A Paradigm Shift in SSTI Management: The Multifunctional Role of Extracellular Vesicles
Abstract
1. Introduction
2. Key Bacterial Pathogens in SSTIs
2.1. Streptococcus aureus
2.2. Group A Streptococcus (Streptococcus pyogenes)
2.3. Clostridium perfringens
2.4. Others
3. Epidemiological Evidence of Antibiotic Resistance in SSTIs
4. Current Treatment for SSTIs
5. Extracellular Vesicles (EVs)
5.1. EVs in Promoting SSTIs
5.2. EVs in Supressing SSTIs
6. Clinical Translation and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sartelli, M.; Coccolini, F.; Kluger, Y.; Agastra, E.; Abu-Zidan, F.M.; Abbas, A.E.S.; Ansaloni, L.; Adesunkanmi, A.K.; Augustin, G.; Bala, M.; et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J. Emerg. Surg. 2022, 17, 3. [Google Scholar] [CrossRef]
- Castaldo, N.; Vena, A.; Limongelli, A.; Giacobbe, D.R.; Bassetti, M. Emerging treatment options for skin and soft tissue infections tailoring drug selection to individual patients. Curr. Opin. Infect. Dis. 2024, 37, 80–86. [Google Scholar] [CrossRef]
- Ling, J.Y.; How, C.H.; Chien, J.M.F.; Poulose, V.; Ng, M.C.W. Skin and soft tissue infections in primary care. Singap. Med. J. 2025, 66, 108–113. [Google Scholar] [CrossRef]
- Toschi, A.; Giannella, M.; Viale, P. Recurrence of skin and soft tissue infections: Identifying risk factors and treatment strategies. Curr. Opin. Infect. Dis. 2025, 38, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vella, V.; Derreumaux, D.; Aris, E.; Pellegrini, M.; Contorni, M.; Scherbakov, M.; Bagnoli, F. The Incidence of Skin and Soft Tissue Infections in the United States and Associated Healthcare Utilization Between 2010 and 2020. Open Forum Infect. Dis. 2024, 11, ofae267. [Google Scholar] [CrossRef]
- Linz, M.S.; Mattappallil, A.; Finkel, D.; Parker, D. Clinical Impact of Staphylococcus aureus Skin and Soft Tissue Infections. Antibiotics 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Anwer, A.; Ghummon, A.K.; Rana, M.S.M.; Rasheed, S.; Hamid, F.; Gul, J. Prevalence of Skin and Soft Tissue Infections in Diabetic Patients in a District Headquarters Hospital of, Pakistan. Pak. J. Med. Health Sci. 2022, 16, 878. [Google Scholar] [CrossRef]
- Lwigale, F.; Kibombo, D.; Kasango, S.D.; Tabajjwa, D.; Atuheire, C.; Kungu, J.; Kalule, J.B.; Otita, M.; Kakooza, F.; Nabukenya, I.; et al. Prevalence, resistance profiles and factors associated with skin and soft-tissue infections at Jinja regional referral hospital: A retrospective study. PLoS Glob. Public Health 2024, 4, e0003582. [Google Scholar] [CrossRef]
- Bereanu, A.S.; Bereanu, R.; Mohor, C.; Vintilă, B.I.; Codru, I.R.; Olteanu, C.; Sava, M. Prevalence of Infections and Antimicrobial Resistance of ESKAPE Group Bacteria Isolated from Patients Admitted to the Intensive Care Unit of a County Emergency Hospital in Romania. Antibiotics 2024, 13, 400. [Google Scholar] [CrossRef]
- Silverberg, B. A Structured Approach to Skin and Soft Tissue Infections (SSTIs) in an Ambulatory Setting. Clin. Pract. 2021, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, Y.; Xu, H.; Lei, Y.; Long, W.; Li, M.; Gu, Y.; Jiang, Z.; Cao, C. Prevalence and clinical characteristics of methicillin-resistant Staphylococcus aureus infections among dermatology inpatients: A 7-year retrospective study at a tertiary care center in southwest China. Front. Public Health 2023, 11, 1124930. [Google Scholar] [CrossRef]
- Loewen, K.; Schreiber, Y.; Kirlew, M.; Bocking, N.; Kelly, L. Community-associated methicillin-resistant Staphylococcus aureus infection: Literature review and clinical update. Can. Fam. Physician 2017, 63, 512–520. [Google Scholar]
- Miller, L.G.; Eisenberg, D.F.; Liu, H.; Chang, C.-L.; Wang, Y.; Luthra, R.; Wallace, A.; Fang, C.; Singer, J.; Suaya, J.A. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect. Dis. 2015, 15, 362. [Google Scholar] [CrossRef] [PubMed]
- Edelsberg, J.; Taneja, C.; Zervos, M.; Haque, N.; Moore, C.; Reyes, K.; Spalding, J.; Jiang, J.; Oster, G. Trends in US hospital admissions for skin and soft tissue infections. Emerg. Infect. Dis. 2009, 15, 1516–1518. [Google Scholar] [CrossRef]
- Shaw, T.D.; Krasnodembskaya, A.D.; Schroeder, G.N.; Zumla, A.; Maeurer, M.; O’Kane, C.M. Mesenchymal Stromal Cells: An Antimicrobial and Host-Directed Therapy for Complex Infectious Diseases. Clin. Microbiol. Rev. 2021, 34, e0006421. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Burnham, J.P.; Kollef, M.H. Treatment of severe skin and soft tissue infections: A review. Curr. Opin. Infect. Dis. 2018, 31, 113–119. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; Chambers, H.F. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46 (Suppl. S5), S368–S377. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.; Fatima, M.; Zaheer, C.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2023, 13, 1067284. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Rawat, S. A genetic regulatory see-saw of biofilm and virulence in MRSA pathogenesis. Front. Microbiol. 2023, 14, 1204428. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Pivard, M.; Moreau, K.; Vandenesch, F. Staphylococcus aureus Arsenal to Conquer the Lower Respiratory Tract. mSphere 2021, 6, e00059-21. [Google Scholar] [CrossRef]
- Ballah, F.M.; Islam, M.S.; Rana, M.L.; Ullah, M.A.; Ferdous, F.B.; Neloy, F.H.; Ievy, S.; Sobur, M.A.; Rahman, A.T.; Khatun, M.M.; et al. Virulence Determinants and Methicillin Resistance in Biofilm-Forming Staphylococcus aureus from Various Food Sources in Bangladesh. Antibiotics 2022, 11, 1666. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, X.; Wang, X.; Du, B.; Xu, K.; Zhang, F.; Jiang, C.; Zhao, Y.; Zhu, Y. Molecular characterization and virulence gene profiling of methicillin-resistant Staphylococcus aureus associated with bloodstream infections in southern China. Front. Microbiol. 2022, 13, 1008052. [Google Scholar] [CrossRef]
- Abraham, N.M.; Jefferson, K.K. Staphylococcus aureus clumping factor B mediates biofilm formation in the absence of calcium. Microbiology 2012, 158 Pt 6, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, F.; Sudagidan, M.; Aydin, A.; Akyazi, I.; Bayrakal, G.M.; Yavuz, O.; Gurel, A. In Vivo Pathogenicity of Methicillin-Susceptible Staphylococcus aureus Strains Carrying Panton-Valentine Leukocidin Gene. Life 2022, 12, 2126. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.R.; Cavaco, L.M.; Nath, G.; Kumar, K.; Gaur, A.; Gokhale, S. Association of Panton Valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: A matter of concern for community infections (a hospital based prospective study). BMC Infect. Dis. 2016, 16, 199. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Ito, T.; Tsubakishita, S.; Sasaki, T.; Takeuchi, F.; Morimoto, Y.; Katayama, Y.; Matsuo, M.; Kuwahara-Arai, K.; Hishinuma, T.; et al. Genomic Basis for Methicillin Resistance in Staphylococcus aureus. Infect. Chemother. 2013, 45, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Wanner, S.; Schade, J.; Keinhörster, D.; Weller, N.; George, S.E.; Kull, L.; Bauer, J.; Grau, T.; Winstel, V.; Stoy, H.; et al. Wall teichoic acids mediate increased virulence in Staphylococcus aureus. Nat. Microbiol. 2017, 2, 16257. [Google Scholar] [CrossRef]
- Liu, G.Y. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr. Res. 2009, 65 Pt 2, 71R–77R. [Google Scholar] [CrossRef]
