Mitochondrial DNA Copy Numbers and Lung Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Source
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
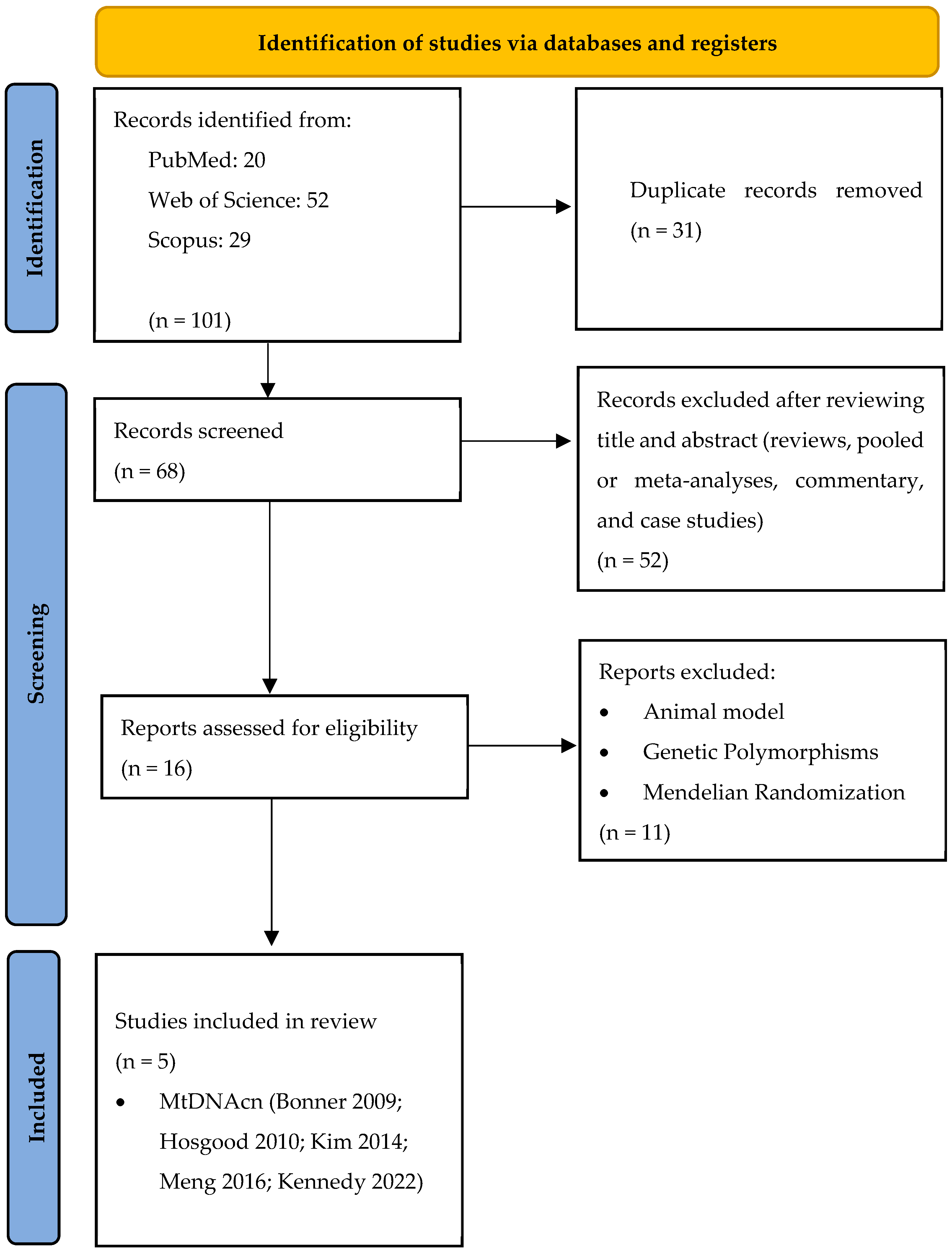
3.1. Study Selection
3.2. Study Characteristics and Quality Assessment
3.3. Meta-Analysis mtDNAcn
3.4. Sensitivity Analysis mtDNAcn
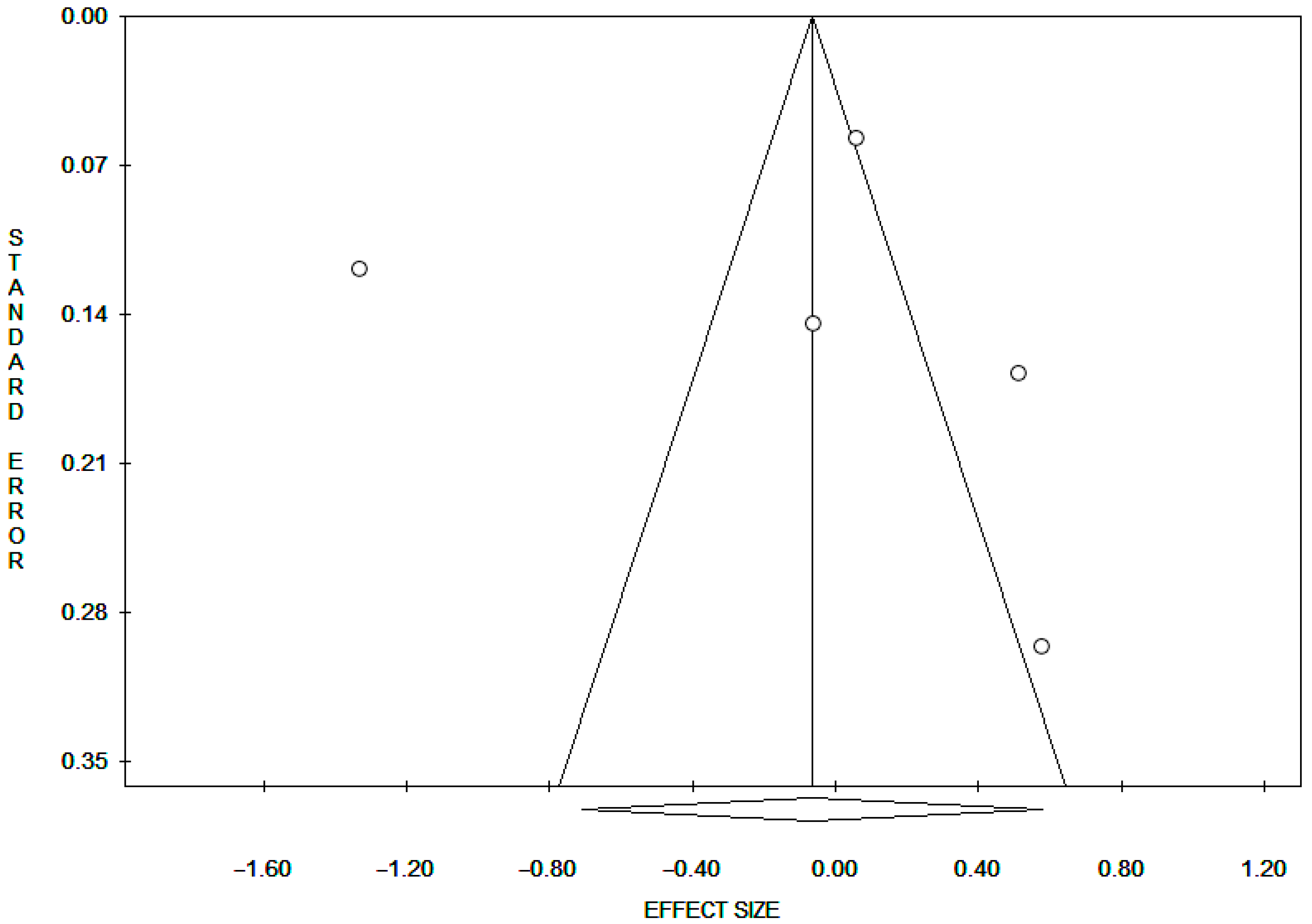
3.5. Publication Bias mtDNAcn
3.6. Dose–Response
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- da Cunha, A.R.; Compton, K.; Xu, R.; Mishra, R.; Drangsholt, M.T.; Antunes, J.L.F.; Kerr, A.R.; Acheson, A.R.; Lu, D.; Wallace, L.E.; et al. The Global, Regional, and National Burden of Adult Lip, Oral, and Pharyngeal Cancer in 204 Countries and Territories: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2023, 9, 1401–1416. [Google Scholar] [CrossRef]
- Alzate, J.D.; Mullen, R.; Mashiach, E.; Bernstein, K.; Vasconcellos, F.D.N.; Rotmann, L.; Berger, A.; Qu, T.; Silverman, J.S.; Golfinos, J.G.; et al. EGFR-mutated non-small lung cancer brain metastases and radiosurgery outcomes with a focus on leptomeningeal disease. J. Neuro-Oncol. 2023, 164, 387–396. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, J.; Yang, R.; Zhou, C.; Liu, Z. Evidence based on Mendelian randomization: Causal relationship between mitochondrial biological function and lung cancer and its subtypes. Neoplasia 2023, 46, 100950. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bandi, P.; Minihan, A.K.; Siegel, R.L.; Islami, F.; Nargis, N.; Jemal, A.; Fedewa, S.A. Updated Review of Major Cancer Risk Factors and Screening Test Use in the United States in 2018 and 2019, with a Focus on Smoking Cessation. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1287–1299. [Google Scholar] [CrossRef]
- Olivares-Hernández, A.; del Portillo, E.G.; Tamayo-Velasco, Á.; Figuero-Pérez, L.; Zhilina-Zhilina, S.; Fonseca-Sánchez, E.; Miramontes-González, J.P. Immune checkpoint inhibitors in non-small cell lung cancer: From current perspectives to future treatments—A systematic review. Ann. Transl. Med. 2023, 11, 354. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, C.; Wei, Z.; Ye, X. Immunotherapy for early-stage non-small cell lung cancer: A system review. J. Cancer Res. Ther. 2023, 19, 849–865. [Google Scholar] [CrossRef]
