High Prevalence of TERT and CTNNB1 Mutations in Brazilian HCC Tissues: Insights into Early Detection and Risk Stratification
Abstract
1. Introduction
2. Results
2.1. TERTp and CTNNB1 Exon 3 Mutations in Tumour Tissue Samples and Their Correlation with Clinicopathological Characteristics
2.2. TERTp and CTNNB1 Exon 3 Mutations Across All Samples and Their Correlation with Liver Disease Tissue Types
2.3. TERTp and CTNNB1 Exon 3 Mutations in Paired Samples and Their Correlation with Liver Disease Tissue Types
2.4. Association Between TERTp and CTNNB1 Exon 3 Mutations
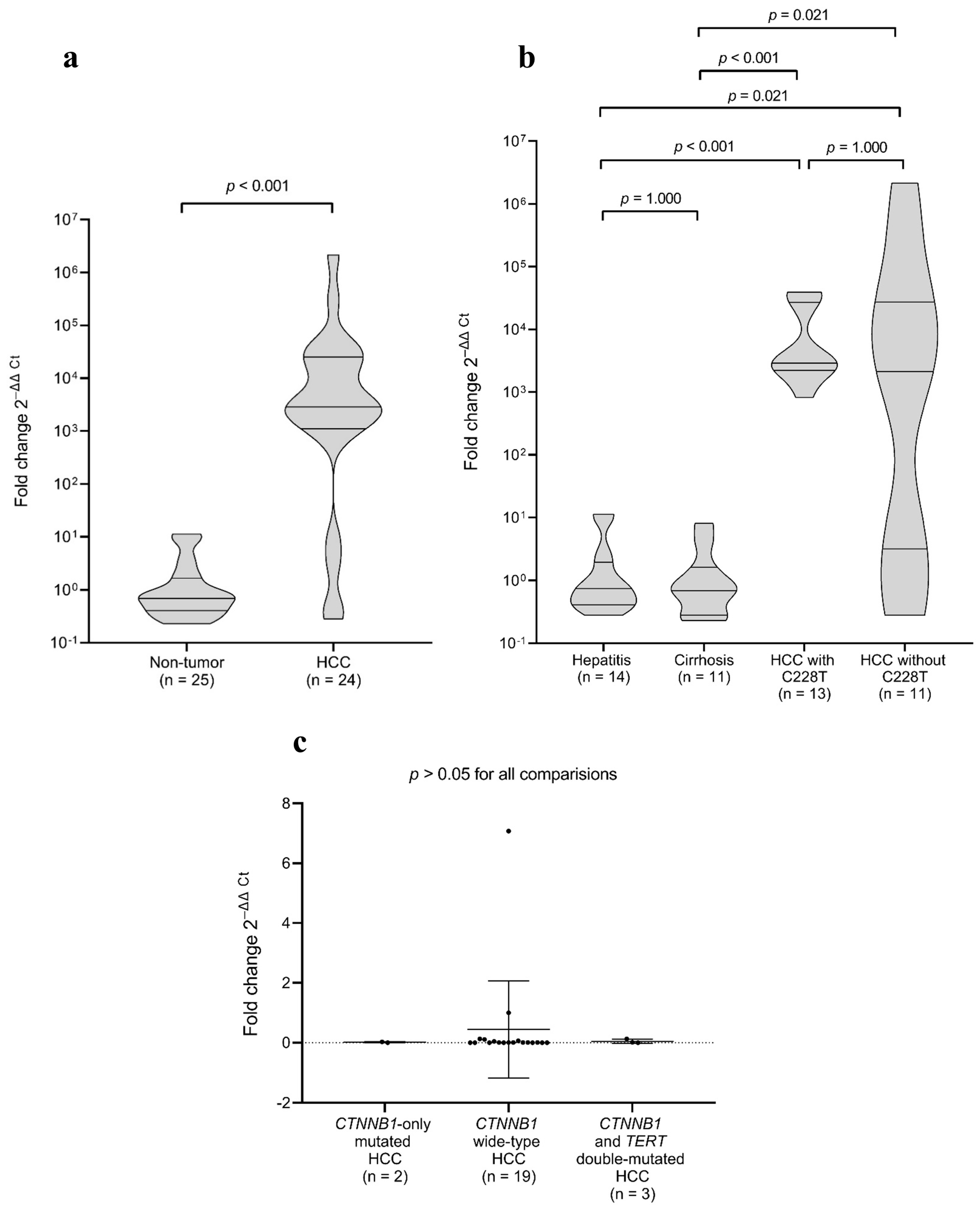
2.5. TERT Gene Expression in Tumour and Non-Tumour Samples
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. DNA Extraction and PCR Amplification
4.3. Nucleotide Sequencing
4.4. RNA Extraction and TERT Reverse Transcription Quantitative PCR (RT-qPCR)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bp | base pair |
| CTNNB1 | catenin beta 1 |
| FFPE | formalin-fixed paraffin-embedded |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| NAFLD | non-alcoholic fatty liver disease |
| PLK1 | polo-like kinase 1 |
| RT-qPCR | reverse transcription quantitative PCR |
| TERTp | telomerase reverse transcriptase promoter |
References
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/11-liver-and-intrahepatic-bile-ducts-fact-sheet.pdf (accessed on 18 January 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef]
- Kotiyal, S.; Evason, K.J. Exploring the Interplay of Telomerase Reverse Transcriptase and beta-Catenin in Hepatocellular Carcinoma. Cancers 2021, 13, 4202. [Google Scholar] [CrossRef]
- Schulze, K.; Nault, J.C.; Villanueva, A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J. Hepatol. 2016, 65, 1031–1042. [Google Scholar] [CrossRef]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Terra, M.L.; Sant’Anna, T.B.F.; de Barros, J.J.F.; de Araujo, N.M. Geographic and Viral Etiology Patterns of TERT Promoter and CTNNB1 Exon 3 Mutations in Hepatocellular Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 2889. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Broaddus, R.; Sun, L.; Xue, F.; Zhang, W. Exon 3 mutations of CTNNB1 drive tumorigenesis: A review. Oncotarget 2018, 9, 5492–5508. [Google Scholar] [CrossRef]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef] [PubMed]
- Totoki, Y.; Tatsuno, K.; Covington, K.R.; Ueda, H.; Creighton, C.J.; Kato, M.; Tsuji, S.; Donehower, L.A.; Slagle, B.L.; Nakamura, H.; et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 2014, 46, 1267–1273. [Google Scholar] [CrossRef]
