Enhancing Functional Recovery After Spinal Cord Injury Through Neuroplasticity: A Comprehensive Review
Abstract
1. Introduction
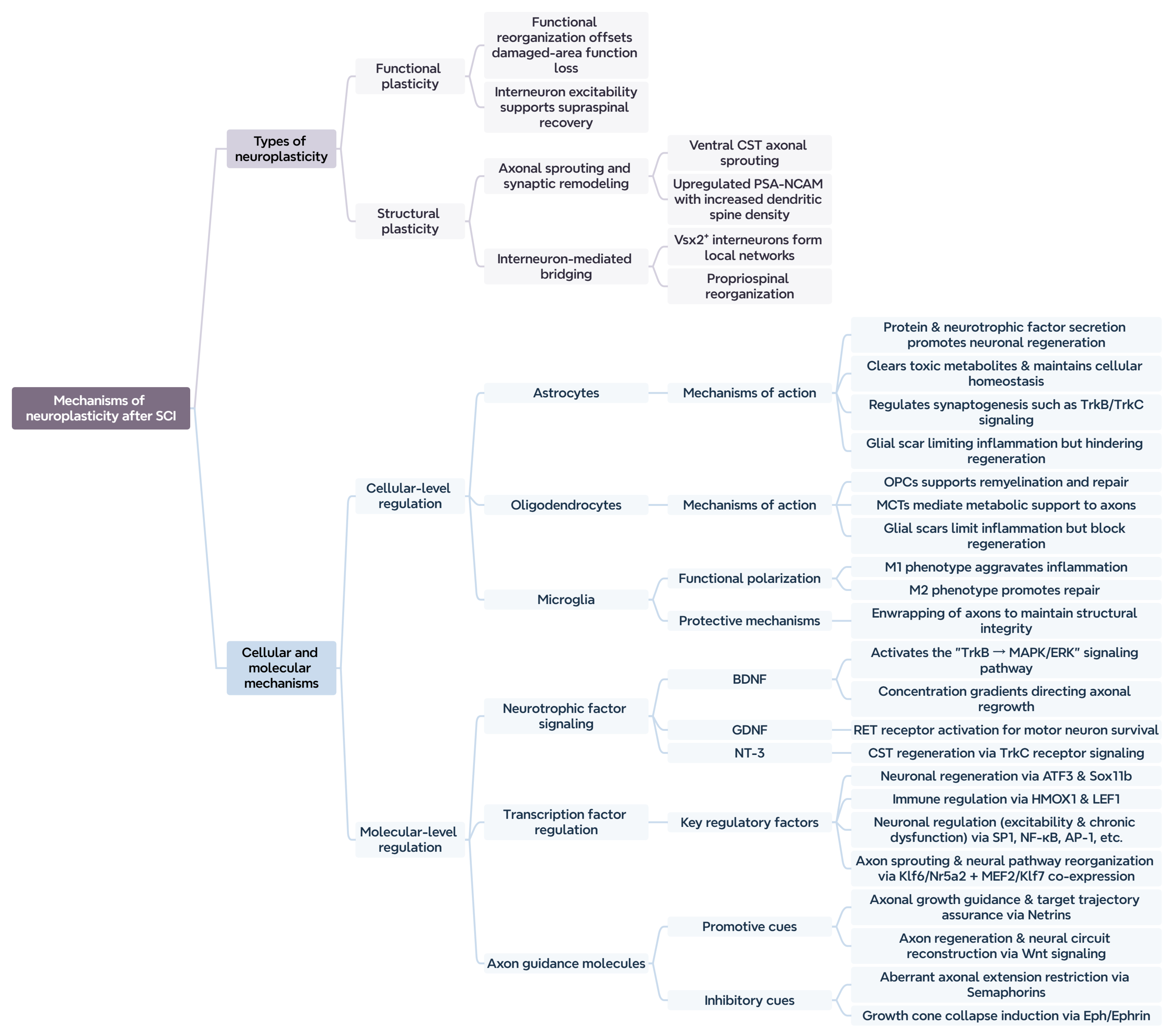
2. Mechanisms of Neuroplasticity
2.1. Definition of Neuroplasticity
2.1.1. Functional Plasticity
2.1.2. Structural Plasticity
Neuronal Changes After SCI
Axonal Changes After SCI
Changes in Dendritic Spines After SCI
Synaptic Changes After SCI
2.2. Mechanisms of Neural Plasticity
2.2.1. Remodeling Mechanisms at the Cellular Level
Astrocytes
Oligodendrocytes
Microglia
2.2.2. Molecular Regulatory Mechanisms
Neurotrophic Factor Signaling Pathways
Regulation of Transcription Factors
Role of Axon Guidance Molecules
2.3. Summary of Neuroplasticity Mechanisms After SCI
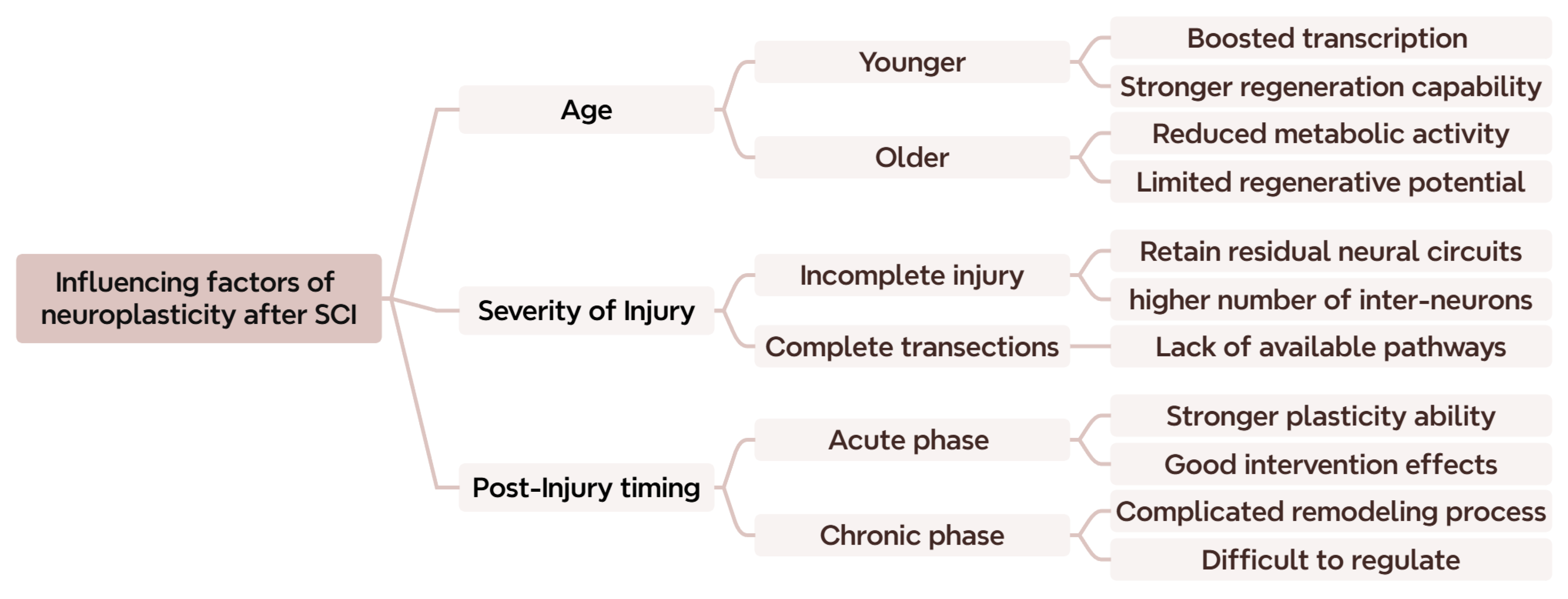
2.4. Other Critical Factors Affecting Neuroplasticity
3. Strategies to Promote Neural Remodeling After SCI
3.1. Pharmacological Interventions
3.2. Biomaterials
3.3. Gene Editing
3.4. Stem Cell Therapy
3.5. Rehabilitation and Physical Therapy
3.5.1. Treadmill Training
3.5.2. Photobiomodulation
3.5.3. Electrical Stimulation
3.5.4. Transcranial Stimulation
3.5.5. Sensory Stimulation
3.5.6. Summary of Rehabilitation and Physical Therapy After SCI
3.6. Comparative Analysis of Therapeutic Strategies Targeting Neuroplasticity
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sachdeva, R.; Gao, F.; Chan, C.C.H.; Krassioukov, A.V. Cognitive function after spinal cord injury: A systematic review. Neurology 2018, 91, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability from the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Krueger, H.; Noonan, V.K.; Trenaman, L.M.; Joshi, P.; Rivers, C.S. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis. Inj. Can. 2013, 33, 113–122. [Google Scholar] [PubMed]
- Diop, M.; Epstein, D.; Gaggero, A. Quality of life, health and social costs of patients with spinal cord injury: A systematic review. Eur. J. Public Health 2021, 31, ckab165.177. [Google Scholar]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review). Mol. Med. Rep. 2021, 23, 417. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Rooney, G.E.; Endo, T.; Ameenuddin, S.; Chen, B.; Vaishya, S.; Gross, L.; Schiefer, T.K.; Currier, B.L.; Spinner, R.J.; Yaszemski, M.J.; et al. Importance of the vasculature in cyst formation after spinal cord injury. J. Neurosurg. Spine 2009, 11, 432–437. [Google Scholar] [CrossRef]
- Shafqat, A.; Albalkhi, I.; Magableh, H.M.; Saleh, T.; Alkattan, K.; Yaqinuddin, A. Tackling the glial scar in spinal cord regeneration: New discoveries and future directions. Front. Cell. Neurosci. 2023, 17, 1180825. [Google Scholar] [CrossRef]
- Wang, D.; Sun, T. Neural plasticity and functional recovery of human central nervous system with special reference to spinal cord injury. Spinal Cord. 2011, 49, 486–492. [Google Scholar] [CrossRef]
- Ding, Y.; Kastin, A.J.; Pan, W. Neural plasticity after spinal cord injury. Curr. Pharm. Des. 2005, 11, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.D.; Harvey, L.A.; Bruce, J.A.; Somers, M.F. Compensation allows recovery of functional independence in people with severe motor impairments following spinal cord injury. J. Rehabil. Med. 2012, 44, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Pirrera, A.; Iannone, A.; Giansanti, D. Development and Use of Assistive Technologies in Spinal Cord Injury: A Narrative Review of Reviews on the Evolution, Opportunities, and Bottlenecks of Their Integration in the Health Domain. Healthcare 2023, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, K.; Wang, Y.; Ge, X.; Ma, Y.; Qin, J.; Zhang, C.; Zhao, Y.; Shi, C. Neurotrophins and neural stem cells in posttraumatic brain injury repair. Anim. Models Exp. Med. 2024, 7, 12–23. [Google Scholar] [CrossRef]
- Aguilar, J.; Humanes-Valera, D.; Alonso-Calviño, E.; Yague, J.G.; Moxon, K.A.; Oliviero, A.; Foffani, G. Spinal cord injury immediately changes the state of the brain. J. Neurosci. 2010, 30, 7528–7537. [Google Scholar] [CrossRef]
- Puderbaugh, M.; Emmady, P.D. Neuroplasticity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Galván, A. Neural plasticity of development and learning. Hum. Brain Mapp. 2010, 31, 879–890. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef]
- Moxon, K.A.; Oliviero, A.; Aguilar, J.; Foffani, G. Cortical reorganization after spinal cord injury: Always for good? Neuroscience 2014, 283, 78–94. [Google Scholar] [CrossRef]
- Lawal, O.; Ulloa Severino, F.P.; Eroglu, C. The role of astrocyte structural plasticity in regulating neural circuit function and behavior. Glia 2022, 70, 1467–1483. [Google Scholar] [CrossRef]
- Buzsáki, G. Neural syntax: Cell assemblies, synapsembles, and readers. Neuron 2010, 68, 362–385. [Google Scholar] [CrossRef]
- Bame, X.; Hill, R.A. Mitochondrial network reorganization and transient expansion during oligodendrocyte generation. Nat. Commun. 2024, 15, 6979. [Google Scholar] [CrossRef] [PubMed]
- Calderone, A.; Cardile, D.; De Luca, R.; Quartarone, A.; Corallo, F.; Calabrò, R.S. Brain Plasticity in Patients with Spinal Cord Injuries: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 2224. [Google Scholar] [CrossRef]
- Villiger, M.; Grabher, P.; Hepp-Reymond, M.C.; Kiper, D.; Curt, A.; Bolliger, M.; Hotz-Boendermaker, S.; Kollias, S.; Eng, K.; Freund, P. Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: A longitudinal pilot study. Front. Hum. Neurosci. 2015, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, A.N.; Selzer, M.E. Neuroplasticity in the spinal cord. Handb. Clin. Neurol. 2013, 110, 23–42. [Google Scholar] [CrossRef]
- Noble, B.T.; Brennan, F.H.; Wang, Y.; Guan, Z.; Mo, X.; Schwab, J.M.; Popovich, P.G. Thoracic VGluT2+ Spinal Interneurons Regulate Structural and Functional Plasticity of Sympathetic Networks after High-Level Spinal Cord Injury. J. Neurosci. 2022, 42, 3659–3675. [Google Scholar] [CrossRef]
- Bassett, D.S.; Sporns, O. Network neuroscience. Nat. Neurosci. 2017, 20, 353–364. [Google Scholar] [CrossRef]
- Trolle, C.; Goldberg, E.; Linnman, C. Spinal cord atrophy after spinal cord injury—A systematic review and meta-analysis. Neuroimage Clin. 2023, 38, 103372. [Google Scholar] [CrossRef]
- Jure, I.; Labombarda, F. Spinal cord injury drives chronic brain changes. Neural Regen. Res. 2017, 12, 1044–1047. [Google Scholar] [CrossRef]
- Ziegler, G.; Grabher, P.; Thompson, A.; Altmann, D.; Hupp, M.; Ashburner, J.; Friston, K.; Weiskopf, N.; Curt, A.; Freund, P. Progressive neurodegeneration following spinal cord injury: Implications for clinical trials. Neurology 2018, 90, e1257–e1266. [Google Scholar] [CrossRef]
- Buss, A.; Pech, K.; Merkler, D.; Kakulas, B.A.; Martin, D.; Schoenen, J.; Noth, J.; Schwab, M.E.; Brook, G.A. Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain 2005, 128, 356–364. [Google Scholar] [CrossRef]
- Lu, Q.; Botchway, B.O.A.; Zhang, Y.; Jin, T.; Liu, X. SARM1 can be a potential therapeutic target for spinal cord injury. Cell Mol. Life Sci. 2022, 79, 161. [Google Scholar] [CrossRef]
