Microgravity Therapy as Treatment for Decelerated Aging and Successful Longevity
Abstract
1. Introduction
1.1. Understanding Aging: A Foundation for Longevity Research
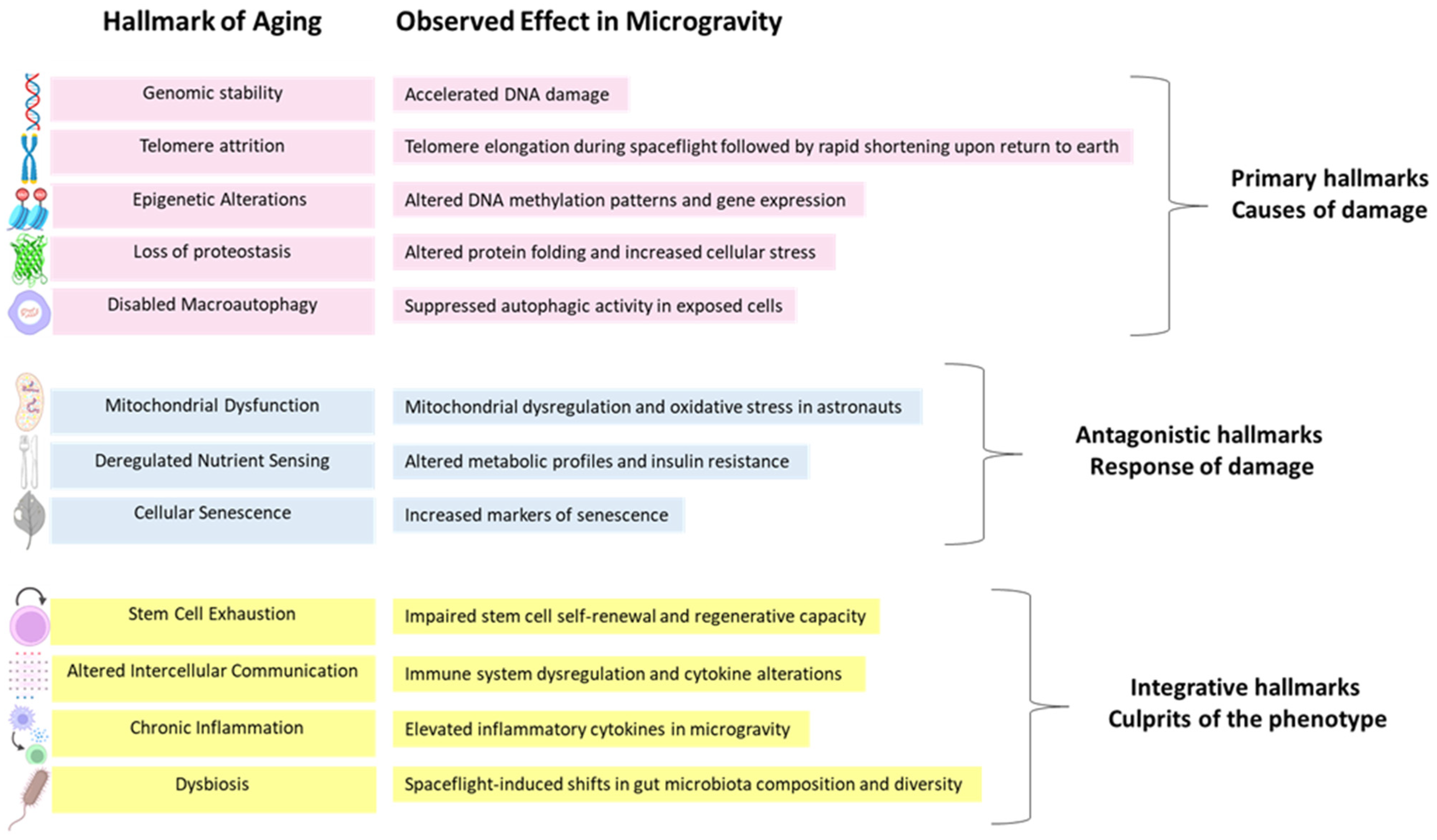
1.2. The Hallmarks of Aging
1.3. Aging Associated Diseases
1.4. Major Aging Treatments
1.5. Microgravity’s Impact on Human Physiology
1.6. NASA’s Twins Study
1.7. Candidate Genes Regulated by Microgravity
1.8. The International Space Station (ISS)
1.9. Microgravity Research: Biological Application
2. Discussion
Author Contributions
Funding
Conflicts of Interest
Correction Statement
Abbreviations
| ISS | International Space Station |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| CVD | Cardiovascular diseases |
| AD | Alzheimer’s disease |
| mTOR | mechanistic target of rapamycin |
| OOC | Organ-on-a-Chip |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Pyrkov, T.V.; Avchaciov, K.; Tarkhov, A.E.; Menshikov, L.I.; Gudkov, A.V.; Fedichev, P.O. Longitudinal analysis of blood markers reveals progressive loss of resilience and predicts human lifespan limit. Nat. Commun. 2021, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, A.; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and oxidative stress: The role of aging. Antioxidants 2021, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-García, N.; Huete-Acevedo, J.; Dromant, M.; Borrás, C. MicroRNA biogenesis pathway alterations in aging. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Iwasaki, K.; Abarca, C.; Aguayo-Mazzucato, C. Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications. Diabetes Metab. J. 2023, 47, 441–453. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Rossman, M.J.; Mahoney, S.A.; Venkatasubramanian, R.; Maurer, G.S.; Hutton, D.A.; VanDongen, N.S.; Greenberg, N.T.; Longtine, A.G.; Ludwig, K.R.; et al. Cellular Senescence Contributes to Large Elastic Artery Stiffening and Endothelial Dysfunction with Aging: Amelioration with Senolytic Treatment. Hypertension 2023, 80, 2072–2087. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Wesselbaum, D. Global evidence of inequality in well-being among older adults. J. Am. Geriatr. Soc. 2024, 72, 842–849. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Waaijer, M.E.C.; Slee-Valentijn, M.S.; Stijnen, T.; Westendorp, R.; Maier, A.B. Cellular senescence and chronological age in various human tissues: A systematic review and meta-analysis. Aging Cell 2020, 19, e13083. [Google Scholar] [CrossRef]
- López-Otín, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, N.; Zhang, H.; Liang, G.; Lin, Y.; Qian, Z.; Yang, X.; Yang, J.; Fu, Y.; Zhang, C.; et al. Systemic aging and aging-related diseases. FASEB J. 2025, 39, e70430. [Google Scholar] [CrossRef]
- Trevisan, K.; Cristina-Pereira, R.; Silva-Amaral, D.; Aversi-Ferreira, T.A. Theories of aging and the prevalence of Alzheimer’s disease. BioMed Res. Int. 2019, 2019, 9171424. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Wong, N.D.; Wang, J. Impact of Aging on Cardiovascular Diseases: From Chronological Observation to Biological Insights: JACC Family Series. JACC Asia 2024, 4, 345–358. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Sadighi Akha, A.A. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Tomasetti, C.; Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef]
