Transcriptomic Alterations of Canine Histiocytic Sarcoma Cells in Response to Different Stressors
Abstract
1. Introduction
2. Results
2.1. Proliferation Assay
2.2. Transcriptomic Changes in Canine Histiocytic Sarcoma Cells
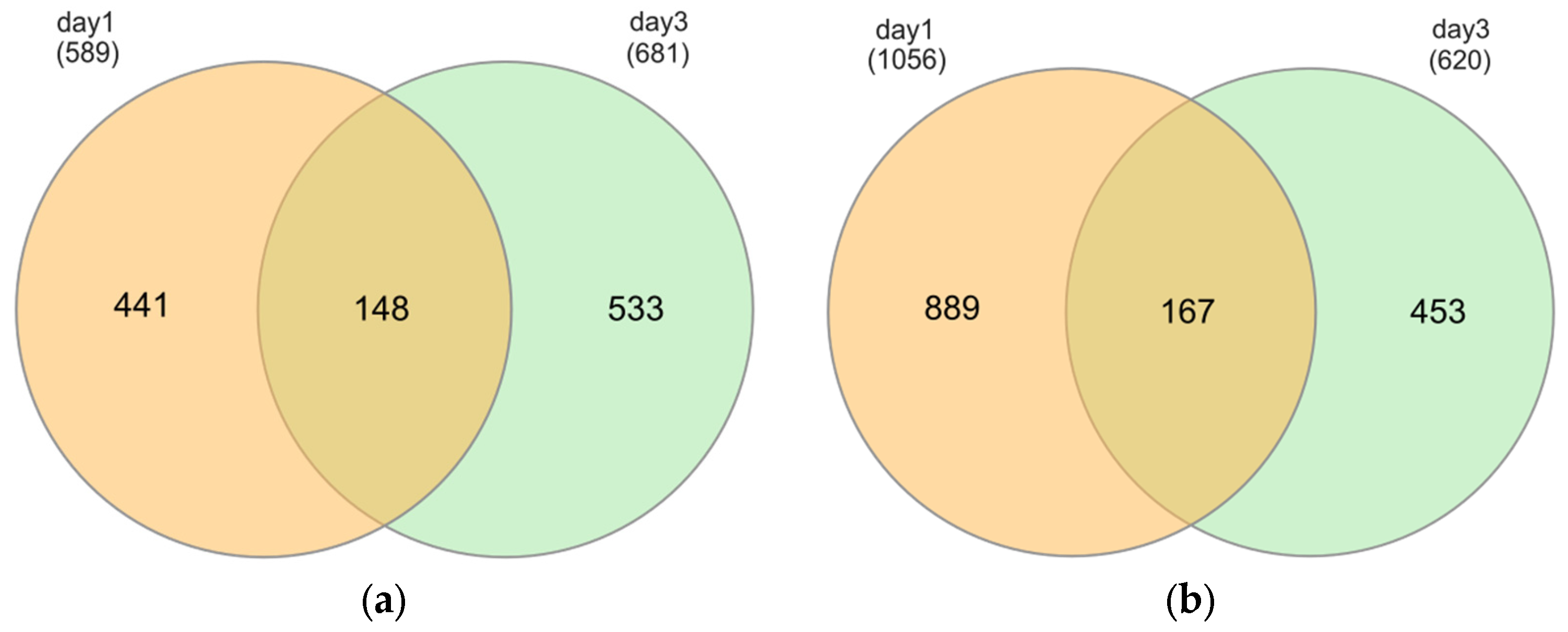
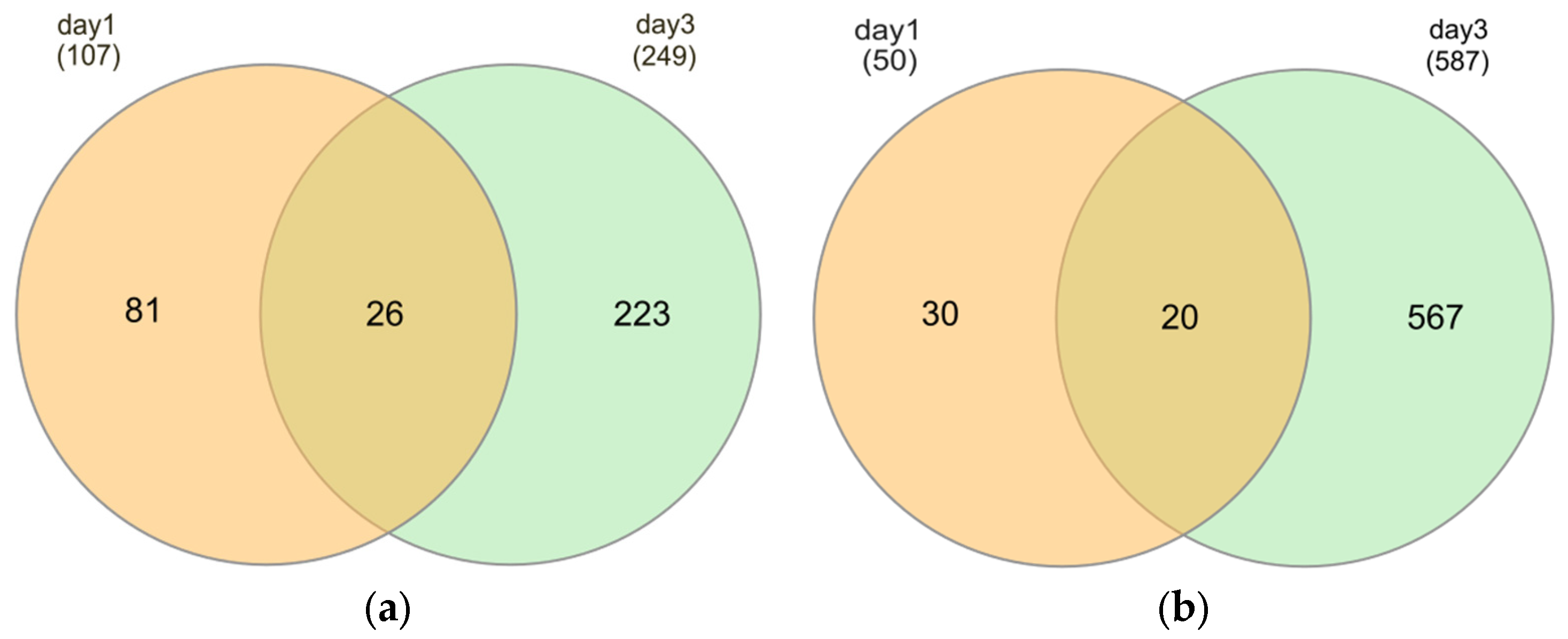
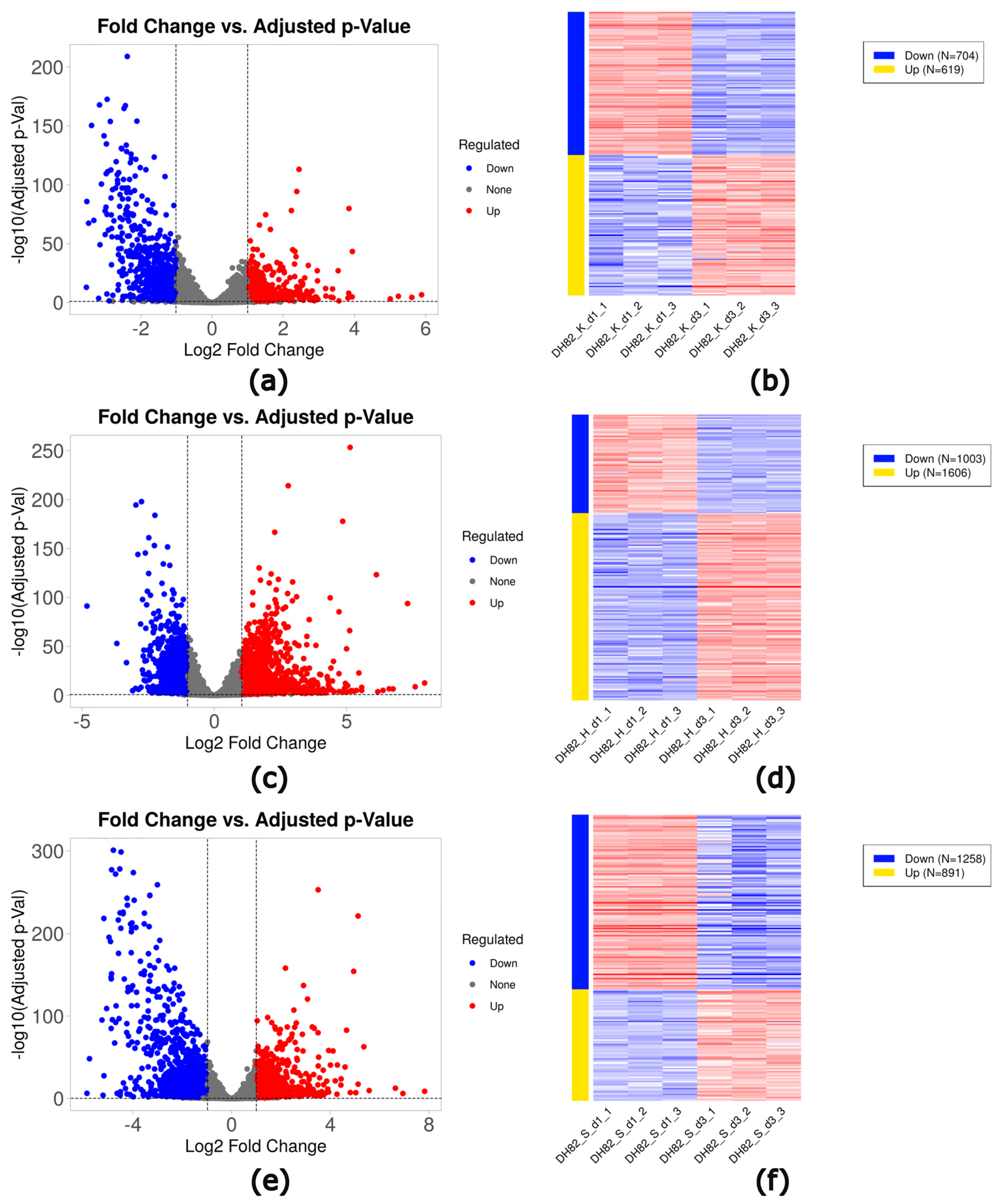
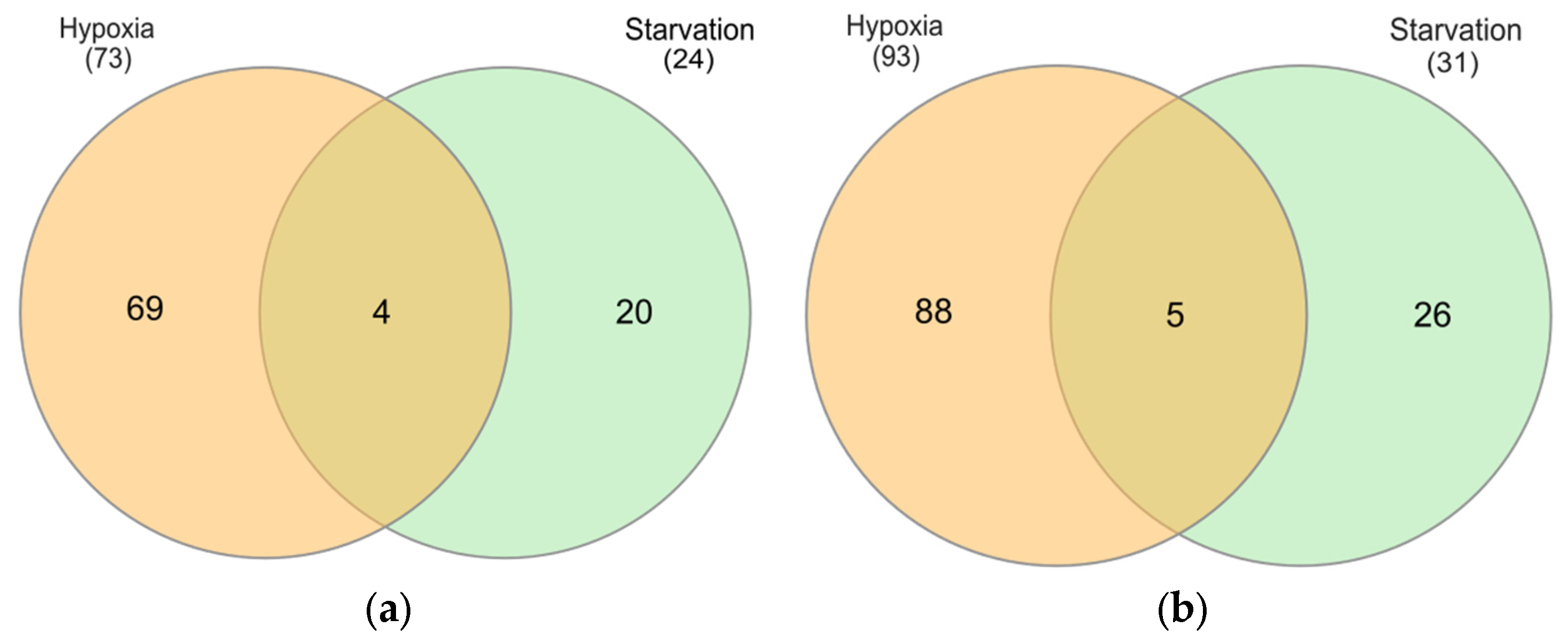
2.3. Identification of Differentially Expressed Genes (DEGs)
2.3.1. Hypoxia
2.3.2. Starvation
2.3.3. Time Effects
2.4. Functional Enrichment Analysis
2.4.1. Hypoxia
2.4.2. Starvation
2.4.3. Time Effect
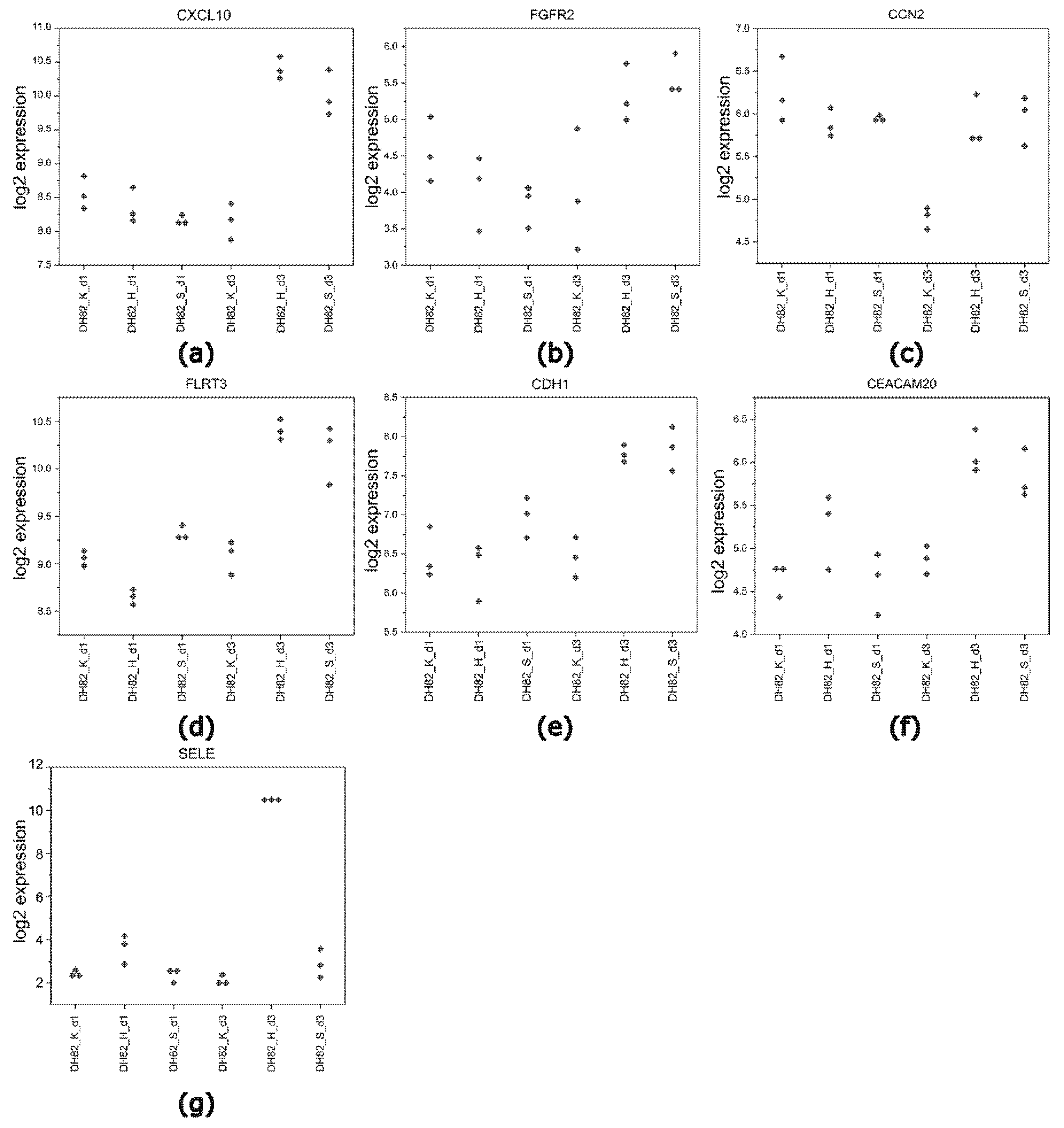
2.4.4. Identification of Common DEGs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Proliferation Assay
4.3. RNA Isolation
4.4. RNA Sequencing
4.5. Data Processing and Data Analysis
4.6. Functional Enrichment Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| DEGs | Differentially expressed genes |
| DH82 | Canine histiocytic sarcoma cells |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| FDR | False discovery rate |
| GO | Gene ontology |
| H | Hypoxia |
| HIF | Hypoxia inducible factor |
| HS | Histiocytic sarcoma |
| IL | Interleukin |
| K | Control |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MRI | Magnetic resonance imaging |
| NF-κB | Nuclear factor kappa |
| PC | Principal component |
| PET | Positron emission tomography |
| pO2 | Oxygen partial pressure |
| S | Starvation |
| SPECT | Single-photon emission computed tomography |
| TME | Tumor microenvironment |
| TNF | Tumor necrosis factor |
References
- Affolter, V.K.; Moore, P.F. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet. Pathol. 2002, 39, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, I.; Vernau, W.; Sturges, B.K.; Vernau, K.M.; Rossmeisl, J.; Zimmerman, K.; Crowe, C.M.; Woolard, K.; Giuffride, M.; Higgins, R.; et al. Clinicopathological characteristics of histiocytic sarcoma affecting the central nervous system in dogs. J. Vet. Intern. Med. 2020, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Dervisis, N.G.; Kiupel, M.; Qin, Q.; Cesario, L. Clinical prognostic factors in canine histiocytic sarcoma. Vet. Comp. Oncol. 2017, 15, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Mariani, C.; Jennings, M.; Olby, N.; Borst, L.; Brown, J., Jr.; Robertson, I.; Seiler, G.S.; MacKillop, E. Histiocytic sarcoma with central nervous system involvement in dogs: 19 cases (2006–2012). J. Vet. Intern. Med. 2015, 29, 607–613. [Google Scholar] [CrossRef]
- Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist 2004, 9, 10–17. [Google Scholar] [CrossRef]
- Rickard, A.G.; Palmer, G.M.; Dewhirst, M.W. Clinical and pre-clinical methods for quantifying tumor hypoxia. In Hypoxia and Cancer Metastasis; Gilkes, D.M., Ed.; Springer: Berlin/Heldelberg, Germany, 2019; Volume 1136, pp. 19–41. [Google Scholar]
- Bruehlmeier, M.; Kaser-Hotz, B.; Achermann, R.; Bley, C.R.; Wergin, M.; Schubiger, P.A.; Ametamey, S.M. Measurement of tumor hypoxia in spontaneous canine sarcomas. Vet. Radiol. Ultrasound 2005, 46, 348–354. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell J. 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Piñeiro Fernández, J.; Luddy, K.A.; Harmon, C.; O’Farrelly, C. Hepatic tumor microenvironments and effects on NK cell phenotype and function. Int. J. Mol. Sci. 2019, 20, 4131. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Angiogenesis as a hallmark of solid tumors-clinical perspectives. Cell. Oncol. 2021, 44, 715–737. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.-F. Hypoxia and the tumor microenvironment. Technol. Cancer Res. Treat. 2021, 20, 15330338211036304. [Google Scholar] [CrossRef]
- Shi, R.; Liao, C.; Zhang, Q. Hypoxia-driven effects in cancer: Characterization, mechanisms, and therapeutic implications. Cells 2021, 10, 678. [Google Scholar] [CrossRef]
- Chapman, J.D.; Engelhardt, E.L.; Stobbe, C.C.; Schneider, R.F.; Hanks, G.E. Measuring hypoxia and predicting tumor radioresistance with nuclear medicine assays. Radiother. Oncol. 1998, 46, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, D.; Young, W.; Vojnovic, B.; Wardman, P.; Lynch, E.; Hill, S.; Chaplin, D.J. Measurement of tumor oxygenation: A comparison between polarographic needle electrodes and a time-resolved luminescence-based optical sensor. Radiat. Res. 1997, 147, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Bergman, K.S.; Rasey, J.S.; Peterson, L.M.; Evans, M.L.; Graham, M.M.; Grierson, J.R.; Lindsley, K.L.; Lewellen, T.K.; Krohn, K.A.; et al. Evaluation of oxygenation status during fractionated radiotherapy in human nonsmall cell lung cancers using [F-18] fluoromisonidazole positron emission tomography. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Parliament, M.; Chapman, J.; Urtasun, R.; McEwan, A.; Golberg, L.; Mercer, J.; Mannan, R.H.; Wiebe, L. Non-invasive assessment of human tumour hypoxia with 123I-iodoazomycin arabinoside: Preliminary report of a clinical study. Br. J. Cancer 1992, 65, 90–95. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef]
