Limited Myelination Capacity in Human Schwann Cells in Experimental Models in Comparison to Rodent and Porcine Schwann Cells
Abstract
1. Introduction
2. Results
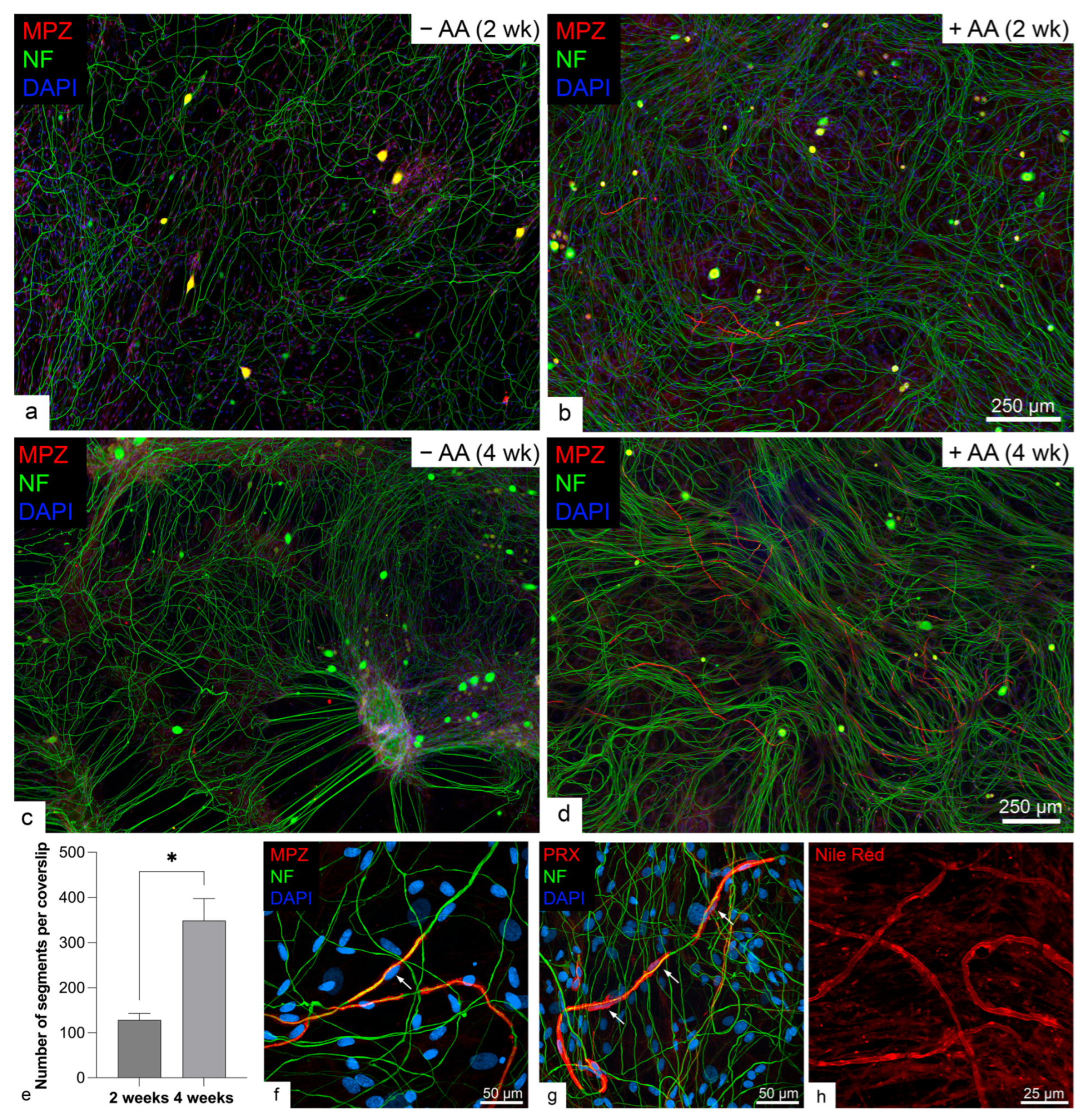
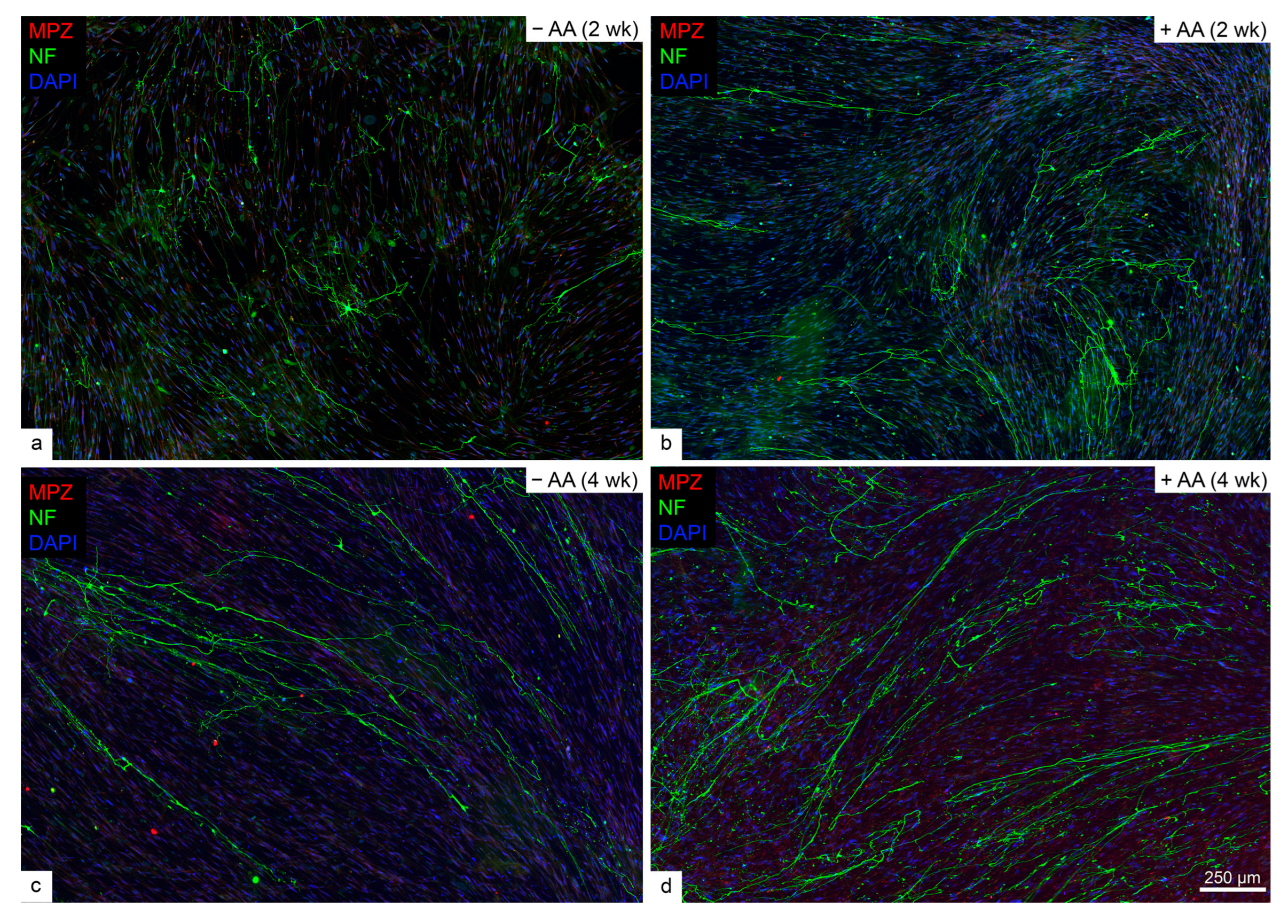
2.1. Rodent but Not Human SCs Myelinated Neurites in the Presence of Ascorbic Acid
2.2. Human SCs Were Unable to Form Myelinate in Cross-Species Co-Cultures
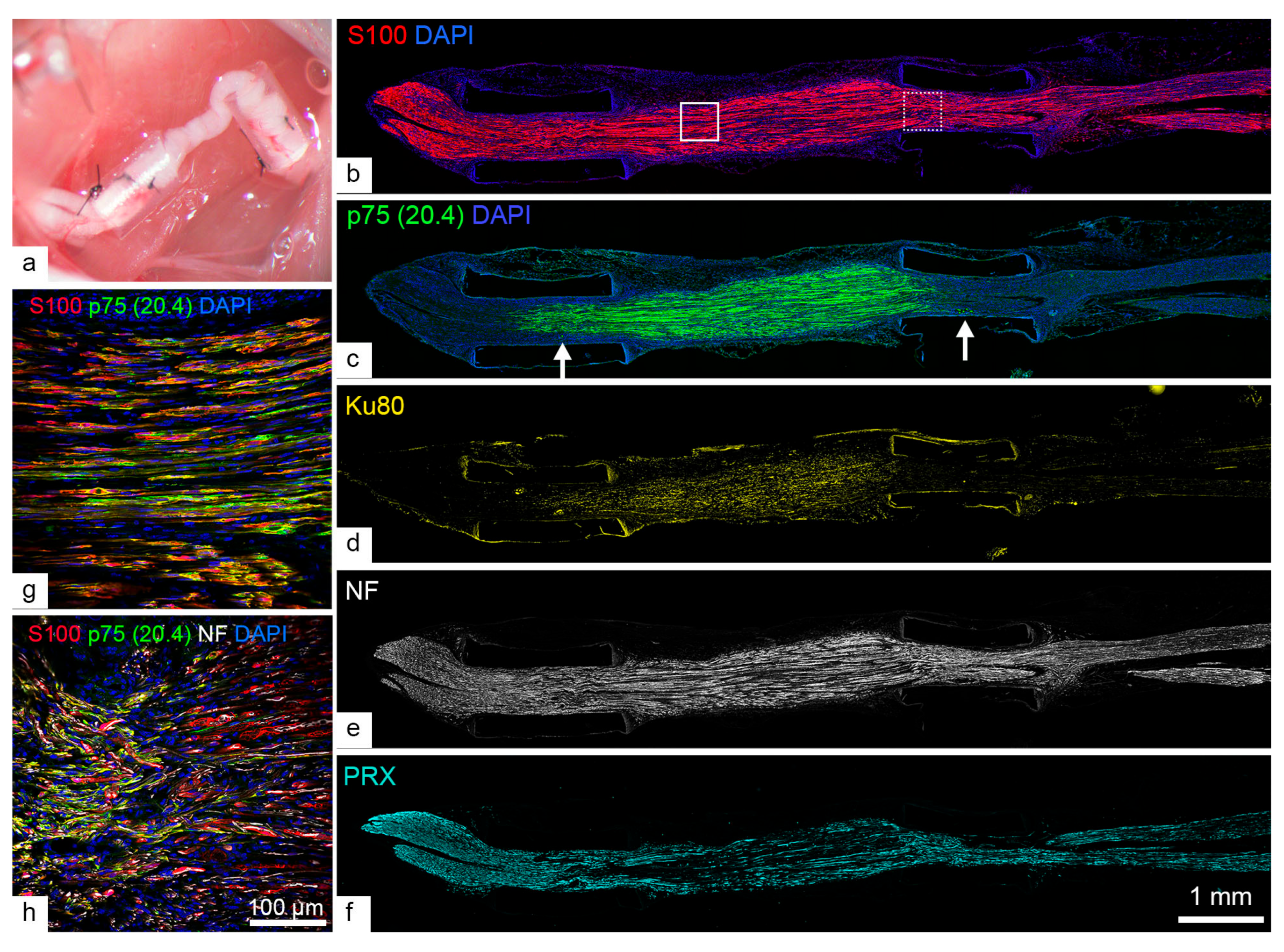
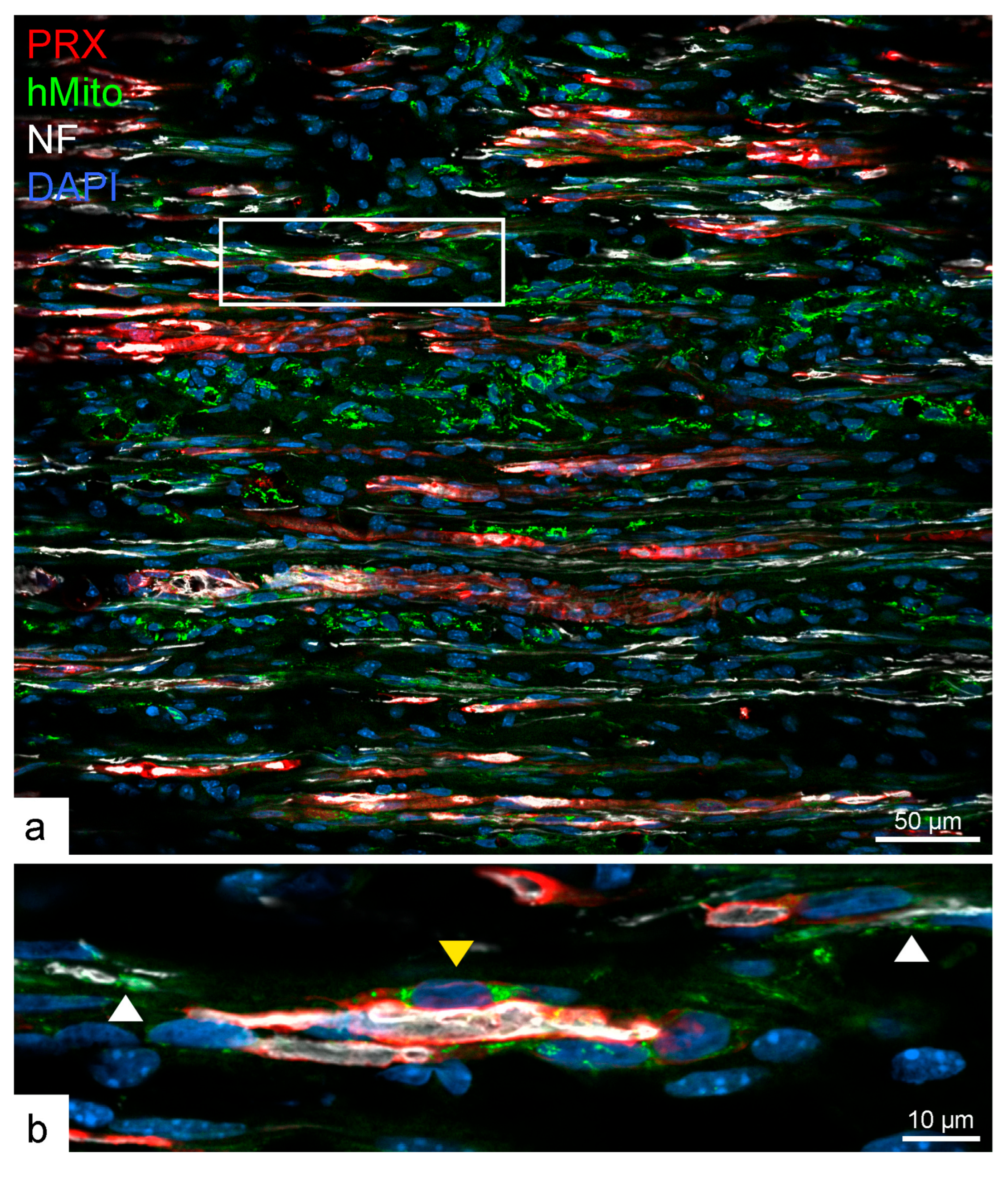
2.3. Human SCs Largely Failed to Myelinate Mouse Regenerating Axons in Human Nerve Grafts
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Human SC and Motor Neuron Culture
4.3. Rodent SC and Dorsal Root Ganglion (DRG) Cell Culture
4.4. Porcine SC and Dorsal Root Ganglion Cell Culture
4.5. Co-Culture Treatments
4.6. Sciatic Nerve Injury, Human Nerve Transplantation, Tissue Harvest, and Immunohistochemistry
4.7. Nile Red Labeling, Immunocytochemistry, and Immunohistochemistry
4.8. Imaging, Myelin Segment Quantification, and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bartlett Bunge, M.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Gersey, Z.C.; Burks, S.S.; Anderson, K.D.; Dididze, M.; Khan, A.; Dietrich, W.D.; Levi, A.D. First Human Experience with Autologous Schwann Cells to Supplement Sciatic Nerve Repair: Report of 2 Cases with Long-Term Follow-Up. Neurosurg. Focus 2017, 42, E2. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, D.; De Martini, I.; Cadoni, A.; Zicca, A.; Tabaton, M.; Schenone, A.; Anfosso, S.; Wattar, A.S.A.; Zaccheo, D.; Mancardi, G.L. GFAP Expression of Human Schwann Cells in Tissue Culture. Brain Res. 1992, 570, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Taschner-Mandl, S.; Bileck, A.; Slany, A.; Kromp, F.; Rifatbegovic, F.; Frech, C.; Windhager, R.; Kitzinger, H.; Tzou, C.H.; et al. Proteomics and Transcriptomics of Peripheral Nerve Tissue and Cells Unravel New Aspects of the Human Schwann Cell Repair Phenotype. Glia 2016, 64, 2133–2153. [Google Scholar] [CrossRef]
- Chu, T.; Baral, K.; Labit, E.; Rosin, N.; Sinha, S.; Umansky, D.; Alzahrani, S.; Arora, R.; Cao, L.; Rancourt, D.; et al. Comparison of Human Skin- and Nerve-Derived Schwann Cells Reveals Many Similarities and Subtle Genomic and Functional Differences. Glia 2022, 70, 2131–2156. [Google Scholar] [CrossRef]
- Mathon, N.F.; Malcolm, D.S.; Harrisingh, M.C.; Cheng, L.; Lloyd, A.C. Lack of Replicative Senescence in Normal Rodent Glia. Science 2001, 291, 872–875. [Google Scholar] [CrossRef]
- Monje, P.V.; Sant, D.; Wang, G. Phenotypic and Functional Characteristics of Human Schwann Cells as Revealed by Cell-Based Assays and RNA-SEQ. Mol. Neurobiol. 2018, 55, 6637–6660. [Google Scholar] [CrossRef]
- Monje, P.V. The Properties of Human Schwann Cells: Lessons from In Vitro Culture and Transplantation Studies. Glia 2020, 68, 797–810. [Google Scholar] [CrossRef]
- Chan, J.R.; Watkins, T.A.; Cosgaya, J.M.; Zhang, C.; Chen, L.; Reichardt, L.F.; Shooter, E.M.; Barres, B.A. NGF Controls Axonal Receptivity to Myelination by Schwann Cells or Oligodendrocytes. Neuron 2004, 43, 183–191. [Google Scholar] [CrossRef]
- Clark, A.J.; Kaller, M.S.; Galino, J.; Willison, H.J.; Rinaldi, S.; Bennett, D.L.H. Co-Cultures with Stem Cell-Derived Human Sensory Neurons Reveal Regulators of Peripheral Myelination. Brain 2017, 140, 898–913. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Guenard, V.; Aebischer, P.; Bunge, R.P. The Functional Characteristics of Schwann Cells Cultured from Human Peripheral Nerve after Transplantation into a Gap within the Rat Sciatic Nerve. J. Neurosci. 1994, 14, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.O.; Bunge, R.P. Studies of Myelin Formation after Transplantation of Human Schwann Cells into the Severe Combined Immunodeficient Mouse. Exp. Neurol. 1994, 130, 41–52. [Google Scholar] [CrossRef]
- Morrissey, T.K.; Kleitman, N.; Bunge, R.P. Human Schwann Cells in Vitro. II. Myelination of Sensory Axons Following Extensive Purification and Heregulin-Induced Expansion. J. Neurobiol. 1995, 28, 190–201. [Google Scholar] [CrossRef]
- Majd, H.; Amin, S.; Ghazizadeh, Z.; Cesiulis, A.; Arroyo, E.; Lankford, K.; Majd, A.; Farahvashi, S.; Chemel, A.K.; Okoye, M.; et al. Deriving Schwann Cells from hPSCs Enables Disease Modeling and Drug Discovery for Diabetic Peripheral Neuropathy. Cell Stem Cell 2023, 30, 632–647.e10. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, J.; Athauda, G.; De La Cruz, G.; Chan, W.M.; Golshani, R.; Berrocal, Y.; Henao, M.; Lalwani, A.; Mannoji, C.; Assi, M.; et al. Human Schwann Cells Exhibit Long-Term Cell Survival, Are Not Tumorigenic and Promote Repair When Transplanted into the Contused Spinal Cord. Glia 2017, 65, 1278–1301. [Google Scholar] [CrossRef]
- Sakaue, M.; Sieber-Blum, M. Human Epidermal Neural Crest Stem Cells as a Source of Schwann Cells. Development 2015, 142, 3188–3197. [Google Scholar] [CrossRef]
- Walters, E.M.; Wells, K.D.; Bryda, E.C.; Schommer, S.; Prather, R.S. Swine Models, Genomic Tools and Services to Enhance Our Understanding of Human Health and Diseases. Lab Anim. 2017, 46, 167–172. [Google Scholar] [CrossRef]
