Hypoxic Conditions Promote Cartilage Repair in a Rat Knee Osteochondral Defect Model via Hypoxia-Inducible Factor-1α
Abstract
1. Introduction
2. Results
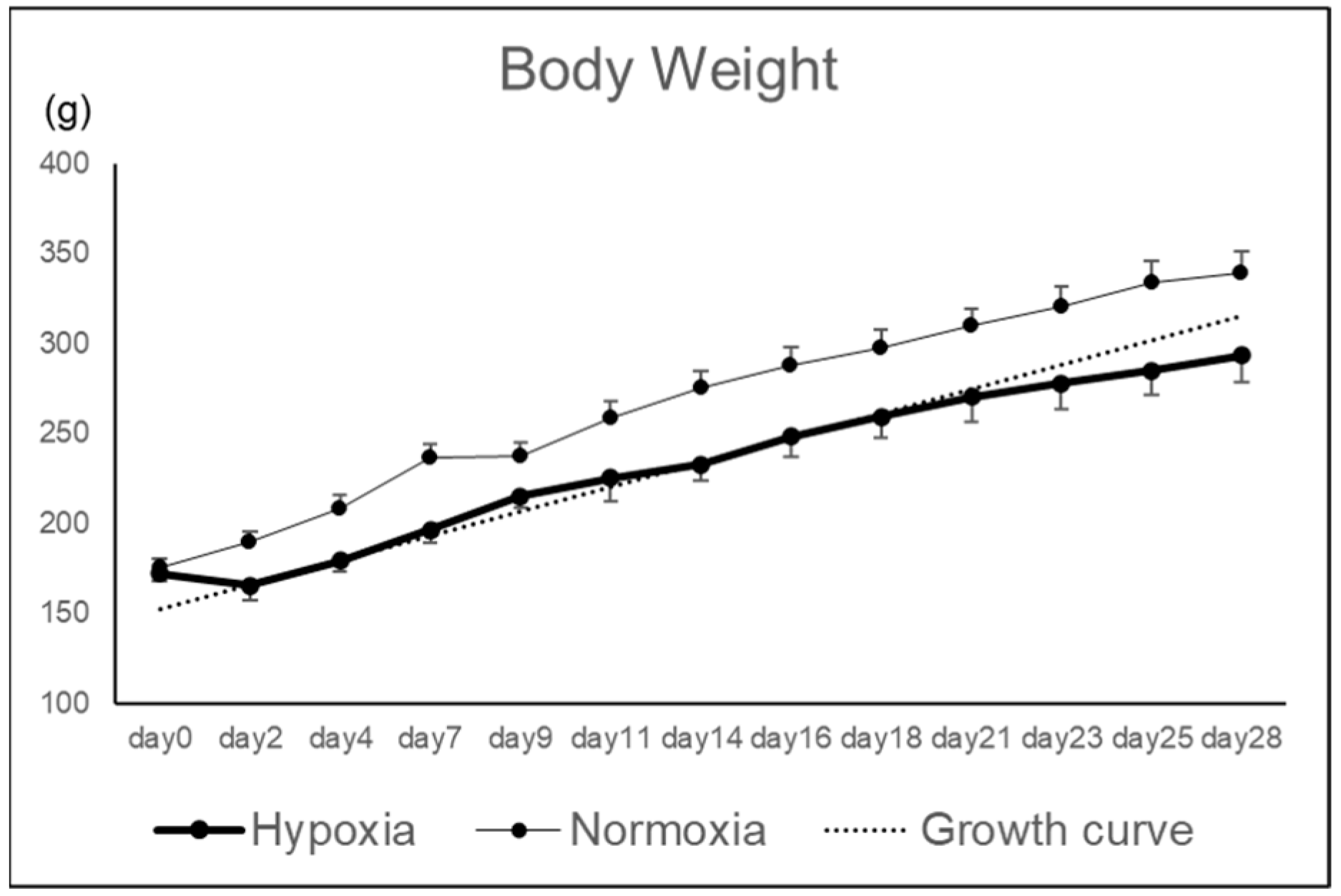
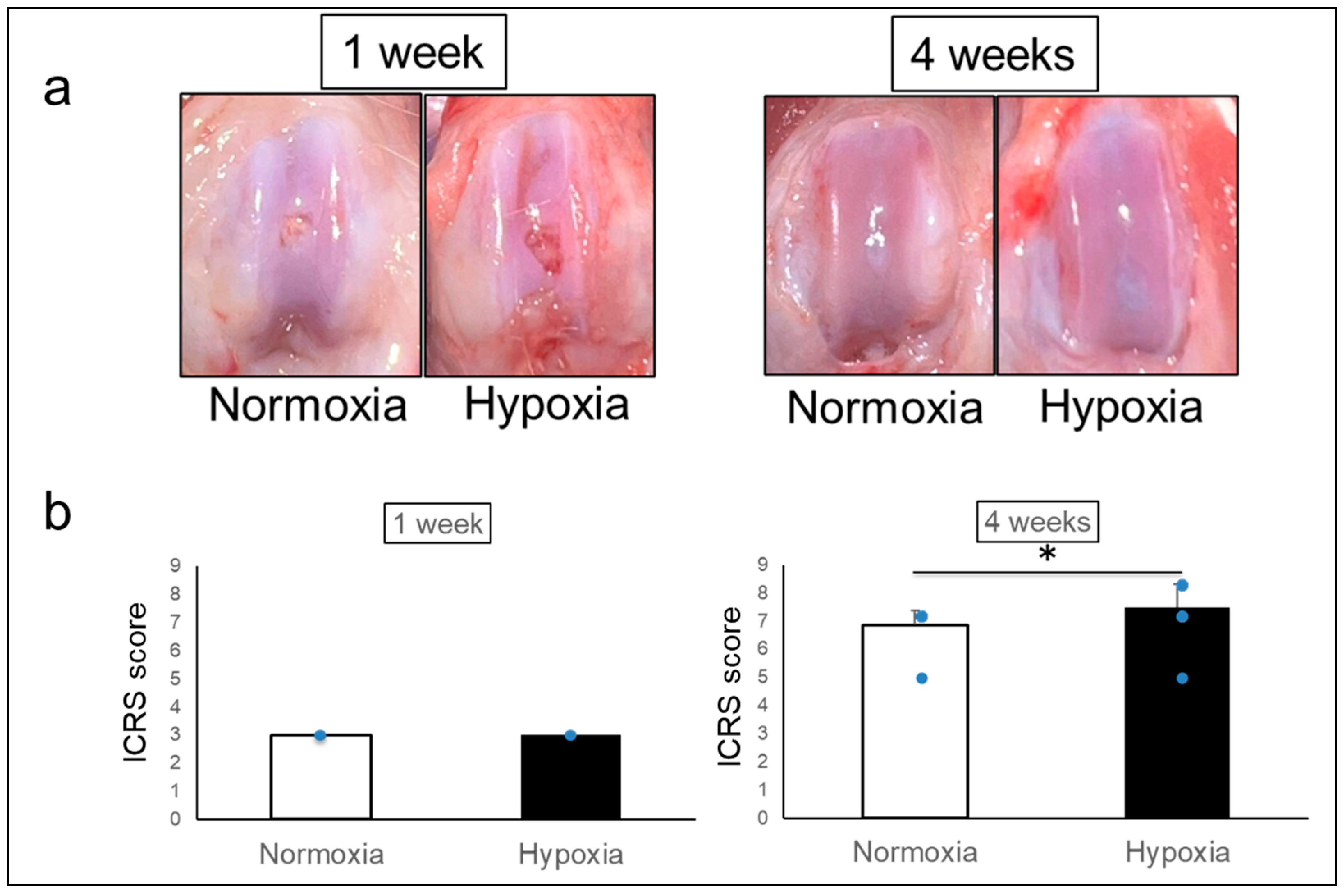
2.1. Effects of Hypoxic Conditions on Rat Knee Osteochondral Defect Model
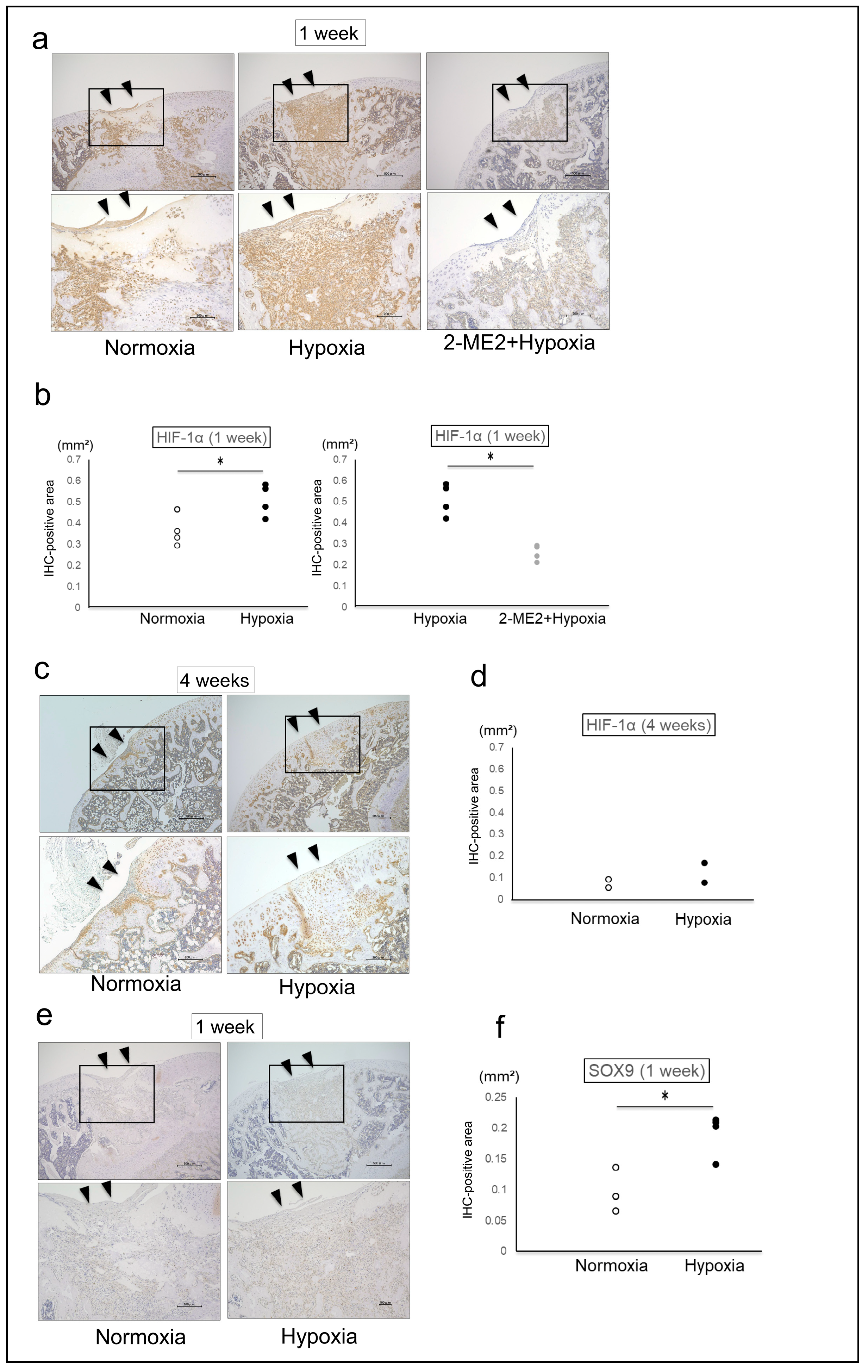
2.2. The Effects of HIF-1α on the Repair Tissue
3. Discussion
Limitations
4. Materials and Methods
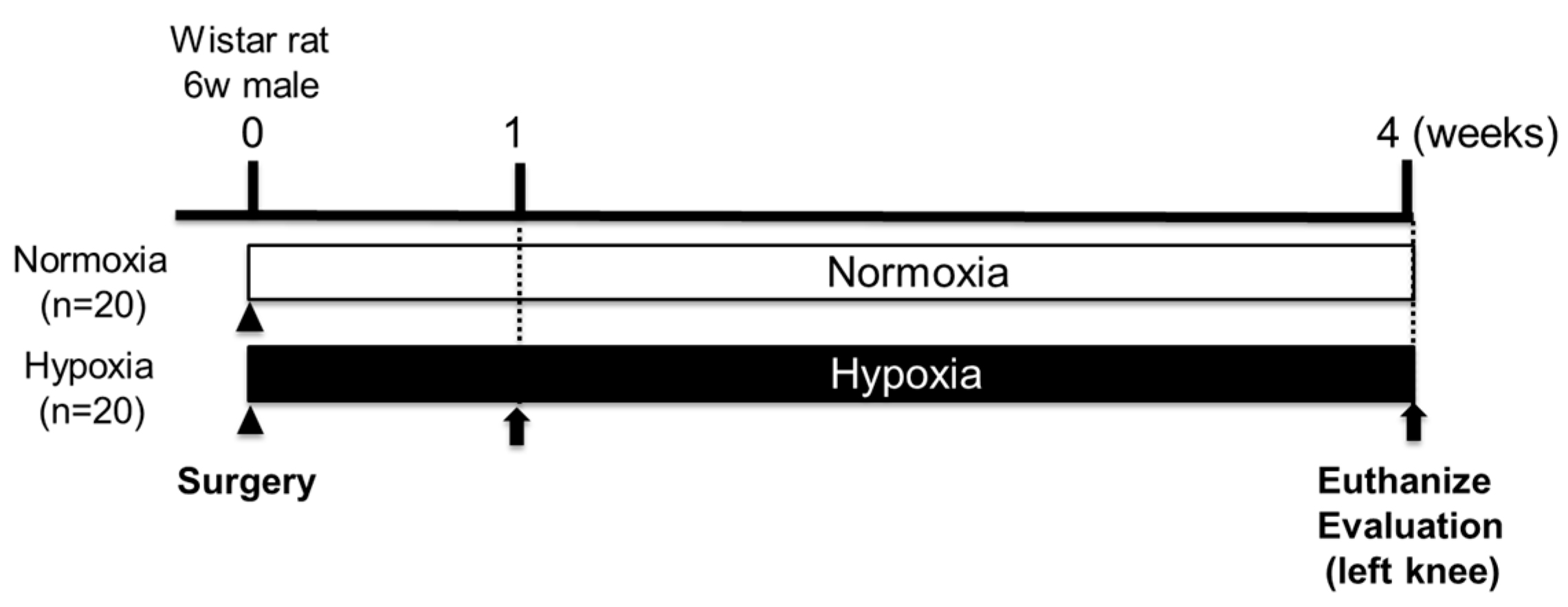
4.1. Animals
4.2. Creation of Rat Knee Osteochondral Defect Model
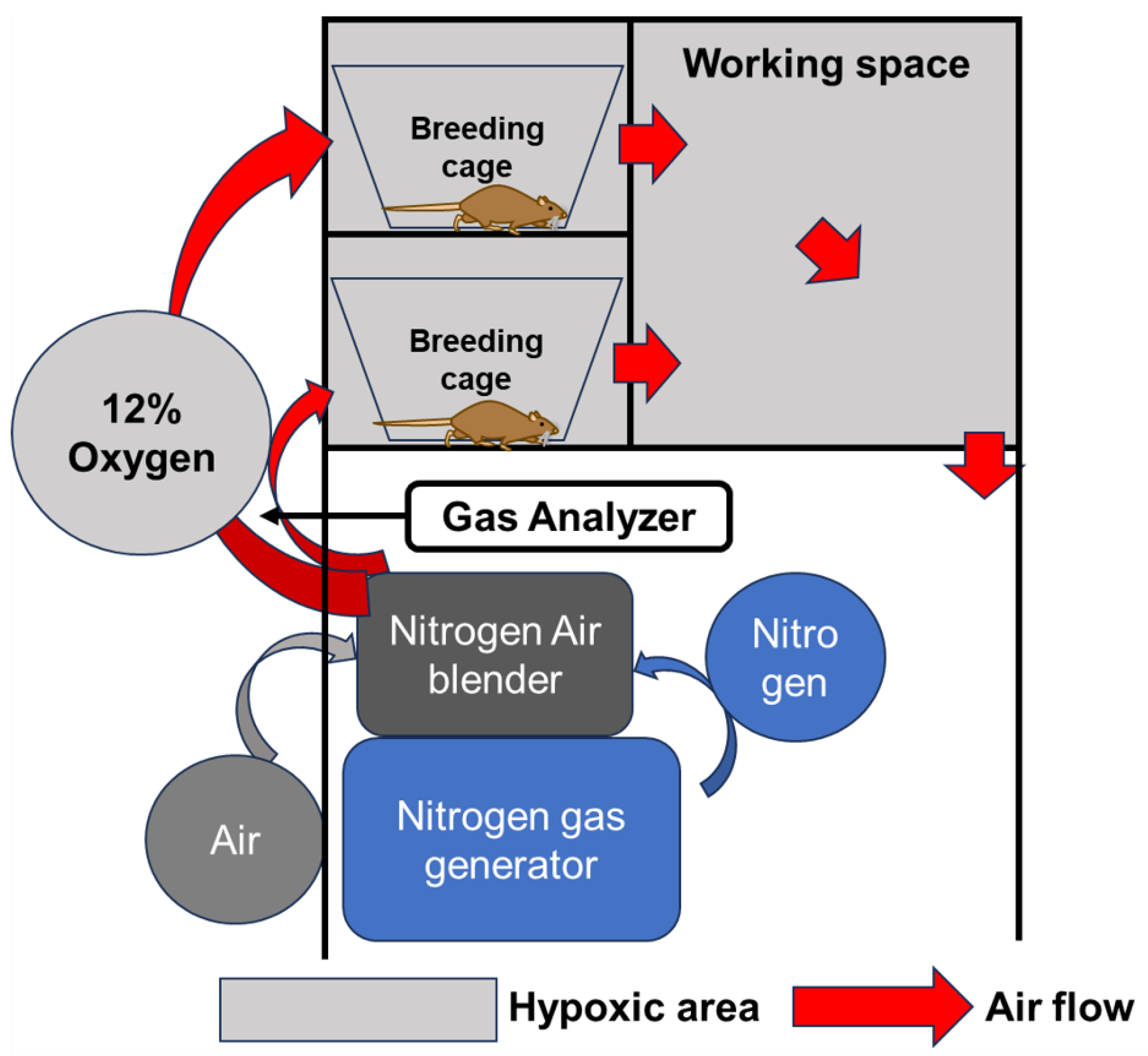
4.3. Breeding in a Hypoxic Chamber
4.4. Effects of Hypoxic Conditions on Rat Knee Osteochondral Defect Model
4.5. HIF-1α Inhibitor Treatment
4.6. Macroscopic Evaluation
4.7. Histological Evaluation
4.8. Immunohistochemical Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Hasson, M.; Fernandes, L.M.; Solomon, H.; Pepper, T.; Huffman, N.L.; Pucha, S.A.; Bariteau, J.T.; Kaiser, J.M.; Patel, J.M. Considering the Cellular Landscape in Marrow Stimulation Techniques for Cartilage Repair. Cells Tissues Organs 2024, 213, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef]
