Autism Spectrum Disorder as a Multifactorial Disorder: The Interplay of Genetic Factors and Inflammation
Abstract
1. Introduction
2. Genetic Factors in ASD
2.1. Categorizing ASD Genes
2.2. Comorbidities of ASD-Related Genes
2.3. Cerebral Folate Deficiency
2.3.1. Folate Metabolism and Brain Development
2.3.2. Genetic Variants Impairing Folate Transport
2.3.3. Folate Receptor Alpha Autoantibodies and Treatments
2.4. Mitochondrial Dysfunction
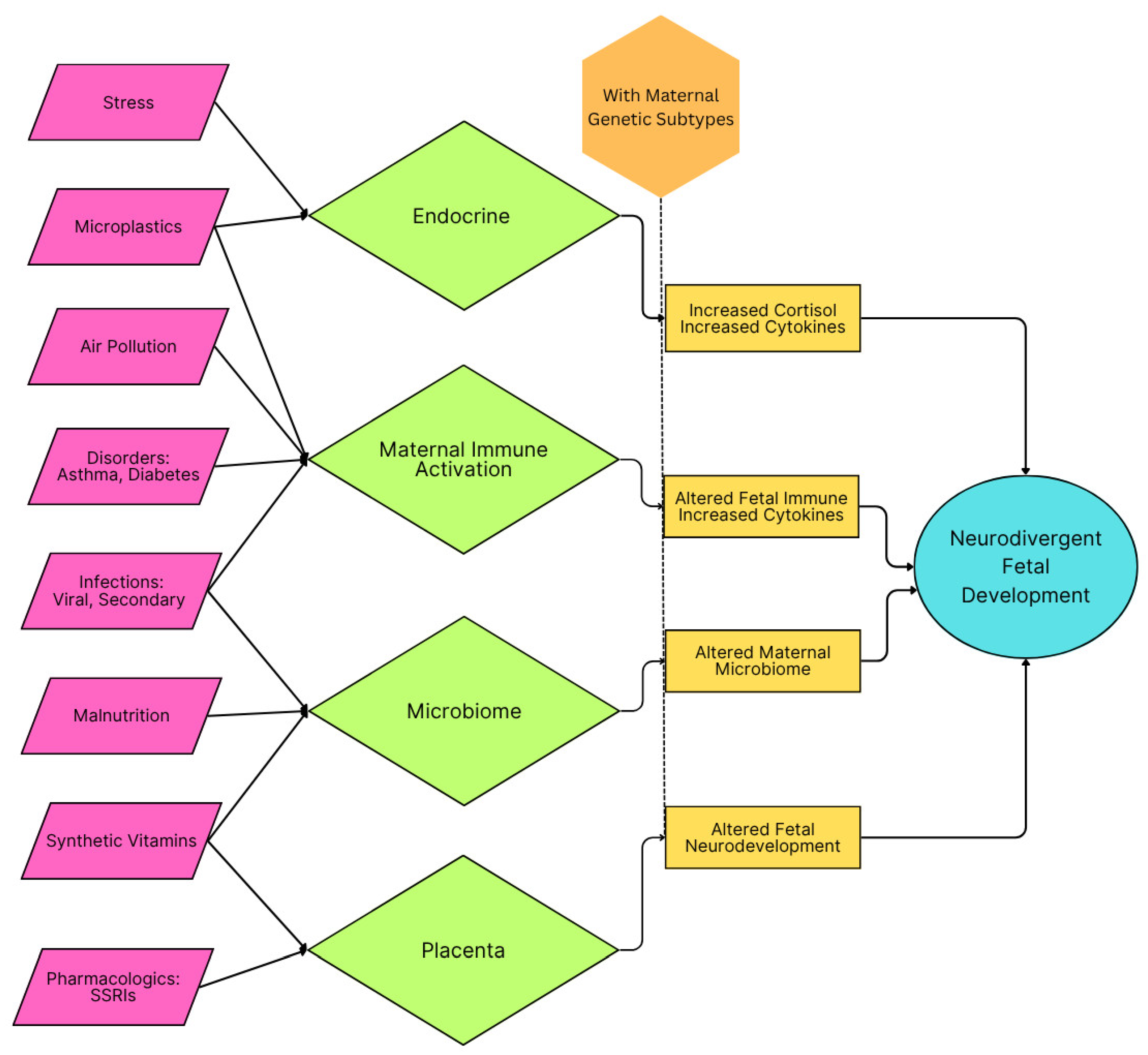
3. Inflammation During Critical Developmental Periods and ASD Risk
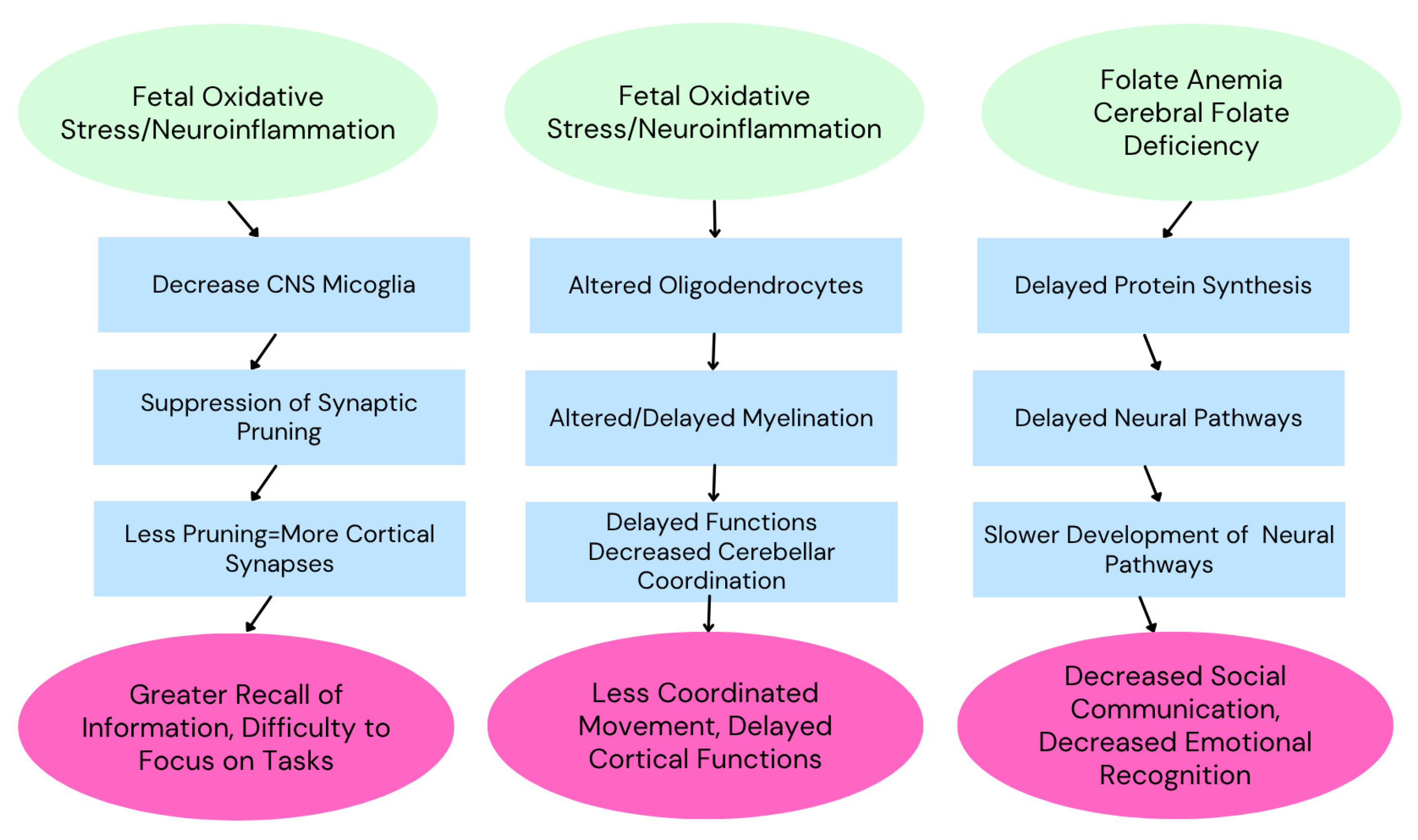
3.1. The Developing Brain and Vulnerability to Inflammation
3.2. Categories of Inflammation
3.2.1. Viral Infections
3.2.2. Air Pollution and Maternal Asthma
3.2.3. Maternal Immune Activation
3.2.4. Microplastics
3.2.5. Malnutrition
3.2.6. Emotional Stress
3.2.7. Pharmaceuticals
3.2.8. Maternal Disorders and Diabetes
3.2.9. Synthetic Vitamins
3.2.10. Vaccines
3.2.11. Microbiome and Metabolic Disorders
3.3. Mechanistic Insights
3.4. Therapeutic Interventions
4. Integrative Perspective: Gene–Environment Interactions in ASD
4.1. Synergistic Effects
4.2. Epigenetic Modifications
4.3. Implications for Prevention and Treatment
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| CFD | Cerebral Folate Deficiency |
| CNS | Central Nervous System |
| FRα | Folate Receptor Alpha |
| FRAA | Folate Receptor Auto Antibody |
| MIA | Maternal Immune Activation |
| RFC | Reduced Folate Carrier |
| SSRI | Selective Serotonin Reuptake Inhibitor |
References
- Volkmar, F.R.; Reichow, B. Autism in DSM-5: Progress and challenges. Mol. Autism 2013, 4, 13. [Google Scholar] [CrossRef]
- Shaw, K.A. Prevalence and Early Identification of Autism Spectrum Disorder Among Children Aged 4 and 8 Years—Autism and Developmental Disabilities Monitoring Network, 16 Sites, United States, 2022. MMWR Surveill. Summ. 2025, 74, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Banerjee-Basu, S.; Packer, A. SFARI Gene: An evolving database for the autism research community. Dis. Models Mech. 2010, 3, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Arpi, M.N.T.; Simpson, T.I. SFARI genes and where to find them; modelling Autism Spectrum Disorder specific gene expression dysregulation with RNA-seq data. Sci. Rep. 2022, 12, 10158. [Google Scholar] [CrossRef]
- Tang, J.; Oroudjev, E.; Wilson, L.; Ayoub, G. Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in MCF7 human breast cancer cells. Integr. Cancer Sci. Ther. 2015, 2, 82–86. [Google Scholar] [CrossRef]
- Kimball, T.N.; Prapiadou, S.; Tack, R.W.P.; Tan, B.Y.-Q.; Senff, J.R.; Kourkoulis, C.; Singh, S.; Rosand, J.; Anderson, C.D. Association of Leucocyte Telomere Length With Stroke, Dementia, and Late-Life Depression. Neurology 2025, 105, e213794. [Google Scholar] [CrossRef]
- Gogate, A.; Kaur, K.; Khalil, R.; Bashtawi, M.; Morris, M.A.; Goodspeed, K.; Evans, P.; Chahrour, M.H. The genetic landscape of autism spectrum disorder in an ancestrally diverse cohort. npj Genom. Med. 2024, 9, 62. [Google Scholar] [CrossRef]
- Havdahl, A.; Niarchou, M.; Starnawska, A.; Uddin, M.; van der Merwe, C.; Warrier, V. Genetic contributions to autism spectrum disorder. Psychol. Med. 2021, 51, 2260–2273. [Google Scholar] [CrossRef]
- Fu, J.M.; Satterstrom, F.K.; Peng, M.; Brand, H.; Collins, R.L.; Dong, S.; Wamsley, B.; Klei, L.; Wang, L.; Hao, S.P.; et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat. Genet. 2022, 54, 1320–1331. [Google Scholar] [CrossRef]
- Khachadourian, V.; Mahjani, B.; Sandin, S.; Kolevzon, A.; Buxbaum, J.D.; Reichenberg, A.; Janecka, M. Comorbidities in autism spectrum disorder and their etiologies. Transl. Psychiatry 2023, 13, 71. [Google Scholar] [CrossRef]
- Rolland, T.; Cliquet, F.; Anney, R.J.L.; Moreau, C.; Traut, N.; Mathieu, A.; Huguet, G.; Duan, J.; Warrier, V.; Portalier, S.; et al. Phenotypic effects of genetic variants associated with autism. Nat. Med. 2023, 29, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Manoli, D.S.; State, M.W. Autism Spectrum Disorder Genetics and the Search for Pathological Mechanisms. Am. J. Psychiatry 2021, 178, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Yang, G.; Yan, Z. Identification of a molecular network regulated by multiple ASD high risk genes. Hum. Mol. Genet. 2024, 33, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Vorstman, J.A.S.; Scherer, S.W. Contemplating syndromic autism. Genet. Med. 2023, 25, 100919. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Zoghbi, H.Y. Lessons learned from studying syndromic autism spectrum disorders. Nat. Neurosci. 2016, 19, 1408–1417. [Google Scholar] [CrossRef]