- Ding, Y.; Mason, R.P.; McColl, R.W.; Yuan, Q.; Hallac, R.R.; Sims, R.D.; Weatherall, P.T. Simultaneous measurement of tissue oxygen level-dependent (TOLD) and blood oxygenation level-dependent (BOLD) effects in abdominal tissue oxygenation level studies. J. Magn. Reson. Imaging 2013, 38, 1230–1236. [Google Scholar] [CrossRef]
- Bane, O.; Besa, C.; Wagner, M.; Oesingmann, N.; Zhu, H.; Fiel, M.I.; Taouli, B. Feasibility and reproducibility of BOLD and TOLD measurements in the liver with oxygen and carbogen gas challenge in healthy volunteers and patients with hepatocellular carcinoma. J. Magn. Reson. Imaging 2016, 43, 866–876. [Google Scholar] [CrossRef]
- Zhao, D.; Ran, S.; Constantinescu, A.; Hahn, E.W.; Mason, R.P. Tumor oxygen dynamics: Correlation of in vivo MRI with histological findings. Neoplasia 2003, 5, 308–318. [Google Scholar] [CrossRef]
- Drew, P.J.; Chatterjee, S.; Turnbull, L.W.; Read, J.; Carleton, P.J.; Fox, J.N.; Monson, J.R.T.; Kerin, M.J. Dynamic contrast enhanced magnetic resonance imaging of the breast is superior to triple assessment for the pre-operative detection of multifocal breast cancer. Ann. Surg. Oncol. 1999, 6, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Egeland, T.A.; Gulliksrud, K.; Gaustad, J.V.; Mathiesen, B.; Rofstad, E.K. Dynamic contrast-enhanced-MRI of tumor hypoxia. Magn. Reson. Med. 2012, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Armando, F.; Gambini, M.; Corradi, A.; Giudice, C.; Pfankuche, V.M.; Brogden, G.; Attig, F.; von Köckritz-Blickwede, M.; Baumgärtner, W.; Puff, C. Oxidative Stress in Canine Histiocytic Sarcoma Cells Induced by an Infection with Canine Distemper Virus Led to a Dysregulation of HIF-1α Downstream Pathway Resulting in a Reduced Expression of VEGF-B in vitro. Viruses 2020, 12, 200. [Google Scholar] [CrossRef]
- Pezzuto, A.; Carico, E. Role of HIF-1 in cancer progression: Novel insights. A review. Curr. Mol. Med. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Hockel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- de Bem Prunes, B.; Nunes, J.S.; da Silva, V.P.; Laureano, N.K.; Gonçalves, D.R.; Machado, I.S.; Barbosa, S.; Lamers, M.L.; Rados, P.V.; Kurth, I.; et al. The role of tumor acidification in aggressiveness, cell dissemination and treatment resistance of oral squamous cell carcinoma. Life Sci. 2022, 288, 120163. [Google Scholar] [CrossRef]
- Andreucci, E.; Peppicelli, S.; Ruzzolini, J.; Bianchini, F.; Biagioni, A.; Papucci, L.; Magnelli, L.; Mazzanti, B.; Stecca, B.; Calorini, L. The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J. Mol. Med. 2020, 98, 1431–1446. [Google Scholar] [CrossRef]
- Yang, L.; Hu, X.; Mo, Y.-Y. Acidosis promotes tumorigenesis by activating AKT/NF-κB signaling. Cancer Metastasis Rev. 2019, 38, 179–188. [Google Scholar] [CrossRef]
- Damaghi, M.; Gillies, R. Phenotypic changes of acid-adapted cancer cells push them toward aggressiveness in their evolution in the tumor microenvironment. Cell Cycle 2017, 16, 1739–1743. [Google Scholar] [CrossRef]
- Püschel, F.; Favaro, F.; Redondo-Pedraza, J.; Lucendo, E.; Iurlaro, R.; Marchetti, S.; Majem, B.; Eldering, E.; Nadal, E.; Ricci, J.E.; et al. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc. Natl. Acad. Sci. USA 2020, 117, 9932–9941. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, T.; Biswas, S.; Mukherjee, A. Cellular response during cellular starvation: A battle for cellular survivability. Cell Biochem. Funct. 2024, 42, e4101. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; De Meyer, G.R.Y.; Andries, L.; Herman, A.G.; Kockx, M.M. In Situ Detection of Starvation-induced Autophagy. J. Histochem. Cytochem. 2006, 54, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, M.; Kemball, C.C.; Flynn, C.T.; Wood, M.R.; Whitton, J.L.; Kiosses, W.B. Short-term fasting induces profound neuronal autophagy. Autophagy 2010, 6, 702–710. [Google Scholar] [CrossRef]
