Abstract
Postpartum depression (PPD) remains a significant health concern worldwide. Both non-pharmacological and pharmacological treatments are available for patients with PPD; however, the standard approach involving selective serotonin reuptake inhibitors (SSRIs) and other antidepressants fails to provide a rapid response. This narrative review presents basic clinical and epidemiological data on PPD, summarizes currently used pharmacotherapies of PPD, highlights their limitations, and discusses new therapies based on a revised understanding of the disease’s pathogenesis. Numerous studies indicate that dysregulation of GABAergic neurotransmission, which may result from fluctuating levels of neuroactive steroids during pregnancy and the postpartum period, plays an important role in the complex pathology of PPD. Considering this, neuroactive steroids, which act as positive allosteric modulators of central GABAA receptors (GABAARs), may offer new promising avenues for treating PPD. The first rapid-acting neurosteroid approved by the FDA to treat PPD in women is brexanolone, although its use is constrained by pharmacokinetic properties. The first oral neuroactive steroid-based antidepressant approved by the FDA for PPD is zuranolone. This review discusses the molecular mechanism of zuranolone action and the results of preclinical and clinical studies regarding the effectiveness and safety of the drug in treating PPD.
1. Postpartum Depression
Depressive disorders are considered to be among the most common mental illnesses worldwide [1]. One of the “faces of depression” is postpartum depression (PPD), also known as perinatal depression. Low mood and apathy right after giving birth are the norm. This phenomenon, called “baby blues” (or postpartum blues), affects 50% to 80% of mothers. Postpartum blues tends to begin 1 to 3 days after parturition, lasting at least 2 weeks, and sometimes continues to develop into PPD [2]. According to the Diagnostic and Statistical Manual of Mental Disorders—Fifth Edition (DSM-5), PPD is an episode of a major depressive disorder (MDD) [3]. For PPD diagnosis the 10-item Edinburgh Postnatal Depression Scale (EPDS), endorsed by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists is also used [4]. Guidelines and definitions of PPD vary with respect to the time period of onset, and usually include symptoms starting during pregnancy or within the period from 4 weeks to 12 months postpartum [3,4]. The peaks of PPD occur during the first 6 months postpartum, especially at 2–3 weeks and 6–8 weeks [5]. PPD is a global health issue—it can affect women from all races, ethnicities, cultures, and educational or economic backgrounds. PPD is one of the most prevalent psychiatric disorders during the peripartum period [6]. The prevalence of PPD varies substantially depending on the definition of the disorder, country, diagnostic tools used, threshold of discrimination chosen for the screening measure, and period over which the prevalence is determined. In a recent systematic review, Bai and coworkers assessed the prevalence of PPD based exclusively on studies using diagnostic interviews. The pooled prevalence of all forms of depression and major depression within one year postpartum was 12.1% and 7.0%, respectively [5]. Despite having a high prevalence, PPD is underdiagnosed and undertreated [7]. Risk factors for PPD include a history of depression, a history of physical or sexual abuse, carrying an unplanned or unwanted pregnancy, younger age, multiparity, obstetric difficulties, poor marital relationship, being unemployed, poor sleep quality, lack of social support, and congenital abnormality [8,9,10,11,12,13,14,15].
PPD is characterized by a mix of symptoms, like low mood. The woman often cries, even for no reason; she is irritated, sad, and depressed for most of the day; she has a lack of energy to perform even basic activities; she shows a loss of interests, anhedonia, lowered self-esteem, and a sense of worthlessness (e.g., thinking about herself, “I am a bad, hopeless mother”, “I am not cut out to be a mother”); she has an excessive or inadequate sense of guilt (e.g., “I do not take care of the child as I should”, “I do not devote myself enough to the child, therefore I am a bad mother”, “I think too much about myself and too little about the child”); she feels a sense of helplessness and inability to cope in the role of a mother (e.g., “Taking care of a child is beyond me”); and she suffers from insomnia, cognitive disturbances, impaired concentration/decision making, memory problems, and anxiety states related to, among other things, often unfounded worries about the child’s health, as well as the need to care for it. Anxiety may be present in approximately 70% of women with PPD [6,16]. Severe PPD symptomatology is correlated with suicidal ideation and significantly increases the risk of completed suicide [17,18]. When compared to MDD, PPD exhibits several distinct characteristics, i.e., the onset of the disease, and more anxiety, psychomotor symptoms, obsessive thoughts, impaired concentration, fatigue, and loss of energy, but less psychoticism, sleep disturbances, and suicidal ideation [6]. PPD is associated with numerous short- and long-term outcomes for the mother, the child, and the entire family, especially if it remains undetected and untreated [19,20,21]. In severe cases, PPD can lead to neglect of the infant or thoughts of harming the infant [22,23]. The disease is associated with poor maternal functioning, worse quality of the mother’s life, poor physical and mental health of the mother, disruption of mother–infant bonding, and relational harms in the mother–child–partner triad. Several studies demonstrate that PPD may have a negative impact on the emotional, cognitive, and social development of a child [24,25,26,27,28,29]. An estimated mean time for a full remission of PPD is 49 weeks, with 30% of patients recovering at 6 months, 66% at 12 months, and 94% at 24-month follow-up [30]. Analysis of longitudinal clinical studies on PPD indicated that at any time point between 4 months and 3 years postpartum, about 30% of mothers diagnosed with PPD still meet the criteria for depression [31].
PPD is a very heterogeneous disorder, and various biological, psychological, and sociocultural factors underlie the etiology of the disease. The pathophysiology of PPD involves a complex interplay between different processes. Despite numerous studies, our understanding of the pathogenesis of PPD still remains incomplete. Recent hypotheses suggest that multiple neurobiological factors, such as hormonal changes, including dysregulation of the hypothalamic–pituitary–gonadal and hypothalamic–pituitary–adrenal axes, neurotransmitter imbalances, disturbances in functioning of neural networks, neuroactive steroids, psychosocial stressors, and inflammation, all play important roles in the pathophysiology of PPD [32].
2. Current Therapeutic Approaches for PPD
There are two basic approaches to treat PPD: non-pharmacological treatment and pharmacotherapy. Non-pharmacological treatments include cognitive and behavioral psychotherapy, psychodynamic psychotherapy, psychoeducation, psychosocial interventions, supportive counseling, interpersonal psychotherapy, creative art therapy, and light therapy. Other non-pharmacological treatments for PPD, like electroconvulsive therapy and repetitive transcranial magnetic stimulation, are also used, but much less often [33,34,35,36,37]. Of these, cognitive behavioral psychotherapy and interpersonal therapy are frequently recommended as first-line treatments for mild-to-moderate PPD [36].
For decades, pharmacological treatment strategies for depression have been based primarily on the monoaminergic deficiency hypothesis. This theory assumes that depression is caused by an imbalance between monoaminergic neurotransmitters in the brain, mainly serotonin and noradrenaline, or their pathologically low levels. According to this hypothesis, the therapeutic effects of antidepressants, including, among others, tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and serotonin and noradrenaline reuptake inhibitors (SNRIs), result from increased serotoninergic/noradrenergic transmission in the brain [38]. However, the currently used antidepressants have three important limitations: (1) although synaptic monoamine concentrations increase rapidly after taking these drugs, the therapeutic effects occur with a long delay of several weeks; (2) about 30% of patients suffer from treatment-resistant depression; and (3) the drugs exert several adverse effects, including, among others, weight gain, sexual dysfunction, cardiovascular disturbances, and sleep problems [38].
