Chemosensory Receptors in Vertebrates: Structure and Computational Modeling Insights
Abstract
1. Introduction
2. Features of GPCRs
2.1. The Classification
2.2. Roles and Functions
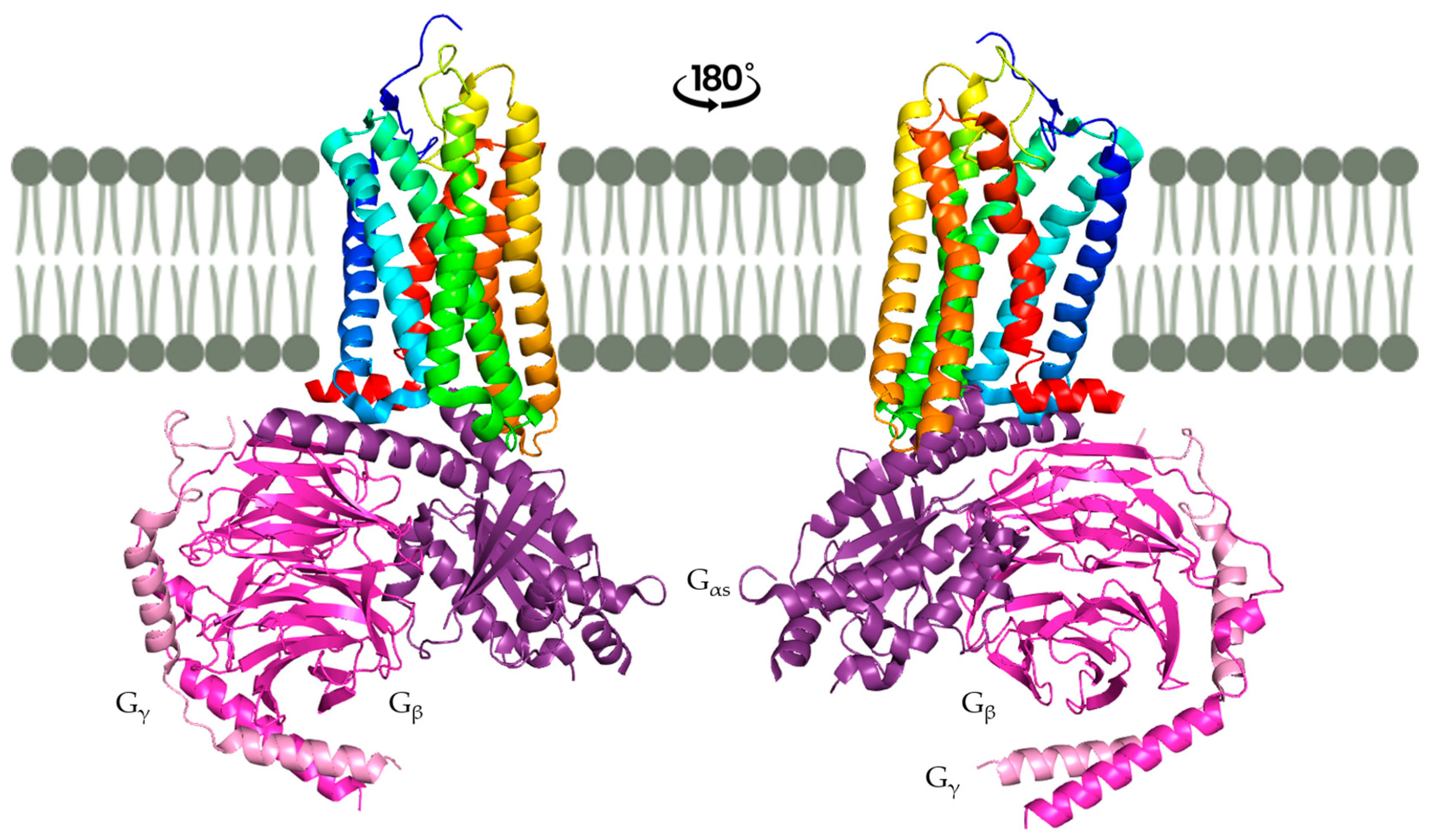
2.3. The Conserved Structure
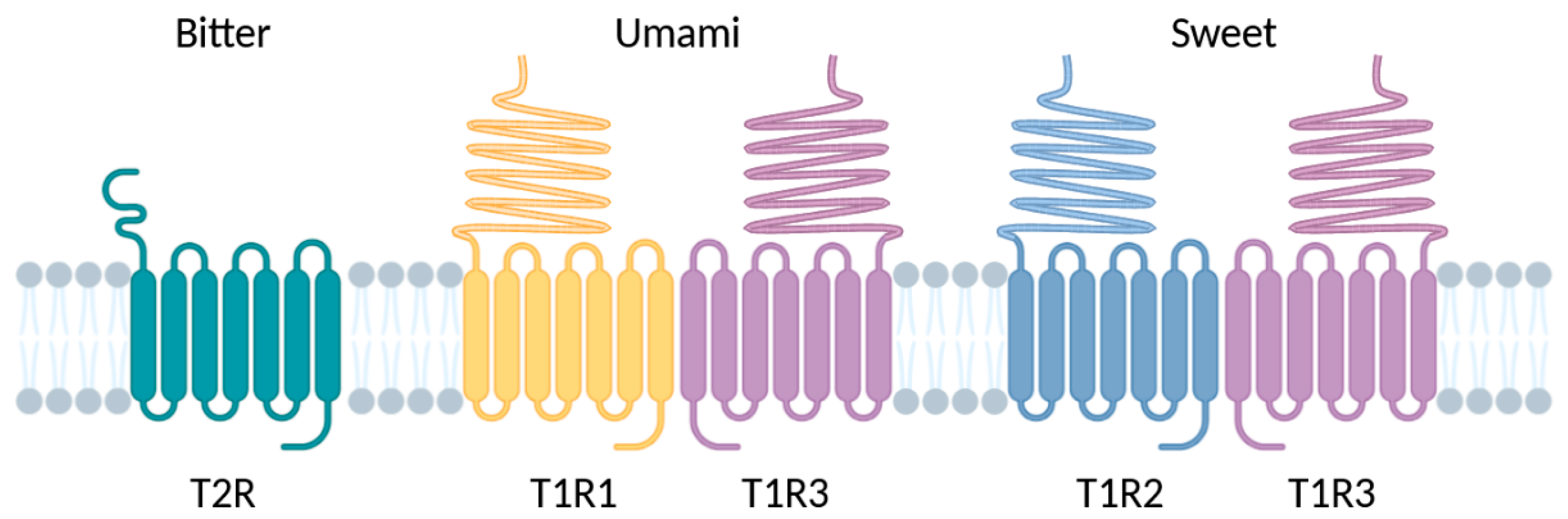
3. Vertebrate Chemosensory Receptors (CRs)
3.1. Olfactory Receptors (ORs)
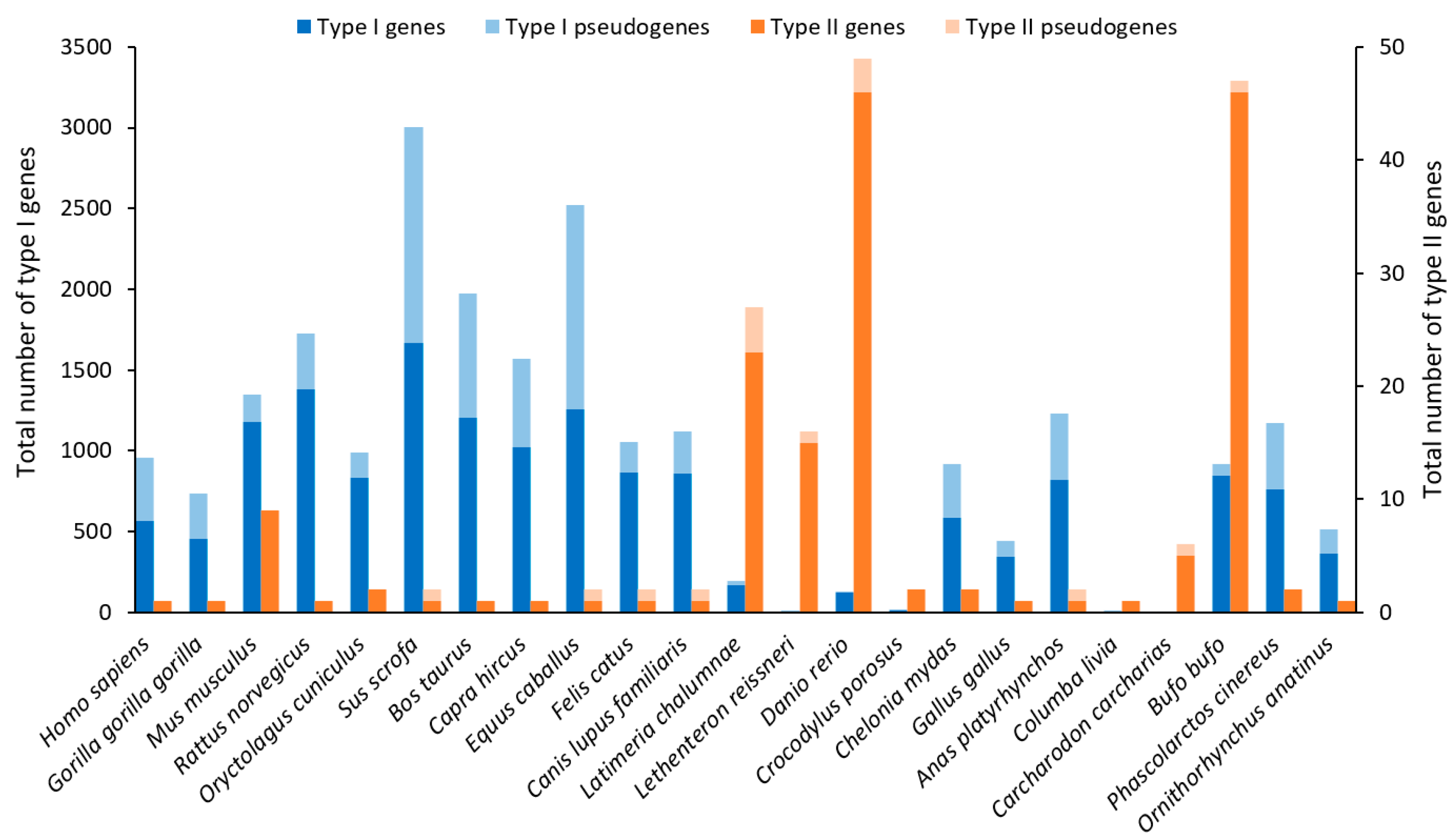
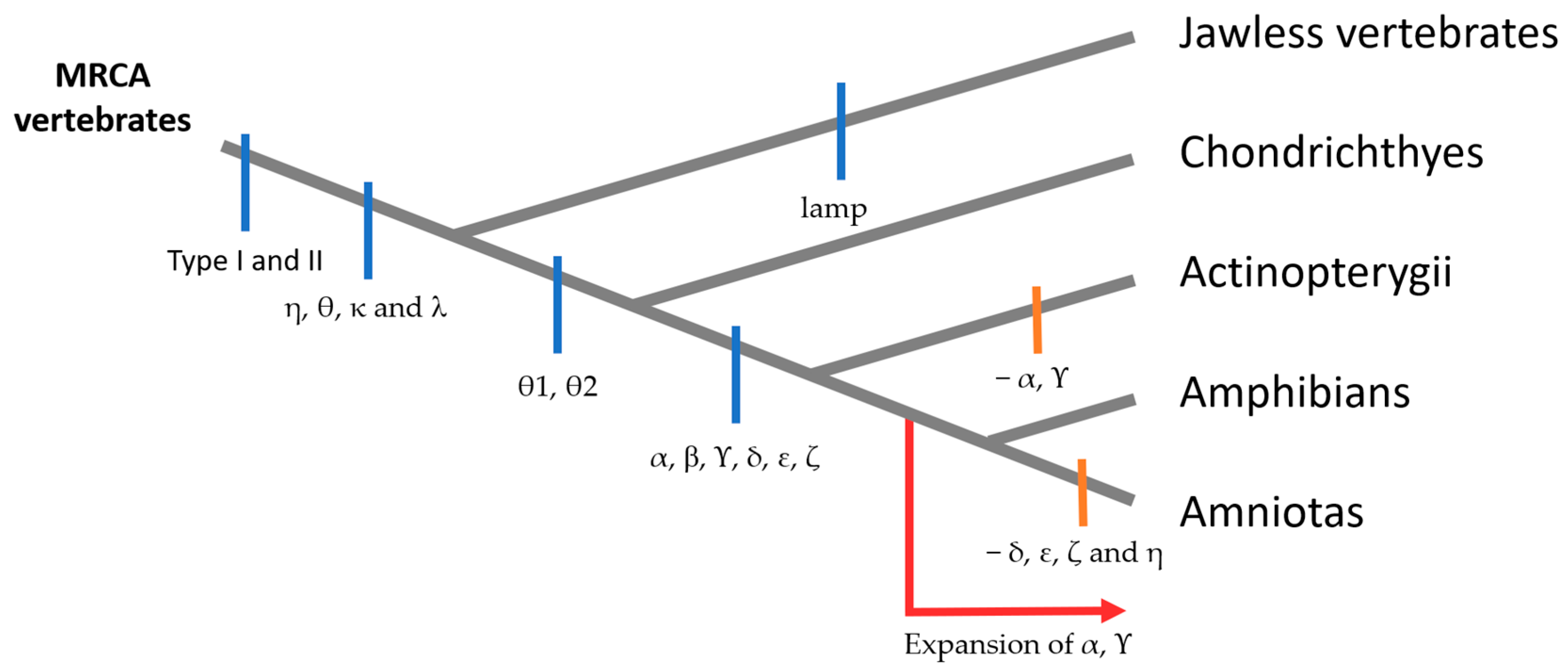
3.1.1. Genes and Evolution of ORs
3.1.2. Expression of ORs
3.1.3. Signaling Pathway of ORs
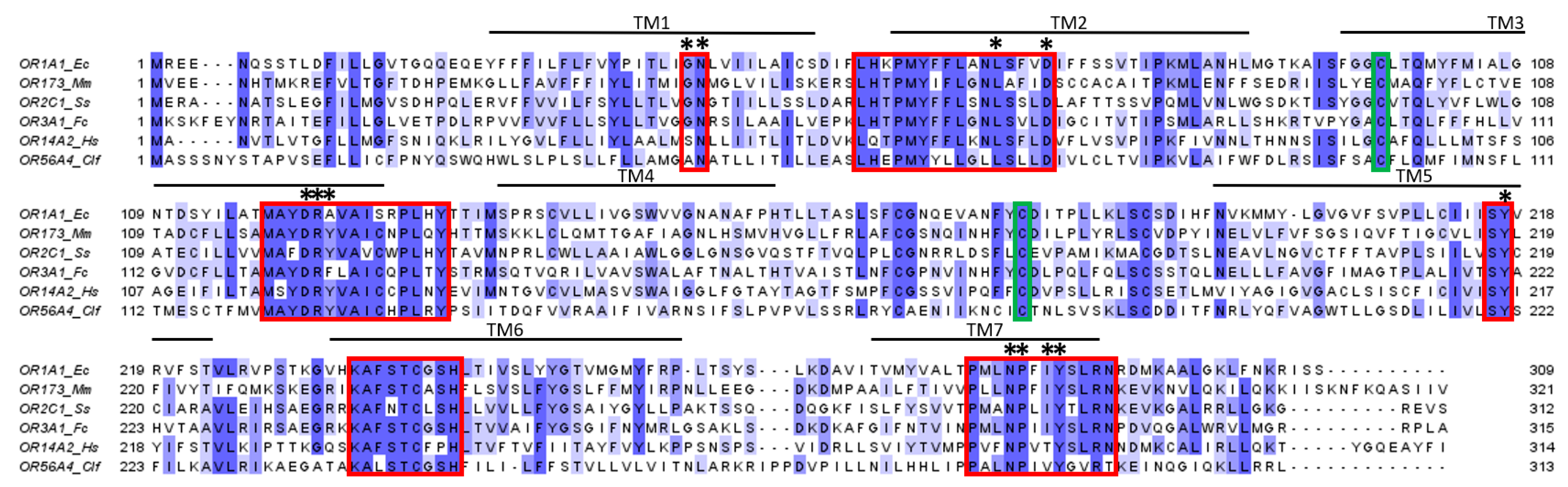
3.1.4. Structure and Binding Site of ORs
- GN in TM1;
- LHxPMYFFLxxLSxxD in TM2;
- MAYD(E)RYVAICxPLxY in TM3;
- SY in TM5;
- KAFSTCxSH in TM6;
- PxLNPxIYSLRN in TM7.