- Garcia, J.P.; Avila, F.R.; Torres, R.A.; Maita, K.C.; Eldaly, A.S.; Rinker, B.D.; Zubair, A.C.; Forte, A.J.; Sarabia-Estrada, R. Hypoxia-preconditioning of human adipose-derived stem cells enhances cellular proliferation and angiogenesis: A systematic review. J. Clin. Transl. Res. 2022, 8, 61–70. [Google Scholar]
- Amarilio, R.; Viukov, S.V.; Sharir, A.; Eshkar-Oren, I.; Johnson, R.S.; Zelzer, E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 2007, 134, 3917–3928. [Google Scholar] [CrossRef]
- Taheem, D.K.; Jell, G.; Gentleman, E. Hypoxia Inducible Factor-1α in Osteochondral Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26, 105–115. [Google Scholar] [CrossRef]
- Shimomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Shin-Ya, M.; Ichimaru, S.; Tsuchida, S.; Mazda, O.; et al. Mechanical stimulation of chondrocytes regulates HIF-1α under hypoxic conditions. Tissue Cell 2021, 71, 101574. [Google Scholar] [CrossRef]
- Kamada, Y.; Arai, Y.; Toyama, S.; Inoue, A.; Nakagawa, S.; Fujii, Y.; Kaihara, K.; Cha, R.; Mazda, O.; Takahashi, K. Hypoxia with or without Treadmill Exercises Affects Slow-Twitch Muscle Atrophy and Joint Destruction in a Rat Model of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 9761. [Google Scholar] [CrossRef]
- Cha, R.; Nakagawa, S.; Arai, Y.; Inoue, A.; Okubo, N.; Fujii, Y.; Kaihara, K.; Nakamura, K.; Kishida, T.; Mazda, O.; et al. Intermittent hypoxic stimulation promotes efficient expression of Hypoxia-inducible factor-1α and exerts a chondroprotective effect in an animal osteoarthritis model. PLoS ONE 2025, 20, e0319976. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Kubo, T.; Coutts, R.D.; Hirasawa, Y. Differences in the repair process of longitudinal and transverse injuries of cartilage in the rat knee. Osteoarthr. Cartil. 1998, 6, 66–75. [Google Scholar] [CrossRef]
- Kiaer, T.; Grønlund, J.; Sørensen, K.H. Subchondral pO2, pCO2, pressure, pH, and lactate in human osteoarthritis of the hip. Clin. Orthop. Relat. Res. 1988, 229, 149–155. [Google Scholar] [CrossRef]
