Deep Proteomics Analysis Unravels the Molecular Signatures of Tonsillar B Cells in PFAPA and OSAS in the Pediatric Population
Abstract
1. Introduction
2. Results
- -
- Enrolled population:
- -
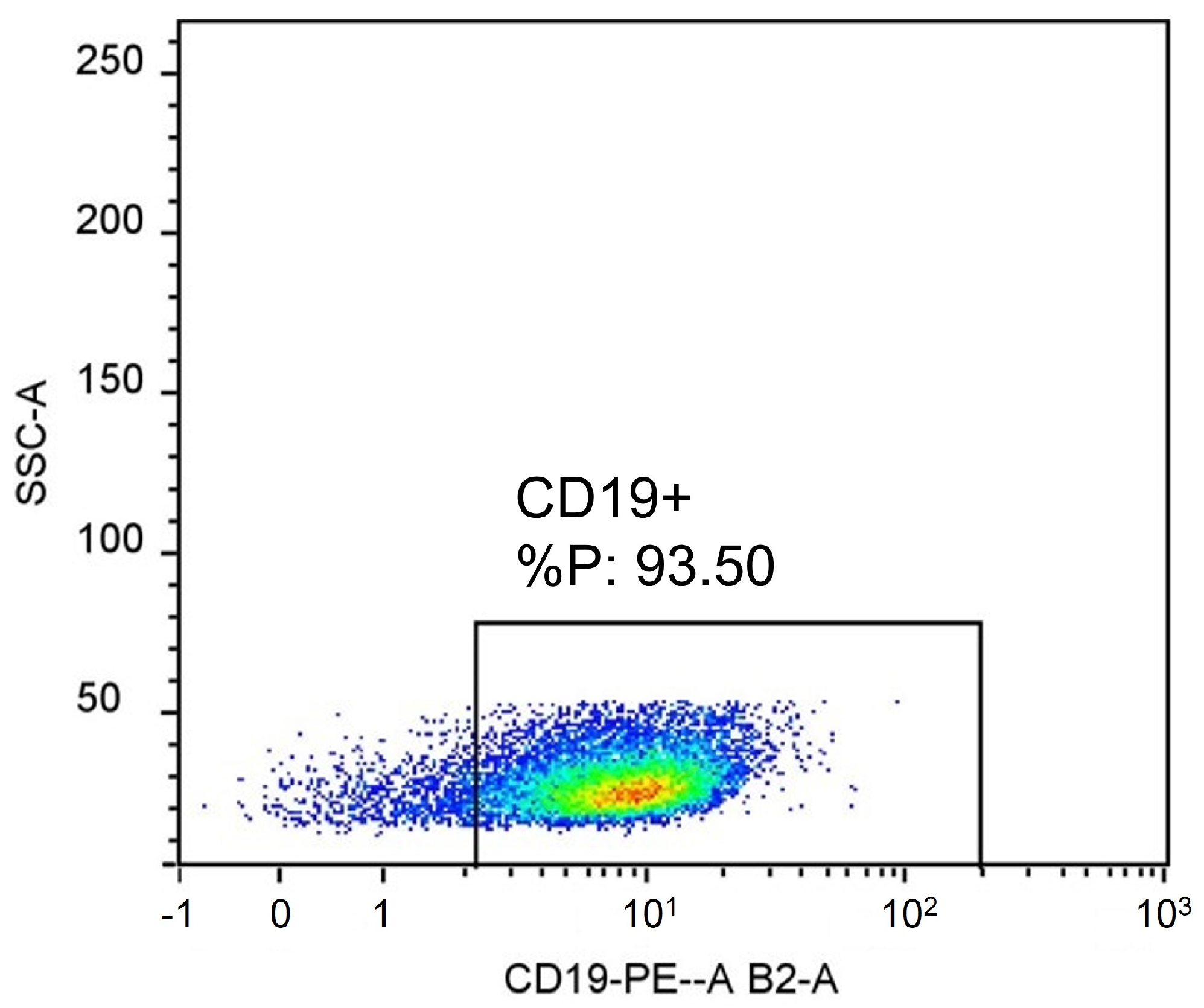
- Flow cytometry:
- -
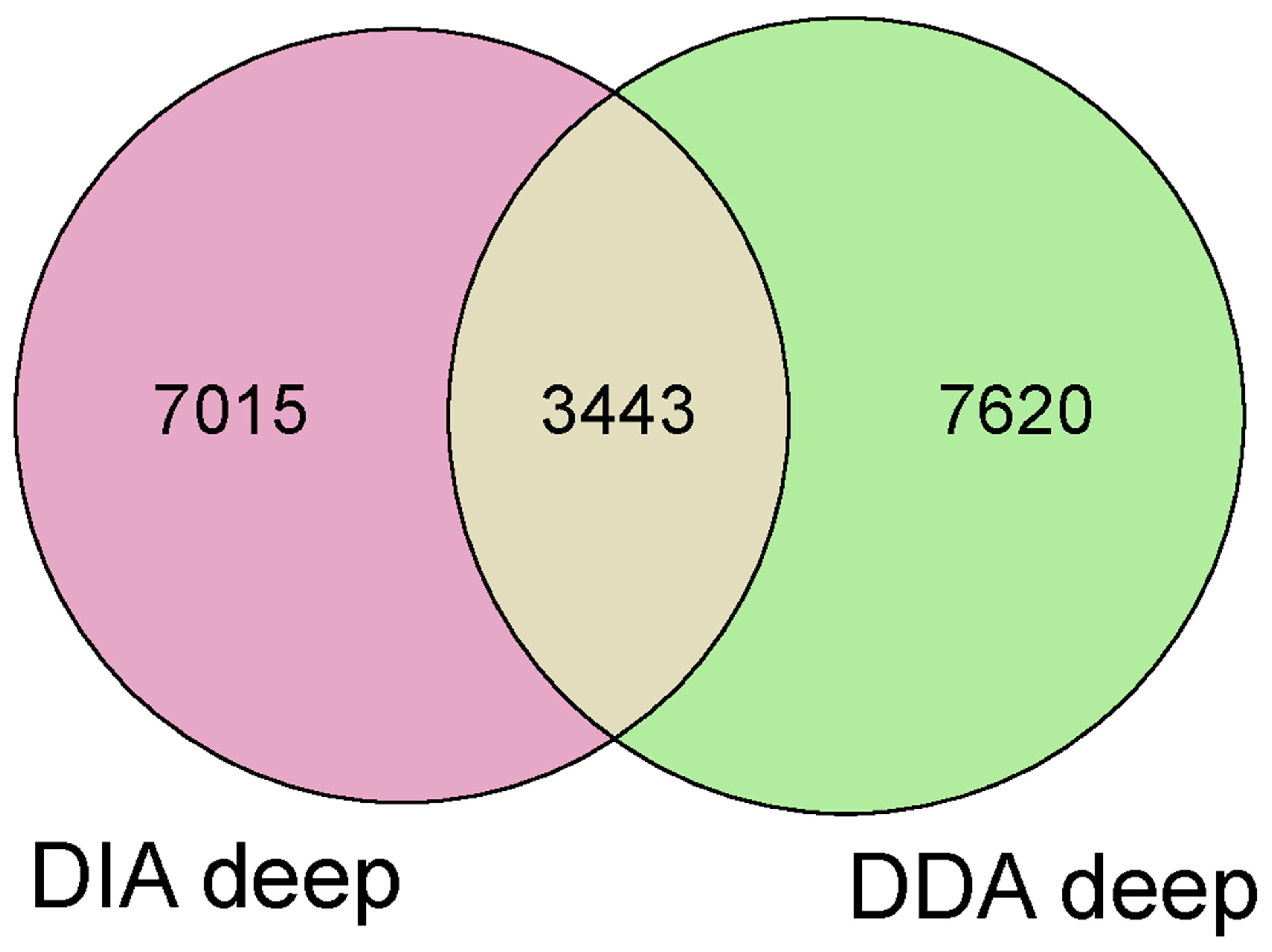
