The Purinergic Receptor P2X5 Modulates Glucose Metabolism and Expression of Thermogenic Genes in Brown Adipose Tissue
Abstract
1. Introduction
2. Results
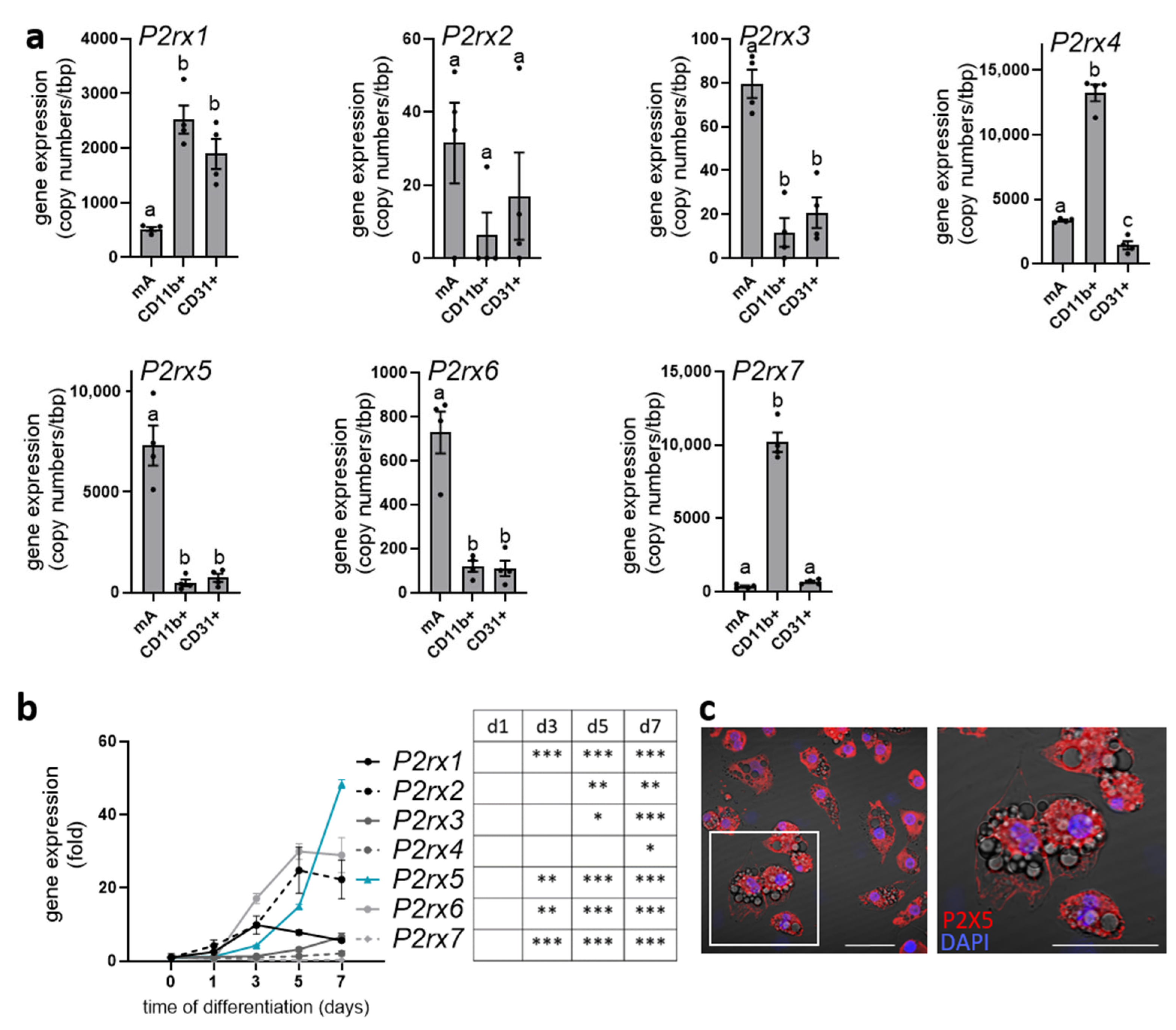
2.1. P2rx5 Is Highly Expressed by Mature Brown Adipocytes
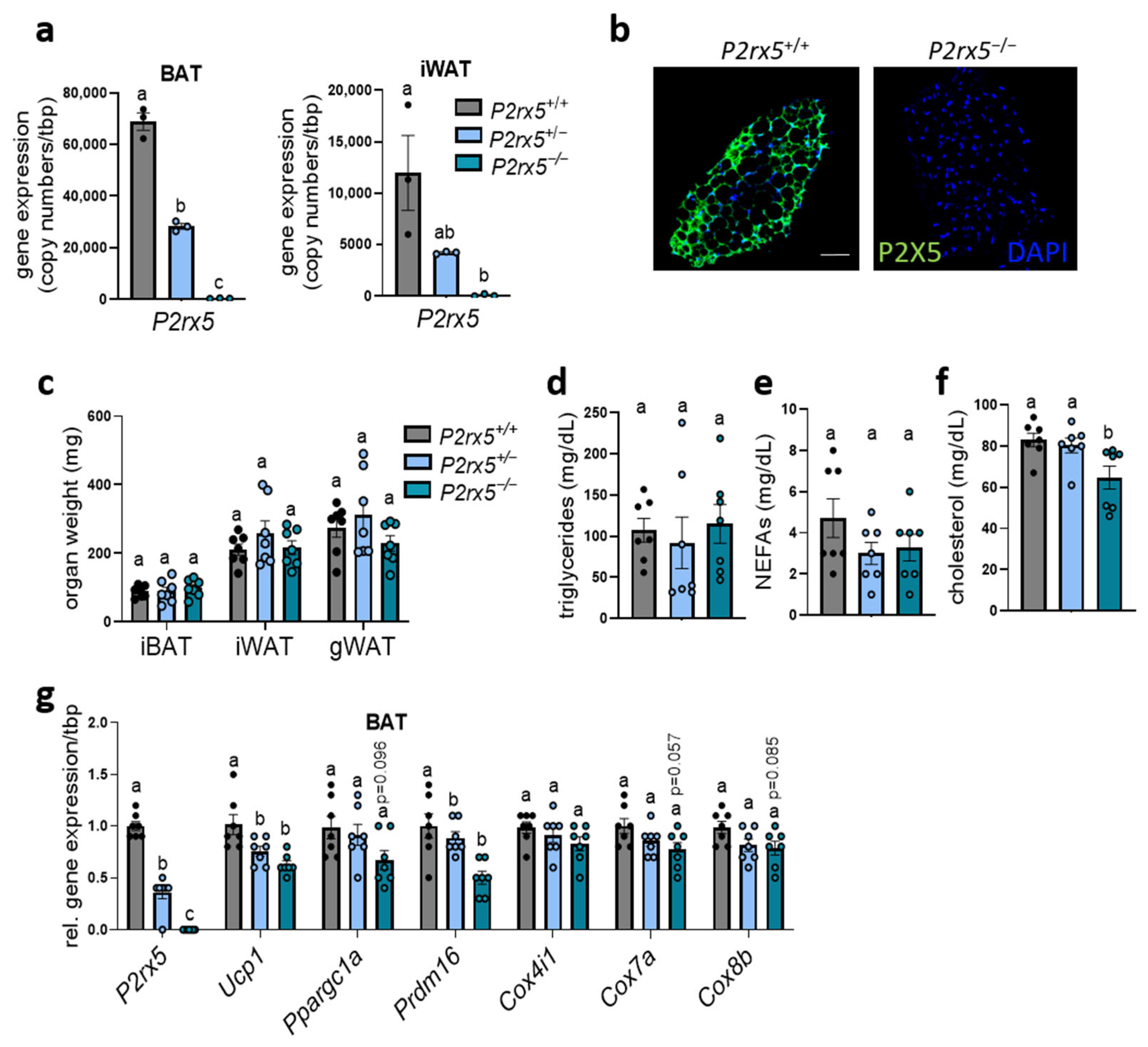
2.2. P2rx5 Deficiency Resulted in Lower Expression of Thermogenic Markers in BAT
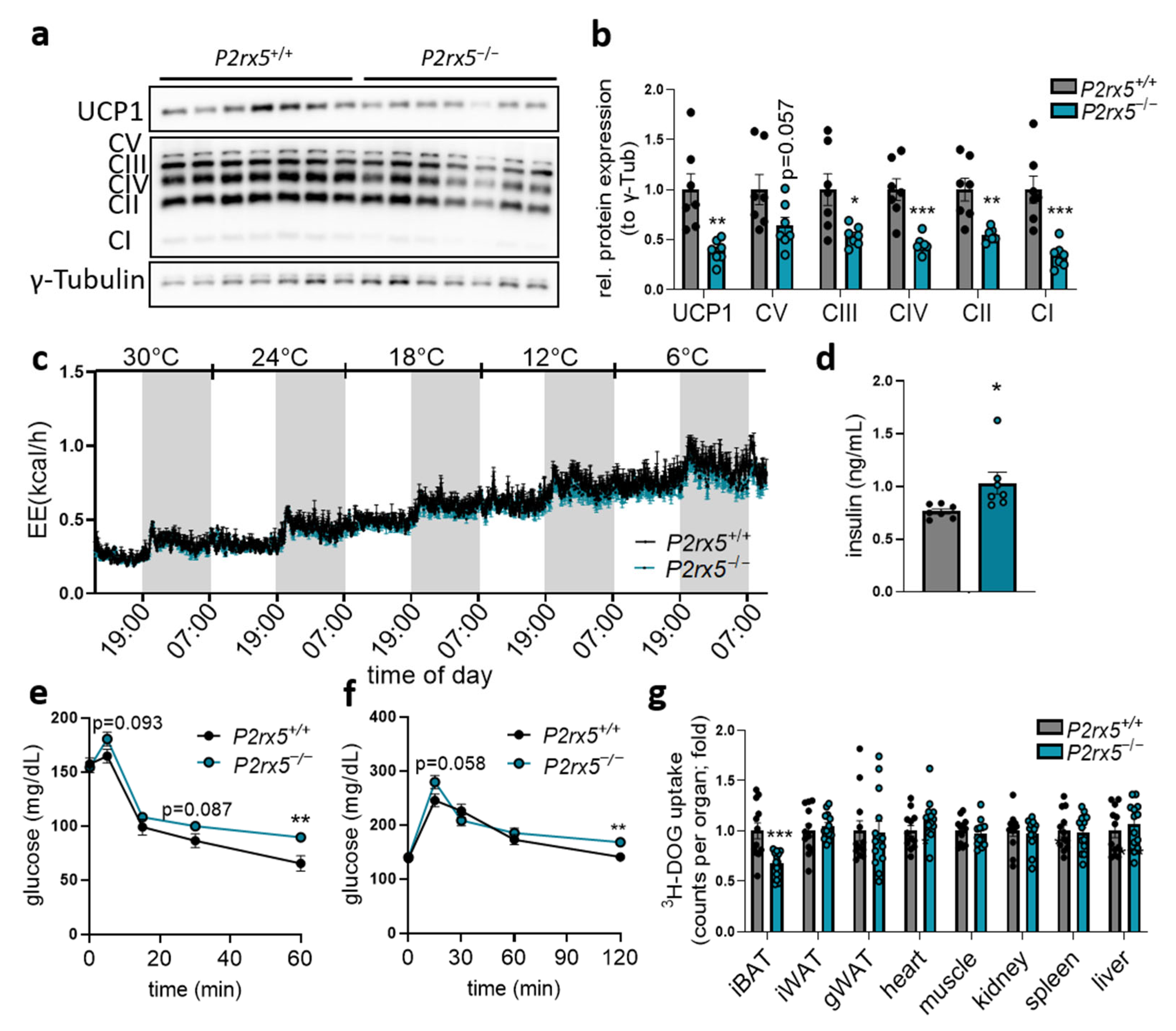
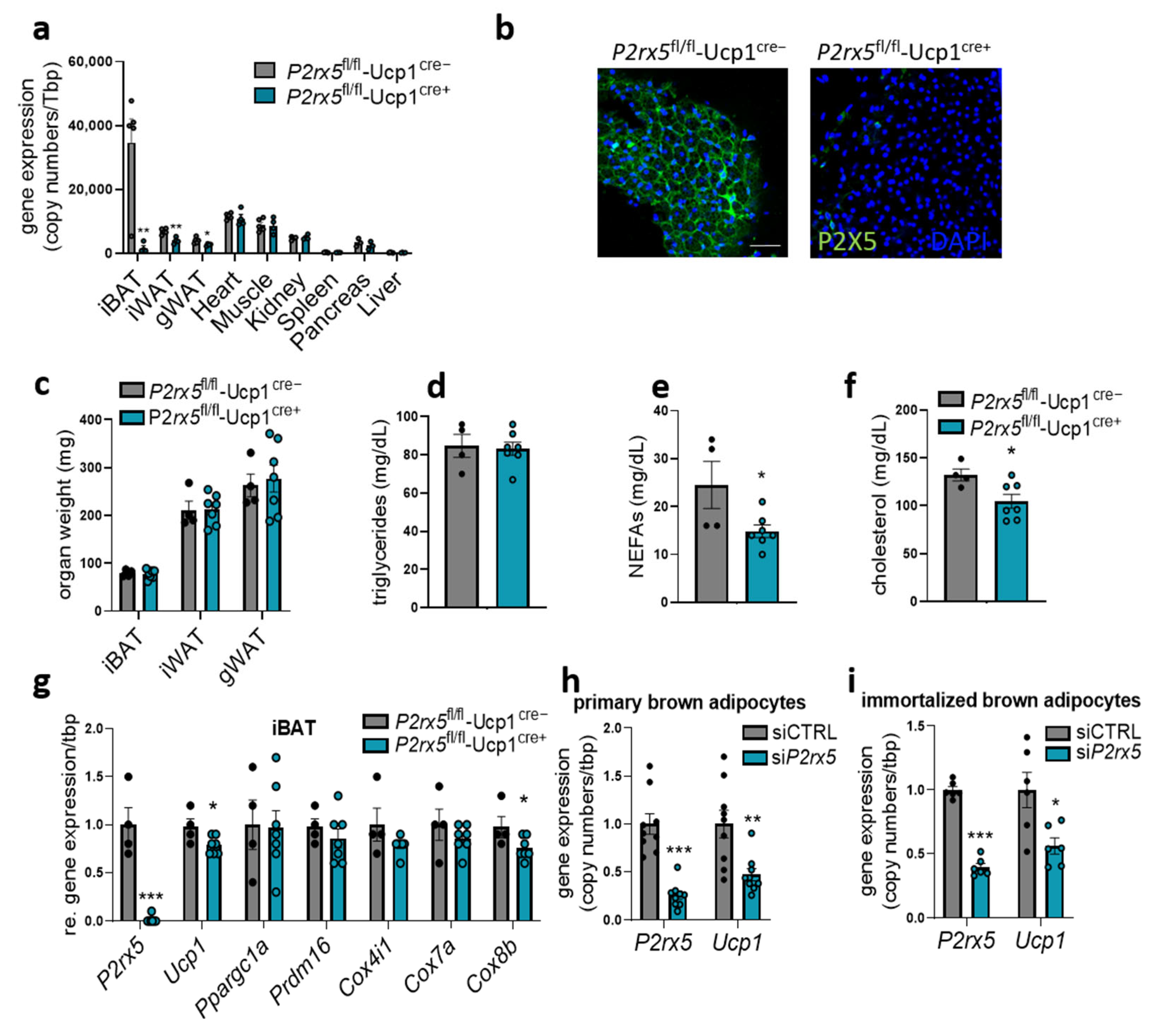
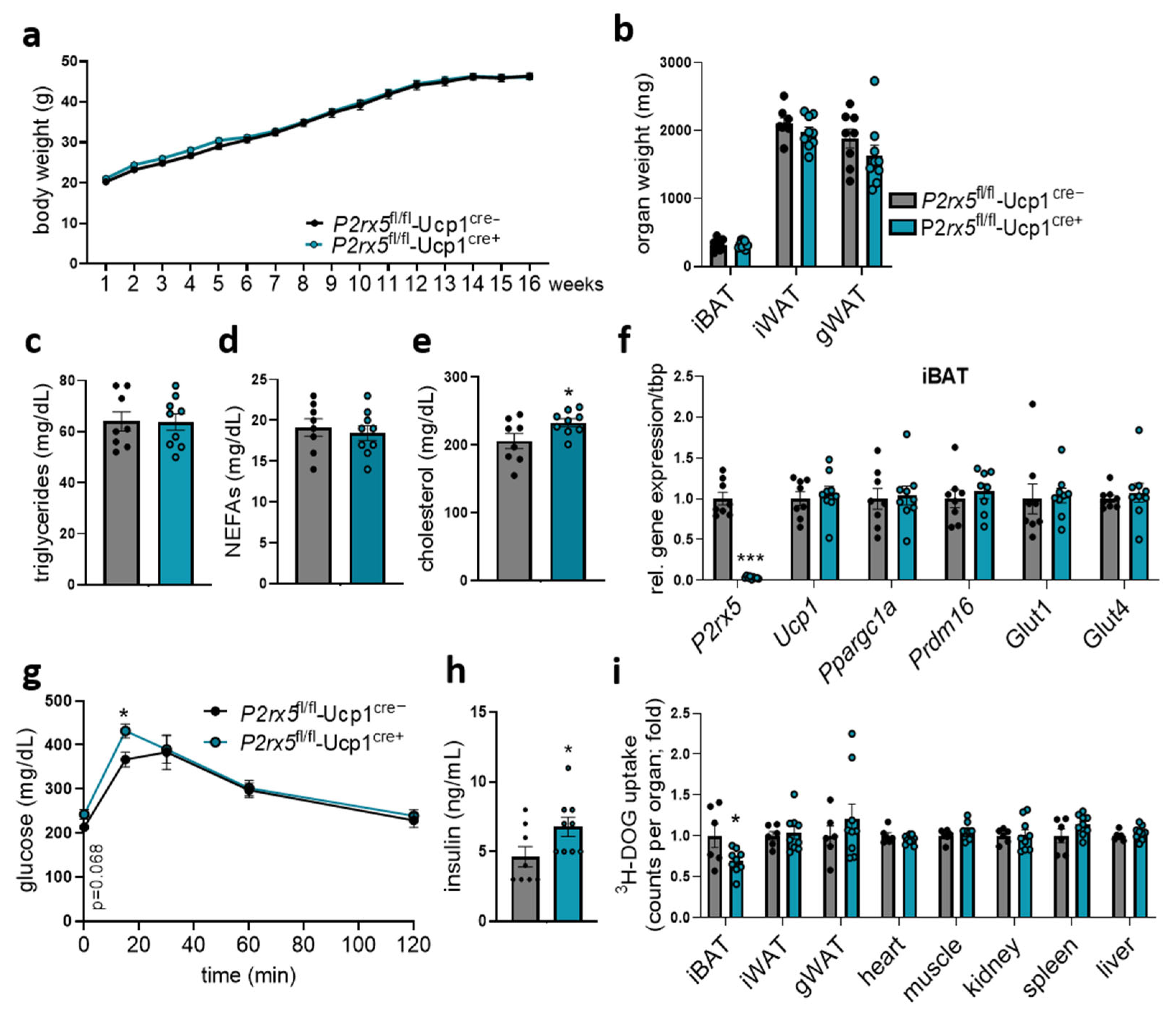
2.3. Brown Adipocyte-Specific P2rx5 Deletion Resulted in Lower UCP1 Expression and Impaired Glucose Tolerance
2.4. Brown Adipocyte-Specific P2rx5 Deficiency Impaired Glucose Tolerance and Uptake into BAT of Diet-Induced Obese Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Isolation of Adipocytes, Tissue-Resident Macrophages and Endothelial Cells
4.3. Gene Expression Analysis
4.4. Glucose Uptake Studies
4.5. Insulin Tolerance Test
4.6. Indirect Calorimetry
4.7. Plasma Parameters
4.8. Cell Culture and siRNA-Mediated Knockdown
4.9. Western Blotting
4.10. Immunofluorescence Staining
4.11. Quantification and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| BAT | Brown adipose tissue |
| DOG | Deoxyglucose |
| WAT | White adipose tissue |
| SVF | Stromal-vascular fractions |
| UCP1 | Uncoupling protein 1 |