- Wang, N.; Lv, L.; Huang, X.; Shi, M.; Dai, Y.; Wei, Y.; Xu, B.; Fu, C.; Huang, H.; Shi, H.; et al. Gene editing in monogenic autism spectrum disorder: Animal models and gene therapies. Front. Mol. Neurosci. 2022, 15, 1043018. [Google Scholar] [CrossRef]
- Weuring, W.; Geerligs, J.; Koeleman, B.P.C. Gene Therapies for Monogenic Autism Spectrum Disorders. Genes 2021, 12, 1667. [Google Scholar] [CrossRef]
- Kereszturi, É. Database-assisted screening of autism spectrum disorder related gene set. Mol. Brain 2024, 17, 55. [Google Scholar] [CrossRef]
- Monteiro, P.; Feng, G. SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017, 18, 147–157. [Google Scholar] [CrossRef]
- Vatsa, N.; Jana, N.R. UBE3A and Its Link with Autism. Front. Mol. Neurosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Tener, S.J.; Lin, Z.; Park, S.J.; Oraedu, K.; Ulgherait, M.; Van Beek, E.; Martínez-Muñiz, A.; Pantalia, M.; Gatto, J.A.; Volpi, J.; et al. Neuronal knockdown of Cullin3 as a Drosophila model of autism spectrum disorder. Sci. Rep. 2024, 14, 1541. [Google Scholar] [CrossRef]
- Vinci, M.; Treccarichi, S.; Galati Rando, R.; Musumeci, A.; Todaro, V.; Federico, C.; Saccone, S.; Elia, M.; Calì, F. A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability. Sci. Rep. 2024, 14, 15848. [Google Scholar] [CrossRef]
- Wen, Y.; Alshikho, M.J.; Herbert, M.R. Pathway Network Analyses for Autism Reveal Multisystem Involvement, Major Overlaps with Other Diseases and Convergence upon MAPK and Calcium Signaling. PLoS ONE 2016, 11, e0153329. [Google Scholar] [CrossRef]
- Parikshak, N.N.; Luo, R.; Zhang, A.; Won, H.; Lowe, J.K.; Chandran, V.; Horvath, S.; Geschwind, D.H. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 2013, 155, 1008–1021. [Google Scholar] [CrossRef]
- Guo, Q.; Xia, L.; Guo, R.; Xu, W.; Zhang, Y.; Zhao, C.; Zhang, P.; Bai, T.; Ni, X.; Hao, C.; et al. Behavioural deficits of autism spectrum disorder and associations with different gene clusters: A study with the whole-genome transmission disequilibrium test. BMJ Paediatr. Open 2023, 7, e001930. [Google Scholar] [CrossRef]
- Betancur, C.; Buxbaum, J.D. SHANK3 haploinsufficiency: A “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol. Autism 2013, 4, 17. [Google Scholar] [CrossRef]
- Glessner, J.T.; Wang, K.; Cai, G.; Korvatska, O.; Kim, C.E.; Wood, S.; Zhang, H.; Estes, A.; Brune, C.W.; Bradfield, J.P.; et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 2009, 459, 569–573. [Google Scholar] [CrossRef]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef]
- Sanders, S.J.; Campbell, A.J.; Cottrell, J.R.; Moller, R.S.; Wagner, F.F.; Auldridge, A.L.; Bernier, R.A.; Catterall, W.A.; Chung, W.K.; Empfield, J.R.; et al. Progress in Understanding and Treating SCN2A-Mediated Disorders. Trends Neurosci. 2018, 41, 442–456. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Mustafin, Z.S.; Lashin, S.A.; Kochetov, A.V. Abnormal mTOR Activity in Pediatric Autoimmune Neuropsychiatric and MIA-Associated Autism Spectrum Disorders. Int. J. Mol. Sci. 2022, 23, 967. [Google Scholar] [CrossRef]
- Curatolo, P.; Moavero, R.; de Vries, P.J. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015, 14, 733–745. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Sig. Transduct. Target Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-van Silfhout, A.T.; et al. Disruptive CHD8 Mutations Define a Subtype of Autism Early in Development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X Syndrome: Loss of Local mRNA Regulation Alters Synaptic Development and Function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef]
- David, M.M.; Enard, D.; Ozturk, A.; Daniels, J.; Jung, J.-Y.; Diaz-Beltran, L.; Wall, D.P. Comorbid Analysis of Genes Associated with Autism Spectrum Disorders Reveals Differential Evolutionary Constraints. PLoS ONE 2016, 11, e0157937. [Google Scholar] [CrossRef]
- Diaz-Beltran, L.; Esteban, F.J.; Varma, M.; Ortuzk, A.; David, M.; Wall, D.P. Cross-disorder comparative analysis of comorbid conditions reveals novel autism candidate genes. BMC Genom. 2017, 18, 315. [Google Scholar] [CrossRef]
- Chen, S.; Xiong, J.; Chen, B.; Zhang, C.; Deng, X.; He, F.; Yang, L.; Chen, C.; Peng, J.; Yin, F. Autism spectrum disorder and comorbid neurodevelopmental disorders (ASD-NDDs): Clinical and genetic profile of a pediatric cohort. Clin. Chim. Acta 2022, 524, 179–186. [Google Scholar] [CrossRef]
- Willsey, A.J.; Morris, M.T.; Wang, S.; Willsey, H.R.; Sun, N.; Teerikorpi, N.; Baum, T.B.; Cagney, G.; Bender, K.J.; Desai, T.A.; et al. The Psychiatric Cell Map Initiative: A Convergent Systems Biological Approach to Illuminating Key Molecular Pathways in Neuropsychiatric Disorders. Cell 2018, 174, 505–520. [Google Scholar] [CrossRef]
