Differences in Tissue Copper and Zinc Content Between Normal Livers and Those with Cirrhosis with or Without Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
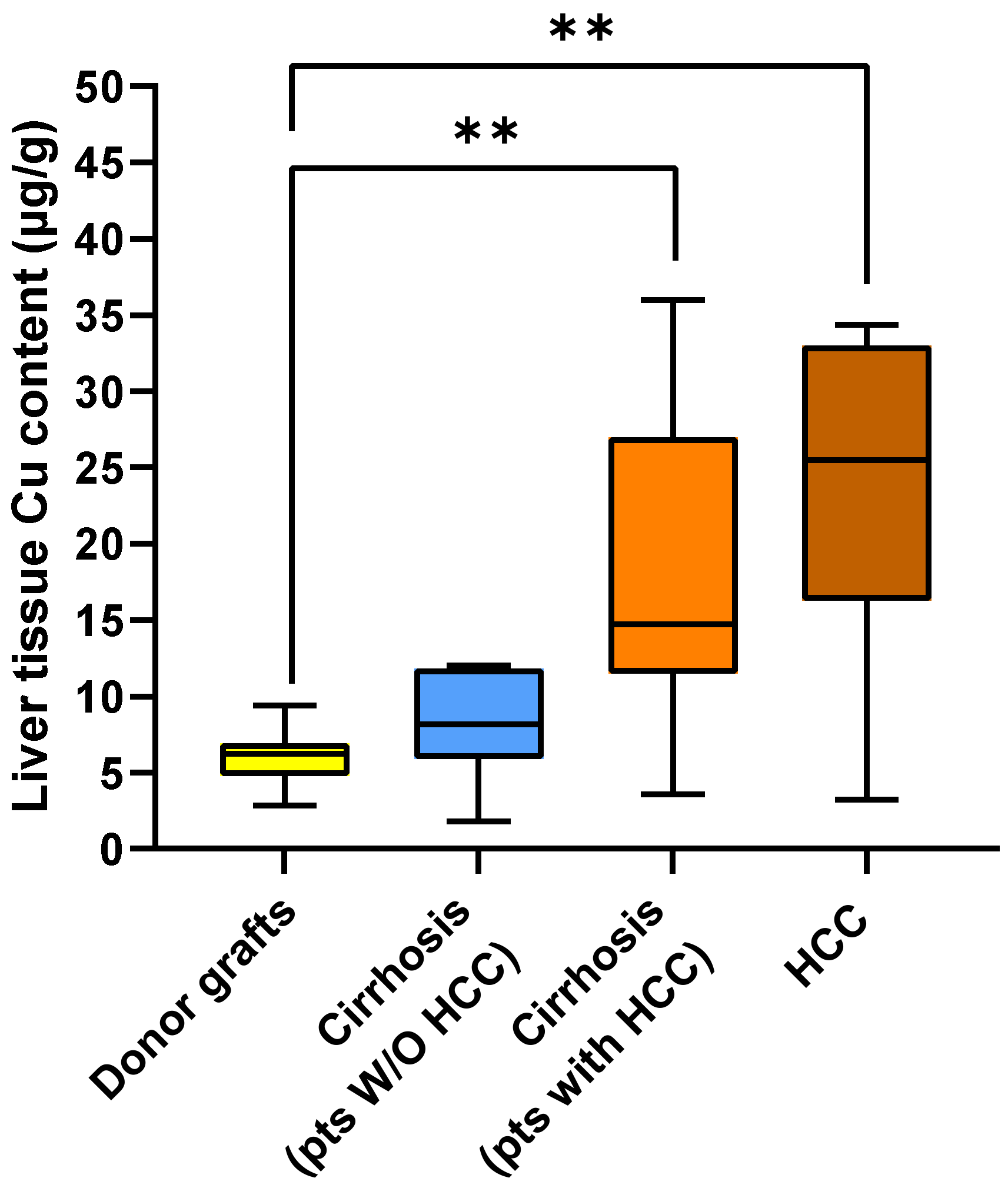
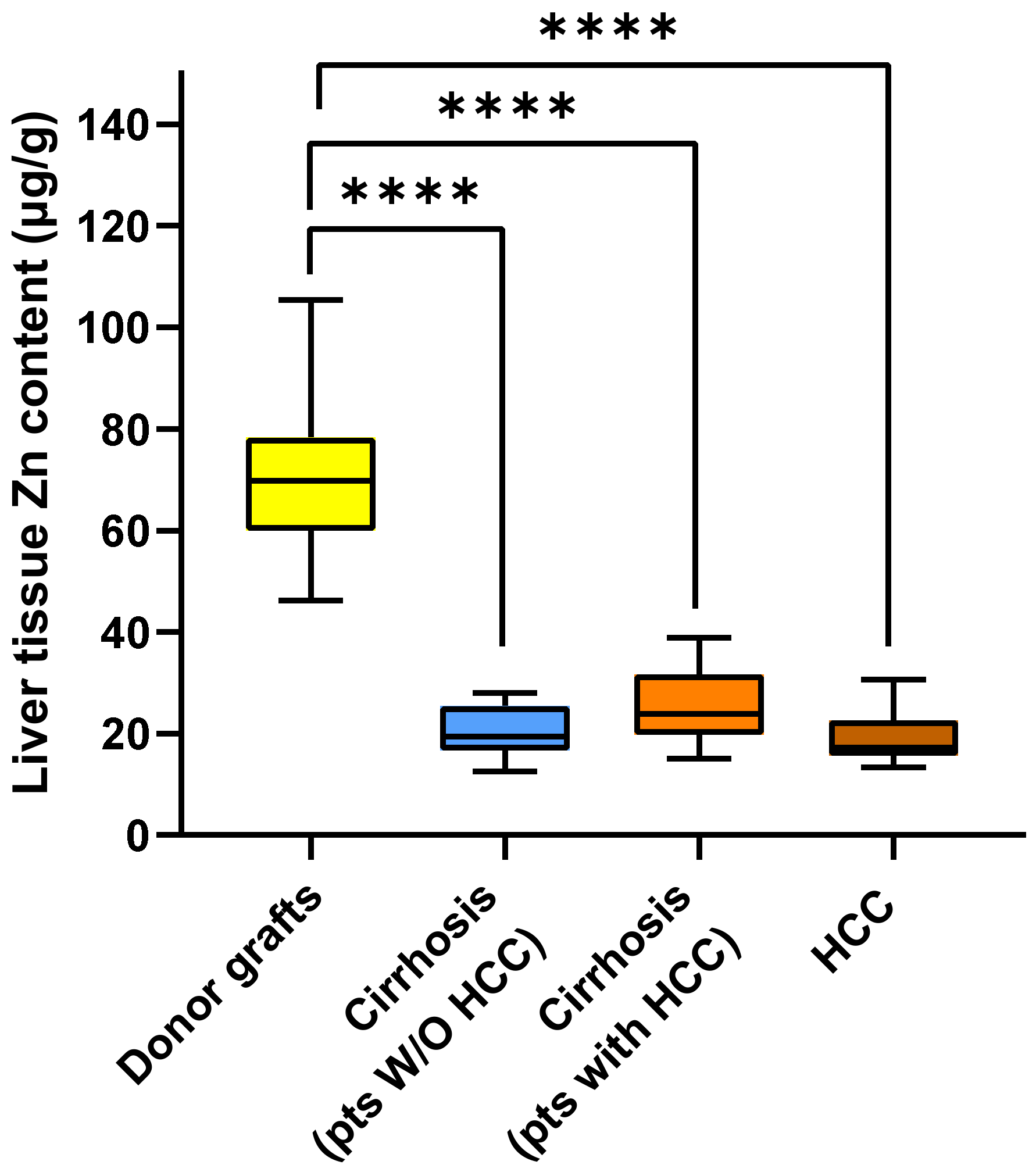
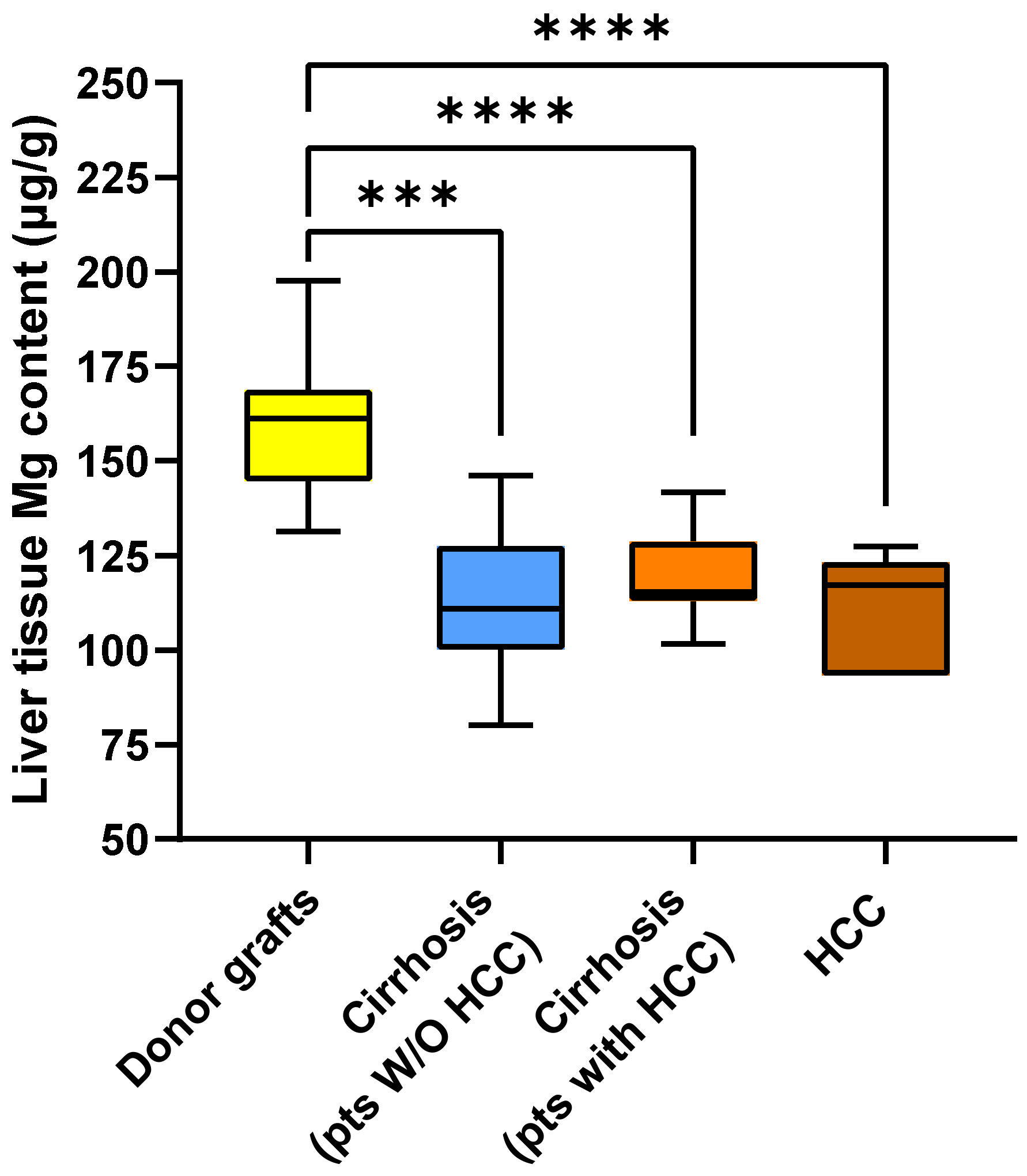
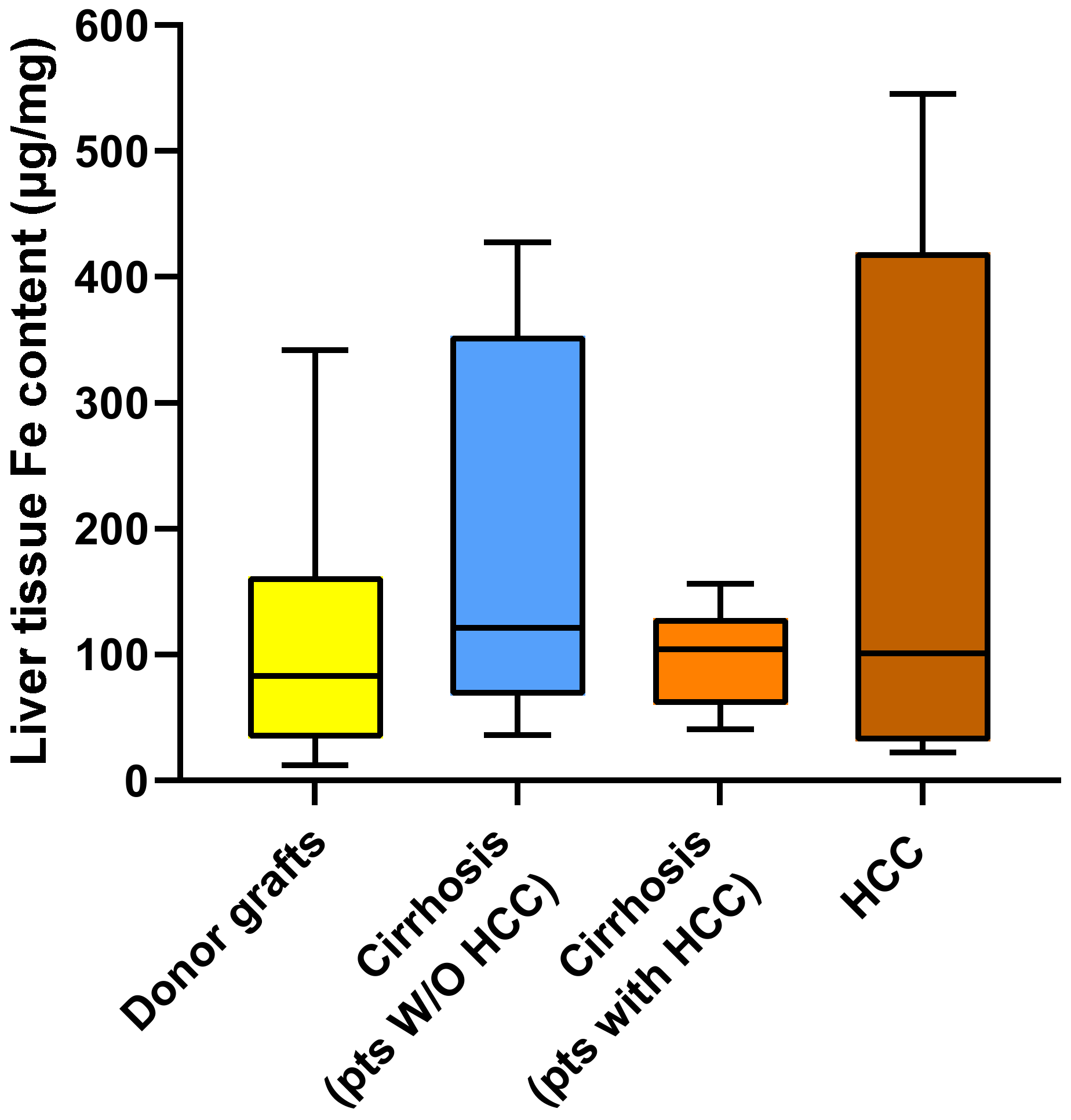
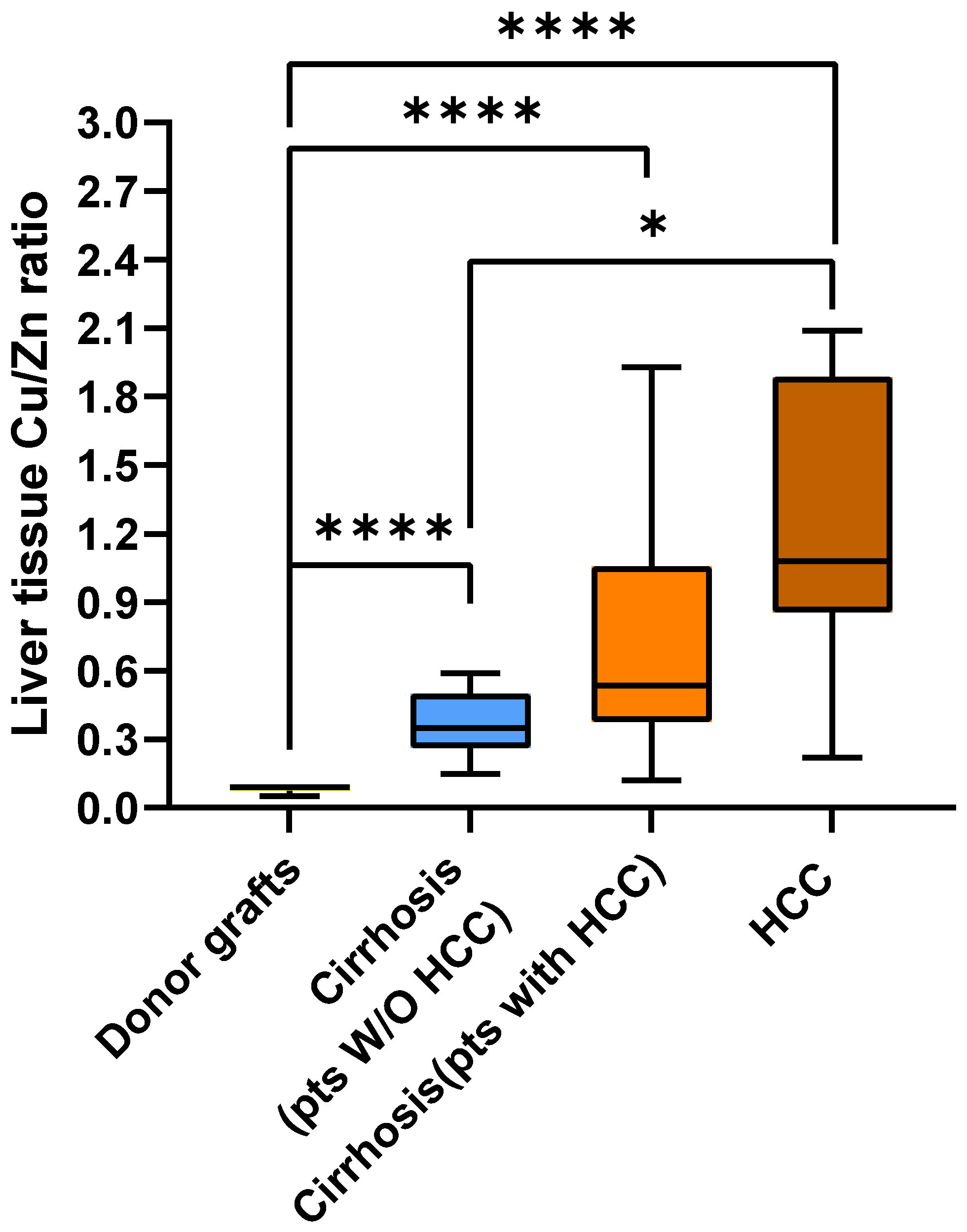
2.2. Metal Content in Liver Tissue
2.3. Correlations Between Tissue Metal Content and Serum ALT and γGT
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Population and Data Collection
4.3. Study Aims
4.4. Metal Quantification in Liver Tissue Using Atomic Absorption Spectrometry
4.5. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| BMI | Body mass index |
| Cu | Copper |
| Fe | Iron |
| IQR | Interquartile range |
| HCC | Hepatocellular carcinoma |
| LT | Liver transplantation |
| MASLD | Metabolic associated steatotic liver disease |
| MELD | Model for End stage Liver Disease |
| Mg | Magnesium |
| Pts | Patients |
| Zn | Zinc |
| W/O | Without |
References
- Mendes, P.M.V.; Bezerra, D.L.C.; dos Santos, L.R.; Santos, R.d.O.; Melo, S.R.d.S.; Morais, J.B.S.; Severo, J.S.; Vieira, S.C.; Marreiro, D.D.N. Magnesium in Breast Cancer: What Is Its Influence on the Progression of This Disease? Biol. Trace Elem. Res. 2018, 184, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef]
- Ho, E.; Song, Y. Zinc and prostatic cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 640–645. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, H.; Han, Z.; Guo, Y.; Yang, W. Zinc Fingers and Homeobox Family in Cancer: A Double-Edged Sword. Int. J. Mol. Sci. 2022, 23, 11167. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, J.; Zheng, N.; Zhang, X.; Dai, X.; Zhang, L.; Hu, C.; Wu, X.; Jiang, Q.; Wu, D.; et al. Copper Promotes Tumorigenesis by Activating the PDK1-AKT Oncogenic Pathway in a Copper Transporter 1 Dependent Manner. Adv. Sci. 2021, 8, 2004303. [Google Scholar] [CrossRef]
