Serum IL-18/IL-13 Ratio Predicts Super Response to Secukinumab in Patients with Psoriasis
Abstract
1. Introduction
2. Results
2.1. Patient Stratification and Baseline Characteristics
2.2. Baseline and Post-Treatment Cytokine Profiles
2.3. Comparison of Cytokine Dynamics Between Groups
2.4. Predictive Value of Baseline Cytokines
2.5. Cytokine-Based Thresholds for Predicting Super Response
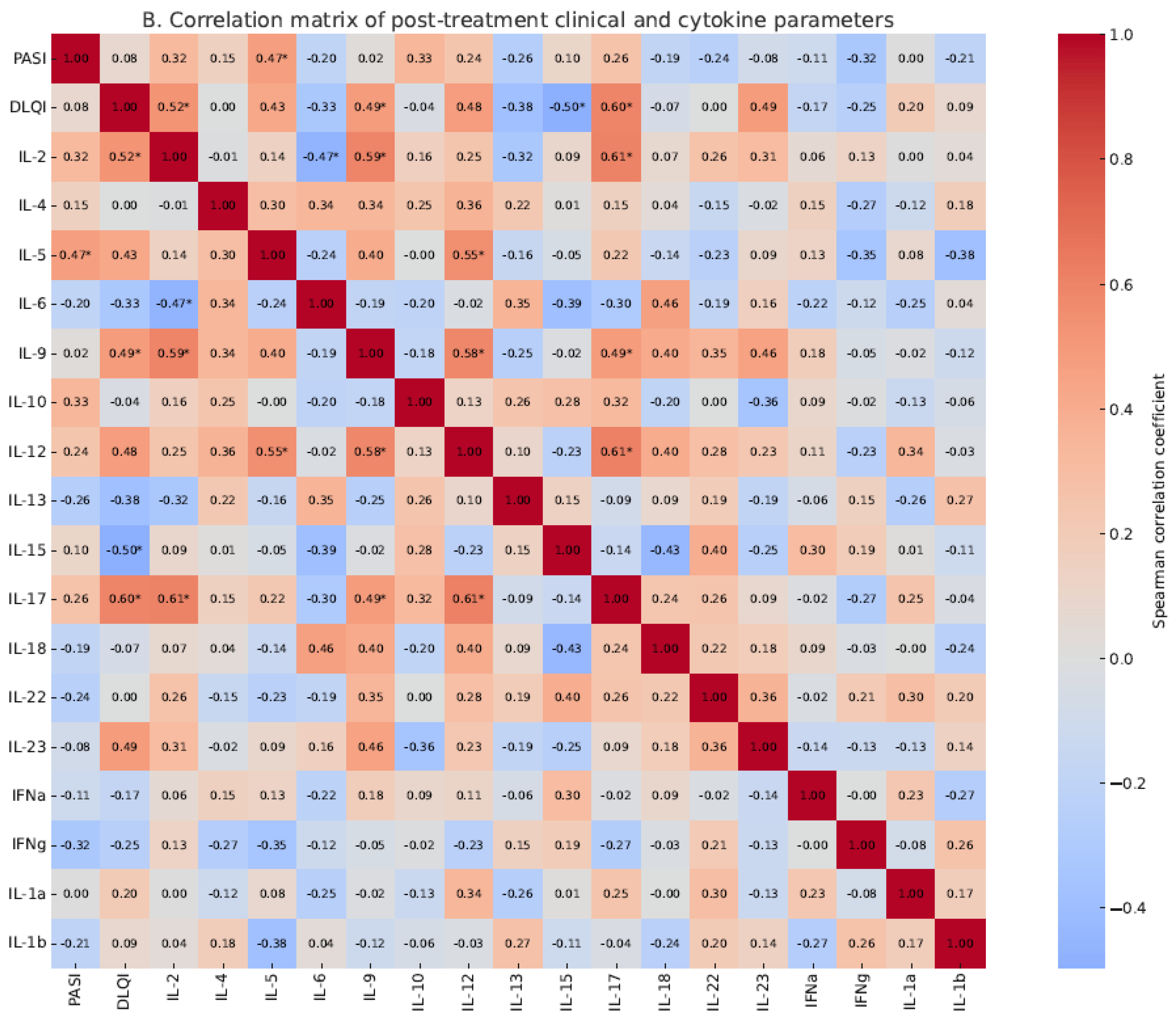
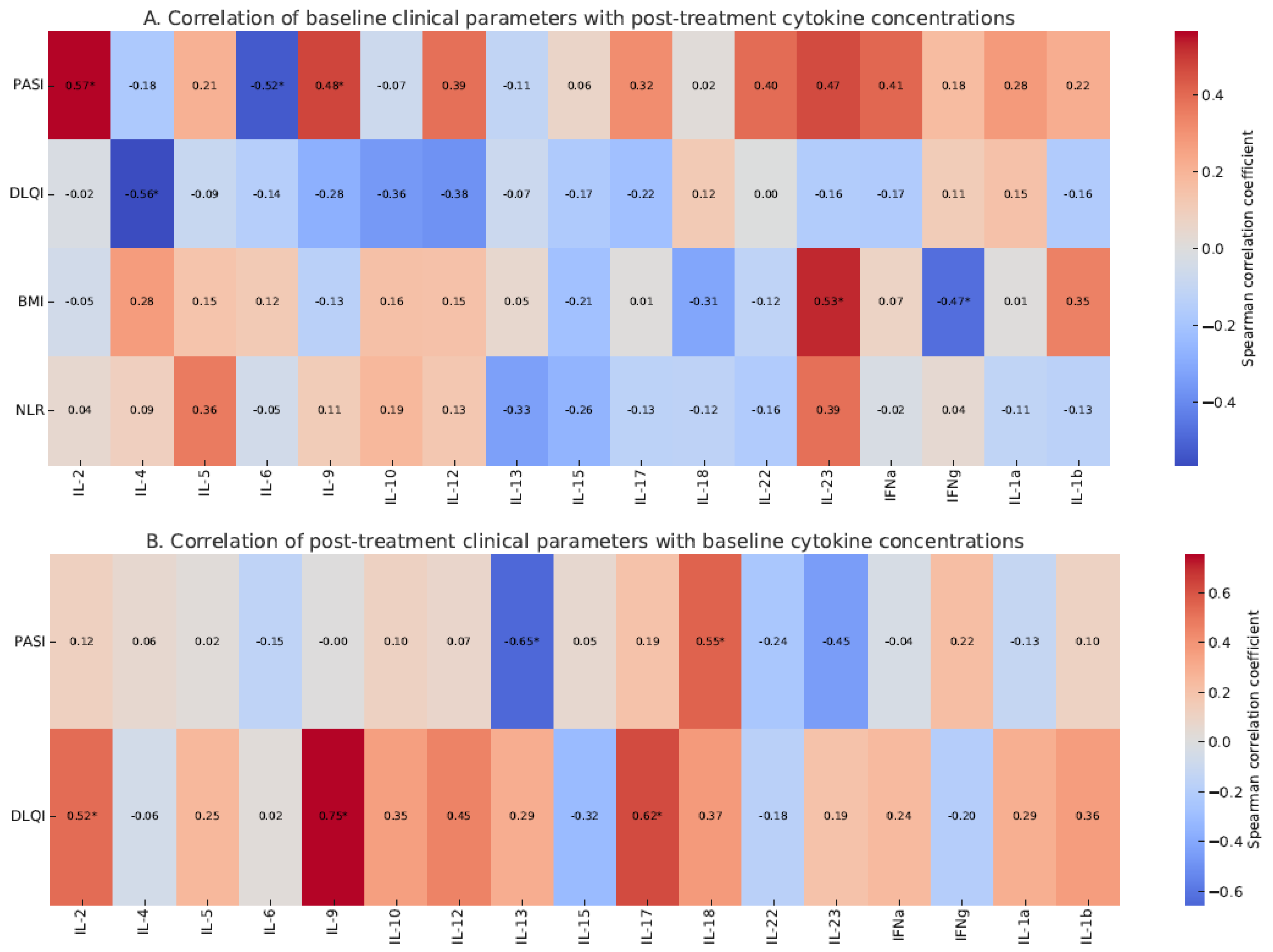
2.6. Correlation Between PASI and Cytokine Concentrations
2.7. Correlation Between IL-18, IL-17, and Monocyte Count
2.8. Cytokine Ratios as Predictors of Treatment Response
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Treatment Regimen
4.3. Data Collection and Sample Handling
4.4. Cytokine Quantification
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar to and distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-T.-Y.; Qiao, Z.-H.; Tian, J.-B.; Lin, J.-L.; Hou, S.-C.; Liu, X.-M. The effect of secukinumab treatment for psoriasis on serum cytokines and correlation with disease severity. Skin Res. Technol. 2023, 29, e13405. [Google Scholar] [CrossRef]
- Solberg, S.M.; de Boer, J.; Langholz, E.; Dyrhol-Riise, A.M.; Skov, L. Serum cytokine measurements and biological therapy of psoriasis—Prospects for individualized therapy. Scand. J. Immunol. 2018, 87, e12662. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Balato, A.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; Dapavo, P.; Dini, V.; et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for moderate-to-severe plaque psoriasis: A retrospective multicenter real-world experience on 5932 treatment courses—IL PSO (Italian landscape psoriasis). Front. Immunol. 2024, 14, 1341708. [Google Scholar] [CrossRef]
- Thomas, S.E.; van den Reek, J.M.P.A.; Seyger, M.M.B.; de Jong, E.M.G.J. How to define a ‘super-responder’ to biologics in psoriasis studies. Br. J. Dermatol. 2023, 189, 621–622. [Google Scholar] [CrossRef]
