Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors
Abstract
1. Introduction
2. Results
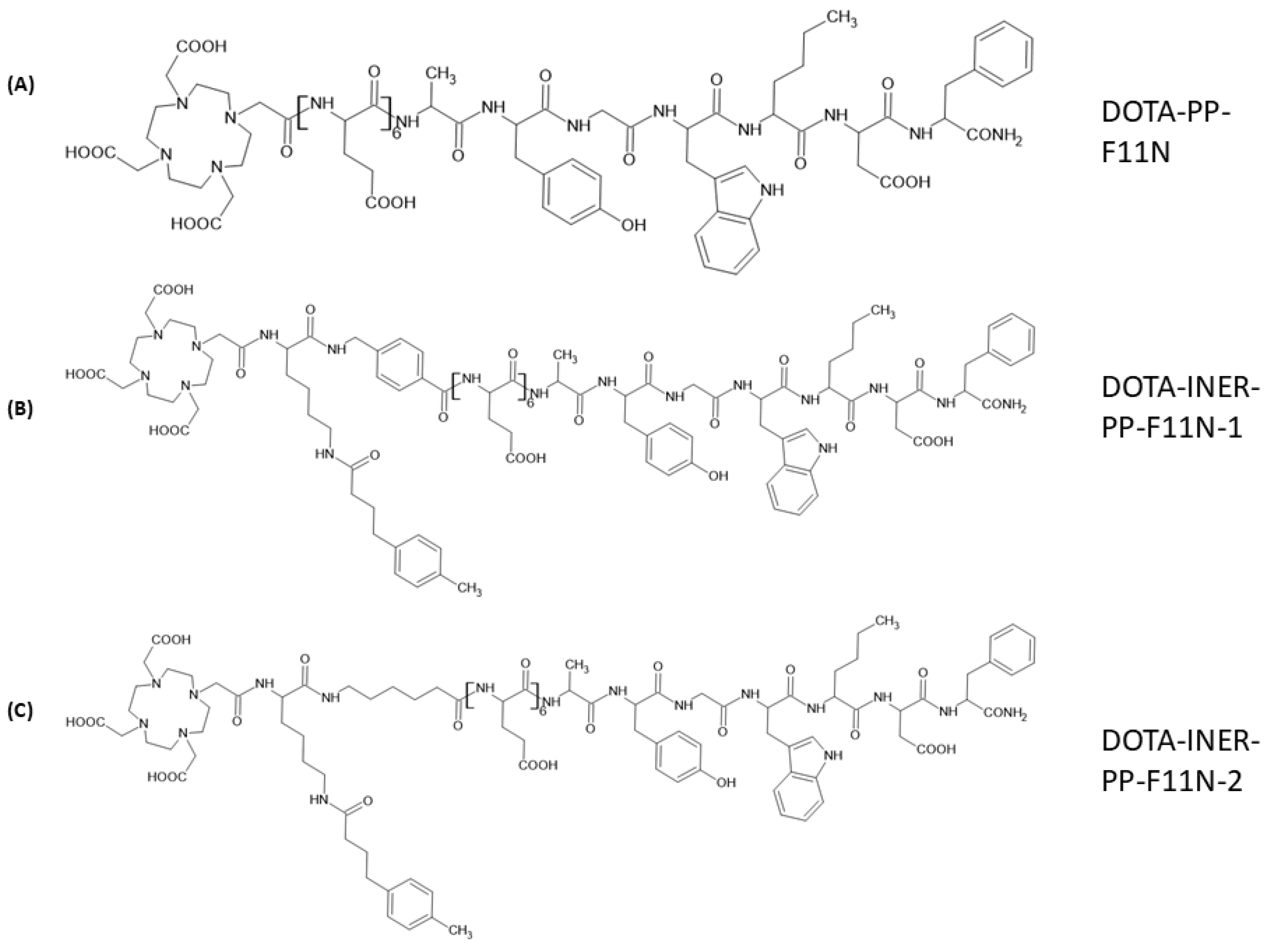
2.1. Structural Designs of Various CCK2R Derivatives Between DOTA and the C-Terminal Binding Sequence
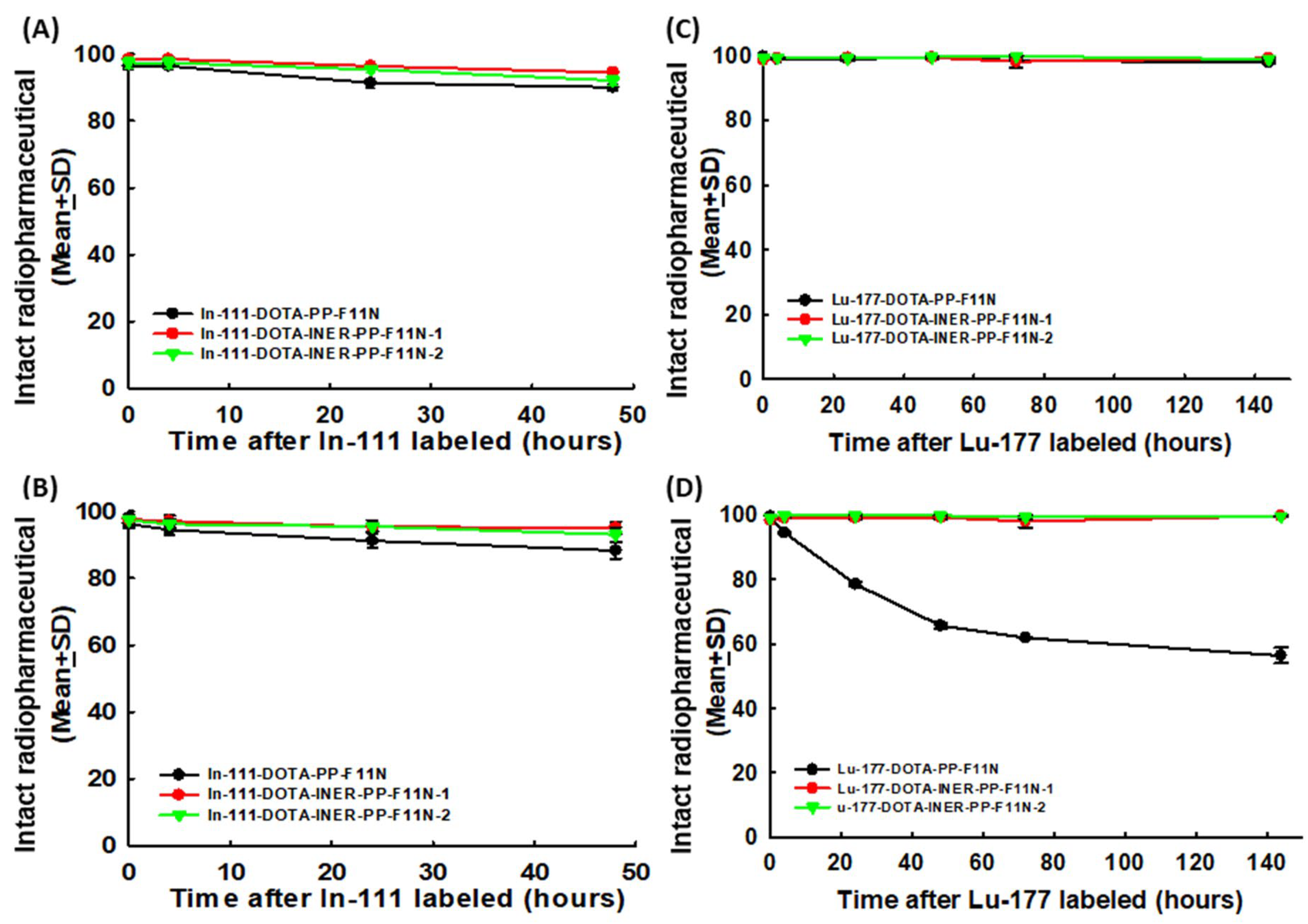
2.2. Radiolabeling DOTA-CCK2R Derivatives Revealed High Efficiency and Reliable Stability
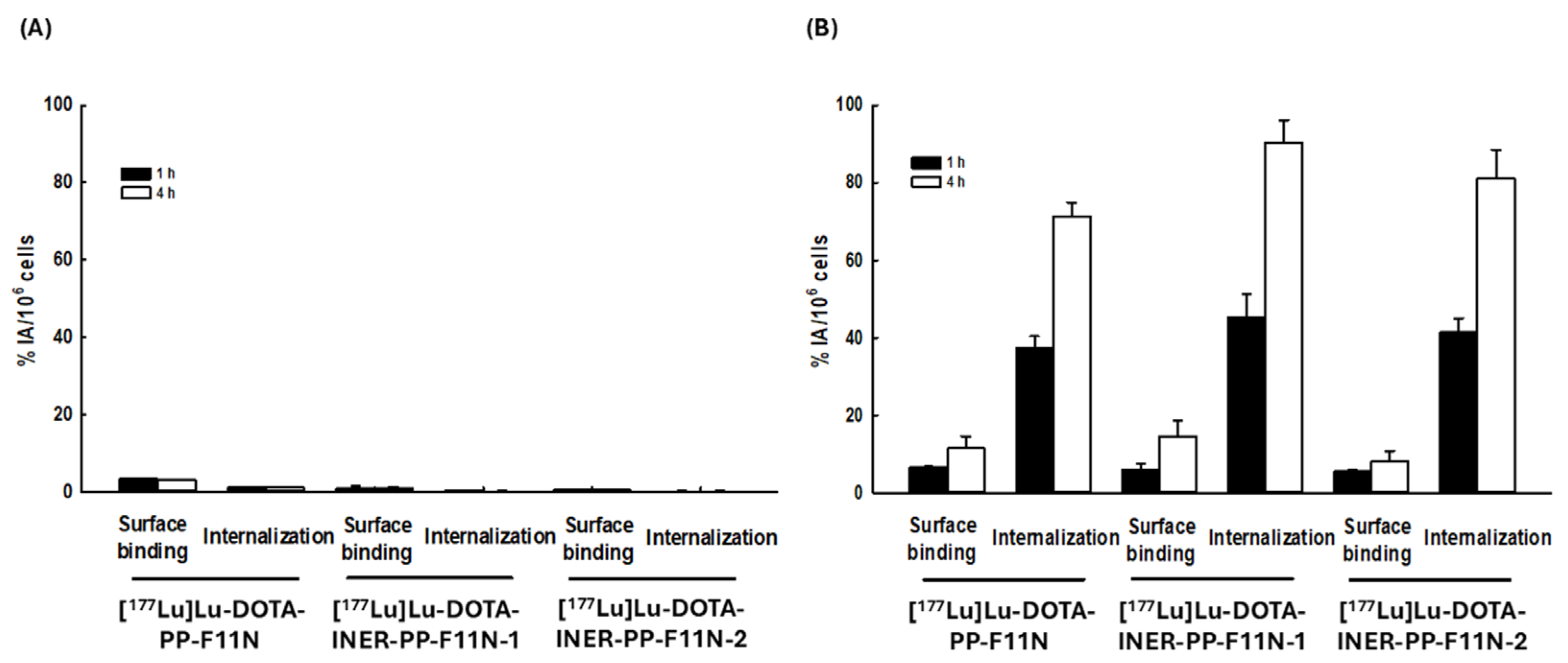
2.3. Lu-177-Labeled DOTA-CCK2R Derivatives Revealed High Cellular Binding and Internalization in A431-CCK2R (+) but Not A431-CCK2R (−) Cells
2.4. NanoSPECT/CT Imaging of Tumor-Bearing Mice and Biodistribution of Radiotracers
2.5. Mice Receiving Lu-177-Labeled DOTA-CCK2R Derivative Therapy Generated Potent Anti-Tumor Effects
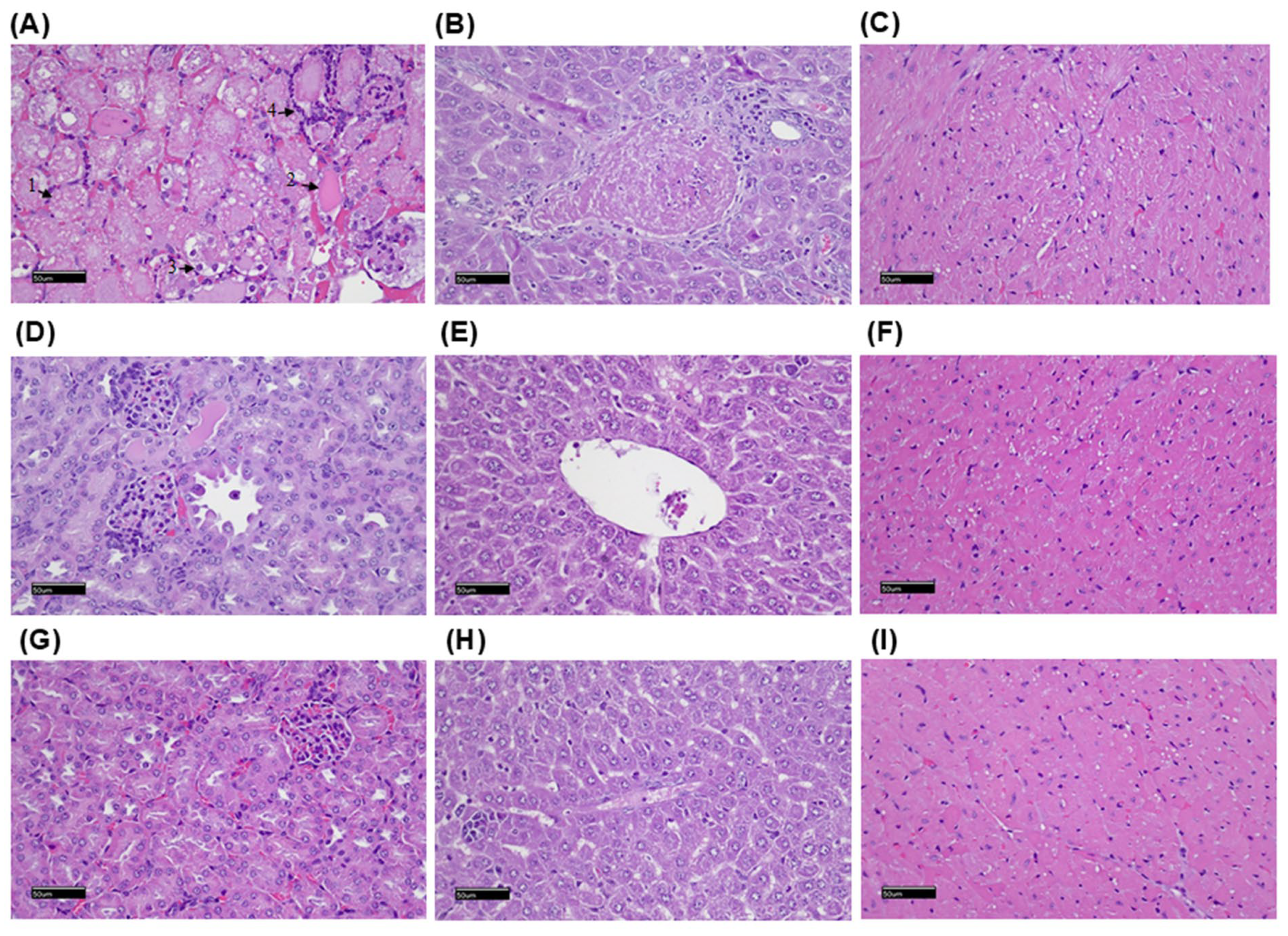
2.6. Histopathological Examination of the Mice After Radiolabeled Drug Administration
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Lines
4.2. Radioisotope Labeling of CCK2R Analogs
4.3. Metabolic Stability Analysis
4.4. Analysis of In Vitro Binding and Internalization Assay
4.5. Animal Tumor Model
4.6. NanoSPECT/CT Imaging
4.7. Biodistribution Study
4.8. Therapeutic Responses to Lu-177-Labeled CCK2R Analogs in CCK2R-Expressing Cancer-Bearing Mice
4.9. Histopathological Examinations
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCK2R | Cholecystokinin receptor subtype 2 |
| DOTA | 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid |
| PP-F11 | (DGlu)6-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| PP-F11N | (DGlu)6-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 |
| DOTA-PP-F11N | DOTA-(Glu)6-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 |
| DOTA-INER-PP-F11N-1 | DOTA-Lys(-4-TBA)-6-AMBA-(Glu)6-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 |
| DOTA-INER-PP-F11N-2 | DOTA-Lys(-4-TBA)-AHA-(Glu)6-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 |
| In-111 | Indium-111 |
| Lu-177 | Lutetium-177 |
| ITLC | instant thin-layer chromatography |
| HPLC | high-performance liquid chromatography |
| OS | overall survival |
References
