Vital Role of Visceral Adipose Tissue in Maintaining Cognitive Functions
Abstract
1. Introduction
2. Effects of Aging on White Adipose Tissue
3. Obesity Accelerates the Aging of White Adipose Tissue
4. Obesity Accelerates Brain Aging
5. BDNF Has a Critical Role in Maintaining Brain Functions
6. Inverse Relationship Between Obesity and BDNF
7. Exercise Increases the Hippocampus BDNF Expression
8. Fasting Augments the Hippocampus BDNF Expression
9. Interim Conclusion
10. Young Visceral Adipose Tissue Upregulates the Hippocampus BDNF Protein Level
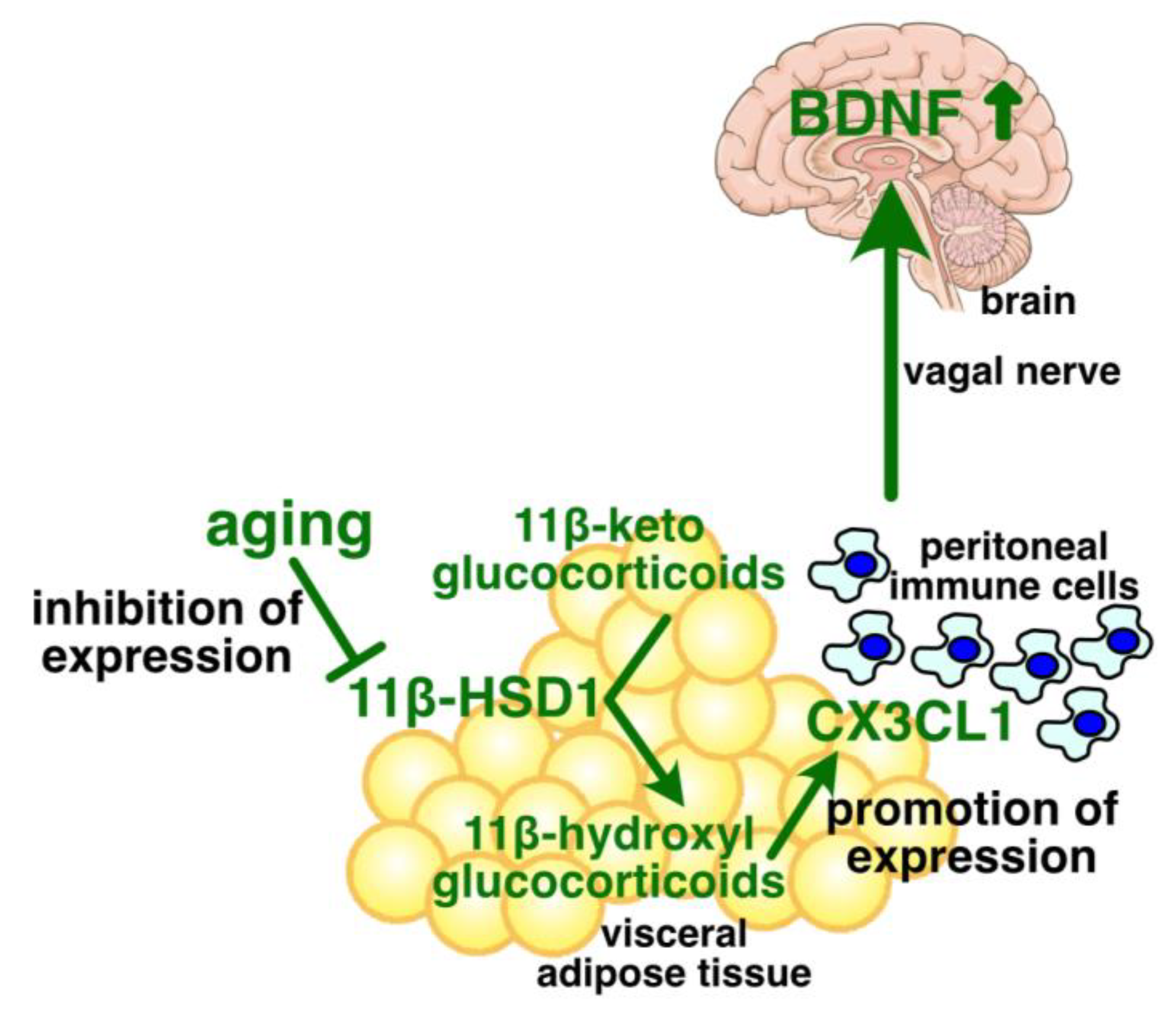
11. Age-Associated Regulation of Adipose CX3CL1 Expression
12. Effects of Obesity on Glucocorticoids and 11β-HSD1 in Adipose Tissue
13. Effects of Obesity on CX3CL1 Expression
14. Exercise Induces Glucocorticoid Secretion and Accelerates Its Termination Process
15. Exercise and CX3CL1
16. Fasting Modulates the Circadian Rhythm of Glucocorticoid Secretion
17. Fasting and CX3CL1
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Lowsky, D.J.; Olshansky, S.J.; Bhattacharya, J.; Goldman, D.P. Heterogeneity in Healthy Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 640–649. [Google Scholar] [CrossRef]
- Tian, Y.E.; Cropley, V.; Maier, A.B.; Lautenschlager, N.T.; Breakspear, M.; Zalesky, A. Heterogeneous Aging across Multiple Organ Systems and Prediction of Chronic Disease and Mortality. Nat. Med. 2023, 29, 1221–1231. [Google Scholar] [CrossRef]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An Update on the Secretory Functions of Brown, White, and Beige Adipose Tissue: Towards Therapeutic Applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Lowell, B.B.; Spiegelman, B.M. Towards a Molecular Understanding of Adaptive Thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, H.; Yonemitsu, N.; Miyabara, S.; Yun, K. Primary Cultures of Unilocular Fat Cells: Characteristics of Growth in Vitro and Changes in Differentiation Properties. Differentiation 1986, 31, 42–49. [Google Scholar] [CrossRef]
- Wu, J.W.; Wang, S.P.; Casavant, S.; Moreau, A.; Yang, G.S.; Mitchell, G.A. Fasting Energy Homeostasis in Mice with Adipose Deficiency of Desnutrin/Adipose Triglyceride Lipase. Endocrinology 2012, 153, 2198–2207. [Google Scholar] [CrossRef]
- Morak, M.; Schmidinger, H.; Riesenhuber, G.; Rechberger, G.N.; Kollroser, M.; Haemmerle, G.; Zechner, R.; Kronenberg, F.; Hermetter, A. Adipose Triglyceride Lipase (ATGL) and Hormone-Sensitive Lipase (HSL) Deficiencies Affect Expression of Lipolytic Activities in Mouse Adipose Tissues. Mol. Cell. Proteom. 2012, 11, 1777–1789. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. The Endocrine Function of Adipose Tissues in Health and Cardiometabolic Disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical Activity and Exercise in the Regulation of Human Adipose Tissue Physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef]
- Hassan, W.U.; Greiser, U.; Wang, W. Role of Adipose-Derived Stem Cells in Wound Healing. Wound Repair Regen. 2014, 22, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gómez, G. Mitochondria and Endocrine Function of Adipose Tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 791–804. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. Biomed Res. Int. 2016, 2016, 2365609. [Google Scholar] [CrossRef]
- Schaum, N.; Lehallier, B.; Hahn, O.; Pálovics, R.; Hosseinzadeh, S.; Lee, S.E.; Sit, R.; Lee, D.P.; Losada, P.M.; Zardeneta, M.E.; et al. Ageing Hallmarks Exhibit Organ-Specific Temporal Signatures. Nature 2020, 583, 596–602. [Google Scholar] [CrossRef]
- Kalathookunnel Antony, A.; Lian, Z.; Wu, H. T Cells in Adipose Tissue in Aging. Front. Immunol. 2018, 9, 2945. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, S.-W.; Tang, Y.; Tang, Q.-Q. The Secretory Function of Adipose Tissues in Metabolic Regulation. Life Metab. 2024, 3, loae003. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The Gut Microbiome as a Modulator of Healthy Ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Amagase, Y.; Kambayashi, R.; Sugiyama, A.; Takei, Y. Peripheral Regulation of Central Brain-Derived Neurotrophic Factor Expression through the Vagus Nerve. Int. J. Mol. Sci. 2023, 24, 3543. [Google Scholar] [CrossRef]
- Spoto, B.; Di Betta, E.; Mattace-Raso, F.; Sijbrands, E.; Vilardi, A.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Vermi, W.; Testa, A.; et al. Pro- and Anti-Inflammatory Cytokine Gene Expression in Subcutaneous and Visceral Fat in Severe Obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Utzschneider, K.M.; Hull, R.L.; Kodama, K.; Retzlaff, B.M.; Brunzell, J.D.; Shofer, J.B.; Fish, B.E.; Knopp, R.H.; Kahn, S.E. Intra-Abdominal Fat Is a Major Determinant of the National Cholesterol Education Program Adult Treatment Panel III Criteria for the Metabolic Syndrome. Diabetes 2004, 53, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Koh-Banerjee, P.; Wang, Y.; Hu, F.B.; Spiegelman, D.; Willett, W.C.; Rimm, E.B. Changes in Body Weight and Body Fat Distribution as Risk Factors for Clinical Diabetes in US Men. Am. J. Epidemiol. 2004, 159, 1150–1159. [Google Scholar] [CrossRef]