- Proteomics study:
- -
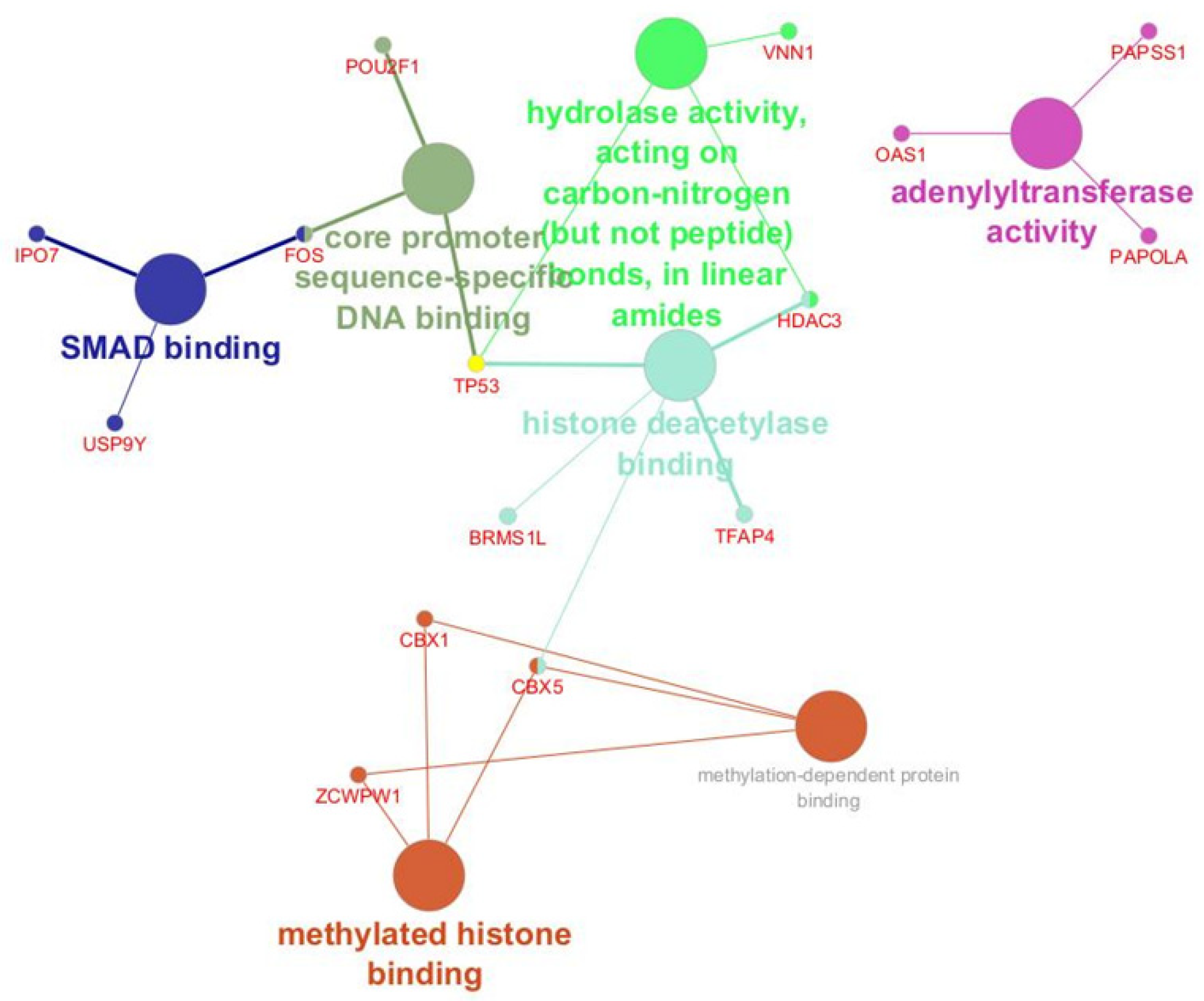
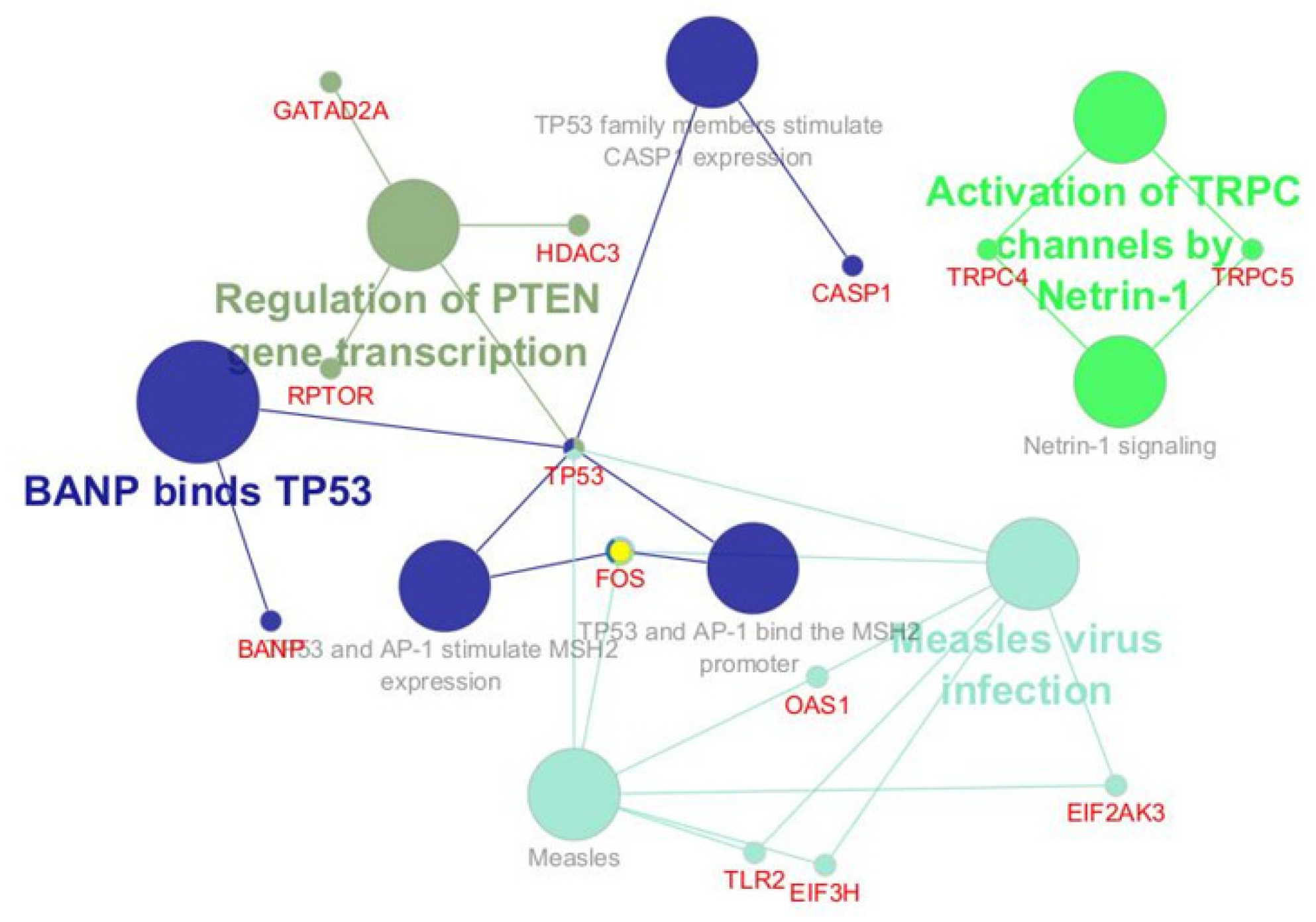
- Bioinformatic Analysis
- -
- Western blotting
3. Discussion
4. Materials and Methods
4.1. Patient Enrolment
- Regularly occurring fevers (without evidence of upper respiratory infection).
