Neuroprotective Terpenoids Derived from Hericium erinaceus Fruiting Bodies: Isolation, Structural Elucidation, and Mechanistic Insights
Abstract
1. Introduction
2. Results
2.1. Structural Elucidation of Compounds 1–5
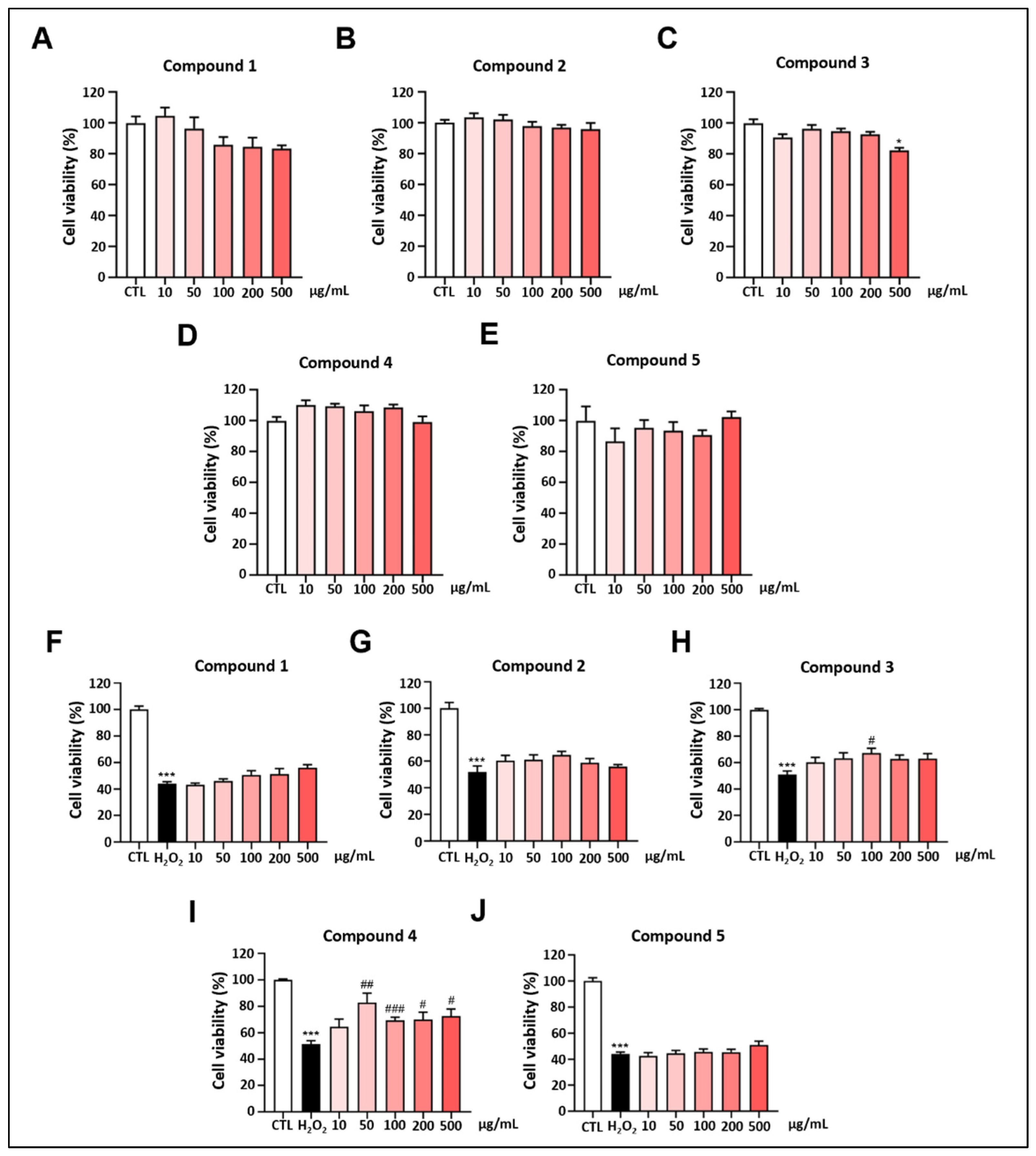
2.2. Protective Effect of Derived Compounds on H2O2-Induced Damage
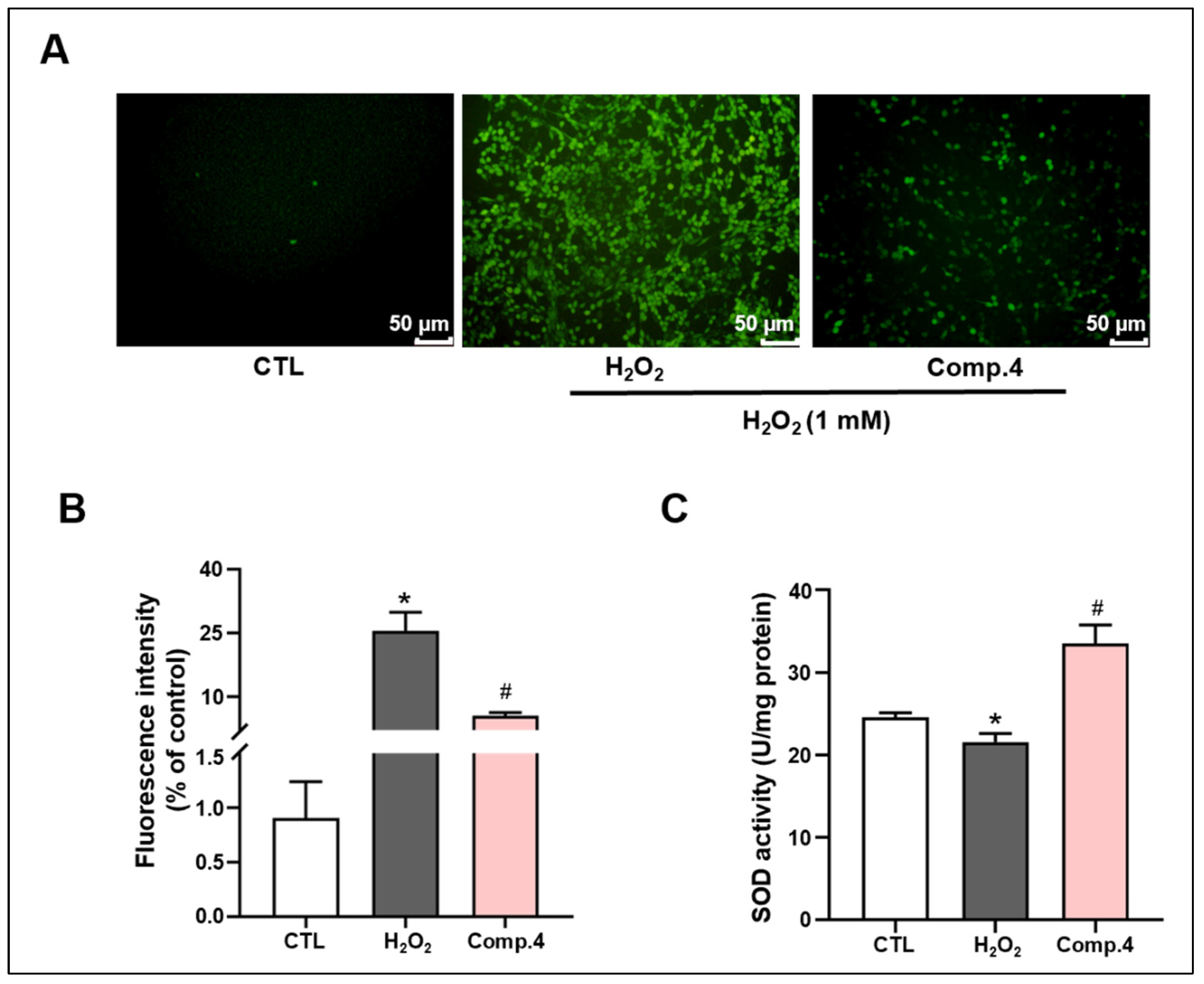
2.3. Protective Effect of Compound 4 on H2O2-Induced Nerve Injury
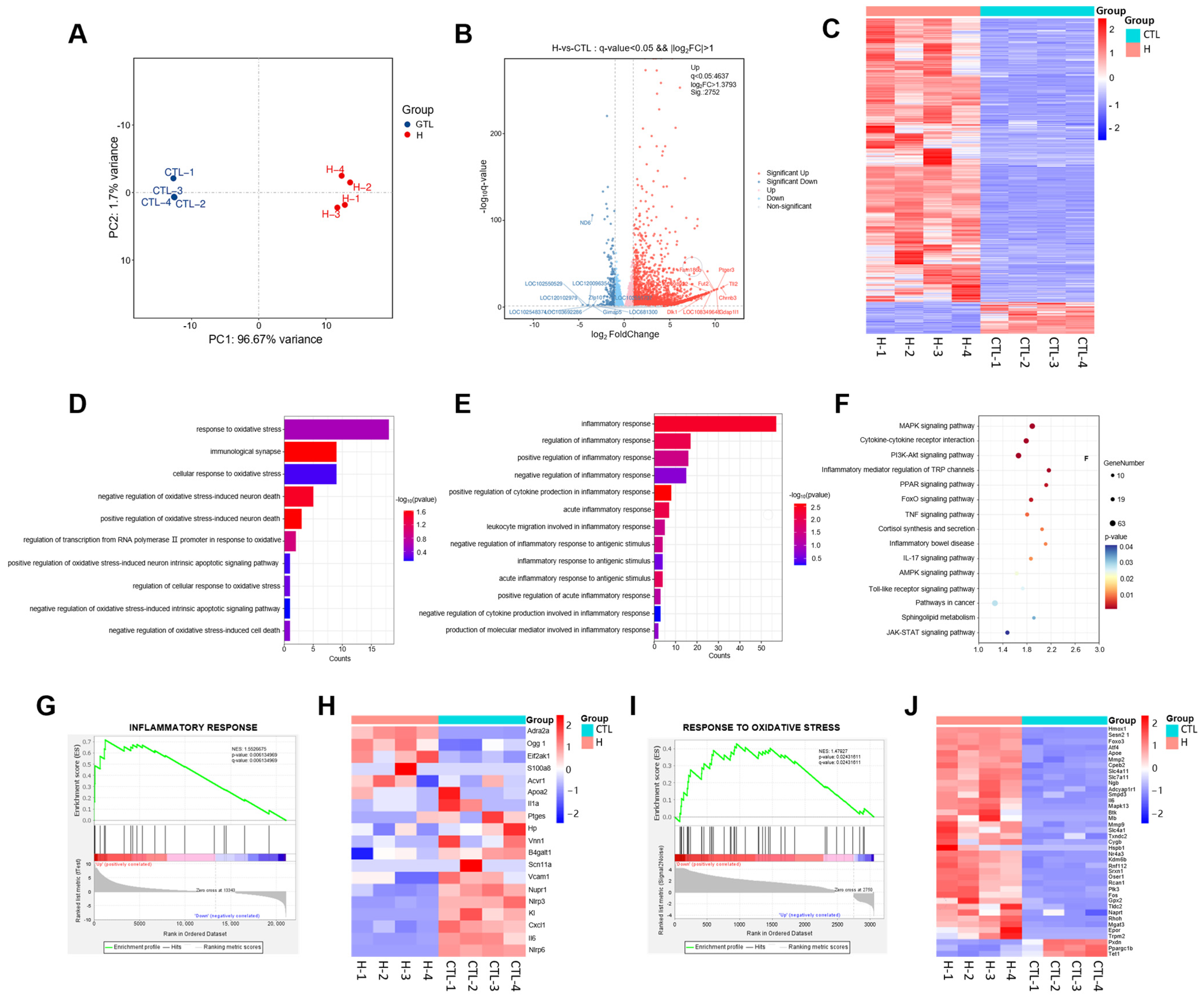
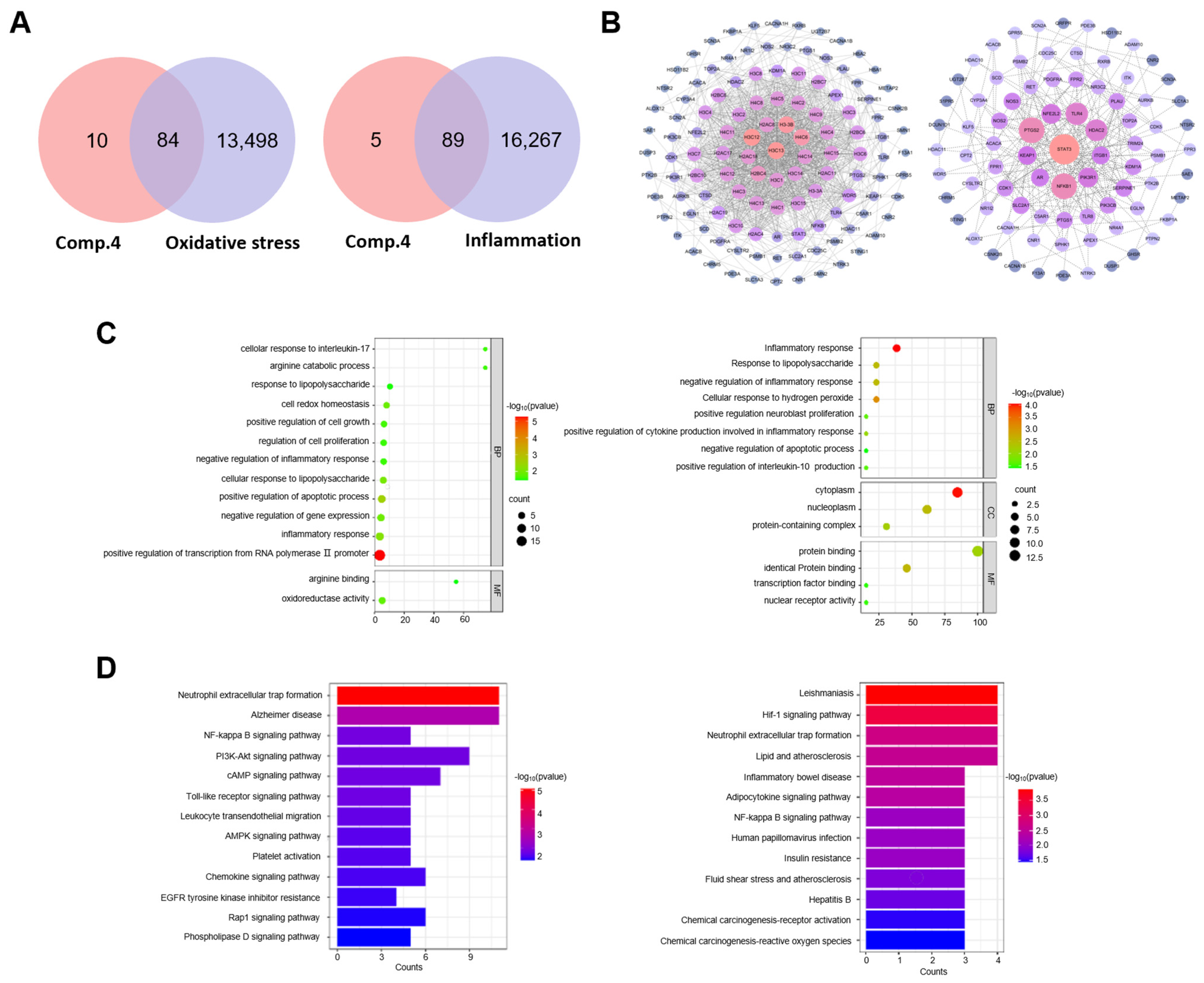
2.4. Transcriptomics Analysis of the Mechanisms of H2O2-Induced Neurological Injury in PC12 Cells
2.5. Interaction Analysis of Compound 4 and Oxidative Stress and Inflammation-Related Target Genes
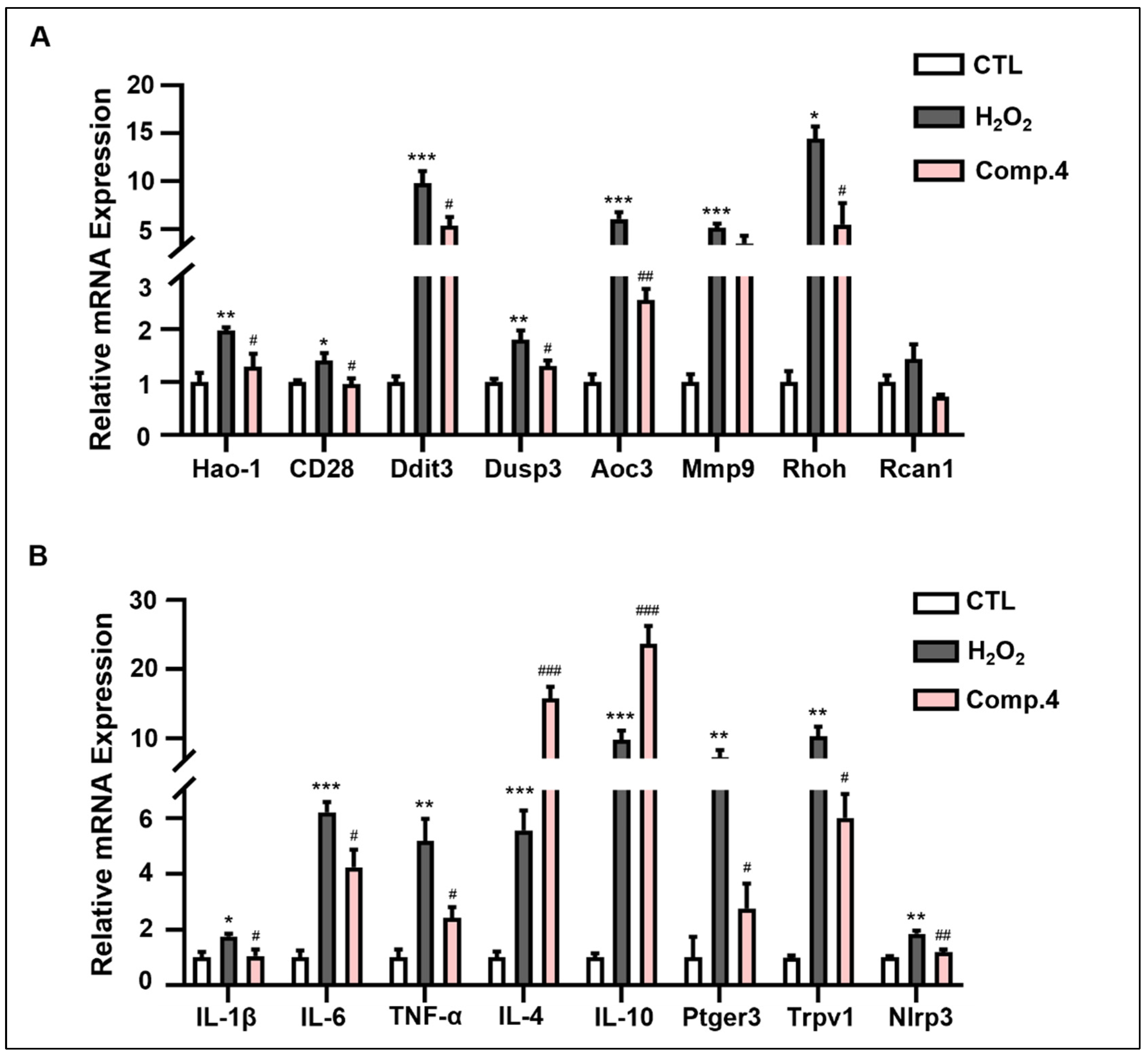
2.6. Compound 4 Attenuates H2O2-Induced Oxidative Stress Damage in PC12 Cells
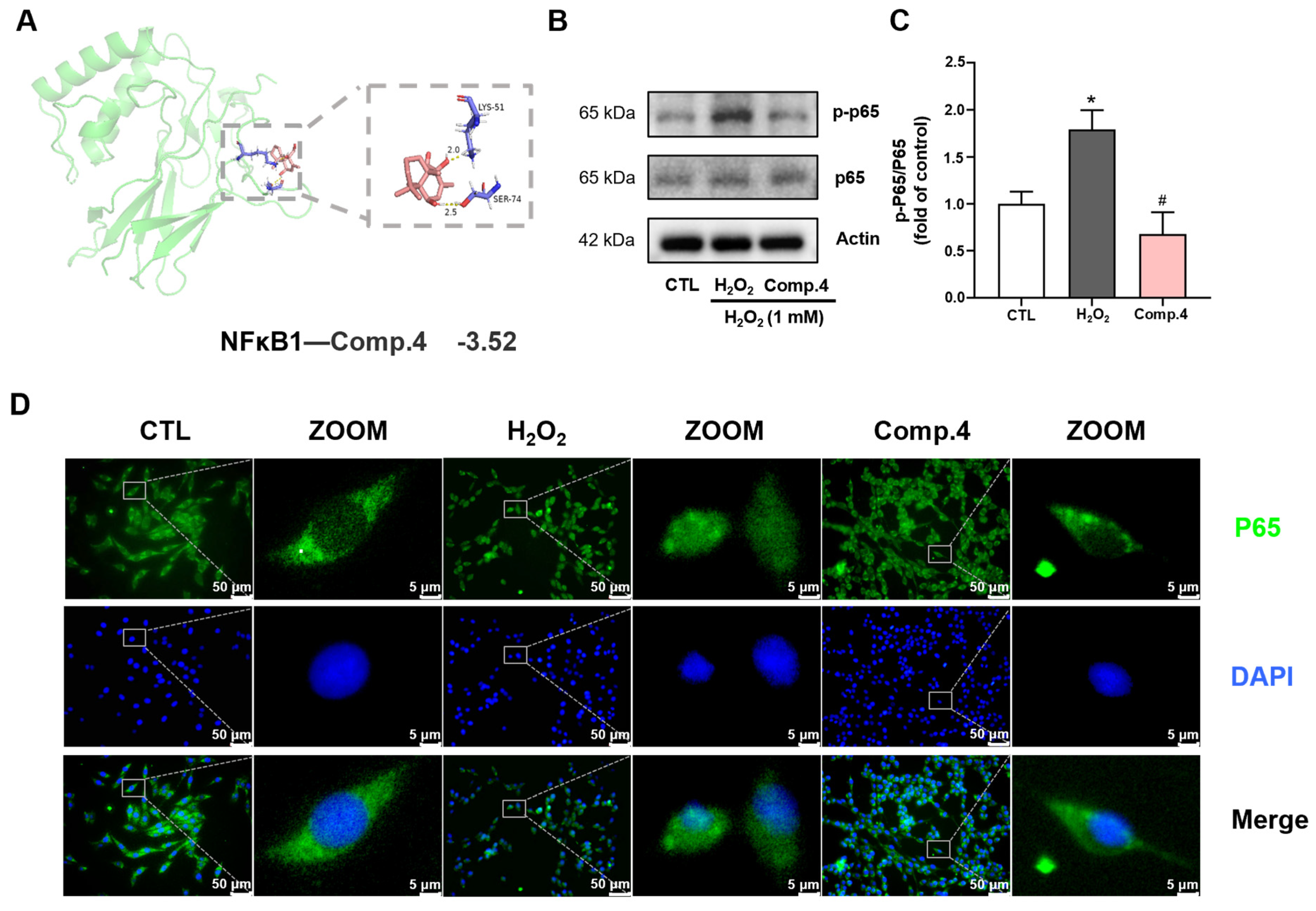
2.7. Mechanism of Compound 4 in Ameliorating H2O2-Induced Neuronal Damage in PC12 Cells
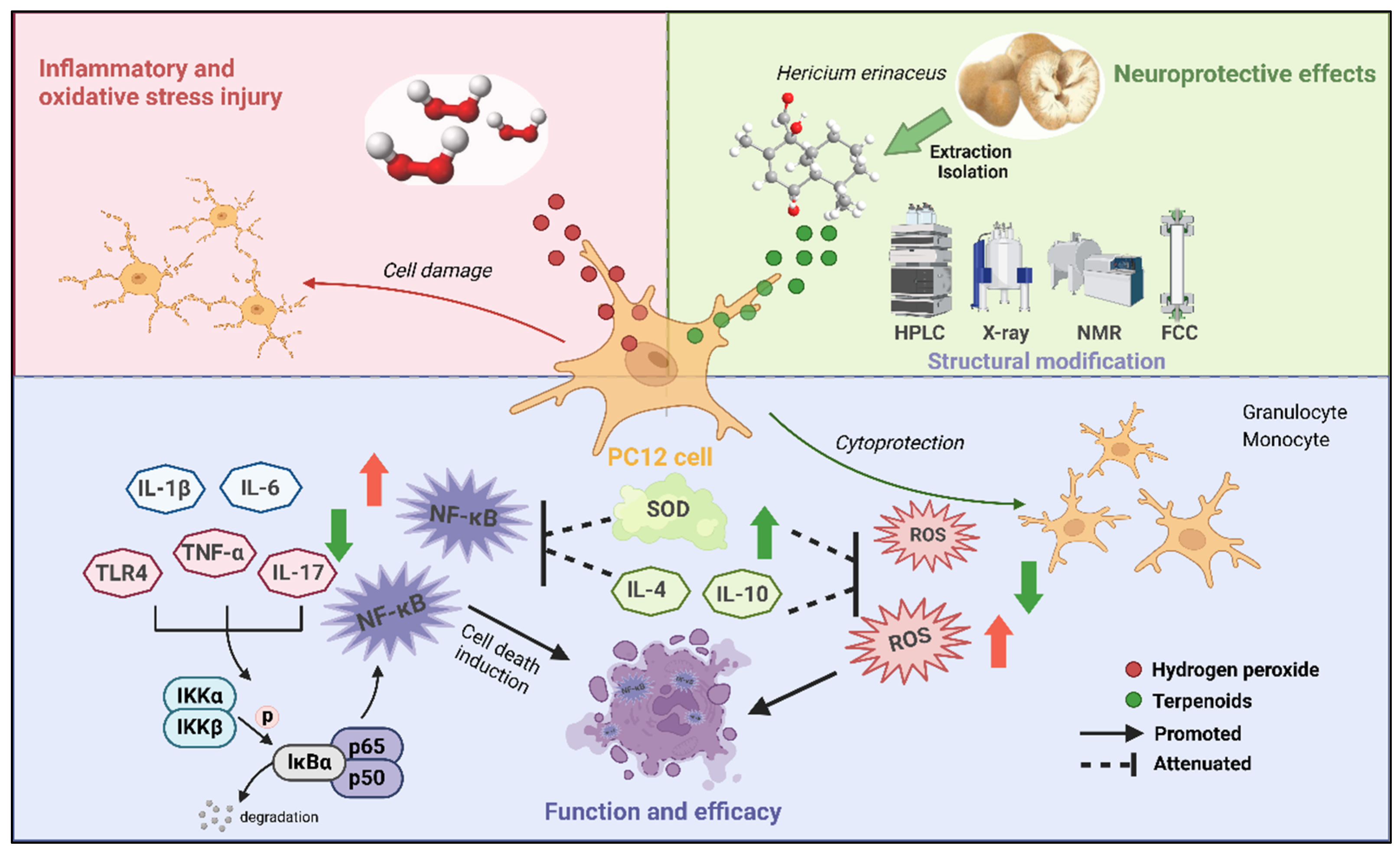
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Fungal Material
4.3. Extraction and Isolation
4.4. Cell Culture
4.5. Cell Viability
4.6. Transcriptome Experiment and Analysis
4.7. Network Pharmacology Analysis
4.7.1. Clustering of Compound 4 and Oxidative Stress-Related Target Genes
4.7.2. Protein–Protein Interaction (PPI) Network Map of Potential Target Genes
4.8. GO and KEGG Pathway Enrichment Analysis
4.9. ROS Staining
4.10. SOD Assay
4.11. Quantitative Real-Time PCR
4.12. Molecular Docking
4.13. Western Blotting
4.14. Immunofluorescence (IF) Staining
4.15. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| COSY | 1H–1H correlation spectroscopy |
| CUMS | Chronic unpredictable mild stress |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| HRMS | High-resolution mass spectrometry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MCAO | Middle cerebral artery occlusion |
