Abstract
Bipolar disorder (BD) may present with neurocognitive dysfunction due to inflammatory alterations through different biological pathways. However, findings are not consistent regarding the patterns of neurocognitive dysfunction and elevation of inflammatory biomarkers during the different mood phases. Therefore, we aimed to determine associations between inflammatory biomarkers, neurocognitive functioning, and clinical outcomes in patients with BD in euthymia. We conducted a cross-sectional study including 109 adults. Serum levels of interleukin-6 (IL-6), high-sensitivity C-reactive protein (hs-CRP), neurocognitive parameters (ACER), number of suicide attempts (SA), and hospitalizations (NH) were measured. We found negative and moderate correlations between IL-6 and ACER total score, language, visuospatial abilities, and orientation/attention. There was a positive and moderate correlation between IL-6 and NH. IL-6 significantly predicted ACER total score, language, memory, orientation/attention, visuospatial abilities, and NH. Overall, IL-6 had an inverse association with neurocognition and clinical variables, whereas hs-CRP did not play a role. Here we demonstrate that IL-6 predicts neurocognitive functioning in adults with BD. BD may be a biological model for studying the relationship between inflammation and neurocognition in severe psychiatric disorders. Prospective studies at different mood phases of the disease must be conducted.
1. Introduction
Bipolar disorder (BD) is a chronic disorder characterized by recurrent mood fluctuations between the poles of depression and mania [1]. It was one of the first psychiatric illnesses to receive attention from areas such as genetics and biology due to the clinical observation that the illness runs in families [2]. In this regard, inflammation has been found to play a cross-cutting role in severe psychiatric disorders, especially BD. To approach the study of the biological basis of multiple neuropsychiatric conditions, endophenotypes have acquired great relevance. This concept designates biomarkers of different nature that are measurable, that have a hereditary component, and that represent the underlying biological mechanisms of neuropsychiatric disorders [3]. Two proposed endophenotypes in BD are neurocognitive and inflammatory alterations [3]. In turn, both could be closely related.
Neurocognition refers to cognitive functions that are closely linked to specific areas, neural pathways, or networks in the brain. It determines multiple clinical outcomes and is a key prognostic factor in severe psychiatric disorders [4]. In BD, neurocognitive disturbances are frequent even in euthymia [5], which disrupt an individual’s ability to live independently. They include alterations in executive functions, processing speed, attention, memory, and social cognition [6,7,8]. Douglas et al. [9] found that 64% of patients with BD during a depressive episode and 57% of euthymic individuals were impaired in one or more cognitive domains. A systematic review including five studies concluded that participants with BD in euthymia showed greater cognitive dysfunction than those with mild cognitive impairment, but less than those with dementia [8]. However, a meta-analysis of longitudinal studies of patients with BD did not corroborate progressive cognitive impairment over a 5-year period [6]. In addition, a 5-year longitudinal study described the trajectories of change in the cognitive profile of people with BD with low, medium, and high cognitive performance. In none of the three groups was there a decline, nor were there significant differences between these trajectories [7]. At baseline, only a minority of participants had low cognitive performance, which was associated with a greater presence of childhood trauma and more psychiatric hospitalizations. These findings do not support the neuroprogression hypothesis in BD, as other authors have stated [10,11,12]. However, they do support the idea that the greater the allostatic load, the greater the cognitive dysfunction. There are different hypotheses that seek to explain how neurocognitive alterations originate in BD. At the neurobiological level, in both unipolar and bipolar mood disorders, morphofunctional changes have been found in brain regions involved in mood regulation and cognitive functioning, including the orbitofrontal cortex, amygdala, and hippocampus [13]. Some research groups have proposed that inflammation is the biological substrate of the brain alterations that account for cognitive dysfunction [14,15].