- Onset prior to age 5 years.
- At least one of the following is associated with fevers: aphthous stomatitis, pharyngitis, cervical adenitis.
- Resolution of symptoms with a single, low dose of Betamethasone (0.1 mg/kg po once in the first 24 h of a febrile episode).
- Normal growth as assessed by World Health Organization growth charts.
- Asymptomatic between episodes.
- Age (2–11).
- Tonsil hypertrophy 3 or 4 according to the Brodsky scale [91].
- Genetically defined autoinflammatory disorders (familial Mediterranean fever, Hyper-IgD syndrome/mevalonic kinase deficiency, TNF receptor-associated periodic syndrome, cryopyrin-associated periodic syndrome).
- Cyclic neutropenia.
- Immunodeficiency or autoimmunity.
- Craniofacial abnormality.
- Neuromuscular disease.
- Chromosomal abnormality.
- Previous adenotonsillar surgery.
- Bleeding disorder.
- Cardiopulmonary disease.
- History of recurrent tonsillitis.
4.2. Sample Collection
4.3. Isolation of Mononuclear Cells (MNCs) from Human Palatine Tonsils
4.4. Isolating CD19+ Cells with the Magnetic Microbeads
4.5. Protein Extraction
4.6. Flow Cytometry
4.7. Proteomics
4.8. Bioinformatic Analysis
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massoni-Badosa, R.; Aguilar-Fernández, S.; Nieto, J.C.; Soler-Vila, P.; Elosua-Bayes, M.; Marchese, D.; Kulis, M.; Vilas-Zornoza, A.; Bühler, M.M.; Rashmi, S.; et al. An atlas of cells in the human tonsil. Immunity 2024, 57, 379–399.e18. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Kambas, I.; Tsaoussoglou, M.; Panaghiotopoulou-Gartagani, P.; Chrousos, G.; Kaditis, A.G. Age-dependent changes in the size of adenotonsillar tissue in childhood: Implications for sleep-disordered breathing. J. Pediatr. 2013, 162, 269–274.e4. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A.; Kara, C.O.; Dagdeviren, E.; Zencir, M. Variation in Tonsil Size in 4- to 17-Year-old Schoolchildren. J. Otolaryngol. 2006, 35, 270. [Google Scholar] [CrossRef]
- Lee, J.; Chang, D.-Y.; Kim, S.-W.; Choi, Y.S.; Jeon, S.-Y.; Racanelli, V.; Kim, D.W.; Shin, E.-C. Age-related differences in human palatine tonsillar B cell subsets and immunoglobulin isotypes. Clin. Exp. Med. 2016, 16, 81–87. [Google Scholar] [CrossRef]
- Huntington, N.D.; Vosshenrich, C.A.J.; Di Santo, J.P. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 2007, 7, 703–714. [Google Scholar] [CrossRef]
- Khan, W.N. Regulation of B lymphocyte development and activation by Bruton’s tyrosine kinase. Immunol. Res. 2001, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Dammeijer, F.; Hendriks, R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 2018, 17, 57. [Google Scholar] [CrossRef]
- Sarmiento Varon, L.; de Rosa, J.; Machicote, A.; Billordo, L.A.; Baz, P.; Fernández, P.M.; Kaimen Maciel, I.; Blanco, A.; Arana, E.I. Characterization of tonsillar IL10 secreting B cells and their role in the pathophysiology of tonsillar hypertrophy. Sci. Rep. 2017, 7, 11077. [Google Scholar] [CrossRef]
- Geißler, K.; Markwart, R.; Requardt, R.P.; Weigel, C.; Schubert, K.; Scherag, A.; Rubio, I.; Guntinas-Lichius, O. Functional characterization of T-cells from palatine tonsils in patients with chronic tonsillitis. PLoS ONE 2017, 12, e0183214. [Google Scholar] [CrossRef]
- Luu, I.; Sharma, A.; Guaderrama, M.; Peru, M.; Nation, J.; Page, N.; Carvalho, D.; Magit, A.; Jiang, W.; Leuin, S.; et al. Immune Dysregulation in the Tonsillar Microenvironment of Periodic Fever, Aphthous Stomatitis, Pharyngitis, Adenitis (PFAPA) Syndrome. J. Clin. Immunol. 2020, 40, 179–190. [Google Scholar] [CrossRef]
- Petra, D.; Petra, K.; Michaela, K.; Daniela, K.; Petr, H.; Ladislav, K.; Rami, K.; Ondrej, H.; Zdenek, K.; Pavla, D.; et al. Polyclonal, newly derived T cells with low expression of inhibitory molecule PD-1 in tonsils define the phenotype of lymphocytes in children with Periodic Fever, Aphtous Stomatitis, Pharyngitis and Adenitis (PFAPA) syndrome. Mol. Immunol. 2015, 65, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kolly, L.; Busso, N.; von Scheven-Gete, A.; Bagnoud, N.; Moix, I.; Holzinger, D.; Simon, G.; Ives, A.; Guarda, G.; So, A.; et al. Periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome is linked to dysregulated monocyte IL-1β production. J. Allergy Clin. Immunol. 2013, 131, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, K.; Vanoni, F.; Hofer, M. Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Cervical Adenitis (PFAPA) Syndrome: A Review of the Pathogenesis. Curr. Rheumatol. Rep. 2016, 18, 18. [Google Scholar] [CrossRef]