- Chiavarini, M.; Rosignoli, P.; Sorbara, B.; Giacchetta, I.; Fabiani, R. Benzene Exposure and Lung Cancer Risk: A Systematic Review and Meta-Analysis of Human Studies. Int. J. Environ. Res. Public Health 2024, 21, 205. [Google Scholar] [CrossRef]
- Fabiani, R.; La Porta, G.; Cavoli, L.L.; Rosignoli, P.; Chiavarini, M. Adherence to Data-Driven Dietary Patterns and Lung Cancer Risk: A Systematic Review and Dose–Response Meta-Analysis. Nutrients 2023, 15, 4406. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Sears, C.R.; Mazzone, P.J. Biomarkers in Lung Cancer. Clin. Chest Med. 2020, 41, 115–127. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef]
- Li, Y.; Sundquist, K.; Zhang, N.; Wang, X.; Sundquist, J.; Memon, A.A. Mitochondrial related genome-wide Mendelian randomization identifies putatively causal genes for multiple cancer types. eBioMedicine 2023, 88, 104432. [Google Scholar] [CrossRef]
- Cai, X.; Liang, C.; Zhang, M.; Dong, Z.; Weng, Y.; Yu, W. Mitochondrial DNA copy number and cancer risks: A comprehensive Mendelian randomization analysis. Int. J. Cancer 2024, 154, 1504–1513. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Kim, K.; Esteves, P.; Trian, T.; Barnes, P.J. Mitochondrial dysfunction in lung ageing and disease. Eur. Respir. Rev. 2020, 29, 200165. [Google Scholar] [CrossRef]
- Quirós, P.M.; Mottis, A.; Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 2016, 17, 213–226. [Google Scholar] [CrossRef]
- Montier, L.L.C.; Deng, J.J.; Bai, Y. Number matters: Control of mammalian mitochondrial DNA copy number. J. Genet. Genom. 2009, 36, 125–131. [Google Scholar] [CrossRef]
- Dolcini, J.; Chiavarini, M.; Firmani, G.; Brennan, K.J.M.; Cardenas, A.; Baccarelli, A.A.; Barbadoro, P. Methylation Biomarkers of Lung Cancer Risk: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 690. [Google Scholar] [CrossRef]
- Dolcini, J.; Wu, H.; Nwanaji-Enwerem, J.C.; Kiomourtozlogu, M.-A.; Cayir, A.; Sanchez-Guerra, M.; Vokonas, P.; Schwarz, J.; Baccarelli, A.A. Mitochondria and aging in older individuals: An analysis of DNA methylation age metrics, leukocyte telomere length, and mitochondrial DNA copy number in the VA normative aging study. Aging 2020, 12, 2070–2083. [Google Scholar] [CrossRef]