- Vilela, J.; Martiniano, H.; Marques, A.R.; Santos, J.X.; Rasga, C.; Oliveira, G.; Vicente, A.M. Disease similarity network analysis of Autism Spectrum Disorder and comorbid brain disorders. Front. Mol. Neurosci. 2022, 15, 932305. [Google Scholar] [CrossRef]
- Iannuccelli, M.; Vitriolo, A.; Licata, L.; Lo Surdo, P.; Contino, S.; Cheroni, C.; Capocefalo, D.; Castagnoli, L.; Testa, G.; Cesareni, G.; et al. Curation of causal interactions mediated by genes associated with autism accelerates the understanding of gene-phenotype relationships underlying neurodevelopmental disorders. Mol. Psychiatry 2024, 29, 186–196. [Google Scholar] [CrossRef]
- Jourdon, A.; Wu, F.; Mariani, J.; Capauto, D.; Norton, S.; Tomasini, L.; Amiri, A.; Suvakov, M.; Schreiner, J.D.; Jang, Y.; et al. Modeling idiopathic autism in forebrain organoids reveals an imbalance of excitatory cortical neuron subtypes during early neurogenesis. Nat. Neurosci. 2023, 26, 1505–1515. [Google Scholar] [CrossRef]
- Ayoub, G. Neurodevelopment of Autism: Critical Periods, Stress and Nutrition. Cells 2024, 13, 1968. [Google Scholar] [CrossRef]
- Qiu, S.; Qiu, Y.; Li, Y.; Cong, X. Genetics of autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Transl. Psychiatry 2022, 12, 249. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Sequeira, J.M.; Blau, N.; Quadros, E.V. A milk-free diet downregulates folate receptor autoimmunity in cerebral folate deficiency syndrome. Dev. Med. Child Neurol. 2008, 50, 346–352. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic acid improves verbal communication in children with autism and language impairment: A randomized double-blind placebo-controlled trial. Mol. Psychiatry 2018, 23, 247–256. [Google Scholar] [CrossRef]
- Renard, E.; Leheup, B.; Guéant-Rodriguez, R.-M.; Oussalah, A.; Quadros, E.V.; Guéant, J.-L. Folinic acid improves the score of Autism in the EFFET placebo-controlled randomized trial. Biochimie 2020, 173, 57–61. [Google Scholar] [CrossRef]
- Panda, P.K.; Sharawat, I.K.; Saha, S.; Gupta, D.; Palayullakandi, A.; Meena, K. Efficacy of oral folinic acid supplementation in children with autism spectrum disorder: A randomized double-blind, placebo-controlled trial. Eur. J. Pediatr. 2024, 183, 4827–4835. [Google Scholar] [CrossRef]
- Soetedjo, F.; Kristijanto, J.A.; Durry, F. Folinic acid and autism spectrum disorder in children: A systematic review and meta-analysis of two double-blind randomized placebo-controlled trials. AcTion Aceh Nutr. J. 2025, 10, 194. [Google Scholar] [CrossRef]
- Frye, R.E.; McCarty, P.J.; Werner, B.A.; Rose, S.; Scheck, A.C. Bioenergetic signatures of neurodevelopmental regression. Front. Physiol. 2024, 15, 1306038. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef] [PubMed]
- Gevezova, M.; Ivanov, Z.; Pacheva, I.; Timova, E.; Kazakova, M.; Kovacheva, E.; Ivanov, I.; Sarafian, V. Bioenergetic and Inflammatory Alterations in Regressed and Non-Regressed Patients with Autism Spectrum Disorder. Int. J. Mol. Sci. 2024, 25, 8211. [Google Scholar] [CrossRef] [PubMed]
- Scott, O.; Shi, D.; Andriashek, D.; Clark, B.; Goez, H.R. Clinical clues for autoimmunity and neuroinflammation in patients with autistic regression. Dev. Med. Child Neurol. 2017, 59, 947–951. [Google Scholar] [CrossRef]
- He, Y.; Xie, K.; Yang, K.; Wang, N.; Zhang, L. Unraveling the Interplay Between Metabolism and Neurodevelopment in Health and Disease. CNS Neurosci. Ther. 2025, 31, e70427. [Google Scholar] [CrossRef] [PubMed]
- The Mystery of Regressive Autism—Mental Health. Available online: https://www.enotalone.com/article/mental-health/the-mystery-of-regressive-autism-r20878/ (accessed on 21 May 2025).
- Yenkoyan, K.; Mkhitaryan, M.; Bjørklund, G. Environmental Risk Factors in Autism Spectrum Disorder: A Narrative Review. Curr. Med. Chem. 2024, 31, 2345–2360. [Google Scholar] [CrossRef]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Chen, Y.; Du, X.; Zhang, X.; Li, F.; Yuan, S.; Wang, W.; Zhu, Z.; Wang, M.; Gu, C. Research trends of inflammation in autism spectrum disorders: A bibliometric analysis. Front. Immunol. 2025, 16, 1534660. [Google Scholar] [CrossRef]
- Ellul, P.; Maruani, A.; Vantalon, V.; Humeau, E.; Amestoy, A.; Anchordoqui, A.; Atzori, P.; Baleyte, J.-M.; Benmansour, S.; Bonnot, O.; et al. Maternal immune activation during pregnancy is associated with more difficulties in socio-adaptive behaviors in autism spectrum disorder. Sci. Rep. 2023, 13, 17687. [Google Scholar] [CrossRef]