Historically, the gold standard pharmacologic treatment for PPD has been focused on using antidepressants indicated for MDD outside of the perinatal period, with SSRIs being the most common. However, classical antidepressants do not offer a rapid treatment response, nor are they specific for PPD. In the United States, conventional antidepressants are not approved by the Food and Drug Administration (FDA) for the treatment of PPD (Table 1), thus their use is considered off-label (for reviews see [39,40,41,42]). The facts presented above clearly indicate that there is an urgent need for novel rapidly acting, specific therapies for PPD. One promising avenue to achieve this goal is based on neuroactive steroids and the gamma aminobutyric acid (GABA) signaling hypothesis. According to this hypothesis, dysfunctional GABAergic signaling related to stress, fluctuations in reproductive hormone levels, and rapid loss of circulating levels of allopregnanolone, a neuroactive steroid, at parturition, may play an important role in the pathophysiology of PPD [43].

Table 1.
FDA status of antidepressant drugs used for PPD treatment.
3. In Search of New Drugs for PPD—Neuroactive Steroids and GABAergic Signaling Hypothesis
Functional communication within and between brain circuits is tightly controlled by a balance between excitatory (glutamatergic) and inhibitory (GABAergic) inputs. Depression is associated with dysregulated excitation–inhibition balance in part due to decreased GABA levels or signaling activity. GABA exerts biological effects by stimulating specific membrane bound receptors, namely ionotropic GABAARs and metabotropic G-protein coupled GABABRs. GABAARs are heteropentamers composed of various subunits, typically a combination of α1-6, β1-4, γ1-3, δ, ε, θ, and ρ1-3, that form ligand-gated chloride channels. Binding of drugs to GABAAR depends on its subunit composition [49]. When activated, the channels open, permitting chloride ion influx, and thus hyperpolarization of the cell membrane. GABA can elicit CNS inhibition as either a short signal in response to activation of synaptic GABAARs (phasic inhibition), or a longer-lasting inhibition in response to activation of extrasynaptic GABAARs (tonic inhibition). Tonic inhibition regulates excitation through long-term hyperpolarization and plays an important role in synaptic plasticity, neurogenesis, and cognitive functions [50].
Steroid hormones play a critical role during pregnancy, and of particular importance is a progesterone metabolite and neuroactive steroid, allopregnanolone (3α-hydroxy-5α-9pregnan-20-one) (Figure 1). Allopregnanolone is generated from progesterone by sequential actions of 5α-reductase and 3α-hydroxysteroid dehydrogenase. Levels of allopregnanolone increase during pregnancy, decline after birth, and remain low up to 6 months postpartum [51,52]. Concomitant with an increase in allopregnanolone levels during pregnancy, a decrease in GABAAR expression occurs, yielding a stable inhibition. Following a rapid decline of allopregnanolone at parturition, the recovery of GABAAR expression is delayed, creating a hyperexcitable state that may persist and participate in the symptomology of PPD [40,51,53].
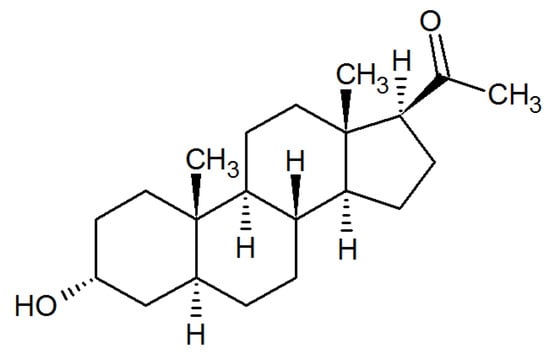
Figure 1.
The chemical structure of allopregnanolone.
Neuroactive steroids are endogenous neuromodulators that rapidly alter neuronal excitability by binding to membrane-bound receptors. They can be de novo synthesized in the brain from cholesterol, particularly within excitatory neurons, but also glial cells, and their synthesis is altered by acute cellular and environmental stressors [54,55]. They can also reach the brain from peripheral steroidogenic organs, such as the adrenal glands and gonads [56]. A major class of these steroids includes allopregnanolone, a progesterone metabolite [57]. Neuroactive steroids powerfully modulate major targets in the brain by affecting ligand-gated and voltage-gated ion channels that regulate neuronal excitability, intercellular communication, and plasticity [58]. They are essential in the regulation of the hypothalamic–pituitary–adrenal axis during acute and chronic stress [59,60]. In addition, they exert anti-inflammatory and neurotrophic effects [59,61,62,63], and induce potent anxiolytic, antidepressant, anticonvulsant, sedative, analgesic, and amnesic effects, mainly through interaction with the GABAARs in the brain [56].
Neuroactive steroids are positive allosteric modulators (PAMs) of GABAARs in the brain. They prolong the opening time of chloride ion channels within GABAARs, thus enhancing inhibitory neurotransmission. It is hypothesized that neuroactive steroids restore excitation–inhibition balance in the brain areas dysregulated in depression [64]. Although they bind to GABAARs containing δ, α4, or α6 subunits localized both at synaptic and extrasynaptic sites, they preferentially interact with extrasynaptic GABAARs containing δ subunits, and at lower concentrations specifically enhance a tonic inhibitory conductance that is mediated by this type of receptors. At higher concentrations, neuroactive steroids also potentiate phasic inhibition mediated by synaptic α1β2γ2 GABAARs [65,66,67]. In contrast, benzodiazepines, another group of positive allosteric modulators of GABAARs, primarily activate synaptic GABAAR receptors by binding to the interface of the ɣ2 subunit and α1-3 and α5 subunits; GABAARs with this composition are localized to synaptic sites (Figure 2) [68].
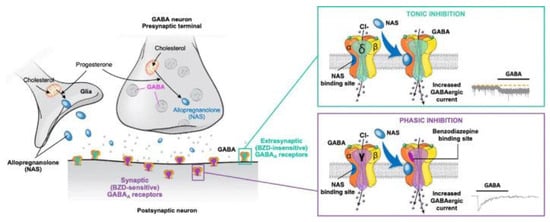
Figure 2.
Mechanism of action of the positive allosteric modulator allopregnanolone on synaptic and extrasynaptic GABAA receptors. BZD, benzodiazepine; GABA, γ-aminobutyric acid; NAS, neuroactive steroid. Figure is reproduced from Gunduz-Bruce et al., 2022 [66] according to the terms of the Creative Commons Attribution License.
The distinct sites of neuroactive steroid action at GABAARs, when compared with other PAMs, offer a new opportunity to explore a GABA-based treatment approach for PPD [69,70]. In line with this, recent studies performed on mice demonstrated that allopregnanolone, but not diazepam (a benzodiazepine drug), increased theta oscillation in the basolateral amygdala and medial prefrontal cortex by stimulating δ-subunit-containing GABAARs. Furthermore, only allopregnanolone exhibited antidepressant-like effects in chronic unpredictable stress and social defeat stress mouse models of depression [55,69,70].