Although the inflammatory theory of mental disorders has occupied a central place in research on the biological basis of severe psychiatric disorders, its relationship to neurocognitive dysfunction is incipient. Recently, the GWAS by Mullins et al. [16] revealed for the first time that the locus associated with the major histocompatibility complex had one of the most significant associations with BD, superior to those loci linked to the mechanisms classically described for the disease. In this line, there are different primary studies that have examined the association between inflammatory biomarkers and some clinical outcomes in BD. Some have concluded that there is a multisystemic inflammation from the early stages of the disease [17]. Two of the most studied inflammatory biomarkers in BD are C-reactive protein (CRP) and interleukin-6 (IL-6). CRP is an acute-phase protein that increases its levels in inflammatory contexts and is induced by IL-6 [18]. High-sensitivity CRP (hs-CRP) is a standardized way of representing the low-grade inflammatory response observed in BD, whose magnitude is greater than in healthy controls, an observation verified by primary studies [19,20] and systematic reviews [21,22,23]. However, this elevation is not a consistent finding in all patients with BD [24] nor in all mood phases of the condition [25,26,27].
It has been proposed that the activation of the HPA axis during manic episodes would generate an initial increase in inflammatory parameters [25]. From a pathophysiological point of view, the increase in CRP promotes the permeability of the blood–brain barrier, allowing the diffusion of proinflammatory cytokines and antibodies that morphofunctionally alter central structures [28], favoring the production of free radicals and altering mitochondrial function, neurotransmitter synthesis, glial function, synaptic remodeling, and neurogenesis [29,30]. Regarding IL-6 levels, a systematic review with meta-analysis found that this biomarker was significantly higher in patients with BD compared to controls, both in mania and euthymia [31]. Consistently, another systematic review concluded that IL-6 remains elevated regardless of the mood phase, indicating that it is a potential biomarker of the disease [23].
In healthy elderly people, increased levels of CRP and IL-6 have been found to be associated with volumetric reductions in hippocampal gray matter [32], structural alterations of the white matter [32], and cortical atrophy [33]. Moreover, inflammatory biomarker levels are associated with cognitive performance in people with major depressive disorder [34,35]. In BD, multiple mood episodes alter the homeostasis between inflammatory mechanisms, oxidative processes, and neuroprotective mechanisms, promoting neuronal apoptosis, which increases the individual’s vulnerability to psychological stress and cognitive impairment [36]. However, the association between inflammatory parameters and cognitive dysfunction has been scarcely studied in samples with BD [35]. Likewise, the potential influence that inflammatory states have on clinical outcomes that account for the severity of the condition, such as suicidality and the number of hospitalizations, is uncertain. The aim of this study is to analyze the association between inflammatory biomarkers, neurocognitive functioning, and clinical outcomes in patients with BD in euthymia. As a hypothesis, we propose that higher levels of inflammatory biomarkers will be related to worse general and specific cognitive functioning and worse clinical outcomes.
2. Results
2.1. Descriptive Analysis
The sample included 109 participants, of whom 72 were women (66%). The mean age of the subjects was 47.4 ± 14 years. Table 1 presents the levels of inflammatory markers, ACER scores, and clinical variables.

Table 1.
Levels of inflammatory biomarkers, ACER scores, and clinical variables.
2.2. Bivariate Analysis
Significant, negative, and moderate correlations were found between IL-6 levels and ACER-T (r = −0.326; p = 0.0007), ACER-L (r = 0.307; p = 0.0015), ACER-VE (r = −0.322; p = 0.0008), and ACER-OA (r = −0.312; p = 0.0011). There was a significant, negative, weak correlation between IL-6 and ACER-M levels (r = −0.236; p = 0.0146). Also, IL-6 had a significant, positive, moderate correlation with NH (r = 0.399; p < 0.001). There were no significant correlations involving hs-CRP levels. Significant correlations were found between ACER-T, ACER-L, ACER-VE, and ACER-VF with clinical outcomes (Table 2).

Table 2.
Correlation between inflammatory biomarkers, ACER scores, and clinical variables.
