Activity of Human-Specific Interlaminar Astrocytes in a Chimeric Mouse Model of Fragile X Syndrome
Abstract
1. Introduction
2. Results
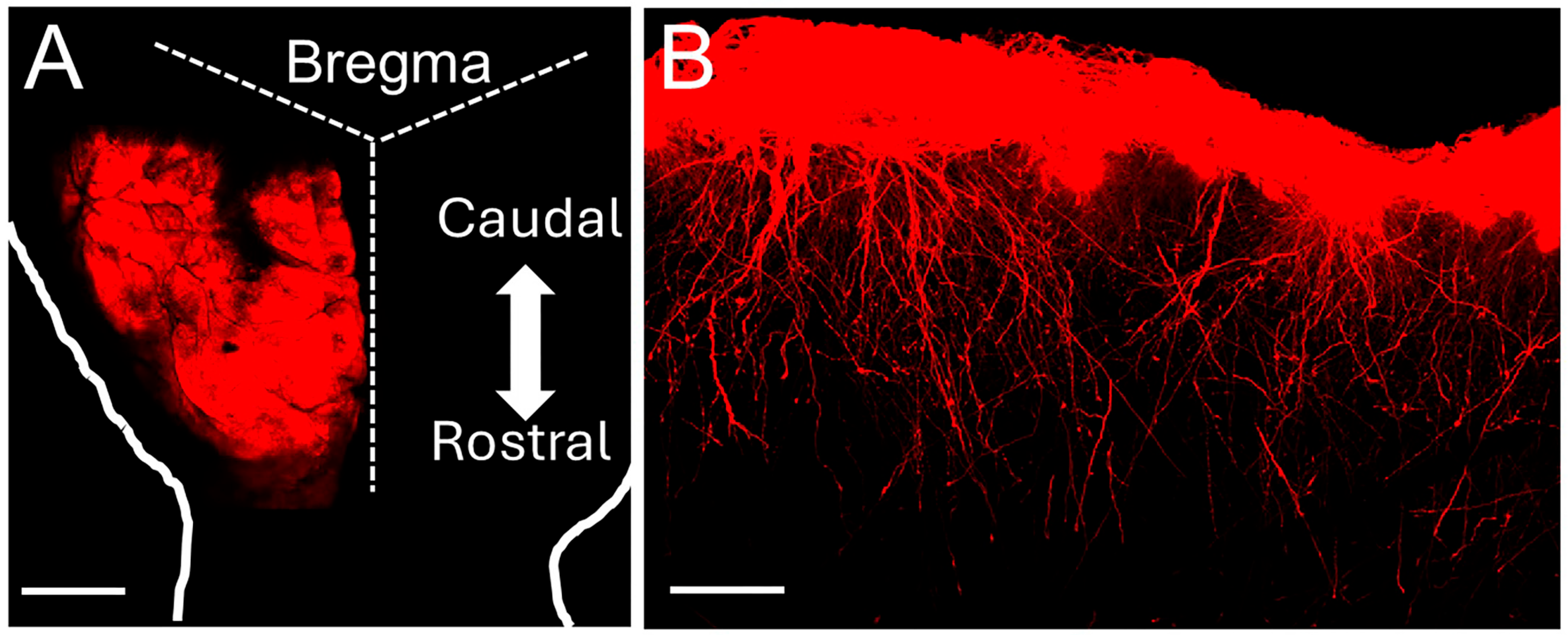
2.1. Generation of hiPSCs-Astrocyte Chimeric Mice
2.2. Calcium Signaling Properties of ILAs
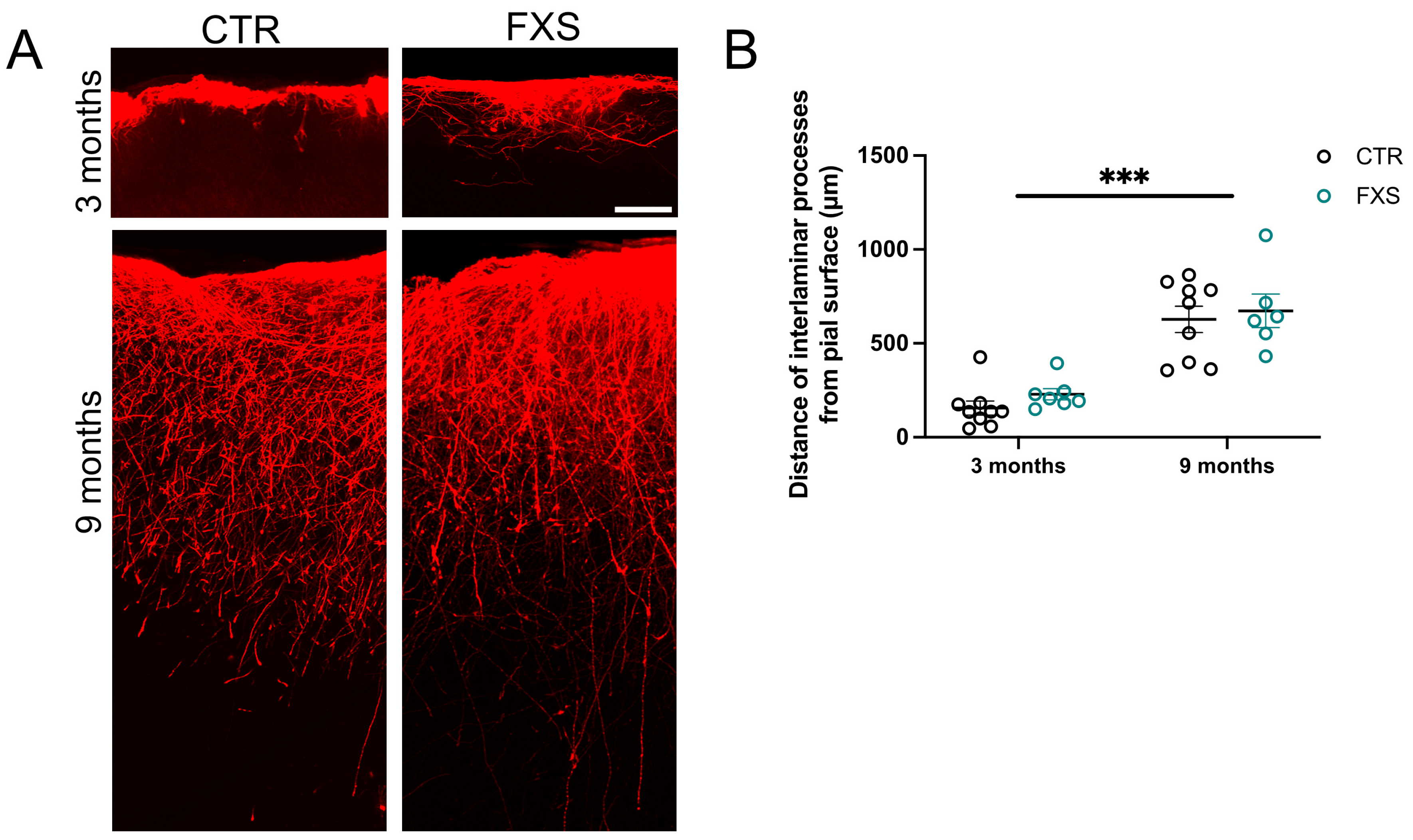
2.3. ILA Process Length Is Not Significantly Altered in FXS
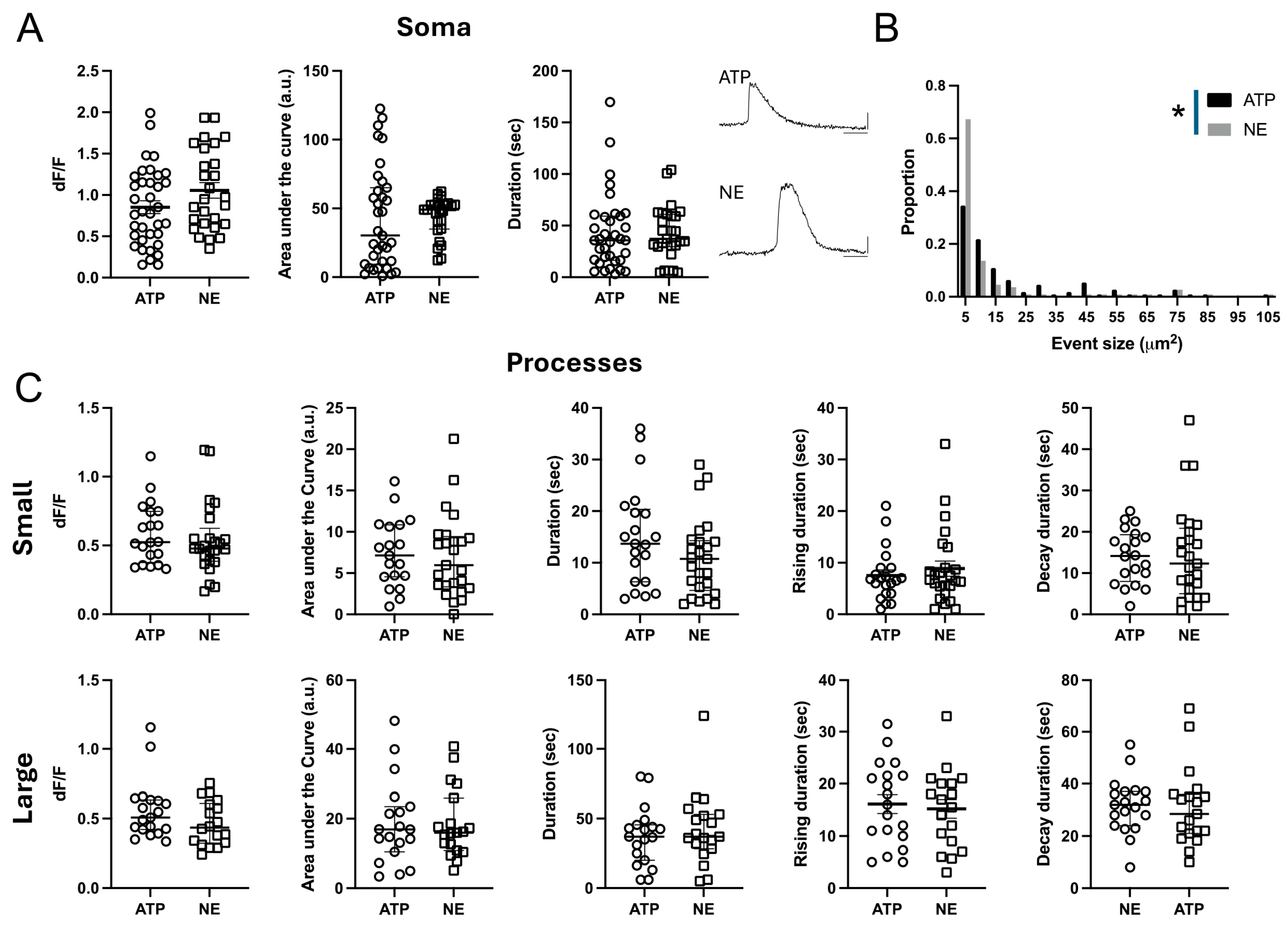
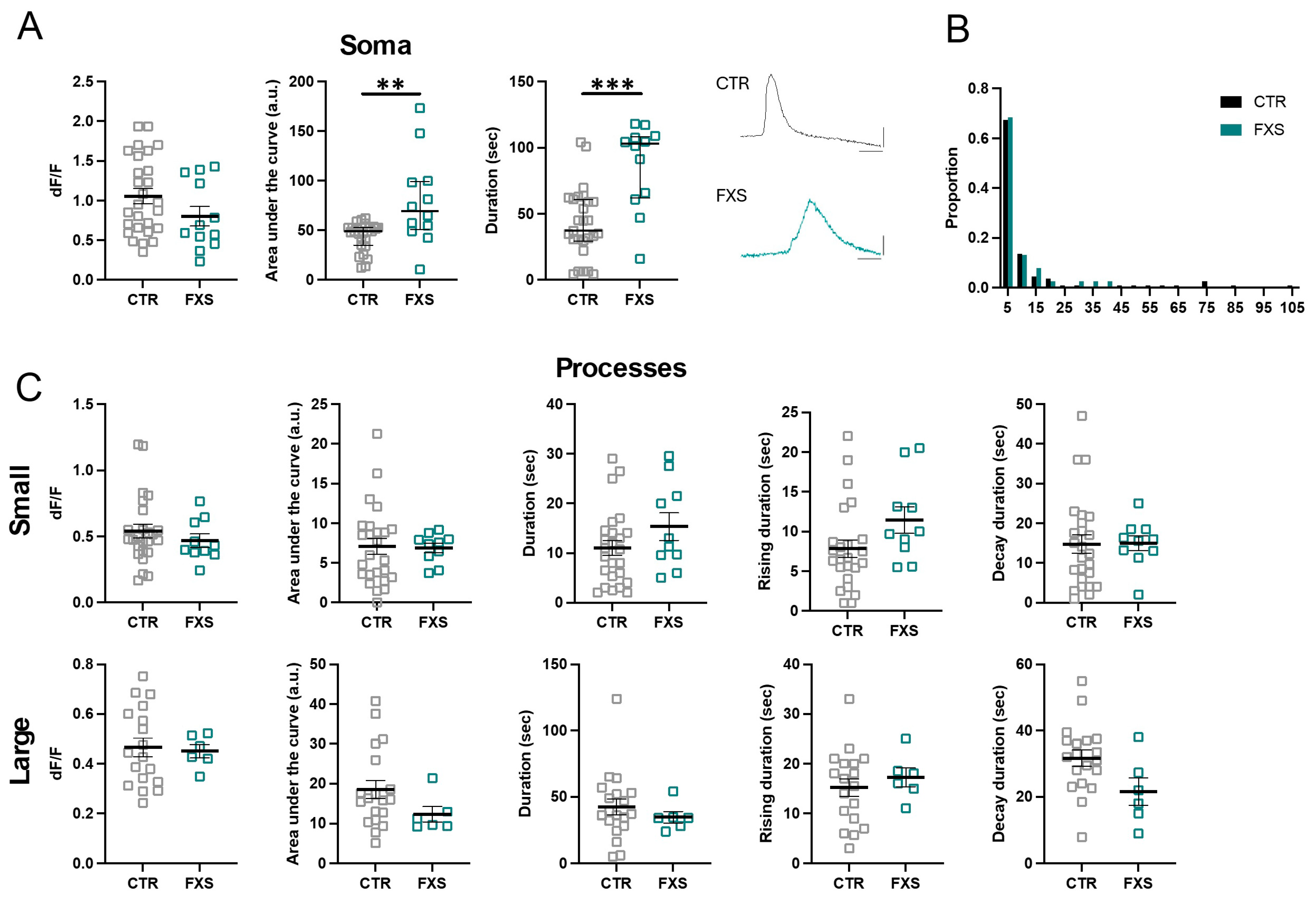
2.4. FXS ILAs Have Increased Calcium Signaling
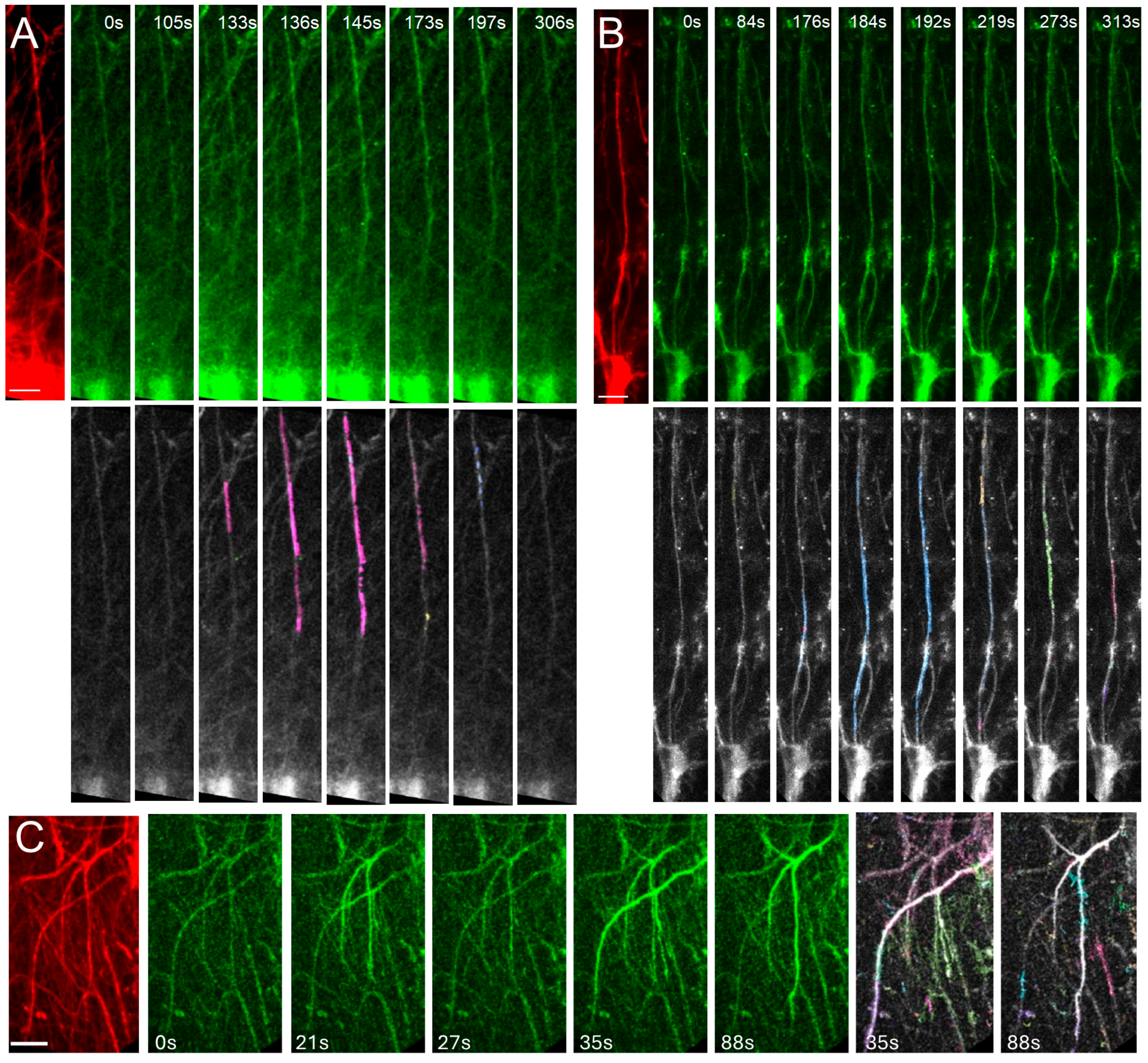
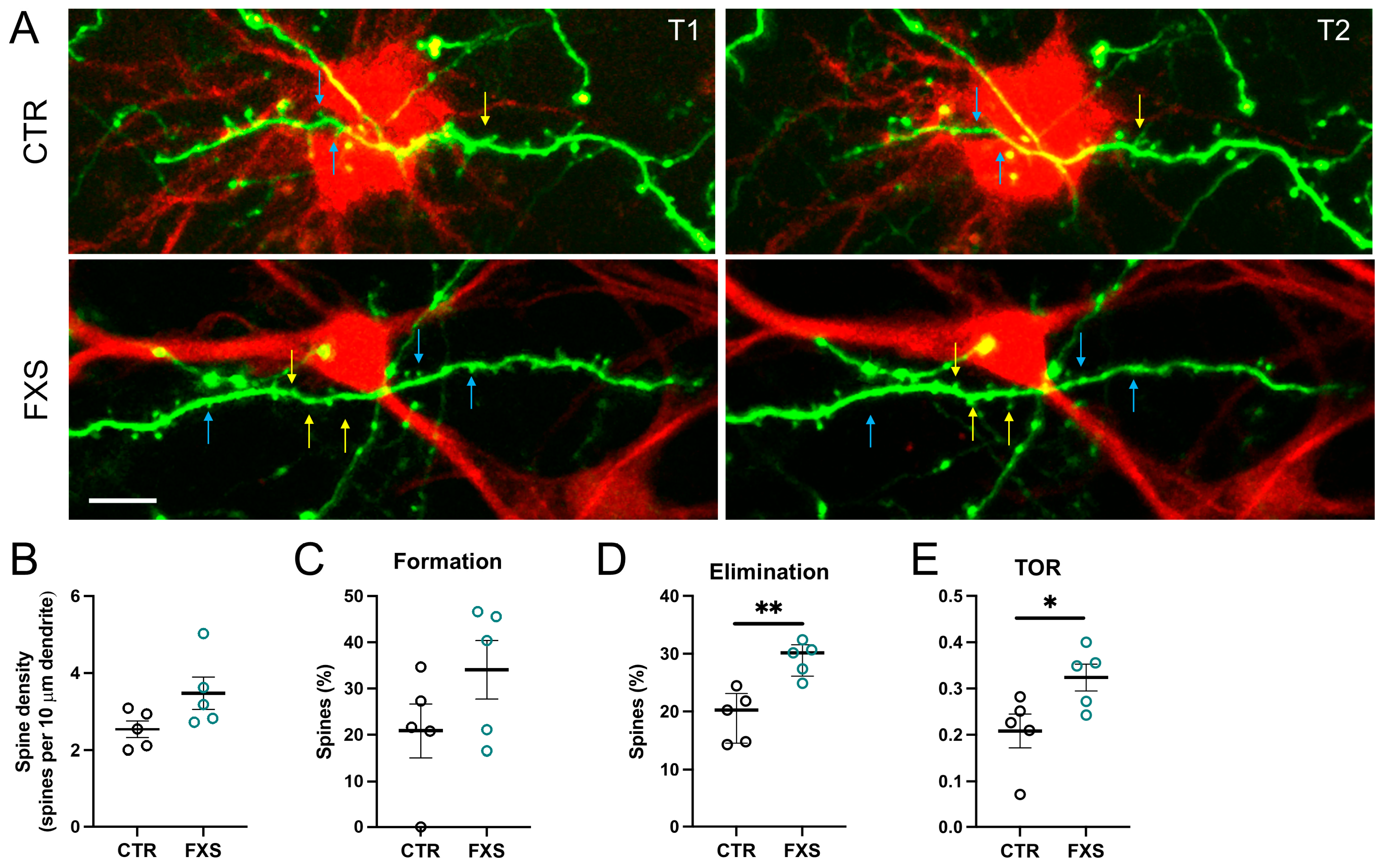
2.5. Increased Dendritic Spine Turnover in Mice Engrafted with FXS ILAs
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Stem Cell Differentiation
4.3. Viral Transduction
4.4. Engraftment of Human iPSC-Derived Astrocytes
4.5. Brain Slice Preparation
4.6. Dye Loading in Slices
4.7. Viral Injection and Cranial Window Implantation
4.8. Multiphoton Imaging
4.8.1. Slice Imaging and Pharmacology
4.8.2. In Vivo Imaging
4.9. Image Analysis
4.9.1. Analysis of Astrocytic Ca2+ Events
4.9.2. Analysis of Spine Plasticity
4.10. Tissue Preparation and Confocal Imaging
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Farizatto, K.L.G.; Baldwin, K.T. Astrocyte-synapse interactions during brain development. Curr. Opin. Neurobiol. 2023, 80, 102704. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.; Ulloa Severino, F.P.; Eroglu, C. The role of astrocyte structural plasticity in regulating neural circuit function and behavior. Glia 2022, 70, 1467–1483. [Google Scholar] [CrossRef] [PubMed]