- Kashif, A.; McClure, J.A.; Lakhundi, S.; Pham, M.; Chen, S.; Conly, J.M.; Zhang, K. Staphylococcus aureus ST398 Virulence Is Associated With Factors Carried on Prophage ϕSa3. Front. Microbiol. 2019, 10, 2219. [Google Scholar] [CrossRef]
- Oliveira, H.B.M.; Selis, N.d.N.; Sampaio, B.A.; Júnior, M.N.S.; de Carvalho, S.P.; de Almeida, J.B.; Almeida, P.P.; da Silva, I.B.S.; Oliveira, C.N.T.; Brito, T.L.S.; et al. Citral modulates virulence factors in methicillin-resistant Staphylococcus aureus. Sci. Rep. 2021, 11, 16482. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Cameron, D.R.; Nethercott, C.; Aires-de-Sousa, M.; Peleg, A.Y. Virulence attributes of successful methicillin-resistant Staphylococcus aureus lineages. Clin. Microbiol. Rev. 2023, 36, e0014822. [Google Scholar] [CrossRef]
- Touaitia, R.; Mairi, A.; Ibrahim, N.A.; Basher, N.S.; Idres, T.; Touati, A. Staphylococcus aureus: A Review of the Pathogenesis and Virulence Mechanisms. Antibiotics 2025, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E. Streptococcus pyogenes Impetigo, Erysipelas, and Cellulitis. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations, 2nd ed.; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft-tissue infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef]
- Nakauyaca, A.V.; Ralph, A.P.; Majoni, W.S.; Kangaharan, N. Case Report: Concurrent Rheumatic Fever and Acute Post-Streptococcal Glomerulonephritis in a High-Burden Setting. Am. J. Trop. Med. Hyg. 2019, 101, 1054–1057. [Google Scholar] [CrossRef]
- Brouwer, S.; Rivera-Hernandez, T.; Curren, B.F.; Harbison-Price, N.; De Oliveira, D.M.P.; Jespersen, M.G.; Davies, M.R.; Walker, M.J. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat. Rev. Microbiol. 2023, 21, 431–447. [Google Scholar] [CrossRef]
- Avire, N.J.; Whiley, H.; Ross, K. A Review of Streptococcus pyogenes: Public Health Risk Factors, Prevention and Control. Pathogens 2021, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Metzgar, D.; Zampolli, A. The M protein of group A Streptococcus is a key virulence factor and a clinically relevant strain identification marker. Virulence 2011, 2, 402–412. [Google Scholar] [CrossRef]
- Smeesters, P.R.; McMillan, D.J.; Sriprakash, K.S. The streptococcal M protein: A highly versatile molecule. Trends Microbiol. 2010, 18, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sierig, G.; Cywes, C.; Wessels, M.R.; Ashbaugh, C.D. Cytotoxic effects of streptolysin o and streptolysin s enhance the virulence of poorly encapsulated group a streptococci. Infect. Immun. 2003, 71, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Shannon, B.A.; McCormick, J.K.; Schlievert, P.M. Toxins and Superantigens of Group A Streptococci. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Huish, S.; Thelwell, C.; Longstaff, C. Activity Regulation by Fibrinogen and Fibrin of Streptokinase from Streptococcus Pyogenes. PLoS ONE 2017, 12, e0170936. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, L. Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiol. 2009, 4, 201–221. [Google Scholar] [CrossRef]
- Happonen, L.; Collin, M. Immunomodulating Enzymes from Streptococcus pyogenes-In Pathogenesis, as Biotechnological Tools, and as Biological Drugs. Microorganisms 2024, 12, 200. [Google Scholar] [CrossRef]
- Hussain, H.; Fadel, A.; Garcia, E.; Hernandez, R.J.; Saadoon, Z.F.; Naseer, L.; Casmartino, E.; Hamad, M.; Schnepp, T.; Sarfraz, R.; et al. Clostridial Myonecrosis: A Comprehensive Review of Toxin Pathophysiology and Management Strategies. Microorganisms 2024, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Camargo, A.; Ramírez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the pathogenic mechanisms of Clostridium perfringens toxins and virulence factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef]
- Verherstraeten, S.; Goossens, E.; Valgaeren, B.; Pardon, B.; Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Deprez, P.; Wade, K.R.; Tweten, R.; et al. Perfringolysin O: The Underrated Clostridium perfringens Toxin? Toxins 2015, 7, 1702–1721. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.W.; Kopari, N.M.; Pham, T.N.; Evans, H.L. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr. Probl. Surg. 2014, 51, 344–362. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Sathe, N.; Beech, P.; Croft, L.; Suphioglu, C.; Kapat, A.; Athan, E. Pseudomonas aeruginosa: Infections and novel approaches to treatment “Knowing the enemy” the threat of Pseudomonas aeruginosa and exploring novel approaches to treatment. Infect. Med. 2023, 2, 178–194. [Google Scholar] [CrossRef]
- Rahal, E.A.; Kazzi, N.; Nassar, F.J.; Matar, G.M. Escherichia coli O157:H7-Clinical aspects and novel treatment approaches. Front. Cell. Infect. Microbiol. 2012, 2, 138. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Ali, S.M.; Singh, P.K. Necrotizing fasciitis and gas gangrene due to Aeromonas hydrophila in an immunocompetent host: A rare entity. IDCases 2022, 28, e01508. [Google Scholar] [CrossRef]
- Abrahamian, F.M.; Goldstein, E.J. Microbiology of animal bite wound infections. Clin. Microbiol. Rev. 2011, 24, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Miyamoto, S.; Tawara, T.; Aoyagi, A.; Oguro, T.; Kobayashi, N.; Suzuki, M.; Takeyama, Y. Capnocytophaga canimorsus infection led to progressively fatal septic shock in an immunocompetent patient. Acute Med. Surg. 2022, 9, e738. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; Abourizk, N.; Gadhiya, K.P.; Hansrivijit, P.; Goldman, J.D. A Bite So Bad: Septic Shock Due to Capnocytophaga Canimorsus Following a Dog Bite. Cureus 2021, 13, e14668. [Google Scholar] [CrossRef]
- Iba, T.; Watanabe, E.; Umemura, Y.; Wada, T.; Hayashida, K.; Kushimoto, S.; Wada, H. Sepsis-associated disseminated intravascular coagulation and its differential diagnoses. J. Intensiv. Care 2019, 7, 32. [Google Scholar] [CrossRef]
- Piorunek, M.; Brajer-Luftmann, B.; Walkowiak, J. Pasteurella multocida Infection in Humans. Pathogens 2023, 12, 1210. [Google Scholar] [CrossRef]
- Stephens, M.A.; Silin, N.; Dakkak, T.; Sathian, S.; Ghosh, A.K.; Singh, H.; Bongu, N. Capnocytophaga canimorsus from Dog Saliva Exposure Causing Severe Sepsis in a Healthy Adult: A Case Report. Am. J. Case Rep. 2025, 26, e946691. [Google Scholar] [CrossRef]
- Kreuger, M.J.; Eshetu, A.; Fanta, E.G. Eikenella corrodens in a patient with septic arthritis: A case report. IDCases 2025, 40, e02222. [Google Scholar] [CrossRef]
- Jones, M.K.; Oliver, J.D. Vibrio vulnificus: Disease and pathogenesis. Infect. Immun. 2009, 77, 1723–1733. [Google Scholar] [CrossRef]
- Hashish, E.; Merwad, A.; Elgaml, S.; Amer, A.; Kamal, H.; Elsadek, A.; Marei, A.; Sitohy, M. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management; a review. Vet. Q. 2018, 38, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Conyers, L.E.; Saunders, B.M. Treatment for non-tuberculous mycobacteria: Challenges and prospects. Front. Microbiol. 2024, 15, 1394220. [Google Scholar] [CrossRef]
- Monk, E.J.M.; Jones, T.P.W.; Bongomin, F.; Kibone, W.; Nsubuga, Y.; Ssewante, N.; Muleya, I.; Nsenga, L.; Rao, V.B.; van Zandvoort, K. Antimicrobial resistance in bacterial wound, skin, soft tissue and surgical site infections in Central, Eastern, Southern and Western Africa: A systematic review and meta-analysis. PLOS Glob. Public Health. 2024, 4, e0003077. [Google Scholar] [CrossRef]