- Carter, M.; Casey, S.; O’Keeffe, G.W.; Gibson, L.; Gallagher, L.; Murray, D.M. Maternal Immune Activation and Interleukin 17A in the Pathogenesis of Autistic Spectrum Disorder and Why It Matters in the COVID-19 Era. Front. Psychiatry 2022, 13, 823096. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Z.; Liu, J.; Li, Y.; Liu, L.; Huang, P.; Wang, W.; Shan, Z.; Sun, R.; Shen, J.; et al. Dysregulation of immune and metabolism pathways in maternal immune activation induces an increased risk of autism spectrum disorders. Life Sci. 2023, 324, 121734. [Google Scholar] [CrossRef]
- Alamoudi, R.A.; Al-Jabri, B.A.; Alsulami, M.A.; Sabbagh, H.J. Prenatal maternal stress and the severity of autism spectrum disorder: A cross-sectional study. Dev. Psychobiol. 2023, 65, e22369. [Google Scholar] [CrossRef]
- Tioleco, N.; Silberman, A.E.; Stratigos, K.; Banerjee-Basu, S.; Spann, M.N.; Whitaker, A.H.; Turner, J.B. Prenatal maternal infection and risk for autism in offspring: A meta-analysis. Autism Res. 2021, 14, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- Nudel, R.; Thompson, W.K.; Børglum, A.D.; Hougaard, D.M.; Mortensen, P.B.; Werge, T.; Nordentoft, M.; Benros, M.E. Maternal pregnancy-related infections and autism spectrum disorder—The genetic perspective. Transl. Psychiatry 2022, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Brynge, M.; Sjöqvist, H.; Gardner, R.M.; Lee, B.K.; Dalman, C.; Karlsson, H. Maternal infection during pregnancy and likelihood of autism and intellectual disability in children in Sweden: A negative control and sibling comparison cohort study. Lancet Psychiatry 2022, 9, 782–791. [Google Scholar] [CrossRef]
- Duque-Cartagena, T.; Dalla, M.D.B.; Mundstock, E.; Neto, F.K.; Espinoza, S.A.R.; de Moura, S.K.; Zanirati, G.; Padoin, A.V.; Jimenez, J.G.P.; Stein, A.T.; et al. Environmental pollutants as risk factors for autism spectrum disorders: A systematic review and meta-analysis of cohort studies. BMC Public Health 2024, 24, 2388. [Google Scholar] [CrossRef]
- Phiri, Y.V.A.; Canty, T.; Nobles, C.; Ring, A.M.; Nie, J.; Mendola, P. Neonatal intensive care admissions and exposure to satellite-derived air pollutants in the United States, 2018. Sci. Rep. 2025, 15, 420. [Google Scholar] [CrossRef]
- Bragg, M.G.; Gorski-Steiner, I.; Song, A.; Chavarro, J.E.; Hart, J.E.; Tabb, L.P.; Weisskopf, M.G.; Volk, H.; Lyall, K. Prenatal air pollution and children’s autism traits score: Examination of joint associations with maternal intake of vitamin D, methyl donors, and polyunsaturated fatty acids using mixture methods. Environ. Epidemiol. 2024, 8, e316. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Sargsyan, S.; Chough, I.; Petrick, L.; Liao, J.; Chen, W.; Pavlovic, N.; Lurmann, F.W.; Martinez, M.P.; McConnell, R.; et al. Dysregulated metabolic pathways associated with air pollution exposure and the risk of autism: Evidence from epidemiological studies. Environ. Pollut. 2024, 361, 124729. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Parasin, N.; Saokaew, S. Exploring the association between early-life air pollution exposure and autism spectrum disorders in children: A systematic review and meta-analysis. Reprod. Toxicol. 2024, 125, 108582. [Google Scholar] [CrossRef]
- Ritz, B.; Liew, Z.; Yan, Q.; Cuia, X.; Virk, J.; Ketzel, M.; Raaschou-Nielsen, O. Air pollution and autism in Denmark. Environ. Epidemiol. 2018, 2, e028. [Google Scholar] [CrossRef]
- Ojha, S.K.; Amal, H. Air pollution: An emerging risk factor for autism spectrum disorder. Brain Med. 2024, 1, 31–34. [Google Scholar] [CrossRef]
- Dutheil, F.; Comptour, A.; Morlon, R.; Mermillod, M.; Pereira, B.; Baker, J.S.; Charkhabi, M.; Clinchamps, M.; Bourdel, N. Autism spectrum disorder and air pollution: A systematic review and meta-analysis. Environ. Pollut. 2021, 278, 116856. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.A.; Rahman, M.M.; Lin, J.C.; Shu, Y.-H.; Chow, T.; Yu, X.; Martinez, M.P.; Eckel, S.P.; Chen, J.-C.; Chen, Z.; et al. In utero exposure to near-roadway air pollution and autism spectrum disorder in children. Environ. Int. 2022, 158, 106898. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.; Malmqvist, E.; Rittner, R.; Gustafsson, P.; Källén, K.; Oudin, A. Exposure to local, source-specific ambient air pollution during pregnancy and autism in children: A cohort study from southern Sweden. Sci. Rep. 2023, 13, 3848. [Google Scholar] [CrossRef]
- Autism Likelihood in Infants Born to Mothers with Asthma Is Associated with Blood Inflammatory Gene Biomarkers in Pregnancy—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2666354624001236 (accessed on 20 May 2025).