When comparing the high and low neurocognitive performance groups, categorized according to the sample mean ACER-T (85.7 ± 9.2), no significant differences were found in hs-CRP or IL-6 levels (Table 3).

Table 3.
Comparison of inflammatory biomarker levels according to ACER-T.
Conversely, when comparing categorizing according to high or low levels of inflammation using the mean IL-6 (8.3 ± 28.4) as a cut-off point, no differences were found in neurocognition, but the NH was significantly higher in the group with high IL-6 levels (2.9 vs. 7.3; p = 0.001) (Table 4).

Table 4.
Comparison of ACER scores and clinical outcomes according to IL-6 level.
There were no differences in ACER scores or clinical outcomes when comparing subjects according to hs-CRP levels above or below the mean (0.2 ± 0.4) (Table 5).

Table 5.
Comparison of ACER scores and clinical outcomes according to hs-CRP level.
2.3. Linear Regression Models
Several regression models were analyzed to explore the influence of IL-6 and hs-CRP levels on neurocognitive functioning and clinical outcomes. Significant models were found to predict levels for ACER-T, ACER-L, ACER-VE, ACER-OA, ACER-M, and NH. Overall, inflammatory variables explained about 10% of the variation in neurocognitive dimensions and NH. IL-6 level was the significant variable in the models that reached statistical significance. An increase in IL-6 values was associated with a decrease in ACER-T, ACER-L, ACER-VE, ACER-OA, and ACER-M, and an increase in the NH (Table 6).

Table 6.
Regression models to predict ACER scores and clinical outcomes from IL-6 and hs-CRP levels.
Taken together, our results show that the sample had high levels of IL-6 and normal levels of hs-CRP. The group with higher-than-average IL-6 levels had a higher SA. This trend was corroborated in the correlation analysis, where the higher the IL-6 levels, the higher the average SA. Conversely, higher IL-6 levels correlated with worse neurocognitive indicators. Indeed, regression models verified that IL-6 levels predicted neurocognitive functioning and NH.
3. Discussion
Our study analyzed the associations between inflammatory biomarkers, neurocognitive functioning, and clinical outcomes in patients with BD type I in euthymia. Significant negative correlations were found between IL-6 levels and neurocognitive functioning, and positive correlations between IL-6 and clinical variables as NH. IL-6 levels significantly predicted total neurocognitive functioning and the dimensions of language, memory, orientation/attention, and visuospatial abilities.
There is sufficient evidence linking inflammation with neurocognitive alterations in mood disorders [36,37], particularly in major depressive disorder [34,38]. However, studies focused on BD are much scarcer, especially those analyzing IL-6 as an inflammatory biomarker. Also, most of the published evidence comes from cross-sectional designs, so they cannot evaluate causal hypotheses that clarify the temporal order of the biological phenomena, that is, whether an inflammatory state precedes a major mood disorder or whether it is this alteration that promotes inflammation.
A theoretical line of research suggests a potential association between IL-6 and cognitive dysfunction in patients with BD in euthymia. This association would indicate that persistently elevated levels of IL-6 generate an inflammatory environment that structurally alters specific brain regions, with an inverse correlation between peripheral inflammatory markers and brain region volume [39,40], which is associated with cognitive deficits [36,41]. In people with BD, some of the areas most sensitive to inflammation are the anterior cingulate cortex, amygdala, prefrontal cortex, hippocampus, and orbitofrontal cortex [40], all involved in the pathophysiology of BD. At the molecular level, IL-6 can inhibit cortical neurotransmitter release through a direct action on the presynaptic neuron [42]. There is also evidence showing that multiple mood episodes alter the homeostasis between inflammatory mechanisms, oxidative processes, and neuroprotective mechanisms, resulting in cognitive alterations [36].