- Zeng, Q.; Ou, L.; Wang, W.; Guo, D.Y. Gastrin, Cholecystokinin, Signaling, and Biological Activities in Cellular Processes. Front. Endocrinol. 2020, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, C.W.; Lindberg, J.E.M.; Fowler, D.K.; Simasko, S.M.; Peters, J.H. Contributing mechanisms underlying desensitization of cholecystokinin-induced activation of primary nodose ganglia neurons. Am. J. Physiol.-Cell Physiol. 2020, 318, C787–C796. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, C.R.; Pappas, T.N.; Vigna, S.R. Localization of cholecystokinin A and cholecystokinin B/gastrin receptors in the canine upper gastrointestinal tract. Gastroenterology 1994, 107, 1019–1030. [Google Scholar] [CrossRef]
- Mezey, E.; Palkovits, M. Localization of targets for anti-ulcer drugs in cells of the immune system. Science 1992, 258, 1662–1665. [Google Scholar] [CrossRef]
- Cayrol, C.; Clerc, P.; Bertrand, C.; Gigoux, V.; Portolan, G.; Fourmy, D.; Dufresne, M.; Seva, C. Cholecystokinin-2 receptor modulates cell adhesion through beta 1-integrin in human pancreatic cancer cells. Oncogene 2006, 25, 4421–4428. [Google Scholar] [CrossRef]
- Ashurst, H.L.; Varro, A.; Dimaline, R. Regulation of mammalian gastrin/CCK receptor (CCK2R) expression in vitro and in vivo. Exp. Physiol. 2008, 93, 223–236. [Google Scholar] [CrossRef]
- Caplin, M.; Savage, K.; Khan, K.; Brett, B.; Rode, J.; Varro, A.; Dhillon, A. Expression and processing of gastrin in pancreatic adenocarcinoma. Br. J. Surg. 2000, 87, 1035–1040. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schaer, J.C.; Waser, B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997, 57, 1377–1386. [Google Scholar]
- Schmitz, F.; Schrader, H.; Otte, J.; Schmitz, H.; Stüber, E.; Herzig, K.; Schmidt, W.E. Identification of CCK-B/gastrin receptor splice variants in human peripheral blood mononuclear cells. Regul. Pept. 2001, 101, 25–33. [Google Scholar] [CrossRef]
- Matsumori, Y.; Katakami, N.; Ito, M.; Taniguchi, T.; Iwata, N.; Takaishi, T.; Chihara, K.; Matsui, T. Cholecystokinin-B/gastrin receptor: A novel molecular probe for human small cell lung cancer. Cancer Res. 1995, 55, 276–279. [Google Scholar]