- Lia, A.; Di Spiezio, A.; Speggiorin, M.; Zonta, M. Two decades of astrocytes in neurovascular coupling. Front. Netw. Physiol. 2023, 3, 1162757. [Google Scholar] [CrossRef]
- Veiga, A.; Abreu, D.S.; Dias, J.D.; Azenha, P.; Barsanti, S.; Oliveira, J.F. Calcium-Dependent Signaling in Astrocytes: Downstream Mechanisms and Implications for Cognition. J. Neurochem. 2025, 169, e70019. [Google Scholar] [CrossRef]
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012, 814, 23–45. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef]
- Colombo, J.A.; Gayol, S.; Yanez, A.; Marco, P. Immunocytochemical and electron microscope observations on astroglial interlaminar processes in the primate neocortex. J. Neurosci. Res. 1997, 48, 352–357. [Google Scholar] [CrossRef]
- Falcone, C.; Wolf-Ochoa, M.; Amina, S.; Hong, T.; Vakilzadeh, G.; Hopkins, W.D.; Hof, P.R.; Sherwood, C.C.; Manger, P.R.; Noctor, S.C.; et al. Cortical interlaminar astrocytes across the therian mammal radiation. J. Comp. Neurol. 2019, 527, 1654–1674. [Google Scholar] [CrossRef] [PubMed]
- Ciani, C.; Falcone, C. Interlaminar and varicose-projection astrocytes: Toward a new understanding of the primate brain. Front. Cell Neurosci. 2024, 18, 1477753. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Reisin, H.D. Interlaminar astroglia of the cerebral cortex: A marker of the primate brain. Brain Res. 2004, 1006, 126–131. [Google Scholar] [CrossRef]
- Padmashri, R.; Ren, B.; Oldham, B.; Jung, Y.; Gough, R.; Dunaevsky, A. Modeling human-specific interlaminar astrocytes in the mouse cerebral cortex. J. Comp. Neurol. 2021, 529, 802–810. [Google Scholar] [CrossRef]