In our study, elevated IL-6 (8.39 ± 28.4) and normal hs-CRP levels (0.24 ± 0.44) were found. This conclusion is variable in the published evidence, since it has been pointed out that CRP levels are higher in subjects with BD [21,22,23] or show no differences with the general population [43]. The systematic review by Bauer et al. [36] found a negative correlation between CRP levels and performance in language, memory, and attention tests in a sample of 107 people with BD. We found no significant correlations among ACER scores and hs-CRP. This lack of association may be partially explained due to the normal hs-CRP levels in our sample, but it is important to consider that IL-6 directly reflects the activity of cytokines, which are the main mediators and regulators of the inflammatory response, while hs-CRP is a more global marker of the acute-phase response, but does not indicate the specific cytokine or molecular pathways involved. In this line, the average IL-6 had a large dispersion in our sample. This may be due to different phenomena beyond psychiatric diagnosis, such as age, sex, genetic variants involved in IL-6 signaling, and concomitant diseases, among others [44]. These factors were not considered in the adjustment of the proposed models, a fact that constitutes a limitation of this study.
IL-6 is a pleiotropic cytokine capable of exerting divergent effects on cellular metabolism [45] and, specifically, on the immune system [46]. The most recently published GWAS of BD highlighted that the main hit was a variant of the gene encoding for the major histocompatibility complex [16]. In addition, individuals with major depressive disorder and BD have alterations of the compensatory immune-regulatory reflex system, a mechanism that regulates the primary immune response to contribute to recovery from the acute phase of the illness; among the alterations found in the reflex system are abnormalities of classical IL-6 signaling [47]. Based on this evidence and the results showing inflammatory alterations in patients with BD in euthymia [23,31,48], it could be suggested that a sustained low-grade inflammatory state may configure an endophenotype of the disease. Our results seem to support this hypothesis. In this line, some authors affirm that neuroinflammation constitutes an endophenotype in BD [3] and, more specifically, IL-6 levels [49]. From a clinical point of view, the immunomodulatory effect of mood stabilizers such as lithium [50] and lamotrigine [51], frequently used in the management of BD, is known. From a cellular perspective, inflammatory states contribute to telomere shortening and thus to aging, characterized by cognitive decline; in the opposite direction, telomeric shortening can induce low-grade inflammatory states, favoring a bidirectional process [52]. However, both telomeric shortening and an increase in inflammatory parameters in patients with BD are not consistently verified in unaffected first-degree relatives [53], so their validity as an endophenotype should be further studied.
The findings of this study demonstrate that an inflammatory state, as indicated by elevated IL-6 levels, significantly predicts a decrease in ACER-T values and the domains of memory, language, visuospatial abilities, and attention/orientation. These findings are consistent with those reported by Wiener et al. [54], where higher IL-6 levels correlated with more severe cognitive dysfunction in a sample of patients with BD. In the study by Barbosa et al. [55], IL-6 levels were found to significantly predict cognitive functioning in patients with BD in euthymia. In a similar study, Poletti et al. [56] employed an elastic net penalized regression on a sample of patients with BD; their findings indicated that higher IL-6 levels were associated with an increased likelihood of having poor cognitive performance. As mentioned above, the proposed mechanisms by which IL-6 levels alter neurocognitive functioning are multiple. Recently, studies of resting-state functional connectivity magnetic resonance imaging have suggested that immune dysregulation is involved in connectivity abnormalities in limbic and somatomotor networks that account for the neurocognitive alterations in BD. These alterations include structural modifications, such as reduced cortical thickness and subcortical volumes [18]. At the cellular level, IL-6 is known to modulate the process of neurogenesis throughout neurodevelopment. However, prolonged exposure at high levels may lead to reduced proliferation and increased neuronal apoptosis. This would be one of the main mechanisms underlying the alterations that occur in the aforementioned structures, all of which are involved in cognitive processes. However, the conclusions on the effects of IL-6 at the cellular level have been obtained from in vitro studies and animal models; therefore, it is necessary to deepen research in this area [57].