4. Neuroactive Steroids as Novel Promising Drugs in Therapy of PPD
4.1. Brexanolone
On 19 March 2019, brexanolone (SAGE-547; ZULRESSO™, Sage Therapeutics, Inc, Cambridge, MA, USA) became the first rapid-acting neuroactive steroid drug approved by the FDA (SAGE-547; ZULRESSO™) specifically to treat PPD in women aged 18–45 years [44]. It was developed by Sage Therapeutics Inc. under a license to the University of California [71]. Brexanolone is prepared as an isotonic solution of 5 mg/mL allopreganolone buffered in 250 mg/mL sulfobutylether-β-cyclodextrin, a solubilizing agent. As brexanolone has poor bioavailability (<5%) when given orally, it is administered as a continuous intravenous infusion over 60 h (2.5 days). During infusion, patients must be accompanied by their infants. The optimal pattern of brexanolone dosing in order to maintain effective levels and cause PPD remission is intravenous administration of 30 μg/kg/h during the first 4 h; this is then increased to 60 μg/kg/h (4 to 24 h) and 90 μg/kg/h (24 to 52 h); decreased to 60 μg/kg/h (52 to 56 h); and finally decreased to 30 μg/kg/h (56 to 60 h) [72]. Three positive randomized, double-blind, placebo-controlled trials of brexanolone versus placebo demonstrated a sustained response to brexanolone infusion through 30 days of follow-up post treatment [73,74]. Significant improvements in depressive symptoms on the Hamilton Depression Rating Scale (HAMD), consistent with effects of other antidepressants but remarkably faster (60 h versus 4 weeks), were reported [75]. In humans, brexanolone is metabolized by non-CYP pathways, mainly ketoreduction, glucuronidation, and sulfation, to inactive metabolites; thus, it is not likely to be a substrate of pharmacokinetic interactions with a concomitant drug used [75]. The most common adverse effects of brexanolone are dizziness, sedation/somnolence, headaches, and dry mouth (xerostomia). Suicidal ideation, presyncope, vertigo, tachycardia, and hot flashes were also reported, but at a very low rate [73,74]. Due to the risk of serious harm in patients treated with brexanolone, during infusion monitoring for excessive sedation and sudden loss of consciousness, continuous pulse oximetry is required. At present, treatment with brexanolone is available only through the FDA-approved restricted Risk Evaluation and Mitigation Strategy (REMS) program [73,75].
4.2. Zuranolone (ZURZUVAE™)—The First FDA-Approved Oral Neuroactive Steroid-Based Antidepressant for PPD
On 4 August 2023, the FDA approved zuranolone (SAGE-217; ZURZUVAE™), developed by Sage Therapeutics Inc. (Cambridge, MA, USA) and Biogen Inc., (Cambridge, MA, USA) as the first oral neuroactive steroid-based drug for the treatment of PPD [45,76]. The chemical structure of zuranolone, 1-[(3α,5β)-3-hydroxy-3-methyl-20-oxo-19-norpregnan-21-yl]-1H-pyrazole-4-carbonitrile, is distinct from brexanolone due to the presence of a cyanopyrazole ring at carbon 21 [77] (Figure 3). When compared with brexanolone, zuranolone has the advantage of being orally bioavailable, and thus offers the ease of once-daily, evening dosing at home.
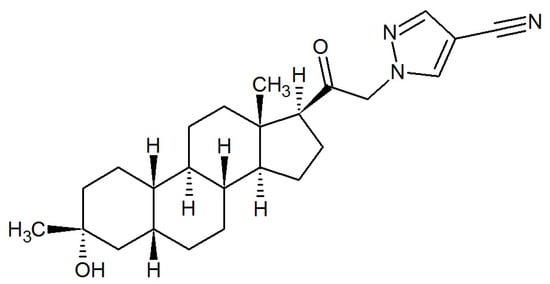
Figure 3.
The chemical structure of zuranolone.
4.2.1. Zuranolone—Preclinical Studies
The effects of zuranolone on GABAAR were examined in vitro using a patch clamp technique. Experiments were performed on recombinant cell lines stably expressing specific receptor subunit combinations: Xenopus oocytes (α1β1γ2 and α6β3δ) and Ltk (α1β2γ2 and α2β2γ2), or Chinese hamster ovary cells transiently expressing α4β3δ. Zuranolone enhanced GABAAR current at α1β2γ2 receptors, with a calculated EC50 of 430 nM and maximum efficacy (Emax) of 1037%, while at α4β3δ receptors with an EC50 of 118 nM and Emax of 556%. Studies conducted on hippocampal slices from postnatal day-21 mice demonstrated that zuranolone enhances both tonic and phasic conductance of GABAARs [78].
The pharmacokinetic parameters of zuranolone (10 mg/kg) were evaluated in mice after a single dose of 10 mg/kg given intraperitoneally (IP) or per os (PO). At both routes of administration, a maximum plasma concentration (Cmax) was observed after 30 min. Oral administration resulted in a lower plasma Cmax (1335 ng/mL) compared to IP one (3197 ng/mL). After oral and IP administration, the bioavailability of zuranolone was 62% and 89%, respectively. The brain–plasma ratio following both administrations was in the range of 1.4–1.6 [78].
Pharmacological activity of zuranolone was analyzed using two animal models: pentylenetetrazol-induced seizures in mice and pharmacoEEG in rats. Zuranolone at doses of 1, 3, and 10 mg/kg given PO one hour before systemic administration of pentylenetetrazol (an antagonist of GABAARs) significantly increased the latency to tonic seizures (≥1688 s) compared to vehicle (751 s). The β-band EEG power (power in the 13–30 Hz frequency range) is a robust in vivo, translatable biomarker of GABAAR PAM activity. Oral administration of zuranolone (3 and 20 mg/kg) resulted in a rapid increase in electroencephalogram β-frequency power in rats [78].
4.2.2. Zuranolone—Clinical Studies
Pharmacokinetic Parameters of Zuranolone in Humans
In adults, the mean apparent clearance (CL/F) of zuranolone was 33 L/h and terminal half-life time (t1/2) was 19.7 to 24.6 hrs. The mean blood-to-plasma concentration ratio ranged from 0.54 to 0.58. Zuranolone has a high binding affinity to plasma proteins >99.5%. In healthy subjects, zuranolone administered once daily at a dose of 30 mg reached a steady state after 3–5 days. It was demonstrated that a fat content of a meal has a significant impact on both the Cmax and AUC values. Following administration of 30 mg of zuranolone with a low-fat meal (25% fat), Cmax and AUClast increased by 3.5-fold and 1.8-fold, respectively, compared to fasted conditions. In the case of a high-fat meal (50% fat), Cmax and AUClast increased by 4.3-fold and 2-fold, respectively. The tmax (time to reach maximum concentration) value was not affected by the fat content in the meal. Following oral administration of radiolabeled zuranolone, 45% of the dose was recovered in urine as metabolites with negligible unchanged zuranolone, and 41% in feces as metabolites with less than 2% as unchanged zuranolone [77].