3.2. Trace Amine-Associated Receptors (TAARs)
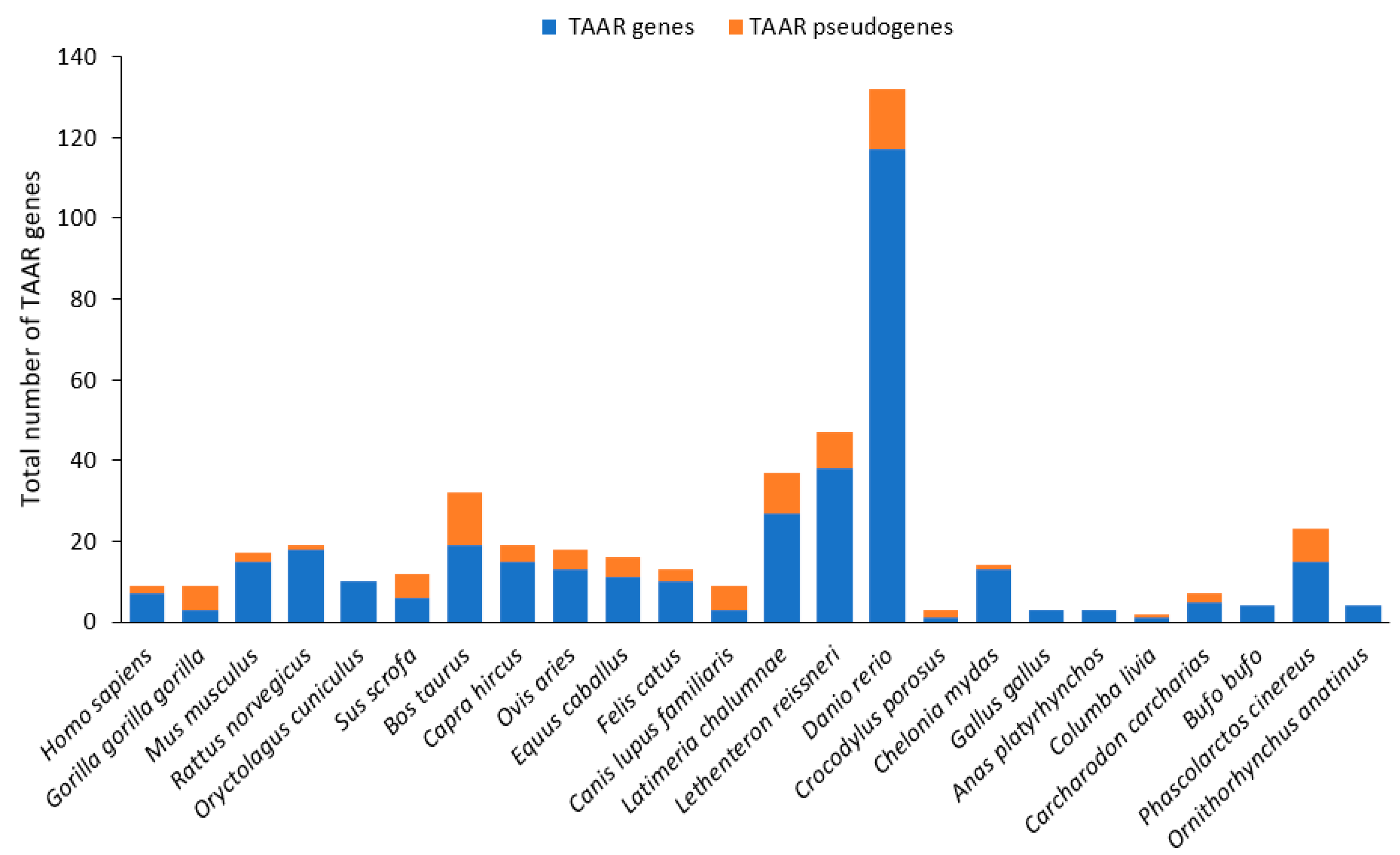
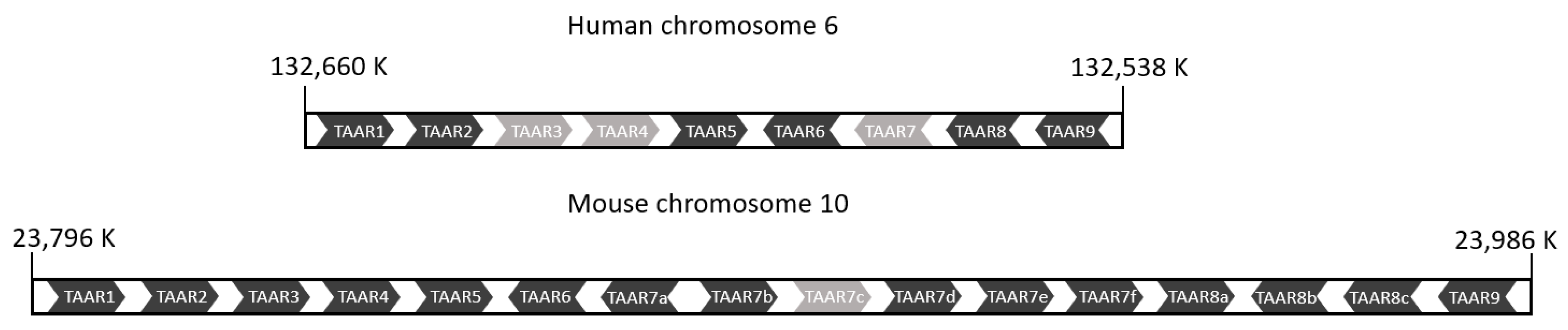
3.2.1. Genes and Evolution of TAARs
3.2.2. Expression of TAARs
3.2.3. Signaling Pathway of TAARs
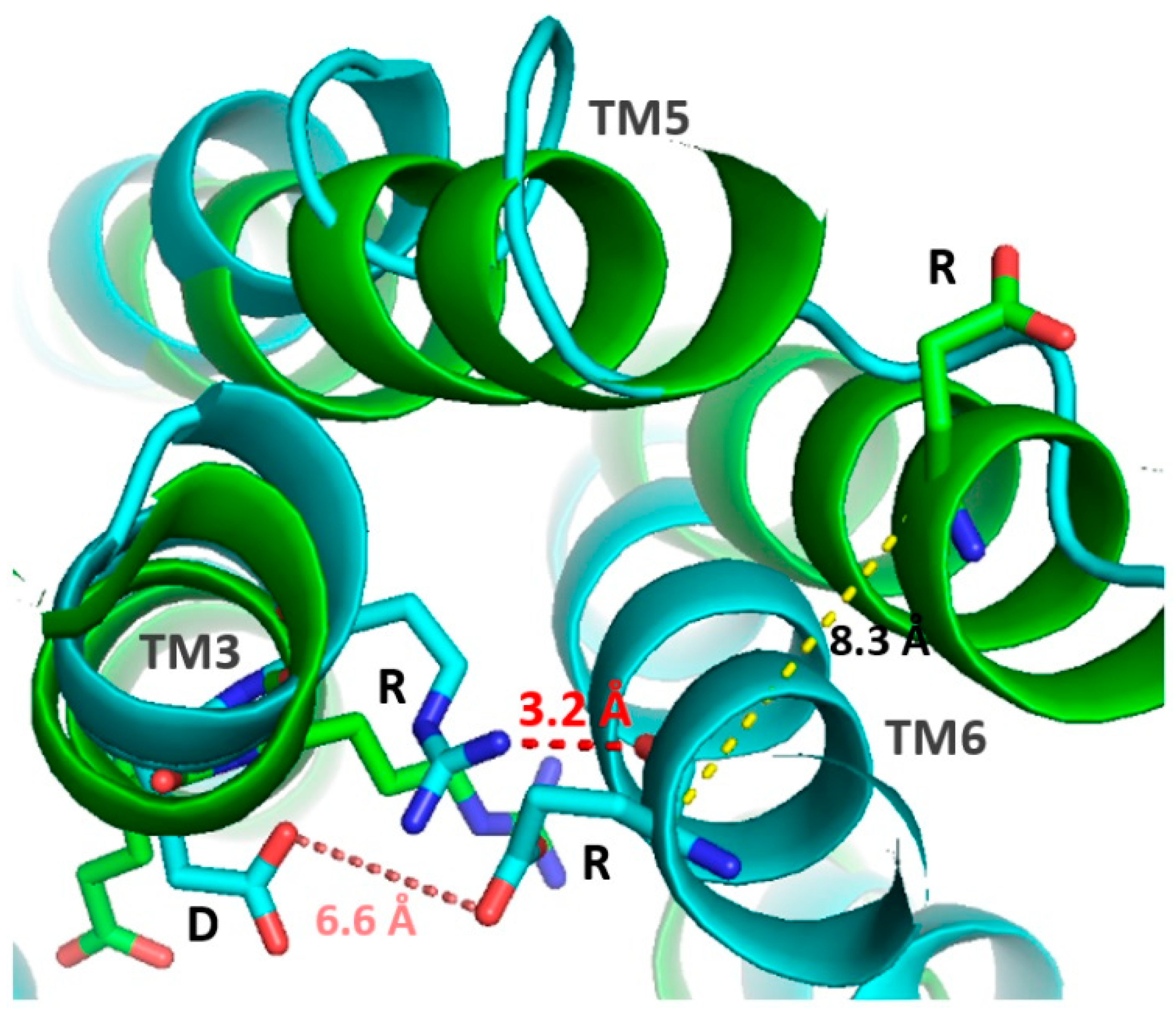
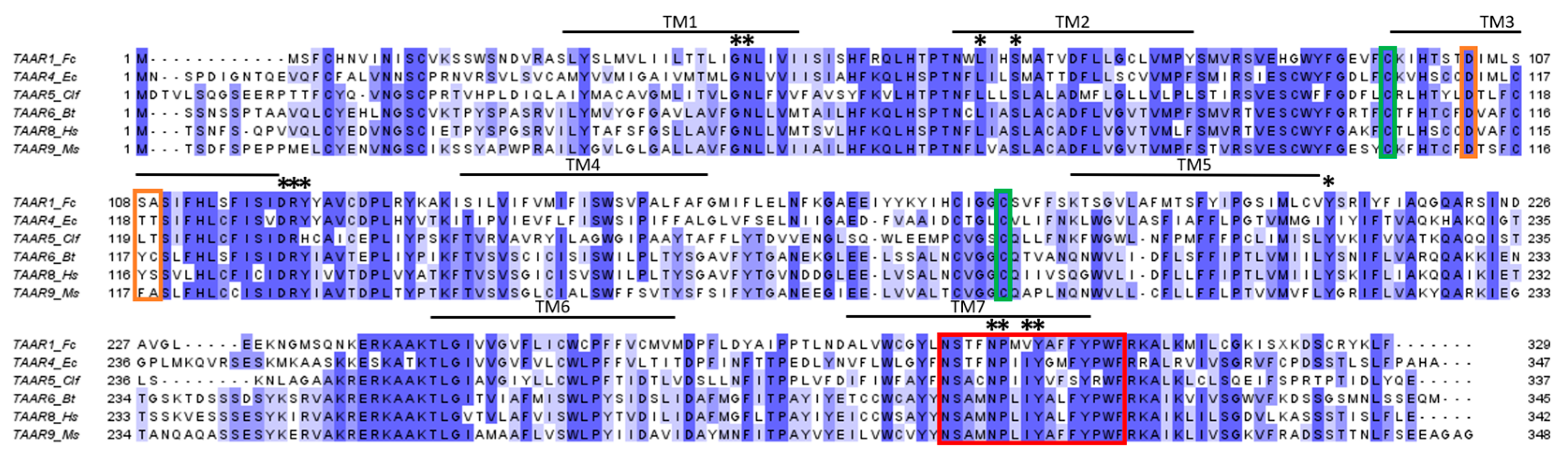
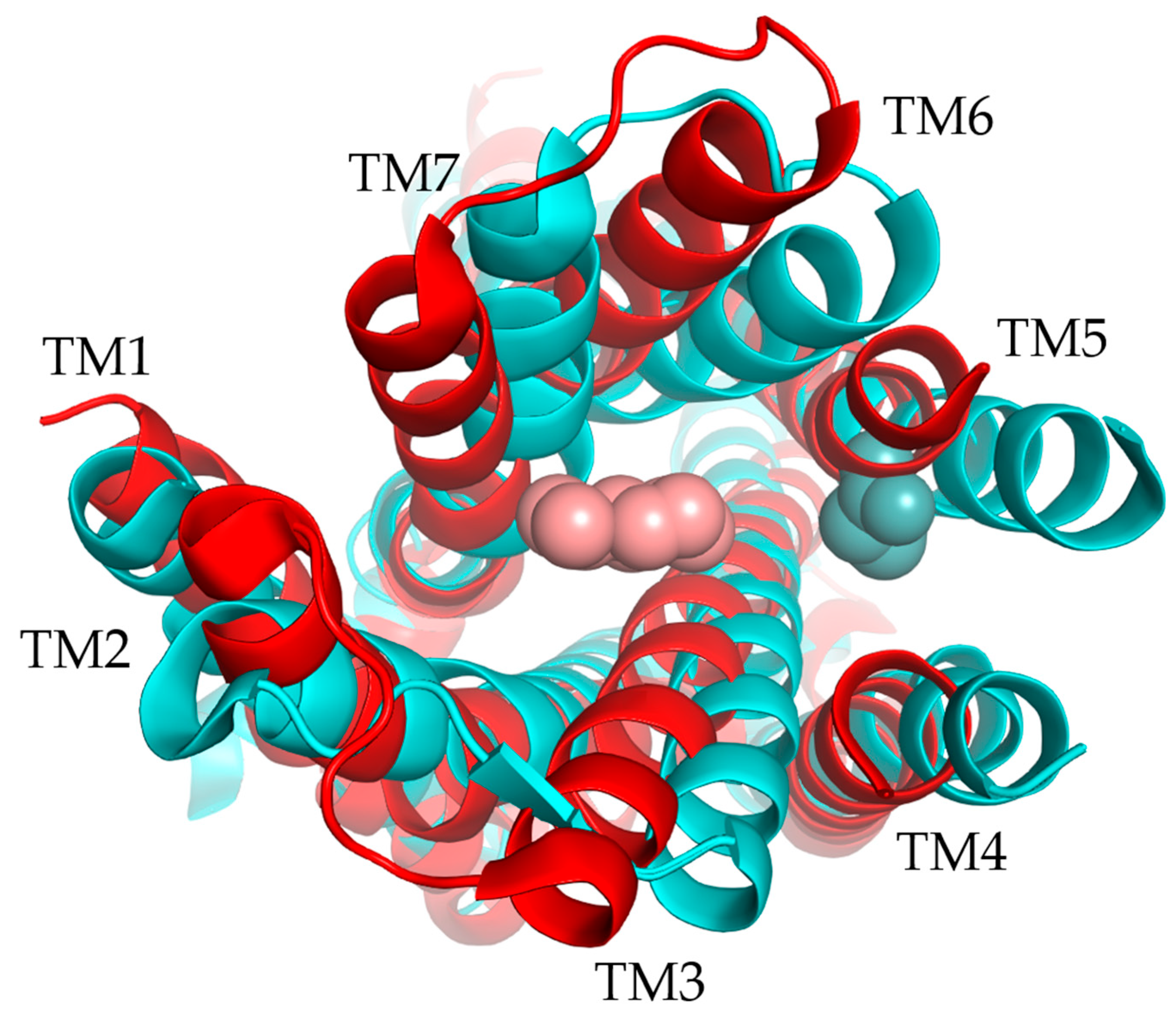
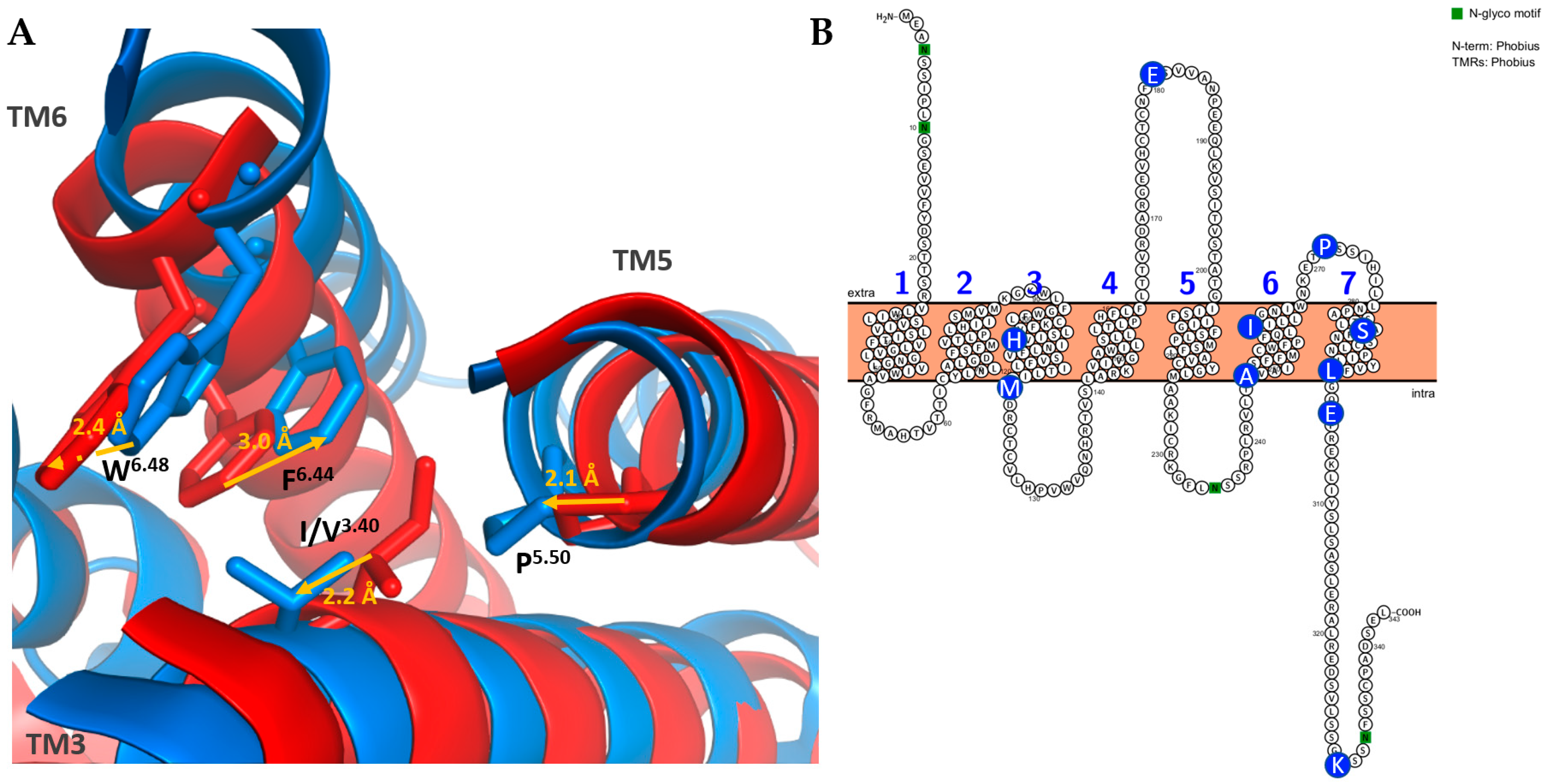
3.2.4. Structure and Binding Site of TAARs
3.3. Vomeronasal Receptors (VRs)
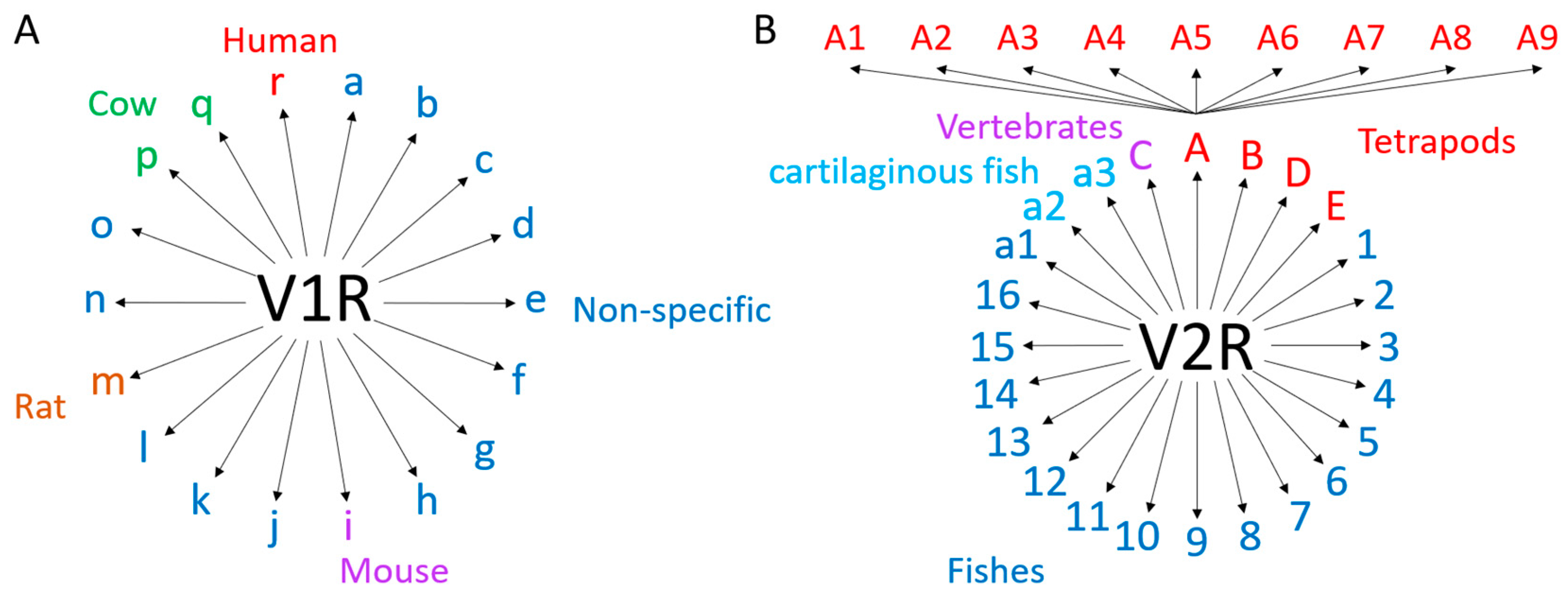
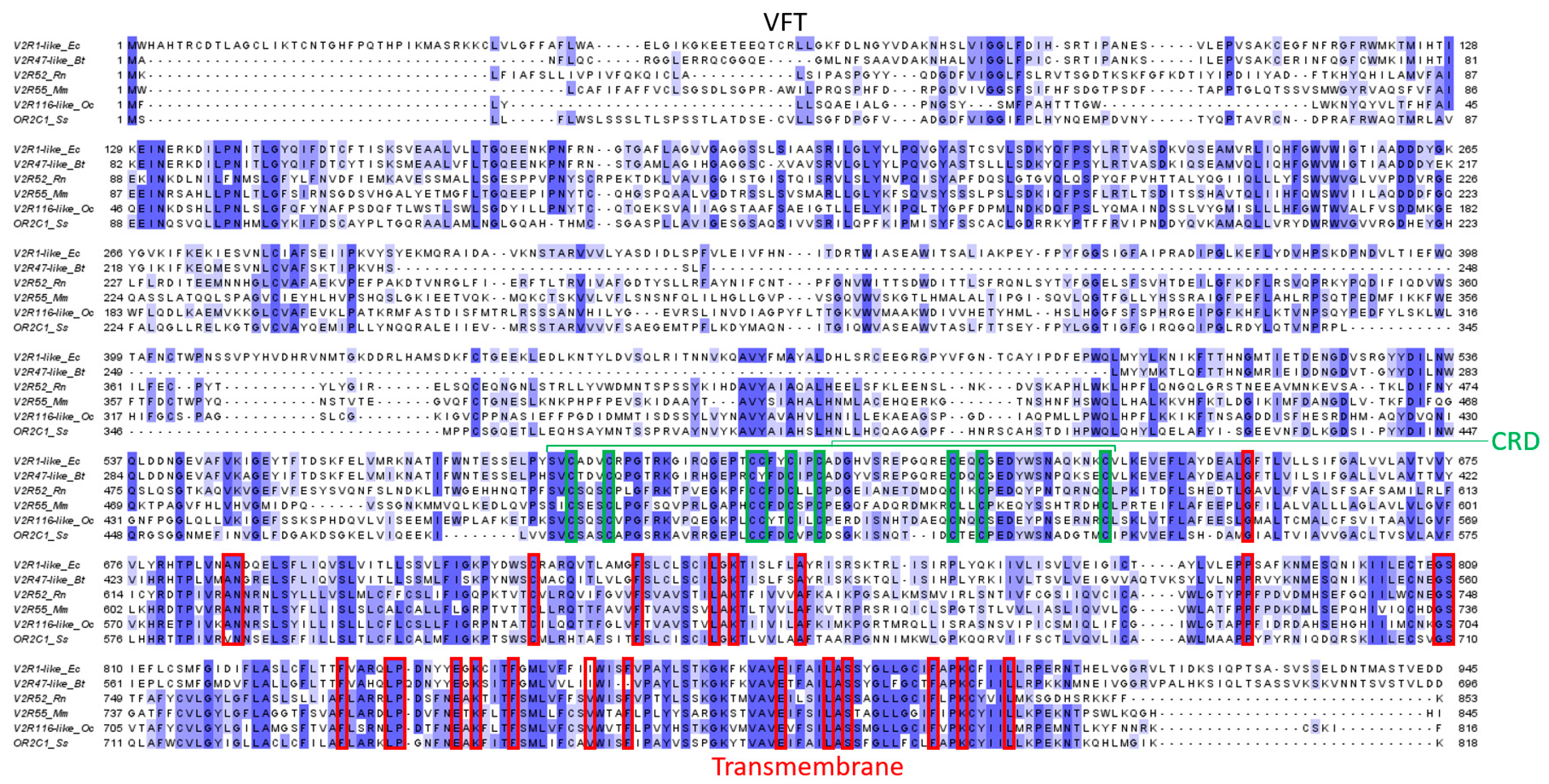
3.3.1. Genes and Evolution of VRs
3.3.2. Expression of VRs
3.3.3. Signaling Pathway of VRs
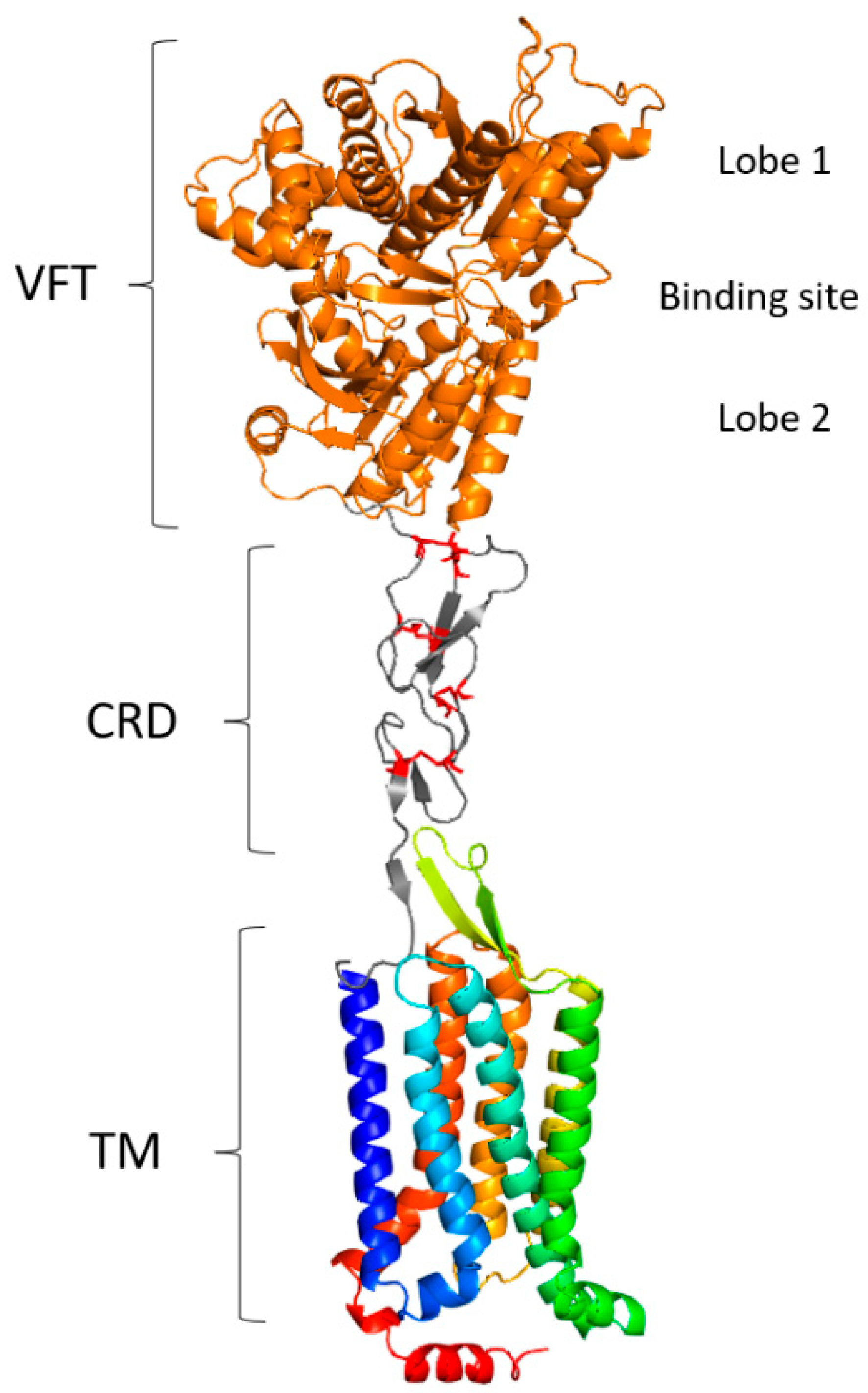
3.3.4. Structure and Binding Site of VRs
- G, M in TM1;
- C, R between TM2 and TM3;
- L in TM3;
- C between TM4 and TM5;
- M, L in TM5;
- H, E, A between TM5 and TM6;
- L, F in TM6;
- P in TM7.
- G in TM1;
- AN between TM1 and TM2;
- C between TM2 and TM3;
- F, LxK, A in TM3;
- P between TM4 and TM5;
- GS in TM5;
