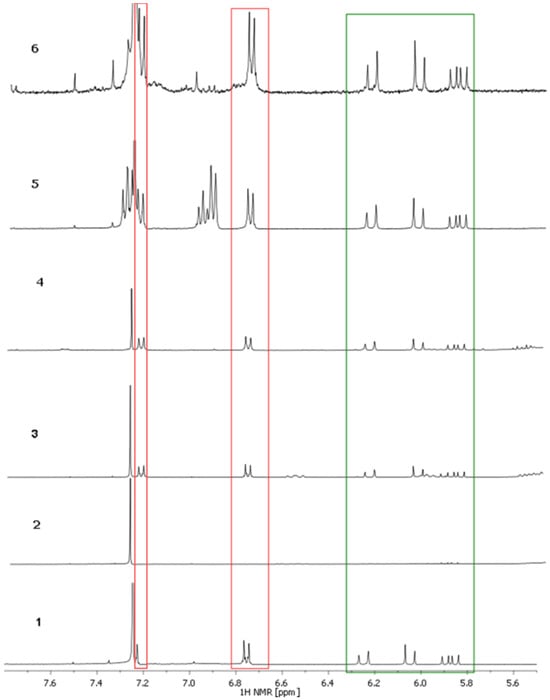
Figure 5.
1H NMR spectra of the samples registered in CDCl3 (300 MHz). The signals of aromatic protons in bakuchiol are marked in red and the signals of hydrogens attached to double bonds are marked in green.
Not all bakuchiol signals are visible. As was found in the HPLC chromatogram, in sample 2 no bakuchiol signal was observed, even though the manufacturer’s declaration stated that the substance was present. This could be due to the fact that the amount of bakuchiol is too low in this sample.
For quantification of bakuchiol in the samples, the signals above 5.5 ppm would be appropriate due to less interference with the other signals of bakuchiol and the signals of possible excipients in the samples.
Nicotinamide (niacinamide, vitamin B3, or vitamin PP) was selected as an internal standard in quantitative NMR due to its availability, lack of reactivity, similarity in solubility as well as its use in pharmaceutical and cosmetic applications (although not in the studied samples), its relatively simple structure, short acquisition time, simplicity of the signals, and the fact that in most cases the signals do not interfere with those of bakuchiol. The assignment of the nicotinamide signals in CDCl3 is presented in Figure S14 (Supplementary Materials). It is worth noting that in CDCl3 the signals of hydrogens of the amide group interfere with possible analytical signals of bakuchiol. This problem can be solved by using the CDCl3:DMSO (5:1, v:v) mixture as a solvent—the signals of nicotinamide and bakuchiol are well separated, as shown in Figure 6. As all the signals of nicotinamide are located in the aromatic region, the analysis could be focused in this region.
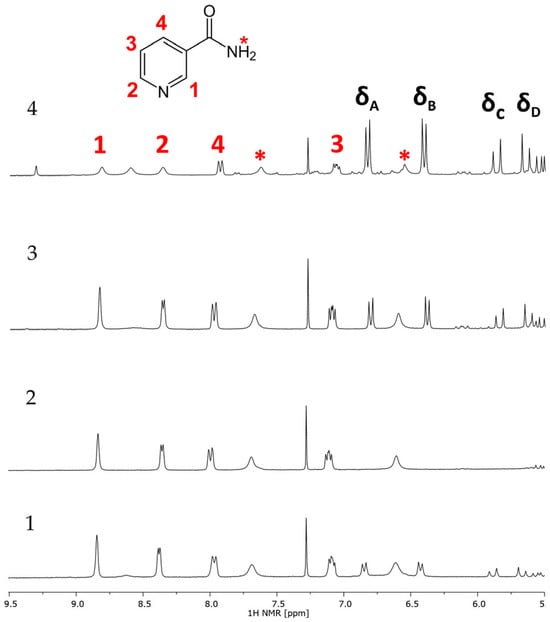
Figure 6.
1H NMR spectra of samples 1, 2, 3, and 4 with nicotinamide added in CDCl3:DMSO (5:1, v:v) (300 Hz). The protons of nicotinamide are assigned in red; analytical signals of bakuchiol are assigned in black. The protons from the NH2 group are marked with a red asterisk.