- Nevo, Z.; Beit-Or, A.; Eilam, Y. Slowing down aging of cultured embryonal chick chondrocytes by maintenance under lowered oxygen tension. Mech. Ageing Dev. 1988, 45, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Henrionnet, C.; Liang, G.; Roeder, E.; Dossot, M.; Wang, H.; Magdalou, J.; Gillet, P.; Pinzano, A. Hypoxia for Mesenchymal Stem Cell Expansion and Differentiation: The Best Way for Enhancing TGFß-Induced Chondrogenesis and Preventing Calcifications in Alginate Beads. Tissue Eng. Part A 2017, 23, 913–922. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef]
- Duval, E.; Baugé, C.; Andriamanalijaona, R.; Bénateau, H.; Leclercq, S.; Dutoit, S.; Poulain, L.; Galéra, P.; Boumédiene, K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials 2012, 33, 6042–6051. [Google Scholar] [CrossRef]
- Li, D.X.; Ma, Z.; Szojka, A.R.; Lan, X.; Kunze, M.; Mulet-Sierra, A.; Westover, L.; Adesida, A.B. Non-hypertrophic chondrogenesis of mesenchymal stem cells through mechano-hypoxia programing. J. Tissue Eng. 2023, 14, 20417314231172574. [Google Scholar] [CrossRef]
- Shimomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Shin-Ya, M.; Ichimaru, S.; Tsuchida, S.; Mazda, O.; et al. Hypoxia promotes differentiation of pure cartilage from human induced pluripotent stem cells. Mol. Med. Rep. 2022, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, S.; Fan, L.; Niu, R.; Yan, M.; Chen, S.; Zheng, M.; Zhang, S. Integrated regulation of chondrogenic differentiation in mesenchymal stem cells and differentiation of cancer cells. Cancer Cell Int. 2022, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Zhang, W.; Zhu, Y.; Fan, Z.; Huang, Y.; Li, F.; Yang, R. Bone marrow mesenchymal stem cells facilitate diabetic wound healing through the restoration of epidermal cell autophagy via the HIF-1α/TGF-β1/SMAD pathway. Stem Cell Res. Ther. 2022, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhou, B.; Zheng, J.; Huang, Y.; Zeng, H.; Xue, L.; Wang, D. Lithium and Copper Induce the Osteogenesis-Angiogenesis Coupling of Bone Marrow Mesenchymal Stem Cells via Crosstalk between Canonical Wnt and HIF-1α Signaling Pathways. Stem Cells Int. 2021, 2021, 6662164. [Google Scholar] [CrossRef]
- Gelse, K.; Pfander, D.; Obier, S.; Knaup, K.X.; Wiesener, M.; Hennig, F.F.; Swoboda, B. Role of hypoxia-inducible factor 1 alpha in the integrity of articular cartilage in murine knee joints. Arthritis Res. Ther. 2008, 10, R111. [Google Scholar] [CrossRef]
- van den Borne, M.P.; Raijmakers, N.J.; Vanlauwe, J.; Victor, J.; de Jong, S.N.; Bellemans, J.; Saris, D.B. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthr. Cartil. 2007, 15, 1397–1402. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.C.; Gertzman, A.A.; Xie, L.; Nizkorodov, A.; Hyzy, S.L.; Truncale, K.; Guldberg, R.E.; Schwartz, Z.; Boyan, B.D. Endogenous regeneration of critical-size chondral defects in immunocompromised rat xiphoid cartilage using decellularized human bone matrix scaffolds. Tissue Eng. Part A 2012, 18, 2332–2342. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Inoue, A.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Cha, R.; Sugie, K.; Hayashi, K.; Kishida, T.; Mazda, O.; et al. Hypoxic Conditions Promote Cartilage Repair in a Rat Knee Osteochondral Defect Model via Hypoxia-Inducible Factor-1α. Int. J. Mol. Sci. 2025, 26, 6370. https://doi.org/10.3390/ijms26136370