- F, LP, ExK between TM5 and TM6;
- F, V, F in TM6;
- E, LxS, FxxK, L in TM7.
3.4. Formyl Peptide Receptors (FPRs)
3.4.1. Genes and Evolution of FPRs
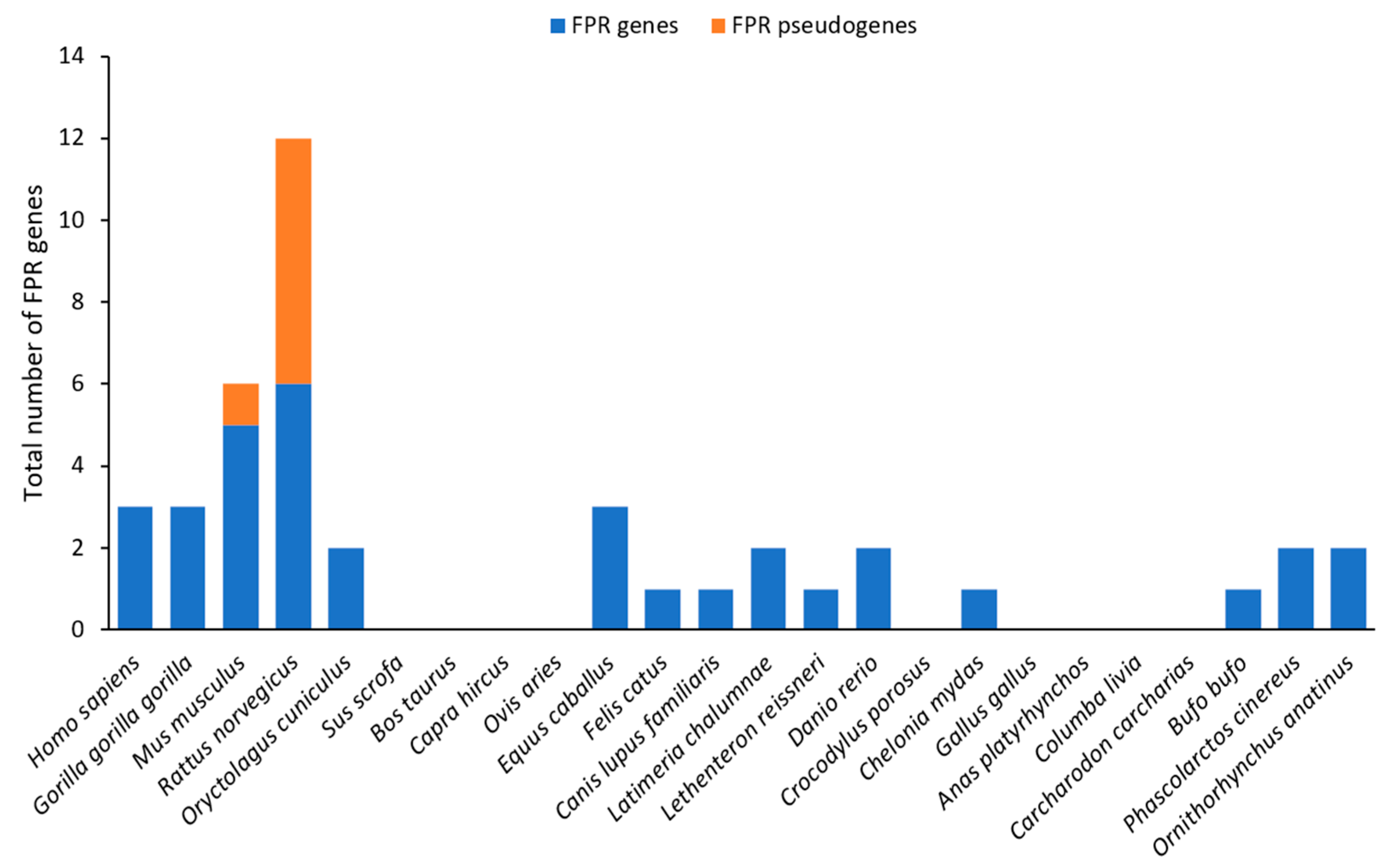
3.4.2. Expression of FPRs
3.4.3. Signaling Pathway of FPRs
3.4.4. Structure and Binding Site of FPRs
3.5. Taste Receptors (TRs)
3.5.1. Genes and Evolution of TRs
3.5.2. Expression of TRs
3.5.3. Signaling Pathway of TRs
3.5.4. Structure and Binding Site of TRs
3.6. Summary of CR Features
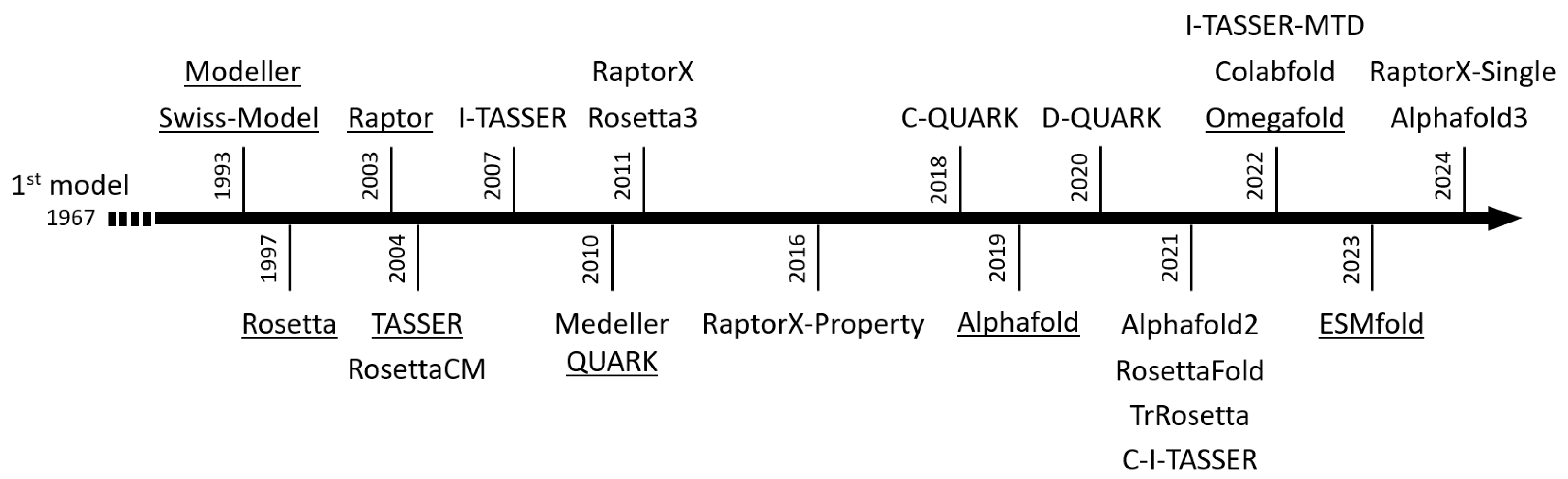
4. In Silico Study of Structures
4.1. Modeling Theories
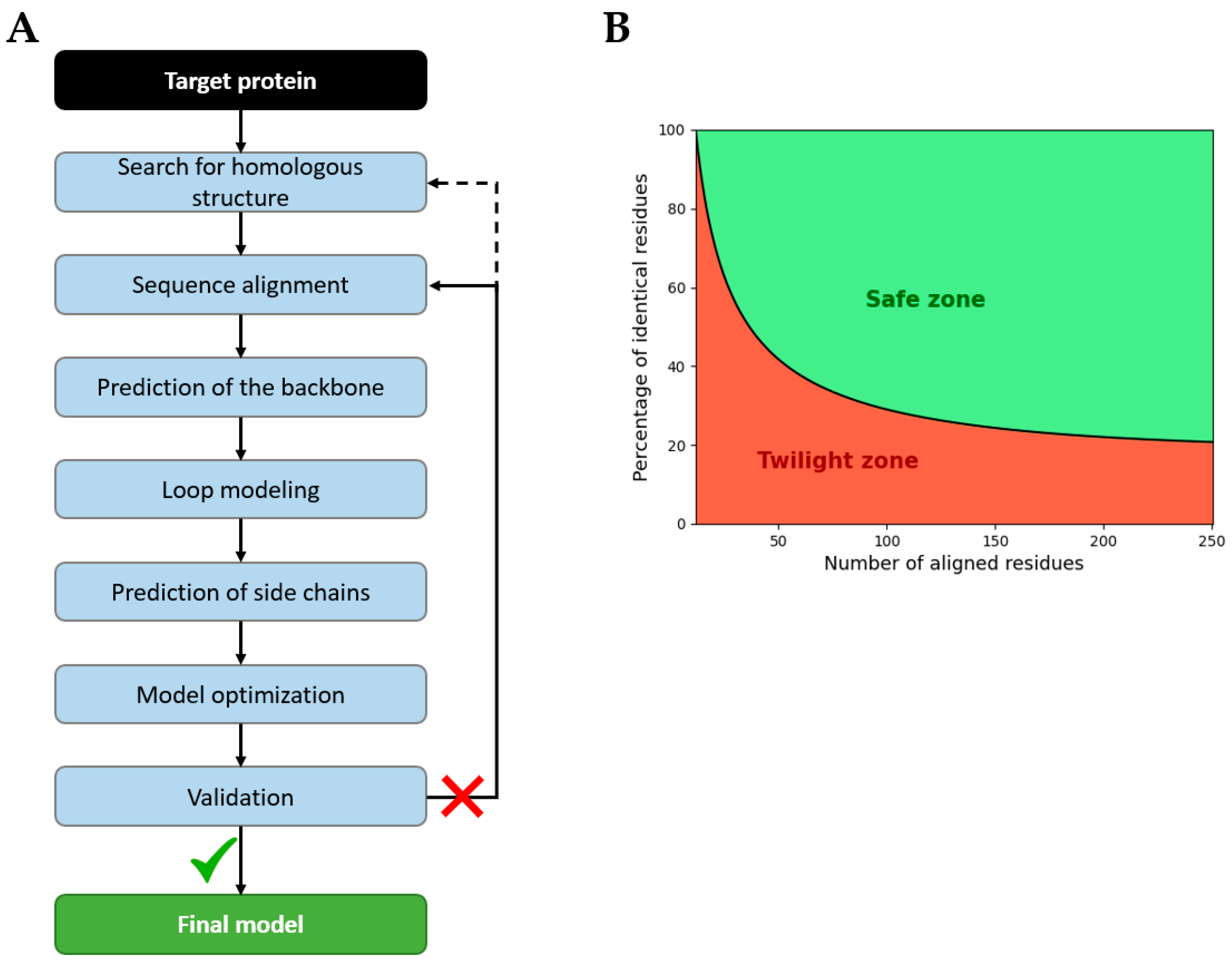
4.1.1. Homology Modeling
- Search for homologous structure by identifying proteins with high identity, common evolutionary origin, a high-quality resolved structure, and biological relevance (ligands, solvent, pH, and conformational state). Sequence identity thresholds depend on protein length (Figure 22B). Under this threshold, many random alignments are found, known as the twilight zone [172].