Phase 1, double-blind, placebo-controlled, single ascending dose (SAD) and multiple ascending dose (MAD) studies were conducted to assess the pharmacokinetic properties of zuranolone. A total of 108 healthy volunteers were enrolled in the studies: 72 subjects in the SAD study and 36 subjects in the MAD study. In the SAD study, subjects received a single dose of zuranolone (0.25, 0.75, 2, 5.5, 11, 22, 44, 55, or 66 mg). In the MAD study, subjects received zuranolone at doses of 15 mg, 35 mg, or 30 mg in the morning for 7 days; control groups received placebo for 7 days. Following single doses of zuranolone, plasma concentrations rapidly increased and reached Cmax approximately one hour post dose. Concentrations of the drug then declined in a biphasic manner and exhibited t1/2 ranging from 16 to 23 hrs. For both single and repeated dose administration, t1/2 and tmax showed dose independence [79].
Zuranolone is extensively metabolized in the liver by the CYP3A4 enzyme. This property of zuranolone indicates a possibility of serious pharmacokinetic interactions with other drugs that affect the activity of CYP3A4. Concomitant use of zuranolone with CYP3A4 inhibitors may increase the risk of its adverse effects, while use with CYP3A4 inducers may decrease the efficacy of zuranolone. Thus, it is recommended that when taking zuranolone concurrently with CYP3A4 inhibitors, the dosage must be adjusted, while usage of CYP3A4 inducers together with zuranolone should be avoided [80].
Therapeutical Effects and Safety of Zuranolone
A multicenter ROBIN study (217-PPD-201) was the first phase 3 randomized, double-blind, placebo-controlled study evaluating the efficacy, safety, and pharmacokinetic parameters of zuranolone (30 mg) in the treatment of women with PPD. The 17-item Hamilton Rating Scale for Depression (HAMD17) scale and the Montgomery–Åsberg Depression Rating Scale (MADRS, a 10-item diagnostic questionnaire) were used to assess the severity of depression. The study was conducted on 153 patients (18–45 years old), six months or less postpartum with severe PPD (HAMD17 ≥ 26). Patients received zuranolone (30 mg) or placebo, once daily for 14 days, and were followed up through day 45. Zuranolone exerted a rapid (by day 3), clinically meaningful, and sustained response as compared to placebo. There was a significantly greater reduction from the baseline in the HAMD17 total score with zuranolone in comparison to placebo at day 15 through day 45. HAMD17 remission at day 15 was 45% in those receiving zuranolone, while it was 23% in the placebo group. There was also a sustained larger reduction from the baseline for the MADRS score with zuranolone at day 15. Anxiety symptoms, assessed by the HAMD17 anxiety/somatization subscale and the Edinburgh Postnatal Depression Scale (EPDS) anxiety subscale, demonstrated a significant improvement with zuranolone at days 3 through 45 compared to placebo. Rates of concurrent remission of depressive and anxiety symptoms were higher with zuranolone in comparison to placebo at days 3, 15, and 45. Furthermore, the rate of sustained concurrent remission at days 15 and 45 was also higher with zuranolone. In addition, zuranolone had beneficial effects on insomnia symptoms in PPD patients [81,82].
The SKYLARK study (217-PPD-301) was an additional phase 3, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of 50 mg zuranolone compared to placebo in women with severe PPD. The study was conducted on 196 patients (18–45 years old) with severe PPD (HAMD17 ≥ 26). Patients received zuranolone or placebo once nightly for 14 days, and then were followed up for an additional 4 weeks. By analogy to the results from the ROBIN trial, the antidepressant effects of zuranolone were rapid, starting at day 3, with a median time to first HAMD17 response of 9 days in the zuranolone group as opposed to 43 days in the placebo group. A significant improvement in depressive symptoms was also observed on days 28 and 45. The HAMD17 remission rate was greater for zuranolone compared with placebo at day 45 (44.0% vs. 29.4%). Patient-reported outcomes as measured by the EPDS and Patient Health Questionnaire-9 correlated well with the overall HAMD17 score. A total of 75.5% of patients participating in the SKYLARK study had moderate to severe anxiety (HAMA score ≥ 20). Improvements in anxiety at day 15, as assessed by a change from the baseline (CFB) in the HAMA score, were significantly greater in the zuranolone group compared with the placebo group. Overall, a significantly greater proportion of women receiving zuranolone achieved concurrent remission of depressive and anxiety symptoms compared with those receiving placebo at day 3 (18.9% versus 2.7%), day 15 (40.5% versus. 19.2%), and day 45 (52.1% versus 23.2%). Beneficial effects of zuranolone on insomnia were also observed. The Clinical Global Impression-Improvement (CGI-I) score at day 15 was also markedly higher in the zuranolone group as compared with placebo, indicating improvements across domains of patient quality of life [83].
Zuranolone was generally well tolerated. Both in the ROBIN and SKYLARK studies, the most common treatment-emergent adverse events (≥5% and greater than in the placebo group) in zuranolone-treated patients were somnolence, dizziness, diarrhea, fatigue, and urinary tract and upper respiratory tract infections. No evidence of withdrawal symptoms or increased suicidal ideation or behavior were identified [81,82].
Integrated functional health and well-being data, as assessed using the 36-Item Short Form Health Survey (SF-36), from three MDD trials (201B, MOUNTAIN, and WATERFALL) and one PPD trial (ROBIN), compared zuranolone (30 and 50 mg) with placebo at day 15 and day 42. SF-36 includes scales for physical functioning, social functioning, role limitations due to physical or emotional problems, mental health, energy, pain, and overall health perception. Participants treated with zuranolone showed early (day 15), clinically meaningful improvements in the analyzed parameters related to mental well-being: vitality, social functioning, role limitations due to emotional problems, and mental health. Importantly, patients also demonstrated improvements in the vitality domain, which is an indicator of improvement in energy, fatigue, and subjective well-being, as early as on day 15. This contrasts with SSRI treatments, where up to 23% of patients can experience reduced energy as the treatment-emergent side effect. Treatment with zuranolone and the time to response appeared to be associated with a return to normative levels of functioning and health-related quality of life. The results of this integrated analysis suggest that zuranolone may help address the unmet need for a fast-acting treatment that can potentially improve mental health, functioning, and well-being for patients with MDD and PPD [84,85].
Recently the group of Meltzer-Body [86], using data from randomized controlled trials, indirectly compared the effectiveness of zuranolone and SSRIs (fluoxetine, sertraline, paroxetine, citalopram, and escitalopram) in reducing depressive symptoms in patients with PPD. The effects of the drugs were assessed by CFB in EPDS and HAMD17 scores. A larger EPDS CFB was observed in zuranolone-treated patients in comparison to SSRI-treated subjects from day 15 onward. Patients treated with zuranolone exhibited a 4.22-point larger reduction in EPDS compared to placebo groups, and a 7.43-point larger reduction at day 45. It should be emphasized that, according to the investigators, “the results of this meta-analysis should be regarded with caution as the overall quality of evidence is low”.