- Mason, K.J.; Alabas, O.A.; Dand, N.; Mason, B.; Lunt, M.; Warren, R.B.; Griffiths, C.E.M.; Ashcroft, D.M.; Smith, C.H.; on behalf of the BADBIR Study Group. Characteristics of ‘super responders’ and ‘super non-responders’ to first biologic monotherapy for psoriasis: A nested case-control study. Br. J. Dermatol. 2024, 190, 441–444. [Google Scholar] [CrossRef]
- Loft, N.; Egeberg, A.; Rasmussen, M.K.; Bryld, L.E.; Skov, L. Prevalence and characterization of treatment-refractory psoriasis and super-responders to biologic treatment: A nationwide study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1284–1291. [Google Scholar] [CrossRef]
- Marcelli, L.; Belcastro, A.; Talamonti, M.; Galluzzo, M.; Giunta, A.; Bavetta, M.; Perino, F.; Cortese, C.; Bianchi, L.; Chimenti, S. Characterization of super-responder profile in chronic plaque psoriatic patients under guselkumab treatment: A long-term real-life experience. J. Clin. Med. 2024, 13, 5175. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, K.; Jian, L.; Duan, Y.; Zhang, M.; Kuang, Y. Comparison between super-responders and non-super-responders in psoriasis under adalimumab treatment: A real-life cohort study on the effectiveness and drug survival over one year. J. Dermatolog. Treat. 2024, 35, 2331782. [Google Scholar] [CrossRef] [PubMed]
- Fratton, Z.; Bighetti, S.; Bettolini, L.; Maione, V.; Arisi, M.; Buligan, C.; Stinco, G.; Errichetti, E. Real-world experience of bimekizumab in a cohort of 109 patients over 48 weeks and identification of predictive factors for an early super response and risk of adverse events. Psoriasis 2025, 15, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Mortato, E.; Talamonti, M.; Marcelli, L.; Megna, M.; Raimondo, A.; Caldarola, G.; Bernardini, N.; Balato, A.; Campanati, A.; Esposito, M.; et al. Predictive factors for super responder status and long-term effectiveness of guselkumab in psoriasis: A multicenter retrospective study. Dermatol. Ther. 2025, 15, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Pirro, F.; Caldarola, G.; Chiricozzi, A.; Burlando, M.; Mariani, M.; Parodi, A.; Peris, K.; De Simone, C. Impact of Body Mass Index on the Efficacy of Biological Therapies in Patients with Psoriasis: A Real-World Study. Clin. Drug Investig. 