- INCA. Instituto Nacional do Câncer, Ministério da Saúde. Tipos de Câncer. Câncer de Fígado. Available online: https://www.gov.br/inca/pt-br/assuntos/cancer/tipos/figado (accessed on 5 May 2025).
- Paranagua-Vezozzo, D.C.; Ono, S.K.; Alvarado-Mora, M.V.; Farias, A.Q.; Cunha-Silva, M.; Franca, J.I.; Alves, V.A.; Sherman, M.; Carrilho, F.J. Epidemiology of HCC in Brazil: Incidence and risk factors in a ten-year cohort. Ann. Hepatol. 2014, 13, 386–393. [Google Scholar] [CrossRef]
- Nault, J.C.; Ningarhari, M.; Rebouissou, S.; Zucman-Rossi, J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 544–558. [Google Scholar] [CrossRef]
- Chianchiano, P.; Pezhouh, M.K.; Kim, A.; Luchini, C.; Cameron, A.; Weiss, M.J.; He, J.; Voltaggio, L.; Oshima, K.; Anders, R.A.; et al. Distinction of intrahepatic metastasis from multicentric carcinogenesis in multifocal hepatocellular carcinoma using molecular alterations. Hum. Pathol. 2018, 72, 127–134. [Google Scholar] [CrossRef]
- Machida, K. HCV and tumor-initiating stem-like cells. Front. Physiol. 2022, 13, 903302. [Google Scholar] [CrossRef]
- An, P.; Xu, J.; Yu, Y.; Winkler, C.A. Host and Viral Genetic Variation in HBV-Related Hepatocellular Carcinoma. Front. Genet. 2018, 9, 261. [Google Scholar] [CrossRef]
- Tu, T.; Budzinska, M.A.; Shackel, N.A.; Urban, S. HBV DNA Integration: Molecular Mechanisms and Clinical Implications. Viruses 2017, 9, 75. [Google Scholar] [CrossRef]
- Sung, W.K.; Zheng, H.; Li, S.; Chen, R.; Liu, X.; Li, Y.; Lee, N.P.; Lee, W.H.; Ariyaratne, P.N.; Tennakoon, C.; et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat. Genet. 2012, 44, 765–769. [Google Scholar] [CrossRef]
- Kawai-Kitahata, F.; Asahina, Y.; Tanaka, S.; Kakinuma, S.; Murakawa, M.; Nitta, S.; Watanabe, T.; Otani, S.; Taniguchi, M.; Goto, F.; et al. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J. Gastroenterol. 2016, 51, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Lin, J.; Song, G.; Chen, M. Prognostic significance of CTNNB1 mutation in hepatocellular carcinoma: A systematic review and meta-analysis. Aging 2023, 15, 9759–9778. [Google Scholar] [CrossRef]
- Mao, T.L.; Chu, J.S.; Jeng, Y.M.; Lai, P.L.; Hsu, H.C. Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J. Pathol. 2001, 193, 95–101. [Google Scholar] [CrossRef]
- Wang, Z.; Sheng, Y.Y.; Gao, X.M.; Wang, C.Q.; Wang, X.Y.; Lu, X.U.; Wei, J.W.; Zhang, K.L.; Dong, Q.Z.; Qin, L.X. beta-catenin mutation is correlated with a favorable prognosis in patients with hepatocellular carcinoma. Mol. Clin. Oncol. 2015, 3, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.M.; Fuentes, D.; Morshid, A.I.; Burke, M.R.; Kaseb, A.O.; Hassan, M.; Hazle, J.D.; Elsayes, K.M. Role of Wnt/beta-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J. Hepatocell. Carcinoma 2018, 5, 61–73. [Google Scholar] [CrossRef]