- Grochmal, J.; Teo, W.; Gambhir, H.; Kumar, R.; Stratton, J.A.; Dhaliwal, R.; Brideau, C.; Biernaskie, J.; Stys, P.K.; Midha, R. A Novel Approach to 32-Channel Peripheral Nervous System Myelin Imaging In Vivo, with Single Axon Resolution. J. Neurosurg. 2019, 130, 163–171. [Google Scholar] [CrossRef]
- Monje, P.V. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells 2020, 9, 1848. [Google Scholar] [CrossRef]
- Mazzara, P.G.; Massimino, L.; Pellegatta, M.; Ronchi, G.; Ricca, A.; Iannielli, A.; Giannelli, S.G.; Cursi, M.; Cancellieri, C.; Sessa, A.; et al. Two Factor-Based Reprogramming of Rodent and Human Fibroblasts into Schwann Cells. Nat. Commun. 2017, 8, 14088. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, M.; Thompson, S.D.; Pollett, J.B.; Usas, A.; Lu, A.; Stolz, D.B.; Clark, K.A.; Sun, B.; Péault, B.; Huard, J. Human Muscle–Derived Stem/Progenitor Cells Promote Functional Murine Peripheral Nerve Regeneration. J. Clin. Investig. 2014, 124, 1745–1756. [Google Scholar] [CrossRef]
- Krause, M.P.; Dworski, S.; Feinberg, K.; Jones, K.; Johnston, A.P.W.; Paul, S.; Paris, M.; Peles, E.; Bagli, D.; Forrest, C.R.; et al. Direct Genesis of Functional Rodent and Human Schwann Cells from Skin Mesenchymal Precursors. Stem Cell Rep. 2014, 3, 85–100. [Google Scholar] [CrossRef]
- Biernaskie, J.A.; McKenzie, I.A.; Toma, J.G.; Miller, F.D. Isolation of Skin-Derived Precursors (SKPs) and Differentiation and Enrichment of Their Schwann Cell Progeny. Nat. Protoc. 2006, 1, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human Dental Pulp Stem Cells Can Differentiate into Schwann Cells and Promote and Guide Neurite Outgrowth in an Aligned Tissue-Engineered Collagen Construct in Vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef]