- Fabiani, R.; Chiavarini, M.; Rosignoli, P.; Giacchetta, I. Leucocyte Telomere Length and Lung Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Cancers 2024, 16, 3218. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Wang, R.; Barcelo, H.; Koh, W.-P.; Yuan, J.-M. Mitochondrial copy number is associated with colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1574–1581. [Google Scholar] [CrossRef]
- Lynch, S.M.; Weinstein, S.J.; Virtamo, J.; Lan, Q.; Liu, C.-S.; Cheng, W.-L.; Rothman, N.; Albanes, D.; Stolzenberg-Solomon, R.Z. Mitochondrial DNA Copy Number and Pancreatic Cancer in the Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) Study. Cancer Prev. Res. 2011, 4, 1912–1919. [Google Scholar] [CrossRef]
- Xing, J.; Chen, M.; Wood, C.G.; Lin, J.; Spitz, M.R.; Ma, J.; Amos, C.I.; Shields, P.G.; Benowitz, N.L.; Gu, J.; et al. Mitochondrial DNA Content: Its Genetic Heritability and Association with Renal Cell Carcinoma. JNCI J. Natl. Cancer Inst. 2008, 100, 1104–1112. [Google Scholar] [CrossRef]
- Cocco, M.P.; White, E.; Xiao, S.; Hu, D.; Mak, A.; Sleiman, P.; Yang, M.; Bobbitt, K.R.; Gui, H.; Levin, A.M.; et al. Asthma and its relationship to mitochondrial copy number: Results from the Asthma Translational Genomics Collaborative (ATGC) of the Trans-Omics for Precision Medicine (TOPMed) program. PLoS ONE 2020, 15, e0242364. [Google Scholar] [CrossRef]
- Liu, S.-F.; Kuo, H.-C.; Tseng, C.-W.; Huang, H.-T.; Chen, Y.-C.; Tseng, C.-C.; Lin, M.-C.; Su, Y. Leukocyte Mitochondrial DNA Copy Number Is Associated with Chronic Obstructive Pulmonary Disease. PLoS ONE 2015, 10, e0138716. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Yu, X.; Zhou, H.; Luo, Y.; Wang, W.; Wang, L. Clinical application of plasma mitochondrial DNA content in patients with lung cancer. Oncol. Lett. 2018, 16, 7074–7081. [Google Scholar] [CrossRef]
- Reznik, E.; Miller, M.L.; Şenbabaoğlu, Y.; Riaz, N.; Sarungbam, J.; Tickoo, S.K.; Al-Ahmadie, H.A.; Lee, W.; Seshan, V.E.; Hakimi, A.A.; et al. Mitochondrial DNA copy number variation across human cancers. eLife 2016, 5, e10769. [Google Scholar] [CrossRef]