- Gong, T.; Lundholm, C.; Rejnö, G.; Bölte, S.; Larsson, H.; D’Onofrio, B.M.; Lichtenstein, P.; Almqvist, C. Parental asthma and risk of autism spectrum disorder in offspring: A population and family-based case-control study. Clin. Exp. Allergy 2019, 49, 883–891. [Google Scholar] [CrossRef]
- Neural Regeneration Research. Available online: https://journals.lww.com/nrronline/fulltext/2025/04000/evidence_supporting_the_relationship_between.26.aspx (accessed on 20 May 2025).
- Liao, J.; Yan, W.; Zhang, Y.; Berhane, K.; Chen, W.; Yang, Z.; Qiu, C.; Ge, Y.; Bai, Z.; Han, B.; et al. Associations of preconception air pollution exposure with growth trajectory in young children: A prospective cohort study. Environ. Res. 2025, 267, 120665. [Google Scholar] [CrossRef]
- Gardner, R.M.; Brynge, M.; Sjöqvist, H.; Dalman, C.; Karlsson, H. Maternal Immune Activation and Autism in Offspring: What Is the Evidence for Causation? Biol. Psychiatry 2024, 97, 1127–1138. [Google Scholar] [CrossRef]
- Brynge, M. Immune Dysregulation in Early Life and Risk of Autism. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2022. [Google Scholar]
- McLellan, J.; Kim, D.H.J.; Bruce, M.; Ramirez-Celis, A.; Van de Water, J. Maternal Immune Dysregulation and Autism–Understanding the Role of Cytokines, Chemokines and Autoantibodies. Front. Psychiatry 2022, 13, 834910. [Google Scholar] [CrossRef]
- Robinson-Agramonte, M.D.L.A.; Noris García, E.; Fraga Guerra, J.; Vega Hurtado, Y.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Kumar, D.; Prajapati, K.B.; Mahajan, A.K.; Pant, D.; Yadav, A.; Giri, A.; Manda, S.; Bhandari, S.; et al. Microplastics influencing aquatic environment and human health: A review of source, determination, distribution, removal, degradation, management strategy and future perspective. J. Environ. Manag. 2025, 375, 124249. [Google Scholar] [CrossRef]
- Thongkorn, S.; Kanlayaprasit, S.; Kasitipradit, K.; Lertpeerapan, P.; Panjabud, P.; Hu, V.W.; Jindatip, D.; Sarachana, T. Investigation of autism-related transcription factors underlying sex differences in the effects of bisphenol A on transcriptome profiles and synaptogenesis in the offspring hippocampus. Biol. Sex Differ. 2023, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Parenti, M.; Schmidt, R.J.; Ozonoff, S.; Shin, H.-M.; Tancredi, D.J.; Krakowiak, P.; Hertz-Picciotto, I.; Walker, C.K.; Slupsky, C.M. Maternal Serum and Placental Metabolomes in Association with Prenatal Phthalate Exposure and Neurodevelopmental Outcomes in the MARBLES Cohort. Metabolites 2022, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Parenti, M.; Slupsky, C.M. Disrupted Prenatal Metabolism May Explain the Etiology of Suboptimal Neurodevelopment: A Focus on Phthalates and Micronutrients and their Relationship to Autism Spectrum Disorder. Adv. Nutr. 2024, 15, 100279. [Google Scholar] [CrossRef]
- Symeonides, C.; Vacy, K.; Thomson, S.; Tanner, S.; Chua, H.K.; Dixit, S.; Mansell, T.; O’Hely, M.; Novakovic, B.; Herbstman, J.B.; et al. Male autism spectrum disorder is linked to brain aromatase disruption by prenatal BPA in multimodal investigations and 10HDA ameliorates the related mouse phenotype. Nat. Commun. 2024, 15, 6367. [Google Scholar] [CrossRef]
- Stevens, S.; McPartland, M.; Bartosova, Z.; Skåland, H.S.; Völker, J.; Wagner, M. Plastic Food Packaging from Five Countries Contains Endocrine- and Metabolism-Disrupting Chemicals. Environ. Sci. Technol. 2024, 58, 4859–4871. [Google Scholar] [CrossRef]
- Su, Z.; Kong, R.; Huang, C.; Wang, K.; Liu, C.; Gu, X.; Wang, H.-L. Exposure to polystyrene nanoplastics causes anxiety and depressive-like behavior and down-regulates EAAT2 expression in mice. Arch. Toxicol. 2025, 99, 2595–2609. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Pan, D.; Zhu, Y.; Shen, B.; Sun, Z.; Zheng, Y.; Yin, Y.; Huang, C.; Wu, W.; Song, Y.; et al. Polystyrene nanoplastics chronic exposure cause zebrafish visual neurobehavior toxicity through TGFβ-crystallin axis. J. Hazard. Mater. 2025, 492, 138255. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.-K.; Choi, E.-J.; Lee, H.-J.; Shim, I.; Woo, H.; Choi, J.; Kim, G.-H.; et al. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ. Int. 2022, 161, 107121. [Google Scholar] [CrossRef]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Siracusano, M.; Riccioni, A.; Abate, R.; Benvenuto, A.; Curatolo, P.; Mazzone, L. Vitamin D Deficiency and Autism Spectrum Disorder. Curr. Pharm. Des. 2020, 26, 2460–2474. [Google Scholar] [CrossRef]
- Zwierz, M.; Suprunowicz, M.; Mrozek, K.; Pietruszkiewicz, J.; Oracz, A.J.; Konarzewska, B.; Waszkiewicz, N. Vitamin B12 and Autism Spectrum Disorder: A Review of Current Evidence. Nutrients 2025, 17, 1220. [Google Scholar] [CrossRef] [PubMed]
- Pancheva, R.; Toneva, A.; Bocheva, Y.; Georgieva, M.; Koleva, K.; Yankov, I. Prevalence of vitamin D deficiency in children with cerebral palsy and autism spectrum disorder: A comparative pilot study. Folia Med. 2024, 66, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Stefanyshyn, V.; Stetsyuk, R.; Hrebeniuk, O.; Ayoub, G.; Fishchuk, L.; Rossokha, Z.; Gorovenko, N. Analysis of the Association Between the SLC19A1 Genetic Variant (rs1051266) and Autism Spectrum Disorders, Cerebral Folate Deficiency, and Clinical and Laboratory Parameters. J. Mol. Neurosci. 2025, 75, 42. [Google Scholar] [CrossRef]