- Pavlidis, N.; Stanta, G.; Audisio, R.A. Cancer prevalence and mortality in centenarians: A systematic review. Crit. Rev. Oncol. Hematol. 2012, 83, 145–152. [Google Scholar] [CrossRef]
- Hashim, D.; Carioli, G.; Malvezzi, M.; Bertuccio, P.; Waxman, S.; Negri, E.; La Vecchia, C.; Boffetta, P. Cancer mortality in the oldest old: A global overview. Peer-Rev. Aging Res. J. 2020, 12, 15. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. MTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of rapamycin on aging and age-related diseases—Past and future. Geroscience 2021, 43, 1135–1158. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Musi, N.; Valentine, J.M.; Sickora, K.R.; Baeuerle, E.; Thompson, C.S.; Shen, Q.; Orr, M.E. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 2018, 17, e12840. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.R.; Daly, L.; Hassibi, S.; Kimura, G.; Nishimoto, Y.; Kizawa, Y.; Ito, K. Senolytic therapy reduces inflammation in epithelial cells from COPD patients and in smoke-exposure mice. Front. Med. 2025, 12, 1451056. [Google Scholar] [CrossRef]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef]
- Gadecka, A.; Nowak, N.; Bulanda, E.; Janiszewska, D.; Dudkowska, M.; Sikora, E.; Bielak-Zmijewska, A. The senolytic cocktail, dasatinib and quercetin, impacts the chromatin structure of both young and senescent vascular smooth muscle cells. GeroScience 2025, 47, 3907–3925. [Google Scholar] [CrossRef]
- Millar, C.L.; Iloputaife, I.; Baldyga, K.; Norling, A.M.; Boulougoura, A.; Vichos, T.; Tchkonia, T.; Deisinger, A.; Pirtskhalava, T.; Kirkland, J.L.; et al. A pilot study of senolytics to improve cognition and mobility in older adults at risk for Alzheimer’s disease. EBioMedicine 2025, 113, 105612. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Fernandes, I.G.; Oliveira, L.d.M.; Andrade, M.M.d.S.; Alberca, R.W.; Lima, J.C.; de Sousa, E.S.A.; Pietrobon, A.J.; Pereira, N.Z.; Branco, A.C.C.C.; Duarte, A.J.d.S.; et al. Resveratrol Upregulates Antioxidant Factors Expression and Downmodulates Interferon-Inducible Antiviral Factors in Aging. Int. J. Mol. Sci. 2025, 26, 2345. [Google Scholar] [CrossRef]
- Salvestrini, V.; Sell, C.; Lorenzini, A. Obesity may accelerate the aging process. Front. Endocrinol. 2019, 10, 266. [Google Scholar] [CrossRef]
- Chang, L.; Fan, W.; Pan, X.; Zhu, X.; Guo, L. Stem cells to reverse aging. Chin. Med. J. 2022, 135, 901–910. [Google Scholar] [CrossRef]
- Bo, M.D.; Gambirasi, M.; Vruzhaj, I.; Cecchin, E.; Pishdadian, A.; Toffoli, G.; Safa, A. Targeting Aging Hallmarks with Monoclonal Antibodies: A New Era in Cancer Immunotherapy and Geriatric Medicine. Int. J. Mol. Sci. 2025, 26, 4982. [Google Scholar] [CrossRef]
- Venkataraman, A.; Kordic, I.; Li, J.; Zhang, N.; Bharadwaj, N.S.; Fang, Z.; Das, S.; Coskun, A.F. Decoding senescence of aging single cells at the nexus of biomaterials, microfluidics, and spatial omics. npj Aging 2024, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Simpkins, J.W.; Ji, X.; Leis, M.; Stambler, I. The critical need to promote research of aging and aging-related diseases to improve health and longevity of the elderly population. Aging Dis. 2015, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N. Small Nonhuman Primates as Potential Models of Human Aging. ILAR J. 1997, 38, 142–147. [Google Scholar] [CrossRef][Green Version]