- Pascot, A.; Lemieux, S.; Lemieux, I.; Prud’homme, D.; Tremblay, A.; Bouchard, C.; Nadeau, A.; Couillard, C.; Tchernof, A.; Bergeron, J.; et al. Age-Related Increase in Visceral Adipose Tissue and Body Fat and the Metabolic Risk Profile of Premenopausal Women. Diabetes Care 1999, 22, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.P.; Rimm, E.B.; Ascherio, A.; Kawachi, I.; Stampfer, M.J.; Willett, W.C. Body Size and Fat Distribution as Predictors of Stroke among US Men. Am. J. Epidemiol. 1996, 144, 1143–1150. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Cesari, M.; Penninx, B.W.J.H.; Kritchevsky, S.B.; Ding, J.; Newman, A.; Kitzman, D.W.; Kanaya, A.M.; Pahor, M.; Harris, T.B. Abdominal Obesity Is an Independent Risk Factor for Chronic Heart Failure in Older People. J. Am. Geriatr. Soc. 2006, 54, 413–420. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gustafson, D.R.; Barrett-Connor, E.; Haan, M.N.; Gunderson, E.P.; Yaffe, K. Central Obesity and Increased Risk of Dementia More than Three Decades Later. Neurology 2008, 71, 1057–1064. [Google Scholar] [CrossRef]
- Razay, G.; Vreugdenhil, A.; Wilcock, G. Obesity, Abdominal Obesity and Alzheimer Disease. Dement. Geriatr. Cogn. Disord. 2006, 22, 173–176. [Google Scholar] [CrossRef]
- Barzilai, N.; She, L.; Liu, B.Q.; Vuguin, P.; Cohen, P.; Wang, J.; Rossetti, L. Surgical Removal of Visceral Fat Reverses Hepatic Insulin Resistance. Diabetes 1999, 48, 94–98. [Google Scholar] [CrossRef]
- Pitombo, C.; Araújo, E.P.; De Souza, C.T.; Pareja, J.C.; Geloneze, B.; Velloso, L.A. Amelioration of Diet-Induced Diabetes Mellitus by Removal of Visceral Fat. J. Endocrinol. 2006, 191, 699–706. [Google Scholar] [CrossRef]
- Klein, S.; Fontana, L.; Young, V.L.; Coggan, A.R.; Kilo, C.; Patterson, B.W.; Mohammed, B.S. Absence of an Effect of Liposuction on Insulin Action and Risk Factors for Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Raguso, C.A.; Kyle, U.; Kossovsky, M.P.; Roynette, C.; Paoloni-Giacobino, A.; Hans, D.; Genton, L.; Pichard, C. A 3-Year Longitudinal Study on Body Composition Changes in the Elderly: Role of Physical Exercise. Clin. Nutr. 2006, 25, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-Related Changes in Total and Regional Fat Distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Gavi, S.; Feiner, J.J.; Melendez, M.M.; Mynarcik, D.C.; Gelato, M.C.; McNurlan, M.A. Limb Fat to Trunk Fat Ratio in Elderly Persons Is a Strong Determinant of Insulin Resistance and Adiponectin Levels. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Gálvez, B.G. “Adipaging”: Ageing and Obesity Share Biological Hallmarks Related to a Dysfunctional Adipose Tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. Adaptive Homeostasis and the Free Radical Theory of Ageing. Free Radic. Biol. Med. 2018, 124, 420–430. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S. Obesity and Aging: Molecular Mechanisms and Therapeutic Approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds 2017, 29, 168–174. [Google Scholar] [PubMed]
- Soto-Gamez, A.; Quax, W.J.; Demaria, M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J. Mol. Biol. 2019, 431, 2629–2643. [Google Scholar] [CrossRef]
- Sakurai, T.; Ogasawara, J.; Shirato, K.; Izawa, T.; Oh-Ishi, S.; Ishibashi, Y.; Radák, Z.; Ohno, H.; Kizaki, T. Exercise Training Attenuates the Dysregulated Expression of Adipokines and Oxidative Stress in White Adipose Tissue. Oxidative Med. Cell. Longev. 2017, 2017, 9410954. [Google Scholar] [CrossRef]
- Palmer, A.K.; Xu, M.; Zhu, Y.; Pirtskhalava, T.; Weivoda, M.M.; Hachfeld, C.M.; Prata, L.G.; van Dijk, T.H.; Verkade, E.; Casaclang-Verzosa, G.; et al. Targeting Senescent Cells Alleviates Obesity-Induced Metabolic Dysfunction. Aging Cell 2019, 18, e12950. [Google Scholar] [CrossRef]
- Lee, H.-C.; Wei, Y.-H. Oxidative Stress, Mitochondrial DNA Mutation, and Apoptosis in Aging. Exp. Biol. Med. 2007, 232, 592–606. [Google Scholar]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A Crucial Role for Adipose Tissue P53 in the Regulation of Insulin Resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef]
- Lee, M.; Martin, H.; Firpo, M.A.; Demerath, E.W. Inverse Association between Adiposity and Telomere Length: The Fels Longitudinal Study. Am. J. Hum. Biol. 2011, 23, 100–106. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed Interplay between Autophagy and Inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, J.S.; Clauson, C.L.; Niedernhofer, L.J.; Robbins, P.D. NF-ΚB in Aging and Disease. Aging Dis. 2011, 2, 449–465. [Google Scholar]
- Tonnessen-Murray, C.A.; Lozano, G.; Jackson, J.G. The Regulation of Cellular Functions by the P53 Protein: Cellular Senescence. Cold Spring Harb. Perspect. Med. 2017, 7, a026112. [Google Scholar] [CrossRef] [PubMed]