- Chen, N.; Wen, S.; Sun, X.; Fang, Q.; Huang, L.; Liu, S.; Li, W.; Qiu, M. Elevated Mitochondrial DNA Copy Number in Peripheral Blood and Tissue Predict the Opposite Outcome of Cancer: A Meta-Analysis. Sci. Rep. 2016, 6, 37404. [Google Scholar] [CrossRef]
- Meng, S.; De Vivo, I.; Liang, L.; Hu, Z.; Christiani, D.C.; Giovannucci, E.; Han, J. Pre-diagnostic leukocyte mitochondrial DNA copy number and risk of lung cancer. Oncotarget 2016, 7, 27307–27312. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.R.; Shen, M.; Liu, C.-S.; DiVita, M.; He, X.; Lan, Q. Mitochondrial DNA content and lung cancer risk in Xuan Wei, China. Lung Cancer 2008, 63, 331–334. [Google Scholar] [CrossRef]
- Hosgood, H.D.; Liu, C.-S.; Rothman, N.; Weinstein, S.J.; Bonner, M.R.; Shen, M.; Lim, U.; Virtamo, J.; Cheng, W.-L.; Albanes, D.; et al. Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis 2010, 31, 847–849. [Google Scholar] [CrossRef]
- Kim, C.; Bassig, B.A.; Seow, W.J.; Hu, W.; Purdue, M.P.; Shu, X.-O.; Huang, W.-Y.; Liu, C.-S.; Cheng, W.-L.; Lin, T.-T.; et al. Pooled analysis of mitochondrial DNA copy number and lung cancer risk in three prospective studies. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2977–2980. [Google Scholar] [CrossRef]
- Kennedy, G.T.; Mitra, N.; Penning, T.M.; Whitehead, A.S.; Vachani, A. Peripheral blood leukocyte mitochondrial DNA content and risk of lung cancer. Transl. Lung Cancer Res. 2022, 11, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking outside the nucleus: Mitochondrial DNA copy number in health and disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sengupta, S.; Scaria, V. Comparative analysis of human mitochondrial methylomes shows distinct patterns of epigenetic regulation in mitochondria. Mitochondrion 2014, 18, 58–62. [Google Scholar] [CrossRef]
- Hartling, L.; Milne, A.; Hamm, M.P.; Vandermeer, B.; Ansari, M.; Tsertsvadze, A.; Dryden, D.M. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 2013, 66, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Oremus, M.; Oremus, C.; Hall, G.B.C.; McKinnon, M.C.; ECT & Cognition Systematic Review Team. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ Open 2012, 2, e001368. [Google Scholar] [CrossRef]