- Gouleu, C.S.; Daouda, M.A.; Oye Bingono, S.O.; McCall, M.B.B.; Alabi, A.S.; Adegnika, A.A.; Schaumburg, F.; Grebe, T. Temporal trends of skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus in Gabon. Antimicrob. Resist. Infect. Control. 2024, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Huang, Y.C.; Chiu, C.H.; Su, L.H.; Lin, T.Y. Clinical features and genotyping analysis of community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children. Pediatr. Infect. Dis. J. 2005, 24, 40–45. [Google Scholar] [CrossRef]
- Lim, W.W.; Wu, P.; Bond, H.S.; Wong, J.Y.; Ni, K.; Seto, W.H.; Jit, M.; Cowling, B.J. Determinants of methicillin-resistant Staphylococcus aureus (MRSA) prevalence in the Asia-Pacific region: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2019, 16, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Mzee, T.; Kazimoto, T.; Madata, J.; Masalu, R.; Bischoff, M.; Matee, M.; Becker, S.L. Prevalence, antimicrobial susceptibility and genotypic characteristics of Staphylococcus aureus in Tanzania: A systematic review. Bull. Natl. Res. Cent. 2021, 45, 162. [Google Scholar] [CrossRef]
- Che Hamzah, A.M.; Yeo, C.C.; Puah, S.M.; Chua, K.H.; Chew, C.H. Staphylococcus aureus Infections in Malaysia: A Review of Antimicrobial Resistance and Characteristics of the Clinical Isolates, 1990–2017. Antibiotics 2019, 8, 128. [Google Scholar] [CrossRef]
- Stenstrom, R.; Grafstein, E.; Romney, M.; Fahimi, J.; Harris, D.; Hunte, G.; Innes, G.; Christenson, J. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus skin and soft tissue infection in a Canadian emergency department. CJEM 2009, 11, 430–438. [Google Scholar] [CrossRef]
- Szakacs, T.A.; Toye, B.; Turnbull, J.M.; Muckle, W.; Roth, V.R. Prevalence of methicillin-resistant Staphylococcus aureus in a Canadian inner-city shelter. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 249–252. [Google Scholar] [CrossRef]
- Berbel, D.; González-Díaz, A.; López de Egea, G.; Càmara, J.; Ardanuy, C. An Overview of Macrolide Resistance in Streptococci: Prevalence, Mobile Elements and Dynamics. Microorganisms 2022, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R. Mechanisms of Resistance to Macrolides and Lincosamides: Nature of the Resistance Elements and Their Clinical Implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef]
- Strich, J.R.; Heil, E.L.; Masur, H. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. J. Infect. Dis. 2020, 222 (Suppl. S2), S119–S131. [Google Scholar] [CrossRef]
- Muhamad, A.N.; Teh, C.S.J.; Draman, M.R.; Adnan, Y.K.; Abbas, A.A.; Khong, T.L.; Narayanan, V.; Tang, S.N.; Karunakaran, R.; Ab Manan, N.; et al. High incidence of multidrug-resistant organisms and modifiable risk factors associated with surgical site infections: A cohort study in a tertiary medical center in Kuala Lumpur, Malaysia from 2020 to 2023. Antimicrob. Resist. Infect. Control 2025, 14, 22. [Google Scholar] [CrossRef]
- Beres, C.; Colobatiu, L.; Tabaran, A.; Mihaiu, R.; Mihaiu, M. Prevalence and Characterisation of Clostridium perfringens Isolates in Food-Producing Animals in Romania. Microorganisms 2023, 11, 1373. [Google Scholar] [CrossRef] [PubMed]
- Mloka, D.; Sangeda, R.Z.; Mwambete, K.D.; Kamuhabwa, A.R. Magnitude of Extended-Spectrum Beta-Lactamase-Producing Gram-Negative and Beta-Lactamase-Producing Gram-Positive Pathogens Isolated from Patients in Dar es Salaam, Tanzania: A Cross-Sectional Study. Cureus 2022, 14, e24451. [Google Scholar] [CrossRef]
- Ragueh, A.A.; Aboubaker, M.H.; Mohamed, S.I.; Rolain, J.M.; Diene, S.M. Emergence of Carbapenem-Resistant Gram-Negative Isolates in Hospital Settings in Djibouti. Antibiotics 2023, 12, 1132. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Dhanapala, P.M.; Kalupahana, R.S.; Kalupahana, A.W.; Wijesekera, D.P.H.; Kottawatta, S.A.; Jayasekera, N.K.; Silva-Fletcher, A.; Jagoda, S.S.S.d.S. Characterization and Antimicrobial Resistance of Environmental and Clinical Aeromonas Species Isolated from Fresh Water Ornamental Fish and Associated Farming Environment in Sri Lanka. Microorganisms 2021, 9, 2106. [Google Scholar] [CrossRef]
- Li, S.; Renick, P.; Senkowsky, J.; Nair, A.; Tang, L. Diagnostics for Wound Infections. Adv. Wound Care 2021, 10, 317–327. [Google Scholar] [CrossRef]
- Mo, Y.; Tan, W.C.; Cooper, B.S. Antibiotic duration for common bacterial infections—A systematic review. JAC-Antimicrob. Resist. 2025, 7, dlae215. [Google Scholar] [CrossRef]
- Bechert, K.; Abraham, S.E. Pain management and wound care. J. Am. Coll. Certif. Wound Spec. 2009, 1, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; DeMott, J.M.; Hallock, M.; Peksa, G.D. Systemic Antibiotics for the Treatment of Skin and Soft Tissue Abscesses: A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2019, 73, 8–16. [Google Scholar] [CrossRef]
- Brindle, R.; Williams, O.M.; Barton, E.; Featherstone, P. Assessment of Antibiotic Treatment of Cellulitis and Erysipelas: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2019, 155, 1033–1040. [Google Scholar] [CrossRef]
- Urbina, T.; Razazi, K.; Ourghanlian, C.; Woerther, P.L.; Chosidow, O.; Lepeule, R.; de Prost, N. Antibiotics in Necrotizing Soft Tissue Infections. Antibiotics 2021, 10, 1104. [Google Scholar] [CrossRef]
- Babiker, A.; Warner, S.; Li, X.; Chishti, E.A.; Saad, E.; Swihart, B.J.; Dekker, J.P.; Walker, M.; Lawandi, A.; Kadri, S.S. NIH-Antimicrobial Resistance Outcomes Research Initiative. Adjunctive linezolid versus clindamycin for toxin inhibition in β-lactam-treated patients with invasive group A streptococcal infections in 195 US hospitals from 2016 to 2021: A retrospective cohort study with target trial emulation. Lancet Infect. Dis. 2025, 25, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Miró, E.M.; Sánchez, N.P. Cutaneous Manifestations of Infectious Diseases. In Atlas of Dermatology in Internal Medicine; Springer: New York, NY, USA, 2011; pp. 77–119. [Google Scholar] [CrossRef]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Carcione, D.; Intra, J.; Andriani, L.; Campanile, F.; Gona, F.; Carletti, S.; Mancini, N.; Brigante, G.; Cattaneo, D.; Baldelli, S.; et al. New Antimicrobials for Gram-Positive Sustained Infections: A Comprehensive Guide for Clinicians. Pharmaceuticals 2023, 16, 1304. [Google Scholar] [CrossRef]
- Siciliano, V.; Sangiorgi, F.; Del Vecchio, P.; Vahedi, L.; Gross, M.M.; Saviano, A.; Ojetti, V. New Frontier on Antimicrobial Therapy: Long-Acting Lipoglycopeptides. Pathogens 2024, 13, 189. [Google Scholar] [CrossRef]
- Diep, B.A.; Equils, O.; Huang, D.B.; Gladue, R. Linezolid effects on bacterial toxin production and host immune response: Review of the evidence. Curr. Ther. Res. 2012, 73, 86–102. [Google Scholar] [CrossRef]
- Greenfield, A.; Deja, E.; Lee, K.; Sastry, S.; Rittmann, B. Linezolid and tedizolid adverse effects: A review on serotonin syndrome, myelosuppression, neuropathies, and lactic acidosis. Antimicrob. Steward. Healthc. Epidemiol. 2025, 5, e20. [Google Scholar] [CrossRef]
- Khilnani, G.C.; Tiwari, P.; Mittal, S.; Kulkarni, A.P.; Chaudhry, D.; Zirpe, K.G.; Todi, S.K.; Mohan, A.; Hegde, A.; Jagiasi, B.G.; et al. Guidelines for Antibiotics Prescription in Critically Ill Patients. Indian J. Crit. Care Med. 2024, 28 (Suppl. S2), S104–S216. [Google Scholar] [CrossRef]