- Lashinger, L.M.; O’Flanagan, C.H.; Dunlap, S.M.; Rasmussen, A.J.; Sweeney, S.; Guo, J.Y.; Lodi, A.; Tiziani, S.; White, E.; Hursting, S.D. Starving cancer from the outside and inside: Separate and combined effects of calorie restriction and autophagy inhibition on Ras-driven tumors. Cancer Metab. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Palm, W. Metabolic plasticity allows cancer cells to thrive under nutrient starvation. Proc. Natl. Acad. Sci. USA 2021, 118, e2102057118. [Google Scholar] [CrossRef]
- Guo, J.Y.; White, E. Role of Tumor Cell Intrinsic and Host Autophagy in Cancer. Cold Spring Harb. Perspect. Med. 2024, 14, a041539. [Google Scholar] [CrossRef]
- Caro-Maldonado, A.; Muñoz-Pinedo, C. Dying for something to eat: How cells respond to starvation. Open Cell Signal. J. 2011, 3, 42–51. [Google Scholar] [CrossRef][Green Version]
- Yu, J.; Wei, Z.; Li, Q.; Wan, F.; Chao, Z.; Zhang, X.; Lin, L.; Meng, H.; Tian, L. Advanced cancer starvation therapy by simultaneous deprivation of lactate and glucose using a MOF nanoplatform. Adv. Sci. 2021, 8, 2101467. [Google Scholar] [CrossRef]
- Yang, B.; Ding, L.; Chen, Y.; Shi, J. Augmenting tumor-starvation therapy by cancer cell autophagy inhibition. Adv. Sci. 2020, 7, 1902847. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Zeng, X.; Chen, X.; Gu, Z. Advances in nanomedicine for cancer starvation therapy. Theranostics 2019, 9, 8026–8047. [Google Scholar] [CrossRef] [PubMed]
- Abou Khouzam, R.; Sharda, M.; Rao, S.P.; Kyerewah-Kersi, S.M.; Zeinelabdin, N.A.; Mahmood, A.S.; Nawafleh, H.; Khan, M.S.; Venkatesh, G.H.; Chouaib, S. Chronic hypoxia is associated with transcriptomic reprogramming and increased genomic instability in cancer cells. Front. Cell Dev. Biol. 2023, 11, 1095419. [Google Scholar] [CrossRef] [PubMed]
- Scheubeck, G.; Berchtold, S.; Smirnow, I.; Schenk, A.; Beil, J.; Lauer, U.M. Starvation-Induced Differential Virotherapy Using an Oncolytic Measles Vaccine Virus. Viruses 2019, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Hahn, N.M.; Ramos-Vara, J.A.; Knapp, D.W. Naturally-occurring canine invasive urothelial carcinoma harbors luminal and basal transcriptional subtypes found in human muscle invasive bladder cancer. PLoS Genet. 2018, 14, e1007571. [Google Scholar] [CrossRef]
- Hernandez, B.; Adissu, H.A.; Wei, B.-R.; Michael, H.T.; Merlino, G.; Simpson, R.M. Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int. J. Mol. Sci. 2018, 19, 394. [Google Scholar] [CrossRef]
- Oh, J.H.; Cho, J.-Y. Comparative oncology: Overcoming human cancer through companion animal studies. Exp. Mol. Med. 2023, 55, 725–734. [Google Scholar] [CrossRef]
- Koehler, J.; Sandey, M.; Prasad, N.; Levy, S.A.; Wang, X.; Wang, X. Differential expression of miRNAs in hypoxia (“HypoxamiRs”) in three canine high-grade glioma cell lines. Front. Vet. Sci. 2020, 7, 104. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef]
- Cassali, G.D.; Nakagaki, K.Y.R.; Salvi, M.; Dos Reys, M.P.; Rocha, M.A.N.; de Campos, C.B.; Ferreira, E.; Rodrigues, A.C.B.; Dos Reis, D.C.; Damasceno, K.A.; et al. Canine, Feline, and Murine Mammary Tumors as a Model for Translational Research in Breast Cancer. Vet. Sci. 2025, 12, 189. [Google Scholar] [CrossRef]
- Asada, H.; Tani, A.; Sakuma, H.; Hirabayashi, M.; Matsumoto, Y.; Watanabe, K.; Tsuboi, M.; Yoshida, S.; Harada, K.; Uchikai, T.; et al. Whole exome and transcriptome analysis revealed the activation of ERK and Akt signaling pathway in canine histiocytic sarcoma. Sci. Rep. 2023, 13, 8512. [Google Scholar] [CrossRef]
- Sakuma, H.; Tomiyasu, H.; Tani, A.; Goto-Koshino, Y.; Tani, H.; Ohno, K.; Tsujimoto, H.; Bonkobara, M.; Okuda, M. Antitumor effects of inhibitors of ERK and Akt pathways in canine histiocytic sarcoma cell lines. Vet. J. 2024, 308, 106264. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, H.; Tomiyasu, H.; Tani, A.; Goto-Koshino, Y.; Bonkobara, M.; Okuda, M. Molecular Classification Based on the Gene Expression Profiles in Canine Histiocytic Sarcoma Cells. Vet. Comp. Oncol. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Martin, J.D.; Snuderl, M.; Mpekris, F.; Jain, S.R.; Jain, R.K. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: Implications for vascular collapse. Cancer Res. 2013, 73, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, Y.; Luo, M.; Hu, X.; Li, H.; Liu, Q.; Zou, Z. Chronic stress in solid tumor development: From mechanisms to interventions. J. Biomed. Sci. 2023, 30, 1–25. [Google Scholar] [CrossRef]
- Nia, H.T.; Datta, M.; Seano, G.; Huang, P.; Munn, L.L.; Jain, R.K. Quantifying solid stress and elastic energy from excised or in situ tumors. Nat. Protoc. 2018, 13, 1091–1105. [Google Scholar] [CrossRef]