- Emmerzaal, T.L.; Kiliaan, A.J.; Gustafson, D.R. 2003-2013: A Decade of Body Mass Index, Alzheimer’s Disease, and Dementia. J. Alzheimers Dis. 2015, 43, 739–755. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, Y.-J.; Jang, W.; Son, K.Y.; Park, H.S.; Kim, Y.S. Body Mass Index Trajectories and the Risk for Alzheimer’s Disease among Older Adults. Sci. Rep. 2021, 11, 3087. [Google Scholar] [CrossRef]
- Wang, R.-T.; Sun, Z.; Tan, C.-C.; Tan, L.; Xu, W.; Alzheimer’s Disease Neuroimaging Initiative. Dynamic Features of Body Mass Index in Late Life Predict Cognitive Trajectories and Alzheimer’s Disease: A Longitudinal Study. J. Alzheimers Dis. 2024, 100, 1365–1378. [Google Scholar] [CrossRef]
- Ronan, L.; Alexander-Bloch, A.F.; Wagstyl, K.; Farooqi, S.; Brayne, C.; Tyler, L.K.; Cam-CAN; Fletcher, P.C. Obesity Associated with Increased Brain Age from Midlife. Neurobiol. Aging 2016, 47, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin Regulation of Neural Circuit Development and Function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.E.; Glover, A.C.; Hoodless, L.; Lim, E.-K.; Beattie, L.; Kirby, A.; Kaye, P.M. The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Leishmania Donovani Infection. PLoS Pathog. 2015, 11, e1004681. [Google Scholar] [CrossRef]
- Linnarsson, S.; Björklund, A.; Ernfors, P. Learning Deficit in BDNF Mutant Mice. Eur. J. Neurosci. 1997, 9, 2581–2587. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence That Brain-Derived Neurotrophic Factor Is Required for Basal Neurogenesis and Mediates, in Part, the Enhancement of Neurogenesis by Dietary Restriction in the Hippocampus of Adult Mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-Specific Deletion of BDNF in Adult Mice Impairs Spatial Memory and Extinction of Aversive Memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef]
- Quesseveur, G.; David, D.J.; Gaillard, M.C.; Pla, P.; Wu, M.V.; Nguyen, H.T.; Nicolas, V.; Auregan, G.; David, I.; Dranovsky, A.; et al. BDNF Overexpression in Mouse Hippocampal Astrocytes Promotes Local Neurogenesis and Elicits Anxiolytic-like Activities. Transl. Psychiatry 2013, 3, e253. [Google Scholar] [CrossRef] [PubMed]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal Long-Term Potentiation Is Impaired in Mice Lacking Brain-Derived Neurotrophic Factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, E.G.; An, J.J.; Orefice, L.L.; Baydyuk, M.; Liao, G.-Y.; Zheng, K.; Lu, B.; Xu, B. BDNF Promotes Differentiation and Maturation of Adult-Born Neurons through GABAergic Transmission. J. Neurosci. 2012, 32, 14318–14330. [Google Scholar] [CrossRef]
- Buchman, A.S.; Yu, L.; Boyle, P.A.; Schneider, J.A.; De Jager, P.L.; Bennett, D.A. Higher Brain BDNF Gene Expression Is Associated with Slower Cognitive Decline in Older Adults. Neurology 2016, 86, 735–741. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H. The Role of Neurotrophin Signaling in Age-Related Cognitive Decline and Cognitive Diseases. Int. J. Mol. Sci. 2022, 23, 7726. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Borovecki, F.; Filipcic, I.; Vuic, B.; Milos, T.; Nedic Erjavec, G.; Konjevod, M.; Tudor, L.; Mimica, N.; Uzun, S.; et al. Relationship between Brain-Derived Neurotrophic Factor and Cognitive Decline in Patients with Mild Cognitive Impairment and Dementia. Biomolecules 2023, 13, 570. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics 2023, 15, 2081. [Google Scholar] [CrossRef]
- Li, Y.; Ning, L.; Yin, Y.; Wang, R.; Zhang, Z.; Hao, L.; Wang, B.; Zhao, X.; Yang, X.; Yin, L.; et al. Age-Related Shifts in Gut Microbiota Contribute to Cognitive Decline in Aged Rats. Aging 2020, 12, 7801–7817. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Holmes, M.C.; Carter, R.N.; Noble, J.; Chitnis, S.; Dutia, A.; Paterson, J.M.; Mullins, J.J.; Seckl, J.R.; Yau, J.L. 11β-Hydroxysteroid Dehydrogenase Type 1 Expression Is Increased in the Aged Mouse Hippocampus and Parietal Cortex and Causes Memory Impairments. J. Neurosci. 2010, 30, 6916–6920. [Google Scholar] [CrossRef]
- Caughey, S.; Harris, A.P.; Seckl, J.R.; Holmes, M.C.; Yau, J.L. Forebrain-Specific Transgene Rescue of 11β-HSD1 Associates with Impaired Spatial Memory and Reduced Hippocampal Brain-Derived Neurotrophic Factor MRNA Levels in Aged 11β-HSD1 Deficient Mice. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef]
- Okamoto, M.; Inoue, K.; Iwamura, H.; Terashima, K.; Soya, H.; Asashima, M.; Kuwabara, T. Reduction in Paracrine Wnt3 Factors during Aging Causes Impaired Adult Neurogenesis. FASEB J. 2011, 25, 3570–3582. [Google Scholar] [CrossRef]