- Lin, J.; Luo, B.; Yu, X.; Yang, Z.; Wang, M.; Cai, W. Copper metabolism patterns and tumor microenvironment characterization in colon adenocarcinoma. Front. Oncol. 2022, 12, 959273. [Google Scholar] [CrossRef]
- Kamiya, T. Copper in the tumor microenvironment and tumor metastasis. J. Clin. Biochem. Nutr. 2022, 71, 22–29. [Google Scholar] [CrossRef]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef]
- Wu, Z.; Lv, G.; Xing, F.; Xiang, W.; Ma, Y.; Feng, Q.; Yang, W.; Wang, H. Copper in hepatocellular carcinoma: A double-edged sword with therapeutic potentials. Cancer Lett. 2023, 571, 216348. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, J.; Liu, T.; Jia, R.; Yang, L.; Sun, P.; Zhao, W. Copper metabolism and hepatocellular carcinoma: Current insights. Front. Oncol. 2023, 13, 1186659. [Google Scholar] [CrossRef]
- Torrez, C.Z.; Easley, A.; Bouamar, H.; Zheng, G.; Gu, X.; Yang, J.; Chiu, Y.-C.; Chen, Y.; Halff, G.A.; Cigarroa, F.G.; et al. STEAP2 promotes hepatocellular carcinoma progression via increased copper levels and stress-activated MAP kinase activity. Sci. Rep. 2024, 14, 12753. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Li, X.; Han, M.; Zhang, H.; Liu, F.; Pan, Y.; Zhu, J.; Liao, Z.; Chen, X.; Zhang, B. Structures and biological functions of zinc finger proteins and their roles in hepatocellular carcinoma. Biomark. Res. 2022, 10, 2. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Shi, H.; Huang, J.; Wang, X.; Li, R.; Shen, Y.; Jiang, B.; Ran, J.; Cai, R.; Guo, F.; Wang, Y.; et al. Development and validation of a copper-related gene prognostic signature in hepatocellular carcinoma. Front. Cell Dev. Biol. 2023, 11, 1157841. [Google Scholar] [CrossRef]
- Kong, L.; Liu, M.; Yang, H.; Yan, P.; Luo, Y.; Xiang, S.; Huang, Z.; Shen, A. Expression of copper metabolism-related genes is associated with the tumor immune microenvironment and predicts the prognosis of hepatocellular carcinoma. Transl. Cancer Res. 2024, 13, 2251–2265. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, R.; Gao, Y.; Xia, F.; Yu, X.F. A prognostic and immune related risk model based on zinc homeostasis in hepatocellular carcinoma. iScience 2024, 27, 109389. [Google Scholar] [CrossRef]