- Schmitz, F.; Otte, J.M.; Stechele, H.U.; Reimann, B.; Banasiewicz, T.; Fölsch, U.R.; Schmidt, W.E.; Herzig, K.H. CCK-B/gastrin receptors in human colorectal cancer. Eur. J. Clin. Investig. 2001, 31, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Nielsen, F.C.; Burcharth, F.; Rehfeld, J.F. Closing the gastrin loop in pancreatic carcinoma: Coexpression of gastrin and its receptor in solid human pancreatic adenocarcinoma. Cancer 2000, 88, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Henwood, M.; Clarke, P.A.; Smith, A.M.; Watson, S.A. Expression of gastrin in developing gastric adenocarcinoma. Br. J. Surg. 2001, 88, 564–568. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int. J. Cancer 1996, 67, 644–647. [Google Scholar] [CrossRef]
- Yu, H.G.; Tong, S.L.; Ding, Y.M.; Ding, J.; Fang, X.M.; Zhang, X.F.; Liu, Z.J.; Zhou, Y.H.; Liu, Q.S.; Luo, H.S.; et al. Enhanced expression of cholecystokinin-2 receptor promotes the progression of colon cancer through activation of focal adhesion kinase. Int. J. Cancer 2006, 119, 2724–2732. [Google Scholar] [CrossRef]
- Asa, S.L. The Current Histologic Classification of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 1–22. [Google Scholar] [CrossRef]
- Laverman, P.; Béhé, M.; Oyen, W.J.; Willems, P.H.; Corstens, F.H.; Behr, T.M.; Boerman, O.C. Two technetium-99m-labeled cholecystokinin-8 (CCK8) peptides for scintigraphic imaging of CCK receptors. Bioconjug. Chem. 2004, 15, 561–568. [Google Scholar] [CrossRef]
- Roosenburg, S.; Laverman, P.; Joosten, L.; Cooper, M.S.; Kolenc-Peitl, P.K.; Foster, J.M.; Hudson, C.; Leyton, J.; Burnet, J.; Oyen, W.J.; et al. PET and SPECT imaging of a radiolabeled minigastrin analogue conjugated with DOTA, NOTA, and NODAGA and labeled with (64)Cu, (68)Ga, and (111)In. Mol. Pharm. 2014, 11, 3930–3937. [Google Scholar] [CrossRef]
- Laverman, P.; Roosenburg, S.; Gotthardt, M.; Park, J.; Oyen, W.J.; de Jong, M.; Hellmich, M.R.; Rutjes, F.P.; van Delft, F.L.; Boerman, O.C. Targeting of a CCK(2) receptor splice variant with (111)In-labelled cholecystokinin-8 (CCK8) and (111)In-labelled minigastrin. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 386–392. [Google Scholar] [CrossRef]
- Ohlsson, B.; Fredäng, N.; Axelson, J. The effect of bombesin, cholecystokinin, gastrin, and their antagonists on proliferation of pancreatic cancer cell lines. Scand. J. Gastroenterol. 1999, 34, 1224–1229. [Google Scholar] [CrossRef]