| NGF | Nerve growth factor |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| PCA | Principal component analysis |
| PPI | Protein–protein interaction |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor |
| XRD | X-ray diffraction |
References
- Yuan, L.L.; Liu, J.K. Hericinosides A–M, Cyathane Diterpene Glycosides with α-Glucosidase Inhibitory Activity from the Medicinal Fungus Hericium erinaceus. J. Agric. Food Chem. 2025, 73, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Zhang, L.; Chen, B.D.; Liu, X.L.; Yang, F.; Ren, Y.Y.; Xiang, H.L.; Wang, P.L.; Li, J. Extraction, purification, structural characteristics, biological activities, modifications, and applications from Hericium erinaceus polysaccharides: A review. Int. J. Bio. Macromol. 2024, 291, 138932. [Google Scholar] [CrossRef]
- Hou, C.; Liu, L.; Ren, J.; Huang, M.; Yuan, E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2021, 375, 131896. [Google Scholar] [CrossRef]
- Xie, G.; Tang, L.; Xie, Y.; Xie, L. Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities. Molecules 2022, 27, 2157. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, M.; Yin, J.-Y.; Song, Y.-H.; Wang, Y.-X.; Nie, S.-P.; Xie, M.-Y. Gastroprotective activity of polysaccharide from the fruiting body of Hericium erinaceus against acetic acid-induced gastric ulcer in rats and structure of one bioactive fraction. Int. J. Bio. Macromol. 2022, 210, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-D.; Ding, Z.-C.; Wang, Y.-Y.; Yang, Y.; Zhang, H.-N.; Yan, J.-K. Hypoglycemic benefit and potential mechanism of a polysaccharide from Hericium erinaceus in streptozotoxin-induced diabetic rats. Process Biochem. 2020, 88, 180–188. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and Neuroprotective Effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef]