- Goltash, S.; Stevens, S.J.; Topcu, E.; Bui, T.V. Changes in synaptic inputs to dI3 INs and MNs after complete transection in adult mice. Front. Neural Circuits 2023, 17, 1176310. [Google Scholar] [CrossRef] [PubMed]
- Oudega, M.; Perez, M.A. Corticospinal reorganization after spinal cord injury. J. Physiol. 2012, 590, 3647–3663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Zhao, J.J.; Chai, W.; Chen, J.Y. Synaptic remodeling in mouse motor cortex after spinal cord injury. Neural Regen. Res. 2021, 16, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.C.; Gerber, Y.N.; Perrin, F.E. Dynamic Diversity of Glial Response Among Species in Spinal Cord Injury. Front. Aging Neurosci. 2021, 13, 769548. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef]
- Klemmensen, M.M.; Borrowman, S.H.; Pearce, C.; Pyles, B.; Chandra, B. Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 2024, 21, e00292. [Google Scholar] [CrossRef]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative Diseases—Is Metabolic Deficiency the Root Cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef]
- Marwaha, A.; Sachdeva, R.; Hunter, D.; Ramer, M.; Krassioukov, A.V. Spinal cord injury leads to atrophy in pelvic ganglia neurons. Exp. Neurol. 2020, 328, 113260. [Google Scholar] [CrossRef]
- Krassioukov, A.V.; Weaver, L.C. Morphological changes in sympathetic preganglionic neurons after spinal cord injury in rats. Neuroscience 1996, 70, 211–225. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Wu, Y.P.; Chen, W.S. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 2012, 135, 287–296. [Google Scholar] [PubMed]
- Liu, X.Z.; Xu, X.M.; Hu, R.; Du, C.; Zhang, S.X.; McDonald, J.W.; Dong, H.X.; Wu, Y.J.; Fan, G.S.; Jacquin, M.F.; et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997, 17, 5395–5406. [Google Scholar] [CrossRef] [PubMed]
- Slater, P.G.; Domínguez-Romero, M.E.; Villarreal, M.; Eisner, V.; Larraín, J. Mitochondrial function in spinal cord injury and regeneration. Cell Mol. Life Sci. 2022, 79, 239. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.R.; Marincu, B.N.; Sorbara, C.D.; Mahler, C.F.; Schumacher, A.M.; Griesbeck, O.; Kerschensteiner, M.; Misgeld, T. A recoverable state of axon injury persists for hours after spinal cord contusion In vivo. Nat. Commun. 2014, 5, 5683. [Google Scholar] [CrossRef]
- Noristani, H.N. Intrinsic regulation of axon regeneration after spinal cord injury: Recent advances and remaining challenges. Exp. Neurol. 2022, 357, 114198. [Google Scholar] [CrossRef]
- Tan, A.M.; Choi, J.S.; Waxman, S.G.; Hains, B.C. Dendritic spine remodeling after spinal cord injury alters neuronal signal processing. J. Neurophysiol. 2009, 102, 2396–2409. [Google Scholar] [CrossRef]
- Jacobi, A.; Bareyre, F.M. Regulation of axonal remodeling following spinal cord injury. Neural Regen. Res. 2015, 10, 1555–1557. [Google Scholar] [CrossRef]
- Debanne, D.; Campanac, E.; Bialowas, A.; Carlier, E.; Alcaraz, G. Axon physiology. Physiol. Rev. 2011, 91, 555–602. [Google Scholar] [CrossRef]
- Mittal, P.; Gupta, R.; Mittal, A.; Mittal, K. MRI findings in a case of spinal cord Wallerian degeneration following trauma. Neurosciences 2016, 21, 372–373. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Yousefifard, M.; Azizi, Y.; Zadegan, S.A.; Sajadi, K.; Sharif-Alhoseini, M.; Shakouri-Motlagh, A.; Mokhatab, M.; Rezvan, M.; Shokraneh, F.; et al. Axonal degeneration and demyelination following traumatic spinal cord injury: A systematic review and meta-analysis. J. Chem. Neuroanat. 2019, 97, 9–22. [Google Scholar] [CrossRef]
- Wu, W.; He, Y.; Chen, Y.; Fu, Y.; He, S.; Liu, K.; Qu, J.Y. Microglial-Mediated Prevention of Axonal Degeneration in the Injured Spinal Cord: Insights from an In Vivo Imaging Study. bioRxiv 2024. [Google Scholar] [CrossRef]
- De Vos, K.J.; Hafezparast, M. Neurobiology of axonal transport defects in motor neuron diseases: Opportunities for translational research? Neurobiol. Dis. 2017, 105, 283–299. [Google Scholar] [CrossRef]
- Seijffers, R.; Mills, C.D.; Woolf, C.J. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J. Neurosci. 2007, 27, 7911–7920. [Google Scholar] [CrossRef] [PubMed]
- Lindå, H.; Sköld, M.K.; Ochsmann, T. Activating transcription factor 3, a useful marker for regenerative response after nerve root injury. Front. Neurol. 2011, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Uyeda, A.; Quan, L.; Tanabe, S.; Kato, Y.; Kawahara, Y.; Muramatsu, R. Synaptotagmin 4 supports spontaneous axon sprouting after spinal cord injury. J. Neurosci. 2024, 44, e1593232024. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, Y.; Xiao, Z.; Chen, X.; Chen, B.; Yang, B.; Shu, M.; Yin, Y.; Wu, S.; Yin, W.; et al. Contralateral Axon Sprouting but Not Ipsilateral Regeneration Is Responsible for Spontaneous Locomotor Recovery Post Spinal Cord Hemisection. Front. Cell. Neurosci. 2021, 15, 730348. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jun, J.; Wang, J.; Bittar, A.; Chung, K.; Chung, J.M. Induction of long-term potentiation and long-term depression is cell-type specific in the spinal cord. Pain 2015, 156, 618–625. [Google Scholar] [CrossRef]
- Taylor, L.; Jones, L.; Tuszynski, M.H.; Blesch, A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J. Neurosci. 2006, 26, 9713–9721. [Google Scholar] [CrossRef]
- Jones, L.L.; Oudega, M.; Bunge, M.B.; Tuszynski, M.H. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J. Physiol. 2001, 533, 83–89. [Google Scholar] [CrossRef]
- Hollis, E.R., 2nd; Tuszynski, M.H. Neurotrophins: Potential therapeutic tools for the treatment of spinal cord injury. Neurotherapeutics 2011, 8, 694–703. [Google Scholar] [CrossRef]
- Lu, P.; Yang, H.; Jones, L.L.; Filbin, M.T.; Tuszynski, M.H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 2004, 24, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, A.E.; Marlow, M.M.; Oudega, M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci. Lett. 2017, 652, 50–55. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, N.; Pathak, Z.; Kumar, H. Extra Cellular Matrix Remodeling: An Adjunctive Target for Spinal Cord Injury and Intervertebral Disc Degeneration. Neurospine 2022, 19, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, X.; Yu, S.; Yan, F.; Chen, J.; Liu, J.; Dong, C. Decellularized extracellular matrix in the treatment of spinal cord injury. Exp. Neurol. 2023, 368, 114506. [Google Scholar] [CrossRef]
- Hirt, J.; Khanteymoori, A.; Hohenhaus, M.; Kopp, M.A.; Howells, D.W.; Schwab, J.M.; Watzlawick, R. Inhibition of the Nogo-pathway in experimental spinal cord injury: A meta-analysis of 76 experimental treatments. Sci. Rep. 2023, 13, 22898. [Google Scholar] [CrossRef]
- Walmsley, A.R.; Mir, A.K. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr. Pharm. Des. 2007, 13, 2470–2484. [Google Scholar] [CrossRef]