- Takei, Y.; Amagase, Y.; Goto, A.; Kambayashi, R.; Izumi-Nakaseko, H.; Hirasawa, A.; Sugiyama, A. Adipose Chemokine Ligand CX3CL1 Contributes to Maintaining the Hippocampal BDNF Level, and the Effect Is Attenuated in Advanced Age. Geroscience 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.B.; Gudbjartsson, D.F.; Steinthorsdottir, V.; Sulem, P.; Helgadottir, A.; Styrkarsdottir, U.; Gretarsdottir, S.; Thorlacius, S.; Jonsdottir, I.; et al. Genome-Wide Association Yields New Sequence Variants at Seven Loci That Associate with Measures of Obesity. Nat. Genet. 2009, 41, 18–24. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model: Gender Difference and Metabolomics Study. Front. Pharmacol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Vanevski, F.; Xu, B. Molecular and Neural Bases Underlying Roles of BDNF in the Control of Body Weight. Front. Neurosci. 2013, 7, 37. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Z.; Kalyani, M.; Janik, J.M.; Shi, H. Effects of Energy Status and Diet on Bdnf Expression in the Ventromedial Hypothalamus of Male and Female Rats. Physiol. Behav. 2014, 130, 99–107. [Google Scholar] [CrossRef]
- de Souza, R.M.; de Souza, L.; Machado, A.E.; de Bem Alves, A.C.; Rodrigues, F.S.; Aguiar, A.S.; Dos Santos, A.R.S.; de Bem, A.F.; Moreira, E.L.G. Behavioural, Metabolic and Neurochemical Effects of Environmental Enrichment in High-Fat Cholesterol-Enriched Diet-Fed Mice. Behav. Brain Res. 2019, 359, 648–656. [Google Scholar] [CrossRef]
- Melgar-Locatelli, S.; de Ceglia, M.; Mañas-Padilla, M.C.; Rodriguez-Pérez, C.; Castilla-Ortega, E.; Castro-Zavala, A.; Rivera, P. Nutrition and Adult Neurogenesis in the Hippocampus: Does What You Eat Help You Remember? Front. Neurosci. 2023, 17, 1147269. [Google Scholar] [CrossRef]
- Criscuolo, C.; Fabiani, C.; Bonadonna, C.; Origlia, N.; Domenici, L. BDNF Prevents Amyloid-Dependent Impairment of LTP in the Entorhinal Cortex by Attenuating P38 MAPK Phosphorylation. Neurobiol. Aging 2015, 36, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Kurhe, Y.; Mahesh, R.; Devadoss, T. Novel 5-HT3 Receptor Antagonist QCM-4 Attenuates Depressive-like Phenotype Associated with Obesity in High-Fat-Diet-Fed Mice. Psychopharmacology 2017, 234, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Park, M.; Choi, J.; Park, K.-Y.; Chung, H.Y.; Lee, J. A High-Fat Diet Impairs Neurogenesis: Involvement of Lipid Peroxidation and Brain-Derived Neurotrophic Factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Maniam, J.; Westbrook, R.F.; Morris, M.J. Dietary-Induced Obesity Disrupts Trace Fear Conditioning and Decreases Hippocampal Reelin Expression. Brain Behav. Immun. 2015, 43, 68–75. [Google Scholar] [CrossRef]
- Abidin, İ.; Aydin-Abidin, S.; Bodur, A.; İnce, İ.; Alver, A. Brain-Derived Neurotropic Factor (BDNF) Heterozygous Mice Are More Susceptible to Synaptic Protein Loss in Cerebral Cortex during High Fat Diet. Arch. Physiol. Biochem. 2018, 124, 442–447. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, L.H.; Yang, W.; Cui, R.J.; Xu, S.B. The Role of BDNF in the Neuroimmune Axis Regulation of Mood Disorders. Front. Neurol. 2019, 10, 515. [Google Scholar] [CrossRef]
- Izquierdo, M.; Morley, J.E.; Lucia, A. Exercise in People over 85. BMJ 2020, 368, m402. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical Activity and Exercise: Strategies to Manage Frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; Izquierdo, M.; Serra-Rexach, J.A.; Santos-Lozano, A.; Lucia, A. Physical Exercise in the Oldest Old. Compr. Physiol. 2019, 9, 1281–1304. [Google Scholar] [CrossRef]
- Kujala, U.M. Is Physical Activity a Cause of Longevity? It Is Not as Straightforward as Some Would Believe. A Critical Analysis. Br. J. Sports Med. 2018, 52, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, D.; Viken, H.; Steinshamn, S.L.; Dalen, H.; Støylen, A.; Loennechen, J.P.; Reitlo, L.S.; Zisko, N.; Bækkerud, F.H.; Tari, A.R.; et al. Effect of Exercise Training for Five Years on All Cause Mortality in Older Adults-the Generation 100 Study: Randomised Controlled Trial. BMJ 2020, 371, m3485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Veeranki, S.P.; Magnussen, C.G.; Xi, B. Recommended Physical Activity and All Cause and Cause Specific Mortality in US Adults: Prospective Cohort Study. BMJ 2020, 370, m2031. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy Intake and Exercise as Determinants of Brain Health and Vulnerability to Injury and Disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Does Vigorous Exercise Have a Neuroprotective Effect in Parkinson Disease? Neurology 2011, 77, 288–294. [Google Scholar] [CrossRef]
- Arida, R.M.; Cavalheiro, E.A.; da Silva, A.C.; Scorza, F.A. Physical Activity and Epilepsy: Proven and Predicted Benefits. Sports Med. 2008, 38, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, P.A.; Yu, L.; Shah, R.C.; Wilson, R.S.; Bennett, D.A. Total Daily Physical Activity and the Risk of AD and Cognitive Decline in Older Adults. Neurology 2012, 78, 1323–1329. [Google Scholar] [CrossRef]