Based on the spectra of the samples recorded in the CDCl3:DMSO mixture, the analytical signals of bakuchiol were selected as δA = 6.88–6.78 ppm, δB = 6.46–6.36 ppm, δC = 5.94–5.80 ppm, and δD = 5.71–5.59 ppm.
The quantitative results obtained using the method with nicotinamide as an internal standard are presented in Table 3.

Table 3.
Bakuchiol content in the products obtained by NMR-IS, UV/VIS, and HPLC-DAD methods, percentage [% w/w] ± SD; ND—not detected.
3. Discussion
The applied methods allowed for the confirmation or denial of bakuchiol content in the examined samples, as well as the quantification of bakuchiol in the samples soluble in the solvents used (Table 3).
Given that bakuchiol is insoluble in water but soluble in vegetable oils, triglycerides, and a wide range of hydrophobic emollients, an oil-in-water emulsion can be used in many preparations to increase the bioavailability of bakuchiol through the skin. This emulsion type was used in cosmetic samples 5 and 6, which were characterized by the presence of water. Consequently, direct determination (lacking additional extraction steps to separate the active substance) was not feasible. This encourages further analysis to optimize the extraction process for quantitative determination.
The correlation between the results of both NMR and HPLC techniques (Figure 7) determined using linear regression indicates that the analytical results are fully convergent and thus can be successfully used interchangeably. The coefficients of determination R2 is close to 1 (0.9917). Hence, the qNMR method is competitive with the HPLC method. However, with regard to the analysis time, the NMR method is superior to HPLC. The analysis using the HPLC method takes about 40 minutes per sample, whereas in the case of NMR the time of analysis is much shorter, of about 4 minutes only.
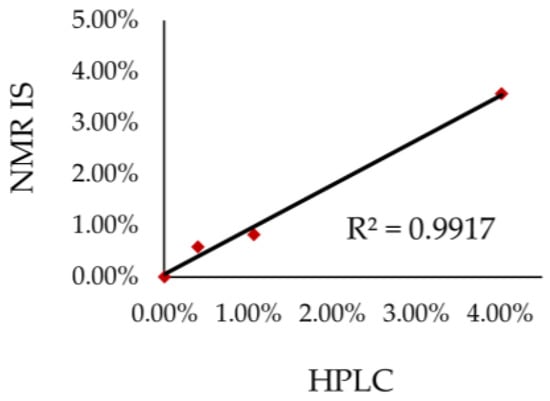
Figure 7.
Correlation between the results obtained using 1H qNMR with internal standard.
The results of this study clearly demonstrate the necessity of examining the quality of cosmetic products and the quantification of the most important ingredient, in this case bakuchiol. Moreover, the results allow one to suspect that there is non-compliance with the manufacturer’s declaration in sample 2, in which bakuchiol content was not confirmed by any of the selective qualitative analysis methods. Likewise, the declared content of 1% bakuchiol in sample 1 could not be confirmed by any of the applied methods, with the results suggesting a content of approximately 0.5–0.6%. For sample 3, the obtained results were consistent with the manufacturer’s declaration, i.e., approximately 1% of bakuchiol content, whereas for sample 4, which had no declaration concerning bakuchiol content in the product label, the result obtained indicates that it contains approximately 4% of the determined meroterpene.
4. Materials and Methods
4.1. Materials
Bakuchiol (Standard) was obtained from Sigma Aldrich (CAS 10309-37-2). Nicotinamide (CAS 98-92-0) was purchased from Merck Polska (Merck KGaA, Darmstadt, Germany). The solvents methanol (CAS 67-56-1), ethanol (CAS 64-17-5), isopropanol (CAS 67-63-0), CDCl3 (CAS 865-49-6), DMSO (CAS 67-68-5, HCOOH (CAS 64-18-6), and acetonitrile (CAS 75-05-8) were obtained from Sigma Aldrich (Steinheim, Germany).
During solubility tests, samples 5 and 6 in the form of water-containing emulsion were excluded from the investigations, thus limiting the use of organic solvents such as chloroform (CDCl3).