- Lovšin, E.; Kovač, J.; Tesovnik, T.; Toplak, N.; Perko, D.; Rozmarič, T.; Debeljak, M.; Avčin, T. PIK3AP1 and SPON2 Genes Are Differentially Methylated in Patients With Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Adenitis (PFAPA) Syndrome. Front. Immunol. 2020, 11, 1322. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bhattacharjee, R.; Dayyat, E.; Snow, A.B.; Kheirandish-Gozal, L.; Goldman, J.L.; Li, R.C.; Serpero, L.D.; Clair, H.B.; Gozal, D. Increased Cellular Proliferation and Inflammatory Cytokines in Tonsils Derived from Children with Obstructive Sleep Apnea. Pediatr. Res. 2009, 66, 423–428. [Google Scholar] [CrossRef]
- Viciani, E.; Montagnani, F.; Tavarini, S.; Tordini, G.; Maccari, S.; Morandi, M.; Faenzi, E.; Biagini, C.; Romano, A.; Salerni, L.; et al. Paediatric obstructive sleep apnoea syndrome (OSAS) is associated with tonsil colonisation by Streptococcus pyogenes. Sci. Rep. 2016, 6, 20609. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Z.; Fu, T.; Liu, S.; Cui, J.; Zhang, B.; Liang, J.; Pang, C.; Ke, Y.; Wang, R.; et al. Parallel comparison of T cell and B cell subpopulations of adenoid hypertrophy and tonsil hypertrophy of children. Nat. Commun. 2025, 16, 3516. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Sharma, E.V.; Jun, J.C.; Polotsky, V.Y. Metabolic dysfunction in obstructive sleep apnea: A critical examination of underlying mechanisms. Sleep Biol. Rhythm. 2015, 13, 2–17. [Google Scholar] [CrossRef]
- Carrasco, A.; Sjölander, I.; van Acker, A.; Dernstedt, A.; Fehrm, J.; Forsell, M.; Friberg, D.; Mjösberg, J.; Rao, A. The Tonsil Lymphocyte Landscape in Pediatric Tonsil Hyperplasia and Obstructive Sleep Apnea. Front. Immunol. 2021, 12, 674080. [Google Scholar] [CrossRef]
- Malicki, M.; Karuga, F.F.; Szmyd, B.; Sochal, M.; Gabryelska, A. Obstructive Sleep Apnea, Circadian Clock Disruption, and Metabolic Consequences. Metabolites 2022, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Turkiewicz, S.; Karuga, F.F.; Sochal, M.; Strzelecki, D.; Białasiewicz, P. Disruption of Circadian Rhythm Genes in Obstructive Sleep Apnea Patients-Possible Mechanisms Involved and Clinical Implication. Int. J. Mol. Sci. 2022, 23, 709. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, F.; Kasap, M.; Yaprak Bayrak, B.; Sarıhan, M.; Şahin, N.; Önal, A.; Akpınar, G.; Bayrak, Y.E.; Sönmez, H.E. The first proteomics analysis of tonsils in patients with periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome (PFAPA). Pediatr. Res. 2024. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Rodriguez-Pastrana, I.; Birli, E.; Coutts, A.S. p53-dependent DNA repair during the DNA damage response requires actin nucleation by JMY. Cell Death Differ. 2023, 30, 1636–1647. [Google Scholar] [CrossRef]
- Meng, Z.; Jia, L.-F.; Gan, Y.-H. PTEN activation through K163 acetylation by inhibiting HDAC6 contributes to tumour inhibition. Oncogene 2016, 35, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Zhang, J.; Zhang, D.; Li, M.; Yan, H.; Zhang, H.; Song, L.; Wang, J.; Hou, Z.; et al. p66α Suppresses Breast Cancer Cell Growth and Migration by Acting as Co-Activator of p53. Cells 2021, 10, 3593. [Google Scholar] [CrossRef]
- Li, K.; Wei, X.; Zhang, L.; Chi, H.; Yang, J. Raptor/mTORC1 Acts as a Modulatory Center to Regulate Anti-bacterial Immune Response in Rockfish. Front. Immunol. 2019, 10, 2953. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Pan, Z.; Hu, K. New insights from integrated bioinformatics analysis: The role of circadian rhythm disruption and immune infiltration in obstructive sleep apnea disease. Front. Immunol. 2023, 14, 1273114. [Google Scholar] [CrossRef]