- Mpekris, F.; Angeli, S.; Pirentis, A.P.; Stylianopoulos, T. Stress-mediated progression of solid tumors: Effect of mechanical stress on tissue oxygenation, cancer cell proliferation, and drug delivery. Biomech. Model. Mechanobiol. 2015, 14, 1391–1402. [Google Scholar] [CrossRef]
- Izuishi, K.; Kato, K.; Ogura, T.; Kinoshita, T.; Esumi, H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000, 60, 6201–6207. [Google Scholar]
- Lü, J.; Zhang, C.; Han, J.; Xu, Z.; Li, Y.; Zhen, L.; Guo, Y.; Wang, Z.; Bischof, E.; Yu, Z. Starvation stress attenuates the miRNA-target interaction in suppressing breast cancer cell proliferation. BMC Cancer 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Buono, R.; Longo, V.D. Starvation, stress resistance, and cancer. Trends Endocrinol. Metab. 2018, 29, 271–280. [Google Scholar] [CrossRef]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012, 4, 124ra27. [Google Scholar] [CrossRef]
- Skopichev, V.; Zharkova, M.; Alistratova, F. Oncoprotective Effect of Short-Term Hypoxic Training in Model of Ehrlich Ascites Carcinoma in Mice. KnE Life Sci. 2021, 32–46. [Google Scholar] [CrossRef]
- Yu, L.; Hales, C.A. Long-term exposure to hypoxia inhibits tumor progression of lung cancer in rats and mice. BMC Cancer 2011, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.-L.; Lin, J.-W.; Hsieh, D.J.-Y.; Yeh, Y.-L.; Shen, C.-Y.; Day, C.-H.; Ho, T.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Long-term hypoxia exposure enhanced IGFBP-3 protein synthesis and secretion resulting in cell apoptosis in H9c2 myocardial cells. Growth Factors 2015, 33, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-d.; Cheng, Y.; Jia, J.-f.; Cao, X.-s.; Yang, Y. Effect of long-term exposure to hypoxia on the proliferation of colon cancer cells in vitro. Tumor 2013, 33, 42–47. [Google Scholar]
- Skjellegrind, H.K.; Fayzullin, A.; Johnsen, E.O.; Eide, L.; Langmoen, I.A.; Moe, M.C.; Vik-Mo, E.O. Short-term differentiation of glioblastoma stem cells induces hypoxia tolerance. Neurochem. Res. 2016, 41, 1545–1558. [Google Scholar] [CrossRef][Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell J. 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Ma, B.; Li, M.; Fuchs, S.; Bischoff, I.; Hofmann, A.; Unger, R.E.; Kirkpatrick, C.J. Short-term hypoxia promotes vascularization in co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. J. Biomed. Mater. Res. 2020, 108, 7–18. [Google Scholar] [CrossRef]
- Philip, B.; Ito, K.; Moreno-Sánchez, R.; Ralph, S.J. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. J. Carcinog. 2013, 34, 1699–1707. [Google Scholar] [CrossRef]
- O’Reilly, D.; Johnson, P.; Buchanan, P.J. Hypoxia induced cancer stem cell enrichment promotes resistance to androgen deprivation therapy in prostate cancer. Steroids 2019, 152, 108497. [Google Scholar] [CrossRef]
- Setty, B.A.; Pillay Smiley, N.; Pool, C.M.; Jin, Y.; Liu, Y.; Nelin, L.D. Hypoxia-induced proliferation of HeLa cells depends on epidermal growth factor receptor-mediated arginase II induction. Physiol. Rep. 2017, 5, e13175. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Lubor, B. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef] [PubMed]
- Yayan, J.; Franke, K.J.; Berger, M.; Windisch, W.; Rasche, K. Adhesion, metastasis, and inhibition of cancer cells: A comprehensive review. Mol. Biol. Rep. 2024, 51, 165. [Google Scholar] [CrossRef] [PubMed]
- Beri, P.; Popravko, A.; Yeoman, B.; Kumar, A.; Chen, K.; Hodzic, E.; Chiang, A.; Banisadr, A.; Placone, J.K.; Carter, H.; et al. Cell adhesiveness serves as a biophysical marker for metastatic potential. Cancer Res. 2020, 80, 901–911. [Google Scholar] [CrossRef]
- Lekarski, P.M.; Towarzystwa, O.P. Cell adhesion molecules and their participation in the process of inflammation and cancerogenesis. Pol. Merkur. Lek. 2008, 24, 177–180. [Google Scholar]
- Yu, C.C.; Chen, L.C.; Lin, V.C.; Huang, C.Y.; Cheng, W.C.; Hsieh, A.R.; Chang, T.Y.; Lu, T.L.; Lee, C.H.; Huang, S.P.; et al. Effect of genetic variants in cell adhesion pathways on the biochemical recurrence in prostate cancer patients with radical prostatectomy. Cancer Med. 2019, 8, 2777–2783. [Google Scholar] [CrossRef]
- Aftab, S.; Shakoori, A.R. Low glucose availability alters the expression of genes involved in initial adhesion of human glioblastoma cancer cell line SF767. J. Cell. Biochem. 2019, 120, 16824–16839. [Google Scholar] [CrossRef]
- Opiłka, M.N.; Lorenc, Z.; Starzewska, M.; Lorenc, J.; Rajs, A. Cell adhesion molecules in terms of carcinogenesis. Pol. J. Surg. 2014, 86, 151–157. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Godlewski, J.; Śliwińska-Jewsiewicka, A.; Kmieć, Z. Cell adhesion molecules in the process of carcinogenesis and metastasis. Pol. Ann. Med. 2009, 16, 128–137. [Google Scholar] [CrossRef]