- Rebouissou, S.; Franconi, A.; Calderaro, J.; Letouze, E.; Imbeaud, S.; Pilati, C.; Nault, J.C.; Couchy, G.; Laurent, A.; Balabaud, C.; et al. Genotype-phenotype correlation of CTNNB1 mutations reveals different ss-catenin activity associated with liver tumor progression. Hepatology 2016, 64, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Quaas, A.; Oldopp, T.; Tharun, L.; Klingenfeld, C.; Krech, T.; Sauter, G.; Grob, T.J. Frequency of TERT promoter mutations in primary tumors of the liver. Virchows Arch. 2014, 465, 673–677. [Google Scholar] [CrossRef]
- Pezzuto, F.; Izzo, F.; Buonaguro, L.; Annunziata, C.; Tatangelo, F.; Botti, G.; Buonaguro, F.M.; Tornesello, M.L. Tumor specific mutations in TERT promoter and CTNNB1 gene in hepatitis B and hepatitis C related hepatocellular carcinoma. Oncotarget 2016, 7, 54253–54262. [Google Scholar] [CrossRef]
- Hafezi, F.; Bercoff, D.P. The Solo Play of TERT Promoter Mutations. Cells 2020, 9, 749. [Google Scholar] [CrossRef]
- Nault, J.C.; Calderaro, J.; Tommaso, L.D.; Balabaud, C.; Zafrani, E.S.; Bioulac-Sage, P.; Roncalli, M.; Zucman-Rossi, J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 2014, 60, 1983–1992. [Google Scholar] [CrossRef]
- Javanmard, D.; Najafi, M.; Babaei, M.R.; Niya, M.H.K.; Esghaei, M.; Panahi, M.; Tameshkel, F.S.; Tavakoli, A.; Jazayeri, S.M.; Ghaffari, H.; et al. Investigation of CTNNB1 gene mutations and expression in hepatocellular carcinoma and cirrhosis in association with hepatitis B virus infection. Infect. Agent. Cancer 2020, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.C.; Shao, Y.Y.; Lee, Y.H.; Hsieh, M.S.; Hsiao, C.H.; Lin, H.H.; Kao, H.F.; Ma, Y.Y.; Yen, F.C.; Cheng, A.L.; et al. beta-catenin (CTNNB1) mutations are not associated with prognosis in advanced hepatocellular carcinoma. Oncology 2014, 87, 159–166. [Google Scholar] [CrossRef]
- Laurent-Puig, P.; Legoix, P.; Bluteau, O.; Belghiti, J.; Franco, D.; Binot, F.; Monges, G.; Thomas, G.; Bioulac-Sage, P.; Zucman-Rossi, J. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology 2001, 120, 1763–1773. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, W.S.; Nam, S.W.; Kim, S.Y.; Lee, S.H.; Yoo, N.J.; Lee, J.Y.; Park, C.K. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int. 2005, 25, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Martin, Y.; Caruso, S.; Hirsch, T.Z.; Bayard, Q.; Calderaro, J.; Charpy, C.; Copie-Bergman, C.; Ziol, M.; Bioulac-Sage, P.; et al. Clinical Impact of Genomic Diversity From Early to Advanced Hepatocellular Carcinoma. Hepatology 2020, 71, 164–182. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S.; Letouze, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Galle, P.R.; Marquardt, J.U. The role of molecular enrichment on future therapies in hepatocellular carcinoma. J. Hepatol. 2018, 69, 237–247. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, G.; Sang, Y.; Chen, Y.; Wei, G.; Zhu, M.; Chen, M.; Li, S.; Liu, R.; Peng, Z. Therapeutic targeting of PLK1 in TERT promoter-mutant hepatocellular carcinoma. Clin. Transl. Med. 2024, 14, e1703. [Google Scholar] [CrossRef]
- de Galarreta, M.R.; Bresnahan, E.; Molina-Sanchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- Gabata, R.; Harada, K.; Mizutani, Y.; Ouchi, H.; Yoshimura, K.; Sato, Y.; Kitao, A.; Kimura, K.; Kouji, H.; Miyashita, T.; et al. Anti-tumor Activity of the Small Molecule Inhibitor PRI-724 Against beta-Catenin-activated Hepatocellular Carcinoma. Anticancer. Res. 2020, 40, 5211–5219. [Google Scholar] [CrossRef]
- Scott, G.A.; Laughlin, T.S.; Rothberg, P.G. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014, 27, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Chang, S.H.; Kim, W.Y.; Lim, S.D.; Kim, W.S.; Hwang, T.S.; Han, H.S. Frequent somatic TERT promoter mutations and CTNNB1 mutations in hepatocellular carcinoma. Oncotarget 2016, 7, 69267–69275. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Variables | TERTp C228T | p-Value | CTNNB1 Exon3 Mutations | p-Value | ||
|---|---|---|---|---|---|---|
| Negative n = 23 (%) | Positive n = 19 (%) | Negative n = 24 (%) | Positive n = 5 (%) | |||
| Age (years) | 0.7080 | 0.2981 | ||||
| ≥60 | 11 (47.8) | 13 (68.4) | 15 (62.5) | 5 (100) | ||
| <60 | 6 (26.1) | 4 (21.1) | 6 (25) | 0 | ||
| Unknown | 6 (26.1) | 2 (10.5) | 3 (12.5) | 0 | ||
| Sex | 0.4384 | 1 | ||||
| Male | 13 (56.5) | 11 (57.9) | 15 (62.5) | 3 (60) | ||
| Female | 4 (17.4) | 6 (31.6) | 6 (25) | 2 (40) | ||
| Unknown | 6 (26.1) | 2 (10.5) | 3 (12.5) | 0 | ||
| Aetiology | 0.07872 | 1 | ||||
| HBV | 3 (13) | 0 | 0 | 0 | ||
| HCV | 4 (17.4) | 8 (42.1) | 6 (25) | 1 (20) | ||
| Non-viral | 16 (69.6) | 11 (57.9) | 18 (75) | 4 (80) | ||
| Tumour size | 0.4795 | 0.6146 | ||||
| ≥5 cm | 10 (43.5) | 7 (36.8) | 12 (50) | 2 (40) | ||
| <5 cm | 6 (26.1) | 8 (42.1) | 7 (29.2) | 3 (60) | ||
| Unknown | 7 (30.4) | 4 (21.1) | 5 (20.8) | 0 | ||
| Tumour differentiation | 0.7854 | 0.3377 | ||||
| Well | 0 | 1 (5.3) | 1 (4.2) | 0 | ||
| Moderately | 14 (60.9) | 12 (63.2) | 17 (70.8) | 3 (60) | ||
| Poorly | 2 (8.7) | 1 (5.3) | 0 | 1 (20) | ||
| Unknown | 7 (30.4) | 5 (26.3) | 6 (25) | 1 (20) | ||