- Ren, B.; Burkovetskaya, M.; Jung, Y.; Bergdolt, L.; Totusek, S.; Martinez-Cerdeno, V.; Stauch, K.; Korade, Z.; Dunaevsky, A. Dysregulated cholesterol metabolism, aberrant excitability and altered cell cycle of astrocytes in fragile X syndrome. Glia 2023, 71, 1176–1196. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, P.; Xue, H.; Peterson, S.E.; Tran, H.T.; McCann, A.E.; Parast, M.M.; Li, S.; Pleasure, D.E.; Laurent, L.C.; et al. Role of astroglia in Down’s syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat. Commun. 2014, 5, 4430. [Google Scholar] [CrossRef]
- Sejourne, G.; Eroglu, C. Astrocyte-neuron crosstalk in neurodevelopmental disorders. Curr. Opin. Neurobiol. 2024, 89, 102925. [Google Scholar] [CrossRef]
- Pan, F.; Aldridge, G.M.; Greenough, W.T.; Gan, W.B. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA 2010, 107, 17768–17773. [Google Scholar] [CrossRef]
- Padmashri, R.; Reiner, B.C.; Suresh, A.; Spartz, E.; Dunaevsky, A. Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J. Neurosci. 2013, 33, 19715–19723. [Google Scholar] [CrossRef]
- Suresh, A.; Dunaevsky, A. Relationship Between Synaptic AMPAR and Spine Dynamics: Impairments in the FXS Mouse. Cereb. Cortex 2017, 27, 4244–4256. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Doering, L.C. Astrocytes prevent abnormal neuronal development in the fragile X mouse. J. Neurosci. 2010, 30, 4508–4514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Feng, B.; Zhang, K.; Guo, Y.Y.; Liu, S.B.; Wu, Y.M.; Li, X.Q.; Zhao, M.G. Excessive astrocyte-derived neurotrophin-3 contributes to the abnormal neuronal dendritic development in a mouse model of fragile X syndrome. PLoS Genet. 2012, 8, e1003172. [Google Scholar] [CrossRef]