- Paschos, K.A.; Canovas, D.; Bird, N.C. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell. Signal. 2009, 21, 665–674. [Google Scholar] [CrossRef]
- Shukla, S.; MacLennan, G.T.; Hartman, D.J.; Fu, P.; Resnick, M.I.; Gupta, S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer 2007, 121, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.W.; Lam, A.K.Y.; Wang, Y.; Hu, H.-F.; Guan, X.Y.; Qin, Y.R.; Saremi, N.; Tsao, S.W.; He, Q.Y.; et al. Significance of PI3K/AKT signaling pathway in metastasis of esophageal squamous cell carcinoma and its potential as a target for anti-metastasis therapy. Oncotarget 2017, 8, 38755–38766. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, L.; Wu, X.; Li, R.; Wen, J.; Sha, J.; Wen, X. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front. Biosci. (Landmark Ed.) 2016, 21, 1084–1091. [Google Scholar] [PubMed]
- Vara, J.Á.F.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Gkotinakou, I.-M.; Kechagia, E.; Pazaitou-Panayiotou, K.; Mylonis, I.; Liakos, P.; Tsakalof, A. Calcitriol suppresses HIF-1 and HIF-2 transcriptional activity by reducing HIF-1/2α protein levels via a VDR-independent mechanism. Cells 2020, 9, 2440. [Google Scholar] [CrossRef]
- Druker, J.; Wilson, J.W.; Child, F.; Shakir, D.; Fasanya, T.; Rocha, S. Role of Hypoxia in the Control of the Cell Cycle. Int. J. Mol. Sci. 2021, 22, 4874. [Google Scholar] [CrossRef]
- Kondo, A.; Safaei, R.; Mishima, M.; Niedner, H.; Lin, X.; Howell, S.B. Hypoxia-induced enrichment and mutagenesis of cells that have lost DNA mismatch repair. Cancer Res. 2001, 61, 7603–7607. [Google Scholar]
- Cowman, S.; Pizer, B.; Sée, V. Downregulation of both mismatch repair and non-homologous end-joining pathways in hypoxic brain tumour cell lines. PeerJ 2021, 9, e11275. [Google Scholar] [CrossRef]
- Tellier, C.; Desmet, D.; Petit, L.; Finet, L.; Graux, C.; Raes, M.; Feron, O.; Michiels, C. Cycling hypoxia induces a specific amplified inflammatory phenotype in endothelial cells and enhances tumor-promoting inflammation in vivo. Neoplasia 2015, 17, 66–78. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: A review of the molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Park, G.-Y.; Bae, M.J.; Kim, J.S.; Jo, W.S.; Lee, C.G. Hypoxia induces immunogenic cell death of cancer cells by enhancing the exposure of cell surface calreticulin in an endoplasmic reticulum stress-dependent manner. Oncol. Lett. 2019, 18, 6269–6274. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Pulskens, W.P.; Sadler, J.J.; Butter, L.M.; Teske, G.J.; Ulland, T.K.; Eisenbarth, S.C.; Florquin, S.; Flavell, R.A.; Leemans, J.C.; et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 20388–20393. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wu, S.; Zhao, B.; Li, Z.; Zhou, Q.; Yu, Y.; Yang, X.; Wang, R.; Wang, X.; Wu, W.; et al. Reprogramming of glucose metabolism: The hallmark of malignant transformation and target for advanced diagnostics and treatments. Biomed. Pharmacother. 2024, 178, 117257. [Google Scholar] [CrossRef]
- Jin, H.-R.; Wang, J.; Wang, Z.-J.; Xi, M.-J.; Xia, B.-H.; Deng, K.; Yang, J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Du, J.; Qin, H. Lipid metabolism dynamics in cancer stem cells: Potential targets for cancers. Front. Pharmacol. 2024, 15, 1367981. [Google Scholar] [CrossRef]
- Anthony, J.; Varalakshmi, S.; Sekar, A.K.; Devarajan, N.; Janakiraman, B.; Peramaiyan, R. Glutaminase-A potential target for cancer treatment. Biomedicines 2024, 14, 29–37. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Li, L.; Zhou, X.; Zhang, Z.; Huang, Y.; Xiao, X. Lipid metabolism reprogramming in head and neck cancer. Front. Oncol. 2023, 13, 1271505. [Google Scholar] [CrossRef]
- Farhat, E.; Weber, J.-M. Hypometabolic responses to chronic hypoxia: A potential role for membrane lipids. Metabolites 2021, 11, 503. [Google Scholar] [CrossRef]
- Agarwala, P.K.; Nie, S.; Reid, G.E.; Kapoor, S. Global lipid remodelling by hypoxia aggravates migratory potential in pancreatic cancer while maintaining plasma membrane homeostasis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159398. [Google Scholar] [CrossRef]
- Yang, M.; Wu, S.; Cai, W.; Ming, X.; Zhou, Y.; Chen, X. Hypoxia-Induced MIF Induces Dysregulation of Lipid Metabolism in Hep2 Laryngocarcinoma Through IL-6/JAK-STAT Pathway. Lipids Health Dis. 2022, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, J.R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, H.Y.; Woo, J.W.; Chung, Y.R.; Park, S.Y. Role of CXCL10 in the progression of in situ to invasive carcinoma of the breast. Sci. Rep. 2021, 11, 18007. [Google Scholar] [CrossRef] [PubMed]