- Galliher-Beckley, A.J.; Lan, L.-Q.; Aono, S.; Wang, L.; Shi, J. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World J. Biol. Chem. 2013, 4, 30–34. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hoes, M.F.; Tromp, J.; Ouwerkerk, W.; Bomer, N.; Oberdorf-Maass, S.U.; Samani, N.J.; Ng, L.L.; Lang, C.C.; van der Harst, P.; Hillege, H.; et al. The role of cathepsin D in the pathophysiology of heart failure and its potentially beneficial properties: A translational approach. Eur. J. Heart Fail. 2020, 22, 2102–2111. [Google Scholar] [CrossRef]
- Dell’Italia, L.J.; Collawn, J.F.; Ferrario, C.M. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ. Res. 2018, 122, 319–336. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.; Laumet, S.; Inyang, K.E.; Hua, H.; Sim, J.; Folger, J.K.; Moeser, A.J.; Laumet, G. Mast cell-derived chymases are essential for the resolution of inflammatory pain in mice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ma, J.; Wei, K.; Liu, J.; Tang, K.; Zhang, H.; Zhu, L.; Chen, J.; Li, F.; Xu, P.; Chen, J.; et al. Glycogen metabolism regulates macrophage-mediated acute inflammatory responses. Nat. Commun. 2020, 11, 1769. [Google Scholar] [CrossRef]
- Schollen, E.; Frank, C.G.; Keldermans, L.; Reyntjens, R.; Grubenmann, C.E.; Clayton, P.T.; Winchester, B.G.; Smeitink, J.; Wevers, R.A.; Aebi, M.; et al. Clinical and molecular features of three patients with congenital disorders of glycosylation type Ih (CDG-Ih) (ALG8 deficiency). J. Med. Genet. 2004, 41, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Chantret, I.; Dancourt, J.; Dupré, T.; Delenda, C.; Bucher, S.; Vuillaumier-Barrot, S.; Ogier de Baulny, H.; Peletan, C.; Danos, O.; Seta, N.; et al. A deficiency in dolichyl-P-glucose:Glc1Man9GlcNAc2-PP-dolichyl alpha3-glucosyltransferase defines a new subtype of congenital disorders of glycosylation. J. Biol. Chem. 2003, 278, 9962–9971. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, B.-F.; Xiao, W.; Yang, X.; Sun, H.-Y.; Zhao, Y.-L.; Yang, Y.-G. Dynamic m 6 A modification and its emerging regulatory role in mRNA splicing. Sci. Bull. 2015, 60, 21–32. [Google Scholar] [CrossRef]
- Diaz-Muñoz, M.D.; Bell, S.E.; Fairfax, K.; Monzon-Casanova, E.; Cunningham, A.F.; Gonzalez-Porta, M.; Andrews, S.R.; Bunik, V.I.; Zarnack, K.; Curk, T.; et al. The RNA-binding protein HuR is essential for the B cell antibody response. Nat. Immunol. 2015, 16, 415–425. [Google Scholar] [CrossRef]
- Sobocińska, J.; Molenda, S.; Machnik, M.; Oleksiewicz, U. KRAB-ZFP Transcriptional Regulators Acting as Oncogenes and Tumor Suppressors: An Overview. Int. J. Mol. Sci. 2021, 22, 2212. [Google Scholar] [CrossRef]
- Lavie, L. Obstructive sleep apnoea syndrome—An oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Urrutia, R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 2003, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kono, H. Heterochromatin protein 1 (HP1): Interactions with itself and chromatin components. Biophys. Rev. 2020, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lu, J.; Pan, B.; Fan, H.; Byrum, S.D.; Xu, C.; Kim, A.; Guo, Y.; Kanchi, K.L.; Gong, W.; et al. TNRC18 engages H3K9me3 to mediate silencing of endogenous retrotransposons. Nature 2023, 623, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-P.; Chen, K.; Feng, Z.; Wen, X.; Sun, H. Synergistic antitumor activity of artesunate and HDAC inhibitors through elevating heme synthesis via synergistic upregulation of ALAS1 expression. Acta Pharm. Sin. B 2019, 9, 937–951. [Google Scholar] [CrossRef]
- Qiu, X.; Yao, Y.; Chen, Y.; Li, Y.; Sun, X.; Zhu, X. TRPC5 Promotes Intermittent Hypoxia-Induced Cardiomyocyte Injury Through Oxidative Stress. Nat. Sci. Sleep 2024, 16, 2125–2141. [Google Scholar] [CrossRef]
- Wen, W.; Yao, Q.; Chen, Y.; Li, Z.; Maitikuerban, B.; Zhang, Y.; Zhang, J.; Simayi, Z.; Xu, X.; Zhang, X. Transient receptor potential canonical 5 channel is involved in the cardiac damage related to obstructive sleep apnea-hypopnea syndrome in rats. Ann. Palliat. Med. 2020, 9, 895–902. [Google Scholar] [CrossRef]
- Abramowitz, J.; Birnbaumer, L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009, 23, 297–328. [Google Scholar] [CrossRef]
- Yu, L.; Yu, T.-T.; Young, K.H. Cross-talk between Myc and p53 in B-cell lymphomas. Chronic Dis. Transl. Med. 2019, 5, 139–154. [Google Scholar] [CrossRef]