- Hernando, F.; Fuentes, J.A.; Roques, B.P.; Ruiz-Gayo, M. The CCKB receptor antagonist, L-365,260, elicits antidepressant-type effects in the forced-swim test in mice. Eur. J. Pharmacol. 1994, 261, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.C.; Greiner, M.; Adams, J.B.; Lydiard, R.B.; Pierce, M.W. Placebo-controlled trial of the CCK-B antagonist, CI-988, in panic disorder. Biol. Psychiatry 1999, 46, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Michaeli, D.; Grimes, S.; Morris, T.M.; Varro, A.; Clarke, P.A.; Smith, A.M.; Justin, T.A.; Hardcastle, J.D. A comparison of an anti-gastrin antibody and cytotoxic drugs in the therapy of human gastric ascites in SCID mice. Int. J. Cancer 1999, 81, 248–254. [Google Scholar] [CrossRef]
- Takács, T.; Nagy, I.; Pap, A.; Varró, V. The effect of CR 1409, a potent CCK receptor antagonist, on basal and stimulated pancreatic secretion in rat. Pancreas 1990, 5, 60–64. [Google Scholar] [CrossRef]
- Carrillo, J.; Agra, N.; Fernández, N.; Pestaña, A.; Alonso, J. Devazepide, a nonpeptide antagonist of CCK receptors, induces apoptosis and inhibits Ewing tumor growth. Anti-Cancer Drugs 2009, 20, 527–533. [Google Scholar] [CrossRef]
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T.; et al. Cholecystokinin 2 Receptor Agonist (177)Lu-PP-F11N for Radionuclide Therapy of Medullary Thyroid Carcinoma: Results of the Lumed Phase 0a Study. J. Nucl. Med. 2020, 61, 520–526. [Google Scholar] [CrossRef]
- Behr, T.M.; Béhé, M.P. Cholecystokinin-B/Gastrin receptor-targeting peptides for staging and therapy of medullary thyroid cancer and other cholecystokinin-B receptor-expressing malignancies. Semin. Nucl. Med. 2002, 32, 97–109. [Google Scholar] [CrossRef]
- Busslinger, S.D.; Becker, A.E.; Vaccarin, C.; Deberle, L.M.; Renz, M.L.; Groehn, V.; Schibli, R.; Müller, C. Investigations Using Albumin Binders to Modify the Tissue Distribution Profile of Radiopharmaceuticals Exemplified with Folate Radioconjugates. Cancers 2023, 15, 4259. [Google Scholar] [CrossRef]