- Xiao, W.P.; Ding, L.L.; Min, Y.J.; Yang, H.Y.; Yao, H.H.; Sun, J.; Zhou, X.; Zeng, X.B.; Yu, W. Electroacupuncture Promoting Axonal Regeneration in Spinal Cord Injury Rats via Suppression of Nogo/NgR and Rho/ROCK Signaling Pathway. Neuropsychiatr. Dis. Treat. 2019, 15, 3429–3442. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front. Neurosci. 2014, 8, 338. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Hale, C.R.; Noble, N.; Luo, J.D.; Fak, J.J.; Saito, M.; Chen, J.; Weissman, J.S.; Darnell, R.B. Neuronal activity rapidly reprograms dendritic translation via eIF4G2:uORF binding. Nat. Neurosci. 2024, 27, 822–835. [Google Scholar] [CrossRef]
- Takata, Y.; Yamanaka, H.; Nakagawa, H.; Takada, M. Morphological changes of large layer V pyramidal neurons in cortical motor-related areas after spinal cord injury in macaque monkeys. Sci. Rep. 2023, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.A.; Reimer, M.L.; Tan, A.M. Dendritic Spines in the Spinal Cord: Live Action Pain. Neurosci. Insights 2020, 15, 2633105520951164. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.A.; Fenrich, K.K.; Olson, K.L.; Patwa, S.; Bangalore, L.; Waxman, S.G.; Tan, A.M. Dendritic Spine Dynamics after Peripheral Nerve Injury: An Intravital Structural Study. J. Neurosci. 2020, 40, 4297–4308. [Google Scholar] [CrossRef] [PubMed]
- Kyi, C.W.; Garcia, V.B.; Garcia, M.L.; Schulz, D.J. Spinal cord injury is associated with changes in synaptic properties of the mouse major pelvic ganglion. J. Neurophysiol. 2022, 128, 892–909. [Google Scholar] [CrossRef]
- Darian-Smith, C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist 2009, 15, 149–165. [Google Scholar] [CrossRef]
- Li, G.L.; Farooque, M.; Isaksson, J.; Olsson, Y. Changes in synapses and axons demonstrated by synaptophysin immunohistochemistry following spinal cord compression trauma in the rat and mouse. Biomed. Environ. Sci. 2004, 17, 281–290. [Google Scholar]
- Quraishe, S.; Forbes, L.H.; Andrews, M.R. The Extracellular Environment of the CNS: Influence on Plasticity, Sprouting, and Axonal Regeneration after Spinal Cord Injury. Neural Plast. 2018, 2018, 2952386. [Google Scholar] [CrossRef]
- Gomes-Leal, W.; Corkill, D.J.; Freire, M.A.; Picanço-Diniz, C.W.; Perry, V.H. Astrocytosis, microglia activation, oligodendrocyte degeneration, and pyknosis following acute spinal cord injury. Exp. Neurol. 2004, 190, 456–467. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, R.; Wu, L.; He, K.; Ma, R. Immunoregulation of Glia after spinal cord injury: A bibliometric analysis. Front. Immunol. 2024, 15, 1402349. [Google Scholar] [CrossRef]
- Wang, H.F.; Liu, X.K.; Li, R.; Zhang, P.; Chu, Z.; Wang, C.L.; Liu, H.R.; Qi, J.; Lv, G.Y.; Wang, G.Y.; et al. Effect of glial cells on remyelination after spinal cord injury. Neural Regen. Res. 2017, 12, 1724–1732. [Google Scholar] [CrossRef]
- Chang, L.; Yamamoto, N. Molecular mechanisms of lesion-induced axonal sprouting in the corticofugal projection: The role of glial cells. Neural Regen. Res. 2023, 18, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.; Janowitz, H.; Dougherty, S.E. Neuronal Redevelopment and the Regeneration of Neuromodulatory Axons in the Adult Mammalian Central Nervous System. Front. Cell. Neurosci. 2022, 16, 872501. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell. Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Risher, W.C.; Eroglu, C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012, 31, 170–177. [Google Scholar] [CrossRef]
- Risher, W.C.; Kim, N.; Koh, S.; Choi, J.E.; Mitev, P.; Spence, E.F.; Pilaz, L.J.; Wang, D.; Feng, G.; Silver, D.L.; et al. Thrombospondin receptor α2δ-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. J. Cell Biol. 2018, 217, 3747–3765. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef]
- Misgeld, T.; Kummer, T.T.; Lichtman, J.W.; Sanes, J.R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. USA 2005, 102, 11088–11093. [Google Scholar] [CrossRef]
- Daniels, M.P. The role of agrin in synaptic development, plasticity and signaling in the central nervous system. Neurochem. Int. 2012, 61, 848–853. [Google Scholar] [CrossRef]
- Lin, P.Y.; Kavalali, E.T.; Monteggia, L.M. Genetic Dissection of Presynaptic and Postsynaptic BDNF-TrkB Signaling in Synaptic Efficacy of CA3-CA1 Synapses. Cell Rep. 2018, 24, 1550–1561. [Google Scholar] [CrossRef]
- Han, Q.; Xu, X.M. Neurotrophin-3-mediated locomotor recovery: A novel therapeutic strategy targeting lumbar neural circuitry after spinal cord injury. Neural Regen. Res. 2020, 15, 2241–2242. [Google Scholar] [CrossRef] [PubMed]
- Sendtner, M.; Arakawa, Y.; Stöckli, K.A.; Kreutzberg, G.W.; Thoenen, H. Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival. J. Cell Sci. Suppl. 1991, 15, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 665795. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research-Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Elmalky, M.I.; Alvarez-Bolado, G.; Younsi, A.; Skutella, T. Axonal Regeneration after Spinal Cord Injury: Molecular Mechanisms, Regulatory Pathways, and Novel Strategies. Biology 2024, 13, 703. [Google Scholar] [CrossRef]
- Lin, B.; Xu, Y.; Zhang, B.; He, Y.; Yan, Y.; He, M.C. MEK inhibition reduces glial scar formation and promotes the recovery of sensorimotor function in rats following spinal cord injury. Exp. Ther. Med. 2014, 7, 66–72. [Google Scholar] [CrossRef]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef]
- Zhao, W.; Gasterich, N.; Clarner, T.; Voelz, C.; Behrens, V.; Beyer, C.; Fragoulis, A.; Zendedel, A. Astrocytic Nrf2 expression protects spinal cord from oxidative stress following spinal cord injury in a male mouse model. J. Neuroinflamm. 2022, 19, 134. [Google Scholar] [CrossRef]
- Duncan, G.J.; Manesh, S.B.; Hilton, B.J.; Assinck, P.; Plemel, J.R.; Tetzlaff, W. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia 2020, 68, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Almad, A.; Sahinkaya, F.R.; McTigue, D.M. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics 2011, 8, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Angulo, M.C. Unraveling the role of oligodendrocytes and myelin in pain. J. Neurochem. 2025, 169, e16206. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Leung, G.K. Oligodendrocyte Precursor Cells in Spinal Cord Injury: A Review and Update. Biomed. Res. Int. 2015, 2015, 235195. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Y.; Ren, Y.; Yin, S.; Yu, L.; Huang, R.; Song, S.; Hu, X.; Zhu, R.; Cheng, L.; et al. Neurogenesis potential of oligodendrocyte precursor cells from oligospheres and injured spinal cord. Front. Cell. Neurosci. 2022, 16, 1049562. [Google Scholar] [CrossRef]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef]
- Duncan, G.J.; Simkins, T.J.; Emery, B. Neuron-Oligodendrocyte Interactions in the Structure and Integrity of Axons. Front. Cell Dev. Biol. 2021, 9, 653101. [Google Scholar] [CrossRef]
- Philips, T.; Mironova, Y.A.; Jouroukhin, Y.; Chew, J.; Vidensky, S.; Farah, M.H.; Pletnikov, M.V.; Bergles, D.E.; Morrison, B.M.; Rothstein, J.D. MCT1 Deletion in Oligodendrocyte Lineage Cells Causes Late-Onset Hypomyelination and Axonal Degeneration. Cell Rep. 2021, 34, 108610. [Google Scholar] [CrossRef]