2021, 41, 917–925. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Yu, S.J.; Lai, K.L.; Chao, W.T.; Yen, C.Y. IFN-gamma, IL-17A, IL-4, and IL-13: Potential biomarkers for prediction of the effectiveness of biologics in psoriasis patients. Biomedicines 2024, 12, 1115. [Google Scholar] [CrossRef]
- Ruscitti, P.; Esposito, M.; Di Cola, I.; Pellegrini, C.; De Berardinis, A.; Mastrangelo, M.; Gianneramo, C.; Barile, A.; Fargnoli, M.C.; Cipriani, P. Cytokine profile characterization of naïve patients with psoriasis and psoriatic arthritis: Implications for a pathogenic disease continuum. Front. Immunol. 2023, 14, 1229516. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Johnson-Huang, L.M.; Suárez-Fariñas, M.; Pierson, K.C.; Fuentes-Duculan, J.; Cueto, I.; Lentini, T.; Sullivan-Whalen, M.; Gilleaudeau, P.; Krueger, J.G.; Haider, A.S.; et al. A single intradermal injection of IFN-γ induces an inflammatory state in both non-lesional psoriatic and healthy skin. J. Investig. Dermatol. 2012, 132, 1177–1187. [Google Scholar] [CrossRef]
- Rusiñol, L.; Puig, L.A. Narrative Review of the IL-18 and IL-37 Implications in the Pathogenesis of Atopic Dermatitis and Psoriasis: Prospective Treatment Targets. Int. J. Mol. Sci. 2024, 25, 8437. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in health and disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Pietrzak, A.; Bartosinska, J.; Chodorowska, G.; Juszkiewicz-Borowiec, M.; Wozniacka, A.; Szepietowski, J. Serum levels of IL-18 in patients with psoriasis. Acta Derm. Venereol. 2003, 83, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Orzan, O.A.; Tutunaru, C.V.; Ianoși, S.L. Understanding the intricate pathophysiology of psoriasis and related skin disorders. Int. J. Mol. Sci. 2025, 26, 749. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, H.; Jabbari Azad, F.; Rastin, M.; Banihashemi, M.; Mahmoudi, M. Expression of Th1 and Th2 cytokine and associated transcription factors in peripheral blood mononuclear cells and correlation with disease severity. Rep. Biochem. Mol. Biol. 2017, 6, 102–111. [Google Scholar] [PubMed] [PubMed Central]