- Li, X.; Meng, F.; Wang, H.; Sun, L.; Chang, S.; Li, G.; Chen, F. Iron accumulation and lipid peroxidation: Implication of ferroptosis in hepatocellular carcinoma. Front. Endocrinol. 2024, 14, 1319969. [Google Scholar] [CrossRef]
- Kowdley, K.V. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004, 127, S79–S86. [Google Scholar] [CrossRef]
- Poznański, J.; Sołdacki, D.; Czarkowska-Pączek, B.; Bonna, A.; Kornasiewicz, O.; Krawczyk, M.; Bal, W.; Pączek, L. Cirrhotic Liver of Liver Transplant Recipients Accumulate Silver and Co-Accumulate Copper. Int. J. Mol. Sci. 2021, 22, 1782. [Google Scholar] [CrossRef]
- Stepien, M.; Hughes, D.J.; Hybsier, S.; Bamia, C.; Tjønneland, A.; Overvad, K.; Affret, A.; His, M.; Boutron-Ruault, M.-C.; Katzke, V.; et al. Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br. J. Cancer 2017, 116, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Yishake, D.; Luo, Y.; Liu, Z.; Chen, Y.; Zhu, H.; Fang, A. Dietary intake and serum levels of copper and zinc and risk of hepatocellular carcinoma: A matched case-control study. Chin. Med. J. 2024, 137, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Tamai, Y.; Iwasa, M.; Eguchi, A.; Shigefuku, R.; Sugimoto, K.; Hasegawa, H.; Takei, Y.; Alpini, G.D. Serum copper, zinc and metallothionein serve as potential biomarkers for hepatocellular carcinoma. PLoS ONE 2020, 15, e0237370. [Google Scholar] [CrossRef]
- Poo, J.L.; Rosas-Romero, R.; Montemayor, A.C.; Isoard, F.; Uribe, M. Diagnostic value of the copper/zinc ratio in hepatocellular carcinoma: A case control study. J. Gastroenterol. 2003, 38, 45–51. [Google Scholar] [CrossRef]
- Fang, A.; Chen, P.; Wang, X.; Liu, Z.; Zhang, D.; Luo, Y.; Liao, G.; Long, J.; Zhong, R.; Zhou, Z.; et al. Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 144, 2823–2832. [Google Scholar] [CrossRef]