- Sidahmed, A.M.; León, A.J.; Bosinger, S.E.; Banner, D.; Danesh, A.; Cameron, M.J.; Kelvin, D.J. CXCL10 contributes to p38-mediated apoptosis in primary T lymphocytes in vitro. Cytokine 2012, 59, 433–441. [Google Scholar] [CrossRef]
- Romagnani, P.; Annunziato, F.; Lazzeri, E.; Cosmi, L.; Beltrame, C.; Lasagni, L.; Galli, G.; Francalanci, M.; Manetti, R.; Marra, F.; et al. Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) αβ+CD8+ single-positive T cells, TCRγδ+T cells, and natural killer–type cells in human thymus. Blood 2001, 97, 601–607. [Google Scholar]
- Li, N.; Xiao, H.; Shen, J.; Qiao, X.; Zhang, F.; Zhang, W.; Gao, Y.; Liu, Y.D. SELE gene as a characteristic prognostic biomarker of colorectal cancer. J. Int. Med. Res. 2021, 49, 1–11. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, W.; Chen, R.; Xiang, B.; Zhou, S.; Lan, L. CXCL10 is a Tumor Microenvironment and Immune Infiltration Related Prognostic Biomarker in Pancreatic Adenocarcinoma. Front. Mol. Biosci. 2021, 8, 611508. [Google Scholar] [CrossRef]
- Ma, R.-X.; Wei, J.-R.; Hu, Y.-W. Characteristics of Carcinoembryonic Antigen-Related Cell Adhesion Molecules and Their Relationship to Cancer. Mol. Cancer Ther. 2024, 23, 939–948. [Google Scholar] [CrossRef]
- Petrova, Y.I.; Schecterson, L.; Gumbiner, B.M. Roles for E-cadherin cell surface regulation in cancer. Mol. Biol. Cell. 2016, 27, 3233–3244. [Google Scholar] [CrossRef]
- Armando, F.; Gambini, M.; Corradi, A.; Becker, K.; Marek, K.; Pfankuche, V.M.; Mergani, A.E.; Brogden, G.; de Buhr, N.; von Köckritz-Blickwede, M.; et al. Mesenchymal to epithelial transition driven by canine distemper virus infection of canine histiocytic sarcoma cells contributes to a reduced cell motility in vitro. J. Cell. Mol. Med. 2020, 24, 9332–9348. [Google Scholar] [CrossRef]
- Bennewith, K.L.; Huang, X.; Ham, C.M.; Graves, E.E.; Erler, J.T.; Kambham, N.; Feazell, J.; Yang, G.P.; Koong, A.; Giaccia, A.J. The Role of Tumor Cell–Derived Connective Tissue Growth Factor (CTGF/CCN2) in Pancreatic Tumor Growth. Cancer Res. 2009, 69, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Ubink, I.; Verhaar, E.R.; Kranenburg, O.; Goldschmeding, R. A potential role for CCN2/CTGF in aggressive colorectal cancer. J. Cell Commun. Signal. 2016, 10, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, T.; Gao, F.; He, H.; Zhu, Y.; Shen, Z. Targeting of CCN2 suppresses tumor progression and improves chemo-sensitivity in urothelial bladder cancer. Oncotarget 2017, 8, 66316–66327. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, T.; Naiel, S.; Vierhout, M.; Otsubo, K.; Ali, P.; Tsubouchi, K.; Yazdanshenas, P.; Kumaran, V.; Dvorkin-Gheva, A.; Kolb, M.R.; et al. Therapeutic strategies to target connective tissue growth factor in fibrotic lung diseases. Pharmacol. Ther. 2024, 253, 108578. [Google Scholar] [CrossRef]
- Barbe, M.F.; Hilliard, B.A.; Amin, M.; Harris, M.Y.; Hobson, L.J.; Cruz, G.E.; Popoff, S.N. Blocking CTGF/CCN2 reduces established skeletal muscle fibrosis in a rat model of overuse injury. FASEB J. 2020, 34, 6554–6569. [Google Scholar] [CrossRef]
- Resovi, A.; Borsotti, P.; Ceruti, T.; Passoni, A.; Zucchetti, M.; Berndt, A.; Riser, B.L.; Taraboletti, G.; Belotti, D. CCN-Based Therapeutic Peptides Modify Pancreatic Ductal Adenocarcinoma Microenvironment and Decrease Tumor Growth in Combination with Chemotherapy. Cells 2020, 9, 952. [Google Scholar] [CrossRef]
- Yang, M.; Li, D.; Jiang, Z.; Li, C.; Ji, S.; Sun, J.; Chang, Y.; Ruan, S.; Wang, Z.; Liang, R.; et al. TGF-β-Induced FLRT3 Attenuation Is Essential for Cancer-Associated Fibroblast–Mediated Epithelial–Mesenchymal Transition in Colorectal Cancer. Mol. Cancer Res. 2022, 20, 1247–1259. [Google Scholar] [CrossRef]
- Adamczyk-Gruszka, O.; Horecka-Lewitowicz, A.; Gruszka, J.; Wawszczak-Kasza, M.; Strzelecka, A.; Lewitowicz, P. FGFR-2 and Epithelial–Mesenchymal Transition in Endometrial Cancer. J. Clin. Med. 2022, 11, 5416. [Google Scholar] [CrossRef]
- Puff, C.; Krudewig, C.; Imbschweiler, I.; Baumgärtner, W.; Alldinger, S. Influence of persistent canine distemper virus infection on expression of RECK, matrix-metalloproteinases and their inhibitors in a canine macrophage/monocytic tumour cell line (DH82). Vet. J. 2009, 182, 100–107. [Google Scholar] [CrossRef]
- FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 May 2024).