In a randomized, phase 1, double-blind, active- and placebo-controlled, four-treatment, four-period crossover study, the effect of zuranolone (50 and 100 mg) on simulated driving performance was examined. In this study, the SDLP (a measure of lane position control) and additional driving performance assessments, such as lane exceedance, excessive speed count, excessive cornering speed threshold, and total number of collisions, were evaluated. One group of participants received 50 mg of zuranolone or placebo once nightly on days 1–7, while another group received zuranolone 50 mg or placebo on days 1–6 and then zuranolone 100 mg or placebo on day 7. Reduction in driving performance was typically observed after the first dose of zuranolone 50 mg. It decreased in magnitude on day 8 after repeated dosing and increased in magnitude on day 8 following administration of zuranolone 100 mg. The most frequently reported treatment-emergent adverse events occurring in ≥10% of participants who received 50 mg of zuranolone on days 1–7 were dizziness, somnolence, fatigue, tremor, headache, asthenia, nausea, insomnia, speech disorder, and constipation [87].
It is well known that the safety of antidepressants during lactation is of a high importance for women with a depressive disorder who choose to breastfeed. The cessation of breastfeeding was required in the clinical studies referenced above [81,82,83].. However, this aspect has not been thoroughly investigated. Taking into account a potential infant exposure to zuranolone via excretion into breast milk, Deligiannidis and coworkers assessed the extent of transfer of five 30 mg drug doses into the breastmilk of 15 healthy, non-pregnant, lactating adult females using RID (relative infant dose) values. RID is the weight-adjusted proportion of the maternal dose consumed by the infant in breast milk over a 24-h period. The RID at day 5 of 30 mg dose was 0.357%. The estimated mean RID of once-daily administration of 50 mg of zuranolone for 14 days was 0.74% and 0.98% with daily milk intake of 150 and 200 mg/kg per day, respectively. These RIDs are below 1% and well below the 10% RID threshold generally considered compatible with breastfeeding [88]. As this was a single study conducted on a small group of participants, additional studies are required to access whether treatment with zuranolone by breast feeding patients may affect the health condition of their infants.
5. Concluding Remarks
PPD is a significant health problem affecting not only mothers but also their children and children’s fathers. The currently used basic pharmacological treatment, including SSRIs, is often not effective. Neuroactive steroids offer several advantages over traditional antidepressants, including rapid onset, unique mechanism of action, and lack of tolerance upon repeated use. Zuranolone, the oral medication, represents a promising advancement in the neurosteroid-based treatment for PPD. Compared to brexanolone, it offers an easier and well-tolerated treatment option. As clinical trials on zuranolone conducted so far have been short-term ones with relatively small sample sizes, there is an urgent need for large-scale clinical studies. Moreover, a high under-representation in clinical trials on zuranolone of several ethnic groups (Table 2) constitutes a significant limitation in the comprehensive assessment of the efficacy and safety of treatment with zuranolone and, as highlighted by Swieczkowski and coworkers [89], clearly indicates the need to include ethnic minorities in future clinical trials.

Table 2.
Characteristics of the population participating in clinical trials of zuranolone in PPD.
Author Contributions
Conceptualization, J.B.Z.; validation, J.B.Z. and E.Z.; writing—original draft preparation, J.B.Z. and E.Z.; writing—review and editing, J.B.Z. and E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Medical University of Lodz, Poland (Grant No. 503/3-011-01/503-31-001).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUC | Area under curve |
| CFB | Change from baseline |
| CGI-I | The Clinical Global Impression-Improvement |
| CL/F | Apparent clearance |
| Cmax | Maximum plasma concentration |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders—Fifth Edition |
| Emax | Maximum efficacy |
| EPDS | Edinburgh Postnatal Depression Scale |
| FDA | Food and Drug Administration |
| GABA | Gamma aminobutyric acid |
| GABAARs | GABAA receptors |
| HAMD17 | 17-item Hamilton Rating Scale for Depression |
| HAMD | Hamilton Depression Rating Scale |
| IP | Intraperitoneally |
| MAD | Multiple ascending dose |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MDD | Major depressive disorder |
| NAS | Neuroactive steroid |
| PAMs | Positive allosteric modulators |
| PO | Per os |
| PPD | Postpartum depression |
| RID | Relative infant dose |
| SAD | Single ascending dose |
| SDLP | Standard deviation of lateral position |
| SF-36 | 36-Item Short Form Health Survey |
| SNRIs | Serotonin and noradrenaline reuptake inhibitors |
| SSRIs | Selective serotonin reuptake inhibitors |
| t1/2 | Half-life time |
| TCAs | Tricyclic antidepressants |
| tmax | Time to reach maximum concentration |
References
- World Health Organization. Depressive Disorder (Depression); WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 14 February 2025).
- Balaram, K.; Marwaha, R. Postpartum Blues. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022; Available online: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425787 (accessed on 14 February 2025).
- ACOG Committee on Clinical Practice Guidelines–Obstetrics. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline No. 4. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2023, 141, 1232–1261. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, Q.; Cheng, K.K.; Caine, E.D.; Tong, Y.; Wu, X.; Gong, W. Prevalence of Postpartum Depression Based on Diagnostic Interviews: A Systematic Review and Meta-Analysis. Depress. Anxiety 2023, 2023, 8403222. [Google Scholar] [CrossRef] [PubMed]
- Radoš, S.N.; Akik, B.K.; Žutić, M.; Rodriguez-Muñoz, M.F.; Uriko, K.; Motrico, E.; Moreno-Peral, P.; Apter, G.; den Berg, M.L. Diagnosis of peripartum depression disorder: A state-of-the-art approach from the COST Action Riseup-PPD. Compr. Psychiatry 2024, 130, 152456. [Google Scholar] [CrossRef]
- Ko, J.Y.; Farr, S.L.; Dietz, P.M.; Robbins, C.L. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005–2009. J. Womens Health 2012, 21, 830–836. [Google Scholar] [CrossRef]