- Parisse, S.; Ferri, F.; Persichetti, M.; Mischitelli, M.; Abbatecola, A.; Di Martino, M.; Lai, Q.; Carnevale, S.; Lucatelli, P.; Bezzi, M.; et al. Low serum magnesium concentration is associated with the presence of viable hepatocellular carcinoma tissue in cirrhotic patients. Sci. Rep. 2021, 11, 15184. [Google Scholar] [CrossRef]
- Voringer, S.; Schreyer, L.; Nadolni, W.; Meier, M.A.; Woerther, K.; Mittermeier, C.; Ferioli, S.; Singer, S.; Holzer, K.; Zierler, S.; et al. Inhibition of TRPM7 blocks MRTF/SRF-dependent transcriptional and tumorigenic activity. Oncogene 2020, 39, 2328–2344. [Google Scholar] [CrossRef]
- Yu, Z.; Song, Y.; Cai, M.; Jiang, B.; Zhang, Z.; Wang, L.; Jiang, Y.; Zou, L.; Liu, X.; Yu, N.; et al. PPM1D is a potential prognostic biomarker and correlates with immune cell infiltration in hepatocellular carcinoma. Aging 2021, 13, 21294–21308. [Google Scholar] [CrossRef]
- Li, Q.; Xiong, D.L.; Wang, H.; Jin, W.L.; Ma, Y.Y.; Fan, X.M. High Expression of SLC41A3 Correlates with Poor Prognosis in Hepatocellular Carcinoma. Onco Targets Ther. 2021, 14, 2975–2988. [Google Scholar] [CrossRef]
- Flannery, P.C.; Abbott, K.L.; Pondugula, S.R. Correlation of PPM1A Downregulation with CYP3A4 Repression in the Tumor Liver Tissue of Hepatocellular Carcinoma Patients. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 297–304. [Google Scholar] [CrossRef]
- Geetha, A.; Saranya, P.; Annie Jeyachristy, S.; Surendran, R.; Sundaram, A. Relevance of Non-ceruloplasmin Copper to Oxidative Stress in Patients with Hepatocellular Carcinoma. Biol. Trace Elem. Res. 2009, 130, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Fukuda, H.; Ebara, M.; Ikota, N.; Saisho, H.; Nakagawa, H.; Ozawa, T.; Yukawa, M.; Kato, K.; Satoh, T.; et al. Evaluation of Distribution Patterns for Copper and Zinc in Metallothionein and Superoxide Dismutase in Chronic Liver