- Klingler, M.; Decristoforo, C.; Rangger, C.; Summer, D.; Foster, J.; Sosabowski, J.K.; von Guggenberg, E. Site-specific stabilization of minigastrin analogs against enzymatic degradation for enhanced cholecystokinin-2 receptor targeting. Theranostics 2018, 8, 2896–2908. [Google Scholar] [CrossRef]
- Sauter, A.W.; Mansi, R.; Hassiepen, U.; Muller, L.; Panigada, T.; Wiehr, S.; Wild, A.M.; Geistlich, S.; Béhé, M.; Rottenburger, C.; et al. Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog (177)Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution? J. Nucl. Med. 2019, 60, 393–399. [Google Scholar] [CrossRef]
- Kuo, H.T.; Lin, K.S.; Zhang, Z.; Uribe, C.F.; Merkens, H.; Zhang, C.; Benard, F. (177)Lu-Labeled Albumin-Binder-Conjugated PSMA-Targeting Agents with Extremely High Tumor Uptake and Enhanced Tumor-to-Kidney Absorbed Dose Ratio. J. Nucl. Med. 2021, 62, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Ichinose, T.; Watanabe, H.; Ono, M. Comparison of carbonic anhydrase-IX-targeted trifunctional radioligands between linear- and branched-chain arrangements. Front. Nucl. Med. 2025, 5, 1585027. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, P.; Ding, J.; Chen, J.; Huo, L.; Liu, Z. Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J. Nucl. Med. 2022, 63, 952–958. [Google Scholar] [CrossRef]
- Kazuta, N.; Nakashima, K.; Watanabe, H.; Ono, M. Effect of Linker Entities on Pharmacokinetics of (111)In-Labeled Prostate-Specific Membrane Antigen-Targeting Ligands with an Albumin Binder. ACS Pharmacol. Transl. Sci. 2024, 7, 2401–2413. [Google Scholar] [CrossRef]
- Beyer, D.; Vaccarin, C.; Schmid, J.V.; Deberle, L.M.; Deupi, X.; Schibli, R.; Muller, C. Design and Preclinical Evaluation of Novel uPAR-Targeting Radiopeptides Modified with an Albumin-Binding Entity. Mol. Pharm. 2025, 22, 3242–3254. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Fuscaldi, L.L.; Townsend, D.M.; Rubello, D.; Barros, A.L.B. Radiolabeled bombesin derivatives for preclinical oncological imaging. Biomed. Pharmacother. 2017, 87, 58–72. [Google Scholar] [CrossRef]
- Holzleitner, N.; Günther, T.; Daoud-Gadieh, A.; Lapa, C.; Wester, H.J. Investigation of the structure-activity relationship at the N-terminal part of minigastrin analogs. EJNMMI Res. 2023, 13, 65. [Google Scholar] [CrossRef]