- Tai, W.; Du, X.; Chen, C.; Xu, X.M.; Zhang, C.L.; Wu, W. NG2 glia reprogramming induces robust axonal regeneration after spinal cord injury. iScience 2024, 27, 108895. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, C.Y.; Lee, H.J.; Kim, J.G.; Han, D.W.; Ko, K.; Walter, J.; Chung, H.M.; Schöler, H.R.; Bae, Y.M.; et al. Two-Step Generation of Oligodendrocyte Progenitor Cells from Mouse Fibroblasts for Spinal Cord Injury. Front. Cell. Neurosci. 2018, 12, 198. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Ding, Y.; Wang, L.; Zhu, Y.J. Current Knowledge of Microglia in Traumatic Spinal Cord Injury. Front. Neurol. 2021, 12, 796704. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.H.; Li, Y.; Wang, C.; Ma, A.; Guo, Q.; Li, Y.; Pukos, N.; Campbell, W.A.; Witcher, K.G.; Guan, Z.; et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 2022, 13, 4096. [Google Scholar] [CrossRef]
- Brennan, F.H.; Swarts, E.A.; Kigerl, K.A.; Mifflin, K.A.; Guan, Z.; Noble, B.T.; Wang, Y.; Witcher, K.G.; Godbout, J.P.; Popovich, P.G. Microglia promote maladaptive plasticity in autonomic circuitry after spinal cord injury in mice. Sci. Transl. Med. 2024, 16, eadi3259. [Google Scholar] [CrossRef]
- Lannes, N.; Eppler, E.; Etemad, S.; Yotovski, P.; Filgueira, L. Microglia at center stage: A comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget 2017, 8, 114393–114413. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Zha, X.; Zheng, G.; Skutella, T.; Kiening, K.; Unterberg, A.; Younsi, A. Microglia: A promising therapeutic target in spinal cord injury. Neural Regen. Res. 2025, 20, 454–463. [Google Scholar] [CrossRef]
- Bedolla, A.; Wegman, E.; Weed, M.; Stevens, M.K.; Ware, K.; Paranjpe, A.; Alkhimovitch, A.; Ifergan, I.; Taranov, A.; Peter, J.D.; et al. Adult microglial TGFβ1 is required for microglia homeostasis via an autocrine mechanism to maintain cognitive function in mice. Nat. Commun. 2024, 15, 5306. [Google Scholar] [CrossRef]
- Yu, H.; Yang, S.; Li, H.; Wu, R.; Lai, B.; Zheng, Q. Activating endogenous neurogenesis for spinal cord injury repair: Recent advances and future prospects. Neurospine 2023, 20, 164–180. [Google Scholar] [CrossRef]
- Muheremu, A.; Shu, L.; Liang, J.; Aili, A.; Jiang, K. Sustained delivery of neurotrophic factors to treat spinal cord injury. Transl. Neurosci. 2021, 12, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Patodia, S.; Raivich, G. Role of transcription factors in peripheral nerve regeneration. Front. Mol. Neurosci. 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Vartak, A.; Goyal, D.; Kumar, H. Role of Axon Guidance Molecules in Ascending and Descending Paths in Spinal Cord Regeneration. Neuroscience 2023, 533, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Hollis, E.R., 2nd. Axon Guidance Molecules and Neural Circuit Remodeling After Spinal Cord Injury. Neurotherapeutics 2016, 13, 360–369. [Google Scholar] [CrossRef]
- Boyce, V.S.; Mendell, L.M. Neurotrophins and spinal circuit function. Front. Neural Circuits 2014, 8, 59. [Google Scholar] [CrossRef]
- Schirò, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef]
- Revest, J.M.; Le Roux, A.; Roullot-Lacarrière, V.; Kaouane, N.; Vallée, M.; Kasanetz, F.; Rougé-Pont, F.; Tronche, F.; Desmedt, A.; Piazza, P.V. BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol. Psychiatry 2014, 19, 1001–1009. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. Involvement of brain-derived neurotrophic factor signaling in the pathogenesis of stress-related brain diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [Google Scholar] [CrossRef]
- Tom, V.J.; Sandrow-Feinberg, H.R.; Miller, K.; Domitrovich, C.; Bouyer, J.; Zhukareva, V.; Klaw, M.C.; Lemay, M.A.; Houlé, J.D. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp. Neurol. 2013, 239, 91–100. [Google Scholar] [CrossRef]
- McCall, J.; Weidner, N.; Blesch, A. Neurotrophic factors in combinatorial approaches for spinal cord regeneration. Cell Tissue Res. 2012, 349, 27–37. [Google Scholar] [CrossRef]
- Rao, J.S.; Zhao, C.; Zhang, A.; Duan, H.; Hao, P.; Wei, R.H.; Shang, J.; Zhao, W.; Liu, Z.; Yu, J.; et al. NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc. Natl. Acad. Sci. USA 2018, 115, E5595–E5604. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Mol, P.; Balaya, R.D.A.; Dagamajalu, S.; Babu, S.; Chandrasekaran, P.; Raghavan, R.; Suresh, S.; Ravishankara, N.; Raju, A.H.; Nair, B.; et al. A network map of GDNF/RET signaling pathway in physiological and pathological conditions. J. Cell Commun. Signal. 2023, 17, 1089–1095. [Google Scholar] [CrossRef]
- Ryge, J.; Winther, O.; Wienecke, J.; Sandelin, A.; Westerdahl, A.C.; Hultborn, H.; Kiehn, O. Transcriptional regulation of gene expression clusters in motor neurons following spinal cord injury. BMC Genom. 2010, 11, 365. [Google Scholar] [CrossRef]
- Moore, D.L.; Goldberg, J.L. Multiple transcription factor families regulate axon growth and regeneration. Dev. Neurobiol. 2011, 71, 1186–1211. [Google Scholar] [CrossRef]
- Avraham, O.; Le, J.; Leahy, K.; Li, T.; Zhao, G.; Cavalli, V. Analysis of neuronal injury transcriptional response identifies CTCF and YY1 as co-operating factors regulating axon regeneration. Front. Mol. Neurosci. 2022, 15, 967472. [Google Scholar] [CrossRef]
- Yu, B.; Yao, C.; Wang, Y.; Mao, S.; Wang, Y.; Wu, R.; Feng, W.; Chen, Y.; Yang, J.; Xue, C.; et al. The Landscape of Gene Expression and Molecular Regulation Following Spinal Cord Hemisection in Rats. Front. Mol. Neurosci. 2019, 12, 287. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, L.; Cristofanilli, M.; Hart, R.P.; Hao, A.; Schachner, M. Transcription factor Sox11b is involved in spinal cord regeneration in adult zebrafish. Neuroscience 2011, 172, 329–341. [Google Scholar] [CrossRef]
- Chen, G.; Fang, X.; Yu, M. Regulation of gene expression in rats with spinal cord injury based on microarray data. Mol. Med. Rep. 2015, 12, 2465–2472. [Google Scholar] [CrossRef]
- Cui, S.Y.; Zhang, W.; Cui, Z.M.; Yi, H.; Xu, D.W.; Liu, W.; Zhu, X.H. Knockdown of long non-coding RNA LEF1-AS1 attenuates apoptosis and inflammatory injury of microglia cells following spinal cord injury. J. Orthop. Surg. Res. 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Han, K.; Hyun, J.K. The Time Sequence of Gene Expression Changes after Spinal Cord Injury. Cells 2022, 11, 2236. [Google Scholar] [CrossRef]
- Venkatesh, I.; Mehra, V.; Wang, Z.; Simpson, M.T.; Eastwood, E.; Chakraborty, A.; Beine, Z.; Gross, D.; Cabahug, M.; Olson, G.; et al. Co-occupancy identifies transcription factor co-operation for axon growth. Nat. Commun. 2021, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Attwell, C.L.; Maldonado-Lasunción, I.; Eggers, R.; Bijleveld, B.A.; Ellenbroek, W.M.; Siersema, N.; Razenberg, L.; Lamme, D.; Fagoe, N.D.; van Kesteren, R.E.; et al. The transcription factor combination MEF2 and KLF7 promotes axonal sprouting in the injured spinal cord with functional improvement and regeneration-associated gene expression. Mol. Neurodegener. 2025, 20, 18. [Google Scholar] [CrossRef]