- Cancino-Díaz, J.C.; Reyes-Maldonado, E.; Bañuelos-Pánuco, C.A.; Jiménez-Zamudio, L.; García-Latorre, E.; León-Dorantes, G.; Blancas-González, F.; Paredes-Cabrera, G.; Cancino-Díaz, M.E. Interleukin-13 Receptor in Psoriatic Keratinocytes: Overexpression of the mRNA and Underexpression of the Protein. J. Investig. Dermatol. 2002, 119, 1114–1120. [Google Scholar] [CrossRef]
- Ziolkowska-Banasik, D.; Hadas, E.; Pastuszczak, M. Blood Monocyte Count Can Predict Early Response to Secukinumab Therapy in Patients With Psoriasis. J. Drugs Dermatol. 2024, 23, 74–77. [Google Scholar] [CrossRef]
- ten Bergen, L.L.; Petrovic, A.; Aarebrot, A.K.; Appel, S. The TNF/IL-23/IL-17 Axis—Head-to-Head Trials Comparing Different Biologics in Psoriasis Treatment. Scand. J. Immunol. 2020, 92, e12946. [Google Scholar] [CrossRef]
- Yamamoto, T.; Akasaka, E.; Hikosaka, A.; Katayama, I. Effects of Cyclosporine on Palmoplantar Pustulosis and Serum Expression of IL-17, IL-23, and TNF-α. J. Dermatol. 2018, 45, 1044–1050. [Google Scholar] [CrossRef]


| Super Responders (SRs), n = 9 | Non-Super Responders (NSRs), n = 19 | p-Value | |
|---|---|---|---|
| females; n (%) | 4 (44.4) | 9 (47.4) | 1.0 |
| males; n (%) | 5 (55.6) | 10 (52.6) | 1.0 |
| age | 30.50 (19.00–57.00) | 45.00 (27.00–57.00) | 0.15 |
| BMI | 23.66 (22.34–27.47) | 26.20 (20.83–29.97) | 0.41 |
| baseline PASI | 35.3 (16.2–50.1) | 24.2 (20–40) | 0.76 |
| post-treatment PASI | 0.0 (0.0–0.0) | 6.6 (2.4–18.0) | 0.0002 |
| disease duration; months | 32.1 (18–108) | 37.9 (12–144) | 0.54 |
| previous treatment with cyclosporine; n (%) | 8 (88.9) | 17 (89.4) | 1.0 |
| previous treatment with methoretxate; n (%) | 9 (100) | 16 (84.2) | 0.53 |
| previous treatment with acitretin; n (%) | 3 (33.3) | 6 (31.6) | 1.0 |
| baseline CRP, mg/dL | 8.25 (3.80–15.90) | 10.40 (4.30–22.10) | 0.63 |
| baseline lymphocytes count; ×103/μL | 3.05 (2.02–4.02) | 2.83 (1.56–3.55) | 0.23 |
| baseline monocytes count; ×103/μL | 0.41 (0.12–0.81) | 0.67 (0.33–0.78) | 0.03 |