- Andrawus, M.; Sharvit, L.; Touitou, N.; Lerrer, B.; Cohen, H.Y.; Atzmon, G. Copy number variation as a tool for implementing pregnancy as an aging model. Aging 2023, 15, 7922–7932. [Google Scholar] [CrossRef]
- Giller, A.; Andrawus, M.; Gutman, D.; Atzmon, G. Pregnancy as a model for aging. Ageing Res. Rev. 2020, 62, 101093. [Google Scholar] [CrossRef]
- Bradbury, P.; Wu, H.; Choi, J.U.; Rowan, A.E.; Zhang, H.; Poole, K.; Lauko, J.; Chou, J. Modeling the Impact of Microgravity at the Cellular Level: Implications for Human Disease. Front. Cell Dev. Biol. 2020, 8, 96. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamura, A.; Shimizu, T. Simulated microgravity accelerates aging of human skeletal muscle myoblasts at the single cell level. Biochem. Biophys. Res. Commun. 2021, 578, 115–121. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Fu, Z.; Xing, C.; Wang, Z.; Yang, H.; Li, J.; Liu, M.; Dong, L.; Zhang, X.; et al. Long-term simulated microgravity fosters carotid aging-like changes via Piezo1. Cardiovasc. Res. 2024, 120, 548–559. [Google Scholar] [CrossRef]
- Gertz, M.L.; Chin, C.R.; Tomoiaga, D.; MacKay, M.; Chang, C.; Butler, D.; Afshinnekoo, E.; Bezdan, D.; Schmidt, M.A.; Mozsary, C.; et al. Multi-omic, Single-Cell, and Biochemical Profiles of Astronauts Guide Pharmacological Strategies for Returning to Gravity. Cell Rep. 2020, 33, 108429. [Google Scholar] [CrossRef]
- Doroshin, A.; Jillings, S.; Jeurissen, B.; Tomilovskaya, E.; Pechenkova, E.; Nosikova, I.; Rumshiskaya, A.; Litvinova, L.; Rukavishnikov, I.; De Laet, C.; et al. Brain Connectometry Changes in Space Travelers After Long-Duration Spaceflight. Front. Neural Circuits 2022, 16, 815838. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA twins study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, E. A review of the effects of microgravity and of hypergravity on aging and longevity. Exp. Gerontol. 1999, 34, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Galčenko, K.; Bourdakou, M.M.; Spyrou, G.M. Exploring the Impact of Microgravity on Gene Expression: Dysregulated Pathways and Candidate Repurposed Drugs. Int. J. Mol. Sci. 2025, 26, 1287. [Google Scholar]
- Hwang, H.; Rampoldi, A.; Forghani, P.; Li, D.; Fite, J.; Boland, G.; Maher, K.; Xu, C. Space microgravity increases expression of genes associated with proliferation and differentiation in human cardiac spheres. npj Microgravity 2023, 9, 88. [Google Scholar] [CrossRef]
- Mair, D.B.; Tsui, J.H.; Higashi, T.; Koenig, P.; Dong, Z.; Chen, J.F.; Meir, J.U.; Smith, A.S.T.; Lee, P.H.U.; Ahn, E.H.; et al. Spaceflight-induced contractile and mitochondrial dysfunction in an automated heart-on-a-chip platform. Proc. Natl. Acad. Sci. USA 2024, 121, e2404644121. [Google Scholar] [CrossRef]
- Wu, F.; Du, H.; Overbey, E.; Kim, J.; Makhijani, P.; Martin, N.; Lerner, C.A.; Nguyen, K.; Baechle, J.; Valentino, T.R.; et al. Single-cell analysis identifies conserved features of immune dysfunction in simulated microgravity and spaceflight. Nat. Commun. 2024, 15, 4795. [Google Scholar] [CrossRef]