- Gusso, D.; Prauchner, G.R.K.; Rieder, A.S.; Wyse, A.T.S. Biological Pathways Associated with Vitamins in Autism Spectrum Disorder. Neurotox. Res. 2023, 41, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Kacimi, F.E.; Ed-Day, S.; Didou, L.; Azzaoui, F.Z.; Ramchoun, M.; Arfaoui, A.; Boulbaroud, S. Narrative Review: The Effect of Vitamin A Deficiency on Gut Microbiota and Their Link with Autism Spectrum Disorder. J. Diet Suppl. 2024, 21, 116–134. [Google Scholar] [CrossRef]
- Kacimi, F.E.; Didou, L.; Ed Day, S.; Azzaoui, F.Z.; Ramchoun, M.; Berrougui, H.; Khalki, H.; Boulbaroud, S. Gut microbiota, vitamin A deficiency and autism spectrum disorder: An interconnected trio—A systematic review. Nutr. Neurosci. 2025, 28, 492–502. [Google Scholar] [CrossRef]
- Savino, R.; Medoro, A.; Ali, S.; Scapagnini, G.; Maes, M.; Davinelli, S. The Emerging Role of Flavonoids in Autism Spectrum Disorder: A Systematic Review. J. Clin. Med. 2023, 12, 3520. [Google Scholar] [CrossRef]
- Schimansky, S.; Jasim, H.; Pope, L.; Hinds, P.; Fernandez, D.; Choleva, P.; Dev Borman, A.; Sharples, P.M.; Smallbone, T.; Atan, D. Nutritional blindness from avoidant-restrictive food intake disorder—Recommendations for the early diagnosis and multidisciplinary management of children at risk from restrictive eating. Arch. Dis. Child 2024, 109, 181–187. [Google Scholar] [CrossRef]
- Panchawagh, S.J.; Kumar, P.; Srikumar, S.; Sarkar, M.; Ashok, T.; Gupta, S.; Shaikh, H.; Soumya, K.S.; Poorvikha, S.; Abhishek, K.; et al. Role of Micronutrients in the Management of Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Indian J. Med. Spec. 2023, 14, 187–196. [Google Scholar] [CrossRef]
- Sumathi, T.; Manivasagam, T.; Thenmozhi, A.J. The Role of Gluten in Autism. Adv. Neurobiol. 2020, 24, 469–479. [Google Scholar] [CrossRef]
- Woo, T.; King, C.; Ahmed, N.I.; Cordes, M.; Nistala, S.; Will, M.J.; Bloomer, C.; Kibiryeva, N.; Rivera, R.M.; Talebizadeh, Z.; et al. microRNA as a Maternal Marker for Prenatal Stress-Associated ASD, Evidence from a Murine Model. J. Pers. Med. 2023, 13, 1412. [Google Scholar] [CrossRef] [PubMed]
- Love, C.; Sominsky, L.; O’Hely, M.; Berk, M.; Vuillermin, P.; Dawson, S.L. Prenatal environmental risk factors for autism spectrum disorder and their potential mechanisms. BMC Med. 2024, 22, 393. [Google Scholar] [CrossRef]
- Subashi, E.; Lemaire, V.; Petroni, V.; Pietropaolo, S. The Impact of Mild Chronic Stress and Maternal Experience in the Fmr1 Mouse Model of Fragile X Syndrome. Int. J. Mol. Sci. 2023, 24, 11398. [Google Scholar] [CrossRef] [PubMed]
- Ahmavaara, K.; Ayoub, G. Stress and Folate Impact Neurodevelopmental Disorders. J. Health Care Res. 2024, 5, 1–6. [Google Scholar] [CrossRef]
- Hoover, D.W.; Kaufman, J. Adverse childhood experiences in children with autism spectrum disorder. Curr. Opin. Psychiatry 2018, 31, 128–132. [Google Scholar] [CrossRef]
- Liu, K.; Garcia, A.; Park, J.J.; Toliver, A.A.; Ramos, L.; Aizenman, C.D. Early Developmental Exposure to Fluoxetine and Citalopram Results in Different Neurodevelopmental Outcomes. Neuroscience 2021, 467, 110–121. [Google Scholar] [CrossRef]
- Bravo, K.; González-Ortiz, M.; Beltrán-Castillo, S.; Cáceres, D.; Eugenín, J. Development of the Placenta and Brain Are Affected by Selective Serotonin Reuptake Inhibitor Exposure During Critical Periods. In Advances in Maternal-Fetal Biomedicine: Cellular and Molecular Mechanisms of Pregnancy Pathologies; Gonzalez-Ortiz, M., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 179–198. [Google Scholar] [CrossRef]
- Arzuaga, A.L.; Teneqexhi, P.; Amodeo, K.; Larson, J.R.; Ragozzino, M.E. Prenatal stress and fluoxetine exposure in BTBR and B6 mice differentially affects autism-like behaviors in adult male and female offspring. Physiol. Behav. 2025, 295, 114891. [Google Scholar] [CrossRef]
- Arzuaga, A.L.; Edmison, D.D.; Mroczek, J.; Larson, J.; Ragozzino, M.E. Prenatal stress and fluoxetine exposure in mice differentially affect repetitive behaviors and synaptic plasticity in adult male and female offspring. Behav. Brain Res. 2023, 436, 114114. [Google Scholar] [CrossRef]
- Lan, Z.; Tachibana, R.O.; Kanno, K. Chronic exposure of female mice to selective serotonin reuptake inhibitors during lactation induces vocal behavior deficits in pre-weaned offspring. Pharmacol. Biochem. Behav. 2023, 230, 173606. [Google Scholar] [CrossRef]