- Steer, A.C.; Lamagni, T.; Curtis, N.; Carapetis, J.R. Invasive group a streptococcal disease: Epidemiology, pathogenesis and management. Drugs 2012, 72, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Hedetoft, M.; Bennett, M.H.; Hyldegaard, O. Adjunctive hyperbaric oxygen treatment for necrotising soft-tissue infections: A systematic review and meta-analysis. Diving Hyperb. Med. J. 2021, 51, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed]
- Onita, T.; Ishihara, N.; Yano, T. PK/PD-Guided Strategies for Appropriate Antibiotic Use in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 92. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular Vesicles: A Review of Their Therapeutic Potentials, Sources, Biodistribution, and Administration Routes. Int. J. Nanomed. 2025, 20, 3175–3199. [Google Scholar] [CrossRef]
- Tew, V.K.; Barathan, M.; Nordin, F.; Law, J.X.; Ng, M.H. Emerging Role of Mesenchymal Stromal Cell and Exosome Therapies in Treating Cognitive Impairment. Pharmaceutics 2025, 17, 284. [Google Scholar] [CrossRef]
- Lee, I.; Choi, Y.; Shin, D.U.; Kwon, M.; Kim, S.; Jung, H.; Nam, G.H.; Kwon, M. Small Extracellular Vesicles as a New Class of Medicines. Pharmaceutics 2023, 15, 325. [Google Scholar] [CrossRef]
- Ma, B.; Barathan, M.; Ng, M.H.; Law, J.X. Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials. Int. J. Mol. Sci. 2025, 26, 3148. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, T.; Zhao, C.; Li, G. The Regulation of Exosome Generation and Function in Physiological and Pathological Processes. Int. J. Mol. Sci. 2024, 25, 255. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barceló, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential Disease Biomarkers and New Therapeutic Targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cell 2019, 8, 727. [Google Scholar] [CrossRef]
- Wu, L.G.; Chan, C.Y. Membrane transformations of fusion and budding. Nat. Commun. 2024, 15, 21. [Google Scholar] [CrossRef]
- Lehne, F.; Bogdan, S. Getting cells into shape by calcium-dependent actin cross-linking proteins. Front. Cell Dev. Biol. 2023, 11, 1171930. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Elieh-Ali-Komi, D. Significance of extracellular vesicles in orchestration of immune responses in Mycobacterium tuberculosis infection. Front. Cell. Infect. Microbiol. 2024, 14, 1398077. [Google Scholar] [CrossRef]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, M.P.; Yoshikawa, F.S.Y.; Aoki, V.; Sato, M.N.; Orfali, R.L. State of the Art on the Role of Staphylococcus aureus Extracellular Vesicles in the Pathogenesis of Atopic Dermatitis. Microorganisms 2024, 12, 531. [Google Scholar] [CrossRef]
- Harvey-Seutcheu, C.; Hopkins, G.; Fairclough, L.C. The Role of Extracellular Vesicles in Atopic Dermatitis. Int. J. Mol. Sci. 2024, 25, 3255. [Google Scholar] [CrossRef]
- Rasquel-Oliveira, F.S.; Ribeiro, J.M.; Martelossi-Cebinelli, G.; Costa, F.B.; Nakazato, G.; Casagrande, R.; Verri, W.A. Staphylococcus aureus in Inflammation and Pain: Update on Pathologic Mechanisms. Pathogens 2025, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L.; Focken, J.; Schlatterer, K.; Kretschmer, D.; Schittek, B. Bacterial membrane vesicles shape Staphylococcus aureus skin colonization and induction of innate immune responses. Exp. Dermatol. 2022, 31, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Ring, S.; Jin, S.; Singh, S.; Mahnke, K. Extracellular Vesicles and Their Role in Skin Inflammatory Diseases: From Pathogenesis to Therapy. Int. J. Mol. Sci. 2025, 26, 3827. [Google Scholar] [CrossRef]
- Im, H.; Lee, S.; Soper, S.A.; Mitchell, R.J. Staphylococcus aureus extracellular vesicles (EVs): Surface-binding antagonists of biofilm formation. Mol. Biosyst. 2017, 13, 2704–2714. [Google Scholar] [CrossRef]
- Jeong, G.J.; Khan, F.; Tabassum, N.; Cho, K.J.; Kim, Y.M. Bacterial extracellular vesicles: Modulation of biofilm and virulence properties. Acta Biomater. 2024, 178, 13–23. [Google Scholar] [CrossRef]
- Uppu, D.S.; Wang, X.; Lee, J.C. Contribution of Extracellular Membrane Vesicles to the Secretome of Staphylococcus aureus. mBio 2023, 14, e0357122. [Google Scholar] [CrossRef]
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704. [Google Scholar] [CrossRef] [PubMed]
- Magaña, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions. Antibiotics 2023, 13, 32. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Fan, G.C. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol. Sin. 2018, 39, 514–533. [Google Scholar] [CrossRef]
- Chen, S.; Lei, Q.; Zou, X.; Ma, D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front. Immunol. 2023, 14, 1157813. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Deo, P.; Chow, S.H.; Hay, I.D.; Kleifeld, O.; Costin, A.; Elgass, K.D.; Jiang, J.H.; Ramm, G.; Gabriel, K.; Dougan, G.; et al. Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog. 2018, 14, e1006945. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Hung, C.F.; Aljuffali, I.A.; Fang, J.Y. The roles of the virulence factor IpaB in Shigella spp. in the escape from immune cells and invasion of epithelial cells. Microbiol. Res. 2015, 181, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Zhou, J.; Zou, S.; Dai, D.; He, L.; Mou, X.; Zhao, N.; Li, H.; Bao, R. Bacterial Outer Membrane Vesicles: From Physics to Clinical. MedComm—Biomater. Appl. 2025, 4, e70013. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X. Fungal extracellular vesicle-mediated regulation: From virulence factor to clinical application. Front. Microbiol. 2023, 14, 1205477. [Google Scholar] [CrossRef]
- Johansson, H.J.; Vallhov, H.; Holm, T.; Gehrmann, U.; Andersson, A.; Johansson, C.; Blom, H.; Carroni, M.; Lehtiö, J.; Scheynius, A. Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci. Rep. 2018, 8, 9182. [Google Scholar] [CrossRef]
- Egea-Jimenez, A.L.; Zimmermann, P. Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles. J. Lipid Res. 2018, 59, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tsavou, A.; Zarnowski, R.; Pforte, R.; Allert, S.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A.; Bui, T.T.T.; Andes, D.R.; et al. Candida albicans biofilm extracellular vesicles deliver candidalysin to epithelial cell membranes and induce host cell responses. Infect. Immun. 2025, 93, e0040424. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Jiang, B.; Hou, F.; Huang, X.; Ling, B.; Lu, H.; Zhong, T.; Huang, J. The emerging role of extracellular vesicles in fungi: A double-edged sword. Front. Microbiol. 2023, 14, 1216895. [Google Scholar] [CrossRef]
- Al-Huthaifi, A.M.; Radman, B.A.; Al-Alawi, A.A.; Mahmood, F.; Liu, T.B. Mechanisms and Virulence Factors of Cryptococcus neoformans Dissemination to the Central Nervous System. J. Fungi 2024, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Karnas, E.; Dudek, P.; Zuba-Surma, E.K. Stem cell-derived extracellular vesicles as new tools in regenerative medicine—Immunomodulatory role and future perspectives. Front. Immunol. 2023, 14, 1120175. [Google Scholar] [CrossRef]
- Che, Z.; Yan, W.; Zhao, Q. Extracellular Vesicles in the Mesenchymal Stem Cell/Macrophage Axis: Potential Targets for Inflammatory Treatment. Int. J. Mol. Sci. 2025, 26, 2827. [Google Scholar] [CrossRef]
- Li, Y.; Baniel, A.; Diaz, D.; Ogawa-Momohara, M.; Ricco, C.; Eldaboush, A.; Bashir, M.; Sharma, M.; Liu, M.L.; Werth, V.P. Keratinocyte derived extracellular vesicles mediated crosstalk between epidermis and dermis in UVB-induced skin inflammation. Cell Commun. Signal. 2024, 22, 461. [Google Scholar] [CrossRef]