- The sequence alignment of target and template sequences; manual correction may be necessary, according to the bibliography.
- The generation of the backbone based on the coordinates of templates.
- Loop modeling for the gap regions or for poorly conserved regions, using either database-driven loop libraries or ab initio energy-based methods.
- Side-chain insertion based on rotamer libraries built from high-resolution structures and steric or energy constraints [173].
- The optimization of the model via energy minimization and/or dynamic simulation to resolve atomic clashes, abnormal bond lengths or angles, incorrect positions of loops, etc. The goal is to find a geometry with the minimum potential energy that corresponds to a state of equilibrium.
- Validation of the model on physics, knowledge, machine learning, or experiments scores, if the model fails, the alignment or template must be revised.
4.1.2. Fold Recognition or Threading
- A structural profile includes information on the environment of each amino acid in a score, including the secondary structure, the fraction exposed to polar atoms, and the fraction buried in the protein. Thus, environment-specific substitution tables have emerged to improve secondary structure predictions [177,181,186,187].
- Hidden Markov models (HMMs) are algorithms that create a probabilistic model, similar to a profile, that incorporates evolutionary events [188,189]. Iterations enhance the model’s ability to find distant homologs of a target and predict secondary structure, that also helps to improve the model’s ability to find real homologs because of the high conservation of the structure.
4.1.3. Ab Initio or Template-Free
4.1.4. Deep Learning Methods
4.1.5. Algorithm Comparison
4.2. Model Assessments
4.3. The Main Modeling Algorithms
4.3.1. Swiss-Model
4.3.2. Modeller
4.3.3. Rosetta
4.3.4. Raptor
4.3.5. TASSER
4.3.6. QUARK
4.3.7. Medeller
4.3.8. AlphaFold
4.3.9. ColabFold
4.3.10. ESMFold
5. CR Structural Studies
5.1. Experimental Structures
5.2. Predicted Models
5.3. Limitations
6. Usefulness of Structural Studies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| 7TM | Seven-transmembrane |
| ACIII | Adenylate cyclase 3 |
| AI | Artificial intelligence |
| ANO | Anoctamin |
| AOB | Accessory olfactory bulb |
| ATP | Adenosine triphosphate |
| β2m | β2-microglobulin |
| CAMEO | Continuous Automated Model Evaluation |
| cAMP | Cyclic adenosine monophosphate |
| CASP | Critical Assessment of protein Structure Prediction |
| CaSR | Calcium-sensing receptor |
| CNG | Cyclic nucleotide-gated |
| CNN | Convolutional neural networks |
| CP | Circumvallate papillae |
| CR | Chemosensory receptor |
| CRD | Cysteine-rich domain |
| Cryo-EM | Cryogenic electron microscopy |
| DAG | Diacylglycerol |
| ECL | Extracellular loop |
| eIF2α | Eukaryotic translation initiation factor 2α |
| ER | Endoplasmic reticulum |
| FDA | Food and Drug Administration |
| FP | Fungiform papillae |
| fP | Foliate papillae |
| FPR | Formyl peptide receptor |
| FZD | Frizzled receptors |
| GABAB | γ-aminobutyric acid B |
| GDP | Guanosine diphosphate |
| GDT_TS | Global distance test total score |
| GG | Grueneberg ganglion |
| GPCR | G-protein-coupled receptor |
| GRAFS | Glutamate, rhodopsin, adhesion, frizzled/taste2, and secretin |
| GRK | G-protein-coupled receptor kinase |
| GS | Gustatory system |
| GTP | Guanosine triphosphate |
| HMM | Hidden Markov model |
| Hsc70t | Heat shock cognate protein 70 testis enriched |
| HSPA6 | Heat shock protein A6 |
| IP3 | Inositol-1,4,5-triphosphate |
| lDDT | Local distance difference test |
| LSD1 | Lysine-specific demethylase 1 |
| MDS | Molecular dynamic simulation |
| mGluR | Metabotropic glutamate receptor |
| MHC | Major histocompatibility complex |
| MOE | Main olfactory epithelium |
| MRCA | Most recent common ancestor |
| MSA | Multiple sequence alignment |
| NHERF1 | Na+/H+ exchanger regulatory factor-1 |
| NKCC | Sodium–potassium–chloride cotransporter |
| NLP | Natural language processing |
| NMR | Nuclear magnetic resonance |
| OB | Olfactory bulb |
| OBP | Odorant binding protein |
| OlfC | Olfactory C family GPCR |
| OR | Olfactory receptor |
| ORA | Olfactory receptors related to class A |
| OS | Olfactory system |
| OSN | Olfactory sensing neuron |
| PCR | Polymerase chain reaction |
| PDB | Protein Data Bank |
| PDZ | Post-synaptic density-95, disks-large and zonula occludens-1 |
| PERK | Protein kinase R (PKR)-like endoplasmic reticulum kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PKA | Protein kinase A |
| PKR | Protein kinase R |
| PLC | Phospholipase C |
| PSSM | Position-specific scoring matrix |
| RNA | Ribonucleic acid |
| RTP | Receptor transporting protein |
| RTP1S | Receptor transporting protein 1 short |
| SMO | Smoothened receptor |
| SOM | Septal organ of Masera |
| STAU2 | Staufen homolog 2 |
| T1R | Taste receptor type 1 |
| T2R | Taste receptor type 2 |
| TAAR | Trace amine-associated receptor |
| TM | Transmembrane |
| TR | Taste receptor |
| TRPC2 | Transient receptor potential channel 2 |
| TRPM | Transient receptor potential cation channel subfamily M |
| UPR | Unfolded protein response |
| V1R | Vomeronasal receptor type 1 |
| V2R | Vomeronasal receptor type 2 |
| VFT | Venus flytrap |
| VNO | Vomeronasal organ |
| VNS | Vomeronasal system |
| VR | Vomeronasal receptor |
| XME | Xenobiotic metabolizing enzymes |
References
- Surridge, A.K.; Osorio, D.; Mundy, N.I. Evolution and Selection of Trichromatic Vision in Primates. Trends Ecol. Evol. 2003, 18, 198–205. [Google Scholar] [CrossRef]
- Keller, M.; Douhard, Q.; Baum, M.J.; Bakker, J. Sexual Experience Does Not Compensate for the Disruptive Effects of Zinc Sulfate—Lesioning of the Main Olfactory Epithelium on Sexual Behavior in Male Mice. Chem. Senses 2006, 31, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Karlson, P.; Lüscher, M. ‘Pheromones’: A New Term for a Class of Biologically Active Substances. Nature 1959, 183, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, A.; Idziak, P.; Rokosz, M. The Importance of Intact Senses in Mating and Social Assessments Made by Deaf Individuals. Arch. Sex. Behav. 2021, 50, 3799–3808. [Google Scholar] [CrossRef]
- Bell, F.R.; Dennis, B.; Sly, J. A Study of Olfaction and Gustatory Senses in Sheep after Olfactory Bulbectomy. Physiol. Behav. 1979, 23, 919–924. [Google Scholar] [CrossRef]
- Bremner, A.J.; Spence, C. The Development of Tactile Perception. Adv. Child Dev. Behav. 2017, 52, 227–268. [Google Scholar]
- Fleischer, J. The Grueneberg Ganglion: Signal Transduction and Coding in an Olfactory and Thermosensory Organ Involved in the Detection of Alarm Pheromones and Predator-Secreted Kairomones. Cell Tissue Res. 2021, 383, 535–548. [Google Scholar] [CrossRef]
- Ma, M. Encoding Olfactory Signals via Multiple Chemosensory Systems. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 463–480. [Google Scholar] [CrossRef]
- Matsunami, H.; Buck, L.B. A Multigene Family Encoding a Diverse Array of Putative Pheromone Receptors in Mammals. Cell 1997, 90, 775–784. [Google Scholar] [CrossRef]
- Slotnick, B.; Restrepo, D.; Schellinck, H.; Archbold, G.; Price, S.; Lin, W. Accessory Olfactory Bulb Function Is Modulated by Input from the Main Olfactory Epithelium. Eur. J. Neurosci. 2010, 31, 1108–1116. [Google Scholar] [CrossRef]
- Tirindelli, R. Coding of Pheromones by Vomeronasal Receptors. Cell Tissue Res. 2021, 383, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Spehr, J.; Ukhanov, K.; Kelliher, K.R.; Leinders-Zufall, T.; Zufall, F. Signaling in the Chemosensory Systems. Cell. Mol. Life Sci. 2006, 63, 1476–1484. [Google Scholar] [CrossRef]
- Dalton, R.P.; Lomvardas, S. Chemosensory Receptor Specificity and Regulation. Annu. Rev. Neurosci. 2015, 38, 331–349. [Google Scholar] [CrossRef]
- Yeagle, P.L.; Albert, A.D. G-Protein Coupled Receptor Structure. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 808–824. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 Ion Channel Determined by Electron Cryo-Microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Earl, L.A.; Falconieri, V.; Milne, J.L.; Subramaniam, S. Cryo-EM: Beyond the Microscope. Curr. Opin. Struct. Biol. 2017, 46, 71–78. [Google Scholar] [CrossRef]
- Thangaratnarajah, C.; Rheinberger, J.; Paulino, C. Cryo-EM Studies of Membrane Proteins at 200 KeV. Curr. Opin. Struct. Biol. 2022, 76, 102440. [Google Scholar] [CrossRef]
- Billesbølle, C.B.; de March, C.A.; van der Velden, W.J.C.; Ma, N.; Tewari, J.; del Torrent, C.L.; Li, L.; Faust, B.; Vaidehi, N.; Matsunami, H.; et al. Structural Basis of Odorant Recognition by a Human Odorant Receptor. Nature 2023, 615, 742–749. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-Scale Prediction of Atomic-Level Protein Structure with a Language Model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G Protein-Coupled Receptors (GPCRs): Advances in Structures, Mechanisms, and Drug Discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef]
- Kolakowski, L.F., Jr. GCRDb: A G-protein-coupled Receptor Database. Recept. Channels 1994, 2, 1–7. [Google Scholar]
- Chun, L.; Zhang, W.; Liu, J. Structure and Ligand Recognition of Class C GPCRs. Acta Pharmacol. Sin. 2012, 33, 312–323. [Google Scholar] [CrossRef]
- Di Pizio, A.; Levit, A.; Slutzki, M.; Behrens, M.; Karaman, R.; Niv, M.Y. Comparing Class A GPCRs to Bitter Taste Receptors. Methods Cell Biol. 2016, 132, 401–427. [Google Scholar]
- Schiöth, H.B.; Fredriksson, R. The GRAFS Classification System of G-Protein Coupled Receptors in Comparative Perspective. Gen. Comp. Endocrinol. 2005, 142, 94–101. [Google Scholar] [CrossRef]
- Alhosaini, K.; Azhar, A.; Alonazi, A.; Al-Zoghaibi, F. GPCRs: The Most Promiscuous Druggable Receptor of the Mankind. Saudi Pharm. J. 2021, 29, 539–551. [Google Scholar] [CrossRef]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Trong, I.L.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal Structure of Rhodopsin: A G Protein-Coupled Receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef]
- Rasmussen, S.G.F.; Choi, H.-J.; Rosenbaum, D.M.; Kobilka, T.S.; Thian, F.S.; Edwards, P.C.; Burghammer, M.; Ratnala, V.R.P.; Sanishvili, R.; Fischetti, R.F.; et al. Crystal Structure of the Human Β2 Adrenergic G-Protein-Coupled Receptor. Nature 2007, 450, 383–387. [Google Scholar] [CrossRef]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.F.; Thian, F.S.; Kobilka, T.S.; Choi, H.-J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-Resolution Crystal Structure of an Engineered Human β2-Adrenergic G Protein–Coupled Receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef]
- Almén, M.S.; Nordström, K.J.; Fredriksson, R.; Schiöth, H.B. Mapping the Human Membrane Proteome: A Majority of the Human Membrane Proteins Can Be Classified According to Function and Evolutionary Origin. BMC Biol. 2009, 7, 50. [Google Scholar] [CrossRef]
- Cvicek, V.; Goddard, W.A.; Abrol, R. Structure-Based Sequence Alignment of the Transmembrane Domains of All Human GPCRs: Phylogenetic, Structural and Functional Implications. PLoS Comput. Biol. 2016, 12, e1004805. [Google Scholar] [CrossRef]
- Buck, L.; Axel, R. A Novel Multigene Family May Encode Odorant Receptors: A Molecular Basis for Odor Recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Touhara, K.; Vosshall, L.B. Sensing Odorants and Pheromones with Chemosensory Receptors. Annu. Rev. Physiol. 2009, 71, 307–332. [Google Scholar] [CrossRef]
- Niimura, Y.; Nei, M. Evolutionary Dynamics of Olfactory Receptor Genes in Fishes and Tetrapods. Proc. Natl. Acad. Sci. USA 2005, 102, 6039–6044. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.; Niimura, Y. Similar Numbers but Different Repertoires of Olfactory Receptor Genes in Humans and Chimpanzees. Mol. Biol. Evol. 2008, 25, 1897–1907. [Google Scholar] [CrossRef]
- Policarpo, M.; Baldwin, M.W.; Casane, D.; Salzburger, W. Diversity and Evolution of the Vertebrate Chemoreceptor Gene Repertoire. Nat. Commun. 2024, 15, 1421. [Google Scholar] [CrossRef]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression Profile of Ectopic Olfactory Receptors Determined by Deep Sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, H.; Fu, N.; Chen, L. The Diversified Function and Potential Therapy of Ectopic Olfactory Receptors in Non-olfactory Tissues. J. Cell Physiol. 2018, 233, 2104–2115. [Google Scholar] [CrossRef]
- Glusman, G.; Bahar, A.; Sharon, D.; Pilpel, Y.; White, J.; Lancet, D. The Olfactory Receptor Gene Superfamily: Data Mining, Classification, and Nomenclature. Mamm. Genome 2000, 11, 1016–1023. [Google Scholar] [CrossRef]
- Silva, M.C.; Chibucos, M.; Munro, J.B.; Daugherty, S.; Coelho, M.M.; Silva, J.C. Signature of Adaptive Evolution in Olfactory Receptor Genes in Cory’s Shearwater Supports Molecular Basis for Smell in Procellariiform Seabirds. Sci. Rep. 2020, 10, 543. [Google Scholar] [CrossRef]
- Freitag, J.; Krieger, J.; Strotmann, J.; Breer, H. Two Classes of Olfactory Receptors in Xenopus laevis. Neuron 1995, 15, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Niimura, Y. On the Origin and Evolution of Vertebrate Olfactory Receptor Genes: Comparative Genome Analysis Among 23 Chordate Species. Genome Biol. Evol. 2009, 1, 34–44. [Google Scholar] [CrossRef]
- Gilad, Y.; Wiebe, V.; Przeworski, M.; Lancet, D.; Pääbo, S. Loss of Olfactory Receptor Genes Coincides with the Acquisition of Full Trichromatic Vision in Primates. PLoS Biol. 2004, 2, e5. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, G.M. The Human Sense of Smell: Are We Better Than We Think? PLoS Biol. 2004, 2, e146. [Google Scholar] [CrossRef]
- Laska, M.; Seibt, A.; Weber, A. “Microsmatic” Primates Revisited: Olfactory Sensitivity in the Squirrel Monkey. Chem. Senses 2000, 25, 47–53. [Google Scholar] [CrossRef]
- Lai, P.C.; Bahl, G.; Gremigni, M.; Matarazzo, V.; Clot-Faybesse, O.; Ronin, C.; Crasto, C.J. An Olfactory Receptor Pseudogene Whose Function Emerged in Humans: A Case Study in the Evolution of Structure–Function in GPCRs. J. Struct. Funct. Genomics 2008, 9, 29–40. [Google Scholar] [CrossRef]