- Tripodi, F.; Falletta, E.; Leri, M.; Angeloni, C.; Beghelli, D.; Giusti, L.; Milanesi, R.; Sampaio-Marques, B.; Ludovico, P.; Goppa, L.; et al. Anti-Aging and Neuroprotective Properties of Grifola frondosa and Hericium erinaceus Extracts. Nutrients 2022, 14, 4368. [Google Scholar] [CrossRef]
- Kim, S. Antioxidant Compounds for the Inhibition of Enzymatic Browning by Polyphenol Oxidases in the Fruiting Body Extract of the Edible Mushroom Hericium erinaceus. Foods 2020, 9, 951. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An insight into the nutritional and medicinal value of edible mushrooms: A natural treasury for human health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Jia, X.; Wang, Y.; Wang, Y.; Zhou, Y.; Li, F.; Qu, Y.; Cheng, H. Structural Analysis and Antioxidant Activity of Alkaline-Extracted Glucans from Hericium erinaceus. Foods 2024, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Chen, Y.-P.; Lin, T.-W.; Li, T.-J.; Chen, Y.-W.; Li, I.C.; Chen, C.-C. Discovery of a New Compound, Erinacerin W, from the Mycelia of Hericium erinaceus, with Immunomodulatory and Neuroprotective Effects. Molecules 2024, 13, 2742. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Cha, J.Y.; Kwon, S.U.; Baek, K.-H.; Shim, S.H.; Lee, Y.M.; Kim, Y.H. Sterols from Hericium erinaceum and their inhibition of TNF-α and NO production in lipopolysaccharide-induced RAW 264.7 cells. Phytochemistry 2015, 115, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gaascht, F.; Schmidt-Dannert, C.; Salomon, C.E. Discovery of Antifungal and Biofilm Preventative Compounds from Mycelial Cultures of a Unique North American Hericium sp. Fungus. Mol. 2020, 25, 963. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.-Y.; Feng, X.-L.; Zhang, Y.; Hu, X.; Hui, H.; Xue, X.; Qi, J. Unprecedented Neoverrucosane and Cyathane Diterpenoids with Anti-Neuroinflammatory Activity from Cultures of the Culinary-Medicinal Mushroom Hericium erinaceus. Molecules 2023, 28, 6380. [Google Scholar] [CrossRef]