- Shi, Z.; Fang, T.; Fan, B.; Ma, J.; Wang, J.; Feng, S. Analysis and experimental validation of genes and their transcription factor prediction in contused rat spinal cord at the intermediate phase. Aging 2024, 16, 9990–10003. [Google Scholar] [CrossRef]
- Zou, Y. Targeting axon guidance cues for neural circuit repair after spinal cord injury. J. Cereb. Blood Flow. Metab. 2021, 41, 197–205. [Google Scholar] [CrossRef]
- Goldshmit, Y.; McLenachan, S.; Turnley, A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res. Rev. 2006, 52, 327–345. [Google Scholar] [CrossRef]
- Geoffroy, C.G.; Hilton, B.J.; Tetzlaff, W.; Zheng, B. Evidence for an Age-Dependent Decline in Axon Regeneration in the Adult Mammalian Central Nervous System. Cell Rep. 2016, 15, 238–246. [Google Scholar] [CrossRef]
- Scivoletto, G.; Morganti, B.; Ditunno, P.; Ditunno, J.F.; Molinari, M. Effects on age on spinal cord lesion patients’ rehabilitation. Spinal Cord 2003, 41, 457–464. [Google Scholar] [CrossRef]
- Liu, H.K. Human adult hippocampal neurogenesis is back, again? Cell Res. 2022, 32, 793–794. [Google Scholar] [CrossRef]
- Surget, A.; Belzung, C. Adult hippocampal neurogenesis shapes adaptation and improves stress response: A mechanistic and integrative perspective. Mol. Psychiatry 2022, 27, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. New neurons are born in the adult human brain. Nat. Med. 2025, 31, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e585. [Google Scholar] [CrossRef]
- Sutherland, T.C.; Geoffroy, C.G. The Influence of Neuron-Extrinsic Factors and Aging on Injury Progression and Axonal Repair in the Central Nervous System. Front. Cell Dev. Biol. 2020, 8, 190. [Google Scholar] [CrossRef]
- Vanacore, G.; Christensen, J.B.; Bayin, N.S. Age-dependent regenerative mechanisms in the brain. Biochem. Soc. Trans. 2024, 52, 2243–2252. [Google Scholar] [CrossRef]
- Mellado, W.; Willis, D.E. Ribosomal S6 kinases determine intrinsic axonal regeneration capacity. PLoS Biol. 2023, 21, e3002094. [Google Scholar] [CrossRef]
- Stewart, A.N.; Jones, L.A.T.; Gensel, J.C. Improving translatability of spinal cord injury research by including age as a demographic variable. Front. Cell. Neurosci. 2022, 16, 1017153. [Google Scholar] [CrossRef]
- Thomas, C.K.; Grumbles, R.M. Age at spinal cord injury determines muscle strength. Front. Integr. Neurosci. 2014, 8, 2. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022, 387, 319–336. [Google Scholar] [CrossRef]
- Curt, A.; Van Hedel, H.J.; Klaus, D.; Dietz, V. Recovery from a spinal cord injury: Significance of compensation, neural plasticity, and repair. J. Neurotrauma 2008, 25, 677–685. [Google Scholar] [CrossRef]
- Beauparlant, J.; van den Brand, R.; Barraud, Q.; Friedli, L.; Musienko, P.; Dietz, V.; Courtine, G. Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain 2013, 136, 3347–3361. [Google Scholar] [CrossRef] [PubMed]
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.; Lukas, L.P.; Kondiles, B.R.; Tong, B.; Lee, J.J.; Gomes, T.; Tetzlaff, W.; Kramer, J.L.K.; Walter, M.; Jutzeler, C.R. Impact of commonly administered drugs on the progression of spinal cord injury: A systematic review. Commun. Med. 2024, 4, 213. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; He, L.; Pang, M.; Luo, C.; Liu, B.; Rong, L. High-dose methylprednisolone for acute traumatic spinal cord injury: A meta-analysis. Neurology 2019, 93, e841–e850. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, K.H.; Yoon, D.H.; Kim, U.J.; Hwang, Y.S.; Park, S.K.; Choi, J.U.; Park, Y.G. Effects of methylprednisolone on the neural conduction of the motor evoked potentials in spinal cord injured rats. J. Korean Med. Sci. 2005, 20, 132–138. [Google Scholar] [CrossRef]
- Chvatal, S.A.; Kim, Y.T.; Bratt-Leal, A.M.; Lee, H.; Bellamkonda, R.V. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials 2008, 29, 1967–1975. [Google Scholar] [CrossRef]
- Bracken, M.B. Steroids for acute spinal cord injury. Cochrane Database Syst. Rev. 2012, 1, Cd001046. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.X.; Zhang, Y.J.; Liu, Y.D.; Liu, Z.J.; Wu, Q.C.; Guan, Y.; Chen, X.M. Melatonin for the treatment of spinal cord injury. Neural Regen. Res. 2018, 13, 1685–1692. [Google Scholar] [CrossRef]
- Xie, L.; Wu, H.; Huang, X.; Yu, T. Melatonin, a natural antioxidant therapy in spinal cord injury. Front. Cell Dev. Biol. 2023, 11, 1218553. [Google Scholar] [CrossRef]
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Curr. Neuropharmacol. 2016, 14, 641–653. [Google Scholar] [CrossRef]
- Chaovipoch, P.; Jelks, K.A.; Gerhold, L.M.; West, E.J.; Chongthammakun, S.; Floyd, C.L. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J. Neurotrauma 2006, 23, 830–852. [Google Scholar] [CrossRef]
- Letaif, O.B.; Cristante, A.F.; Barros Filho, T.E.; Ferreira, R.; Santos, G.B.; Rocha, I.D.; Marcon, R.M. Effects of estrogen on functional and neurological recovery after spinal cord injury: An experimental study with rats. Clinics 2015, 70, 700–705. [Google Scholar] [CrossRef]
- Nazli, Y.; Colak, N.; Alpay, M.F.; Uysal, S.; Uzunlar, A.K.; Cakir, O. Neuroprotective effect of atorvastatin in spinal cord ischemia-reperfusion injury. Clinics 2015, 70, 52–60. [Google Scholar] [CrossRef]
- Evaniew, N.; Noonan, V.K.; Fallah, N.; Kwon, B.K.; Rivers, C.S.; Ahn, H.; Bailey, C.S.; Christie, S.D.; Fourney, D.R.; Hurlbert, R.J.; et al. Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Propensity Score-Matched Cohort Study from a Canadian Multi-Center Spinal Cord Injury Registry. J. Neurotrauma 2015, 32, 1674–1683. [Google Scholar] [CrossRef]
- Sultan, I.; Lamba, N.; Liew, A.; Doung, P.; Tewarie, I.; Amamoo, J.J.; Gannu, L.; Chawla, S.; Doucette, J.; Cerecedo-Lopez, C.D.; et al. The safety and efficacy of steroid treatment for acute spinal cord injury: A Systematic Review and meta-analysis. Heliyon 2020, 6, e03414. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Patil, A.A.; Chamczuk, A.J.; Agrawal, D.K. Hormonal therapy in traumatic spinal cord injury. Am. J. Transl. Res. 2017, 9, 3881–3895. [Google Scholar]
- Khong, S.; Savic, G.; Gardner, B.P.; Ashworth, F. Hormone replacement therapy in women with spinal cord injury—A survey with literature review. Spinal Cord 2005, 43, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, J.; Samadi Motlagh, P.; Salehpour, F.; Meshkini, A.; Fatehi, M.; Mirzaei, F.; Naseri Alavi, S.A. Effects of Atorvastatin in Patients with Acute Spinal Cord Injury. Asian Spine J. 2017, 11, 903–907. [Google Scholar] [CrossRef]
- Triplet, E.M.; Scarisbrick, I.A. Statin use is associated with reduced motor recovery after spinal cord injury. Spinal Cord Ser. Cases 2021, 7, 8. [Google Scholar] [CrossRef]
- Chen, K.; Yu, W.; Zheng, G.; Xu, Z.; Yang, C.; Wang, Y.; Yue, Z.; Yuan, W.; Hu, B.; Chen, H. Biomaterial-based regenerative therapeutic strategies for spinal cord injury. NPG Asia Mater. 2024, 16, 5. [Google Scholar]