Diseases and Hepatocellular Carcinoma Using High-Performance Liquid Chromatography (HPLC). Biol. Pharm. Bull. 2005, 28, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Fukuda, H.; Hatano, R.; Yoshikawa, M.; Sugiura, N.; Saisho, H.; Kondo, F.; Yukawa, M. Metal Contents in the Liver of Patients with Chronic Liver Disease Caused by Hepatitis C Virus. Oncology 2003, 65, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Kawamoto, T.; Okubo, T.; Koide, O. Variation in the Distribution of Trace Elements in Hepatoma. Biol. Trace Elem. Res. 2003, 95, 49–64. [Google Scholar] [CrossRef]
- Tashiro-Itoh, T.; Ichida, T.; Matsuda, Y.; Satoh, T.; Sugiyama, M.; Tanaka, Y.; Ishikawa, T.; Itoh, S.; Nomoto, M.; Asakura, H. Metallothionein expression and concentrations of copper and zinc are associated with tumor differentiation in hepatocellular carcinoma. Liver 1997, 17, 300–306. [Google Scholar] [CrossRef]
- Ebara, M.; Fukuda, H.; Hatano, R.; Saisho, H.; Nagato, Y.; Suzuki, K.; Nakajima, K.; Yukawa, M.; Kondo, F.; Nakayama, A.; et al. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J. Hepatol. 2000, 33, 415–422. [Google Scholar] [CrossRef]
- Kew, M.C.; Mallett, R.C. Hepatic zinc concentrations in primary cancer of the liver. Br. J. Cancer 1974, 29, 80–83. [Google Scholar] [CrossRef]
- Angelico, M.; Cillo, U.; Fagiuoli, S.; Gasbarrini, A.; Gavrila, C.; Marianelli, T.; Costa, A.N.; Nardi, A.; Strazzabosco, M.; Burra, P.; et al. Liver Match, a prospective observational cohort study on liver transplantation in Italy: Study design and current practice of donor–recipient matching. Dig. Liver Dis. 2011, 43, 155–164. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef]
- Goldberg, D.; French, B.; Newcomb, C.; Liu, Q.; Sahota, G.; Wallace, A.E.; Forde, K.A.; Lewis, J.D.; Halpern, S.D. Patients with Hepatocellular Carcinoma Have Highest Rates of Wait-listing for Liver Transplantation Among Patients with End-Stage Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1638–1646.e2. [Google Scholar] [CrossRef]