- Bumbaca, B.; Li, Z.; Shah, D.K. Pharmacokinetics of protein and peptide conjugates. Drug Metab. Pharmacokinet. 2019, 34, 42–54. [Google Scholar] [CrossRef]
- Kletting, P.; Thieme, A.; Eberhardt, N.; Rinscheid, A.; D’Alessandria, C.; Allmann, J.; Wester, H.J.; Tauber, R.; Beer, A.J.; Glatting, G.; et al. Modeling and Predicting Tumor Response in Radioligand Therapy. J. Nucl. Med. 2019, 60, 65–70. [Google Scholar] [CrossRef]
- Barone, R.; Borson-Chazot, F.; Valkema, R.; Walrand, S.; Chauvin, F.; Gogou, L.; Kvols, L.K.; Krenning, E.P.; Jamar, F.; Pauwels, S. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: Relevance of kidney volume and dose rate in finding a dose-effect relationship. J. Nucl. Med. 2005, 46 (Suppl. 1), 99S–106S. [Google Scholar]
- Klingler, M.; Hörmann, A.A.; Guggenberg, E.V. Cholecystokinin-2 Receptor Targeting with Radiolabeled Peptides: Current Status and Future Directions. Curr. Med. Chem. 2020, 27, 7112–7132. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, A.A.; Klingler, M.; Rezaeianpour, M.; Hörmann, N.; Gust, R.; Shahhosseini, S.; Guggenberg, E.V. Initial In Vitro and In Vivo Evaluation of a Novel CCK2R Targeting Peptide Analog Labeled with Lutetium-177. Molecules 2020, 25, 4585. [Google Scholar] [CrossRef] [PubMed]
- Kolff, W.J. Early years of artificial organs at the Cleveland Clinic: Part II: Open heart surgery and artificial hearts. ASAIO J. 1998, 44, 123–128. [Google Scholar] [CrossRef]
- Iozzo, P.; Gastaldelli, A.; Jarvisalo, M.J.; Kiss, J.; Borra, R.; Buzzigoli, E.; Viljanen, A.; Naum, G.; Viljanen, T.; Oikonen, V.; et al. 18F-FDG assessment of glucose disposal and production rates during fasting and insulin stimulation: A validation study. J. Nucl. Med. 2006, 47, 1016–1022. [Google Scholar]
- de Roode, K.E.; Joosten, L.; Behe, M. Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands. Pharmaceuticals 2024, 17, 256. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Giarika, A.; Kulkarni, H.R.; Klette, I.; Singh, A.; Krenning, E.P.; de Jong, M.; Maina, T.; et al. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. J. Nucl. Med. 2017, 58, 75–80. [Google Scholar] [CrossRef]