- Yue, Q.; Genglan, L.; Weiming, L.; Fuming, Z.; Robert, J.L.; Xingli, W.; Anqiang, Z. Bioactive substances in Hericium erinaceus and their biological properties: A review. Food Sci. Hum. Wellness 2024, 13, 1825–1844. [Google Scholar]
- Tzeng, T.-T.; Chen, C.-C.; Chen, C.-C.; Tsay, H.-J.; Lee, L.-Y.; Chen, W.-P.; Shen, C.-C.; Shiao, Y.-J. The Cyanthin Diterpenoid and Sesterterpene Constituents of Hericium erinaceus Mycelium Ameliorate Alzheimer’s Disease-Related Pathologies in APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Cao, C.-Y.; Kubo, M.; Harada, K.; Yan, X.-T.; Fukuyama, Y.; Gao, J.-M. Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway. Int. J. Mol. Sci. 2017, 18, 1659. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Bao, L.; Yang, Y.; Ma, K.; Liu, H. Three new cyathane diterpenes with neurotrophic activity from the liquid cultures of Hericium erinaceus. J. Antibiot. 2018, 71, 818–821. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Gong, X.; Hu, G.; Ge, J.; Wu, J.; Gao, X. Investigating the Regulation of Neural Differentiation and Injury in PC12 Cells Using Microstructure Topographic Cues. Biosensors 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Wu, J.; Kang, S.; Gao, J.; Hirokazu, K.; Liu, H.; Liu, C. The chemical structures, biosynthesis, and biological activities of secondary metabolites from the culinary-medicinal mushrooms of the genus Hericium: A review. Chin. J. Nat. Med. 2024, 22, 676–698. [Google Scholar] [CrossRef] [PubMed]
- Grabley, S.; Thiericke, R.; Zerlin, M.; Gohrt, A.; Philipps, S.; Zeeck, A. New albrassitriols from Aspergillus sp. (FH-A 6357). J. Antibiot. 1996, 49, 593–595. [Google Scholar] [CrossRef]