- TrimGalore: A Wrapper Around Cutadapt and FastQC to Consistently Apply Adapter and Quality Trimming to FastQ Files, with Extra Functionality for RRBS Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 15 May 2024).
- Dobin, A.; Gingeras, T.R. Mapping RNA-seq reads with STAR. Curr. Protoc. Bioinform. 2015, 51, 11.14.1–11.14.19. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [PubMed]
- Ge, X. iDEP Web Application for RNA-Seq Data Analysis. In RNA Bioinformatics; Picardi, E., Ed.; Humana: New York, NY, USA, 2021; Volume 2284, pp. 417–442. [Google Scholar]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, 1–11. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Barrett, T.; Suzek, T.O.; Troup, D.B.; Wilhite, S.E.; Ngau, W.C.; Ledoux, P.; Rudnev, D.; Lash, A.E.; Fujibuchi, W.; Edgar, R. NCBI GEO: Mining millions of expression profiles—Database and tools. Nucleic Acids Res. 2005, 33, D562–D566. [Google Scholar] [CrossRef]

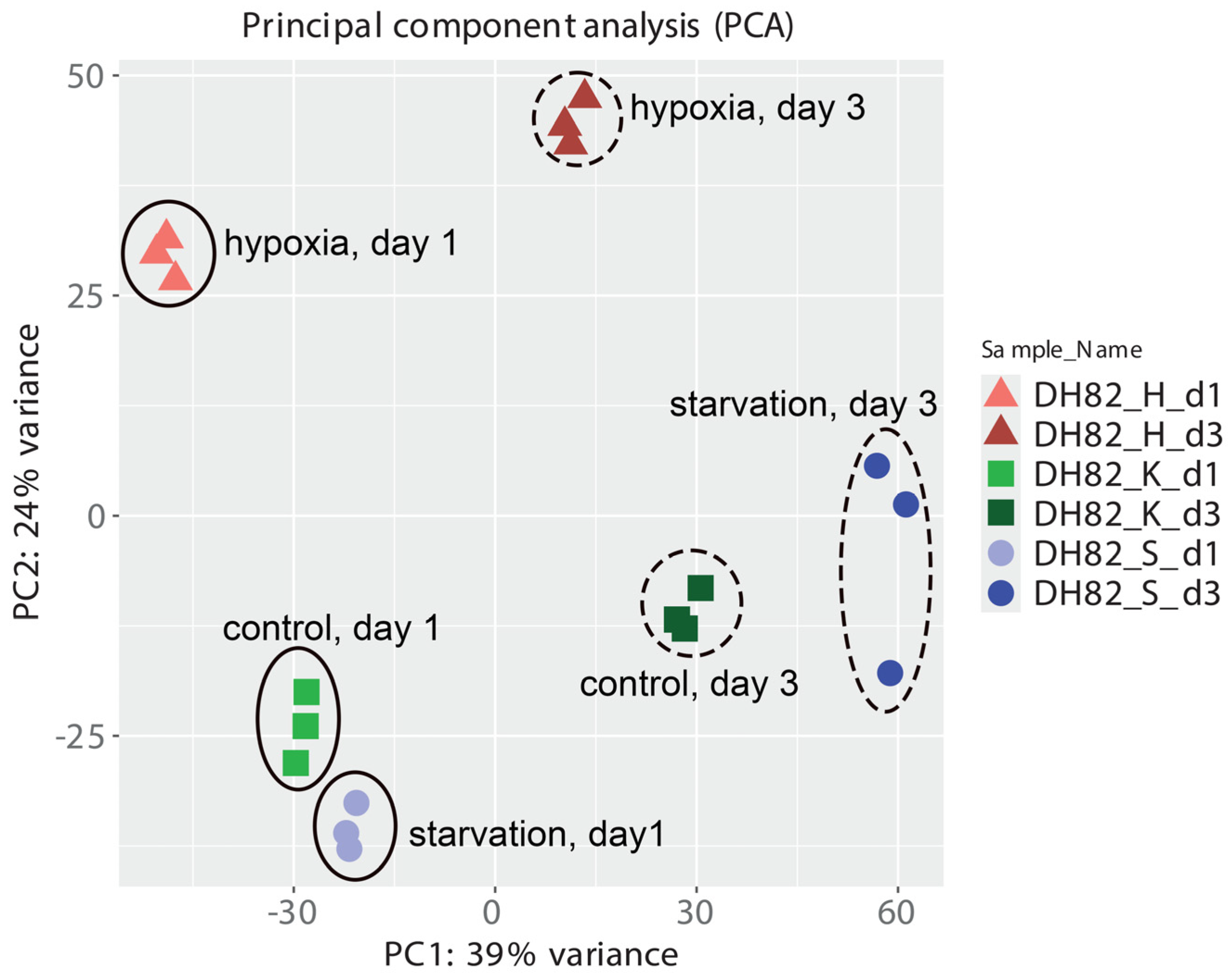


| Conditions | Total Cell Number (×106 cells/T25) | |||
|---|---|---|---|---|
| Day 1 | Day 3 | |||
| Median | Range | Median | Range | |
| Control | 6.58 | 5.50–6.99 | 6.58 | 5.75–7.85 |
| Hypoxia | 4.28 | 3.40–5.25 | 4.28 | 0.65–1.25 |
| Starvation | 5.85 | 4.65–8.05 | 5.85 | 6.00–7.75 |
| Conditions | Pairwise Comparisons | Number of DEGs | ||
|---|---|---|---|---|
| Upregulated | Downregulated | Total | ||
| Short-term hypoxia (1d) | K_d1 versus H_d1 | 589 | 1056 | 1645 |
| Short-term starvation (1d) | K_d1 versus S_d1 | 107 | 50 | 157 |
| Prolonged hypoxia (3d) | K_d3 versus H_d3 | 681 | 620 | 1301 |
| Prolonged starvation (3d) | K_d3 versus S_d3 | 249 | 587 | 836 |
| Control over time | K_d1 versus K_d3 | 619 | 704 | 1323 |
| Hypoxia over time | H_d1 versus H_d3 | 1606 | 1003 | 2609 |
| Starvation over time | S_d1 versus S_d3 | 891 | 1258 | 2149 |
| Comparisons | Number of DEGs | Functional Annotation Clusters: GO Terms (FDR Value) |
|---|---|---|
| Short-term hypoxia (1d) (total: 1645) | Up: 589 | Circulatory system development (<0.001), regulation of cell communication (<0.001), blood vessel morphogenesis (<0.001), vasculature development (<0.001), regulation of signal transduction (<0.001), cell adhesion (<0.001), angiogenesis (<0.001), regulation of cell migration (<0.001), regulation of cell motility (<0.001), positive regulation of angiogenesis (<0.001) |
| Down: 1056 | Cell cycle process (<0.001), cell cycle (<0.001), mitotic cell cycle process (<0.001), nuclear division (<0.001), chromosome segregation (<0.001), DNA metabolic process (<0.001), nuclear chromosome segregation (<0.001), DNA replication (<0.001), cell division (<0.001), DNA repair (<0.001) | |
| Short-term starvation (1d) (total: 157) | Up: 107 | Cellular response to chemical stimulus (<0.001), cell surface receptor signaling pathway (<0.05), negative regulation of response to stimulus (<0.05), second messenger-mediated signaling (<0.05), calcium-mediated signaling (<0.05) |
| Down: 50 | Cellular response to fibroblast growth factor stimulus (<0.05), response to fibroblast growth factor (<0.05) | |
| Prolonged hypoxia (3d) (total: 1301) | Up: 681 | Regulation of signal transduction (<0.001), cell surface receptor signaling pathway (<0.001), regulation of cell communication (<0.001), immune response (<0.001), positive regulation of response to stimulus (<0.001), cell adhesion (<0.001), biological adhesion (<0.001), inflammatory response (<0.001), vasculature development (<0.001), circulatory system development (<0.001) |
| Down: 620 | Lipid metabolic process (<0.001), cellular lipid metabolic process (<0.001), cell activation (<0.01), lipid biosynthetic process (<0.01), response to external stimulus (<0.01), cellular response to chemical stimulus (<0.01), fatty acid metabolic process (<0.01), regulation of cell communication (<0.05), regulation of lipid metabolic process (<0.05), membrane lipid metabolic process (<0.05) | |
| Prolonged starvation (3d) (total: 836) | Up: 249 | Cell surface receptor signaling pathway (<0.05), cell adhesion (<0.05), biological adhesion (<0.05), ERK1 and ERK2 cascade (<0.05), regulation of ERK1 and ERK2 cascade (<0.05), circulatory system development (<0.05), positive regulation of response to stimulus (<0.05), response to external stimulus (<0.05) |
| Down: 587 | Mitotic cell cycle (<0.001), mitotic cell cycle process (<0.001), cell cycle (<0.001), cell cycle process (<0.001), nuclear division (<0.001), chromosome segregation (<0.001), mitotic nuclear division (<0.001), mitotic sister chromatid segregation (<0.001), sister chromatid segregation (<0.001), cell division (<0.001) | |
| Control, d1 vs. d3 (total: 1323) | Up: 619 | n.s. |
| Down: 704 | Cell cycle process (<0.001), cell cycle (<0.001), mitotic cell cycle (<0.001), mitotic cell cycle process (<0.001), nuclear division (<0.001), mitotic nuclear division (<0.001), chromosome segregation (<0.001), chromosome organization (<0.001), DNA replication (<0.001), DNA metabolic process (<0.001) | |
| Hypoxia, d1 vs. d3 (total: 2609) | Up: 1606 | Regulation of gene expression (<0.001), transcription, DNA-templated (<0.001), regulation of RNA biosynthetic process (<0.001), RNA metabolic process (<0.001), RNA biosynthetic process (<0.001), regulation of response to stress (<0.001), positive regulation of metabolic process (<0.001), regulation of signal transduction (<0.001), regulation of apoptotic process (<0.001), regulation of defense response (<0.001) |