- Samuel, D.; De Martin, E.; Berg, T.; Berenguer, M.; Burra, P.; Fondevila, C.; Heimbach, J.K.; Pageaux, G.-P.; Sanchez-Fueyo, A.; Toso, C. EASL Clinical Practice Guidelines on liver transplantation. J. Hepatol. 2024, 81, 1040–1086. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Coilly, A. Management of patients with liver diseases on the waiting list for transplantation: A major impact to the success of liver transplantation. BMC Med. 2018, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.I.; Akkiz, H.; Bag, H.G.; Karaoğullarından, U.; Yalçın, K.; Ekin, N.; Özakyol, A.; Altıntaş, E.; Balaban, H.Y.; Şimşek, H.; et al. Serum levels of gamma-glutamyl transpeptidase in relation to HCC human biology and prognosis. J. Transl. Sci. 2021, 7, 10-15761. [Google Scholar]
- Hu, G.; Tuomilehto, J.; Pukkala, E.; Hakulinen, T.; Antikainen, R.; Vartiainen, E.; Jousilahti, P. Joint effects of coffee consumption and serum gamma-glutamyltransferase on the risk of liver cancer†. Hepatology 2008, 48, 129–136. [Google Scholar] [CrossRef]
- Sun, P.; Li, Y.; Chang, L.; Tian, X. Prognostic and clinicopathological significance of Gamma-Glutamyltransferase in patients with hepatocellular carcinoma. Medicine 2019, 98, e15603. [Google Scholar] [CrossRef]
- Lee, D.H.; Blomhoff, R.; Jacobs, D.R. ReviewIs Serum Gamma Glutamyltransferase a Marker of Oxidative Stress? Free Radic Res. 2004, 38, 535–539. [Google Scholar] [CrossRef]
- Himoto, T.; Masaki, T. Current Trends on the Involvement of Zinc, Copper, and Selenium in the Process of Hepatocarcinogenesis. Nutrients 2024, 16, 472. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, X.; Tang, H.; Chen, Y.; Bai, L. Emerging roles of cuproptosis in liver diseases. Dig. Liver Dis. 2025; ahead of print. [Google Scholar] [CrossRef]
- Ullah, M.I.; Alameen, A.A.M.; Al-Oanzi, Z.H.; Eltayeb, L.B.; Atif, M.; Munir, M.U.; Ejaz, H. Biological Role of Zinc in Liver Cirrhosis: An Updated Review. Biomedicines 2023, 11, 1094. [Google Scholar] [CrossRef]