4.2. Methods
4.2.1. UV-Vis
The spectra were recorded using a UV/Vis Thermo Scientific Evolution 60S spectrometer, in the range of wavelength 200–300 nm, with ethanol as a solvent. Based on standard spectra, the wavelength of 262 nm was selected for quantification, in agreement with the literature [21]. A series of bakuchiol standard concentrations were prepared in the range of 5–20 μg/mL. Based on the measured absorbance, a calibration curve was obtained (Table 4).

Table 4.
Validation parameters for the UV-Vis method.
Then, 25 mg of each sample was dissolved in 1.5 mL of ethanol and diluted. The concentration of each sample was calculated using the calibration curve.
4.2.2. HPLC
Qualitative and quantitative analyses were performed using a Chromaster HPLC system (Hitachi, Tokyo, Japan) equipped with a gradient pump, a diode-array detector, an autosampler with a thermostat, and a column thermostat. A Merck A Purospher STAR RP-18e (5 µm, 250 mm × 4.6 mm) column was used with a flow rate of 1 mL/min at the temperature of 35 °C. The mobile phase consists of 1% formic acid and acetonitrile, 35/65 (v/v); the analysis was carried out under isocratic conditions. The injected volume was 20 µL, wavelength range 220–380 nm, and detection at λ = 260 nm.
A stock solution of bakuchiol (standard), 10 mg/mL in 2:1 (v:v) methanol/isopropanol, was prepared, and dilutions were used to obtain a calibration curve (Table 5).

Table 5.
Concentration range, limit of detection (LOD), and limit of quantification (LOQ) parameters obtained by the HPLC-DAD method.
For quantitative analysis, 20–30 mg of each sample was dissolved in 1.5 mL of 2:1 (v:v) methanol/isopropanol. The chromatograms were recorded at 260 nm. Bakuchiol content was calculated using the calibration curve.
4.2.3. 1H and 13C NMR
The 1H and 13C NMR spectra (Figure 4), as well as the 2D experiments COSY, HMQC (Figure S1), HSQC, and HMBC were recorded using a VARIAN VNMRS spectrometer operating at 299.61 (1H) and 75.13 (13C) MHz, with a gradient probe (AutoSw PFG 4 Nuc type, 5 mm) direct type with an axis gradient. The used frequency was 100 MHz. SW = 16 ppm, taq = 2.05 s, delay D1 = 1 s, and NS = 64. NMR tubes of 5 mm were used with CDCl3 as solvent.
Based on the spectra of bakuchiol (standard), the signals of bakuchiol were assigned. The samples were dissolved in CDCl3 (samples 5 and 6 only partially) and the 1H spectra were recorded (Figure 5 and Figure S2 in Supplementary Materials).
4.2.4. qNMR Internal Standard (IS)
Nicotinamide was chosen as the internal standard as it would minimally interfere with the analytical signals of bakuchiol and the signals from other components (oils). First, 250 mg of each sample was dissolved in 1 mL of 5:1 (v:v) CDCl3:DMSO and 800 µL of each of the obtained solutions was placed in NMR tubes. Then, 50 µL of the nicotinamide standard solution (45.73 mg/mL) was added to every tube. Each sample was stirred and the 1H spectra were recorded. After FT and baseline correction, all analytical signals of bakuchiol (δA = 6.88–6.78 ppm; δB = 6.46–6.36 ppm; δC = 5.94–5.80 ppm; δD = 5.71–5.59 ppm) and nicotinamide (δIS1 = 8.88–8.75 ppm; δIS2 = 8.42–8.30 ppm; δIS4 = 8.04–7.89 ppm; δIS3 = 7.15–7.02 ppm) that did not interfere with any other signal were manually integrated. Only in sample 1 the integration of all signals was possible. In sample 3, the δD signal interfered with the signal from other ingredients contained in the sample, which was also the case in sample 4, with the signals δA, δD, δIS1, and δIS3 interfering.
Using the area under the curve of all integrated signals, the concentration of each sample was calculated with the following formula:
where IBAK and IIS are integrities of bakuchiol and internal standard (nicotinamide) signals, respectively; nIS and nBAK are numbers of equivalent protons producing the integrated signals; MBAK and MIS are molar masses of the examined compounds; mIS and mBAK are the mass of internal standard and bakuchiol, respectively in the NMR tube, and P is the purity of internal standard used.