| Down: 1003 | Cell adhesion (<0.001), cell migration (<0.001), cell motility (<0.001), regulation of immune system process (<0.001), endocytosis (<0.001), cell activation (<0.001), response to external stimulus (<0.001), vasculature development (<0.001), angiogenesis (<0.001), phagocytosis (<0.001) | |
| Starvation, d1 vs. d3 (total: 2149) | Up: 891 | Lipid metabolic process (<0.001), single-organism catabolic process (<0.001), ion transport (<0.05), cell–cell signaling (<0.05), movement of cell or subcellular component (<0.05), transmembrane transport (<0.05), regulation of phosphate metabolic process (<0.05), regulation of protein phosphorylation (<0.05), regulation of cell communication (<0.05), regulation of cellular component movement (<0.05) |
| Down: 1258 | Cell cycle (<0.001), cell cycle process (<0.001), mitotic cell cycle (<0.001), nuclear division (<0.001), chromosome segregation (<0.001), chromosome organization (<0.001), DNA metabolic process (<0.001), DNA replication (<0.001), DNA repair (<0.001), cell migration (<0.001) |
| Comparisons | Number of DEGs | Functional Annotation Clusters: KEGG Pathways (FDR Value) |
|---|---|---|
| Short-term hypoxia (1d) (total: 1645) | Up: 589 | AGE-RAGE signaling pathway in diabetic complications (<0.001), Pathways in cancer (<0.001), HIF-1 signaling pathway (<0.001), TNF signaling pathway (<0.01), MAPK signaling pathway (<0.01), NF-κB signaling pathway (<0.01), Focal adhesion (<0.01), PI3K-Akt signaling pathway (<0.05), Endocytosis (<0.05), MicroRNAs in cancer (<0.05) |
| Down: 1056 | Cell cycle (<0.001), Fanconi anemia pathway (<0.001), DNA replication (<0.001), Homologous recombination (<0.01), Mismatch repair (<0.01) | |
| Short-term starvation (1d) (total: 157) | Up: 107 | n.s. |
| Down: 50 | Complement and coagulation cascades (<0.001), Pathways in cancer (<0.05) | |
| Prolonged hypoxia (3d) (total: 1301) | Up: 681 | IL-17 signaling pathway (<0.001), TNF signaling pathway (<0.001), Cytokine–cytokine receptor interaction (<0.001), Pathways in cancer (<0.001), NF-κB signaling pathway (<0.001), C-type lectin receptor signaling pathway (<0.001), Cell adhesion molecules (<0.001), MAPK signaling pathway (<0.001), JAK-STAT signaling pathway (<0.001), NOD-like receptor signaling pathway (<0.001) |
| Down: 620 | Metabolic pathways (<0.001) | |
| Prolonged starvation (3d) (total: 836) | Up: 249 | Complement and coagulation cascades (<0.05) |
| Down: 587 | Cell cycle (<0.001), Pathways in cancer (<0.05) | |
| Control, d1 vs. d3 (total: 1323) | Up: 619 | n.s. |
| Down: 704 | Cell cycle (<0.001), DNA replication (<0.001), Homologous recombination (<0.001), Mismatch repair (<0.001), Steroid biosynthesis (<0.01), Base excision repair (<0.01), Pyrimidine metabolism (<0.01), p53 signaling pathway (<0.05), Transcriptional misregulation in cancer (<0.05), IL-17 signaling pathway (<0.05) | |
| Hypoxia, d1 vs. d3 (total: 2609) | Up: 1606 | Cytokine–cytokine receptor interaction (<0.01), JAK-STAT signaling pathway (<0.05), Neurotrophin signaling pathway (<0.05), TNF signaling pathway (<0.05) |
| Down: 1003 | Focal adhesion (<0.001), ECM–receptor interaction (<0.001), Steroid biosynthesis (<0.001), Proteoglycans in cancer (<0.01), Phagosome (<0.01), Rap1 signaling pathway (<0.01), Endocytosis (<0.01), PI3K-Akt signaling pathway (<0.01), Metabolic pathways (<0.05), Glutathione metabolism (<0.05) | |
| Starvation, d1 vs. d3 (total: 2149) | Up: 891 | n.s. |
| Down: 1258 | Cell cycle (<0.001), DNA replication (<0.001), ECM–receptor interaction (<0.01), Cytokine–cytokine receptor interaction (<0.01), PI3K-Akt signaling pathway (<0.05), Motor proteins (<0.05), Pyrimidine metabolism (<0.05), Mismatch repair (<0.05), Base excision repair (<0.05), Steroid biosynthesis (<0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asawapattanakul, T.; Schughart, K.; von Köckritz-Blickwede, M.; Armando, F.; Claus, P.; Baumgärtner, W.; Puff, C. Transcriptomic Alterations of Canine Histiocytic Sarcoma Cells in Response to Different Stressors. Int. J. Mol. Sci. 2025, 26, 6629. https://doi.org/10.3390/ijms26146629
Asawapattanakul T, Schughart K, von Köckritz-Blickwede M, Armando F, Claus P, Baumgärtner W, Puff C. Transcriptomic Alterations of Canine Histiocytic Sarcoma Cells in Response to Different Stressors. International Journal of Molecular Sciences. 2025; 26(14):6629. https://doi.org/10.3390/ijms26146629
Chicago/Turabian StyleAsawapattanakul, Thanaporn, Klaus Schughart, Maren von Köckritz-Blickwede, Federico Armando, Peter Claus, Wolfgang Baumgärtner, and Christina Puff. 2025. "Transcriptomic Alterations of Canine Histiocytic Sarcoma Cells in Response to Different Stressors" International Journal of Molecular Sciences 26, no. 14: 6629. https://doi.org/10.3390/ijms26146629
APA StyleAsawapattanakul, T., Schughart, K., von Köckritz-Blickwede, M., Armando, F., Claus, P., Baumgärtner, W., & Puff, C. (2025). Transcriptomic Alterations of Canine Histiocytic Sarcoma Cells in Response to Different Stressors. International Journal of Molecular Sciences, 26(14), 6629. https://doi.org/10.3390/ijms26146629