| Author, Year, Reference | Cohort 1, Location | Study Design | N | Age (M: Mean, Mdn: Median) | Sex (% Male) | Race (% White) | BMI (% or M: Mean, [kg/m2] <25) | Type of LC 2 | Tissue Type | Smoking Status (% Never) | Matched or Adjusted Variables | NOS 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kennedy GT, 2022 [41] | UPHS, USA | Case–control | Cases: 465 Controls: 378 | Cases: 66 (Mdn) Controls: 58 (Mdn) | Cases: 55.7 Controls: 51.3 | Cases: 81.3 Controls: 63.8 | NSCLC and SCLC | Peripheral blood | Cases: 9.7 Controls: 24.3 | Age, race, gender, tobacco use, and tobacco pack-years | 8 | |
| Meng S, 2016 [31] | NHS, USA | Nested case–control | Cases: 285 Controls: 285 | Cases: 59.8 (M) Controls: 59.5 (M) | Cases: 60.9 (%) Controls: 57.3 (%) | LC | Peripheral blood | BMI, physical activity, alcohol consumption. and health eating index score | 9 | |||
| HPFS, USA | Nested case–control | Cases: 178 Controls: 178 | Cases: 66.0 (M) Controls: 65.7 (M) | Cases: 42.9 (%) Controls: 31.8 (%) | LC | Peripheral blood | BMI, physical activity, alcohol consumption, and health eating index score | 9 | ||||
| Kim C, 2014 [40] | PLCO, USA | Nested case–control | Cases: 426 Controls: 436 | Cases: 64.1 (M) Controls: 63.7 (M) | Cases: 60.8 Controls: 61.2 | Cases: 93.7 Controls: 92.7 | Cases: 26.8 (M) Controls: 27.4 (M) | LC | Peripheral blood | Cases: 10.8 Controls: 45.4 | Age, BMI, pack-years, race, sex, date of enrollment, center (if applicable), and study | 7 |
| SWHS, China | Nested case–control | Cases: 221 Controls: 222 | Cases: 59.2 (M) Controls: 59.2 (M) | Cases: 24.6 (M) Controls: 25.0 (M) | LC | Peripheral blood | Cases: 92.3 Controls: 95.0 | Age, BMI, pack-years, race, sex, date of enrollment, center (if applicable), and study | 7 | |||
| ATBC, Finland | Nested case–control | Cases: 227 Controls: 227 | Cases: 56.7 (M) Controls: 58.4 (M) | Cases: 25.6 (M) Controls: 26.3 (M) | LC | Peripheral blood | Cases: 0.0 Controls: 0.0 | Age, BMI, pack-years, race, sex, date of enrollment, center (if applicable), and study | 7 | |||
| Hosgood HD III, 2010 [39] | ATBC, Finland | Case–control | Cases: 227 Controls: 227 | Cases: 58.7 (M) Controls: 58.4 (M) | Cases: 100 Controls: 100 | Cases: 25.6 (M) Controls: 26.3 (M) | LAUD and LUSC | Peripheral blood | Cases: 0.0 Controls: 0.0 | Age, number of years smoking, and number of cigarettes per day | 7 | |