- Hodges, J.L.; Yu, X.; Gilmore, A.; Bennett, H.; Tjia, M.; Perna, J.F.; Chen, C.C.; Li, X.; Lu, J.; Zuo, Y. Astrocytic Contributions to Synaptic and Learning Abnormalities in a Mouse Model of Fragile X Syndrome. Biol. Psychiatry 2016, 82, 139–149. [Google Scholar] [CrossRef]
- Higashimori, H.; Schin, C.S.; Chiang, M.S.; Morel, L.; Shoneye, T.A.; Nelson, D.L.; Yang, Y. Selective Deletion of Astroglial FMRP Dysregulates Glutamate Transporter GLT1 and Contributes to Fragile X Syndrome Phenotypes In Vivo. J. Neurosci. 2016, 36, 7079–7094. [Google Scholar] [CrossRef]
- Padmashri, R.; Tyner, K.; Dunaevsky, A. Implantation of a Cranial Window for Repeated In Vivo Imaging in Awake Mice. J. Vis. Exp. 2021, 169, e62633. [Google Scholar] [CrossRef]
- Holtmaat, A.J.; Trachtenberg, J.T.; Wilbrecht, L.; Shepherd, G.M.; Zhang, X.; Knott, G.W.; Svoboda, K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 2005, 45, 279–291. [Google Scholar] [CrossRef]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; Xu, Q.; Oberheim, N.A.; Bekar, L.; Betstadt, S.; et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013, 12, 342–353. [Google Scholar] [CrossRef]
- Shigetomi, E.; Bushong, E.A.; Haustein, M.D.; Tong, X.; Jackson-Weaver, O.; Kracun, S.; Xu, J.; Sofroniew, M.V.; Ellisman, M.H.; Khakh, B.S. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 2013, 141, 633–647. [Google Scholar] [CrossRef]