- Das, K.; Paul, S.; Mukherjee, T.; Ghosh, A.; Sharma, A.; Shankar, P.; Gupta, S.; Keshava, S.; Parashar, D. Beyond Macromolecules: Extracellular Vesicles as Regulators of Inflammatory Diseases. Cells 2023, 12, 1963. [Google Scholar] [CrossRef]
- Zeng, Z.; Vadivel, C.K.; Gluud, M.; Namini, M.R.J.; Yan, L.; Ahmad, S.; Hansen, M.B.; Coquet, J.; Mustelin, T.; Koralov, S.B.; et al. Keratinocytes Present Staphylococcus aureus Enterotoxins and Promote Malignant and Nonmalignant T Cell Proliferation in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2024, 144, 2789–2804.e10. [Google Scholar] [CrossRef]
- Acevedo-Sánchez, V.; Rodríguez-Hernández, R.M.; Aguilar-Ruíz, S.R.; Torres-Aguilar, H.; Pina-Canseco, S.; Chávez-Olmos, P.; Garrido, E.; Baltiérrez-Hoyos, R.; Romero-Tlalolini, M.A. Keratinocyte-derived extracellular vesicles induce macrophage polarization toward an M1-like phenotype. Biochem. Biophys. Res. Commun. 2025, 758, 151659. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, G.; Azarpira, N.; Alizadeh, A.; Goshtasbi, S.; Tayebi, L. Shedding light on the role of keratinocyte-derived extracellular vesicles on skin-homing cells. Stem Cell Res. Ther. 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955. [Google Scholar] [CrossRef]
- Hüser, L.; Chhabra, Y.; Gololobova, O.; Wang, V.; Liu, G.; Dixit, A.; Rocha, M.R.; Harper, E.I.; Fane, M.E.; Marino-Bravante, G.E.; et al. Aged fibroblast-derived extracellular vesicles promote angiogenesis in melanoma. Cell Rep. 2024, 43, 114721. [Google Scholar] [CrossRef]
- McCoy, S.S.; Reed, T.J.; Berthier, C.C.; Tsou, P.S.; Liu, J.; Gudjonsson, J.E.; Khanna, D.; Kahlenberg, J.M. Scleroderma keratinocytes promote fibroblast activation independent of transforming growth factor beta. Rheumatology 2017, 56, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Diederichs, S.; Melnik, S.; Riegger, J.; Trivanović, D.; Li, S.; Jenei-Lanzl, Z.; Brenner, R.E.; Huber-Lang, M.; Zaucke, F.; et al. Extracellular Vesicles in Musculoskeletal Pathologies and Regeneration. Front. Bioeng. Biotechnol. 2021, 8, 624096. [Google Scholar] [CrossRef]
- Liu, W.; Liu, T.; Zhao, Q.; Ma, J.; Jiang, J.; Shi, H. Adipose Tissue-Derived Extracellular Vesicles: A Promising Biomarker and Therapeutic Strategy for Metabolic Disorders. Stem Cells Int. 2023, 2023, 9517826. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Q.; Zhang, Q.; Tian, W.; Chen, T.; Liu, Z. Therapeutic potential of adipose-derived stem cell extracellular vesicles: From inflammation regulation to tissue repair. Stem Cell Res. Ther. 2024, 15, 249. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, Y.; Yang, Q.; Chen, J.; Liu, L.; Jin, J.; Zhu, S. Adipose-derived stem cells derived decellularized extracellular matrix enabled skin regeneration and remodeling. Front. Bioeng. Biotechnol. 2024, 12, 1347995. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles alter disease outcomes via endorsement of macrophage polarization. Stem Cell Res. Ther. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Bannazadeh Baghi, H.; Bayat, M.; Mehrasa, P.; Alavi, S.M.A.; Lotfalizadeh, M.H.; Memar, M.Y.; Taghavi, S.P.; Zarepour, F.; Hamblin, M.R.; Sadri Nahand, J.; et al. Regulatory role of microRNAs in virus-mediated inflammation. J. Inflamm. 2024, 21, 43. [Google Scholar] [CrossRef]
- Gui, Q.; Ding, N.; Yao, Z.; Wu, M.; Fu, R.; Wang, Y.; Zhao, Y.; Zhu, L. Extracellular vesicles derived from mesenchymal stem cells: The wine in Hebe’s hands to treat skin aging. Precis. Clin. Med. 2024, 7, pbae004. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jung, Y.; Seo, J.; Bae, Y.; Kim, H.S.; Jeong, W. Roles of extracellular vesicles from mesenchymal stem cells in regeneration. Mol. Cells 2024, 47, 100151. [Google Scholar] [CrossRef]
- Li, R.; Wang, H.; Wang, X.; Yang, Y.; Zhong, K.; Zhang, X.; Li, H. MSC-EVs and UCB-EVs promote skin wound healing and spatial transcriptome analysis. Sci. Rep. 2025, 15, 4006. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.L.; Baxter, A.A.; Poon, I.K.H. Gift bags from the sentinel cells of the immune system: The diverse role of dendritic cell-derived extracellular vesicles. J. Leukoc. Biol. 2022, 111, 903–920. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhu, Y.Z.; Hu, X.; Nie, J.Y.; Wang, Z.H.; Wu, S.; Yi, Y.Y. Extracellular Vesicles Derived from Adipose-Derived Stem Cells Accelerate Diabetic Wound Healing by Suppressing the Expression of Matrix Metalloproteinase-9. Curr. Pharm. Biotechnol. 2022, 23, 894–901. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, H.S.; Hong, I.S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef]
- Mittal, S.; Gupta, P.; Chaluvally-Raghavan, P.; Pradeep, S. Emerging Role of Extracellular Vesicles in Immune Regulation and Cancer Progression. Cancers 2020, 12, 3563. [Google Scholar] [CrossRef] [PubMed]
- Debes, G.F.; McGettigan, S.E. Skin-Associated B Cells in Health and Inflammation. J. Immunol. 2019, 202, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Vayalil, J.; Lee, G.; Wang, Y.; Peng, G. Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J. Immunother. Cancer 2021, 9, e003217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef]
- Zhou, Y.; Bréchard, S. Neutrophil Extracellular Vesicles: A Delicate Balance between Pro-Inflammatory Responses and Anti-Inflammatory Therapies. Cells 2022, 11, 3318. [Google Scholar] [CrossRef]
- Glémain, A.; Néel, M.; Néel, A.; André-Grégoire, G.; Gavard, J.; Martinet, B.; Le Bloas, R.; Riquin, K.; Hamidou, M.; Fakhouri, F.; et al. Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J. Autoimmun. 2022, 129, 102826. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Caillon, A.; Trimaille, A.; Favre, J.; Jesel, L.; Morel, O.; Kauffenstein, G. Role of neutrophils, platelets, and extracellular vesicles and their interactions in COVID-19-associated thrombopathy. J. Thromb. Haemost. 2022, 20, 17–31. [Google Scholar] [CrossRef]
- Li, P.; Hong, G.; Zhan, W.; Deng, M.; Tu, C.; Wei, J.; Lin, H. Endothelial progenitor cell derived exosomes mediated miR-182-5p delivery accelerate diabetic wound healing via down-regulating PPARG. Int. J. Med. Sci. 2023, 20, 468–481. [Google Scholar] [CrossRef]
- Petkovic, M.; Sørensen, A.E.; Leal, E.C.; Carvalho, E.; Dalgaard, L.T. Mechanistic Actions of microRNAs in Diabetic Wound Healing. Cells 2020, 9, 2228. [Google Scholar] [CrossRef]
- Chen, D.X.; Lu, C.H.; Na, N.; Yin, R.X.; Huang, F. Endothelial progenitor cell-derived extracellular vesicles: The world of potential prospects for the treatment of cardiovascular diseases. Cell Biosci. 2024, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Terriaca, S.; Fiorelli, E.; Scioli, M.G.; Fabbri, G.; Storti, G.; Cervelli, V.; Orlandi, A. Endothelial Progenitor Cell-Derived Extracellular Vesicles: Potential Therapeutic Application in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 6375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, P.K.; Ahmed, Z.; Singh, S.; Kumar, A. Essential Role of NLRP3 Inflammasome in Mediating IL-1β Production and the Pathobiology of Staphylococcus aureus Endophthalmitis. Infect. Immun. 2022, 90, e0010322. [Google Scholar] [CrossRef]