References
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef]
- Chondronikola, M.; Volpi, E.; Borsheim, E.; Porter, C.; Annamalai, P.; Enerback, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef]
- Chondronikola, M.; Volpi, E.; Borsheim, E.; Porter, C.; Saraf, M.K.; Annamalai, P.; Yfanti, C.; Chao, T.; Wong, D.; Shinoda, K.; et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab. 2016, 23, 1200–1206. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Heine, M.; Fischer, A.W.; Schlein, C.; Jung, C.; Straub, L.G.; Gottschling, K.; Mangels, N.; Yuan, Y.; Nilsson, S.K.; Liebscher, G.; et al. Lipolysis Triggers a Systemic Insulin Response Essential for Efficient Energy Replenishment of Activated Brown Adipose Tissue in Mice. Cell Metab. 2018, 28, 644-655.e4. [Google Scholar] [CrossRef]
- Khedoe, P.P.; Hoeke, G.; Kooijman, S.; Dijk, W.; Buijs, J.T.; Kersten, S.; Havekes, L.M.; Hiemstra, P.S.; Berbee, J.F.; Boon, M.R.; et al. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J. Lipid Res. 2015, 56, 51–59. [Google Scholar] [CrossRef]
- Gavalda-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The endocrine role of brown adipose tissue: An update on actors and actions. Rev. Endocr. Metab. Disord. 2022, 23, 31–41. [Google Scholar] [CrossRef]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2020, 41, 53–65. [Google Scholar] [CrossRef]