- Ding, F.; O’Donnell, J.; Thrane, A.S.; Zeppenfeld, D.; Kang, H.; Xie, L.; Wang, F.; Nedergaard, M. alpha1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013, 54, 387–394. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Mukamel, E.A.; Schnitzer, M.J. Motor behavior activates Bergmann glial networks. Neuron 2009, 62, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Paukert, M.; Agarwal, A.; Cha, J.; Doze, V.A.; Kang, J.U.; Bergles, D.E. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014, 82, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D.; Burnstock, G. Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 2006, 7, 423–436. [Google Scholar] [CrossRef]
- Amadio, S.; Montilli, C.; Magliozzi, R.; Bernardi, G.; Reynolds, R.; Volonte, C. P2Y12 receptor protein in cortical gray matter lesions in multiple sclerosis. Cereb. Cortex 2010, 20, 1263–1273. [Google Scholar] [CrossRef]
- Bekar, L.K.; He, W.; Nedergaard, M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb. Cortex 2008, 18, 2789–2795. [Google Scholar] [CrossRef]
- Srinivasan, R.; Huang, B.S.; Venugopal, S.; Johnston, A.D.; Chai, H.; Zeng, H.; Golshani, P.; Khakh, B.S. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat. Neurosci. 2015, 18, 708–717. [Google Scholar] [CrossRef]
- Ramos, B.P.; Arnsten, A.F. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol. Ther. 2007, 113, 523–536. [Google Scholar] [CrossRef]
- Shelton, M.K.; McCarthy, K.D. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J. Neurochem. 2000, 74, 555–563. [Google Scholar] [CrossRef]