- Cai, S.; Tsui, Y.-P.; Tam, K.-W.; Shea, G.K.-H.; Chang, R.S.-K.; Ao, Q.; Shum, D.K.-Y.; Chan, Y.-S. Directed Differentiation of Human Bone Marrow Stromal Cells to Fate-Committed Schwann Cells. Stem Cell Rep. 2017, 9, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Differentiate Into Functional Schwann Cells That Sustain Peripheral Nerve Regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar] [CrossRef]
- Tomita, K.; Madura, T.; Sakai, Y.; Yano, K.; Terenghi, G.; Hosokawa, K. Glial Differentiation of Human Adipose-Derived Stem Cells: Implications for Cell-Based Transplantation Therapy. Neuroscience 2013, 236, 55–65. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.; Lee, D.Y.; Kim, Y.D.; Kim, J.Y.; Lim, H.J.; Lim, S.; Cho, Y.S. Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Rep. 2017, 8, 1714–1726. [Google Scholar] [CrossRef]
- Liu, Q.; Spusta, S.C.; Mi, R.; Lassiter, R.N.T.; Stark, M.R.; Höke, A.; Rao, M.S.; Zeng, X. Human Neural Crest Stem Cells Derived from Human ESCs and Induced Pluripotent Stem Cells: Induction, Maintenance, and Differentiation into Functional Schwann Cells. Stem Cells Transl. Med. 2012, 1, 266–278. [Google Scholar] [CrossRef]
- Birchmeier, C.; Nave, K. Neuregulin-1, a Key Axonal Signal That Drives Schwann Cell Growth and Differentiation. Glia 2008, 56, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Zanazzi, G.; Einheber, S.; Westreich, R.; Hannocks, M.J.; Bedell-Hogan, D.; Marchionni, M.A.; Salzer, J.L. Glial Growth Factor/Neuregulin Inhibits Schwann Cell Myelination and Induces Demyelination. J. Cell Biol. 2001, 152, 1289–1299. [Google Scholar] [CrossRef]