The published evidence analyzing the effect of inflammatory status on clinical outcomes in BD is even scarcer. The systematic review and meta-analysis by Miola et al. [58] included 21 studies and 7682 participants with a psychiatric diagnosis (7445 with mood disorders), finding a large association between CRP concentrations and suicidal ideation (SMD 1.145, 95% CI 0.273–2.018), and a medium association with suicide attempts (SMD 0.549, 95% CI 0.363–0.735). Our results did not corroborate a relevant role of hs-CRP in the prediction of SA, nor of neurocognitive functioning. On the other hand, IL-6 levels have received more attention as a biomarker of suicidal behavior [59]. Indeed, the meta-analysis by González-Castro et al. [60] (n = nine studies) showed that suicidal behaviors were associated with higher levels of IL-6 in serum and cerebrospinal fluid, while the meta-regression analysis indicated an association between plasma and serum IL-6 levels with male sex. These conclusions are not concordant with our findings, since, in our study, IL-6 levels were significantly associated with a higher NH but not SA. However, it is important to consider that the results of Miola et al. [58] and González-Castro et al. [60] incorporate samples with a wide variety of psychiatric diagnoses, so they are not directly comparable with our sample. Inflammatory burden has been clearly recognized as a preponderant factor in immune-mediated diseases [61], but in psychiatric conditions, this relationship has been scarcely studied. In unipolar depression, a correlation between inflammatory burden and depressive outcomes has been corroborated [62], including hospital variables such as those observed in our results. In BD, Queissner et al. [63] concluded that individuals who were exposed to a higher level of inflammation over time suffered from more manic symptoms in this period. This finding allows us to indirectly hypothesize that people with higher levels of inflammation would require a greater NH. However, the relationship between inflammation and NH is still statistical and requires further theoretical understanding. Despite its biological plausibility, the clinical utility of pharmacological targets that seek to modulate the IL-6-mediated inflammatory pathway is a matter of debate. To date, there are few clinical trials with highly variable results regarding the pro-cognitive effect of IL-6 modulators; most of these studies have been conducted in samples of patients with Alzheimer’s disease [64], making direct extrapolation to our population of interest difficult. In parallel, other interventions of a non-pharmacological nature, such as dietary interventions, have received some degree of interest as modulators of the IL-6-related inflammatory pathway, but evidence is still scarce [65].
Among the limitations of this study, its cross-sectional design does not allow us to establish a causal hypothesis regarding the phenomena studied. The sample size was limited, and there was a disparity between the proportion of men and women. We did not incorporate a control group constituted by participants without BD, an aspect to be considered in future designs; however, we focused on the concept of “intra-group differences,” emphasizing the analysis of the clinical heterogeneity that results from the classification based on the traditional diagnostic categories. Regarding the measurement of neurocognition, ACER offers a first global assessment, so other instruments can complement a more in-depth evaluation of this construct. Additionally, there is great heterogeneity in the instruments used to measure neurocognition, which makes it difficult to compare our own results. We also did not have measurements of inflammation and cognition in active mood phases of the disease, nor did we control for factors that may have modified the results, such as the use of antipsychotics, the presence of chronic diseases related to inflammation, and body weight. On the other hand, the regression models explained only a small proportion of the variance of the cognitive and clinical outcomes, which could be since other relevant variables were not considered, especially clinical variables. Nevertheless, there is sufficient evidence from in vitro analyses, in vivo models, clinical studies, and meta-analyses to support that inflammation has a significant impact on clinically meaningful outcomes. Our results allow a detailed analysis of different dimensions of neurocognition and its relationship with inflammatory biomarkers, not always present in the published evidence, where neurocognition is mostly reported as a global outcome.
4. Materials and Methods
4.1. Design
We developed an exploratory, analytical, cross-sectional study from the Ríos cohort [66]. Our study presents a methodological design similar to that used in other studies with comparable objectives and samples [20,67,68].