- Hinton, V.J.; Brown, W.T.; Wisniewski, K.; Rudelli, R.D. Analysis of neocortex in three males with the fragile X syndrome. Am. J. Med. Genet. 1991, 41, 289–294. [Google Scholar] [CrossRef]
- Irwin, S.A.; Patel, B.; Idupulapati, M.; Harris, J.B.; Crisostomo, R.A.; Larsen, B.P.; Kooy, F.; Willems, P.J.; Cras, P.; Kozlowski, P.B.; et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am. J. Med. Genet. 2001, 98, 161–167. [Google Scholar] [CrossRef]
- Comery, T.A.; Harris, J.B.; Willems, P.J.; Oostra, B.A.; Irwin, S.A.; Weiler, I.J.; Greenough, W.T. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc. Natl. Acad. Sci. USA 1997, 94, 5401–5404. [Google Scholar] [CrossRef] [PubMed]
- McKinney, B.C.; Grossman, A.W.; Elisseou, N.M.; Greenough, W.T. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 136, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martin, A.; Crespo, M.; Portera-Cailliau, C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 2010, 30, 7793–7803. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Lau, S.K.; Doering, L.C. Astrocyte-secreted thrombospondin-1 modulates synapse and spine defects in the fragile X mouse model. Mol. Brain 2016, 9, 74. [Google Scholar] [CrossRef]
- Wallingford, J.; Scott, A.L.; Rodrigues, K.; Doering, L.C. Altered Developmental Expression of the Astrocyte-Secreted Factors Hevin and SPARC in the Fragile X Mouse Model. Front. Mol. Neurosci. 2017, 10, 268. [Google Scholar] [CrossRef]
- Krasovska, V.; Doering, L.C. Regulation of IL-6 Secretion by Astrocytes via TLR4 in the Fragile X Mouse Model. Front. Mol. Neurosci. 2018, 11, 272. [Google Scholar] [CrossRef]