- Villarroya, J.; Cereijo, R.; Gavalda-Navarro, A.; Peyrou, M.; Giralt, M.; Villarroya, F. New insights into the secretory functions of brown adipose tissue. J. Endocrinol. 2019, 243, R19–R27. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Sharma, A.K.; Khandelwal, R.; Wolfrum, C. Futile cycles: Emerging utility from apparent futility. Cell Metab. 2024, 36, 1184–1203. [Google Scholar] [CrossRef]
- Razzoli, M.; Frontini, A.; Gurney, A.; Mondini, E.; Cubuk, C.; Katz, L.S.; Cero, C.; Bolan, P.J.; Dopazo, J.; Vidal-Puig, A.; et al. Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol. Metab. 2016, 5, 19–33. [Google Scholar] [CrossRef]
- Gnad, T.; Scheibler, S.; von Kugelgen, I.; Scheele, C.; Kilic, A.; Glode, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef]
- Sneddon, P.; Westfall, D.P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J. Physiol. 1984, 347, 561–580. [Google Scholar] [CrossRef]
- Vizi, E.S.; Burnstock, G. Origin of ATP release in the rat vas deferens: Concomitant measurement of [3H]noradrenaline and [14C]ATP. Eur. J. Pharmacol. 1988, 158, 69–77. [Google Scholar] [CrossRef]