| baseline neutrophils count; ×103/μL | 5.22 (2.14–7.05) | 5.44 (4.12–8.77) | 0.59 |
| baseline NLR | 1.7 (0.9–3.1) | 2.1 (1.4–5.6) | 0.31 |
| Baseline Levels | Post-Treatment Levels | |||||
|---|---|---|---|---|---|---|
| Super Responder (SR) Group (n = 9) | Non-Super Responder (NSR) Group (n = 19) | p-Value | Super Responder (SR) Group (n = 9) | Non-Super Responder (NSR) Group (n = 19) | p-Value | |
| IL-2; pg/mL | 2.3 (0.1–17.4) | 3.1 (0.1–16.9) | 1.0 | 0.2 (0.1–20.9) | 4.0 (0.1–13.8) | 0.30 |
| IL-4; pg/mL | 0.1 (0.1–67.9) | 0.1 (0.1–14.8) | 0.6 | 0.1 (0.1–45.2) | 0.1 (0.1–10.3) | 0.34 |
| IL-5; pg/mL | 2.6 (0.1–23.1) | 0.45 (0.1–34.9) | 0.75 | 0.8 (0.1–8.1) | 7.05 (0.1–15.8) | 0.2 |
| IL-6; pg/mL | 0.2 (0.1–11.9) | 0.1 (0.1–0.9) | 0.35 | 0.1 (0.1–0.4) | 0.1 (0.1–0.3) | 1.0 |
| IL-9; pg/mL | 0.1 (0.1–4.5) | 0.1 (0.1–5.2) | 0.75 | 0.1 (0.1–5.9) | 0.1 (0.1–3.1) | 1.0 |
| IL-10; pg/mL | 0.2 (0.1–3.2) | 0.15 (0.1–14.5) | 1.0 | 0.25 (0.1–3.1) | 0.7 (0.1–13.5) | 0.34 |
| IL-12; pg/mL | 1.5 (0.1–3.7) | 1.2 (0.1–4.1) | 0.93 | 0.9 (0.1–2.2) | 1.05 (0.1–3.1) | 0.53 |
| IL-13; pg/mL | 1.2 (0.6–1.6) | 0.35 (0.1–1.1) | 0.002 | 0.8 (0.0–1.7) | 0.35 (0.1–1.1) | 0.17 |
| IL-15; pg/mL | 5.8 (0.5–55.4) | 7.1 (0.1–45.2) | 0.96 | 8.1 (0.1–39.2) | 7.5 (0.4–34.1) | 1.0 |
| IL-17; pg/mL | 3.3 (0.4–11.4) | 4.0 (0.5–14.9) | 0.35 | 2.7 (0.5–10.2) | 3.1 (1.1–19.3) | 0.42 |
| IL-18; pg/mL | 0.15 (0.1–0.4) | 2.05 (0.2–8.1) | 0.007 | 0.5 (0.1–1.7) | 0.25 (0.1–11.2) | 0.82 |
| IL-22; pg/mL | 0.1 (0.1–0.3) | 0.1 (0.1–0.1) | 0.31 | 0.1 (0.1–0.4) | 0.1 (0.1–0.1) | 0.31 |
| IL-23; pg/mL | 345.0 (98.0–1004.0) | 129.0 (34.0–871.0) | 0.12 | 145.0 (6.0–801.0) | 121.0 (32.0–892.0) | 0.92 |
| TNF-α; pg/mL | 3.1 (0.1–9.1) | 1.1 (0.1–5.1) | 0.2 | 2.1 (0.1–3.9) | 0.4 (0.1–2.9) | 0.26 |
| IFN-α; pg/mL | 0.3 (0.1–1.2) | 0.25 (0.1–0.5) | 0.68 | 0.25 (0.1–1.9) | 0.2 (0.1–2.2) | 0.93 |
| IFN-γ; pg/mL | 35.5 (7.0–118.0) | 71.5 (7.0–142.0) | 0.37 | 52.0 (28.0–145.0) | 28.0 (7.0–88.0) | 0.04 |
| IL-1α; pg/mL | 0.25 (0.1–1.8) | 0.1 (0.1–4.3) | 0.51 | 0.1 (0.1–0.9) | 0.1 (0.1–0.4) | 0.91 |