- He, R.; He, Z.; Zhang, T.; Liu, B.; Gao, M.; Li, N.; Geng, Q. HDAC3 in action: Expanding roles in inflammation and inflammatory diseases. Cell Prolif. 2025, 58, e13731. [Google Scholar] [CrossRef]
- Stengel, K.R.; Bhaskara, S.; Wang, J.; Liu, Q.; Ellis, J.D.; Sampathi, S.; Hiebert, S.W. Histone deacetylase 3 controls a transcriptional network required for B cell maturation. Nucleic Acids Res. 2019, 47, 10612–10627. [Google Scholar] [CrossRef] [PubMed]
- Tonc, E.; Takeuchi, Y.; Chou, C.; Xia, Y.; Holmgren, M.; Fujii, C.; Raju, S.; Chang, G.S.; Iwamoto, M.; Egawa, T. Unexpected suppression of tumorigenesis by c-MYC via TFAP4-dependent restriction of stemness in B lymphocytes. Blood 2021, 138, 2526–2538. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.C.; Welch, D.R. BRMS1: A multifunctional signaling molecule in metastasis. Cancer Metastasis Rev. 2020, 39, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Harioudh, M.K.; Perez, J.; Chong, Z.; Nair, S.; So, L.; McCormick, K.D.; Ghosh, A.; Shao, L.; Srivastava, R.; Soveg, F.; et al. Oligoadenylate synthetase 1 displays dual antiviral mechanisms in driving translational shutdown and protecting interferon production. Immunity 2024, 57, 446–461.e7. [Google Scholar] [CrossRef]
- Venkatachalam, K.V. Human 3’-phosphoadenosine 5’-phosphosulfate (PAPS) synthase: Biochemistry, molecular biology and genetic deficiency. IUBMB Life 2003, 55, 1–11. [Google Scholar] [CrossRef]
- Brown, K.L.; Maiti, A.; Johnson, P. Role of sulfation in CD44-mediated hyaluronan binding induced by inflammatory mediators in human CD14(+) peripheral blood monocytes. J. Immunol. 2001, 167, 5367–5374. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Turner, M. Regulation and function of poised mRNAs in lymphocytes. Bioessays 2023, 45, e2200236. [Google Scholar] [CrossRef]
- Neve, J.; Patel, R.; Wang, Z.; Louey, A.; Furger, A.M. Cleavage and polyadenylation: Ending the message expands gene regulation. RNA Biol. 2017, 14, 865–890. [Google Scholar] [CrossRef]
- Zhang, Y.; Good-Jacobson, K.L. Epigenetic regulation of B cell fate and function during an immune response. Immunol. Rev. 2019, 288, 75–84. [Google Scholar] [CrossRef]
- Horvath, R.; Chinnery, P.F. Modifying mitochondrial tRNAs: Delivering what the cell needs. Cell Metab. 2015, 21, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Mitochondrial function in immune cells in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165845. [Google Scholar] [CrossRef] [PubMed]
- Marchingo, J.M. tRNA methylation—A new level of control for T cell immunity. Nat. Immunol. 2022, 23, 1401–1402. [Google Scholar] [CrossRef] [PubMed]
- Hori, H. Methylated nucleosides in tRNA and tRNA methyltransferases. Front. Genet. 2014, 5, 144. [Google Scholar] [CrossRef]
- Peterson, M.L. Mechanisms controlling production of membrane and secreted immunoglobulin during B cell development. Immunol. Res. 2007, 37, 33–46. [Google Scholar] [CrossRef]
- Hollerer, I.; Grund, K.; Hentze, M.W.; Kulozik, A.E. mRNA 3′end processing: A tale of the tail reaches the clinic. EMBO Mol. Med. 2014, 6, 16–26. [Google Scholar] [CrossRef]
- Lam, J.H.; Baumgarth, N. Toll-like receptor mediated inflammation directs B cells towards protective antiviral extrafollicular responses. Nat. Commun. 2023, 14, 3979. [Google Scholar] [CrossRef]
- Browne, E.P. Regulation of B-cell responses by Toll-like receptors. Immunology 2012, 136, 370–379. [Google Scholar] [CrossRef]
- Xia, X.; Cui, J.; Wang, H.Y.; Zhu, L.; Matsueda, S.; Wang, Q.; Yang, X.; Hong, J.; Songyang, Z.; Chen, Z.J.; et al. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 2011, 34, 843–853. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kalai, M.; Saelens, X.; Declercq, W.; Vandenabeele, P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J. Biol. Chem. 2004, 279, 24785–24793. [Google Scholar] [CrossRef]