5. Conclusions
Because of the difficulty of working with this type of research material (complex mixtures—cosmetic serum) as well as a wide variety of potential samples, it might be valuable to consider developing a method for extracting bakuchiol (an active substance which has a partially terpenoid structure) in order to extend the possibilities of using the methods presented. Thus, it would be possible to include a much more diverse material both in composition and form, and also to increase the reliability of the obtained results. However, the principal aim of this study was to assess the usefulness of qualitative and quantitative analysis methods by which the identity of bakuchiol could be confirmed and quantified in a complex material such as serum-type cosmetics in a relatively short time and at low cost. UV-Vis spectrophotometry, NMR spectroscopy, and HPLC chromatography were successfully employed to unambiguously assess the quality and verify the manufacturer’s declarations of selected cosmetic products with diversified compositions and prices available on the Polish market.
The present study demonstrated that the composition of cosmetic products can prove challenging to analyze. Samples 5 and 6 contained bakuchiol, but were biphasic systems. In this case, it was impossible to analyze the product directly, which would make it necessary to search for an alternative procedure. One potential method that was planned for use in future studies was lyophilization of the emulsion [22], followed by determination by liquid or gas chromatography. In the present study, the HPLC method was used; however, gas chromatography could also be applied [23]. This would obviate the need for an extraction step, enabling the bakuchiol content to be determined using a small amount of sample processing. It is also conceivable to use a direct determination method derived from nanoemulsion products [24].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26146638/s1.
Author Contributions
Conceptualization, M.G., U.H. and K.P.; methodology, M.G., U.H. and K.P.; software, M.G. and P.S.; validation, M.G., L.T. and P.S.; formal analysis, M.G., P.S. and K.P.; investigation, M.G., N.T. and L.T.; resources, K.P.; data curation, M.G., P.S. and K.P.; writing—original draft preparation, M.G., P.S. and K.P.; writing—review and editing, M.G., P.S., K.P. and U.H.; visualization, M.G.; supervision, K.P. and U.H.; project administration, K.P.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Medical University of Warsaw.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Glass, G.E. Cosmeceuticals: The Principles and Practice of Skin Rejuvenation by Nonprescription Topical Therapy. Aesthet. Surg. J. Open Forum 2020, 2, ojaa038. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Voorhees, J.J. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996, 10, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cai, S.-Y.; Boyer, J.L. The role of the retinoid receptor, RAR/RXR heterodimer, in liver physiology. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166085. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Joshi, R.; Patro, B.S.; Ghanty, T.K.; Chintalwar, G.J.; Sharma, A.; Chattopadhyay, S.; Mukherjee, T. Antioxidant activity of bakuchiol: Experimental evidences and theoretical treatments on the possible involvement of the terpenoid chain. Chem. Res. Toxicol. 2003, 16, 1062–1069. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef]
- Mehta, G.N.U.R.; Nayak, U.R.; Dev, S. Bakuchiol, a novel monoterpenoid. Tetrahedron Lett. 1966, 7, 4561–4567. [Google Scholar] [CrossRef]
- Prakasa Rao, A.S.C.; Bhalla, V.K.; Nayak, U.R. Meroterpenoids—II: Psoralea corylifolia Linn.—2. Absolute configuration of (+)-bakuchiol. Tetrahedron 1973, 29, 1127–1130. [Google Scholar]
- Banerji, A.; Chintalwar, G.J. Biosynthesis of bakuchiol from cinnamic and p-coumaric acids. Phytochemistry 1984, 23, 1605–1606. [Google Scholar] [CrossRef]