- Russo-Neustadt, A.; Beard, R.C.; Cotman, C.W. Exercise, Antidepressant Medications, and Enhanced Brain Derived Neurotrophic Factor Expression. Neuropsychopharmacology 1999, 21, 679–682. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Zhang, P.; Sha, H.; Jia, J.; Hu, Y.; Zhu, J. Exercise Induces Mitochondrial Biogenesis after Brain Ischemia in Rats. Neuroscience 2012, 205, 10–17. [Google Scholar] [CrossRef]
- van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise Enhances Learning and Hippocampal Neurogenesis in Aged Mice. J. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and Brain Neurotrophins. Nature 1995, 373, 109. [Google Scholar]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF Mediates the Efficacy of Exercise on Synaptic Plasticity and Cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Vaynman, S.S.; Ying, Z.; Yin, D.; Gomez-Pinilla, F. Exercise Differentially Regulates Synaptic Proteins Associated to the Function of BDNF. Brain Res. 2006, 1070, 124–130. [Google Scholar] [CrossRef] [PubMed]
- So, J.H.; Huang, C.; Ge, M.; Cai, G.; Zhang, L.; Lu, Y.; Mu, Y. Intense Exercise Promotes Adult Hippocampal Neurogenesis But Not Spatial Discrimination. Front. Cell. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef]
- Zouhal, H.; Saeidi, A.; Salhi, A.; Li, H.; Essop, M.F.; Laher, I.; Rhibi, F.; Amani-Shalamzari, S.; Ben Abderrahman, A. Exercise Training and Fasting: Current Insights. Open Access J. Sports Med. 2020, 11, 1–28. [Google Scholar] [CrossRef]
- Wegman, M.P.; Guo, M.H.; Bennion, D.M.; Shankar, M.N.; Chrzanowski, S.M.; Goldberg, L.A.; Xu, J.; Williams, T.A.; Lu, X.; Hsu, S.I.; et al. Practicality of Intermittent Fasting in Humans and Its Effect on Oxidative Stress and Genes Related to Aging and Metabolism. Rejuvenation Res. 2015, 18, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Witte, A.V.; Fobker, M.; Gellner, R.; Knecht, S.; Flöel, A. Caloric Restriction Improves Memory in Elderly Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 1255–1260. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S422–S428. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-Restricted Feeding and Risk of Metabolic Disease: A Review of Human and Animal Studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef]
- Anton, S.; Leeuwenburgh, C. Fasting or Caloric Restriction for Healthy Aging. Exp. Gerontol. 2013, 48, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent Fasting vs Daily Calorie Restriction for Type 2 Diabetes Prevention: A Review of Human Findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus Daily Calorie Restriction: Which Diet Regimen Is More Effective for Weight Loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Brandhorst, S.; Levine, M.E.; Wei, M.; Shelehchi, M.; Morgan, T.E.; Nayak, K.S.; Dorff, T.; Hong, K.; Crimmins, E.M.; Cohen, P.; et al. Fasting-Mimicking Diet Causes Hepatic and Blood Markers Changes Indicating Reduced Biological Age and Disease Risk. Nat. Commun. 2024, 15, 1309. [Google Scholar] [CrossRef]
- Fontana, L.; Klein, S.; Holloszy, J.O. Long-Term Low-Protein, Low-Calorie Diet and Endurance Exercise Modulate Metabolic Factors Associated with Cancer Risk. Am. J. Clin. Nutr. 2006, 84, 1456–1462. [Google Scholar] [CrossRef]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of Intermittent Fasting and Refeeding on Insulin Action in Healthy Men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Solianik, R.; Sujeta, A.; Čekanauskaitė, A. Effects of 2-Day Calorie Restriction on Cardiovascular Autonomic Response, Mood, and Cognitive and Motor Functions in Obese Young Adult Women. Exp. Brain Res. 2018, 236, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, S.; Hu, C.; Wang, M.; Zhou, T.; Zhou, Y. Neuroprotective Effects of Intermittent Fasting in the Aging Brain. Ann. Nutr. Metab. 2024, 80, 175–185. [Google Scholar] [CrossRef]
- Ouellette, A.R.; Hadad, N.; Deighan, A.; Robinson, L.; O’Connell, K.; Freund, A.; Churchill, G.A.; Kaczorowski, C.C. Life-Long Dietary Restrictions Have Negligible or Damaging Effects on Late-Life Cognitive Performance: A Key Role for Genetics in Outcomes. Neurobiol. Aging 2022, 118, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.E.; Roberts, S.B.; Kramer, A.F.; Chui, K.K.H.; Das, S.K. No Effect of Calorie Restriction or Dietary Patterns on Spatial Working Memory During a 2-Year Intervention: A Secondary Analysis of the CALERIE Trial. J. Nutr. 2023, 153, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Kackley, M.L.; Buga, A.; Crabtree, C.D.; Sapper, T.N.; McElroy, C.A.; Focht, B.C.; Kraemer, W.J.; Volek, J.S. Influence of Nutritional Ketosis Achieved through Various Methods on Plasma Concentrations of Brain Derived Neurotropic Factor. Brain Sci. 2022, 12, 1143. [Google Scholar] [CrossRef]