- Yim, I.S.; Tanner Stapleton, L.R.; Guardino, C.M.; Hahn-Holbrook, J.; Dunkel Schetter, C. Biological and psychosocial predictors of postpartum depression: Systematic review and call for integration. Annu. Rev. Clin. Psychol. 2015, 11, 99–137. [Google Scholar] [CrossRef]
- Matsumura, K.; Hamazaki, K.; Tsuchida, A.; Kasamatsu, H.; Inadera, H.; Japan Environment and Children’s Study (JECS) Group. Education level and risk of postpartum depression: Results from the Japan Environment and Children’s Study (JECS). BMC Psychiatry 2019, 19, 419. [Google Scholar] [CrossRef]
- Bauman, B.L.; Ko, J.Y.; Cox, S.; D’Angelo, D.V.; Warner, L.; Folger, S.; Tevendale, H.D.; Coy, K.C.; Harrison, L.; Barfield, W.D. Vital Signs: Postpartum Depressive Symptoms and Provider Discussions About Perinatal Depression—United States, 2018. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 575–581. [Google Scholar] [CrossRef]
- Shi, X.; Ying, Y.; Yu, Z.; Xing, M.; Zhu, J.; Feng, W.; Xu, D.; Zhang, W.; Zhou, M.; Wang, J.; et al. Risk factors for postpartum depression in Chinese women: A cross-sectional study at 6 weeks postpartum. J. Psychosom. Res. 2021, 140, 110295. [Google Scholar] [CrossRef]
- Walker, A.L.; de Rooij, S.R.; Dimitrova, M.V.; Witteveen, A.B.; Verhoeven, C.J.; de Jonge, A.; Vrijkotte, T.G.; Henrichs, J. Psychosocial and peripartum determinants of postpartum depression: Findings from a prospective population-based cohort. The ABCD study. Compr. Psychiatry 2021, 108, 152239. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, G. Prevalence and risk factors of postpartum depression in women: A systematic review and meta-analysis. J. Clin. Nurs. 2022, 31, 2665–2677. [Google Scholar] [CrossRef]
- Della Corte, L.; La Rosa, V.L.; Cassinese, E.; Ciebiera, M.; Zaręba, K.; De Rosa, N.; Verrazzo, P.; Improda, F.P.; Vitale, S.G.; Giampaolino, P.; et al. Prevalence and associated psychological risk factors of postpartum depression: A cross-sectional study. J. Obstet. Gynaecol. 2022, 42, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Justesen, K.; Jourdaine, D. Peripartum Depression: Detection and Treatment. Am. Fam. Physician 2023, 108, 267–272. [Google Scholar] [PubMed]
- Sharma, V. Peripartum anxiety: Parsing heterogeneity in clinical settings. Braz. J. Psychiatry 2022, 441, 4–5. [Google Scholar] [CrossRef]
- Sit, D.; Luther, J.; Buysse, D.; Dills, J.L.; Eng, H.; Okun, M.; Wisniewski, S.; Wisner, K.L. Suicidal ideation in depressed postpartum women: Associations with childhood trauma, sleep disturbance and anxiety. J. Psychiatr. Res. 2015, 66–67, 95–104. [Google Scholar] [CrossRef]
- Orsolini, L.; Valchera, A.; Vecchiotti, R.; Tomasetti, C.; Iasevoli, F.; Fornaro, M.; De Berardis, D.; Perna, G.; Pompili, M.; Bellantuono, C. Suicide during Perinatal Period: Epidemiology, Risk Factors, and Clinical Correlates. Front. Psychiatry 2016, 7, 138. [Google Scholar] [CrossRef]
- Eastwood, J.G.; Jalaludin, B.B.; Kemp, L.A.; Phung, H.N.; Barnett, B.E. Relationship of postnatal depressive symptoms to infant temperament, maternal expectations, social support and other potential risk factors: Findings from a large Australian cross-sectional study. BMC Pregnancy Childbirth 2012, 12, 148. [Google Scholar] [CrossRef]
- Lilja, G.; Edhborg, M.; Nissen, E. Depressive mood in women at childbirth predicts their mood and relationship with infant and partner during the first year postpartum. Scand. J. Caring Sci. 2012, 26, 245–253. [Google Scholar] [CrossRef]
- Vismara, L.; Rollè, L.; Agostini, F.; Sechi, C.; Fenaroli, V.; Molgora, S.; Neri, E.; Prino, L.E.; Odorisio, F.; Trovato, A.; et al. Perinatal Parenting Stress, Anxiety, and Depression Outcomes in First-Time Mothers and Fathers: A 3- to 6-Months Postpartum Follow-Up Study. Front. Psychol. 2016, 7, 938. [Google Scholar] [CrossRef]
- Kerstis, B.; Aarts, C.; Tillman, C.; Persson, H.; Engström, G.; Edlund, B.; Öhrvik, J.; Sylvén, S.; Skalkidou, A. Association between parental depressive symptoms and impaired bonding with the infant. Arch. Womens Ment. Health 2016, 19, 87–94. [Google Scholar] [CrossRef]
- Saharoy, R.; Potdukhe, A.; Wanjari, M.; Taksande, A.B. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus 2023, 15, e41381. [Google Scholar] [CrossRef]
- Pearson, R.M.; Evans, J.; Kounali, D.; Lewis, G.; Heron, J.; Ramchandani, P.G.; O’Connor, T.G.; Stein, A. Maternal depression during pregnancy and the postnatal period: Risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry 2013, 70, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, H.; Gartland, D.; Mensah, F.; Giallo, R.; Brown, S. Maternal depression from pregnancy to 4 years postpartum and emotional/behavioural difficulties in children: Results from a prospective pregnancy cohort study. Arch. Womens Ment. Health 2016, 19, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Netsi, E.; Pearson, R.M.; Murray, L.; Cooper, P.; Craske, M.G.; Stein, A. Association of Persistent and Severe Postnatal Depression with Child Outcomes. JAMA Psychiatry 2018, 75, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Slomian, J.; Honvo, G.; Emonts, P.; Reginster, J.Y.; Bruyère, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health 2019, 15, 1745506519844044. [Google Scholar] [CrossRef]
- Rogers, A.; Obst, S.; Teague, S.J.; Rossen, L.; Spry, E.A.; Macdonald, J.A.; Sunderland, M.; Olsson, C.A.; Youssef, G.; Hutchinson, D. Association Between Maternal Perinatal Depression and Anxiety and Child and Adolescent Development: A Meta-analysis. JAMA Pediatr. 2020, 174, 1082–1092. [Google Scholar] [CrossRef]
- Schwarze, C.E.; Lerche, V.; Wallwiener, S.; Pauen, S. Partnership quality and maternal depressive symptoms in the transition to parenthood: A prospective cohort study. BMC Pregnancy Childbirth. 2024, 4, 664. [Google Scholar] [CrossRef]
- Torres, A.; Gelabert, E.; Roca, A.; Navarro, P.; Plaza, A.; Subirà, S.; Martin-Santos, R.; Ascaso, C.; Garcia-Esteve, L. Course of a major postpartum depressive episode: A prospective 2 years naturalistic follow-up study. J. Affect. Disord. 2019, 245, 965–970. [Google Scholar] [CrossRef]
- Vliegen, N.; Casalin, S.; Luyten, P. The course of postpartum depression: A review of longitudinal studies. Harv. Rev. Psychiatry 2014, 22, 1–22. [Google Scholar] [CrossRef]
- Zhang, K.; He, L.; Li, Z.; Ding, R.; Han, X.; Chen, B.; Cao, G.; Ye, J.H.; Li, T.; Fu, R. Bridging Neurobiological Insights and Clinical Biomarkers in Postpartum Depression: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 8835. [Google Scholar] [CrossRef]