- Fliegert, R.; Heeren, J.; Koch-Nolte, F.; Nikolaev, V.O.; Lohr, C.; Meier, C.; Guse, A.H. Adenine nucleotides as paracrine mediators and intracellular second messengers in immunity and inflammation. Biochem. Soc. Trans. 2019, 47, 329–337. [Google Scholar] [CrossRef]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef]
- Burnstock, G. P2X ion channel receptors and inflammation. Purinergic Signal 2016, 12, 59–67. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- North, R.A. P2X receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150427. [Google Scholar] [CrossRef]
- Tian, T.; Heine, M.; Evangelakos, I.; Jaeckstein, M.Y.; Schaltenberg, N.; Stahler, T.; Koch-Nolte, F.; Kumari, M.; Heeren, J. The P2X7 ion channel is dispensable for energy and metabolic homeostasis of white and brown adipose tissues. Purinergic Signal 2020, 16, 529–542. [Google Scholar] [CrossRef]
- Ussar, S.; Lee, K.Y.; Dankel, S.N.; Boucher, J.; Haering, M.F.; Kleinridders, A.; Thomou, T.; Xue, R.; Macotela, Y.; Cypess, A.M.; et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 2014, 6, 247ra103. [Google Scholar] [CrossRef]
- Le, K.T.; Boue-Grabot, E.; Archambault, V.; Seguela, P. Functional and biochemical evidence for heteromeric ATP-gated channels composed of P2X1 and P2X5 subunits. J. Biol. Chem. 1999, 274, 15415–15419. [Google Scholar] [CrossRef]
- Torres, G.E.; Egan, T.M.; Voigt, M.M. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J. Biol. Chem. 1999, 274, 6653–6659. [Google Scholar] [CrossRef]