- Walsh, J.J.; Tschakovsky, M.E. Exercise and Circulating BDNF: Mechanisms of Release and Implications for the Design of Exercise Interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF Concentrations Reflect Brain-Tissue BDNF Levels across Species. Int. J. Neuropsychopharmacol. 2011, 14, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Amagase, Y.; Iida, K.; Sagawa, T.; Goto, A.; Kambayashi, R.; Izumi-Nakaseko, H.; Matsumoto, A.; Kawai, S.; Sugiyama, A.; et al. Alteration in Peritoneal Cells with the Chemokine CX3CL1 Reverses Age-Associated Impairment of Recognition Memory. Geroscience 2022, 44, 2305–2318. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Morgan, S.A.; McCabe, E.L.; Gathercole, L.L.; Hassan-Smith, Z.K.; Larner, D.P.; Bujalska, I.J.; Stewart, P.M.; Tomlinson, J.W.; Lavery, G.G. 11β-HSD1 Is the Major Regulator of the Tissue-Specific Effects of Circulating Glucocorticoid Excess. Proc. Natl. Acad. Sci. USA 2014, 111, E2482–E2491. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, H.; Paterson, J.; Shinyama, H.; Morton, N.M.; Mullins, J.J.; Seckl, J.R.; Flier, J.S. A Transgenic Model of Visceral Obesity and the Metabolic Syndrome. Science 2001, 294, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Green, B.B.; Armstrong, D.A.; Lesseur, C.; Paquette, A.G.; Guerin, D.J.; Kwan, L.E.; Marsit, C.J. The Role of Placental 11-Beta Hydroxysteroid Dehydrogenase Type 1 and Type 2 Methylation on Gene Expression and Infant Birth Weight. Biol. Reprod. 2015, 92, 149. [Google Scholar] [CrossRef]
- Inder, W.J.; Obeyesekere, V.R.; Jang, C.; Saffery, R. Evidence for Transcript-Specific Epigenetic Regulation of Glucocorticoid-Stimulated Skeletal Muscle 11β-Hydroxysteroid Dehydrogenase-1 Activity in Type 2 Diabetes. Clin. Epigenet. 2012, 4, 24. [Google Scholar] [CrossRef]
- Takeda, Y.; Demura, M.; Kometani, M.; Karashima, S.; Aono, D.; Konishi, S.; Horike, S.-I.; Meguro-Horike, M.; Yoneda, T.; Takeda, Y. Epigenetic Alterations of 11beta-Hydroxysteroid Dehydrogenase 1 Gene in the Adipose Tissue of Patients with Primary Aldosteronism. Endocr. J. 2024, 71, 245–252. [Google Scholar] [CrossRef]
- Fraga, M.F.; Esteller, M. Epigenetics and Aging: The Targets and the Marks. Trends Genet. 2007, 23, 413–418. [Google Scholar] [CrossRef]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suñer, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. Epigenetic Differences Arise during the Lifetime of Monozygotic Twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.C.; Houseman, E.A.; Marsit, C.J.; Zheng, S.; Wrensch, M.R.; Wiemels, J.L.; Nelson, H.H.; Karagas, M.R.; Padbury, J.F.; Bueno, R.; et al. Aging and Environmental Exposures Alter Tissue-Specific DNA Methylation Dependent upon CpG Island Context. PLoS Genet. 2009, 5, e1000602. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sánchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E. Epigenetic Predictor of Age. PLoS ONE 2011, 6, e14821. [Google Scholar] [CrossRef]
- Koch, C.M.; Wagner, W. Epigenetic-Aging-Signature to Determine Age in Different Tissues. Aging 2011, 3, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Hewagalamulage, S.D.; Lee, T.K.; Clarke, I.J.; Henry, B.A. Stress, Cortisol, and Obesity: A Role for Cortisol Responsiveness in Identifying Individuals Prone to Obesity. Domest. Anim. Endocrinol. 2016, 56, S112–S120. [Google Scholar] [CrossRef]
- Kumar, R.; Rizvi, M.R.; Saraswat, S. Obesity and Stress: A Contingent Paralysis. Int. J. Prev. Med. 2022, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Kirschbaum, C.; Steptoe, A. Perceived Weight Discrimination and Chronic Biochemical Stress: A Population-Based Study Using Cortisol in Scalp Hair. Obesity 2016, 24, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Wester, V.L.; Staufenbiel, S.M.; Veldhorst, M.A.B.; Visser, J.A.; Manenschijn, L.; Koper, J.W.; Klessens-Godfroy, F.J.M.; van den Akker, E.L.T.; van Rossum, E.F.C. Long-Term Cortisol Levels Measured in Scalp Hair of Obese Patients. Obesity 2014, 22, 1956–1958. [Google Scholar] [CrossRef]
- Savas, M.; Wester, V.L.; de Rijke, Y.B.; Rubinstein, G.; Zopp, S.; Dorst, K.; van den Berg, S.A.A.; Beuschlein, F.; Feelders, R.A.; Reincke, M.; et al. Hair Glucocorticoids as a Biomarker for Endogenous Cushing’s Syndrome: Validation in Two Independent Cohorts. Neuroendocrinology 2019, 109, 171–178. [Google Scholar] [CrossRef]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-Related and Basic Determinants of Hair Cortisol in Humans: A Meta-Analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef]
- Rodriguez, A.C.I.; Epel, E.S.; White, M.L.; Standen, E.C.; Seckl, J.R.; Tomiyama, A.J. Hypothalamic-Pituitary-Adrenal Axis Dysregulation and Cortisol Activity in Obesity: A Systematic Review. Psychoneuroendocrinology 2015, 62, 301–318. [Google Scholar] [CrossRef]
- Manenschijn, L.; Koper, J.W.; Lamberts, S.W.J.; van Rossum, E.F.C. Evaluation of a Method to Measure Long Term Cortisol Levels. Steroids 2011, 76, 1032–1036. [Google Scholar] [CrossRef]