- Ruyters, G.; Friedrich, U. From the Bremen Drop Tower to the international space station ISS—Ways to weightlessness in the German Space Life Sciences Program. Signal Transduct. 2006, 6, 397–405. [Google Scholar] [CrossRef]
- Amselem, S. Remote Controlled Autonomous Microgravity Lab Platforms for Drug Research in Space. Pharm. Res. 2019, 36, 183. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Bhusnure, O.G.; Satpute, V.; Gholve, S.B.; Giram, P.S.; Jagtap, S.; Chakure, S.S. Organs-On-A-Chip: A New Tool for Drug Discovery. Int. J. ChemTech Res. 2017, 10, 35–49. [Google Scholar]
- de Mello, C.P.P.; Rumsey, J.; Slaughter, V.; Hickman, J.J. A human-on-a-chip approach to tackling rare diseases. Drug Discov. Today 2019, 24, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Tagle, D.A. Tissue chips to aid drug development and modeling for rare diseases. Expert Opin. Orphan Drugs 2016, 4, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef]
- Amselem, S.; Kogan, D.; Loboda, O.; Levy, A.; Feuchtwanger, Y.; Bavli, D. Monoclonal Antibodies from Space: Improved Crystallization Under Microgravity During Manufacturing in Orbit. J. Explor. Res. Pharmacol. 2024, 9, 96–105. [Google Scholar] [CrossRef]
- Cohen, A.A. Aging across the tree of life: The importance of a comparative perspective for the use of animal models in aging. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 2680–2689. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Nie, H.-Y.; Ge, J.; Liu, K.-G.; Yue, Y.; Li, H.; Lin, H.-G.; Yan, H.-F.; Zhang, T.; Sun, H.-W.; Yang, J.-W.; et al. The effects of microgravity on stem cells and the new insights it brings to tissue engineering and regenerative medicine. Life Sci. Space Res. 2024, 41, 1–17. [Google Scholar] [CrossRef]
- Sharma, A.; Clemens, R.A.; Garcia, O.; Taylor, D.L.; Wagner, N.L.; Shepard, K.A.; Gupta, A.; Malany, S.; Grodzinsky, A.J.; Kearns-Jonker, M.; et al. Biomanufacturing in low Earth orbit for regenerative medicine. Stem Cell Rep. 2022, 17, 1–13. [Google Scholar] [CrossRef]
- Weber, B.M.; Panzirsch, M.; Pleintinger, B.; Stelzer, M.; Arand, S.; Schöttler, C.; Bayer, R.; Hagengruber, A.; Proske, U. Disturbances in human position sense during alterations in gravity: A parabolic flight experiment. Exp. Brain Res. 2025, 243, 127. [Google Scholar] [CrossRef]
- Salatino, A.; Iacono, C.; Gammeri, R.; Chiadò, S.T.; Lambert, J.; Sulcova, D.; Mouraux, A.; George, M.S.; Roberts, D.R.; Berti, A.; et al. Zero gravity induced by parabolic flight enhances automatic capture and weakens voluntary maintenance of visuospatial attention. npj Microgravity 2021, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Clément, G.; Macaulay, T.R.; Bollinger, A.; Weiss, H.; Wood, S.J. Functional activities essential for space exploration performed in partial gravity during parabolic flight. npj Microgravity 2024, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human pathophysiological adaptations to the space environment. Front. Physiol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
| Treatment/Approach | Therapeutic Category | Associated Effect | Reference |
|---|---|---|---|
| Rapamycin | Pharmacological | mTOR inhibitor enhanced resilience | [29] |