- Adamson, S.E.; Meher, A.K.; Chiu, Y.H.; Sandilos, J.K.; Oberholtzer, N.P.; Walker, N.N.; Hargett, S.R.; Seaman, S.A.; Peirce-Cottler, S.M.; Isakson, B.E.; et al. Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes. Mol. Metab. 2015, 4, 610–618. [Google Scholar] [CrossRef]
- Svensson, K.J.; Long, J.Z.; Jedrychowski, M.P.; Cohen, P.; Lo, J.C.; Serag, S.; Kir, S.; Shinoda, K.; Tartaglia, J.A.; Rao, R.R.; et al. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab. 2016, 23, 454–466. [Google Scholar] [CrossRef]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef]
- Tozzi, M.; Novak, I. Purinergic Receptors in Adipose Tissue As Potential Targets in Metabolic Disorders. Front. Pharmacol. 2017, 8, 878. [Google Scholar] [CrossRef]
- Gnad, T.; Navarro, G.; Lahesmaa, M.; Reverte-Salisa, L.; Copperi, F.; Cordomi, A.; Naumann, J.; Hochhauser, A.; Haufs-Brusberg, S.; Wenzel, D.; et al. Adenosine/A2B Receptor Signaling Ameliorates the Effects of Aging and Counteracts Obesity. Cell Metab. 2020, 32, 56-70.e7. [Google Scholar] [CrossRef]
- Niemann, B.; Haufs-Brusberg, S.; Puetz, L.; Feickert, M.; Jaeckstein, M.Y.; Hoffmann, A.; Zurkovic, J.; Heine, M.; Trautmann, E.M.; Muller, C.E.; et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature 2022, 609, 361–368. [Google Scholar] [CrossRef]