- Croen, L.A.; Ames, J.L.; Qian, Y.; Alexeeff, S.; Ashwood, P.; Gunderson, E.P.; Wu, Y.W.; Boghossian, A.S.; Yolken, R.; Van de Water, J.; et al. Inflammatory Conditions During Pregnancy and Risk of Autism and Other Neurodevelopmental Disorders. Biol. Psychiatry Glob. Open Sci. 2024, 4, 39–50. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Zhou, J.; Liang, X.; Wen, J.; Huang, J.; Zeng, Y.; Wu, Y.; Gao, Y.; Liu, Z.; et al. Association between maternal diabetes and neurodevelopmental outcomes in children: A systematic review and meta-analysis of 202 observational studies comprising 56·1 million pregnancies. Lancet Diabetes Endocrinol. 2025, 13, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Fardous, A.M.; Heydari, A.R. Uncovering the Hidden Dangers and Molecular Mechanisms of Excess Folate: A Narrative Review. Nutrients 2023, 15, 4699. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, N.; Dehay, E. Folate Supplementation in Fertility and Pregnancy: The Advantages of (6S)5-Methyltetrahydrofolate. Altern Ther. Health Med. 2022, 28, 12–17. [Google Scholar] [PubMed]
- Xu, X.; Zhang, Z.; Lin, Y.; Xie, H. Risk of Excess Maternal Folic Acid Supplementation in Offspring. Nutrients 2024, 16, 755. [Google Scholar] [CrossRef]
- Ledowsky, C.J.; Schloss, J.; Steel, A. Variations in folate prescriptions for patients with the MTHFR genetic polymorphisms: A case series study. Explor. Res. Clin. Soc. Pharm. 2023, 10, 100277. [Google Scholar] [CrossRef]
- Maguire, G. Vaccine Induced Autoimmunity May Cause Autism and Neurological Disorders. Arch. Microbiol. Immunol. 2025, 9, 103–132. [Google Scholar] [CrossRef]
- Sotelo-Orozco, J.; Schmidt, R.J.; Slupsky, C.M.; Hertz-Picciotto, I. Investigating the Urinary Metabolome in the First Year of Life and Its Association with Later Diagnosis of Autism Spectrum Disorder or Non-Typical Neurodevelopment in the MARBLES Study. Int. J. Mol. Sci. 2023, 24, 9454. [Google Scholar] [CrossRef]
- Parenti, M.; Shoff, S.; Sotelo-Orozco, J.; Hertz-Picciotto, I.; Slupsky, C.M. Metabolomics of mothers of children with autism, idiopathic developmental delay, and Down syndrome. Sci. Rep. 2024, 14, 31981. [Google Scholar] [CrossRef]
- Parenti, M.; Schmidt, R.J.; Tancredi, D.J.; Hertz-Picciotto, I.; Walker, C.K.; Slupsky, C.M. Neurodevelopment and Metabolism in the Maternal-Placental-Fetal Unit. JAMA Netw. Open 2024, 7, e2413399. [Google Scholar] [CrossRef]
- Merchak, A.R.; Bolen, M.L.; Tansey, M.G.; Menees, K.B. Thinking outside the brain: Gut microbiome influence on innate immunity within neurodegenerative disease. Neurotherapeutics 2024, 21, e00476. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Dossaji, Z.; Khattak, A.; Tun, K.M.; Hsu, M.; Batra, K.; Hong, A.S. Efficacy of Fecal Microbiota Transplant on Behavioral and Gastrointestinal Symptoms in Pediatric Autism: A Systematic Review. Microorganisms 2023, 11, 806. [Google Scholar] [CrossRef]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The Role of Gut Microbiota in Various Neurological and Psychiatric Disorders-An Evidence Mapping Based on Quantified Evidence. Mediat. Inflamm. 2023, 2023, 5127157. [Google Scholar] [CrossRef]
- Lagod, P.P.; Abdelli, L.S.; Naser, S.A. An In Vivo Model of Propionic Acid-Rich Diet-Induced Gliosis and Neuro-Inflammation in Mice (FVB/N-Tg(GFAPGFP)14Mes/J): A Potential Link to Autism Spectrum Disorder. Int. J. Mol. Sci. 2024, 25, 8093. [Google Scholar] [CrossRef] [PubMed]
- Retuerto, M.; Al-Shakhshir, H.; Herrada, J.; McCormick, T.S.; Ghannoum, M.A. Analysis of Gut Bacterial and Fungal Microbiota in Children with Autism Spectrum Disorder and Their Non-Autistic Siblings. Nutrients 2024, 16, 3004. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Gómez-Fernández, A.; Chueca, N.; Torre-Aguilar, M.J.D.L.; Gil, Á.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism Spectrum Disorder (ASD) with and without Mental Regression is Associated with Changes in the Fecal Microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Brister, D.; Rose, S.; Delhey, L.; Tippett, M.; Jin, Y.; Gu, H.; Frye, R.E. Metabolomic Signatures of Autism Spectrum Disorder. J. Pers. Med. 2022, 12, 1727. [Google Scholar] [CrossRef]
- Che, X.; Roy, A.; Bresnahan, M.; Mjaaland, S.; Reichborn-Kjennerud, T.; Magnus, P.; Stoltenberg, C.; Shang, Y.; Zhang, K.; Susser, E.; et al. Metabolomic analysis of maternal mid-gestation plasma and cord blood in autism spectrum disorders. Mol. Psychiatry 2023, 28, 2355–2369. [Google Scholar] [CrossRef]
- Vacy, K.; Thomson, S.; Moore, A.; Eisner, A.; Tanner, S.; Pham, C.; Saffery, R.; Mansell, T.; Burgner, D.; Collier, F.; et al. Cord blood lipid correlation network profiles are associated with subsequent attention-deficit/hyperactivity disorder and autism spectrum disorder symptoms at 2 years: A prospective birth cohort study. eBioMedicine 2024, 100, 104949. [Google Scholar] [CrossRef]
- Nabetani, M.; Mukai, T.; Taguchi, A. Cell Therapies for Autism Spectrum Disorder Based on New Pathophysiology: A Review. Cell Transpl. 2023, 32, 9636897231163217. [Google Scholar] [CrossRef]
- Ahrens, A.P.; Hyötyläinen, T.; Petrone, J.R.; Igelström, K.; George, C.D.; Garrett, T.J.; Orešič, M.; Triplett, E.W.; Ludvigsson, J. Infant microbes and metabolites point to childhood neurodevelopmental disorders. Cell 2024, 187, 1853–1873.e15. [Google Scholar] [CrossRef] [PubMed]