- Yerneni, S.S.; Werner, S.; Azambuja, J.H.; Ludwig, N.; Eutsey, R.; Aggarwal, S.D.; Lucas, P.C.; Bailey, N.; Whiteside, T.L.; Campbell, P.G.; et al. Pneumococcal Extracellular Vesicles Modulate Host Immunity. mBio 2021, 12, e0165721. [Google Scholar] [CrossRef] [PubMed]
- Vdovikova, S.; Luhr, M.; Szalai, P.; Nygård Skalman, L.; Francis, M.K.; Lundmark, R.; Engedal, N.; Johansson, J.; Wai, S.N. A Novel Role of Listeria monocytogenes Membrane Vesicles in Inhibition of Autophagy and Cell Death. Front. Cell. Infect. Microbiol. 2017, 7, 154. [Google Scholar] [CrossRef]
- Rivera, J.; Cordero, R.J.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef]
- Su, D.; Li, M.; Xie, Y.; Xu, Z.; Lv, G.; Jiu, Y.; Lin, J.; Chang, C.J.; Chen, H.; Cheng, F. Gut commensal bacteria Parabacteroides goldsteinii-derived outer membrane vesicles suppress skin inflammation in psoriasis. J. Control. Release 2025, 377, 127–145. [Google Scholar] [CrossRef]
- Shen, Y.; Giardino Torchia, M.L.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef]
- Costantini, P.E.; Vanpouille, C.; Firrincieli, A.; Cappelletti, M.; Margolis, L.; Ñahui Palomino, R.A. Extracellular Vesicles Generated by Gram-Positive Bacteria Protect Human Tissues Ex Vivo From HIV-1 Infection. Front. Cell. Infect. Microbiol. 2022, 11, 822882. [Google Scholar] [CrossRef]
- Cros, M.P.; Mir-Pedrol, J.; Toloza, L.; Knödlseder, N.; Maruotti, J.; Zouboulis, C.C.; Güell, M.; Fábrega, M.J. New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders. Sci. Rep. 2023, 13, 16058. [Google Scholar] [CrossRef]
- García-Gómez, E.; Miranda-Ozuna, J.F.T.; Díaz-Cedillo, F.; Vázquez-Sánchez, E.A.; Rodríguez-Martínez, S.; Jan-Roblero, J.; Cancino-Diaz, M.E.; Cancino-Diaz, J.C. Staphylococcus epidermidis lipoteichoic acid: Exocellular release and ltaS gene expression in clinical and commensal isolates. J. Med. Microbiol. 2017, 66, 864–873. [Google Scholar] [CrossRef]
- Kim, W.; Lee, E.J.; Bae, I.H.; Myoung, K.; Kim, S.T.; Park, P.J.; Lee, K.H.; Pham, A.V.Q.; Ko, J.; Oh, S.H.; et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell. Vesicles 2020, 9, 1793514. [Google Scholar] [CrossRef]
- Gómez-Chávez, F.; Cedillo-Peláez, C.; Zapi-Colín, L.A.; Gutiérrez-González, G.; Martínez-Torres, I.; Peralta, H.; Chavez-Galan, L.; Avila-Calderón, E.D.; Contreras-Rodríguez, A.; Bartolo-Aguilar, Y.; et al. The Extracellular Vesicles from the Commensal Staphylococcus Epidermidis ATCC12228 Strain Regulate Skin Inflammation in the Imiquimod-Induced Psoriasis Murine Model. Int. J. Mol. Sci. 2021, 22, 13029. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Ding, H.; Qiu, M.; Xue, L.; Ge, D.; Wen, G.; Ren, H.; Li, P.; Wang, J. Applications of plant-derived extracellular vesicles in medicine. MedComm 2024, 5, e741. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Saroj, S.; Saha, S.; Ali, A.; Gupta, S.K.; Bharadwaj, A.; Agrawal, T.; Pal, S.; Rakshit, T. Plant extracellular nanovesicle-loaded hydrogel for topical antibacterial wound healing in vivo. ACS Appl. Bio Mater. 2025, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Liu, Z.; Cong, M.; Zhong, X.; Mao, Y.; Fan, M.; Jiao, F.; Qiao, H. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing Staphylococcus aureus exotoxins for the care of invasive wounds. J. Control. Release 2024, 368, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Mu, J.; Li, L.; Hu, J.; Lin, H.; Cao, J.; Gao, J. An antioxidative sophora exosome-encapsulated hydrogel promotes spinal cord repair by regulating oxidative stress microenvironment. Nanomed. Nanotechnol. Biol. Med. 2023, 47, 102625. [Google Scholar] [CrossRef]
- Valentino, A.; Conte, R.; Bousta, D.; Bekkari, H.; Di Salle, A.; Calarco, A.; Peluso, G. Extracellular Vesicles Derived from Opuntia ficus-indica Fruit (OFI-EVs) Speed Up the Normal Wound Healing Processes by Modulating Cellular Responses. Int. J. Mol. Sci. 2024, 25, 7103. [Google Scholar] [CrossRef]
- Urzì, O.; Cafora, M.; Ganji, N.R.; Tinnirello, V.; Gasparro, R.; Raccosta, S.; Manno, M.; Corsale, A.M.; Conigliaro, A.; Pistocchi, A.; et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience 2023, 26, 107041. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Guo, Y.; Zeng, W.; Li, J.; Wu, J.; Li, N.; Zhu, A.; Li, J.; Di, L.; et al. Plant-derived nanovesicles: Promising therapeutics and drug delivery nanoplatforms for brain disorders. Fundam. Res. 2023, 5, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.R.; Kraus, R.B.; da Silva Nascente, P. Exploring the potential of bovine colostrum as a bioactive agent in human tissue regeneration: A comprehensive analysis of mechanisms of action and challenges to be overcome. Cell Biochem. Funct. 2024, 42, e4021. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.E.; Han, G.; Lim, N.R.; Kim, E.H.; Jang, Y.; Cho, H.; Jang, H.; Kim, K.H.; Kim, S.H.; et al. Harnessing the Natural Healing Power of Colostrum: Bovine Milk-Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102027. [Google Scholar] [CrossRef] [PubMed]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Milk-Derived Extracellular Vesicles: A Novel Perspective on Comparative Therapeutics and Targeted Nanocarrier Application. Vaccines 2024, 12, 1282. [Google Scholar] [CrossRef]
- Muttiah, B.; Law, J.X. Milk-derived extracellular vesicles and gut health. npj Sci. Food 2025, 9, 12. [Google Scholar] [CrossRef]
- Lou, C.; Cai, X. The emerging roles of platelet-derived extracellular vesicles in disease. Ann. Med. 2025, 57, 2499029. [Google Scholar] [CrossRef]
- Muttiah, B.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles. Biomedicines 2024, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, A.; Yeganeh, P.M.; Nazari, M.; Esmaeilzadeh, K. Platelet-derived extracellular vesicles: A new-generation nanostructured tool for chronic wound healing. Nanomedicine 2024, 19, 915–941. [Google Scholar] [CrossRef]
- Rezaei, S.; Nilforoushzadeh, M.A.; Amirkhani, M.A.; Moghadasali, R.; Taghiabadi, E.; Nasrabadi, D. Preclinical and Clinical Studies on the Use of Extracellular Vesicles Derived from Mesenchymal Stem Cells in the Treatment of Chronic Wounds. Mol. Pharm. 2024, 21, 2637–2658. [Google Scholar] [CrossRef]
- Bailey, A.J.M.; Li, H.; Kirkham, A.M.; Tieu, A.; Maganti, H.B.; Shorr, R.; Fergusson, D.A.; Lalu, M.M.; Elomazzen, H.; Allan, D.S. MSC-Derived Extracellular Vesicles to Heal Diabetic Wounds: A Systematic Review and Meta-Analysis of Preclinical Animal Studies. Stem Cell Rev. Rep. 2022, 18, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, R.; Cen, X.; Dong, W.; Chen, Q.; Lin, J.; Wang, X.; Ling, Y.; Mao, R.; Sun, H.; et al. Preclinical study of engineering MSCs promoting diabetic wound healing and other inflammatory diseases through M2 polarization. Stem Cell Res. Ther. 2025, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-Y.; Chen, M.-J.; Wu, L.-F.; Shu, G.-F.; Fang, S.-J.; Li, Z.-Y.; Chu, X.-R.; Li, X.-K.; Wang, Z.-G.; Ji, J.-S. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: Roles, opportunities and challenges. Mil. Med. Res. 2023, 10, 36. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Inoue, Y.; Matsuura, N.; Sunami, H.; Sowa, Y. Optimizing mesenchymal stem cell extracellular vesicles for chronic wound healing: Bioengineering, standardization, and safety. Regen. Ther. 2024, 26, 260–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Lan, M.; Chen, Y. Minimal Information for Studies of Extracellular Vesicles (MISEV): Ten-Year Evolution (2014-2023). Pharmaceutics 2024, 16, 1394. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Takakura, Y.; Hanayama, R.; Akiyoshi, K.; Futaki, S.; Hida, K.; Ichiki, T.; Ishii-Watabe, A.; Kuroda, M.; Maki, K.; Miura, Y.; et al. Quality and Safety Considerations for Therapeutic Products Based on Extracellular Vesicles. Pharm. Res. 2024, 41, 1573–1594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nazary Abrbekoh, F.; Salimi, L.; Saghati, S.; Amini, H.; Fathi Karkan, S.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Y.; Davis, E. Nanoplatforms for Targeted Stimuli-Responsive Drug Delivery: A Review of Platform Materials and Stimuli-Responsive Release and Targeting Mechanisms. Nanomaterials 2021, 11, 746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, Y.; Liu, X.; Sun, M.; Xiong, S.; Xiao, N.; Li, J.; He, X.; Xie, J. Recent Progress in Extracellular Vesicle-Based Carriers for Targeted Drug Delivery in Cancer Therapy. Pharmaceutics 2023, 15, 1902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, Y.; Xu, C.; Zhu, Y.; Gu, Z. Extracellular vesicle as a next-generation drug delivery platform for rheumatoid arthritis therapy. J. Control. Release 2025, 381, 113610. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, W.; Su, J. Research Progress of Extracellular Vesicles-Loaded Microneedle Technology. Pharmaceutics 2024, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Aafreen, S.; Feng, J.; Wang, W.; Liu, G. Theranostic extracellular vesicles: A concise review of current imaging technologies and labeling strategies. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 107–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacNair, C.R.; Rutherford, S.T.; Tan, M.W. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nat. Rev. Microbiol. 2024, 22, 262–275. [Google Scholar] [CrossRef] [PubMed]

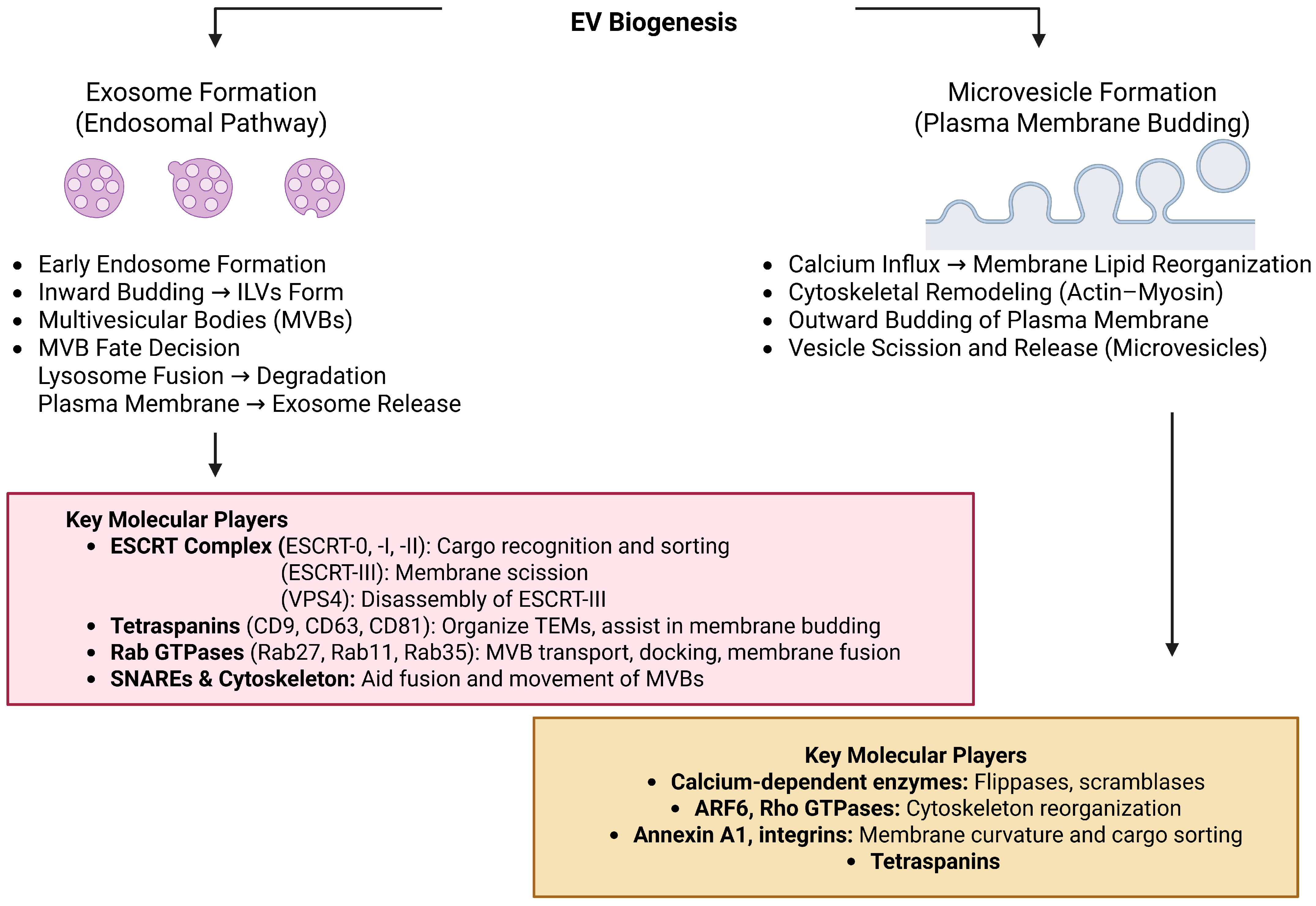
| Pathogen | Common Infections | Key Virulence Factors | Resistance Mechanisms/Notable Strains | Treatment Considerations |
|---|---|---|---|---|
| Staphylococcus aureus (incl. MRSA) | Impetigo, cellulitis, abscesses, necrotizing fasciitis | Adhesins (ClfA/B, FnBPs), Protein A, α-toxin, biofilm, PVL (CA-MRSA), TSST-1, wall teichoic acid | MRSA (mecA gene, SCCmec, ACME), multidrug resistance | Clindamycin, doxycycline, TMP-SMX, vancomycin; monitor resistance |
| Streptococcus pyogenes (GAS) | Cellulitis, erysipelas, necrotizing fasciitis, STSS | M protein, streptolysins O/S, SpeA/B/C, streptokinase, DNase, C5a peptidase, hyaluronidase | Limited resistance; strain diversity (Class I: rheumatic fever, Class II: APSGN) | High-dose penicillin + clindamycin; early intervention critical |
| Clostridium perfringens | Gas gangrene (clostridial myonecrosis), deep wound infections | Alpha-toxin (lecithinase), theta-toxin (PFO), spore formation | Rare resistance, but rapid progression increases risk | Surgical debridement + high-dose IV penicillin; consider hyperbaric O2 |
| Pseudomonas aeruginosa | Burn wound infections, hot tub folliculitis | Biofilm, elastases, exotoxin A, pyocyanin | MDR, efflux pumps, β-lactamases | Piperacillin–tazobactam, ceftazidime, carbapenems; tailored by sensitivity |
| Escherichia coli | SSTIs in contaminated water exposure | Endotoxins, adhesins, invasins (in pathogenic strains) | ESBL-producing strains (esp. O157:H7) | Empirical broad-spectrum antibiotics; adjust per susceptibility |
| Aeromonas hydrophila | Water-exposed cellulitis, necrotizing fasciitis | Hemolysins, aerolysin, proteases | Emerging MDR | Fluoroquinolones or TMP-SMX; debridement if severe |
| Pasteurella multocida | Cat bite infections (rapid onset cellulitis) | Capsule, adhesins, LPS | Beta-lactamase production (occasionally) | Penicillin or amoxicillin–clavulanate; early treatment key |
| Capnocytophaga canimorsus | Dog bite-related systemic infections, sepsis | Sialidase, capsule, immune evasion enzymes | Beta-lactamase; high risk in asplenic hosts | IV penicillin G or β-lactam/β-lactamase inhibitor; urgent care required |
| Eikenella corrodens | Human bite/clenched fist injuries | Lysins, biofilm-forming capacity | Beta-lactamase in some isolates | Amoxicillin–clavulanate; surgical drainage if abscess forms |
| Vibrio vulnificus | Necrotizing fasciitis, wound sepsis (saltwater exposure) | Cytolysin, metalloproteases, capsule | Intrinsic resistance to some beta-lactams | Doxycycline + third-gen cephalosporin; urgent debridement |
| Mycobacterium marinum | Chronic granulomatous infection (“fish tank granuloma”) | Slow growth, granuloma formation | Intrinsic resistance; slow response to therapy | Clarithromycin + rifampin or ethambutol; prolonged therapy |
| SSTI Type | First-Line Antimicrobials | Adjunctive Therapies | Recent Advances |
|---|---|---|---|
| Purulent (e.g., abscess) | I&D ± TMP-SMX, doxycycline, or clindamycin | Wound care, hygiene education, follow-up | Long-acting lipoglycopeptides (dalbavancin, oritavancin) |
| Nonpurulent cellulitis | Cephalexin or IV cefazolin; vancomycin if MRSA suspected | Limb elevation, hygiene education | PK/PD-optimized dosing, serum drug monitoring |
| Necrotizing fasciitis | IV vancomycin + cefepime or piperacillin–tazobactam + clindamycin/linezolid | Urgent surgical debridement, IVIG, HBOT | Prolonged/continuous β-lactam infusion, MSCs/EVs |
| Animal bites | Amoxicillin–clavulanate; doxycycline (penicillin allergy) | Wound cleansing, tetanus prophylaxis | Awareness of zoonotic infections, resistance patterns |
| Folliculitis | Topical agents; systemic if extensive | Hygiene education, topical antibiotics | Improved topical agents, resistance surveillance |
| Resistant/toxin-producing | Delafloxacin, omadacycline; linezolid, tedizolid | Toxin suppression (clindamycin, linezolid, tedizolid) | Next-gen oxazolidinones (tedizolid) |
| Polymicrobial infections | Broad-spectrum β-lactam + clindamycin or linezolid | Combination therapy, supportive care | Regenerative strategies (MSCs, EVs) |
| Feature | Small EVs (Exosomes) | Large EVs (Microvesicles/Ectosomes) |
|---|---|---|
| Size | 40–150 nm | 100–1000 nm (can exceed 1 µm) |
| Origin | Endosomal system (MVB fusion) | Plasma membrane (direct budding) |
| Biogenesis | ESCRT-dependent/independent pathways | Calcium-triggered membrane blebbing and cytoskeletal remodeling |
| Key Regulators | Rab GTPases (RAB27a/b, RAB11), SNAREs | ARF6, Rho GTPases |
| Markers | CD9, CD63, CD81, ALIX, TSG101, HSP70 | Annexin A1, ARF6, integrins |
| Cargo | Nucleic acids, tetraspanins, signaling proteins | Ribosomal proteins, RNA biogenesis factors, cytoplasmic contents |
| Functions | Intercellular communication, immune modulation, adhesion | Inflammation, coagulation, metastasis, cell signaling |
| Sedimentation (UC) | ~100,000× g | ~10,000–20,000× g |
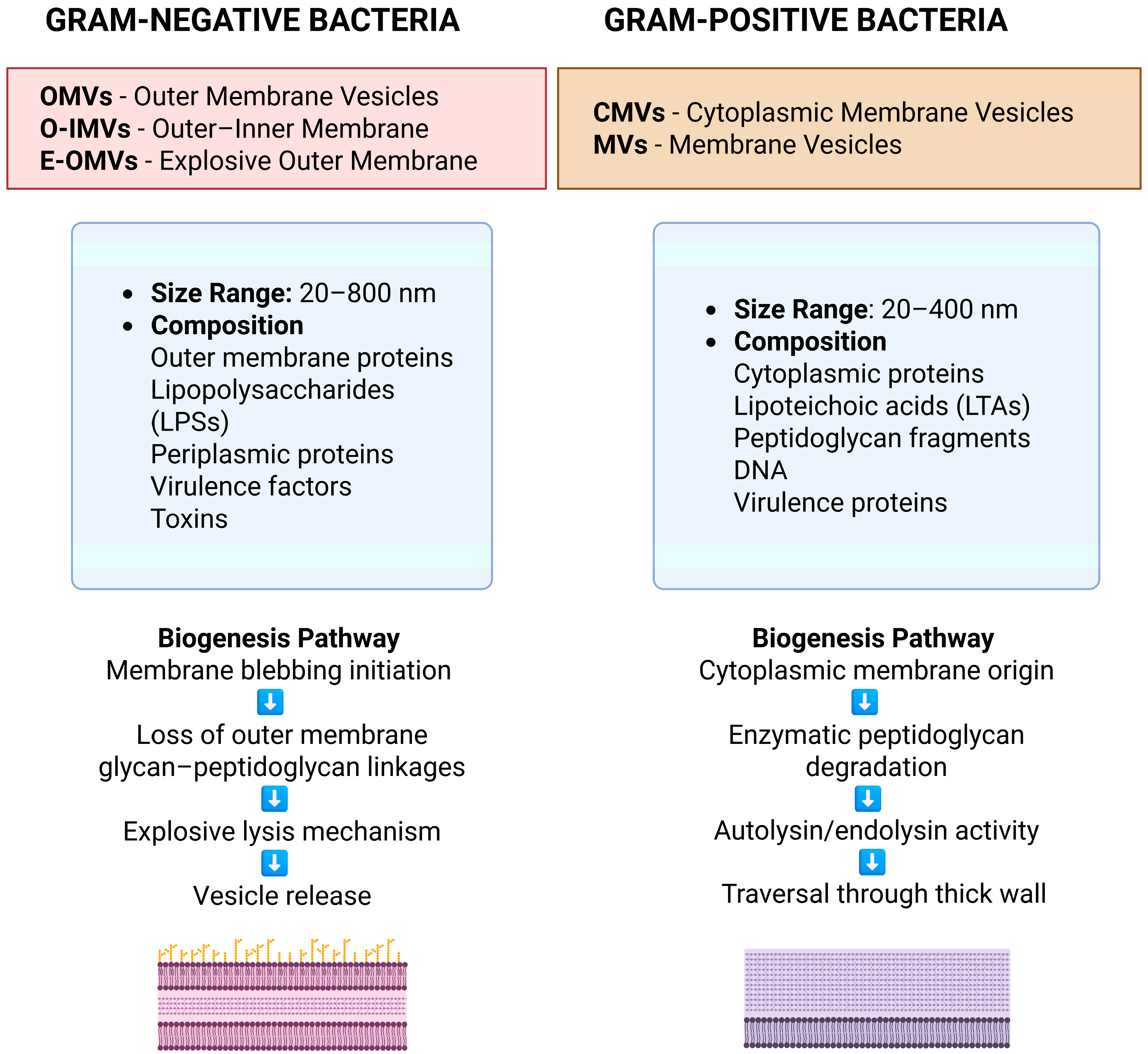
| Microorganism | EV Type | Cargo | Pathogenic Roles in SSTIs | References |
|---|---|---|---|---|
| Staphylococcus aureus (Gram-positive) | Cytoplasmic membrane vesicles (CMVs)/SA-EVs | α-Hemolysin, Protein A, β-lactamases, Proteases, DNA | Disrupts epidermal barrier; induces proinflammatory cytokines (IL-6, TNF-α), biofilm formation, immune evasion, antibiotic resistance | [123,124,125,126,127,128,129,130] |
| Pseudomonas aeruginosa (Gram-negative) | Outer membrane vesicles (OMVs) | Quorum-sensing molecules, extracellular matrix proteins | Supports polymicrobial biofilm maturation, modulates host immunity, enhances antibiotic resistance in chronic wounds | [131] |