- Jaeckstein, M.Y.; Schulze, I.; Zajac, M.W.; Heine, M.; Mann, O.; Pfeifer, A.; Heeren, J. CD73-dependent generation of extracellular adenosine by vascular endothelial cells modulates de novo lipogenesis in adipose tissue. Front. Immunol. 2023, 14, 1308456. [Google Scholar] [CrossRef]
- Razzoli, M.; McGonigle, S.; Sahu, B.S.; Rodriguez, P.; Svedberg, D.; Rao, L.; Ruocco, C.; Nisoli, E.; Vezzani, B.; Frontini, A.; et al. A key role for P2RX5 in brown adipocyte differentiation and energy homeostasis. Adipocyte 2024, 13, 2421745. [Google Scholar] [CrossRef]
- Bo, X.; Jiang, L.H.; Wilson, H.L.; Kim, M.; Burnstock, G.; Surprenant, A.; North, R.A. Pharmacological and biophysical properties of the human P2X5 receptor. Mol. Pharmacol. 2003, 63, 1407–1416. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, X.; Huang, X.; Serag, S.; Woolf, C.J.; Spiegelman, B.M. Crosstalk between KCNK3-Mediated Ion Current and Adrenergic Signaling Regulates Adipose Thermogenesis and Obesity. Cell 2017, 171, 836-848.e13. [Google Scholar] [CrossRef]
- Sun, W.; Luo, Y.; Zhang, F.; Tang, S.; Zhu, T. Involvement of TRP Channels in Adipocyte Thermogenesis: An Update. Front. Cell Dev. Biol. 2021, 9, 686173. [Google Scholar] [CrossRef]