- Gao, Y.; Wang, Y.; Wu, Y.; Liu, S. Biomaterials targeting the microenvironment for spinal cord injury repair: Progression and perspectives. Front. Cell. Neurosci. 2024, 18, 1362494. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Bao, Q.; Saijilahu; Chimedtseren, C.; Tumurbaatar, K.; Saijilafu. Research Progress on Biomaterials for Spinal Cord Repair. Int. J. Nanomed. 2025, 20, 1773–1787. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Y.Y.; Wang, B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res. 2019, 14, 1352–1363. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, A.; Duan, H.; Zhang, S.; Hao, P.; Ye, K.; Sun, Y.E.; Li, X. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2015, 112, 13354–13359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Rao, J.S.; Duan, H.; Hao, P.; Shang, J.; Fan, Y.; Zhao, W.; Gao, Y.; Yang, Z.; Sun, Y.E.; et al. Chronic spinal cord injury repair by NT3-chitosan only occurs after clearance of the lesion scar. Signal Transduct. Target. Ther. 2022, 7, 184. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, X.; Xiao, Z.; Yao, Y.; Chu, Y.; Farkas, B.; Romano, I.; Brandi, F.; Dai, J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Healthc. Mater. 2018, 7, e1800315. [Google Scholar] [CrossRef]
- Feng, C.; Deng, L.; Yong, Y.-Y.; Wu, J.-M.; Qin, D.-L.; Yu, L.; Zhou, X.-G.; Wu, A.-G. The application of biomaterials in spinal cord injury. Int. J. Mol. Sci. 2023, 24, 816. [Google Scholar] [CrossRef]
- Zeng, C.W.; Zhang, C.L. Neuronal regeneration after injury: A new perspective on gene therapy. Front. Neurosci. 2023, 17, 1181816. [Google Scholar] [CrossRef]
- Aljović, A.; Jacobi, A.; Marcantoni, M.; Kagerer, F.; Loy, K.; Kendirli, A.; Bräutigam, J.; Fabbio, L.; Van Steenbergen, V.; Pleśniar, K.; et al. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol. Med. 2023, 15, e16111. [Google Scholar] [CrossRef]
- Paschon, V.; Correia, F.F.; Morena, B.C.; da Silva, V.A.; Dos Santos, G.B.; da Silva, M.C.C.; Cristante, A.F.; Willerth, S.M.; Perrin, F.E.; Kihara, A.H. CRISPR, Prime Editing, Optogenetics, and DREADDs: New Therapeutic Approaches Provided by Emerging Technologies in the Treatment of Spinal Cord Injury. Mol. Neurobiol. 2020, 57, 2085–2100. [Google Scholar] [CrossRef]
- Metcalfe, M.; Steward, O. PTEN deletion in spinal pathways via retrograde transduction with AAV-RG enhances forelimb motor recovery after cervical spinal cord injury; Sex differences and late-onset pathophysiologies. Exp. Neurol. 2023, 370, 114551. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, G.; Chuang, H.; Chiu, C.; Abdelmaksoud, A.; Ye, Y.; Zhao, L. Portrait of glial scar in neurological diseases. Int. J. Immunopathol. Pharmacol. 2018, 31, 2058738418801406. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. Immune response following traumatic spinal cord injury: Pathophysiology and therapies. Front. Immunol. 2022, 13, 1084101. [Google Scholar] [CrossRef]
- Freyermuth-Trujillo, X.; Segura-Uribe, J.J.; Salgado-Ceballos, H.; Orozco-Barrios, C.E.; Coyoy-Salgado, A. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells 2022, 11, 2692. [Google Scholar] [CrossRef]
- Stewart, A.N.; Gensel, J.C.; Jones, L.; Fouad, K. Challenges in Translating Regenerative Therapies for Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2023, 29, 23–43. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, S.; Yang, M. Recent progress and challenges in the treatment of spinal cord injury. Protein Cell 2023, 14, 635–652. [Google Scholar] [CrossRef]
- Islam, A.; Tom, V.J. The use of viral vectors to promote repair after spinal cord injury. Exp. Neurol. 2022, 354, 114102. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Viskontas, M.; Janowicz, K.; Sani, Y.; Håkansson, M.E.; Heidari, A.; Huang, W.; Bo, X. The potential of gene therapies for spinal cord injury repair: A systematic review and meta-analysis of pre-clinical studies. Neural Regen. Res. 2023, 18, 299–305. [Google Scholar] [CrossRef]
- Zeng, C.W. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30, 963689721989266. [Google Scholar] [CrossRef]
- Suzuki, H.; Imajo, Y.; Funaba, M.; Nishida, N.; Sakamoto, T.; Sakai, T. Current Concepts of Neural Stem/Progenitor Cell Therapy for Chronic Spinal Cord Injury. Front. Cell. Neurosci. 2021, 15, 794692. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, H.; Joo, K.M.; Lee, S.H. Advances in Neural Stem Cell Therapy for Spinal Cord Injury: Safety, Efficacy, and Future Perspectives. Neurospine 2022, 19, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Zheng, J.; Kim, I.Y.; Kang, P.J.; Park, K.; Priscilla, L.; Hong, W.; Yoon, B.S.; Park, G.; Yoo, J.E.; et al. Human induced neural stem cells support functional recovery in spinal cord injury models. Exp. Mol. Med. 2023, 55, 1182–1192. [Google Scholar] [CrossRef]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef]
- Shinozaki, M.; Nagoshi, N.; Nakamura, M.; Okano, H. Mechanisms of Stem Cell Therapy in Spinal Cord Injuries. Cells 2021, 10, 2676. [Google Scholar] [CrossRef]
- Głowacka, A.; Ji, B.; Szczepankiewicz, A.A.; Skup, M.; Gajewska-Woźniak, O. BDNF Spinal Overexpression after Spinal Cord Injury Partially Protects Soleus Neuromuscular Junction from Disintegration, Increasing VAChT and AChE Transcripts in Soleus but Not Tibialis Anterior Motoneurons. Biomedicines 2022, 10, 2851. [Google Scholar] [CrossRef]
- Punjani, N.; Deska-Gauthier, D.; Hachem, L.D.; Abramian, M.; Fehlings, M.G. Neuroplasticity and regeneration after spinal cord injury. N. Am. Spine Soc. J. 2023, 15, 100235. [Google Scholar] [CrossRef]
- Jagrit, V.; Koffler, J.; Dulin, J.N. Combinatorial strategies for cell transplantation in traumatic spinal cord injury. Front. Neurosci. 2024, 18, 1349446. [Google Scholar] [CrossRef]
- Tashiro, S.; Nishimura, S.; Iwai, H.; Sugai, K.; Zhang, L.; Shinozaki, M.; Iwanami, A.; Toyama, Y.; Liu, M.; Okano, H.; et al. Functional Recovery from Neural Stem/Progenitor Cell Transplantation Combined with Treadmill Training in Mice with Chronic Spinal Cord Injury. Sci. Rep. 2016, 6, 30898. [Google Scholar] [CrossRef]
- Bydon, M.; Qu, W.; Moinuddin, F.M.; Hunt, C.L.; Garlanger, K.L.; Reeves, R.K.; Windebank, A.J.; Zhao, K.D.; Jarrah, R.; Trammell, B.C.; et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial. Nat. Commun. 2024, 15, 2201. [Google Scholar] [CrossRef]
- Muheremu, A.; Wu, J. Restoring neural circuits after spinal cord injury. Front. Mol. Neurosci. 2024, 17, 1428164. [Google Scholar] [CrossRef]
- Khan, S.I.; Ahmed, N.; Ahsan, K.; Abbasi, M.; Maugeri, R.; Chowdhury, D.; Bonosi, L.; Brunasso, L.; Costanzo, R.; Iacopino, D.G.; et al. An Insight into the Prospects and Drawbacks of Stem Cell Therapy for Spinal Cord Injuries: Ongoing Trials and Future Directions. Brain Sci. 2023, 13, 1697. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, J.; Yang, R.; Wang, H.; Li, Y.; Fu, C. Mesenchymal stem cells in the treatment of spinal cord injury: Mechanisms, current advances and future challenges. Front. Immunol. 2023, 14, 1141601. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.A.; Pedro, K.M.; Quddusi, A.I.; Fehlings, M.G. Advances and Challenges in Spinal Cord Injury Treatments. J. Clin. Med. 2024, 13, 4101. [Google Scholar] [CrossRef]