| IL-1β; pg/mL | 0.2 (0.1–1.1) | 0.3 (0.1–1.6) | 0.70 | 0.2 (0.1–1.4) | 0.1 (0.1–1.1) | 0.37 |
| Baseline Levels | Post-Treatment Levels | |||||
|---|---|---|---|---|---|---|
| Super Responder (SR) Group (n = 9) | Non-Super Responder (NSR) Group (n = 19) | p-Value | Super Responder (SR) Group (n = 9) | Non-Super Responder (NSR) Group (n = 19) | p-Value | |
| IL-17/IL-4; pg/mL | 27.5 (0.01–114.0) | 22.3 (0.05–128.0) | 1.0 | 21.5 (0.1–102.0) | 20.5 (0.2–147.0) | 0.76 |
| IL-17/IL-13; pg/mL | 2.5 (0.36–10.36) | 13.3 (1.7–53.0) | 0.004 | 3.1 (0.56–inf) | 14.9 (1–96.3) | 0.17 |
| IL-23/IL-4; pg/mL | 1965.0 (8.3–10040) | 650.0 (2.3–5610.0) | 0.11 | 1450.0 (0.62–8010.0) | 430.0 (20.9–3990.0) | 0.39 |
| IL-23/IL-13; pg/mL | 337.5 (80.6–912.7) | 231.0 (90–5620.0) | 0.74 | 270.8 (12–inf) | 356.7 (80.0–3990.0) | 0.54 |
| IL-18/IL-13; pg/mL | 0.18 (0.06–0.33) | 3.46 (1.5–30.0) | 0.00001 | 0.76 (0.2–inf) | 2.0 (0.09–37.3) | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziolkowska-Banasik, D.; Pastuszczak, M.; Zawadzinska-Halat, K.; Hadas, E.; Bozek, A. Serum IL-18/IL-13 Ratio Predicts Super Response to Secukinumab in Patients with Psoriasis. Int. J. Mol. Sci. 2025, 26, 6432. https://doi.org/10.3390/ijms26136432
Ziolkowska-Banasik D, Pastuszczak M, Zawadzinska-Halat K, Hadas E, Bozek A. Serum IL-18/IL-13 Ratio Predicts Super Response to Secukinumab in Patients with Psoriasis. International Journal of Molecular Sciences. 2025; 26(13):6432. https://doi.org/10.3390/ijms26136432
Chicago/Turabian StyleZiolkowska-Banasik, Dominika, Maciej Pastuszczak, Kamila Zawadzinska-Halat, Ewa Hadas, and Andrzej Bozek. 2025. "Serum IL-18/IL-13 Ratio Predicts Super Response to Secukinumab in Patients with Psoriasis" International Journal of Molecular Sciences 26, no. 13: 6432. https://doi.org/10.3390/ijms26136432
APA StyleZiolkowska-Banasik, D., Pastuszczak, M., Zawadzinska-Halat, K., Hadas, E., & Bozek, A. (2025). Serum IL-18/IL-13 Ratio Predicts Super Response to Secukinumab in Patients with Psoriasis. International Journal of Molecular Sciences, 26(13), 6432. https://doi.org/10.3390/ijms26136432