- Dennis, C.L.; Dowswell, T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst. Rev. 2013, 2, Cd001134. [Google Scholar] [CrossRef]
- Zlotnick, C.; Tzilos, G.; Miller, I.; Seifer, R.; Stout, R. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J. Affect. Disord. 2016, 189, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Genovez, M.; Vanderkruik, R.; Lemon, E.; Dimidjian, S. Psychotherapeutic treatments for depression during pregnancy. Clin. Obstet. Gynecol. 2018, 61, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Motrico, E.; Moreno-Peral, P.; Uriko, K.; Hancheva, C.; Brekalo, M.; Ajaz, E.; Apter, G.; Bramante, A.; Conejo-Cerón, S.; Christoforou, A.; et al. Clinical practice guidelines with recommendations for peripartum depression: A European systematic review. Acta Psychiatr. Scand. 2022, 146, 325–339. [Google Scholar] [CrossRef]
- Sushmitha, G.; Eashwar, V.M.A.; Pandian, S.; Albert Sekhar, M.; Pricilla, S.E. Non-pharmacological Radical Methods for Treating Postpartum Depression Around the Globe: A Narrative Review. Cureus 2024, 16, e76052. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef]
- McEvoy, K.; Payne, J.L.; Osborne, L.M. Neuroactive steroids and perinatal depression: A review of recent literature. Curr. Psychiatry Rep. 2018, 20, 78. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Kanes, S.J. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol. Stress 2020, 12, 100212. [Google Scholar] [CrossRef]
- Brown, J.V.E.; Wilson, C.A.; Ayre, K.; Robertson, L.; South, E.; Molyneaux, E.; Trevillion, K.; Howard, L.M.; Khalifeh, H. Antidepressant treatment for postnatal depression. Cochrane Database Syst. Rev. 2021, 2, CD013560. [Google Scholar] [CrossRef]
- Giannopoulos, A.; Singh, J.; Deligiannidis, K.M. Clinical Utility of Zuranolone for Postpartum Depression: A Narrative Review. Neuropsychiatr. Dis. Treat. 2025, 21, 93–105. [Google Scholar] [CrossRef]
- Patterson, R.; Balan, I.; Morrow, A.L.; Meltzer-Brody, S. Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology 2024, 49, 67–72. [Google Scholar] [CrossRef]
- Powell, J.G.; Garland, S.; Preston, K.; Piszczatoski, C. Brexanolone (Zulresso): Finally, an FDA-approved treatment for postpartum depression. Ann. Pharmacother. 2020, 54, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Zuranolone: First approval. Drugs 2023, 83, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Wadhwa, R. Selective Serotonin Reuptake Inhibitors. In StatPearls [Internet]; [Updated 1 May 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- SNRIs (Serotonin and Norepinephrine Reuptake Inhibitors). Available online: https://my.clevelandclinic.org/health/treatments/24797-snri (accessed on 26 June 2025).
- Moraczewski, J.; Awosika, A.O.; Aedma, K.K. Tricyclic Antidepressants. In StatPearls [Internet]; [Updated 17 August 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mohamad, F.H.; Mohamad Jamali, M.A.; Che Has, A.T. Structure-function Studies of GABA (A) Receptors and Related computer-aided Studies. J. Mol. Neurosci. 2023, 73, 804–817. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Pinna, G.; Almeida, F.B.; Davis, M. Allopregnanolone in Postpartum Depression. Front. Glob. Womens Health 2022, 3, 823616. [Google Scholar] [CrossRef]
- Grötsch, M.K.; Ehlert, U. Allopregnanolone in the peripartum: Correlates, concentrations, and challenges—A systematic review. Psychoneuroendocrinolgy 2024, 166, 107081. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Kroll-Desrosiers, A.R.; Mo, S.; Nguyen, H.; Svenson, A.; Jaitly, N.; Hall, J.E.; Barton, B.A.; Rothschild, A.J.; Shaffer, S.A. Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology 2016, 70, 98–107. [Google Scholar] [CrossRef]
- Purdy, R.H.; Morrow, A.L.; Moore, P.H., Jr.; Paul, S.M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4553–4557. [Google Scholar] [CrossRef]
- Walton, N.L.; Antonoudiou, P.; Barros, L.; Dargan, T.; DiLeo, A.; Evans-Strong, A.; Gabby, J.; Howard, S.; Paracha, R.; Sánchez, E.J.; et al. Impaired Endogenous Neurosteroid Signaling Contributes to Behavioral Deficits Associated with Chronic Stress. Biol. Psychiatry 2023, 94, 249–261. [Google Scholar] [CrossRef]
- Porcu, P.; Barron, A.M.; Frye, C.A.; Walf, A.A.; Yang, S.Y.; He, X.Y.; Morrow, A.L.; Panzica, G.C.; Melcangi, R.C. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. J. Neuroendocrinol. 2016, 28, 12351. [Google Scholar] [CrossRef]
- Paul, S.M.; Purdy, R.H. Neuroactive steroids. FASEB J. 1992, 6, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Zorumski, C.F.; Covey, D.F.; Izumi, Y.; Evers, A.S.; Maguire, J.L.; Mennerick, S.J. New directions in neurosteroid therapeutics in neuropsychiatry. Neurosci. Biobehav. Rev. 2025, 172, 106119. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Wakefield, S.; MacKenzie, G.; Moss, S.J.; Maguire, J. Neurosteroidogenesis is required for the physiological response to stress: Role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 2011, 31, 18198–18210. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.K.; Girdler, S.S. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: What is the current state of knowledge in humans? Psychopharmacology 2014, 231, 3619–3634. [Google Scholar] [CrossRef]
- Diotel, N.; Charlierm, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid transport, local synthesis, and signaling within the brain: Roles in neurogenesis, neuroprotection, and sexual behaviors. Front. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef]
- Murugan, S.; Jakka, P.; Namani, S.; Mujumdar, V.; Radhakrishnan, G. The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. J. Biol. Chem. 2019, 294, 4596–4607. [Google Scholar] [CrossRef]
- Balan, I.; Beattie, M.C.; O’Buckley, T.K.; Aurelian, L.; Morrow, A.L. Endogenous neurosteroid (3⍺,5⍺)3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci. Rep. 2019, 9, 1220. [Google Scholar] [CrossRef]
- Lambert, J.J.; Cooper, M.A.; Simmons, R.D.; Weir, C.J.; Belelli, D. Neurosteroids: Endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S48–S58. [Google Scholar] [CrossRef]
- Carver, C.M.; Reddy, D.S. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal excitability. Psychopharmacology 2013, 230, 151–188. [Google Scholar] [CrossRef]
- Gunduz-Bruce, H.; Takahashi, K.; Huang, M.Y. Development of neuroactive steroids for the treatment of postpartum depression. J. Neuroendocrinol. 2022, 34, e13019. [Google Scholar] [CrossRef]