- Zhang, X.; Rodriguez, I.; Mombaerts, P.; Firestein, S. Odorant and Vomeronasal Receptor Genes in Two Mouse Genome Assemblies. Genomics 2004, 83, 802–811. [Google Scholar] [CrossRef]
- Mombaerts, P. Odorant Receptor Gene Choice in Olfactory Sensory Neurons: The One Receptor–One Neuron Hypothesis Revisited. Curr. Opin. Neurobiol. 2004, 14, 31–36. [Google Scholar] [CrossRef]
- Magklara, A.; Yen, A.; Colquitt, B.M.; Clowney, E.J.; Allen, W.; Markenscoff-Papadimitriou, E.; Evans, Z.A.; Kheradpour, P.; Mountoufaris, G.; Carey, C.; et al. An Epigenetic Signature for Monoallelic Olfactory Receptor Expression. Cell 2011, 145, 555–570. [Google Scholar] [CrossRef]
- Dalton, R.P.; Lyons, D.B.; Lomvardas, S. Co-Opting the Unfolded Protein Response to Elicit Olfactory Receptor Feedback. Cell 2013, 155, 321–332. [Google Scholar] [CrossRef]
- Lyons, D.B.; Allen, W.E.; Goh, T.; Tsai, L.; Barnea, G.; Lomvardas, S. An Epigenetic Trap Stabilizes Singular Olfactory Receptor Expression. Cell 2013, 154, 325–336. [Google Scholar] [CrossRef]
- Saito, H.; Kubota, M.; Roberts, R.W.; Chi, Q.; Matsunami, H. RTP Family Members Induce Functional Expression of Mammalian Odorant Receptors. Cell 2004, 119, 679–691. [Google Scholar] [CrossRef]
- Wu, L.; Pan, Y.; Chen, G.-Q.; Matsunami, H.; Zhuang, H. Receptor-Transporting Protein 1 Short (RTP1S) Mediates Translocation and Activation of Odorant Receptors by Acting through Multiple Steps. J. Biol. Chem. 2012, 287, 22287–22294. [Google Scholar] [CrossRef]
- Inoue, R.; Fukutani, Y.; Niwa, T.; Matsunami, H.; Yohda, M. Identification and Characterization of Proteins That Are Involved in RTP1S-Dependent Transport of Olfactory Receptors. Int. J. Mol. Sci. 2023, 24, 7829. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional Characterization of a Mouse Testicular Olfactory Receptor and Its Role in Chemosensing and in Regulation of Sperm Motility. J. Cell Sci. 2004, 117, 5835–5845. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, E.M. A Specific Heat Shock Protein Enhances the Expression of Mammalian Olfactory Receptor Proteins. Chem. Senses 2006, 31, 445–452. [Google Scholar] [CrossRef]
- Heydel, J.; Coelho, A.; Thiebaud, N.; Legendre, A.; Bon, A.L.; Faure, P.; Neiers, F.; Artur, Y.; Golebiowski, J.; Briand, L. Odorant-Binding Proteins and Xenobiotic Metabolizing Enzymes: Implications in Olfactory Perireceptor Events. Anat. Rec. 2013, 296, 1333–1345. [Google Scholar] [CrossRef]
- Firestein, S. How the Olfactory System Makes Sense of Scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Stephan, A.B.; Shum, E.Y.; Hirsh, S.; Cygnar, K.D.; Reisert, J.; Zhao, H. ANO2 Is the Cilial Calcium-Activated Chloride Channel That May Mediate Olfactory Amplification. Proc. Natl. Acad. Sci. USA 2009, 106, 11776–11781. [Google Scholar] [CrossRef]
- Sagheddu, C.; Boccaccio, A.; Dibattista, M.; Montani, G.; Tirindelli, R.; Menini, A. Calcium Concentration Jumps Reveal Dynamic Ion Selectivity of Calcium-Activated Chloride Currents in Mouse Olfactory Sensory Neurons and TMEM16b-Transfected HEK 293T Cells. J. Physiol. 2010, 588, 4189–4204. [Google Scholar] [CrossRef]
- Pietra, G.; Dibattista, M.; Menini, A.; Reisert, J.; Boccaccio, A. The Ca2+-Activated Cl− Channel TMEM16B Regulates Action Potential Firing and Axonal Targeting in Olfactory Sensory Neurons. J. Gen. Physiol. 2016, 148, 293–311. [Google Scholar] [CrossRef]
- Henkel, B.; Drose, D.R.; Ackels, T.; Oberland, S.; Spehr, M.; Neuhaus, E.M. Co-Expression of Anoctamins in Cilia of Olfactory Sensory Neurons. Chem. Senses 2015, 40, 73–87. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, Z.; Pacalon, J.; Xu, L.; Li, W.; Belloir, C.; Topin, J.; Briand, L.; Golebiowski, J.; Cong, X. Extracellular Loop 2 of G Protein–Coupled Olfactory Receptors Is Critical for Odorant Recognition. J. Biol. Chem. 2022, 298, 102331. [Google Scholar] [CrossRef] [PubMed]
- de March, C.A.; Kim, S.; Antonczak, S.; Goddard, W.A.; Golebiowski, J. G Protein-coupled Odorant Receptors: From Sequence to Structure. Protein Sci. 2015, 24, 1543–1548. [Google Scholar] [CrossRef]
- de March, C.A.; Yu, Y.; Ni, M.J.; Adipietro, K.A.; Matsunami, H.; Ma, M.; Golebiowski, J. Conserved Residues Control Activation of Mammalian G Protein-Coupled Odorant Receptors. J. Am. Chem. Soc. 2015, 137, 8611–8616. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace Amines: Identification of a Family of Mammalian G Protein-Coupled Receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-Methylenedioxymethamphetamine, Lysergic Acid Diethylamide, and Metabolites of the Catecholamine Neurotransmitters Are Agonists of a Rat Trace Amine Receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Liberles, S.D.; Buck, L.B. A Second Class of Chemosensory Receptors in the Olfactory Epithelium. Nature 2006, 442, 645–650. [Google Scholar] [CrossRef]
- Ferrero, D.M.; Lemon, J.K.; Fluegge, D.; Pashkovski, S.L.; Korzan, W.J.; Datta, S.R.; Spehr, M.; Fendt, M.; Liberles, S.D. Detection and Avoidance of a Carnivore Odor by Prey. Proc. Natl. Acad. Sci. USA 2011, 108, 11235–11240. [Google Scholar] [CrossRef]
- Nair, P.C.; Shajan, B.; Bastiampillai, T. Newly Identified Structures of Trace-Amine Associated Receptor-1 (TAAR1) Will Aid Discovery of next Generation Neuropsychiatric Drugs. Mol. Psychiatry 2024, 29, 1925–1928. [Google Scholar] [CrossRef]
- Lindemann, L.; Ebeling, M.; Kratochwil, N.A.; Bunzow, J.R.; Grandy, D.K.; Hoener, M.C. Trace Amine-Associated Receptors Form Structurally and Functionally Distinct Subfamilies of Novel G Protein-Coupled Receptors. Genomics 2005, 85, 372–385. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Battey, J.; Benson, H.E.; Benya, R.V.; Bonner, T.I.; Davenport, A.P.; Dhanachandra Singh, K.; Eguchi, S.; Harmar, A.; Holliday, N.; et al. Class A Orphans in GtoPdb v.2023.1. IUPHAR/BPS Guide Pharmacol. CITE 2023, 2023. [Google Scholar] [CrossRef]
- Eyun, S.; Moriyama, H.; Hoffmann, F.G.; Moriyama, E.N. Molecular Evolution and Functional Divergence of Trace Amine–Associated Receptors. PLoS ONE 2016, 11, e0151023. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Nishida, M. Evolution of Trace Amine–Associated Receptor (TAAR) Gene Family in Vertebrates: Lineage-Specific Expansions and Degradations of a Second Class of Vertebrate Chemosensory Receptors Expressed in the Olfactory Epithelium. Mol. Biol. Evol. 2007, 24, 2099–2107. [Google Scholar] [CrossRef]
- Ferrero, D.M.; Wacker, D.; Roque, M.A.; Baldwin, M.W.; Stevens, R.C.; Liberles, S.D. Agonists for 13 Trace Amine-Associated Receptors Provide Insight into the Molecular Basis of Odor Selectivity. ACS Chem. Biol. 2012, 7, 1184–1189. [Google Scholar] [CrossRef]
- Azzouzi, N.; Barloy-Hubler, F.; Galibert, F. Identification and Characterization of Cichlid TAAR Genes and Comparison with Other Teleost TAAR Repertoires. BMC Genom. 2015, 16, 335. [Google Scholar] [CrossRef][Green Version]
- Fleischer, J.; Schwarzenbacher, K.; Breer, H. Expression of Trace Amine-Associated Receptors in the Grueneberg Ganglion. Chem. Senses 2007, 32, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Tsai, L.; Roy, D.S.; Valenzuela, D.H.; Mosley, C.; Magklara, A.; Lomvardas, S.; Liberles, S.D.; Barnea, G. Neurons Expressing Trace Amine-Associated Receptors Project to Discrete Glomeruli and Constitute an Olfactory Subsystem. Proc. Natl. Acad. Sci. USA 2012, 109, 13410–13415. [Google Scholar] [CrossRef]
- Shah, A.; Ratkowski, M.; Rosa, A.; Feinstein, P.; Bozza, T. Olfactory Expression of Trace Amine-Associated Receptors Requires Cooperative Cis-Acting Enhancers. Nat. Commun. 2021, 12, 3797. [Google Scholar] [CrossRef]
- Gusach, A.; Lee, Y.; Khoshgrudi, A.N.; Mukhaleva, E.; Ma, N.; Koers, E.J.; Chen, Q.; Edwards, P.C.; Huang, F.; Kim, J.; et al. Molecular Recognition of an Odorant by the Murine Trace Amine-Associated Receptor TAAR7f. Nat. Commun. 2024, 15, 7555. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. Receptors. In Methods in Neurosciences; Academic Press: Cambridge, MA, USA, 1995; Volume 25, pp. 366–428. [Google Scholar]
- Nicoli, A.; Weber, V.; Bon, C.; Steuer, A.; Gustincich, S.; Gainetdinov, R.R.; Lang, R.; Espinoza, S.; Di Pizio, A. Structure-Based Discovery of Mouse Trace Amine-Associated Receptor 5 Antagonists. J. Chem. Inf. Model. 2023, 63, 6667–6680. [Google Scholar] [CrossRef]
- Zilberg, G.; Parpounas, A.K.; Warren, A.L.; Yang, S.; Wacker, D. Molecular Basis of Human Trace Amine-Associated Receptor 1 Activation. Nat. Commun. 2024, 15, 108. [Google Scholar] [CrossRef]
- Dulac, C.; Axel, R. A Novel Family of Genes Encoding Putative Pheromone Receptors in Mammals. Cell 1995, 83, 195–206. [Google Scholar] [CrossRef]
- Rodriguez, I.; Greer, C.A.; Mok, M.Y.; Mombaerts, P. A Putative Pheromone Receptor Gene Expressed in Human Olfactory Mucosa. Nat. Genet. 2000, 26, 18–19. [Google Scholar] [CrossRef]
- Rodriguez, I.; Mombaerts, P. Novel Human Vomeronasal Receptor-like Genes Reveal Species-Specific Families. Curr. Biol. 2002, 12, R409–R411. [Google Scholar] [CrossRef]
- Herrada, G.; Dulac, C. A Novel Family of Putative Pheromone Receptors in Mammals with a Topographically Organized and Sexually Dimorphic Distribution. Cell 1997, 90, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Date-Ito, A.; Ohara, H.; Ichikawa, M.; Mori, Y.; Hagino-Yamagishi, K. Xenopus V1R Vomeronasal Receptor Family Is Expressed in the Main Olfactory System. Chem. Senses 2008, 33, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.S.; Sansone, A.; Nadler, W.; Manzini, I.; Korsching, S.I. Ancestral Amphibian V2rs Are Expressed in the Main Olfactory Epithelium. Proc. Natl. Acad. Sci. USA 2013, 110, 7714–7719. [Google Scholar] [CrossRef]
- Fleischer, J.; Schwarzenbacher, K.; Besser, S.; Hass, N.; Breer, H. Olfactory Receptors and Signalling Elements in the Grueneberg Ganglion. J. Neurochem. 2006, 98, 543–554. [Google Scholar] [CrossRef]
- Pfister, P.; Rodriguez, I. Olfactory Expression of a Single and Highly Variable V1r Pheromone Receptor-like Gene in Fish Species. Proc. Natl. Acad. Sci. USA 2005, 102, 5489–5494. [Google Scholar] [CrossRef]
- Saraiva, L.R.; Korsching, S.I. A Novel Olfactory Receptor Gene Family in Teleost Fish. Genome Res. 2007, 17, 1448–1457. [Google Scholar] [CrossRef]
- Cao, Y.; Oh, B.C.; Stryer, L. Cloning and Localization of Two Multigene Receptor Families in Goldfish Olfactory Epithelium. Proc. Natl. Acad. Sci. USA 1998, 95, 11987–11992. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Saito, Y.; Yamamoto, J.; Nozaki, Y.; Tomura, K.; Hazama, M.; Nakanishi, S.; Brenner, S. Putative Pheromone Receptors Related to the Ca2+-Sensing Receptor in Fugu. Proc. Natl. Acad. Sci. USA 1998, 95, 5178–5181. [Google Scholar] [CrossRef]
- Grus, W.E.; Zhang, J. Origin and Evolution of the Vertebrate Vomeronasal System Viewed through System-specific Genes. BioEssays 2006, 28, 709–718. [Google Scholar] [CrossRef]
- Alioto, T.S.; Ngai, J. The Repertoire of Olfactory C Family G Protein-Coupled Receptors in Zebrafish: Candidate Chemosensory Receptors for Amino Acids. BMC Genom. 2006, 7, 309. [Google Scholar] [CrossRef]
- Ryba, N.J.P.; Tirindelli, R. A New Multigene Family of Putative Pheromone Receptors. Neuron 1997, 19, 371–379. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y. Genomic Organization and Sequence Analysis of the Vomeronasal Receptor V2R Genes in Mouse Genome. Chin. Sci. Bull. 2007, 52, 336–342. [Google Scholar] [CrossRef]
- Grus, W.E.; Shi, P.; Zhang, Y.; Zhang, J. Dramatic Variation of the Vomeronasal Pheromone Receptor Gene Repertoire among Five Orders of Placental and Marsupial Mammals. Proc. Natl. Acad. Sci. USA 2005, 102, 5767–5772. [Google Scholar] [CrossRef]
- Young, J.M.; Kambere, M.; Trask, B.J.; Lane, R.P. Divergent V1R Repertoires in Five Species: Amplification in Rodents, Decimation in Primates, and a Surprisingly Small Repertoire in Dogs. Genome Res. 2005, 15, 231–240. [Google Scholar] [CrossRef][Green Version]
- Grus, W.E.; Zhang, J. Rapid Turnover and Species-Specificity of Vomeronasal Pheromone Receptor Genes in Mice and Rats. Gene 2004, 340, 303–312. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Robledo, D.; Fernández, C.; Gullón, J.; Quintela, L.; Sánchez-Quinteiro, P.; Martínez, P. Analysis of the Vomeronasal Organ Transcriptome Reveals Variable Gene Expression Depending on Age and Function in Rabbits. Genomics 2021, 113, 2240–2252. [Google Scholar] [CrossRef]
- Yang, H.; Shi, P.; Zhang, Y.-P.; Zhang, J. Composition and Evolution of the V2r Vomeronasal Receptor Gene Repertoire in Mice and Rats. Genomics 2005, 86, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Francia, S.; Silvotti, L.; Ghirardi, F.; Catzeflis, F.; Percudani, R.; Tirindelli, R. Evolution of Spatially Coexpressed Families of Type-2 Vomeronasal Receptors in Rodents. Genome Biol. Evol. 2015, 7, 272–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belluscio, L.; Koentges, G.; Axel, R.; Dulac, C. A Map of Pheromone Receptor Activation in the Mammalian Brain. Cell 1999, 97, 209–220. [Google Scholar] [CrossRef]
- Rodriguez, I.; Feinstein, P.; Mombaerts, P. Variable Patterns of Axonal Projections of Sensory Neurons in the Mouse Vomeronasal System. Cell 1999, 97, 199–208. [Google Scholar] [CrossRef]
- Martini, S.; Silvotti, L.; Shirazi, A.; Ryba, N.J.P.; Tirindelli, R. Co-Expression of Putative Pheromone Receptors in the Sensory Neurons of the Vomeronasal Organ. J. Neurosci. 2001, 21, 843–848. [Google Scholar] [CrossRef]
- Ishii, T.; Mombaerts, P. Coordinated Coexpression of Two Vomeronasal Receptor V2R Genes per Neuron in the Mouse. Mol. Cell. Neurosci. 2011, 46, 397–408. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Nishida, M. Evolution and Origin of Vomeronasal-Type Odorant Receptor Gene Repertoire in Fishes. BMC Evol. Biol. 2006, 6, 76. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Sakuma, A.; Kuraku, S.; Nikaido, M. Remarkable Diversity of Vomeronasal Type 2 Receptor (OlfC) Genes of Basal Ray-Finned Fish and Its Evolutionary Trajectory in Jawed Vertebrates. Sci. Rep. 2022, 12, 6455. [Google Scholar] [CrossRef]