- Wester, V.L.; van Rossum, E.F.C. Clinical Applications of Cortisol Measurements in Hair. Eur. J. Endocrinol. 2015, 173, M1–M10. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Alexander, N.; Bornstein, S.R.; Gao, W.; Miller, R.; Stark, S.; Bosch, J.A.; Fischer, J.E. Cortisol in Hair and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, Z.; Jensen, M.D.; Dumesic, D.A.; Chapman, K.E.; Seckl, J.R.; Walker, B.R.; Morton, N.M. Omental 11β-Hydroxysteroid Dehydrogenase 1 Correlates with Fat Cell Size Independently of Obesity. Obesity 2007, 15, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.M.; Seckl, J.R. 11β-Hydroxysteroid Dehydrogenase Type 1 and Obesity. Front. Horm. Res. 2008, 36, 146–164. [Google Scholar] [CrossRef]
- Stewart, P.M.; Boulton, A.; Kumar, S.; Clark, P.M.; Shackleton, C.H. Cortisol Metabolism in Human Obesity: Impaired Cortisone → cortisol Conversion in Subjects with Central Adiposity. J. Clin. Endocrinol. Metab. 1999, 84, 1022–1027. [Google Scholar] [CrossRef][Green Version]
- Rask, E.; Olsson, T.; Söderberg, S.; Andrew, R.; Livingstone, D.E.; Johnson, O.; Walker, B.R. Tissue-Specific Dysregulation of Cortisol Metabolism in Human Obesity. J. Clin. Endocrinol. Metab. 2001, 86, 1418–1421. [Google Scholar] [CrossRef]
- Tomlinson, J.W.; Moore, J.S.; Clark, P.M.S.; Holder, G.; Shakespeare, L.; Stewart, P.M. Weight Loss Increases 11β-Hydroxysteroid Dehydrogenase Type 1 Expression in Human Adipose Tissue. J. Clin. Endocrinol. Metab. 2004, 89, 2711–2716. [Google Scholar] [CrossRef]
- Shah, R.; Hinkle, C.C.; Ferguson, J.F.; Mehta, N.N.; Li, M.; Qu, L.; Lu, Y.; Putt, M.E.; Ahima, R.S.; Reilly, M.P. Fractalkine Is a Novel Human Adipochemokine Associated with Type 2 Diabetes. Diabetes 2011, 60, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Oatmen, K.E.; Wang, T.; DelProposto, J.L.; Lumeng, C.N. CX3CR1 Deficiency Does Not Influence Trafficking of Adipose Tissue Macrophages in Mice with Diet-Induced Obesity. Obesity 2012, 20, 1189–1199. [Google Scholar] [CrossRef]
- Nagashimada, M.; Sawamoto, K.; Ni, Y.; Kitade, H.; Nagata, N.; Xu, L.; Kobori, M.; Mukaida, N.; Yamashita, T.; Kaneko, S.; et al. CX3CL1-CX3CR1 Signaling Deficiency Exacerbates Obesity-Induced Inflammation and Insulin Resistance in Male Mice. Endocrinology 2021, 162, bqab064. [Google Scholar] [CrossRef]
- Lazarov, T.; Juarez-Carreño, S.; Cox, N.; Geissmann, F. Physiology and Diseases of Tissue-Resident Macrophages. Nature 2023, 618, 698–707. [Google Scholar] [CrossRef]
- Heijnen, S.; Hommel, B.; Kibele, A.; Colzato, L.S. Neuromodulation of Aerobic Exercise—A Review. Front. Psychol. 2015, 6, 1890. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth With Exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Strahler, J.; Skoluda, N.; Kappert, M.B.; Nater, U.M. Simultaneous Measurement of Salivary Cortisol and Alpha-Amylase: Application and Recommendations. Neurosci. Biobehav. Rev. 2017, 83, 657–677. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Patton, J.F.; Gordon, S.E.; Harman, E.A.; Deschenes, M.R.; Reynolds, K.; Newton, R.U.; Triplett, N.T.; Dziados, J.E. Compatibility of High-Intensity Strength and Endurance Training on Hormonal and Skeletal Muscle Adaptations. J. Appl. Physiol. 1995, 78, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and Circulating Cortisol Levels: The Intensity Threshold Effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef]
- Wood, C.J.; Clow, A.; Hucklebridge, F.; Law, R.; Smyth, N. Physical Fitness and Prior Physical Activity Are Both Associated with Less Cortisol Secretion during Psychosocial Stress. Anxiety Stress. Coping 2018, 31, 135–145. [Google Scholar] [CrossRef]
- Strahler, J.; Wurst, R.; Fuchs, R.; Wunsch, K. Joint Associations of Regular Exercise and Healthy Diet with Psychobiological Stress Reactivity in a Healthy Male Sample. Stress 2021, 24, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Gouarné, C.; Groussard, C.; Gratas-Delamarche, A.; Delamarche, P.; Duclos, M. Overnight Urinary Cortisol and Cortisone Add New Insights into Adaptation to Training. Med. Sci. Sports Exerc. 2005, 37, 1157–1167. [Google Scholar] [CrossRef]
- Catoire, M.; Mensink, M.; Kalkhoven, E.; Schrauwen, P.; Kersten, S. Identification of Human Exercise-Induced Myokines Using Secretome Analysis. Physiol. Genom. 2014, 46, 256–267. [Google Scholar] [CrossRef]
- Strömberg, A.; Olsson, K.; Dijksterhuis, J.P.; Rullman, E.; Schulte, G.; Gustafsson, T. CX3CL1—A Macrophage Chemoattractant Induced by a Single Bout of Exercise in Human Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R297–R304. [Google Scholar] [CrossRef]
- Hashida, R.; Matsuse, H.; Kawaguchi, T.; Yoshio, S.; Bekki, M.; Iwanaga, S.; Sugimoto, T.; Hara, K.; Koya, S.; Hirota, K.; et al. Effects of a Low-Intensity Resistance Exercise Program on Serum MiR-630, MiR-5703, and Fractalkine/CX3CL1 Expressions in Subjects with No Exercise Habits: A Preliminary Study. Hepatol. Res. 2021, 51, 823–833. [Google Scholar] [CrossRef]