| Senolytics (Dasatinib, Quercetin) | Pharmacological | Reduced senescence, improved tissue function | [32] |
| Metformin | Pharmacological | Improved mitochondrial function, reduced oxidative stress, and inflammation | [38] |
| Resveratrol | Pharmacological | Antioxidant effects, activation of longevity-associated genes | [38] |
| Caloric restriction | Non- Pharmacological | Reduced inflammation, improved metabolic health | [39] |
| Physical exercise | Non- Pharmacological | Enhanced metabolic, cardiovascular health | [39] |
| Stem cell therapies | Biologics | Tissue regeneration, immune modulation | [40] |
| Monoclonal antibodies (anti-senescence) | Biologics | Targeting senescent cells, modulation of age-related pathways | [41] |
| Biomaterials, scaffolds | Materials | Tissue engineering | [42] |
| Gene Symbol | Regulation | Associated Function | Reference |
|---|---|---|---|
| CSGALNACT2 | Upregulated | Biosynthesis, inflammation response | [54] |
| CSNK2A2 | Upregulated | Cell cycle regulation, DNA repair, and apoptosis | [54] |
| HIPK1 | Upregulated | Apoptosis, development, and stress response pathways | [54] |
| MBNL2 | Upregulated | RNA-binding protein, alternative splicing regulation | [54] |
| PHF21A | Upregulated | Transcriptional repression, chromatin remodeling | [54] |
| RAP1A | Upregulated | Cell adhesion, proliferation, and differentiation | [54] |
| DNPH1 | Downregulated | Catabolism of deoxynucleotides | [54] |
| EXOSC5 | Downregulated | RNA processing | [54] |
| L3MBTL2 | Downregulated | Regulation of gene expression, chromatin organization | [54] |
| LGALS3BP | Downregulated | Immune response, cell adhesion | [54] |
| SPRYD4 | Downregulated | Cell cycle regulation | [54] |
| CCND1 | Upregulated | Cell cycle, cell proliferation | [55] |
| CCND2 | Upregulated | Cell cycle, cell proliferation | [55] |
| IGF2 | Upregulated | Cell cycle, cell proliferation | [55] |
| TBX3 | Upregulated | Cardiac differentiation | [55] |
| Contractile and calcium genes | Downregulated | Muscle contraction, calcium handling | [56] |
| Oxidative stress and inflammatory genes | Upregulated | Inflammation, mitochondrial dysfunction | [56] |
| IL-6 pathway genes | Altered | Inflammation, immune regulation | [57] |
| Sirtuin-regulated genes | Altered | Metabolic control, aging resilience | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozalbat, N.; Sharvit, L.; Atzmon, G. Microgravity Therapy as Treatment for Decelerated Aging and Successful Longevity. Int. J. Mol. Sci. 2025, 26, 6544. https://doi.org/10.3390/ijms26136544
Mozalbat N, Sharvit L, Atzmon G. Microgravity Therapy as Treatment for Decelerated Aging and Successful Longevity. International Journal of Molecular Sciences. 2025; 26(13):6544. https://doi.org/10.3390/ijms26136544
Chicago/Turabian StyleMozalbat, Nadine, Lital Sharvit, and Gil Atzmon. 2025. "Microgravity Therapy as Treatment for Decelerated Aging and Successful Longevity" International Journal of Molecular Sciences 26, no. 13: 6544. https://doi.org/10.3390/ijms26136544
APA StyleMozalbat, N., Sharvit, L., & Atzmon, G. (2025). Microgravity Therapy as Treatment for Decelerated Aging and Successful Longevity. International Journal of Molecular Sciences, 26(13), 6544. https://doi.org/10.3390/ijms26136544