- McKee, K.K.; Yang, D.-H.; Patel, R.; Chen, Z.-L.; Strickland, S.; Takagi, J.; Sekiguchi, K.; Yurchenco, P.D. Schwann Cell Myelination Requires Integration of Laminin Activities. J. Cell Sci. 2012, 125, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Chernousov, M.A.; Rothblum, K.; Stahl, R.C.; Evans, A.; Prentiss, L.; Carey, D.J. Glypican-1 and A4(V) Collagen Are Required for Schwann Cell Myelination. J. Neurosci. 2006, 26, 508–517. [Google Scholar] [CrossRef]
- Sances, S.; Bruijn, L.I.; Chandran, S.; Eggan, K.; Ho, R.; Klim, J.R.; Livesey, M.R.; Lowry, E.; Macklis, J.D.; Rushton, D.; et al. Modeling ALS with Motor Neurons Derived from Human Induced Pluripotent Stem Cells. Nat. Neurosci. 2016, 19, 542–553. [Google Scholar] [CrossRef] [PubMed]
- McEachin, Z.T.; Gendron, T.F.; Raj, N.; García-Murias, M.; Banerjee, A.; Purcell, R.H.; Ward, P.J.; Todd, T.W.; Merritt-Garza, M.E.; Jansen-West, K.; et al. Chimeric Peptide Species Contribute to Divergent Dipeptide Repeat Pathology in c9ALS/FTD and SCA36. Neuron 2020, 107, 292–305.e6. [Google Scholar] [CrossRef]
- Stassart, R.M.; Fledrich, R.; Velanac, V.; Brinkmann, B.G.; Schwab, M.H.; Meijer, D.; Sereda, M.W.; Nave, K.A. A Role for Schwann Cell-Derived Neuregulin-1 in Remyelination. Nat. Neurosci. 2013, 16, 48–54. [Google Scholar] [CrossRef]
- Ciceri, G.; Baggiolini, A.; Cho, H.S.; Kshirsagar, M.; Benito-Kwiecinski, S.; Walsh, R.M.; Aromolaran, K.A.; Gonzalez-Hernandez, A.J.; Munguba, H.; Koo, S.Y.; et al. An Epigenetic Barrier Sets the Timing of Human Neuronal Maturation. Nature 2024, 626, 881–890. [Google Scholar] [CrossRef]
- Takazawa, T.; Croft, G.F.; Amoroso, M.W.; Studer, L.; Wichterle, H.; MacDermott, A.B. Maturation of Spinal Motor Neurons Derived from Human Embryonic Stem Cells. PLoS ONE 2012, 7, e40154. [Google Scholar] [CrossRef]