- Bergendahl, M.; Vance, M.L.; Iranmanesh, A.; Thorner, M.O.; Veldhuis, J.D. Fasting as a Metabolic Stress Paradigm Selectively Amplifies Cortisol Secretory Burst Mass and Delays the Time of Maximal Nyctohemeral Cortisol Concentrations in Healthy Men. J. Clin. Endocrinol. Metab. 1996, 81, 692–699. [Google Scholar] [CrossRef][Green Version]
- Poursalehian, M.; Mohseni, S.; Shadman, Z.; Mohajeri-Tehrani, M.; Atlasi, R.; Khoshniat Nikoo, M.; Qorbani, M.; Larijani, B. Impact of Ramadan Fasting on Serum Levels of Major Endocrinology Hormonal and Biochemical Parameters in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analyses. PLoS ONE 2024, 19, e0299695. [Google Scholar] [CrossRef]
- Michalsen, A.; Schneider, S.; Rodenbeck, A.; Lüdtke, R.; Huether, G.; Dobos, G.J. The Short-Term Effects of Fasting on the Neuroendocrine System in Patients with Chronic Pain Syndromes. Nutr. Neurosci. 2003, 6, 11–18. [Google Scholar] [CrossRef]
- Kim, B.H.; Joo, Y.; Kim, M.-S.; Choe, H.K.; Tong, Q.; Kwon, O. Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones. Endocrinol. Metab. 2021, 36, 745–756. [Google Scholar] [CrossRef]
- Morimoto, Y.; Arisue, K.; Yamamura, Y. Relationship between Circadian Rhythm of Food Intake and That of Plasma Corticosterone and Effect of Food Restriction on Circadian Adrenocortical Rhythm in the Rat. Neuroendocrinology 1977, 23, 212–222. [Google Scholar] [CrossRef]
- Wilkinson, C.W.; Shinsako, J.; Dallman, M.F. Daily Rhythms in Adrenal Responsiveness to Adrenocorticotropin Are Determined Primarily by the Time of Feeding in the Rat. Endocrinology 1979, 104, 350–359. [Google Scholar] [CrossRef]
- Hojlund, K.; Wildner-Christensen, M.; Eshøj, O.; Skjaerbaek, C.; Holst, J.J.; Koldkjaer, O.; Møller Jensen, D.; Beck-Nielsen, H. Reference Intervals for Glucose, β-Cell Polypeptides, and Counterregulatory Factors during Prolonged Fasting. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E50–E58. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Faber, P.; Andrew, R.; Gibney, E.R.; Elia, M.; Lobley, G.; Stubbs, R.J.; Walker, B.R. Influence of Short-Term Dietary Weight Loss on Cortisol Secretion and Metabolism in Obese Men. Eur. J. Endocrinol. 2004, 150, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Schurgin, S.; Canavan, B.; Koutkia, P.; Depaoli, A.M.; Grinspoon, S. Endocrine and Metabolic Effects of Physiologic R-MetHuLeptin Administration during Acute Caloric Deprivation in Normal-Weight Women. J. Clin. Endocrinol. Metab. 2004, 89, 5402–5409. [Google Scholar] [CrossRef][Green Version]
- Veldhuis, J.D.; Iranmanesh, A.; Evans, W.S.; Lizarralde, G.; Thorner, M.O.; Vance, M.L. Amplitude Suppression of the Pulsatile Mode of Immunoradiometric Luteinizing Hormone Release in Fasting-Induced Hypoandrogenemia in Normal Men. J. Clin. Endocrinol. Metab. 1993, 76, 587–593. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Kawamura, N.; Katsuura, G.; Yamada-Goto, N.; Nakama, R.; Kambe, Y.; Miyata, A.; Furuyashiki, T.; Narumiya, S.; Ogawa, Y.; Inui, A. Brain Fractalkine-CX3CR1 Signalling Is Anti-Obesity System as Anorexigenic and Anti-Inflammatory Actions in Diet-Induced Obese Mice. Sci. Rep. 2022, 12, 12604. [Google Scholar] [CrossRef]
- Deuschle, M.; Gotthardt, U.; Schweiger, U.; Weber, B.; Körner, A.; Schmider, J.; Standhardt, H.; Lammers, C.H.; Heuser, I. With Aging in Humans the Activity of the Hypothalamus-Pituitary-Adrenal System Increases and Its Diurnal Amplitude Flattens. Life Sci. 1997, 61, 2239–2246. [Google Scholar] [CrossRef]
- Van Cauter, E.; Leproult, R.; Kupfer, D.J. Effects of Gender and Age on the Levels and Circadian Rhythmicity of Plasma Cortisol. J. Clin. Endocrinol. Metab. 1996, 81, 2468–2473. [Google Scholar] [CrossRef]
| Aging | Obesity | Exercise | Fasting | |
|---|---|---|---|---|
| Mass of visceral adipose tissue | ↑ | ↑ | ↓ | ↓ |
| Cognition | ↓ | ↓ | ↑ | ↑ *5 |
| Circulating glucocorticoids | ± *1 | ↑ | ↑ *3 | ↑ |
| 11β-HSD1 in visceral adipose tissue | ↓ | ↑ | unknown | unknown |
| CX3CL1 in visceral adipose tissue | ↓ | ↑ *2 | ↑(?) *4 | unknown |
| BDNF in the hippocampus | ↓ | ↓ | ↑ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirafuji, R.; Amagase, Y.; Goto, A.; Takei, Y. Vital Role of Visceral Adipose Tissue in Maintaining Cognitive Functions. Int. J. Mol. Sci. 2025, 26, 6597. https://doi.org/10.3390/ijms26146597
Shirafuji R, Amagase Y, Goto A, Takei Y. Vital Role of Visceral Adipose Tissue in Maintaining Cognitive Functions. International Journal of Molecular Sciences. 2025; 26(14):6597. https://doi.org/10.3390/ijms26146597
Chicago/Turabian StyleShirafuji, Rina, Yoko Amagase, Ai Goto, and Yoshinori Takei. 2025. "Vital Role of Visceral Adipose Tissue in Maintaining Cognitive Functions" International Journal of Molecular Sciences 26, no. 14: 6597. https://doi.org/10.3390/ijms26146597
APA StyleShirafuji, R., Amagase, Y., Goto, A., & Takei, Y. (2025). Vital Role of Visceral Adipose Tissue in Maintaining Cognitive Functions. International Journal of Molecular Sciences, 26(14), 6597. https://doi.org/10.3390/ijms26146597