- Sun, W.; Uchida, K.; Suzuki, Y.; Zhou, Y.; Kim, M.; Takayama, Y.; Takahashi, N.; Goto, T.; Wakabayashi, S.; Kawada, T.; et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016, 17, 383–399. [Google Scholar] [CrossRef]
- Benzi, A.; Heine, M.; Spinelli, S.; Salis, A.; Worthmann, A.; Diercks, B.; Astigiano, C.; Perez Mato, R.; Memushaj, A.; Sturla, L.; et al. The TRPM2 ion channel regulates metabolic and thermogenic adaptations in adipose tissue of cold-exposed mice. Front. Endocrinol. 2024, 14, 1251351. [Google Scholar] [CrossRef]
- King, B.F. Rehabilitation of the P2X5 receptor: A re-evaluation of structure and function. Purinergic Signal 2023, 19, 421–439. [Google Scholar] [CrossRef]
- Duckwitz, W.; Hausmann, R.; Aschrafi, A.; Schmalzing, G. P2X5 subunit assembly requires scaffolding by the second transmembrane domain and a conserved aspartate. J. Biol. Chem. 2006, 281, 39561–39572. [Google Scholar] [CrossRef]
- Sellayah, D. The Impact of Early Human Migration on Brown Adipose Tissue Evolution and Its Relevance to the Modern Obesity Pandemic. J. Endocr. Soc. 2019, 3, 372–386. [Google Scholar] [CrossRef]
- Klein, J.; Fasshauer, M.; Ito, M.; Lowell, B.B.; Benito, M.; Kahn, C.R. Beta(3)-Adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J. Biol. Chem. 1999, 274, 34795–34802. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeckstein, M.Y.; Miegel, L.; Behrens, J.; Stähler, T.; Diercks, B.