- Parisse, S.; Gianoncelli, A.; Isani, G.; Gambaro, F.L.; Andreani, G.; Malucelli, E.; Aquilanti, G.; Carlomagno, I.; Carletti, R.; Mischitelli, M.; et al. Severity of Hepatocyte Damage and Prognosis in Cirrhotic Patients Correlate with Hepatocyte Magnesium Depletion. Nutrients 2023, 15, 2626. [Google Scholar] [CrossRef]
- Falcone, E.; Okafor, M.; Vitale, N.; Raibaut, L.; Sour, A.; Faller, P. Extracellular Cu2+ pools and their detection: From current knowledge to next-generation probes. Coord. Chem. Rev. 2021, 433, 213727. [Google Scholar] [CrossRef]
- Hara, T.; Takeda Taki Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
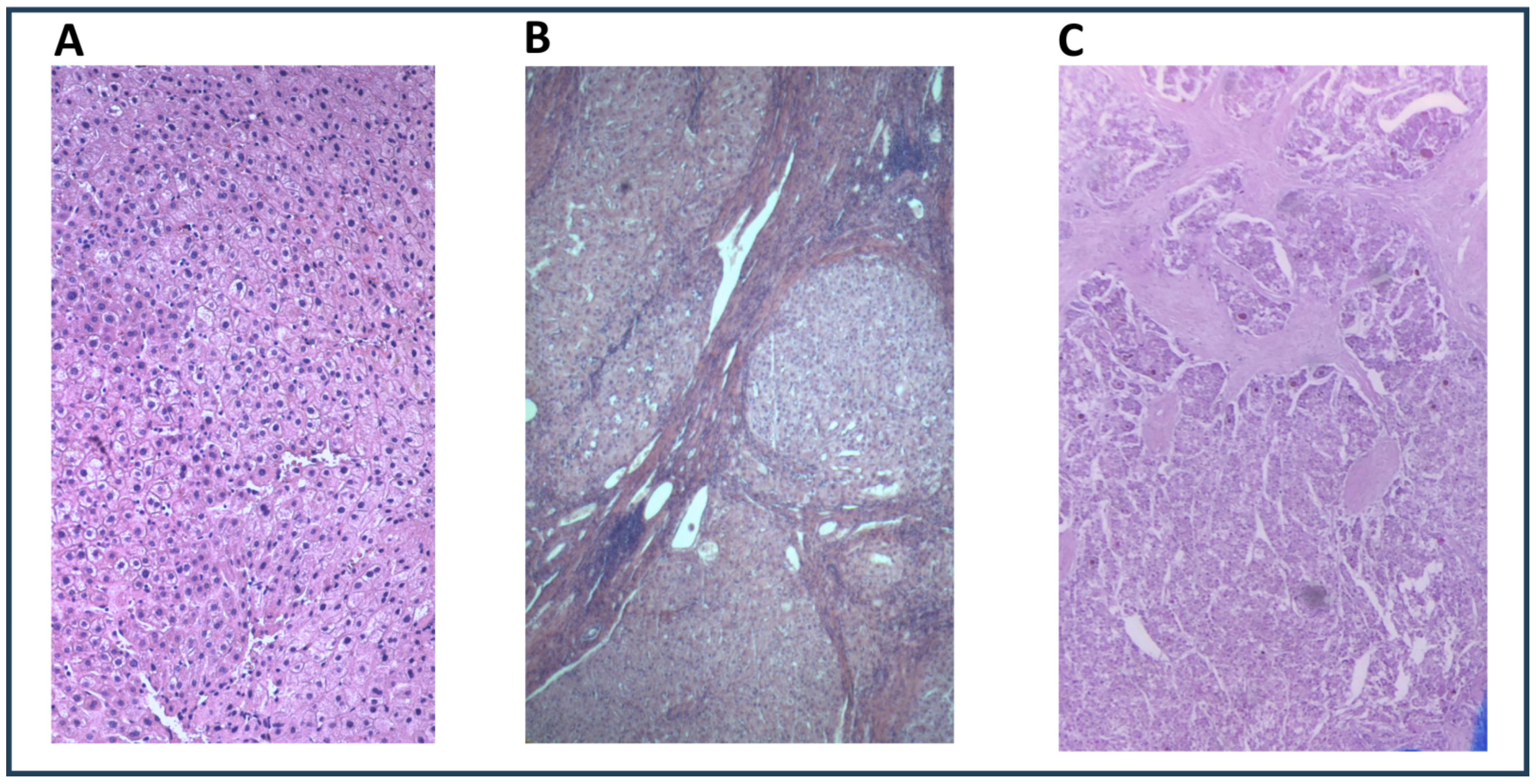
| Liver Graft Donors (n = 18) | Cirrhotic Patients Without HCC (n = 14) | Cirrhotic Patients with HCC (n = 14) | p Value Donors vs. Cirrhotic Patients Without HCC | p Value Donors vs. Cirrhotic Patients with HCC | p Value Cirrhotic Patients Without HCC vs. Those with HCC | |
|---|---|---|---|---|---|---|
| Age (years) | 53.00 (37.00–62.00) | 52.00 (47.25–56.00) | 53.50 (48.00–63.75) | 0.892 | 0.296 | 0.168 |
| Sex M (%) | 12 (66.7) | 10 (71.4) | 13 (92.9) | 1.000 | 0.104 | 0.326 |
| BMI (kg/m2) | 25.70 (24.95–27.31) | 24.20 (22.89–27.42) | 24.85 (22.65–29.98) | 0.184 | 0.569 | 0.818 |
| Serum AST (IU/L) | 33.50 (22.00–43.75) | 58.50 (41.75–92.00) | 75.50 (39.75–117.25) | 0.006 | 0.006 | 0.520 |
| Serum ALT (IU/L) | 23.00 (18.00–29.50) | 48.00 (25.00–59.25) | 51.00 (36.75–117.00) | 0.008 | 0.001 | 0.232 |
| Serum ALP (IU/L) | Not available | 137.00 (105.00–176.50) | 110.00 (83.50–204.25) | _ | _ | 0.713 |
| Serum γGT (IU/L) | Not available | 40.00 (29.75–50.00) | 104.00 (58.25–129.50) | _ | _ | 0.0004 |
| MELD score | Not applicable | 19.06 (12.70–22.83) | 10.36 (7.75–14.12) | _ | _ | 0.008 |
| Etiology of Liver Cirrhosis, n (%) | ||||||
| Not applicable | 8 (57.1) | 12 (85.7) | _ | _ | 0.103 |
| 6 (42.9) | 5 (35.7) | _ | _ | 1.000 | |