- Lau, J.; Lee, H.; Rousseau, J.; Bénard, F.; Lin, K.S. Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies. Molecules 2022, 27, 4959. [Google Scholar] [CrossRef]
- Rizvi, S.F.A.; Zhang, L.; Zhang, H.; Fang, Q. Peptide-Drug Conjugates: Design, Chemistry, and Drug Delivery System as a Novel Cancer Theranostic. ACS Pharmacol. Transl. Sci. 2024, 7, 309–334. [Google Scholar] [CrossRef]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin Receptor Antagonists for Imaging and Therapy. J. Nucl. Med. 2017, 58 (Suppl. 2), 61S–66S. [Google Scholar] [CrossRef]
- Roy, J.; Putt, K.S.; Coppola, D.; Leon, M.E.; Khalil, F.K.; Centeno, B.A.; Clark, N.; Stark, V.E.; Morse, D.L.; Low, P.S. Assessment of cholecystokinin 2 receptor (CCK2R) in neoplastic tissue. Oncotarget 2016, 7, 14605–14615. [Google Scholar] [CrossRef]
- Kaloudi, A.; Kanellopoulos, P.; Radolf, T.; Chepurny, O.G.; Rouchota, M.; Loudos, G.; Andreae, F.; Holz, G.G.; Nock, B.A.; Maina, T. [(99m)Tc]Tc-DGA1, a Promising CCK(2)R-Antagonist-Based Tracer for Tumor Diagnosis with Single-Photon Emission Computed Tomography. Mol. Pharm. 2020, 17, 3116–3128. [Google Scholar] [CrossRef] [PubMed]
- Ogbomo, S.M.; Shi, W.; Wagh, N.K.; Zhou, Z.; Brusnahan, S.K.; Garrison, J.C. 177Lu-labeled HPMA copolymers utilizing cathepsin B and S cleavable linkers: Synthesis, characterization and preliminary in vivo investigation in a pancreatic cancer model. Nucl. Med. Biol. 2013, 40, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Cheng, W.F.; Chang, M.C.; Lu, T.P.; Kuo, K.T.; Lin, H.P.; Hsieh, C.Y.; Chen, C.A. Establishment of a New Ovarian Cancer Cell Line CA5171. Reprod. Sci. 2015, 22, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Lo, S.N.; Lee, W.C.; Hsu, W.C.; Lee, T.W.; Chang, C.H. Evaluation of Nanotargeted (111)In-Cyclic RGDfK-Liposome in a Human Melanoma Xenotransplantation Model. Int. J. Mol. Sci. 2021, 22, 1099. [Google Scholar] [CrossRef]
- Chang, M.C.; Chiang, P.F.; Kuo, Y.J.; Peng, C.L.; Chen, I.C.; Huang, C.Y.; Chen, C.A.; Chiang, Y.C. Develop companion radiopharmaceutical YKL40 antibodies as potential theranostic agents for epithelial ovarian cancer. Biomed. Pharmacother. 2022, 155, 113668. [Google Scholar] [CrossRef]