- Reynolds, K.E.; Wong, C.R.; Scott, A.L. Astrocyte-mediated purinergic signaling is upregulated in a mouse model of Fragile X syndrome. Glia 2021, 69, 1816–1832. [Google Scholar] [CrossRef]
- Colombo, J.A.; Reisin, H.D.; Jones, M.; Bentham, C. Development of interlaminar astroglial processes in the cerebral cortex of control and Down’s syndrome human cases. Exp. Neurol. 2005, 193, 207–217. [Google Scholar] [CrossRef]
- Doers, M.E.; Musser, M.T.; Nichol, R.; Berndt, E.R.; Baker, M.; Gomez, T.M.; Zhang, S.C.; Abbeduto, L.; Bhattacharyya, A. iPSC-derived forebrain neurons from FXS individuals show defects in initial neurite outgrowth. Stem Cells Dev. 2014, 23, 1777–1787. [Google Scholar] [CrossRef]
- Utami, K.H.; Skotte, N.H.; Colaco, A.R.; Yusof, N.; Sim, B.; Yeo, X.Y.; Bae, H.G.; Garcia-Miralles, M.; Radulescu, C.I.; Chen, Q.; et al. Integrative Analysis Identifies Key Molecular Signatures Underlying Neurodevelopmental Deficits in Fragile X Syndrome. Biol. Psychiatry 2020, 88, 500–511. [Google Scholar] [CrossRef]
- Raj, N.; McEachin, Z.T.; Harousseau, W.; Zhou, Y.; Zhang, F.; Merritt-Garza, M.E.; Taliaferro, J.M.; Kalinowska, M.; Marro, S.G.; Hales, C.M.; et al. Cell-type-specific profiling of human cellular models of fragile X syndrome reveal PI3K-dependent defects in translation and neurogenesis. Cell Rep. 2021, 35, 108991. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.T.; Lee, B.R.; Chong, P.; Soler-Llavina, G.; Cobbs, C.; Koch, C.; Zeng, H.; Lein, E. Preparation of Acute Brain Slices Using an Optimized N-Methyl-D-glucamine Protective Recovery Method. J. Vis. Exp. 2018, 132, e53825. [Google Scholar] [CrossRef]