- Hicks, A.L.; Martin Ginis, K.A.; Pelletier, C.A.; Ditor, D.S.; Foulon, B.; Wolfe, D.L. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: A systematic review. Spinal Cord 2011, 49, 1103–1127. [Google Scholar] [CrossRef]
- Ben, M.; Glinsky, J.V.; Chu, J.; Spooren, A.I.; Roberts, S.; Chen, L.W.; Denis, S.; Lorusso, M.; Jorgensen, V.; Gollan, E.J.; et al. Early and intensive Motor Training for people with spinal cord injuries (the SCI-MT Trial): Description of the intervention. Spinal Cord 2023, 61, 600–607. [Google Scholar] [CrossRef]
- Nas, K.; Yazmalar, L.; Şah, V.; Aydın, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef]
- Duan, R.; Qu, M.; Yuan, Y.; Lin, M.; Liu, T.; Huang, W.; Gao, J.; Zhang, M.; Yu, X. Clinical Benefit of Rehabilitation Training in Spinal Cord Injury: A Systematic Review and Meta-Analysis. Spine 2021, 46, E398–E410. [Google Scholar] [CrossRef]
- Sun, T.; Ye, C.; Wu, J.; Zhang, Z.; Cai, Y.; Yue, F. Treadmill step training promotes spinal cord neural plasticity after incomplete spinal cord injury. Neural Regen. Res. 2013, 8, 2540–2547. [Google Scholar] [CrossRef]
- Wang, H.; Liu, N.K.; Zhang, Y.P.; Deng, L.; Lu, Q.B.; Shields, C.B.; Walker, M.J.; Li, J.; Xu, X.M. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Exp. Neurol. 2015, 271, 368–378. [Google Scholar] [CrossRef]
- Frood, R.T. The use of treadmill training to recover locomotor ability in patients with spinal cord injury. Biosci. Horiz. Int. J. Stud. Res. 2011, 4, 108–117. [Google Scholar] [CrossRef]
- Pelletier, C. Exercise prescription for persons with spinal cord injury: A review of physiological considerations and evidence-based guidelines. Appl. Physiol. Nutr. Metab. 2023, 48, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Patathong, T.; Klaewkasikum, K.; Woratanarat, P.; Rattanasiri, S.; Anothaisintawee, T.; Woratanarat, T.; Thakkinstian, A. The efficacy of gait rehabilitations for the treatment of incomplete spinal cord injury: A systematic review and network meta-analysis. J. Orthop. Surg. Res. 2023, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.L.; Ginis, K.A. Treadmill training after spinal cord injury: It’s not just about the walking. J. Rehabil. Res. Dev. 2008, 45, 241–248. [Google Scholar] [CrossRef]
- Giangregorio, L.M.; Hicks, A.L.; Webber, C.E.; Phillips, S.M.; Craven, B.C.; Bugaresti, J.M.; McCartney, N. Body weight supported treadmill training in acute spinal cord injury: Impact on muscle and bone. Spinal Cord 2005, 43, 649–657. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, Y.W.; Lee, S.J.; Shin, J.C. Robot-Assisted Gait Training in Individuals with Spinal Cord Injury: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Rehabil. Med. 2024, 48, 171–191. [Google Scholar] [CrossRef]
- Hornby, T.G.; Zemon, D.H.; Campbell, D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys. Ther. 2005, 85, 52–66. [Google Scholar]
- Alashram, A.R.; Annino, G.; Padua, E. Robot-assisted gait training in individuals with spinal cord injury: A systematic review for the clinical effectiveness of Lokomat. J. Clin. Neurosci. 2021, 91, 260–269. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Lythgo, N.; Galea, M.P.; Turnley, A.M. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J. Neurotrauma 2008, 25, 449–465. [Google Scholar] [CrossRef]
- De Leon, R.D.; Hodgson, J.A.; Roy, R.R.; Edgerton, V.R. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J. Neurophysiol. 1999, 81, 85–94. [Google Scholar] [CrossRef]
- Ramezani, F.; Razmgir, M.; Tanha, K.; Nasirinezhad, F.; Neshastehriz, A.; Bahrami-Ahmadi, A.; Hamblin, M.R.; Janzadeh, A. Photobiomodulation for spinal cord injury: A systematic review and meta-analysis. Physiol. Behav. 2020, 224, 112977. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Ma, Y.G.; Zuo, X.S.; Wang, X.K.; Song, Z.W.; Zhang, Z.H.; Zhu, Z.J.; Li, X.; Liang, Z.W.; Ding, T.; et al. Potential targets and mechanisms of photobiomodulation for spinal cord injury. Neural Regen. Res. 2023, 18, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.R.; Hadis, M.; Alldrit, H.; Milward, M.R.; Di Pietro, V.; Gendoo, D.M.A.; Belli, A.; Palin, W.; Davies, D.J.; Ahmed, Z. Evaluation of transcriptomic changes after photobiomodulation in spinal cord injury. Sci. Rep. 2025, 15, 3193. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Zhu, Z.; Liang, Z.; Zuo, X.; Ju, C.; Song, Z.; Li, X.; Hu, X.; Wang, Z. Photobiomodulation Promotes Repair Following Spinal Cord Injury by Regulating the Transformation of A1/A2 Reactive Astrocytes. Front. Neurosci. 2021, 15, 768262. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, X.; Song, Z.; Zuo, X.; Ma, Y.; Zhang, Z.; Ju, C.; Liang, Z.; Li, K.; Hu, X.; et al. Photobiomodulation promotes repair following spinal cord injury by restoring neuronal mitochondrial bioenergetics via AMPK/PGC-1α/TFAM pathway. Front. Pharmacol. 2022, 13, 991421. [Google Scholar] [CrossRef]
- da Cruz Tobelem, D.; Silva, T.; Araujo, T.; Andreo, L.; Malavazzi, T.; Horliana, A.; Fernandes, K.P.S.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of photobiomodulation in experimental spinal cord injury models: A systematic review. J. Biophotonics 2022, 15, e202200059. [Google Scholar] [CrossRef]
- Stevens, A.R.; Hadis, M.; Phillips, A.; Thareja, A.; Milward, M.; Belli, A.; Palin, W.; Davies, D.J.; Ahmed, Z. Implantable and transcutaneous photobiomodulation promote neuroregeneration and recovery of lost function after spinal cord injury. Bioeng. Transl. Med. 2024, 9, e10674. [Google Scholar] [CrossRef]
- Dorrian, R.M.; Berryman, C.F.; Lauto, A.; Leonard, A.V. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front. Cell. Neurosci. 2023, 17, 1095259. [Google Scholar] [CrossRef]
- Ho, C.H.; Triolo, R.J.; Elias, A.L.; Kilgore, K.L.; DiMarco, A.F.; Bogie, K.; Vette, A.H.; Audu, M.L.; Kobetic, R.; Chang, S.R.; et al. Functional electrical stimulation and spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 631. [Google Scholar] [CrossRef]
- Kanakis, A.K.; Benetos, I.S.; Evangelopoulos, D.S.; Vlamis, J.; Vasiliadis, E.S.; Kotroni, A.; Pneumaticos, S.G. Electrical Stimulation and Motor Function Rehabilitation in Spinal Cord Injury: A Systematic Review. Cureus 2024, 16, e61436. [Google Scholar] [CrossRef]
- Jo, H.J.; Perez, M.A. Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 2020, 143, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Greiner, N.; Barra, B.; Schiavone, G.; Lorach, H.; James, N.; Conti, S.; Kaeser, M.; Fallegger, F.; Borgognon, S.; Lacour, S.; et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat. Commun. 2021, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Zhao, Z.; Chang, J.Q.; He, L.W.; Su, W.N.; Feng, T.; Zhao, C.; Xu, M.; Rao, J.S. Epidural combined optical and electrical stimulation induces high-specificity activation of target muscles in spinal cord injured rats. Front. Neurosci. 2023, 17, 1282558. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; He, L.W.; Chang, J.Q.; Su, W.N.; Feng, T.; Gao, Y.M.; Wu, Y.Y.; Zhao, C.; Rao, J.S. Epidural electrical stimulation combined with photobiomodulation restores hindlimb motor function in rats with thoracic spinal cord injury. Exp. Neurol. 2025, 385, 115112. [Google Scholar] [CrossRef]