| Bonner MR, 2009 [38] | China (Xuan Wei hospitals) | Case–control | Cases: 113 Controls: 107 | Cases: 54.9 (M) Controls: 54.5 (M) | Cases: 65 Controls: 64 | LC | Sputum | Age, type of current fuel use, sex, pack-years smoking, and lifetime smoky coal use | 6 |
| Combined Risk Estimate | Test of Heterogeneity | Publication Bias | |||||
|---|---|---|---|---|---|---|---|
| N. b | Value (95% CI) | Q | I2 % | p | p (Egger Test) | p (Begg Test) | |
| ALL (5art) 1 | 33 | 0.94 (0.49–1.78) | 132.48 | 96.98 | 0.00 | 0.93 | 1.00 |
| Excluding Bonner et al. [38] 2 | 31 | 0.81 (0.39–1.67) | 126.82 | 97.63 | 0.00 | 0.75 | 1.00 |
| SEX | |||||||
| M | 4 | 1.13 (0.65–1.98) | 10.63 | 71.77 | 0.014 | 0.51 | 0.17 |
| F | 4 | 1.36 (0.93–2.01) | 4.75 | 36.84 | 0.19 | 0.35 | 0.50 |
| Smoking Status | |||||||
| CURRENT | 4 | 1.37 (0.90–2.08) | 5.38 | 44.25 | 0.15 | 0.18 | 0.17 |
| EVER | 5 | 1.10 (0.81–1.50) | 5.21 | 23.30 | 0.27 | 0.62 | 0.33 |
| NEVER | 4 | 1.06 (0.76–1.48) | 0.82 | 0.00 | 0.85 | 0.14 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavarini, M.; Dolcini, J.; Firmani, G.; Brennan, K.J.M.; Cardenas, A.; Baccarelli, A.A.; Barbadoro, P. Mitochondrial DNA Copy Numbers and Lung Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 6610. https://doi.org/10.3390/ijms26146610
Chiavarini M, Dolcini J, Firmani G, Brennan KJM, Cardenas A, Baccarelli AA, Barbadoro P. Mitochondrial DNA Copy Numbers and Lung Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(14):6610. https://doi.org/10.3390/ijms26146610
Chicago/Turabian StyleChiavarini, Manuela, Jacopo Dolcini, Giorgio Firmani, Kasey J. M. Brennan, Andrès Cardenas, Andrea A. Baccarelli, and Pamela Barbadoro. 2025. "Mitochondrial DNA Copy Numbers and Lung Cancer: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 14: 6610. https://doi.org/10.3390/ijms26146610
APA StyleChiavarini, M., Dolcini, J., Firmani, G., Brennan, K. J. M., Cardenas, A., Baccarelli, A. A., & Barbadoro, P. (2025). Mitochondrial DNA Copy Numbers and Lung Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(14), 6610. https://doi.org/10.3390/ijms26146610