| 2 (14.3) | 4 (28.6) | _ | _ | 0.648 |
| Tissue Cu Content | Serum ALT | Serum γGT | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Donor grafts | 0.520 | 0.027 | N.A. | N.A. |
| Cirrhotic tissue (pts W/O HCC) | 0.873 | <0.001 | 0.846 | <0.001 |
| Cirrhotic tissue (pts with HCC) | −0.006 | 0.983 | −0.004 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisse, S.; Andreani, G.; Mischitelli, M.; Gianoncelli, A.; Malucelli, E.; Fratini, M.; Ferri, F.; Carlucci, M.; Lai, Q.; Ascione, A.; et al. Differences in Tissue Copper and Zinc Content Between Normal Livers and Those with Cirrhosis with or Without Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 6571. https://doi.org/10.3390/ijms26146571
Parisse S, Andreani G, Mischitelli M, Gianoncelli A, Malucelli E, Fratini M, Ferri F, Carlucci M, Lai Q, Ascione A, et al. Differences in Tissue Copper and Zinc Content Between Normal Livers and Those with Cirrhosis with or Without Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2025; 26(14):6571. https://doi.org/10.3390/ijms26146571
Chicago/Turabian StyleParisse, Simona, Giulia Andreani, Monica Mischitelli, Alessandra Gianoncelli, Emil Malucelli, Michela Fratini, Flaminia Ferri, Maria Carlucci, Quirino Lai, Andrea Ascione, and et al. 2025. "Differences in Tissue Copper and Zinc Content Between Normal Livers and Those with Cirrhosis with or Without Hepatocellular Carcinoma" International Journal of Molecular Sciences 26, no. 14: 6571. https://doi.org/10.3390/ijms26146571
APA StyleParisse, S., Andreani, G., Mischitelli, M., Gianoncelli, A., Malucelli, E., Fratini, M., Ferri, F., Carlucci, M., Lai, Q., Ascione, A., Mennini, G., Rossi, M., Iotti, S., Isani, G., & Ginanni Corradini, S. (2025). Differences in Tissue Copper and Zinc Content Between Normal Livers and Those with Cirrhosis with or Without Hepatocellular Carcinoma. International Journal of Molecular Sciences, 26(14), 6571. https://doi.org/10.3390/ijms26146571