- Roppolo, D.; Vollery, S.; Kan, C.-D.; Lüscher, C.; Broillet, M.-C.; Rodriguez, I. Gene Cluster Lock after Pheromone Receptor Gene Choice. EMBO J. 2007, 26, 3423–3430. [Google Scholar] [CrossRef]
- Dietschi, Q.; Tuberosa, J.; Fodoulian, L.; Boillat, M.; Kan, C.; Codourey, J.; Pauli, V.; Feinstein, P.; Carleton, A.; Rodriguez, I. Clustering of Vomeronasal Receptor Genes Is Required for Transcriptional Stability but Not for Choice. Sci. Adv. 2022, 8, 7450. [Google Scholar] [CrossRef]
- Capello, L.; Roppolo, D.; Jungo, V.P.; Feinstein, P.; Rodriguez, I. A Common Gene Exclusion Mechanism Used by Two Chemosensory Systems. Eur. J. Neurosci. 2009, 29, 671–678. [Google Scholar] [CrossRef]
- DeMaria, S.; Berke, A.P.; Van Name, E.; Heravian, A.; Ferreira, T.; Ngai, J. Role of a Ubiquitously Expressed Receptor in the Vertebrate Olfactory System. J. Neurosci. 2013, 33, 15235–15247. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Matsunami, H. Calreticulin Chaperones Regulate Functional Expression of Vomeronasal Type 2 Pheromone Receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 16651–16656. [Google Scholar] [CrossRef]
- Loconto, J.; Papes, F.; Chang, E.; Stowers, L.; Jones, E.P.; Takada, T.; Kumánovics, A.; Lindahl, K.F.; Dulac, C. Functional Expression of Murine V2R Pheromone Receptors Involves Selective Association with the M10 and M1 Families of MHC Class Ib Molecules. Cell 2003, 112, 607–618. [Google Scholar] [CrossRef]
- Ishii, T.; Mombaerts, P. Expression of Nonclassical Class I Major Histocompatibility Genes Defines a Tripartite Organization of the Mouse Vomeronasal System. J. Neurosci. 2008, 28, 2332–2341. [Google Scholar] [CrossRef]
- Holy, T.E.; Dulac, C.; Meister, M. Responses of Vomeronasal Neurons to Natural Stimuli. Science 2000, 289, 1569–1572. [Google Scholar] [CrossRef]
- Liman, E.R.; Corey, D.P.; Dulac, C. TRP2: A Candidate Transduction Channel for Mammalian Pheromone Sensory Signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 5791–5796. [Google Scholar] [CrossRef]
- Lucas, P.; Ukhanov, K.; Leinders-Zufall, T.; Zufall, F. A Diacylglycerol-Gated Cation Channel in Vomeronasal Neuron Dendrites Is Impaired in TRPC2 Mutant Mice. Neuron 2003, 40, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Chamero, P.; Weiss, J.; Alonso, M.T.; Rodríguez-Prados, M.; Hisatsune, C.; Mikoshiba, K.; Leinders-Zufall, T.; Zufall, F. Type 3 Inositol 1,4,5-Trisphosphate Receptor Is Dispensable for Sensory Activation of the Mammalian Vomeronasal Organ. Sci. Rep. 2017, 7, 10260. [Google Scholar] [CrossRef]
- Yang, C.; Delay, R.J. Calcium-Activated Chloride Current Amplifies the Response to Urine in Mouse Vomeronasal Sensory Neurons. J. Gen. Physiol. 2010, 135, 3–13. [Google Scholar] [CrossRef]
- Dibattista, M.; Amjad, A.; Maurya, D.K.; Sagheddu, C.; Montani, G.; Tirindelli, R.; Menini, A. Calcium-Activated Chloride Channels in the Apical Region of Mouse Vomeronasal Sensory Neurons. J. Gen. Physiol. 2012, 140, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, K.R.; Spehr, M.; Li, X.; Zufall, F.; Leinders-Zufall, T. Pheromonal Recognition Memory Induced by TRPC2-independent Vomeronasal Sensing. Eur. J. Neurosci. 2006, 23, 3385–3390. [Google Scholar] [CrossRef]
- Kim, S.; Ma, L.; Yu, C.R. Requirement of Calcium-Activated Chloride Channels in the Activation of Mouse Vomeronasal Neurons. Nat. Commun. 2011, 2, 365. [Google Scholar] [CrossRef]
- Henkel, B.; Bintig, W.; Bhat, S.S.; Spehr, M.; Neuhaus, E.M. NHERF1 in Microvilli of Vomeronasal Sensory Neurons. Chem. Senses 2016, 42, bjw094. [Google Scholar] [CrossRef] [PubMed]
- Spehr, J.; Hagendorf, S.; Weiss, J.; Spehr, M.; Leinders-Zufall, T.; Zufall, F. Ca2+–Calmodulin Feedback Mediates Sensory Adaptation and Inhibits Pheromone-Sensitive Ion Channels in the Vomeronasal Organ. J. Neurosci. 2009, 29, 2125–2135. [Google Scholar] [CrossRef]
- Sarno, N.; Hernandez-Clavijo, A.; Boccaccio, A.; Menini, A.; Pifferi, S. Slow Inactivation of Sodium Channels Contributes to Short-Term Adaptation in Vomeronasal Sensory Neurons. eNeuro 2022, 9, ENEURO.0471-21.2022. [Google Scholar] [CrossRef]
- Rodriguez, I.; Del Punta, K.; Rothman, A.; Ishii, T.; Mombaerts, P. Multiple New and Isolated Families within the Mouse Superfamily of V1r Vomeronasal Receptors. Nat. Neurosci. 2002, 5, 134–140. [Google Scholar] [CrossRef]
- O’Hara, P.J.; Sheppard, P.O.; Thógersen, H.; Venezia, D.; Haldeman, B.A.; McGrane, V.; Houamed, K.M.; Thomsen, C.; Gilbert, T.L.; Mulvihill, E.R. The Ligand-Binding Domain in Metabotropic Glutamate Receptors Is Related to Bacterial Periplasmic Binding Proteins. Neuron 1993, 11, 41–52. [Google Scholar] [CrossRef]
- Leach, K.; Gregory, K.J. Molecular Insights into Allosteric Modulation of Class C G Protein-Coupled Receptors. Pharmacol. Res. 2017, 116, 105–118. [Google Scholar] [CrossRef]
- Williams, L.T.; Snydermantt, R.; Piket, M.C.; Lefkowitzt, R.J. Specific Receptor Sites for Chemotactic Peptides on Human Polymorphonuclear Leukocytes. Proc. Natl. Acad. Sci. USA 1977, 74, 1204–1208. [Google Scholar] [CrossRef]
- Migeotte, I.; Communi, D.; Parmentier, M. Formyl Peptide Receptors: A Promiscuous Subfamily of G Protein-Coupled Receptors Controlling Immune Responses. Cytokine Growth Factor. Rev. 2006, 17, 501–519. [Google Scholar] [CrossRef]
- Liberles, S.D.; Horowitz, L.F.; Kuang, D.; Contos, J.J.; Wilson, K.L.; Siltberg-Liberles, J.; Liberles, D.A.; Buck, L.B. Formyl Peptide Receptors Are Candidate Chemosensory Receptors in the Vomeronasal Organ. Proc. Natl. Acad. Sci. USA 2009, 106, 9842–9847. [Google Scholar] [CrossRef]
- Rivière, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl Peptide Receptor-like Proteins Are a Novel Family of Vomeronasal Chemosensors. Nature 2009, 459, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Guindon, S.; Umemura, T.; Kőhidai, L.; Ueda, H. Adaptive Evolution of Formyl Peptide Receptors in Mammals. J. Mol. Evol. 2015, 80, 130–141. [Google Scholar] [CrossRef]
- Gao, J.-L.; Chen, H.; Filie, J.D.; Kozak, C.A.; Murphy, P.M. Differential Expansion of the N-Formylpeptide Receptor Gene Cluster in Human and Mouse. Genomics 1998, 51, 270–276. [Google Scholar] [CrossRef]
- Silva, L.; Mendes, T.; Antunes, A. Acquisition of Social Behavior in Mammalian Lineages Is Related with Duplication Events of FPR Genes. Genomics 2020, 112, 2778–2783. [Google Scholar] [CrossRef] [PubMed]
- Dietschi, Q.; Tuberosa, J.; Rösingh, L.; Loichot, G.; Ruedi, M.; Carleton, A.; Rodriguez, I. Evolution of Immune Chemoreceptors into Sensors of the Outside World. Proc. Natl. Acad. Sci. USA 2017, 114, 7397–7402. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the Formyl Peptide Receptor (FPR) Family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Liao, Q.; Ge, Y.; Jiao, H.; Chen, Q.; Liu, Y.; Lyu, W.; Zhu, L.; van Zundert, G.C.P.; et al. Structural Basis for Recognition of N-Formyl Peptides as Pathogen-Associated Molecular Patterns. Nat. Commun. 2022, 13, 5232. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Zong, X.; Han, S.; Wang, M.; Su, Y.; Ma, L.; Chu, X.; Yi, C.; Zhao, Q.; et al. Structural Basis of FPR2 in Recognition of Aβ42 and Neuroprotection by Humanin. Nat. Commun. 2022, 13, 1775. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, M.; Zong, X.; Ge, Y.; Zhang, H.; Wang, M.; Won Han, G.; Yi, C.; Ma, L.; Ye, R.D.; et al. Structural Basis of Ligand Binding Modes at the Human Formyl Peptide Receptor 2. Nat. Commun. 2020, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.A.; Adler, E.; Lindemeier, J.; Battey, J.F.; Ryba, N.J.P.; Zuker, C.S. Putative Mammalian Taste Receptors. Cell 1999, 96, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Adler, E.; Hoon, M.A.; Mueller, K.L.; Chandrashekar, J.; Ryba, N.J.P.; Zuker, C.S. A Novel Family of Mammalian Taste Receptors. Cell 2000, 100, 693–702. [Google Scholar] [CrossRef]
- Kooistra, A.J.; Mordalski, S.; Pándy-Szekeres, G.; Esguerra, M.; Mamyrbekov, A.; Munk, C.; Keserű, G.M.; Gloriam, D.E. GPCRdb in 2021: Integrating GPCR Sequence, Structure and Function. Nucleic Acids Res. 2021, 49, 335–343. [Google Scholar] [CrossRef]
- Huang, L.; Shanker, Y.G.; Dubauskaite, J.; Zheng, J.Z.; Yan, W.; Rosenweig, S.; Spielman, A.I.; Max, M.; Margolskee, R.F. Ggamma13 Colocalizes with Gustducin in Taste Receptor Cells and Mediates IP3 Responses to Bitter Denatonium. Nat. Neurosci. 1999, 2, 1055–1062. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Lin, C.; Matsumoto, I.; Ohmoto, M.; Reed, D.R.; Nelson, T.M. Genetics of Taste Receptors. Curr. Pharm. Des. 2014, 20, 2669. [Google Scholar] [CrossRef]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.P.; Zuker, C.S. Mammalian Sweet Taste Receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human Receptors for Sweet and Umami Taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef]
- Choi, H.-J.; Cho, Y.-K.; Chung, K.-M.; Kim, K.-N. Differential Expression of Taste Receptors in Tongue Papillae of DBA Mouse. Int. J. Oral Biol. 2016, 41, 25–32. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J.P. T2Rs Function as Bitter Taste Receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef]
- Avenet, P.; Hofmann, F.; Lindemann, B. Transduction in Taste Receptor Cells Requires CAMP-Dependent Protein Kinase. Nature 1988, 331, 351–354. [Google Scholar] [CrossRef]
- Margolskee, R.F. Molecular Mechanisms of Bitter and Sweet Taste Transduction. J. Biol. Chem. 2002, 277, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J.P. Coding of Sweet, Bitter, and Umami Tastes: Different Receptor Cells Sharing Similar Signaling Pathways. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Dutta Banik, D.; Martin, L.E.; Freichel, M.; Torregrossa, A.-M.; Medler, K.F. TRPM4 and TRPM5 Are Both Required for Normal Signaling in Taste Receptor Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E772–E781. [Google Scholar] [CrossRef] [PubMed]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Pérez, C.A.; Shigemura, N.; Yoshida, R.; Mosinger, B.; Glendinning, J.I.; Ninomiya, Y.; et al. Trpm5 Null Mice Respond to Bitter, Sweet, and Umami Compounds. Chem. Senses 2006, 31, 253–264. [Google Scholar] [CrossRef]
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat Activation of TRPM5 Underlies Thermal Sensitivity of Sweet Taste. Nature 2005, 438, 1022–1025. [Google Scholar] [CrossRef]
- Xu, W.; Wu, L.; Liu, S.; Liu, X.; Cao, X.; Zhou, C.; Zhang, J.; Fu, Y.; Guo, Y.; Wu, Y.; et al. Structural Basis for Strychnine Activation of Human Bitter Taste Receptor TAS2R46. Science 2022, 377, 1298–1304. [Google Scholar] [CrossRef]
- Kim, Y.; Gumpper, R.H.; Liu, Y.; Kocak, D.D.; Xiong, Y.; Cao, C.; Deng, Z.; Krumm, B.E.; Jain, M.K.; Zhang, S.; et al. Bitter Taste Receptor Activation by Cholesterol and an Intracellular Tastant. Nature 2024, 628, 664–671. [Google Scholar] [CrossRef]
- Sanvictores, T.; Farci, F. Biochemistry, Primary Protein Structure; StatPearls Publishing: St. Petersburg, FL, USA, 2025. [Google Scholar]
- Jisna, V.A.; Jayaraj, P.B. Protein Structure Prediction: Conventional and Deep Learning Perspectives. Protein J. 2021, 40, 522–544. [Google Scholar] [CrossRef]
- Browne, W.J.; North, A.C.T.; Phillips, D.C.; Brew, K.; Vanaman, T.C.; Hill, R.L. A Possible Three-Dimensional Structure of Bovine Alpha-Lactalbumin Based on That of Hen’s Egg-White Lysozyme. J. Mol. Biol. 1969, 42, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Epstein, C.J.; Goldberger, R.F.; Anfinsen, C.B. The Genetic Control of Tertiary Protein Structure: Studies with Model Systems. Cold Spring Harb. Symp. Quant. Biol. 1963, 28, 439–449. [Google Scholar] [CrossRef]
- Lesk, A.M.; Chothia, C.H. The Response of Protein Structures to Amino-Acid Sequence Changes. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1986, 317, 345–356. [Google Scholar] [CrossRef]
- Kaczanowski, S.; Zielenkiewicz, P. Why Similar Protein Sequences Encode Similar Three-Dimensional Structures? Theor. Chem. Acc. 2010, 125, 643–650. [Google Scholar] [CrossRef]
- Krieger, E.; Nabuurs, S.B.; Vriend, G. Homology Modeling. Methods Biochem. Anal. 2003, 44, 509–523. [Google Scholar] [PubMed]
- Sánchez, R.; Šali, A. Comparative Protein Structure Modeling: Introduction and Practical Examples with Modeller. In Protein Structure Prediction; Humana Press: Totowa, NJ, USA, 2000; Volume 143, pp. 97–129. [Google Scholar]
- Sánchez, R.; Šali, A. Advances in Comparative Protein-Structure Modelling. Curr. Opin. Struct. Biol. 1997, 7, 206–214. [Google Scholar] [CrossRef]
- Haddad, Y.; Adam, V.; Heger, Z. Ten Quick Tips for Homology Modeling of High-Resolution Protein 3D Structures. PLoS Comput. Biol. 2020, 16, e1007449. [Google Scholar] [CrossRef] [PubMed]
- Wiltgen, M. Algorithms for Structure Comparison and Analysis: Homology Modelling of Proteins. In Encyclopedia of Bioinformatics and Computational Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1–3, pp. 38–61. [Google Scholar]
- Rost, B. Twilight Zone of Protein Sequence Alignments. Protein Eng. Des. Sel. 1999, 12, 85–94. [Google Scholar] [CrossRef]
- Dunbrack, R.L.; Karplus, M. Conformational Analysis of the Backbone-Dependent Rotamer Preferences of Protein Sidechains. Nat. Struct. Biol. 1994, 1, 334–340. [Google Scholar] [CrossRef]
- Argos, P.; Siezen, R.J. Structural Homology of Lens Crystallins. A Method to Detect Protein Structural Homology from Primary Sequences. Eur. J. Biochem. 1983, 131, 143–148. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylort, W.R.; Thornton, J.M. A New Approach to Protein Fold Recognition. Nature 1992, 358, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.V.; Argos, P. Residue Contacts in Protein Structures and Implications for Protein Folding. Int. J. Pept. Protein Res. 1984, 24, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.U.; Lüthy, R.; Eisenberg, D. A Method to Identify Protein Sequences That Fold into a Known Three-Dimensional Structure. Science 1991, 253, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Casadio, R.; Fariselli, P.; Martelli, P.L.; Tasco, G. Thinking the Impossible: How to Solve the Protein Folding Problem with and without Homologous Structures and More. Methods Mol. Biol. 2007, 350, 305–320. [Google Scholar] [CrossRef]