- Doughty, C.A.; Bleiman, B.F.; Wagner, D.J.; Dufort, F.J.; Mataraza, J.M.; Roberts, M.F.; Chiles, T.C. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: Role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 2006, 107, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Strasser, A.; Kelly, G.L. Tumor-Suppressor Functions of the TP53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026062. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yang, L.; Guan, F.; Miller, H.; Camara, N.O.S.; James, L.K.; Benlagha, K.; Kubo, M.; Heegaard, S.; Lee, P.; et al. The role of Raptor in lymphocytes differentiation and function. Front. Immunol. 2023, 14, 1146628. [Google Scholar] [CrossRef]
- Iwata, T.N.; Ramírez-Komo, J.A.; Park, H.; Iritani, B.M. Control of B lymphocyte development and functions by the mTOR signaling pathways. Cytokine Growth Factor Rev. 2017, 35, 47–62. [Google Scholar] [CrossRef]
- McCaughey, J.; Stevenson, N.L.; Mantell, J.M.; Neal, C.R.; Paterson, A.; Heesom, K.; Stephens, D.J. A general role for TANGO1, encoded by MIA3, in secretory pathway organization and function. J. Cell Sci. 2021, 134, jcs259075. [Google Scholar] [CrossRef]
- Puzio-Kuter, A.M. The Role of p53 in Metabolic Regulation. Genes Cancer 2011, 2, 385–391. [Google Scholar] [CrossRef]
- Kim, H.-M.; Zheng, X.; Lee, E. The Interplay between Histone Modifiers and p53 in Regulating Gene Expression. Int. J. Mol. Sci. 2023, 24, 11032. [Google Scholar] [CrossRef] [PubMed]
- Oh-hora, M.; Rao, A. Calcium signaling in lymphocytes. Curr. Opin. Immunol. 2008, 20, 250–258. [Google Scholar] [CrossRef]
- Scharenberg, A.M.; Humphries, L.A.; Rawlings, D.J. Calcium signalling and cell-fate choice in B cells. Nat. Rev. Immunol. 2007, 7, 778–789. [Google Scholar] [CrossRef]
- Froghi, S.; Grant, C.R.; Tandon, R.; Quaglia, A.; Davidson, B.; Fuller, B. New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice. Clin. Rev. Allergy Immunol. 2021, 60, 271–292. [Google Scholar] [CrossRef]
- Kushnir, A.; Santulli, G.; Reiken, S.R.; Coromilas, E.; Godfrey, S.J.; Brunjes, D.L.; Colombo, P.C.; Yuzefpolskaya, M.; Sokol, S.I.; Kitsis, R.N.; et al. Ryanodine Receptor Calcium Leak in Circulating B-Lymphocytes as a Biomarker in Heart Failure. Circulation 2018, 138, 1144–1154. [Google Scholar] [CrossRef]
- Getahun, A.; Wemlinger, S.M.; Rudra, P.; Santiago, M.L.; van Dyk, L.F.; Cambier, J.C. Impaired B cell function during viral infections due to PTEN-mediated inhibition of the PI3K pathway. J. Exp. Med. 2017, 214, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Setz, C.S.; Khadour, A.; Renna, V.; Iype, J.; Gentner, E.; He, X.; Datta, M.; Young, M.; Nitschke, L.; Wienands, J.; et al. Pten controls B-cell responsiveness and germinal center reaction by regulating the expression of IgD BCR. EMBO J. 2019, 38, e100249. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.J.; Li, A.G.; Wei, G.; Li, H.-H.; Kertesz, N.; Lesche, R.; Whale, A.D.; Martinez-Diaz, H.; Rozengurt, N.; Cardiff, R.D.; et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 2003, 3, 117–130. [Google Scholar] [CrossRef]
- Grand, R.S.; Burger, L.; Gräwe, C.; Michael, A.K.; Isbel, L.; Hess, D.; Hoerner, L.; Iesmantavicius, V.; Durdu, S.; Pregnolato, M.; et al. BANP opens chromatin and activates CpG-island-regulated genes. Nature 2021, 596, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kaileh, M.; Sen, R. NF-κB function in B lymphocytes. Immunol. Rev. 2012, 246, 254–271. [Google Scholar] [CrossRef]
- Hemmers, S.; Rudensky, A.Y. The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Rep. 2015, 11, 1339–1349. [Google Scholar] [CrossRef]
- Downton, P.; Early, J.O.; Gibbs, J.E. Circadian rhythms in adaptive immunity. Immunology 2020, 161, 268–277. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Li, C.; Cao, H.; Chen, Y.; Zhou, W.; Yang, G.; Yang, H. Circadian protein CLOCK modulates regulatory B cell functions of nurses engaging day-night shift rotation. Cell Signal. 2022, 96, 110362. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Niu, Z.; Peng, J.; Li, Q.; Xiong, W.; Langnas, A.N.; Ma, M.Y.; Zhao, Y. MOP3, a component of the molecular clock, regulates the development of B cells. Immunology 2006, 119, 451–460. [Google Scholar] [CrossRef]
- Brodsky, L. Modern Assessment of Tonsils and Adenoids. Pediatr. Clin. N. Am. 1989, 36, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Assadian, F.; Sandström, K.; Laurell, G.; Svensson, C.; Akusjärvi, G.; Punga, T. Efficient Isolation Protocol for B and T Lymphocytes from Human Palatine Tonsils. J. Vis. Exp. 2015, e53374. [Google Scholar] [CrossRef]