- Krohn, K.; Flörke, U.; Rao, M.S.; Steingröver, K.; Aust, H.J.; Draeger, S.; Schulz, B. Metabolites from fungi 15. New isocoumarins from an endophytic fungus isolated from the Canadian thistle Cirsium arvense. Nat. Prod. Lett. 2002, 15, 353–361. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, C.; Li, L.; Dai, X.; Liu, Y.; Hsiang, T.; Liu, S.; Wang, D. Structural characterization of Hericium coralloides polysaccharide and its neuroprotective function in Alzheimer’s disease. Int. J. Biol. Macromol. 2024, 277, 133865. [Google Scholar] [CrossRef] [PubMed]
- Bureau, J.-A.; Oliva, M.E.; Dong, Y.; Ignea, C. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Nat. Prod. Rep. 2023, 40, 1822–1848. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, M.; Fu, A.; Li, Q.; Chen, C.; Zhu, H.; Zhang, Y. Natural Sesquiterpenoids, Diterpenoids, Sesterterpenoids, and Triterpenoids with Intriguing Structures from 2017 to 2022. Chin. J. Chem. 2023, 41, 3115–3132. [Google Scholar]
- Wang, Y.; Zhang, S.; Ma, Y.; Du, X.; Zong, Q.; Lin, D.; Lai, M.; Huang, T.; Luo, Q.; Yang, L.; et al. Solvent effects on terpenoid compositions and antioxidant activities of Cinnamomum camphora (L.) J. Presl extracts and the main antioxidant agent evaluation through in vitro and in vivo assay. Chem. Biol. Technol. Agric. 2024, 11, 2. [Google Scholar] [CrossRef]
- Tong-De, Z.; Xue-Qiong, Y.; Ting, W.; Ju-Cheng, Z.; Ya-Bin, Y.; Le, C.; Zhong-Tao, D. Neuroprotective Guanacastane Diterpenoids from the Endophytic Fungus Candolleomyces sp. J. Agric. Food Chem. 2025, 73, 4687–4699. [Google Scholar]
- Li, Z.-Y.; Zhang, J.-B.; Cheng, Y.-X. Anti-inflammatory 3,4-seco-triterpenoids from Ganoderma cochlear. Tetrahedron 2024, 167, 134240. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, Q.L.; He, G.H.; Long, K.; Tang, X.Q.; Su, Y.; Wu, Z.L. HcCnAα regulates NF-κB signaling in Hyriopsis cumingii by interacting with HcIKK and facilitating IκB phosphorylation. Int. J. Biol. Macromol. 2024, 289, 138787. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.Y.; Zhang, X.; Li, X.; Wang, X.T.; Yang, J.; Liu, G.Y. Design, synthesis and biological evaluation of diarylmethyl amine derivatives with anti-ulcerative colitis activity via inhibiting inflammation and oxidative stress. Eur. J. Med. Chem. 2025, 289, 117433. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-κB Function in the Nervous System. Front. Immunol. 2019, 13, 860977. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Jodo, A.; Shibazaki, A.; Onuma, A.; Kaisho, T.; Tanaka, T. PDLIM7 Synergizes with PDLIM2 and p62/Sqstm1 to Inhibit Inflammatory Signaling by Promoting Degradation of the p65 Subunit of NF-κB. Front. Immunol. 2020, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Liu, R.; Miao, J.; Lv, J.; Yang, S.; Zhu, Y.; Chen, Y.; Lu, K.; Huang, C.; et al. Activation of the Nrf2 Signaling Pathway by Tetrahydroberberine Suppresses Ferroptosis and Enhances Functional Recovery Following Spinal Cord Injury. Mol. Neurobiol. 2025, 62, 8439–8456. [Google Scholar] [CrossRef]
- Li, C.; Ge, H.; Huang, J.; Si, L.; Sun, L.; Wu, L.; Xiao, L.; Xie, Y.; Wang, G. Resveratrol alleviates depression-like behaviors by inhibiting ferroptosis via AKT/NRF2 pathway. Brain Res. Bull. 2024, 220, 111136. [Google Scholar] [CrossRef]
- Su, W.; Chen, H.; Li, Y.; Wang, Y.; Chen, T.; Shi, H.; Yang, J.; Zhang, C.; Wang, T.; Xiong, L. Design, synthesis and biological evaluation of 2-arylbenzo[b]furan-4-vinylcarbonyl derivatives based on Salvianolic acid C as antioxidant neuroprotective agents for the treatment of Ischemic stroke. Eur. J. Med. Chem. 2025, 290, 117506. [Google Scholar] [CrossRef]