-P.; Heine, M.; Koch-Nolte, F.; Heeren, J. The Purinergic Receptor P2X5 Modulates Glucose Metabolism and Expression of Thermogenic Genes in Brown Adipose Tissue. Int. J. Mol. Sci. 2025, 26, 6474. https://doi.org/10.3390/ijms26136474
Jaeckstein MY, Miegel L, Behrens J, Stähler T, Diercks B-P, Heine M, Koch-Nolte F, Heeren J. The Purinergic Receptor P2X5 Modulates Glucose Metabolism and Expression of Thermogenic Genes in Brown Adipose Tissue. International Journal of Molecular Sciences. 2025; 26(13):6474. https://doi.org/10.3390/ijms26136474
Chicago/Turabian StyleJaeckstein, Michelle Y., Lisa Miegel, Janina Behrens, Tobias Stähler, Björn-Philipp Diercks, Markus Heine, Friedrich Koch-Nolte, and Joerg Heeren. 2025. "The Purinergic Receptor P2X5 Modulates Glucose Metabolism and Expression of Thermogenic Genes in Brown Adipose Tissue" International Journal of Molecular Sciences 26, no. 13: 6474. https://doi.org/10.3390/ijms26136474
APA StyleJaeckstein, M. Y., Miegel, L., Behrens, J., Stähler, T., Diercks, B.-P., Heine, M., Koch-Nolte, F., & Heeren, J. (2025). The Purinergic Receptor P2X5 Modulates Glucose Metabolism and Expression of Thermogenic Genes in Brown Adipose Tissue. International Journal of Molecular Sciences, 26(13), 6474. https://doi.org/10.3390/ijms26136474