- Hsu, W.C.; Cheng, C.N.; Lee, T.W.; Hwang, J.J. Cytotoxic Effects of PEGylated Anti-EGFR Immunoliposomes Combined with Doxorubicin and Rhenium-188 Against Cancer Cells. Anticancer Res. 2015, 35, 4777–4788. [Google Scholar]
- Shih, Y.H.; Luo, T.Y.; Chiang, P.F.; Yao, C.J.; Lin, W.J.; Peng, C.L.; Shieh, M.J. EGFR-targeted micelles containing near-infrared dye for enhanced photothermal therapy in colorectal cancer. J. Control. Release 2017, 258, 196–207. [Google Scholar] [CrossRef]
- Guan, S.S.; Wu, C.T.; Liao, T.Z.; Lin, K.L.; Peng, C.L.; Shih, Y.H.; Weng, M.F.; Chen, C.T.; Yeh, C.H.; Wang, Y.C.; et al. A novel (111)indium-labeled dual carbonic anhydrase 9-targeted probe as a potential SPECT imaging radiotracer for detection of hypoxic colorectal cancer cells. Eur. J. Pharm. Biopharm. 2021, 168, 38–52. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Albumin-Binding Evans Blue Derivatives for Diagnostic Imaging and Production of Long-Acting Therapeutics. Bioconjug. Chem. 2016, 27, 2239–2247. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-C.; Chen, C.-T.; Chiang, P.-F.; Tang, I.-C.; Peng, C.-L.; Wang, Y.-F.; Tai, Y.-J.; Chiang, Y.-C. Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors. Int. J. Mol. Sci. 2025, 26, 6565. https://doi.org/10.3390/ijms26146565
Chang M-C, Chen C-T, Chiang P-F, Tang I-C, Peng C-L, Wang Y-F, Tai Y-J, Chiang Y-C. Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors. International Journal of Molecular Sciences. 2025; 26(14):6565. https://doi.org/10.3390/ijms26146565
Chicago/Turabian StyleChang, Ming-Cheng, Chun-Tang Chen, Ping-Fang Chiang, I-Chung Tang, Cheng-Liang Peng, Yuh-Feng Wang, Yi-Jou Tai, and Ying-Cheng Chiang. 2025. "Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors" International Journal of Molecular Sciences 26, no. 14: 6565. https://doi.org/10.3390/ijms26146565
APA StyleChang, M.-C., Chen, C.-T., Chiang, P.-F., Tang, I.-C., Peng, C.-L., Wang, Y.-F., Tai, Y.-J., & Chiang, Y.-C. (2025). Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors. International Journal of Molecular Sciences, 26(14), 6565. https://doi.org/10.3390/ijms26146565