- Wang, T.-T.; Zhou, M.-Y.; Gong, X.-N.; Huang, Y.; Li, F.-L.; Gu, S.-L.; Zhang, M.-Y.; Li, L.-L.; Xu, Z.-S.; Li, R.; et al. Eupalinolide B alleviates corticosterone-induced PC12 cell injury and improves depression-like behaviors in CUMS rats by regulating the GSK-3β/β-catenin pathway. Biochem. Pharmacol. 2025, 235, 116831. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Su, L.; Shi, M.; Sun, L.; Chen, W.; Geng, J.; Li, J.; Zong, Y.; He, Z.; Du, R. Network Pharmacology and Transcriptomics to Explore the Pharmacological Mechanisms of 20(S)-Protopanaxatriol in the Treatment of Depression. Int. J. Mol. Sci. 2024, 25, 7574. [Google Scholar] [CrossRef] [PubMed]
- den Haan, M.C.; van Zuylen, V.-L.; Pluijmert, N.J.; Schutte, C.I.; Fibbe, W.E.; Schalij, M.J.; Roelofs, H.; Atsma, D.E. Discrepant Results of Experimental Human Mesenchymal Stromal Cell Therapy after Myocardial Infarction: Are Animal Models Robust Enough? PLoS ONE 2016, 11, e0152938. [Google Scholar] [CrossRef] [PubMed]
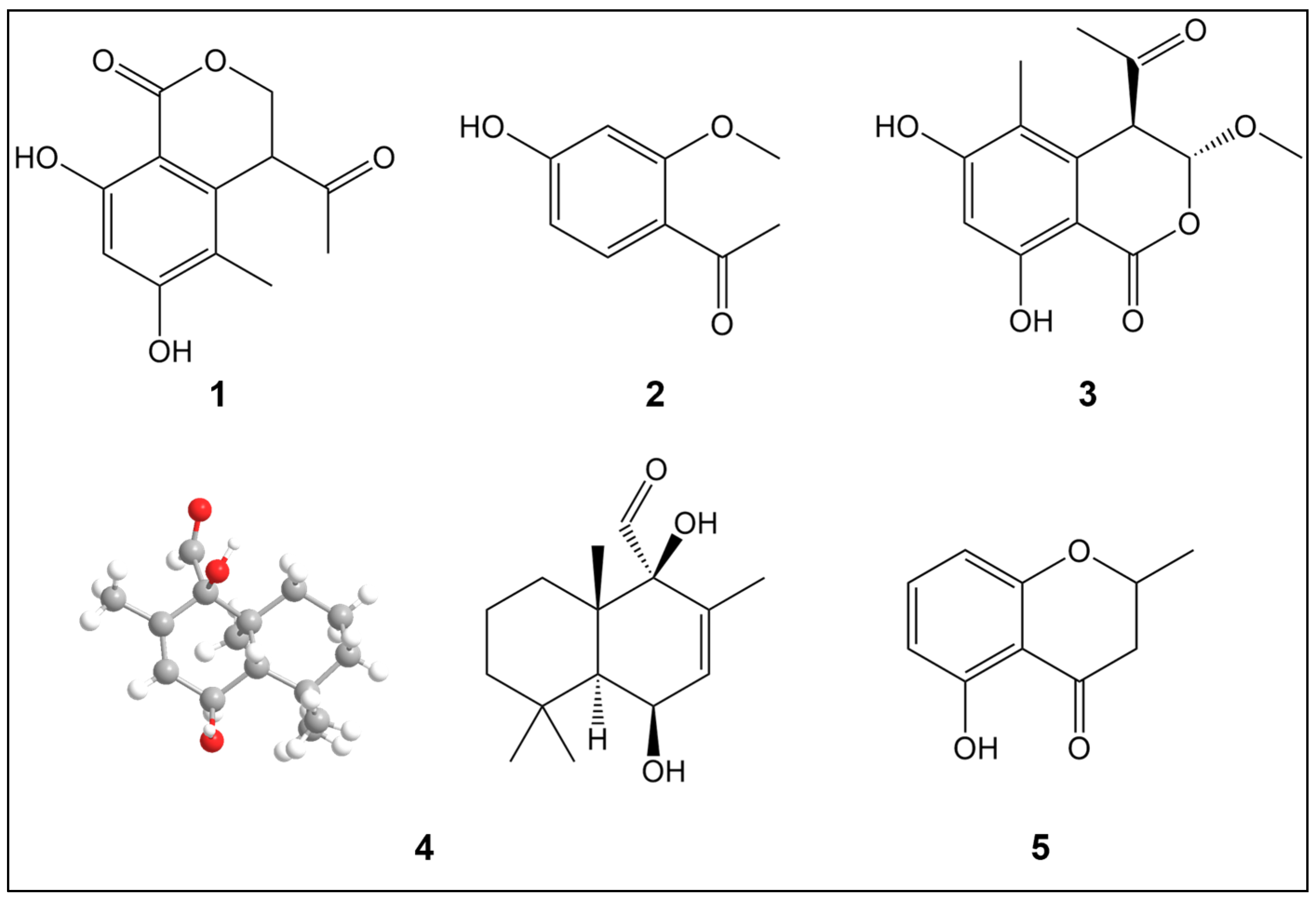
| Pos. | δH | δC | Pos. | δH | δC |
|---|---|---|---|---|---|
| 1 | 1.46 (1H, m), 1.50 (1H, m) | 43 | 9 | 82.6 | |
| 2 | 1.54 (1H, m), 1.72 (1H, m) | 33.9 | 10 | 43.5 | |
| 3 | 1.28 (1H, m), 1.37 (1H, m) | 18.1 | -CHO | 9.79 (H, s) | 205.9 |
| 4 | 33.3 | 12 | 1.60 (3H, d, J = 1.7 Hz) | 36.3 | |
| 5 | 1.64 (1H, d, J = 2.4 Hz) | 49.8 | 13 | 1.12 (3H, s) | 23 |
| 6 | 4.28 (1H, dt, J = 10.2, 2.4 Hz) | 70 | 14 | 1.29 (3H, s) | 18.1 |
| 7 | 5.83 (1H, dq, J = 10.2, 1.7 Hz) | 133.8 | 15 | 1.18 (3H, s) | 18.5 |
| 8 | 130.9 |
| Gene ID | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Aoc3 | CCAGCAGTTCTAGCATCTACAACC | CCAAGTCCTCGCCAGCAATG |
| CD28 | TTGGCTCTCAGCTTCTTCTCAG | GTAAAGGGATGCCCGGAACT |
| Ddit3 | ATCTTCATACACCACCACACCTG | TAGGGATGCAGGGTCAAGAGT |
| Dusp3 | AGGCAGAATCGTGAGATCGG | TTCCTAAGTAGGGCAGCCAG |
| Hao-1 | ACCTCACTGCCCATTGTTGTAAAG | TAAGATCCCATCCACACCATGTTTAAC |
| IL-1β | TCAGACAGCACGAGGCATTT | AGCTTCAGGAAGGCAGTGTC |
| IL-4 | GTACCAGACGTCCTTACGGC | TCAGACCGCTGACACCTCTA |
| IL-6 | CACTTCACAAGTCGGAGGCT | TCTGACAGTGCATCATCGCT |