- Chen, Z.W.; Bracamontes, J.R.; Budelier, M.M.; Germann, A.L.; Shin, D.J.; Kathiresan, K.; Qian, M.X.; Manion, B.; Cheng, W.W.L.; Reichert, D.E.; et al. Multiple functional neurosteroid binding sites on GABAA receptors. PLoS Biol. 2019, 17, e3000157. [Google Scholar] [CrossRef] [PubMed]
- Goldschen-Ohm, M.P. Benzodiazepine Modulation of GABAA Receptors: A Mechanistic Perspective. Biomolecules 2022, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Yawata, Y.; Tashima, R.; Aritomi, H.; Shimada, S.; Onodera, T.; Taishi, T.; Ogawa, K. Distinct mechanisms of allopregnanolone and diazepam underlie neuronal oscillations and differential antidepressant effect. Front. Cell. Neurosci. 2024, 17, 1274459. [Google Scholar] [CrossRef] [PubMed]
- Antonoudiou, P.; Colmers, P.L.W.; Walton, N.L.; Weiss, G.L.; Smith, A.C.; Nguyen, D.P.; Lewis, M.; Quirk, M.C.; Barros, L.; Melon, L.C.; et al. Allopregnanolone Mediates Affective Switching Through Modulation of Oscillatory States in the Basolateral Amygdala. Biol. Psychiatry 2022, 91, 283–293. [Google Scholar] [CrossRef]
- Lüscher, B.; Möhler, H. Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience. F1000Res. 2019, 8, F1000, Faculty Rev-751. [Google Scholar] [CrossRef]
- Kanes, S.; Colquhoun, H.; Gunduz-Bruce, H.; Raines, S.; Arnold, R.; Schacterle, A.; Doherty, J.; Epperson, C.N.; Deligiannidis, K.M.; Riesenberg, R.; et al. Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. Lancet 2017, 390, 480–489. [Google Scholar] [CrossRef]
- Epperson, C.N.; Rubinow, D.R.; Meltzer-Brody, S.; Deligiannidis, K.M.; Riesenberg, R.; Krystal, A.D.; Bankole, K.; Huang, M.Y.; Li, H.; Brown, C.; et al. Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: Pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. J. Affect. Disord. 2023, 320, 353–359. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Colquhoun, H.; Riesenberg, R.; Epperson, C.N.; Deligiannidis, K.M.; Rubinow, D.R.; Li, H.; Sankoh, A.J.; Clemso, C.; Schacterle, A.; et al. Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018, 392, 1058–1070. [Google Scholar] [CrossRef]
- Reddy, D.S.; Mbilinyi, R.H.; Estes, E. Preclinical and clinical pharmacology of brexanolone (allopregnanolone) for postpartum depression: A landmark journey from concept to clinic in neurosteroid replacement therapy. Psychopharmacology 2023, 240, 1841–1863. [Google Scholar] [CrossRef]
- Martinez, B.G.; Salituro, F.G.; Harrison, B.L.; Beresis, R.T.; Bai, Z.; Blanco, M.J.; Belfort, G.M.; Dai, J.; Loya, C.M.; Ackle, M.A.; et al. Neuroactive Steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1’-yl)-19-nor-5β-pregnan-20-one (SAGE-217): A Clinical Next Generation Neuroactive Steroid Positive Allosteric Modulator of the (γ-Aminobutyric Acid)A Receptor. J. Med. Chem. 2017, 60, 7810–7819. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. (2025) PubChem Compound Summary for CID 86294073, Zuranolone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Zuranolone (accessed on 10 April 2025).
- Althaus, A.L.; Ackley, M.A.; Belfort, G.M.; Gee, S.M.; Dai, J.; Nguyen, D.P.; Kazdoba, T.M.; Modgil, A.; Davies, P.A.; Moss, S.J.; et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology 2020, 181, 108333. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Nomikos, G.G.; Kaul, I.; Raines, S.; Wald, J.; Bullock, A.; Sankoh, A.J.; Doherty, J.; Kanes, S.J.; Colquhoun, H. SAGE-217, A Novel GABAA Receptor Positive Allosteric Modulator: Clinical Pharmacology and Tolerability in Randomized Phase I Dose-Finding Studies. Clin. Pharmacokinet. 2020, 59, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Biogen Inc. Zurzuvae (Zuranolone) [Package Insert]. FDA. Revised March 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf (accessed on 25 March 2025).
- Deligiannidis, K.M.; Citrome, L.; Huang, M.Y.; Acaster, S.; Fridman, M.; Bonthapally, V.; Lasser, R.; Kanes, S.J. Effect of Zuranolone on Concurrent Anxiety and Insomnia Symptoms in Women with Postpartum Depression. J. Clin. Psychiatry 2023, 84(1), 22m14475. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Meltzer-Brody, S.; Gunduz-Bruce, H.; Doherty, J.; Jonas, J.; Li, S.; Sankoh, A.J.; Silber, C.; Campbell, A.D.; Werneburg, B.; et al. Effect of Zuranolone vs. Placebo in Postpartum Depression: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 951–959. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Meltzer-Brody, S.; Maximos, B.; Peeper, E.Q.; Freeman, M.; Lasser, R.; Bullock, A.; Kotecha, M.; Li, S.; Forrestal, F.; et al. Zuranolone for the Treatment of Postpartum Depression. Am. J. Psychiatry 2023, 180, 668–675. [Google Scholar] [CrossRef]
- Clayton, A.H.; Suthoff, E.; Jain, R.; Kosinski, M.; Fridman, M.; Deligiannidis, K.M.; Meltzer-Brody, S.; Chen, S.Y.; Gervitz, L.; Huang, M.Y.; et al. The magnitude and sustainability of treatment benefit of zuranolone on function and well-being as assessed by the SF-36 in adult patients with MDD and PPD: An integrated analysis of 4 randomized clinical trials. J. Affect. Disord. 2024, 351, 904–914. [Google Scholar] [CrossRef]
- Raja, A.; Ahmed, S.; Basit Ali Siddiqui, M.; Lamiya Mir, S.; Kumar, R.; Ahmed, M.; Raja, S.; Bin Amin, S.; Alim Ur Rahman, H.; Deepak, F.; et al. Evaluating the safety and efficacy of zuranolone in the management of major depressive disorder and postpartum depression, with or without concurrent insomnia: A rigorous systematic review and meta-analysis. Front. Psychiatry 2024, 15, 1425295. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Gerbasi, M.E.; Mak, C.; Toubouti, Y.; Smith, S.; Roskell, N.; Tan, R.; Chen, S.S.; Deligiannidis, K.M. Indirect comparisons of relative efficacy estimates of zuranolone and selective serotonin reuptake inhibitors for postpartum depression. J. Med. Econ. 2024, 27, 582–595. [Google Scholar] [CrossRef]
- Dunbar, J.; Morelli, G.; Jain, R.; Vaudreuil, C.; Nandy, I.; Ona, V.; Moseley, M.K.; Levin, S.; Kay, G. Effects of zuranolone on next-day simulated driving in healthy adults. Psychopharmacology 2025, 242, 389–400. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Bullock, A.; Nandy, I.; Dunbar, J.; Lasser, R.; Witte, M.; Leclair, B.; Wald, J. Zuranolone Concentrations in the Breast Milk of Healthy, Lactating Individuals: Results from a Phase 1 Open-Label Study. J. Clin. Psychopharmacol. 2024, 44, 337–344. [Google Scholar] [CrossRef]
- Swieczkowski, D.; Kwaśny, A.; Pruc, M.; Szarpak, L.; Cubała, W.J. Racial and ethnic diversity in zuranolone and brexanolone clinical trials for postpartum depression: A cross-sectional analysis of ClinicalTrials.gov. Eur. Neuropsychopharmacol. 2025, 96, 5–6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).