- Pologruto, T.A.; Sabatini, B.L.; Svoboda, K. ScanImage: Flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2003, 2, 13. [Google Scholar] [CrossRef]
- Wang, Y.; DelRosso, N.V.; Vaidyanathan, T.V.; Cahill, M.K.; Reitman, M.E.; Pittolo, S.; Mi, X.; Yu, G.; Poskanzer, K.E. Accurate quantification of astrocyte and neurotransmitter fluorescence dynamics for single-cell and population-level physiology. Nat. Neurosci. 2019, 22, 1936–1944. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anding, A.; Ren, B.; Padmashri, R.; Burkovetskaya, M.; Dunaevsky, A. Activity of Human-Specific Interlaminar Astrocytes in a Chimeric Mouse Model of Fragile X Syndrome. Int. J. Mol. Sci. 2025, 26, 6510. https://doi.org/10.3390/ijms26136510
Anding A, Ren B, Padmashri R, Burkovetskaya M, Dunaevsky A. Activity of Human-Specific Interlaminar Astrocytes in a Chimeric Mouse Model of Fragile X Syndrome. International Journal of Molecular Sciences. 2025; 26(13):6510. https://doi.org/10.3390/ijms26136510
Chicago/Turabian StyleAnding, Alexandria, Baiyan Ren, Ragunathan Padmashri, Maria Burkovetskaya, and Anna Dunaevsky. 2025. "Activity of Human-Specific Interlaminar Astrocytes in a Chimeric Mouse Model of Fragile X Syndrome" International Journal of Molecular Sciences 26, no. 13: 6510. https://doi.org/10.3390/ijms26136510
APA StyleAnding, A., Ren, B., Padmashri, R., Burkovetskaya, M., & Dunaevsky, A. (2025). Activity of Human-Specific Interlaminar Astrocytes in a Chimeric Mouse Model of Fragile X Syndrome. International Journal of Molecular Sciences, 26(13), 6510. https://doi.org/10.3390/ijms26136510