- Zhang, Y. Progress and Challenges in Protein Structure Prediction. Curr. Opin. Struct. Biol. 2008, 18, 342–348. [Google Scholar] [CrossRef]
- Szilagyi, A.; Zhang, Y. Template-Based Structure Modeling of Protein–Protein Interactions. Curr. Opin. Struct. Biol. 2014, 24, 10–23. [Google Scholar] [CrossRef]
- Ginalski, K.; Grishin, N.V.; Godzik, A.; Rychlewski, L. Practical Lessons from Protein Structure Prediction. Nucleic Acids Res. 2005, 33, 1874–1891. [Google Scholar] [CrossRef]
- Dorit, R.L.; Schoenbach, L.; Gilbert, W. How Big Is the Universe of Exons? Science 1990, 250, 1377–1382. [Google Scholar] [CrossRef]
- Bowie, J.U.; Reidhaar-Olson, J.F.; Lim, W.A.; Sauer, R.T. Deciphering the Message in Protein Sequences: Tolerance to Amino Acid Substitutions. Science 1990, 247, 1306–1310. [Google Scholar] [CrossRef]
- Gribskov, M.; McLachlan, A.D.; Eisenberg, D. Profile Analysis: Detection of Distantly Related Proteins. Proc. Natl. Acad. Sci. USA 1987, 84, 4355–4358. [Google Scholar] [CrossRef]
- Jaroszewski, L.; Rychlewski, L.; Li, Z.; Li, W.; Godzik, A. FFAS03: A Server for Profile-Profile Sequence Alignments. Nucleic Acids Res. 2005, 33, W284–W288. [Google Scholar] [CrossRef] [PubMed]
- Azginoglu, N.; Aydin, Z.; Celik, M. Structural Profile Matrices for Predicting Structural Properties of Proteins. J. Bioinform. Comput. Biol. 2020, 18, 2050022. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Blundell, T.L.; Mizuguchi, K. FUGUE: Sequence-Structure Homology Recognition Using Environment-Specific Substitution Tables and Structure-Dependent Gap Penalties. J. Mol. Biol. 2001, 310, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Karplus, K.; Barrett, C.; Hughey, R. Hidden Markov Models for Detecting Remote Protein Homologies. Bioinformatics 1998, 14, 846–856. [Google Scholar] [CrossRef]
- Söding, J. Protein Homology Detection by HMM–HMM Comparison. Bioinformatics 2005, 21, 951–960. [Google Scholar] [CrossRef]
- Jones, D.T. GenTHREADER: An Efficient and Reliable Protein Fold Recognition Method for Genomic Sequences. J. Mol. Biol. 1999, 287, 797–815. [Google Scholar] [CrossRef]
- Cheng, J.; Baldi, P. A Machine Learning Information Retrieval Approach to Protein Fold Recognition. Bioinformatics 2006, 22, 1456–1463. [Google Scholar] [CrossRef]
- Ewbank, J.J.; Creighton, T.E. Protein Folding by Stages. Curr. Biol. 1992, 2, 347–349. [Google Scholar] [CrossRef]
- Anfinsen, C.B. Principles That Govern the Folding of Protein Chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef]
- Govindarajan, S.; Goldstein, R.A. On the Thermodynamic Hypothesis of Protein Folding. Proc. Natl. Acad. Sci. USA 1998, 95, 5545–5549. [Google Scholar] [CrossRef]
- Tanaka, S.; Scheraga, H.A. Model of Protein Folding: Incorporation of a One-Dimensional Short-Range (Ising) Model into a Three-Dimensional Model. Proc. Natl. Acad. Sci. USA 1977, 74, 1320–1323. [Google Scholar] [CrossRef]
- Li, Z.; Scheraga, H.A. Monte Carlo-Minimization Approach to the Multiple-Minima Problem in Protein Folding. Proc. Natl. Acad. Sci. USA 1987, 84, 6611–6615. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liwo, A.; Scheraga, H.A. Energy-Based de Novo Protein Folding by Conformational Space Annealing and an off-Lattice United-Residue Force Field: Application to the 10-55 Fragment of Staphylococcal Protein A and to Apo Calbindin D9K. Proc. Natl. Acad. Sci. USA 1999, 96, 2025–2030. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Dror, R.O.; Shaw, D.E. How Fast-Folding Proteins Fold. Science 2011, 334, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Maier, J.; Huang, H.; Perrone, V.; Simmerling, C. Folding for Proteins with Diverse Topologies Accessible in Days with a Physics-Based Force Field and Implicit. J. Am. Chem. Soc. 2014, 136, 13959. [Google Scholar] [CrossRef]
- Levitt, M.; Warshel, A. Computer Simulation of Protein Folding. Nature 1975, 253, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Okamoto, Y. Replica-Exchange Molecular Dynamics Method for Protein Folding. Chem. Phys. Lett. 1999, 314, 141–151. [Google Scholar] [CrossRef]
- Hinds, D.A.; Levitt, M. A Lattice Model for Protein Structure Prediction at Low Resolution. Proc. Natl. Acad. Sci. USA 1992, 89, 2536–2540. [Google Scholar] [CrossRef]
- Nagano, K. Logical Analysis of the Mechanism of Protein Folding. J. Mol. Biol. 1973, 75, 401–420. [Google Scholar] [CrossRef]
- Robson, B.; Pain, R.H. Analysis of the Code Relating Sequence to Conformation in Globular Proteins. An Informational Analysis of the Role of the Residue in Determining the Conformation of Its Neighbours in the Primary Sequence. Biochem. J. 1974, 141, 883. [Google Scholar] [CrossRef]
- Chou, P.Y.; Fasman, G.D. Prediction of Protein Conformation. Biochemistry 1974, 13, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Lim, V.I. Algorithms for Prediction of Alpha-Helical and Beta-Structural Regions in Globular Proteins. J. Mol. Biol. 1974, 88, 873–894. [Google Scholar] [CrossRef]
- Bohr, H.; Bohr, J.; Brunak, S.; Cotterill, R.M.J.; Lautrup, B.; Nørskov, L.; Olsen, O.H.; Petersen, S.B. Protein Secondary Structure and Homology by Neural Networks. The Alpha-Helices in Rhodopsin. FEBS Lett. 1988, 241, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mesirov, J.P.; Waltz, D.L. Hybrid System for Protein Secondary Structure Prediction. J. Mol. Biol. 1992, 225, 1049–1063. [Google Scholar] [CrossRef]
- Salzberg, S.; Cost, S. Predicting Protein Secondary Structure with a Nearest-Neighbor Algorithm. J. Mol. Biol. 1992, 227, 371–374. [Google Scholar] [CrossRef]
- Holley, L.H.; Karplus, M. Protein Secondary Structure Prediction with a Neural Network. Proc. Natl. Acad. Sci. USA 1989, 86, 152–156. [Google Scholar] [CrossRef]
- Bowie, J.U.; Eisenberg, D. An Evolutionary Approach to Folding Small Alpha-Helical Proteins That Uses Sequence Information and an Empirical Guiding Fitness Function. Proc. Natl. Acad. Sci. USA 1994, 91, 4436–4440. [Google Scholar] [CrossRef]
- Simons, K.T.; Kooperberg, C.; Huang, E.; Baker, D. Assembly of Protein Tertiary Structures from Fragments with Similar Local Sequences Using Simulated Annealing and Bayesian Scoring Functions. J. Mol. Biol. 1997, 268, 209–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. The Protein Structure Prediction Problem Could Be Solved Using the Current PDB Library. Proc. Natl. Acad. Sci. USA 2005, 102, 1029–1034. [Google Scholar] [CrossRef]
- Bonneau, R.; Tsai, J.; Ruczinski, I.; Chivian, D.; Rohl, C.; Strauss, C.E.M.; Baker, D. Rosetta in CASP4: Progress in Ab Initio Protein Structure Prediction. Proteins Struct. Funct. Genet. 2001, 45, 119–126. [Google Scholar] [CrossRef]
- Khatib, F.; Cooper, S.; Tyka, M.D.; Xu, K.; Makedon, I.; Popovic, Z.; Baker, D.; Players, F. Algorithm Discovery by Protein Folding Game Players. Proc. Natl. Acad. Sci. USA 2011, 108, 18949–18953. [Google Scholar] [CrossRef]
- Wang, T.; Qiao, Y.; Ding, W.; Mao, W.; Zhou, Y.; Gong, H. Improved Fragment Sampling for Ab Initio Protein Structure Prediction Using Deep Neural Networks. Nat. Mach. Intell. 2019, 1, 347–355. [Google Scholar] [CrossRef]
- de Oliveira, S.H.P.; Deane, C.M. Combining Co-Evolution and Secondary Structure Prediction to Improve Fragment Library Generation. Bioinformatics 2018, 34, 2219–2227. [Google Scholar] [CrossRef]
- Vendruscolo, M.; Kussell, E.; Domany, E. Recovery of Protein Structure from Contact Maps. Fold. Des. 1997, 2, 295–306. [Google Scholar] [CrossRef]
- Vassura, M.; Margara, L.; Di Lena, P.; Medri, F.; Fariselli, P.; Casadio, R. Reconstruction of 3D Structures from Protein Contact Maps. IEEE/ACM Trans. Comput. Biol. Bioinform. 2008, 5, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Ghahramani, Z.; Podtelezhnikov, A.; Wild, D.L. Bayesian Segmental Models with Multiple Sequence Alignment Profiles for Protein Secondary Structure and Contact Map Prediction. IEEE/ACM Trans. Comput. Biol. Bioinform. 2006, 3, 98–113. [Google Scholar] [CrossRef]
- Luttrell, J.; Liu, T.; Zhang, C.; Wang, Z. Predicting Protein Residue-Residue Contacts Using Random Forests and Deep Networks. BMC Bioinform. 2019, 20, 100. [Google Scholar] [CrossRef]
- Eickholt, J.; Cheng, J. Predicting Protein Residue–Residue Contacts Using Deep Networks and Boosting. Bioinformatics 2012, 28, 3066–3072. [Google Scholar] [CrossRef]
- Liu, Y.; Palmedo, P.; Ye, Q.; Berger, B.; Peng, J. Enhancing Evolutionary Couplings with Deep Convolutional Neural Networks. Cell Syst. 2018, 6, 65–74.e3. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Y.; Elofsson, A.; Yao, Y. Protein Contact Map Prediction Based on ResNet and DenseNet. Biomed. Res. Int. 2020, 2020, 7584968. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Zeng, J.; Gong, H. A Deep Learning Framework for Improving Long-Range Residue–Residue Contact Prediction Using a Hierarchical Strategy. Bioinformatics 2017, 33, 2675–2683. [Google Scholar] [CrossRef]
- Xu, J. Distance-Based Protein Folding Powered by Deep Learning. Proc. Natl. Acad. Sci. USA 2019, 116, 16856–16865. [Google Scholar] [CrossRef]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved Protein Structure Prediction Using Potentials from Deep Learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef]
- Rives, A.; Meier, J.; Sercu, T.; Goyal, S.; Lin, Z.; Liu, J.; Guo, D.; Ott, M.; Zitnick, C.L.; Ma, J.; et al. Biological Structure and Function Emerge from Scaling Unsupervised Learning to 250 Million Protein Sequences. Proc. Natl. Acad. Sci. USA 2021, 118, e2016239118. [Google Scholar] [CrossRef]
- Vig, J.; Madani, A.; Varshney, L.R.; Xiong, C.; Socher, R.; Rajani, N.F. BERTology Meets Biology: Interpreting Attention in Protein Language Models. In Proceedings of the ICLR 2021—9th International Conference on Learning Representations, Virtual, 3–7 May 2021. [Google Scholar]
- Elnaggar, A.; Heinzinger, M.; Dallago, C.; Rehawi, G.; Wang, Y.; Jones, L.; Gibbs, T.; Feher, T.; Angerer, C.; Steinegger, M.; et al. ProtTrans: Toward Understanding the Language of Life Through Self-Supervised Learning. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 7112–7127. [Google Scholar] [CrossRef] [PubMed]
- Asgari, E.; Mofrad, M.R.K. Continuous Distributed Representation of Biological Sequences for Deep Proteomics and Genomics. PLoS ONE 2015, 10, e0141287. [Google Scholar] [CrossRef] [PubMed]
- Glasmachers, T. Limits of End-to-End Learning. J. Mach. Learn. Res. 2017, 77, 17–32. [Google Scholar]
- AlQuraishi, M. End-to-End Differentiable Learning of Protein Structure. Cell Syst. 2019, 8, 292–301.e3. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate Prediction of Protein Structures and Interactions Using a Three-Track Neural Network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ding, F.; Wang, R.; Shen, R.; Zhang, X.; Luo, S.; Su, C.; Wu, Z.; Xie, Q.; Berger, B.; et al. High-Resolution de Novo Structure Prediction from Primary Sequence. bioRxiv 2022. [Google Scholar]
- Fang, X.; Wang, F.; Liu, L.; He, J.; Lin, D.; Xiang, Y.; Zhu, K.; Zhang, X.; Wu, H.; Li, H.; et al. A Method for Multiple-Sequence-Alignment-Free Protein Structure Prediction Using a Protein Language Model. Nat. Mach. Intell. 2023, 5, 1087–1096. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Liu, D.; Xing, Y.; Gong, H. An End-to-End Framework for the Prediction of Protein Structure and Fitness from Single Sequence. Nat. Commun. 2024, 15, 7400. [Google Scholar] [CrossRef] [PubMed]
- Wuyun, Q.; Chen, Y.; Shen, Y.; Cao, Y.; Hu, G.; Cui, W.; Gao, J.; Zheng, W. Recent Progress of Protein Tertiary Structure Prediction. Molecules 2024, 29, 832. [Google Scholar] [CrossRef]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M.; Mostaguir, K.; Gumienny, R.; Schwede, T. Continuous Automated Model EvaluatiOn (CAMEO) Complementing the Critical Assessment of Structure Prediction in CASP12. Proteins Struct. Funct. Bioinform. 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Robin, X.; Haas, J.; Gumienny, R.; Smolinski, A.; Tauriello, G.; Schwede, T. Continuous Automated Model EvaluatiOn (CAMEO)—Perspectives on the Future of Fully Automated Evaluation of Structure Prediction Methods. Proteins Struct. Funct. Bioinform. 2021, 89, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Zemla, A. LGA: A Method for Finding 3D Similarities in Protein Structures. Nucleic Acids Res. 2003, 31, 3370–3374. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Nasif, K.F.A.; Xie, Y.; Deng, B.; Niu, S.; Pouriyeh, S.; Dai, Z.; Chen, J.; Xie, C.Y. AI-Driven Deep Learning Techniques in Protein Structure Prediction. Int. J. Mol. Sci. 2024, 25, 8426. [Google Scholar] [CrossRef]
- Morris, A.L.; MacArthur, M.W.; Hutchinson, E.G.; Thornton, J.M. Stereochemical Quality of Protein Structure Coordinates. Proteins Struct. Funct. Bioinform. 1992, 12, 345–364. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Web Serv. Issue Publ. Online 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Marti-Renom, M.A.; Madhusudhan, M.S.; Sali, A. Alignment of Protein Sequences by Their Profiles. Protein Sci. 2004, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Simons, K.T.; Ruczinski, I.; Kooperberg, C.; Fox, B.A.; Bystroff, C.; Baker, D. Improved Recognition of Native-like Protein Structures Using a Combination of Sequence-Dependent and Sequence-Independent Features of Proteins. Proteins 1999, 34, 82–95. [Google Scholar] [CrossRef]
- Simons, K.T.; Bonneau, R.; Ruczinski, I.; Baker, D. Ab Initio Protein Structure Prediction of CASP III Targets Using ROSETTA. Proteins 1999, 37 (Suppl. 3), 171–176. [Google Scholar] [CrossRef]
- Du, Z.; Su, H.; Wang, W.; Ye, L.; Wei, H.; Peng, Z.; Anishchenko, I.; Baker, D.; Yang, J. The TrRosetta Server for Fast and Accurate Protein Structure Prediction. Nat. Protoc. 2021, 16, 5634–5651. [Google Scholar] [CrossRef]