- Freitas, B.C.; Beltrão-Braga, P.C.B.; Marchetto, M.C. Modeling Inflammation on Neurodevelopmental Disorders Using Pluripotent Stem Cells. Adv. Neurobiol. 2020, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, T.; Shimada, H.; Sakata-Haga, H.; Iizuka, H.; Hatta, T. Molecular mechanisms underlying the models of neurodevelopmental disorders in maternal immune activation relevant to the placenta. Congenit. Anom. 2019, 59, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rincon, N.; McCarty, P.J.; Brister, D.; Scheck, A.C.; Rossignol, D.A. Biomarkers of mitochondrial dysfunction in autism spectrum disorder: A systematic review and meta-analysis. Neurobiol. Dis. 2024, 197, 106520. [Google Scholar] [CrossRef]
- Amini-Khoei, H.; Taei, N.; Dehkordi, H.T.; Lorigooini, Z.; Bijad, E.; Farahzad, A.; Madiseh, M.R. Therapeutic Potential of Ocimum basilicum L. Extract in Alleviating Autistic-Like Behaviors Induced by Maternal Separation Stress in Mice: Role of Neuroinflammation and Oxidative Stress. Phytother. Res. 2025, 39, 64–76. [Google Scholar] [CrossRef]
- Nguyen, V.; Tang, J.; Oroudjev, E.; Lee, C.J.; Marasigan, C.; Wilson, L.; Ayoub, G. Cytotoxic Effects of Bilberry Extract on MCF7-GFP-Tubulin Breast Cancer Cells. J. Med. Food 2010, 13, 278–285. [Google Scholar] [CrossRef]
- Lai, M.; Lee, J.; Chiu, S.; Charm, J.; So, W.Y.; Yuen, F.P.; Kwok, C.; Tsoi, J.; Lin, Y.; Zee, B. A machine learning approach for retinal images analysis as an objective screening method for children with autism spectrum disorder. eClinicalMedicine 2020, 28, 100588. [Google Scholar] [CrossRef]
- Farooq, M.S.; Tehseen, R.; Sabir, M.; Atal, Z. Detection of autism spectrum disorder (ASD) in children and adults using machine learning. Sci. Rep. 2023, 13, 9605. [Google Scholar] [CrossRef]

| Category | Genes | Developmental Impact |
|---|---|---|
| Synaptic | ADNP, UBE3A, GABRB3, MECP2, NRXN1, SHANK3, GRIN2B | Cell Junction Organization |
| ADNP, UBE3A, GABRB3, MECP2, NRXN1, SHANK3, GRIN2B | Synapse Organization | |
| ADNP, STXBP1, GABRB3, MECP2, NRXN1, SHANK3, GRIN2B | Chemical Synaptic Transmission | |
| ADNP, GABRB3, MECP2, NRXN1, SHANK3 | Synapse Assembly | |
| Social/ Behavioral | CHD8, MECP2, NRXN1, SHANK3 | Biological Processes in Intraspecies Interaction |
| CHD8, MECP2, NRXN1, SHANK3 | Social Behavior | |
| Neuronal/ Cellular | TRIO, ADNP, UBE3A, STXBP1, AUTS2, MECP2, NRXN1, TCF4, SHANK3 | Neuron Differentiation |
| TRIO, ADNP, UBE3A, STXBP1, AUTS2, MECP2, NRXN1, SHANK3 | Neuron Projection Development | |
| TRIO, ADNP, UBE3A, STXBP1, AUTS2, NRXN1, SHANK3 | Cell Morphogenesis in Differentiation | |
| TRIO, ADNP, UBE3A, STXBP1, AUTS2, NRXN1, SHANK3 | Cell Part Morphogenesis |
| Category | Gene | Pathways and References |
|---|---|---|
| Neuronal/ Cellular | MAPK1 | MAPK signaling, Calcium signaling [23] |
| MAPK3 | MAPK signaling, Calcium signaling [23] | |
| HRAS | MAPK signaling, Calcium signaling [23] | |
| PRKCB | Calcium signaling, MAPK signaling [23] | |
| BRAF | MAPK signaling, Calcium signaling [23] | |
| CORO1A | Neuron function, Immune response [4] | |
| Synaptic | SCN2A | Synaptic development (M16 module) [24] |
| SHANK2 | Synaptic development (M16 module) [24] | |
| NRXN1 | Synaptic development (M16 module) [24] | |
| GRIN2B | Synaptic transmission [4] | |
| Cellular/ Metabolic | CNDP1 | mTOR pathway (neurodevelopment) [25] |
| PDE4D | mTOR pathway (neurodevelopment) [25] | |
| ULK2 | mTOR pathway (neurodevelopment) [25] | |
| CHD8 | Gene expression in development [4] | |
| PTEN | Cellular development [4] |
| Comorbid Condition | SFARI ASD Gene |
|---|---|
| ADHD | PPP3CB, PRKG1 |
| Anxiety Disorder | ADCYAP1R1, DLGAP4, NPPB, BRP1, VIPR2 |
| Bipolar Disorder | ADAM10, ADCY9, ADCYAP1R1, AKT1, DLGAP4, HSPA1L, MEGF10, NDE1, NPPB, BRP1, VIPR2 |
| Depressive Disorder | ADCY9, AKT1, DGCR8, HSPA1L, VIPR2 |
| Epilepsy | ADCY9, AKT1, ATN1, KCNH2, MMP2, NDE1, SLC29A2, SMARCA2, VIPR2 |
| OCD | ADCYAP1R1, DLGAP4, NPPB, NRP1, BIPR2 |
| Panic Disorder | ADCYAP1R1, DLGAP4, NPPB, NRP1, BIPR2 |
| Schizophrenia | ADCY9, AKT1, ATN1, DGCR8, DLGAP4, HSPA1L, KCNH2, MEGF10, NDE1, PPP3CB, PRKG1, SMARCA2, VIPR2 |
| Sleep Disorders | ADAM10, ADCY9, ATN1, DGCR8, DLGAP4, KCNH2, MEGF10, NPPB, NRP1, PPP3CB, PRKG1, SLC29A2, SMARCA2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, G. Autism Spectrum Disorder as a Multifactorial Disorder: The Interplay of Genetic Factors and Inflammation. Int. J. Mol. Sci. 2025, 26, 6483. https://doi.org/10.3390/ijms26136483
Ayoub G. Autism Spectrum Disorder as a Multifactorial Disorder: The Interplay of Genetic Factors and Inflammation. International Journal of Molecular Sciences. 2025; 26(13):6483. https://doi.org/10.3390/ijms26136483
Chicago/Turabian StyleAyoub, George. 2025. "Autism Spectrum Disorder as a Multifactorial Disorder: The Interplay of Genetic Factors and Inflammation" International Journal of Molecular Sciences 26, no. 13: 6483. https://doi.org/10.3390/ijms26136483
APA StyleAyoub, G. (2025). Autism Spectrum Disorder as a Multifactorial Disorder: The Interplay of Genetic Factors and Inflammation. International Journal of Molecular Sciences, 26(13), 6483. https://doi.org/10.3390/ijms26136483