- Kharrat, F.; Capaci, V.; Conti, A.; Golino, V.; Campiglia, P.; Balasan, N.; Aloisio, M.; Licastro, D.; Monasta, L.; Caponneto, F.; et al. A Pilot Study of Exosome Proteomic Profiling Reveals Dysregulated Metabolic Pathways in Endometrial Cancer. Biomedicines 2025, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- del Vecchio, C.; Gratton, R.; Nait-Meddour, C.; Nardacchione, E.M.; Moura, R.; Sommella, E.; Moltrasio, C.; Marzano, A.V.; Ura, B.; Mentino, D.; et al. Dysregulation of Aquaporin-3 and Glyceryl Glucoside Restoring Action in Hidradenitis Suppurativa in Vitro Models. Cell Physiol. Biochem. 2024, 58, 404–417. [Google Scholar]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]

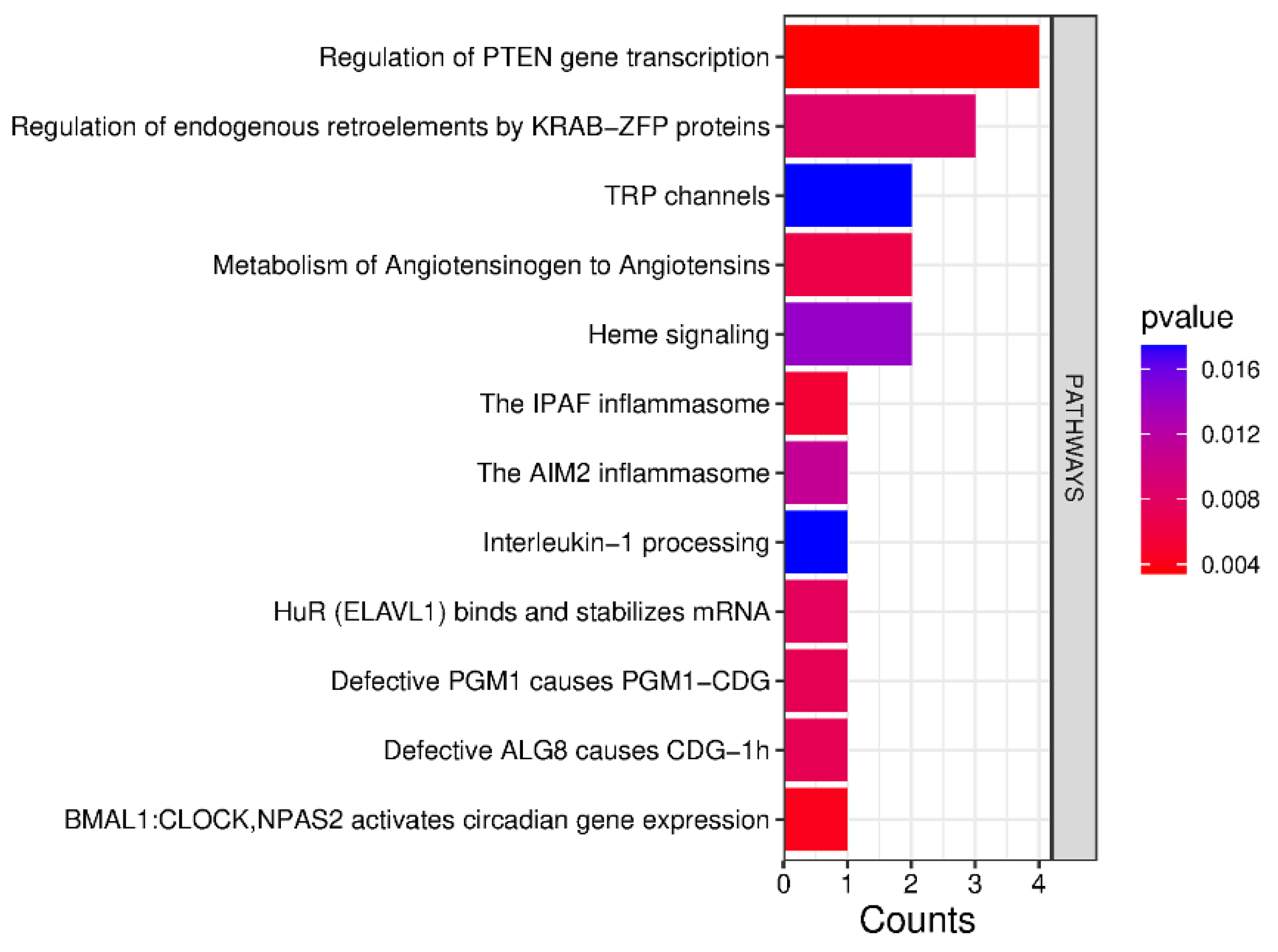
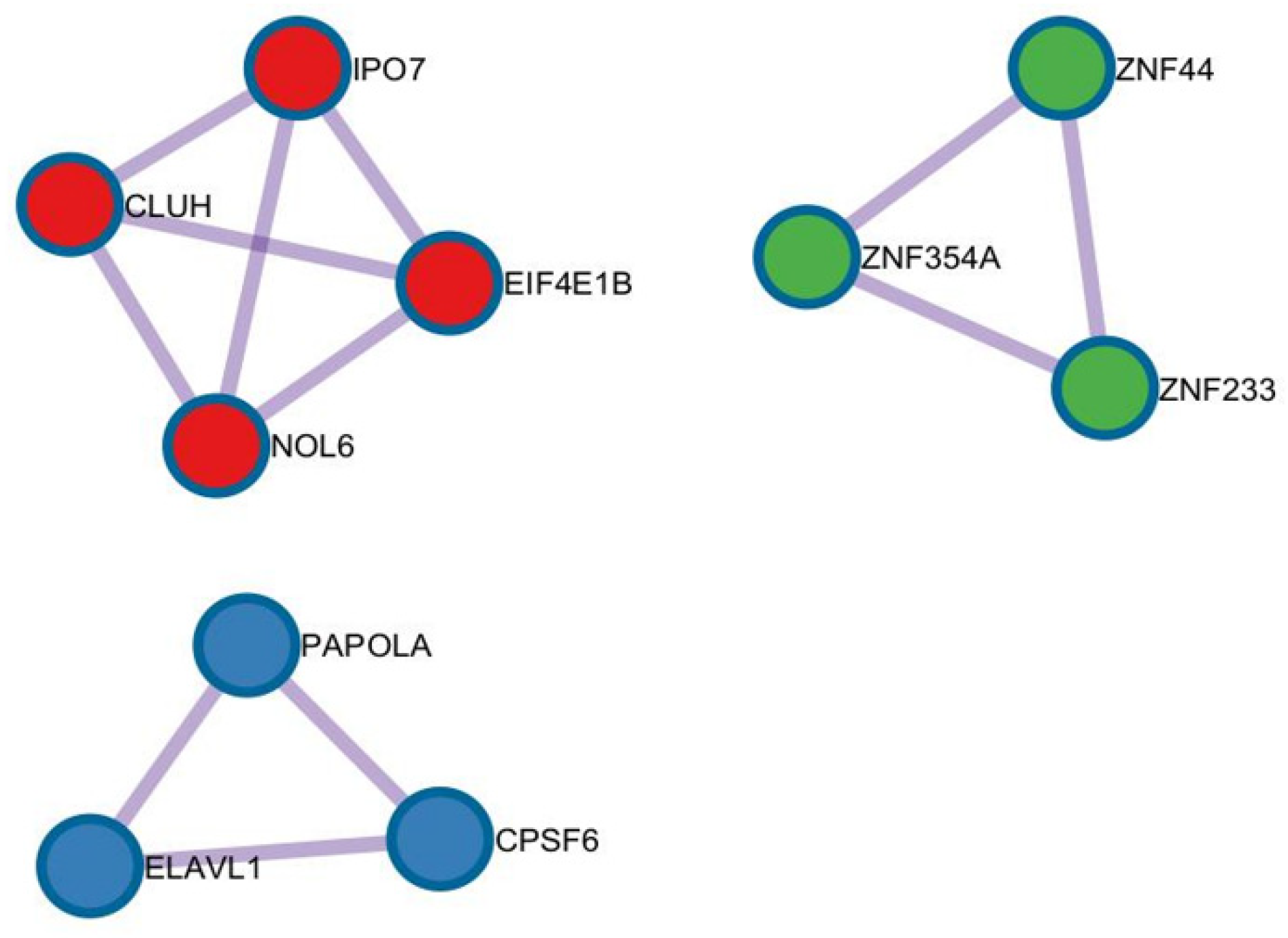
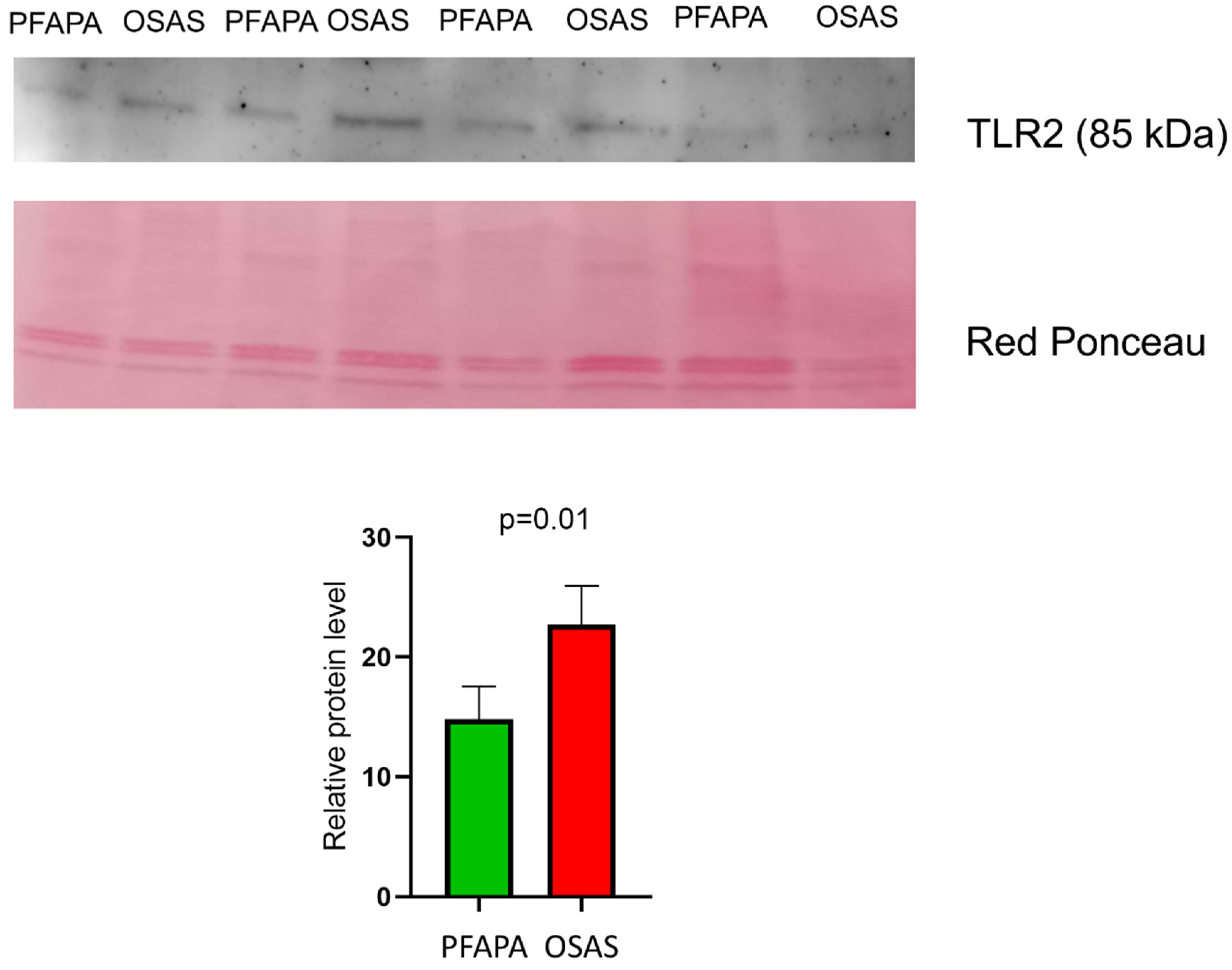
| Pathway | Protein | UniProt Code | PFAPA | OSAS |
|---|---|---|---|---|
| Regulation of PTEN gene transcription | Cellular tumor antigen p53 | P04637 | - | Increase |
| Histone deacetylase 3 | O15379 | - | Increase | |
| Transcriptional repressor p66-alpha | Q86YP4 | - | Increase | |
| Regulatory-associated protein of mTOR | Q8N122 | Increase | - | |
| BMAL1:CLOCK, NPAS2 activates circadian gene expression | Mediator of RNA polymerase II transcription subunit 1 | Q15648 | - | Increase |
| IPAF and AIM2 inflammasomes | Caspase-1 | P29466 | Increase | - |
| Metabolism of Angiotensinogen to Angiotensins | Cathepsin D | P07339 | - | Increase |
| Chymase | P23946 | Increase | - | |
| Defective PGM1 causes PGM1-CDG | Phosphoglucomutase-1 | P36871 | Increase | |
| Defective ALG8 causes CDG-1h | Dolichyl pyrophosphate Glc1Man9GlcNAc2 alpha-1,3-glucosyltransferase | Q9BVK2 | - | Increase |
| HuR (ELAVL1) binds and stabilizes mRNA | ELAV-like protein 1 | Q15717 | - | Increase |
| Regulation of endogenous retroelements by KRAB-ZFP proteins | Zinc finger protein 354A | O60765 | - | Increase |
| Transcriptional repressor p66-alpha | Q86YP4 | - | Increase | |
| Chromobox protein homolog 5 | P45973 | Increase | - | |
| Heme signaling | Mediator of RNA polymerase II transcription subunit 1 | Q15648 | - | Increase |
| Histone deacetylase 3 | O15379 | - | Increase | |
| Interleukin-1 processing | Caspase-1 | P29466 | Increase | - |
| TRP channels | Short transient receptor potential channel 4 | Q9UBN4 | - | Increase |
| Short transient receptor potential channel 5 | Q9UL62 | - | Increase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharrat, F.; Balasan, N.; Ura, B.; Golino, V.; Campiglia, P.; Peri, G.; Valencic, E.; Qaisiya, M.; de Moura, R.; Di Stazio, M.; et al. Deep Proteomics Analysis Unravels the Molecular Signatures of Tonsillar B Cells in PFAPA and OSAS in the Pediatric Population. Int. J. Mol. Sci. 2025, 26, 6621. https://doi.org/10.3390/ijms26146621
Kharrat F, Balasan N, Ura B, Golino V, Campiglia P, Peri G, Valencic E, Qaisiya M, de Moura R, Di Stazio M, et al. Deep Proteomics Analysis Unravels the Molecular Signatures of Tonsillar B Cells in PFAPA and OSAS in the Pediatric Population. International Journal of Molecular Sciences. 2025; 26(14):6621. https://doi.org/10.3390/ijms26146621
Chicago/Turabian StyleKharrat, Feras, Nour Balasan, Blendi Ura, Valentina Golino, Pietro Campiglia, Giulia Peri, Erica Valencic, Mohammed Qaisiya, Ronald de Moura, Mariateresa Di Stazio, and et al. 2025. "Deep Proteomics Analysis Unravels the Molecular Signatures of Tonsillar B Cells in PFAPA and OSAS in the Pediatric Population" International Journal of Molecular Sciences 26, no. 14: 6621. https://doi.org/10.3390/ijms26146621
APA StyleKharrat, F., Balasan, N., Ura, B., Golino, V., Campiglia, P., Peri, G., Valencic, E., Qaisiya, M., de Moura, R., Di Stazio, M., Bortot, B., Tommasini, A., d’Adamo, A. P., Barbi, E., & Grasso, D. L. (2025). Deep Proteomics Analysis Unravels the Molecular Signatures of Tonsillar B Cells in PFAPA and OSAS in the Pediatric Population. International Journal of Molecular Sciences, 26(14), 6621. https://doi.org/10.3390/ijms26146621