- Bonneau, R.; Strauss, C.E.M.; Rohl, C.A.; Chivian, D.; Bradley, P.; Malmström, L.; Robertson, T.; Baker, D. De Novo Prediction of Three-Dimensional Structures for Major Protein Families. J. Mol. Biol. 2002, 322, 65–78. [Google Scholar] [CrossRef]
- Kuhlman, B.; Dantas, G.; Ireton, G.C.; Varani, G.; Stoddard, B.L.; Baker, D. Design of a Novel Globular Protein Fold with Atomic-Level Accuracy. Science 2003, 302, 1364–1368. [Google Scholar] [CrossRef]
- Dantas, G.; Kuhlman, B.; Callender, D.; Wong, M.; Baker, D. A Large Scale Test of Computational Protein Design: Folding and Stability of Nine Completely Redesigned Globular Proteins. J. Mol. Biol. 2003, 332, 449–460. [Google Scholar] [CrossRef]
- Song, Y.; DiMaio, F.; Wang, R.Y.-R.; Kim, D.; Miles, C.; Brunette, T.; Thompson, J.; Baker, D. High-Resolution Comparative Modeling with RosettaCM. Structure 2013, 21, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Rohl, C.A.; Strauss, C.E.M.; Chivian, D.; Baker, D. Modeling Structurally Variable Regions in Homologous Proteins with Rosetta. Proteins Struct. Funct. Bioinform. 2004, 55, 656–677. [Google Scholar] [CrossRef]
- Leaver-Fay, A.; Tyka, M.; Lewis, S.M.; Lange, O.F.; Thompson, J.; Jacak, R.; Kaufman, K.W.; Renfrew, P.D.; Smith, C.A.; Sheffler, W.; et al. Rosetta3. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 487, pp. 545–574. [Google Scholar]
- Hiranuma, N.; Park, H.; Baek, M.; Anishchenko, I.; Dauparas, J.; Baker, D. Improved Protein Structure Refinement Guided by Deep Learning Based Accuracy Estimation. Nat. Commun. 2021, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved Protein Structure Prediction Using Predicted Interresidue Orientations. Proc. Natl. Acad. Sci. USA 2020, 117, 1496–1503. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Kim, D.; Xu, Y. RAPTOR: Optimal Protein Threading by Linear Programming. J. Bioinform. Comput. Biol. 2003, 1, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, J. RaptorX: Exploiting Structure Information for Protein Alignment by Statistical Inference. Proteins 2011, 79 (Suppl. S10), 161–171. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Liu, S.; Xu, J. RaptorX-Property: A Web Server for Protein Structure Property Prediction. Nucleic Acids Res. 2016, 44, W430–W435. [Google Scholar] [CrossRef]
- Wu, F.; Xu, J. Deep Template-Based Protein Structure Prediction. PLoS Comput. Biol. 2021, 17, e1008954. [Google Scholar] [CrossRef]
- Jing, X.; Wu, F.; Luo, X.; Xu, J. Single-Sequence Protein Structure Prediction by Integrating Protein Language Models. Proc. Natl. Acad. Sci. USA 2024, 121, e2308788121. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. Automated Structure Prediction of Weakly Homologous Proteins on a Genomic Scale. Proc. Natl. Acad. Sci. USA 2004, 101, 7594–7599. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Skolnick, J.; Zhang, Y. Ab Initio Modeling of Small Proteins by Iterative TASSER Simulations. BMC Biol. 2007, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y. I-TASSER Server: New Development for Protein Structure and Function Predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding Non-Homologous Proteins by Coupling Deep-Learning Contact Maps with I-TASSER Assembly Simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, W.; Li, Y.; Pearce, R.; Zhang, C.; Bell, E.W.; Zhang, G.; Zhang, Y. I-TASSER-MTD: A Deep-Learning-Based Platform for Multi-Domain Protein Structure and Function Prediction. Nat. Protoc. 2022, 17, 2326–2353. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A Unified Platform for Automated Protein Structure and Function Prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y. Ab Initio Protein Structure Assembly Using Continuous Structure Fragments and Optimized Knowledge-based Force Field. Proteins Struct. Funct. Bioinform. 2012, 80, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Mortuza, S.M.; Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Zhang, Y. Improving Fragment-Based Ab Initio Protein Structure Assembly Using Low-Accuracy Contact-Map Predictions. Nat. Commun. 2021, 12, 5011. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Y.; Zhang, C.; Zhou, X.; Pearce, R.; Bell, E.W.; Huang, X.; Zhang, Y. Protein Structure Prediction Using Deep Learning Distance and Hydrogen-bonding Restraints in CASP14. Proteins Struct. Funct. Bioinform. 2021, 89, 1734–1751. [Google Scholar] [CrossRef]
- Choi, Y.; Deane, C.M. FREAD Revisited: Accurate Loop Structure Prediction Using a Database Search Algorithm. Proteins Struct. Funct. Bioinform. 2010, 78, 1431–1440. [Google Scholar] [CrossRef]
- Kelm, S.; Shi, J.; Deane, C.M. MEDELLER: Homology-Based Coordinate Generation for Membrane Proteins. Bioinformatics 2010, 26, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Simpkin, A.J.; Hartmann, M.D.; Rigden, D.J.; Keegan, R.M.; Lupas, A.N. High-accuracy Protein Structure Prediction in CASP14. Proteins Struct. Funct. Bioinform. 2021, 89, 1687–1699. [Google Scholar] [CrossRef]
- AlQuraishi, M. AlphaFold at CASP13. Bioinformatics 2019, 35, 4862–4865. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Steinegger, M.; Söding, J. MMseqs2 Enables Sensitive Protein Sequence Searching for the Analysis of Massive Data Sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Choi, C.; Bae, J.; Kim, S.; Lee, S.; Kang, H.; Kim, J.; Bang, I.; Kim, K.; Huh, W.K.; Seok, C.; et al. Understanding the Molecular Mechanisms of Odorant Binding and Activation of the Human OR52 Family. Nat. Commun. 2023, 14, 8105. [Google Scholar] [CrossRef]
- de March, C.A.; Ma, N.; Billesbølle, C.B.; Tewari, J.; Llinas del Torrent, C.; van der Velden, W.J.C.; Ojiro, I.; Takayama, I.; Faust, B.; Li, L.; et al. Engineered Odorant Receptors Illuminate the Basis of Odour Discrimination. Nature 2024, 635, 499–508. [Google Scholar] [CrossRef]
- Peri, L.; Matzov, D.; Huxley, D.R.; Rainish, A.; Fierro, F.; Sapir, L.; Pfeiffer, T.; Waterloo, L.; Hübner, H.; Peleg, Y.; et al. A Bitter Anti-Inflammatory Drug Binds at Two Distinct Sites of a Human Bitter Taste GPCR. Nat. Commun. 2024, 15, 9991. [Google Scholar] [CrossRef]
- Juen, Z.; Lu, Z.; Yu, R.; Chang, A.N.; Wang, B.; Fitzpatrick, A.W.P.; Zuker, C.S. The Structure of Human Sweetness. Cell 2025, 188, 1–13. [Google Scholar] [CrossRef]
- Lin, H.; Ma, C.; Cai, K.; Guo, L.; Wang, X.; Lv, L.; Zhang, C.; Lin, J.; Zhang, D.; Ye, C.; et al. Metabolic Signaling of Ceramides through the FPR2 Receptor Inhibits Adipocyte Thermogenesis. Science 2025, 388, eado4188. [Google Scholar] [CrossRef]
- Shang, P.; Rong, N.; Jiang, J.J.; Cheng, J.; Zhang, M.H.; Kang, D.; Qi, L.; Guo, L.; Yang, G.M.; Liu, Q.; et al. Structural and Signaling Mechanisms of TAAR1 Enabled Preferential Agonist Design. Cell 2023, 186, 5347–5362.e24. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, L.; Yu, J.; Shen, S.; Wu, C.; Zhang, W.; Zhao, C.; Deng, Y.; Tian, X.; Feng, Y.; et al. Ligand Recognition and G-Protein Coupling of Trace Amine Receptor TAAR1. Nature 2023, 624, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zheng, Y.; Zeng, L.; Wang, L.; Li, F.; Pu, J.; Lu, Y.; Zhao, S.; Xu, F. The Versatile Binding Landscape of the TAAR1 Pocket for LSD and Other Antipsychotic Drug Molecules. Cell Rep. 2024, 43, 114505. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, Y.; Wang, Y.; Wang, Y.; He, X.; Xu, P.; Huang, S.; Yuan, Q.; Zhang, X.; Wang, L.; et al. Recognition of Methamphetamine and Other Amines by Trace Amine Receptor TAAR1. Nature 2023, 624, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Shi, F.; Yang, L.; Wu, S.; Qiao, A.; Ye, S. Molecular Mechanisms of Methamphetamine-Induced Addiction via TAAR1 Activation. J. Med. Chem. 2024, 67, 18593–18605. [Google Scholar] [CrossRef]
- Guo, L.; Cheng, J.; Lian, S.; Liu, Q.; Lu, Y.; Zheng, Y.; Zhu, K.; Zhang, M.; Kong, Y.; Zhang, C.; et al. Structural Basis of Amine Odorant Perception by a Mammal Olfactory Receptor. Nature 2023, 618, 193–200. [Google Scholar] [CrossRef]
- García-Nafría, J.; Tate, C.G. Structure Determination of GPCRs: Cryo-EM Compared with X-Ray Crystallography. Biochem. Soc. Trans. 2021, 49, 2345–2355. [Google Scholar] [CrossRef]
- Sleator, R.D. Solving the Protein Folding Problem. FEBS Lett. 2024, 598, 2831–2835. [Google Scholar] [CrossRef]
- Marcu, Ş.-B.; Tăbîrcă, S.; Tangney, M. An Overview of Alphafold’s Breakthrough. Front. Artif. Intell. 2022, 5, 875587. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Zhang, X.; Shen, C.; Zhang, O.; Wang, J.; Wu, J.; Jin, R.; Zhou, D.; Chen, S.; et al. Comprehensive Assessment of Protein Loop Modeling Programs on Large-Scale Datasets: Prediction Accuracy and Efficiency. Brief. Bioinform. 2023, 25, bbad486. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.; Leman, J.K.; Meiler, J. Modeling Membrane Geometries Implicitly in Rosetta. Protein Sci. 2024, 33, e4908. [Google Scholar] [CrossRef]
- de March, C.A.; Ryu, S.; Sicard, G.; Moon, C.; Golebiowski, J. Structure–Odour Relationships Reviewed in the Postgenomic Era. Flavour. Fragr. J. 2015, 30, 342–361. [Google Scholar] [CrossRef]
- Jabeen, A.; Vijayram, R.; Ranganathan, S. A Two-Stage Computational Approach to Predict Novel Ligands for a Chemosensory Receptor. Curr. Res. Struct. Biol. 2020, 2, 213–221. [Google Scholar] [CrossRef]
- Shehata, M.A.; Nøhr, A.C.; Lissa, D.; Bisig, C.; Isberg, V.; Andersen, K.B.; Harpsøe, K.; Björkling, F.; Bräuner-Osborne, H.; Gloriam, D.E. Novel Agonist Bioisosteres and Common Structure-Activity Relationships for the Orphan G Protein-Coupled Receptor GPR139. Sci. Rep. 2016, 6, 36681. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Varadwaj, P. Developing Human Olfactory Network and Exploring Olfactory Receptor-Odorant Interaction. J. Biomol. Struct. Dyn. 2023, 41, 8941–8960. [Google Scholar] [CrossRef]
- Hassan Baig, M.; Ahmad, K.; Roy, S.; Mohammad Ashraf, J.; Adil, M.; Haris Siddiqui, M.; Khan, S.; Amjad Kamal, M.; Provazník, I.; Choi, I. Computer Aided Drug Design: Success and Limitations. Curr. Pharm. Des. 2016, 22, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Mackerell, A.D. Computer-Aided Drug Design Methods. Methods Mol. Biol. 2017, 1520, 85. [Google Scholar] [CrossRef]
- Creton, B.; Pageat, P.; Robejean, M.; Lafont-Lecuelle, C.; Cozzi, A. Protection of Horse Ears against Simulid Parasitism: Efficacy of a Mammal Semiochemical Solution over 10 Hours. Vet. Parasitol. 2016, 227, 15–19. [Google Scholar] [CrossRef]
- Madec, I.; Gabarrou, J.-F.; Pageat, P. Influence of a Maternal Odorant on Copying Strategies in Chicks Facing Isolation and Novelty during a Standardized Test. Neuro Endocrinol. Lett. 2008, 29, 507–511. [Google Scholar]
- Asproni, P.; Bienboire-Frosini, C.; Barthélémy, H.; Mechin, V.; Teruel, E.; Leclercq, J.; Cozzi, A.; Pageat, P. Single Fluff-Spray Application of Mother Hen Uropygial Secretion Analogue Positively Influences Bursa of Fabricius Development and the Heterophil-to-Lymphocyte Ratio in ROSS 308 Chicks. Poult. Sci. 2020, 99, 6300–6306. [Google Scholar] [CrossRef] [PubMed]
- DePorter, T.L.; Bledsoe, D.L.; Beck, A.; Ollivier, E. Evaluation of the Efficacy of an Appeasing Pheromone Diffuser Product vs Placebo for Management of Feline Aggression in Multi-Cat Households: A Pilot Study. J. Feline Med. Surg. 2019, 21, 293–305. [Google Scholar] [CrossRef] [PubMed]
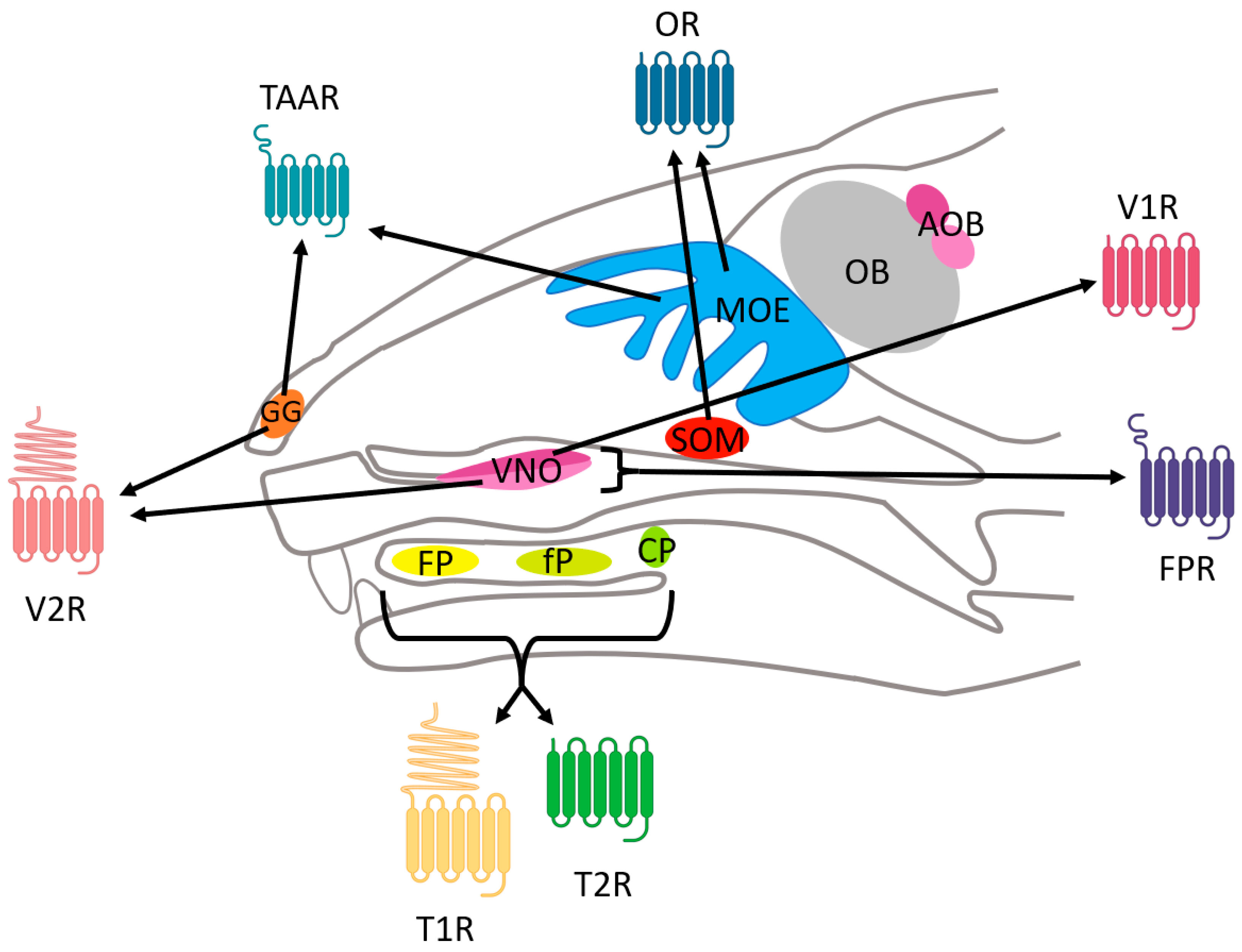



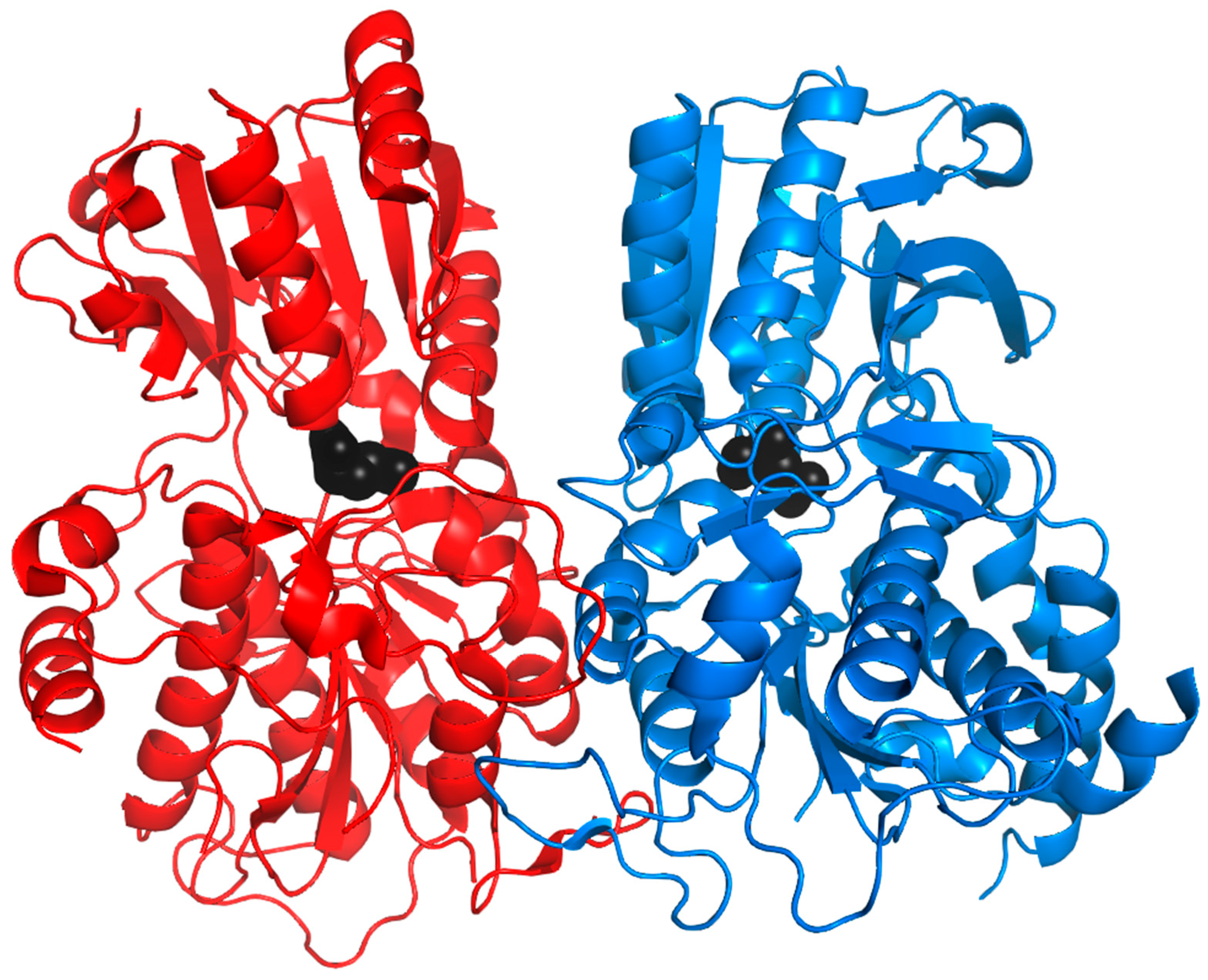

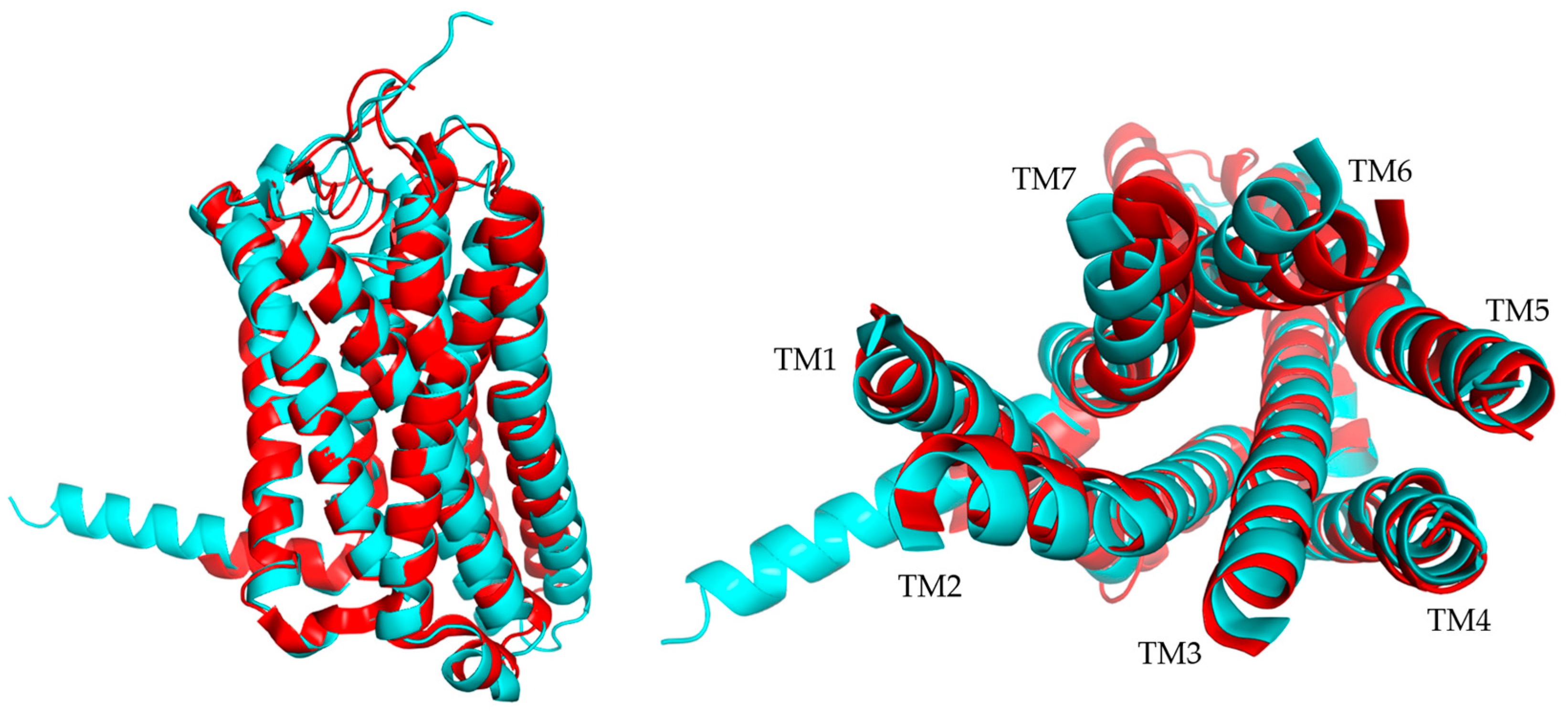
| VR | TR | ||||||
|---|---|---|---|---|---|---|---|
| OR | TAAR | V1R | V2R | FPR | T1R | T2R | |
| GPCR Class | A | A | A | C | A | C | T |
| Length | ~310 | ~350 | ~310 | ~850 | ~350 | ~850 | ~300–330 |
| N-terminal | short | short | short | long | short | long | short |
| Exon | 1 | 1–2 | 1 | ~6 | ~1–3 | 6 | 1 |
| Expression involved in sensing | MOE/SOM | MOE/GG | VNO apical | VNO basal/GG | VNO | FP, fP, CP | FP, fP, CP |
| Binding site | TM3 TM5 TM6 ECL2 | TM3 TM5–7 ECL2 | TM3 TM5 TM6 ECL2 | VFT | TM3 TM5 | VFT | TM3 TM7 |
| Type of Algorithm | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Homology | Modeling by comparison with structures of homologous proteins | Most accurate and reliable results when homologs are available | Limited to available structures Mistakes can arise from errors in alignment |
| Modeller, Swiss-model, RosettaCM, Medeller | |||
| Threading | Use both structures of similar proteins and sequences with structural information | Less limitation in sequence similarity than homology | Limited to available folds |
| Raptor, Tasser, I-Tasser | |||
| Ab initio | Create models based on biophysical principles (total energy, interactions, angles, …) | Can create new types of structures and folds Give information on the folding process | Requires a lot of computational resources |
| Rosetta, QUARK | |||
| Deep learning | Use known structures to learn how to fold proteins | Can create new types of structures and folds High accuracy Fast | Lacks transparency Requires extensive computational resources to train |
| RaptorX, RaptorX-Single, AlphaFold2, AlphaFold3, TrRosetta, RosettaFold, C-I-Tasser, I-TASSER-MTD, Omegafold, ESMfold, C-QUARK, D-QUARK | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamy, A.; Durairaj, R.; Pageat, P. Chemosensory Receptors in Vertebrates: Structure and Computational Modeling Insights. Int. J. Mol. Sci. 2025, 26, 6605. https://doi.org/10.3390/ijms26146605
Lamy A, Durairaj R, Pageat P. Chemosensory Receptors in Vertebrates: Structure and Computational Modeling Insights. International Journal of Molecular Sciences. 2025; 26(14):6605. https://doi.org/10.3390/ijms26146605
Chicago/Turabian StyleLamy, Aurore, Rajesh Durairaj, and Patrick Pageat. 2025. "Chemosensory Receptors in Vertebrates: Structure and Computational Modeling Insights" International Journal of Molecular Sciences 26, no. 14: 6605. https://doi.org/10.3390/ijms26146605
APA StyleLamy, A., Durairaj, R., & Pageat, P. (2025). Chemosensory Receptors in Vertebrates: Structure and Computational Modeling Insights. International Journal of Molecular Sciences, 26(14), 6605. https://doi.org/10.3390/ijms26146605