- Andersen, N.D.; Srinivas, S.; Piñero, G.; Monje, P.V. A Rapid and Versatile Method for the Isolation, Purification and Cryogenic Storage of Schwann Cells from Adult Rodent Nerves. Sci. Rep. 2016, 6, 31781. [Google Scholar] [CrossRef]
- Aparicio, G.; Monje, P. Human Schwann Cells in Vitro I. Nerve Tissue Processing, Pre-Degeneration, Isolation, and Culturing of Primary Cells. BIO-Protocol 2023, 13, e4748. [Google Scholar] [CrossRef] [PubMed]
- Kidd, G.J.; Ohno, N.; Trapp, B.D. Biology of Schwann Cells. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 115, pp. 55–79. [Google Scholar]
- Owen, D.E.; Egerton, J. Culture of Dissociated Sensory Neurons from Dorsal Root Ganglia of Postnatal and Adult Rats. In Neurotrophic Factors: Methods and Protocols; Skaper, S.D., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 179–187. ISBN 978-1-61779-536-7. [Google Scholar]
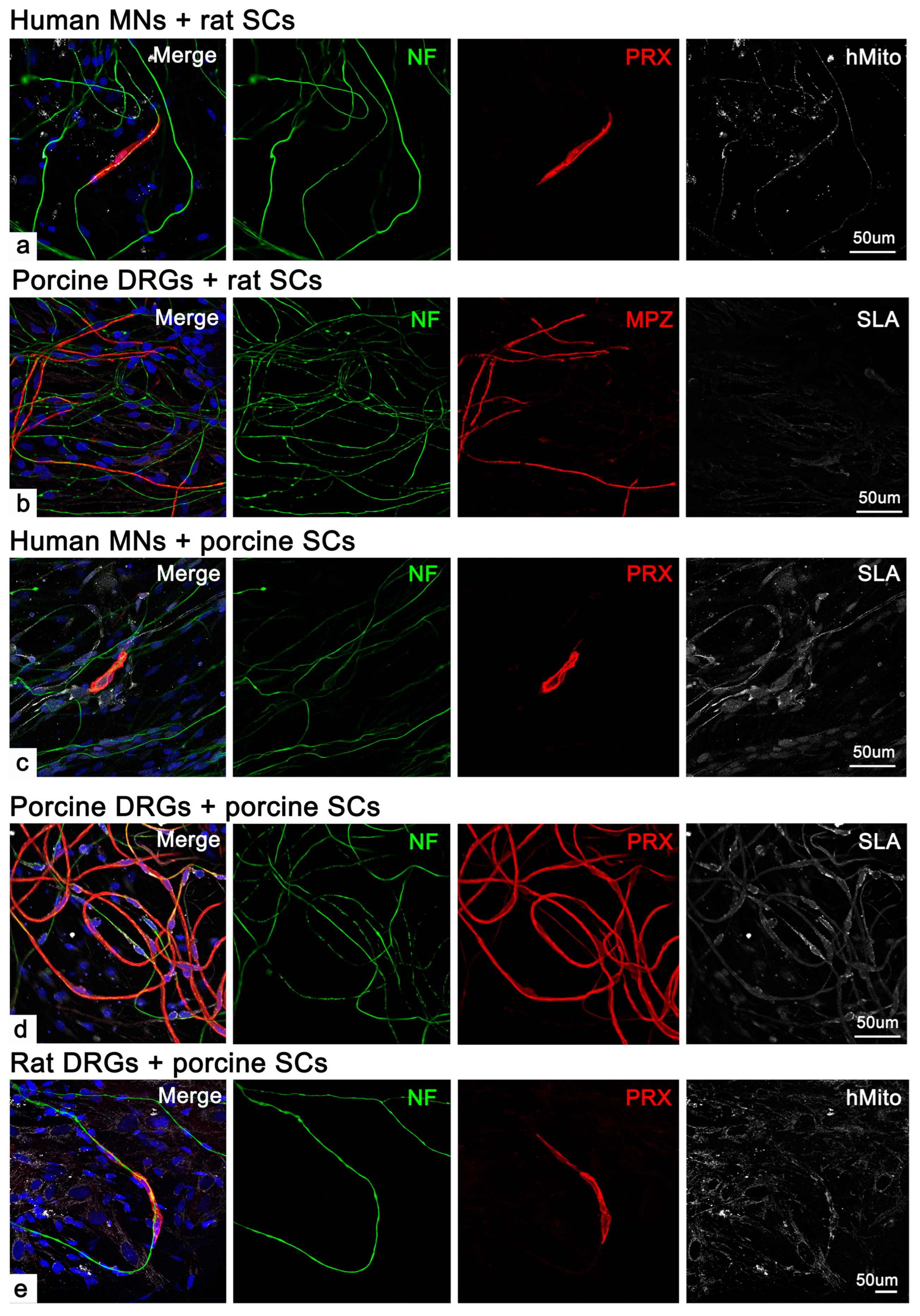
| Co-Culture With | Human SCs | Rat SCs | Porcine SCs |
|---|---|---|---|
| Rat DRGs | Nil | 13.3 ± 3.5 *** | 0.6 ± 0.4 |
| Human MNs | Nil | 2 ± 0.9 | 4.4 ± 1.6 * |
| Porcine DRGs | Nil | 70 ± 13.2 ** | 67.1 ± 20.6 ** |
| Primary Antibody | Brand | Catalog Number | Dilution | Purpose |
|---|---|---|---|---|
| Rabbit anti-periaxin (PRX) | Novus Biologicals | NBP-1-89598 | 1:500 | To label myelinating Schwann cells (all species *) |
| Rabbit anti-myelin protein zero (MPZ) | Abcam | ab31851 | 1:500 | To identify rodent and human myelin segments, negative in porcine myelin |
| Chicken anti-neurofilament 200 | BioLegend | 822601 | 1:10,000 | To label neuronal processes (all species *) |
| Mouse anti-human mitochondria | Millipore | MAB1273 | 1:500 | To identify human and porcine cells |