| IL-10 | TTGAACCACCCGGCATCTAC | CCAAGGAGTTGCTCCCGTTA |
| Mmp9 | CAAACCCTGCGTATTTCCATTCATC | GATAACCATCCGAGCGACCTTTAG |
| Nlrp3 | TGGACCTCAACAGACGCTACAC | GTCCTGCCAATGGTCAAGAGTTC |
| Ptger3 | GCAATTCCTTCCTAATCGCCG | AGGTTGTTCATCATCTGGCA |
| Rcan1 | GCCCTTCGCACCCTTCTCC | CACCTCCTCCATCTCGCAGTC |
| Rhoh | AGGCAGATGTGGTACTAATGTGTTAC | TCCTGACCTCACTAATCCATTTGTTC |
| TNF-α | GGAGGGAGAACAGCAACTCC | GCCAGTGTATGAGAGGGACG |
| Trpv1 | TTATGTTCGTCTACCTCGTGTTCTTG | CATAGGCAGAGAGTTATTCTTCCCATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Wang, Q.; Li, L.; Jiang, H.; Zhang, B.; Wu, Y.; Zhou, F.; Hua, C.; Huo, G.; Li, S.; et al. Neuroprotective Terpenoids Derived from Hericium erinaceus Fruiting Bodies: Isolation, Structural Elucidation, and Mechanistic Insights. Int. J. Mol. Sci. 2025, 26, 6606. https://doi.org/10.3390/ijms26146606
Cao Y, Wang Q, Li L, Jiang H, Zhang B, Wu Y, Zhou F, Hua C, Huo G, Li S, et al. Neuroprotective Terpenoids Derived from Hericium erinaceus Fruiting Bodies: Isolation, Structural Elucidation, and Mechanistic Insights. International Journal of Molecular Sciences. 2025; 26(14):6606. https://doi.org/10.3390/ijms26146606
Chicago/Turabian StyleCao, Ying, Qiaona Wang, Lu Li, Haitao Jiang, Bianjiang Zhang, Yulong Wu, Feng Zhou, Chun Hua, Guangming Huo, Shengjie Li, and et al. 2025. "Neuroprotective Terpenoids Derived from Hericium erinaceus Fruiting Bodies: Isolation, Structural Elucidation, and Mechanistic Insights" International Journal of Molecular Sciences 26, no. 14: 6606. https://doi.org/10.3390/ijms26146606
APA StyleCao, Y., Wang, Q., Li, L., Jiang, H., Zhang, B., Wu, Y., Zhou, F., Hua, C., Huo, G., Li, S., & Li, J. (2025). Neuroprotective Terpenoids Derived from Hericium erinaceus Fruiting Bodies: Isolation, Structural Elucidation, and Mechanistic Insights. International Journal of Molecular Sciences, 26(14), 6606. https://doi.org/10.3390/ijms26146606