Nakamura K, Inoue A, Arai Y, Nakagawa S, Fujii Y, Cha R, Sugie K, Hayashi K, Kishida T, Mazda O, et al. Hypoxic Conditions Promote Cartilage Repair in a Rat Knee Osteochondral Defect Model via Hypoxia-Inducible Factor-1α. International Journal of Molecular Sciences. 2025; 26(13):6370. https://doi.org/10.3390/ijms26136370
Chicago/Turabian StyleNakamura, Kei, Atsuo Inoue, Yuji Arai, Shuji Nakagawa, Yuta Fujii, Ryota Cha, Keisuke Sugie, Kentaro Hayashi, Tsunao Kishida, Osam Mazda, and et al. 2025. "Hypoxic Conditions Promote Cartilage Repair in a Rat Knee Osteochondral Defect Model via Hypoxia-Inducible Factor-1α" International Journal of Molecular Sciences 26, no. 13: 6370. https://doi.org/10.3390/ijms26136370
APA StyleNakamura, K., Inoue, A., Arai, Y., Nakagawa, S., Fujii, Y., Cha, R., Sugie, K., Hayashi, K., Kishida, T., Mazda, O., & Takahashi, K. (2025). Hypoxic Conditions Promote Cartilage Repair in a Rat Knee Osteochondral Defect Model via Hypoxia-Inducible Factor-1α. International Journal of Molecular Sciences, 26(13), 6370. https://doi.org/10.3390/ijms26136370