| Mouse anti-swine leukocyte antigen-Class II | Bio-Rad | MCA2314GA | 1:200 | To label porcine cells |
| Mouse anti-primate and porcine-specific p75 | N/A | Hybridoma supernatant | As is | To identify human and porcine Schwann cells |
| Rabbit anti-S100 | Dako | Z0311 | 1:6 | To label Schwann cells (all species *) |
| Rabbit anti-Ku80 | Cell Signaling | 2180 | 1:200 | To identify human nuclei |
| Mouse anti-HB9 | DSHB | 81.5C10 | 1:200 | To identify motor neurons |
| Mouse anti-Islet 1/2 | DSHB | 39.4D5 | 1:200 | To identify motor neurons |
| Goat anti-choline acetyltransferase | Sigma | AB144P | 1:500 | To identify motor neurons |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, T.-H.; Midha, R. Limited Myelination Capacity in Human Schwann Cells in Experimental Models in Comparison to Rodent and Porcine Schwann Cells. Int. J. Mol. Sci. 2025, 26, 6457. https://doi.org/10.3390/ijms26136457
Chu T-H, Midha R. Limited Myelination Capacity in Human Schwann Cells in Experimental Models in Comparison to Rodent and Porcine Schwann Cells. International Journal of Molecular Sciences. 2025; 26(13):6457. https://doi.org/10.3390/ijms26136457
Chicago/Turabian StyleChu, Tak-Ho, and Rajiv Midha. 2025. "Limited Myelination Capacity in Human Schwann Cells in Experimental Models in Comparison to Rodent and Porcine Schwann Cells" International Journal of Molecular Sciences 26, no. 13: 6457. https://doi.org/10.3390/ijms26136457
APA StyleChu, T.-H., & Midha, R. (2025). Limited Myelination Capacity in Human Schwann Cells in Experimental Models in Comparison to Rodent and Porcine Schwann Cells. International Journal of Molecular Sciences, 26(13), 6457. https://doi.org/10.3390/ijms26136457