- Ruan, B.; Kong, L.-Y.; Takaya, Y.; Niwa, M. Studies on the chemical constituents of Psoralea corylifolia L. J. Asian Nat. Prod. Res. 2007, 9, 41–44. [Google Scholar] [CrossRef]
- Yao, S.; Yang, B.; Xu, Z. Determination of bakuchiol in the fruit of Psoralea corylifolia L. China J. Chin. Mater. Medica 1995, 20, 681–683. [Google Scholar]
- Rao, G.V.; Kavitha, K.; Gopalakrishnan, M.; Mukhopadhyay, T. Isolation and characterization of a potent antimicrobial compound from Aerva sanguinolenta Blume: An alternative source of bakuchiol. J. Pharm. Res. 2012, 5, 174–176. [Google Scholar]
- Ohno, O.; Watabe, T.; Nakamura, K.; Kawagoshi, M.; Uotsu, N.; Chiba, T.; Yamada, M.; Yamaguchi, K.; Yamada, K.; Miyamoto, K.; et al. Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from Piper longum on melanin production in B16 mouse melanoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lee, S.; Choi, W.H.; Lee, Y.; Jo, Y.O.; Ha, T.Y. Isolation and anti-inflammatory activity of Bakuchiol from Ulmus davidiana var. japonica. J. Med. Food 2010, 13, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Krenisky, J.M.; Luo, J.; Reed, M.J.; Carney, J.R. Isolation and antihyperglycemic activity of bakuchiol from Otholobium pubescens (Fabaceae), a Peruvian medicinal plant used for the treatment of diabetes. Biol. Pharm. Bull. 1999, 22, 1137–1140. [Google Scholar] [CrossRef]
- Lystvan, K.; Belokurova, V.; Sheludko, Y.; Ingham, J.L.; Prykhodko, V.; Kishchenko, O.; Paton, E.; Kuchuk, M. Production of bakuchiol by in vitro systems of Psoralea drupacea Bge. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 99–103. [Google Scholar] [CrossRef]
- Backhouse, N.; Delporte, C.; Negrete, R.; Salinas, P.; Pinto, A.; Aravena, S.; Cassels, B.K. Active constituents isolated from Psoralea glandulosa L. with antiinflammatory and antipyretic activities. J. Ethnopharmacol. 2001, 78, 27–31. [Google Scholar] [CrossRef]
- Hsu, P.J.; Miller, J.S.; Berger, J.M. Bakuchiol, an antibacterial component of Psoralidium tenuiflorum. Nat. Prod. Res. 2009, 23, 781–788. [Google Scholar] [CrossRef]
- Chaudhuri, R.K. Bakuchiol: A retinol-like functional compound, modulating multiple retinol and non-retinol targets. In Cosmeceuticals and Active Cosmetics, 3rd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 1–8. [Google Scholar]
- Mascarenhas-Melo, F.; Ribeiro, M.M.; Kahkesh, K.H.; Parida, S.; Pawar, K.D.; Velsankar, K.; Jha, N.K.; Damiri, F.; Costa, G.; Veiga, F.; et al. Comprehensive review of the skin use of bakuchiol: Physicochemical properties, sources, bioactivities, nanotechnology delivery systems, regulatory and toxicological concerns. Phytochem. Rev. 2024, 23, 1377–1413. [Google Scholar] [CrossRef]
- Raj, M.G.; Rani, M.P.; Rameshkumar, K. Detection of coconut oil adulteration with palm oil through NMR spectroscopic method. Grasas Y Aceites 2024, 75, 2012. [Google Scholar]
- Jeong, M.; Hong, T.; Lee, K.; Hwangbo, H.; Kim, M.; Ma, W.; Zahn, M. HPLC method for simultaneous quantification of bakuchiol and minor furocoumarins in bakuchiol extract from Psoralea corylifolia. J. AOAC Int. 2015, 98, 902–906. [Google Scholar] [CrossRef]
- Sanches Filho, P.J.; Silveira, L.A.; Betemps, G.R.; Oliveira, P.K.; Sampaio, D.M.; de los Santos, D.G. Use of lyophilization as analytical strategy for chromatographic characterization of aqueous phase of bio-oil produced by rice husk pyrolysis. Microchem. J. 2020, 152, 104457. [Google Scholar] [CrossRef]
- Kurpet, K.; Chwatko, G. Development of a new chromatographic method for the determination of bakuchiol in cosmetic products. Sci. Rep. 2023, 13, 13893. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Ilyas, U.K.; Kamal, Y.T.; Ahmad, S.; Ansari, S.H.; Ali, J. Development and validation of RP-HPLC-PDA method for the quantification of eugenol in developed nanoemulsion gel and nanoparticles. J. Anal. Sci. Technol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).