- Oishi, R.; Takeda, I.; Ode, Y.; Okada, Y.; Kato, D.; Nakashima, H.; Imagama, S.; Wake, H. Neuromodulation with transcranial direct current stimulation contributes to motor function recovery via microglia in spinal cord injury. Sci. Rep. 2024, 14, 18031. [Google Scholar] [CrossRef]
- Kumru, H.; Flores, A.; Rodríguez-Cañón, M.; Soriano, I.; Garcia, L.; Vidal-Samso, J. Non-invasive brain and spinal cord stimulation for motor and functional recovery after a spinal cord injury. Rev. Neurol. 2020, 70, 461–477. [Google Scholar] [CrossRef]
- Cho, N.; Squair, J.W.; Aureli, V.; James, N.D.; Bole-Feysot, L.; Dewany, I.; Hankov, N.; Baud, L.; Leonhartsberger, A.; Sveistyte, K.; et al. Hypothalamic deep brain stimulation augments walking after spinal cord injury. Nat. Med. 2024, 30, 3676–3686. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, T.; Li, X.; Zhao, H.; Yang, L.; Xu, R.; Fu, Y.; Li, L.; Gai, X.; Qin, D. Research progress on the application of transcranial magnetic stimulation in spinal cord injury rehabilitation: A narrative review. Front. Neurol. 2023, 14, 1219590. [Google Scholar] [CrossRef]
- Arora, T.; Desai, N.; Kirshblum, S.; Chen, R. Utility of transcranial magnetic stimulation in the assessment of spinal cord injury: Current status and future directions. Front. Rehabil. Sci. 2022, 3, 1005111. [Google Scholar] [CrossRef]
- Wincek, A.; Huber, J.; Leszczyńska, K.; Fortuna, W.; Okurowski, S.; Chmielak, K.; Tabakow, P. The Long-Term Effect of Treatment Using the Transcranial Magnetic Stimulation rTMS in Patients after Incomplete Cervical or Thoracic Spinal Cord Injury. J. Clin. Med. 2021, 10, 2975. [Google Scholar] [CrossRef]
- Krogh, S.; Aagaard, P.; Jønsson, A.B.; Figlewski, K.; Kasch, H. Effects of repetitive transcranial magnetic stimulation on recovery in lower limb muscle strength and gait function following spinal cord injury: A randomized controlled trial. Spinal Cord 2022, 60, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Luo, X.; Zhang, C.; Xie, Y.J.; Wang, L.; Wan, T.; Chen, R.; Xu, F.; Wang, J.X. Effects of repeated transcranial magnetic stimulation in the dorsolateral prefrontal cortex versus motor cortex in patients with neuropathic pain after spinal cord injury: A study protocol. BMJ Open 2022, 12, e053476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Zhou, K.; Wei, W.; Liu, Y. Restoring Sensorimotor Function Through Neuromodulation After Spinal Cord Injury: Progress and Remaining Challenges. Front. Neurosci. 2021, 15, 749465. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, A.; Vollenweider, I.; Courtine, G.; Arber, S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 2014, 159, 1626–1639. [Google Scholar] [CrossRef]
- Yadav, A.P.; Li, S.; Krucoff, M.O.; Lebedev, M.A.; Abd-El-Barr, M.M.; Nicolelis, M.A.L. Generating artificial sensations with spinal cord stimulation in primates and rodents. Brain Stimul. 2021, 14, 825–836. [Google Scholar] [CrossRef]
- Vastano, R.; Costantini, M.; Alexander, W.H.; Widerstrom-Noga, E. Multisensory integration in humans with spinal cord injury. Sci. Rep. 2022, 12, 22156. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Hasnan, N.; Abdul Wahab, A.K.; Davis, G.M. Strategies for Rapid Muscle Fatigue Reduction during FES Exercise in Individuals with Spinal Cord Injury: A Systematic Review. PLoS ONE 2016, 11, e0149024. [Google Scholar] [CrossRef]
- Fan, S.; Wang, W.; Zheng, X. Repetitive Transcranial Magnetic Stimulation for the Treatment of Spinal Cord Injury: Current Status and Perspective. Int. J. Mol. Sci. 2025, 26, 825. [Google Scholar] [CrossRef]
- Zavvarian, M.-M.; Toossi, A.; Khazaei, M.; Hong, J.; Fehlings, M. Novel innovations in cell and gene therapies for spinal cord injury. F1000Research 2020, 9, 279. [Google Scholar] [CrossRef]
| Type of Intervention | Effects on Neuroplasticity | Advantages | Limitations |
|---|---|---|---|
| Pharmacological Interventions | Indirectly activate neuroplasticity through anti-inflammatory and neuron protection | (i) The operation is relatively simple and has a wide range of applications (ii) Suppress inflammation, reduce tissue damage, and alleviate complications such as pain and spasms | (i) The side effects are significant (ii) Difficult to reverse neural damage |
| Biomaterials | Provide dual support of physical scaffolding and biochemical signaling to directly promote structural plasticity and neural guidance | (i) Good biocompatibility and degradability (ii) Promotes cell adhesion and axon regeneration (iii) Serve as a carrier for drugs or cells to achieve targeted therapy | (i) Induce inflammatory reactions or degradation toxicity (ii) Challenges in material design |
| Gene Editing | Activate the mechanisms of axonal regeneration and synapse formation at the molecular level | (i) Precisely regulating key genes that promote neuroprotection and regeneration at the molecular level (ii) Potential for long-term therapeutic application | (i) Still in the pre-clinical stage (ii) Low delivery efficiency, off-target effects, and immature technology |
| Stem Cell Therapy | Achieve the reconstruction of structural and functional plasticity by implanting cells to construct conduction pathways | (i) Differentiating into neurons / glial cells (ii) Improving the microenvironment (iii) promoting regeneration and regulating immunity | (i) Ethical controversies and tumor risks (ii) Therapeutic effect is unstable (iii) Need to optimize the strategy for the source and use of stem cells |
| Rehabilitation and Physical Therapy | Activate activity-dependent pathways, induce synaptic remodeling, and neural network function reconstruction | (i) Significantly improve motor function and quality of life (ii) Alleviate chronic pain | (i) The ability to repair is limited (ii) The treatment is contingent upon time and resources (iii) Significant variations among individuals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Y.; Gao, Y.-M.; Feng, T.; Rao, J.-S.; Zhao, C. Enhancing Functional Recovery After Spinal Cord Injury Through Neuroplasticity: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 6596. https://doi.org/10.3390/ijms26146596
Wu Y-Y, Gao Y-M, Feng T, Rao J-S, Zhao C. Enhancing Functional Recovery After Spinal Cord Injury Through Neuroplasticity: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(14):6596. https://doi.org/10.3390/ijms26146596
Chicago/Turabian StyleWu, Yuan-Yuan, Yi-Meng Gao, Ting Feng, Jia-Sheng Rao, and Can Zhao. 2025. "Enhancing Functional Recovery After Spinal Cord Injury Through Neuroplasticity: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 14: 6596. https://doi.org/10.3390/ijms26146596
APA StyleWu, Y.-Y., Gao, Y.-M., Feng, T., Rao, J.-S., & Zhao, C. (2025). Enhancing Functional Recovery After Spinal Cord Injury Through Neuroplasticity: A Comprehensive Review. International Journal of Molecular Sciences, 26(14), 6596. https://doi.org/10.3390/ijms26146596