| Mutation | Tissue Type (n, %) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Non-Tumour | Tumour | Tumour X Non-Tumour | Hepatitis X Cirrhosis | HCC X Hepatitis | HCC X Cirrhosis | ||
| Hepatitis | Cirrhosis | HCC | |||||
| TERTp C228T | 0/22 (0) | 4/21 (19) | 19/42 (45.2) | 0.0005 | 0.0485 | 0.0001 | 0.0542 |
| CTNNB1 exon 3 | 0/20 (0) | 1/18 (5.6) | 5/29 (17.2) | 0.0776 | 0.4737 | 0.0704 | 0.3839 |
| Mutation | Paired Tissue (n, %) | p-Value | |
|---|---|---|---|
| Tumour 1 | Non-Tumour 1 | ||
| TERTp C228T | 12/26 (46.2) | 0/26 (0) | 0.0001 |
| CTNNB1 exon 3 | 4/24 (16.7) | 0/24 (0) | 0.1092 |
| Mutation | CTNNB1 Mutations Positive | CTNNB1 Mutations Negative | p-Value |
|---|---|---|---|
| TERTp C228T positive | 4/6 (66.7%) | 13/54 (24.1%) | 0.0485 |
| TERTp C228T negative | 2/6 (33.3%) | 41/54 (75.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sant’Anna, T.B.F.; Terra, M.L.; de Barros, J.J.F.; Ruivo, L.A.d.S.; Fernandes, A.; Begnami, M.D.F.d.S.; Pannain, V.L.N.; Campos, A.H.J.F.M.; Moreira, O.d.C.; de Araujo, N.M. High Prevalence of TERT and CTNNB1 Mutations in Brazilian HCC Tissues: Insights into Early Detection and Risk Stratification. Int. J. Mol. Sci. 2025, 26, 6503. https://doi.org/10.3390/ijms26136503
Sant’Anna TBF, Terra ML, de Barros JJF, Ruivo LAdS, Fernandes A, Begnami MDFdS, Pannain VLN, Campos AHJFM, Moreira OdC, de Araujo NM. High Prevalence of TERT and CTNNB1 Mutations in Brazilian HCC Tissues: Insights into Early Detection and Risk Stratification. International Journal of Molecular Sciences. 2025; 26(13):6503. https://doi.org/10.3390/ijms26136503
Chicago/Turabian StyleSant’Anna, Thaís Barbosa Ferreira, Mariana Leonardo Terra, Jose Junior França de Barros, Leonardo Alexandre de Souza Ruivo, Arlete Fernandes, Maria Dirlei Ferreira de Souza Begnami, Vera Lucia Nunes Pannain, Antônio Hugo José Fróes Marques Campos, Otacilio da Cruz Moreira, and Natalia Motta de Araujo. 2025. "High Prevalence of TERT and CTNNB1 Mutations in Brazilian HCC Tissues: Insights into Early Detection and Risk Stratification" International Journal of Molecular Sciences 26, no. 13: 6503. https://doi.org/10.3390/ijms26136503
APA StyleSant’Anna, T. B. F., Terra, M. L., de Barros, J. J. F., Ruivo, L. A. d. S., Fernandes, A., Begnami, M. D. F. d. S., Pannain, V. L. N., Campos, A. H. J. F. M., Moreira, O. d. C., & de Araujo, N. M. (2025). High Prevalence of TERT and CTNNB1 Mutations in Brazilian HCC Tissues: Insights into Early Detection and Risk Stratification. International Journal of Molecular Sciences, 26(13), 6503. https://doi.org/10.3390/ijms26136503