| Helicobacter pylori, Porphyromonas gingivalis | OMVs | LPS, OmpA, toxins, small RNAs | Disrupts mucin layers and tight junctions, promotes colonization and tissue invasion, induces NF-κB/MAPK pathways and pyroptosis | [134,135,136,137] |
| Neisseria gonorrhoeae | OMVs | PorB protein | Induces macrophage apoptosis, impairs immune clearance | [138] |
| Escherichia coli, Shigella flexneri | OMVs | Adhesion/invasion proteins (Ail, Ipa) | Promotes adhesion, host invasion, and immune modulation | [139,140] |
| Fungal: Malassezia sympodialis | EVs | Mala s1, nucleic acids, lysophospholipases | Triggers IL-4 and ICAM-1 in keratinocytes, disrupts barrier function via lipid degradation | [143,144] |
| Fungal: Candida albicans | EVs | Proteins, lipids, RNAs, enzymes | Promotes biofilm formation, drug resistance, cytokine production (IL-6, IL-10, TNF-α), impairs wound healing | [145,146] |
| Fungal: Cryptococcus neoformans | EVs | Virulence factors | Modulates macrophage response, enhances inflammation and fungal dissemination in cutaneous cryptococcosis | [147] |
| EV Source | Key Components/Cargo | Primary Mechanisms | Therapeutic Functions | Specific Applications in SSTIs | Clinical Outcomes |
|---|---|---|---|---|---|
| Human Cell-Derived EVs | |||||
| Keratinocyte-EVs | IL-10, TGF-β, miR-146a, miR-21, β-defensin 2, S100A12, CXCLs, MHC molecules | Immune cell modulation, M1→M2 polarization, T cell proliferation, AMP delivery | Anti-inflammatory, antimicrobial, barrier enhancement, re-epithelialization | Antibacterial action against S. aureus, keratinocyte proliferation/migration, ECM remodeling | Reduced inflammation, enhanced barrier, accelerated healing, chronic inflammation prevention |
| Fibroblast-EVs | Cathepsin B, MMP-1, TGF-binding proteins, collagen-related miRNAs | ECM remodeling, keratinocyte migration, oxidative stress protection | Tissue repair, antioxidant effects, cellular migration support | Wound healing, matrix remodeling in infected tissue, PAMP/cytokine response | Enhanced regeneration, inflammatory regulation, adaptive wound healing |
| MSC-EVs | miR-21, miR-223, Type III collagen, TGF-β3, Wnt components | M1→M2 polarization, cell proliferation, anti-fibrosis, angiogenesis | Immunomodulation, tissue regeneration, anti-scarring | Wound closure, re-epithelialization, scar reduction | IL-6/TNF-α suppression, improved skin architecture, reduced scar width |
| Adipocyte-EVs | IL-10, MMPs, collagen regulators | Proinflammatory suppression, ECM remodeling, vascularization support | Inflammation control, tissue protection, barrier restoration | Chronic wound therapy, diabetic wounds, infection resistance | Reduced tissue damage, improved healing, barrier function restoration |
| DC-EVs | Osteopontin, MMP-9, anti-inflammatory mediators | MSC recruitment, immune microenvironment regulation, M2 polarization | Cellular recruitment, tissue repair coordination, immune regulation | MSC homing, immune surveillance, wound repair processes | Enhanced MSC recruitment, coordinated healing, regulated immune response |
| Neutrophil-EVs | Antimicrobial proteins, granule enzymes, S100A8/A9, MPO, Annexin 1 | Microbial inhibition, platelet interaction, macrophage autophagy, endothelial regulation | Antimicrobial action, inflammation modulation, vascular regulation | Pathogen elimination, skin barrier maintenance, psoriasis/eczema therapy | Enhanced clearance, dual inflammatory effects, preserved barrier |
| EPC-EVs | miR-182-5p, miR-221-3p, fibronectin, MMPs, IL-6, IL-8 | Endothelial migration, tube formation, ERK1/2 & RAF/ERK activation | Angiogenesis, tissue repair, diabetic wound healing | Keratinocyte activation, neovascularization, chronic wound treatment | Accelerated healing, improved collagen alignment, reduced scarring |
| Bacterial EVs | |||||
| Gram-positive CMVs | TLR2 ligands, IL-1β, IL-18, NF-κB activators, IgM-inducing components | TLR2/NLRP3 activation, NF-κB signaling, autophagy/lysosome pathways | Innate immunity, adaptive immunity, immune cell recruitment | Immune signaling (e.g., S. aureus, S. pneumoniae), pathogen elimination (L. monocytogenes, B. anthracis) | Enhanced immune response, pathogen clearance, improved survival rates |
| Commensal Bacterial EVs | Anti-inflammatory factors, Treg activators, β-defensins 2 and 3 | Regulatory T cell activation, cytokine production, pathogen inhibition | Skin homeostasis, microbiome balance, inflammation suppression | Pg OMVs for psoriasis, S. epidermidis in AD, C. acnes in acne, HIV protection | Restored skin integrity, reduced inflammation, microbiome improvement |
| Plant-Derived EVs | Proteins, lipids, RNAs, metabolites, IL-10 inducers, Nrf2 activators | Antimicrobial action, keratinocyte activation, angiogenesis, oxidative stress modulation | Wound healing, antioxidant protection, antimicrobial therapy | Mint (MENV-HG), dandelion (TH-EVNs), grapefruit (GEVs), carrot/lemon antioxidants | ~99% healing in 10 days, reduced inflammation, enhanced regeneration |
| Animal-Derived EVs | |||||
| Bovine Colostrum EVs | Growth factors, anti-inflammatory cytokines, miR-148a, miR-21, TGF-β, VEGF | Fibroblast proliferation, angiogenesis, ECM remodeling | Neovascularization, re-epithelialization, anti-inflammatory support | Acute/chronic wound repair, inflammatory to proliferative phase transition | Improved vascularization, reduced inflammation, faster healing |
| Platelet EVs | PDGF, VEGF, TGF-β, chemokines, hemostatic factors | Fibroblast proliferation, immune cell recruitment, angiogenesis, hemostasis | Immune modulation, tissue regeneration, granulation tissue support | Diabetic ulcer therapy, chronic wound healing, re-epithelialization | Accelerated healing, enhanced granulation tissue, inflammation resolution |
| EV Source | Immunomodulation | Antimicrobial Cargo Delivery | Tissue Repair |
|---|---|---|---|
| Keratinocyte-Derived EVs |
|
|
|
| Fibroblast-Derived EVs |
|
|
|
| Adipocyte-Derived EVs |
|
|
|
| MSC-Derived EVs |
|
|
|
| Dendritic-Cell-Derived EVs |
|
|
|
| B Cell-Derived EVs |
|
|
|
| T Cell-Derived EVs |
|
|
|
| Macrophage-Derived EVs |
|
|
|
| Neutrophil-Derived EVs |
|
|
|
| Endothelial Progenitor Cell EVs |
|
|
|
| Gram+ Bacterial EVs (CMVs) |
|
|
|
| Commensal Bacterial EVs |
|
|
|
| Plant-Derived EVs |
|
|
|
| Animal-Derived EVs |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttiah, B.; Hanafiah, A. A Paradigm Shift in SSTI Management: The Multifunctional Role of Extracellular Vesicles. Int. J. Mol. Sci. 2025, 26, 6481. https://doi.org/10.3390/ijms26136481
Muttiah B, Hanafiah A. A Paradigm Shift in SSTI Management: The Multifunctional Role of Extracellular Vesicles. International Journal of Molecular Sciences. 2025; 26(13):6481. https://doi.org/10.3390/ijms26136481
Chicago/Turabian StyleMuttiah, Barathan, and Alfizah Hanafiah. 2025. "A Paradigm Shift in SSTI Management: The Multifunctional Role of Extracellular Vesicles" International Journal of Molecular Sciences 26, no. 13: 6481. https://doi.org/10.3390/ijms26136481
APA StyleMuttiah, B., & Hanafiah, A. (2025). A Paradigm Shift in SSTI Management: The Multifunctional Role of Extracellular Vesicles. International Journal of Molecular Sciences, 26(13), 6481. https://doi.org/10.3390/ijms26136481