4.2. Participants
We included adults (18 to 65 years old) with a diagnosis of BD type I according to DSM-IV-TR [69] in euthymia for three months or more, assessed with the Young Mania Rating Scale (≤6 points) [70] and the Hamilton Depression Rating Scale (≤6 points) [71]. Subjects with active substance use or who had received electroconvulsive therapy during the previous three months were excluded. We did not exclude patients with medical comorbidities or use of specific drugs.
4.3. Instruments
The Addenbrooke’s Cognitive Examination Revised (ACER) was administered to assess neurocognitive performance. This instrument is designed to detect different types of neurocognitive disorders, and its performance would not be affected by mood symptoms or mood disorders. It evaluates five cognitive domains: orientation/attention (18 points), memory (26 points), verbal fluency (14 points), language (26 points), and visuospatial abilities (16 points). We used the version of the instrument validated in the Chilean population [72].
4.4. Clinical Variables
Three clinical variables of relevance in the course of BD were analyzed: age of disease onset (according to age at diagnosis), number of suicide attempts (SA), and number of hospitalizations (NH). Data were collected from the clinical records of each patient.
4.5. Inflammatory Variables
Inflammatory analyses were conducted at the Toxicology Laboratory Universidad de Valparaíso. Quantitative determination of IL-6 and hs-CRP in serum was performed using peripheral venous blood samples (forearm vein) by standard venipuncture. All blood samples were collected between 07:00 and 09:00 A.M. into vacuum blood-collecting tubes containing 1 mg/mL of anticoagulant ethylenediaminetetraacetic acid (EDTA), and the samples were obtained by centrifugation immediately for 10 min at 3500 rpm.
4.5.1. hs-CRP
The determination and quantification of hs-CRP was performed using BN ProSpec® (Erlangen, Germany) automated equipment, and the reagent used for this measurement was CardioPhase® (Erlangen, Germany) hs-CRP. The technique used was immunonephelometry with intensifying particles, which consists of the use of polystyrene particles coated with a specific monoclonal antibody against human CRP, which, when mixed with samples containing CRP, form aggregates that scatter the incident light beam. The intensity of the scattered light depends on the concentration of the corresponding protein in the sample. The final titration is made by comparison with a standard curve of known concentration, with the lower limit of this curve being the sensitivity (LOD) of the assay, which typically corresponds to 0.0175 mg/dL. The following reference values were used according to the manufacturer’s instructions for CardioPhase® hs-CRP with BN ProSpec® equipment: <0.10 mg/L (low risk), 0.10–0.30 mg/L (moderate risk), >0.30 mg/L (high risk), and >1.00 mg/L (very high risk).
4.5.2. IL-6
Quantitative determination of IL-6 was performed through a quantitative enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Quantikine, R&D Systems, Minneapolis, MN, USA), taking IL-6 < 3.5 pg/mL as the reference value according to the manufacturers’ instructions. Briefly, this assay employs the quantitative sandwich enzyme immunoassay technique, in which the microplate is pre-coated with a monoclonal antibody specific for human IL-6. A total of 100 μL of standards or the plasma sample was added to each well and incubated for 2 h at RT, and any IL-6 present was bound by the immobilized antibody. To construct a calibration curve, we diluted Human IL-6 Standard with the 1× Assay diluent to 300, 100, 50, 25, 12.5, 6.25, 3.13, and 0 pg/mL and used them as standard samples. After washing away any unbound substances, 200 μL of human IL-6 conjugate (enzyme-linked polyclonal antibody specific for human IL-6) was added to the wells, followed by a 2 h incubation at room temperature. Then, 200 μL of substrate solution was added to each well and incubated for 20 min at RT, with the color developing in proportion to the amount of IL-6 bound. The color development is stopped with 50 μL of the stop solution, and the intensity of the color is measured. Absorbance was interpolated from a calibration curve. The sensitivity was 0.7 pg/mL in a range of 0–300 pg/mL with intra-assay and inter-assay coefficients from 4.2% to 7%, respectively. There were no undetectable values. All samples were over the detection limit [73].
4.6. Statistical Analysis
For descriptive statistics, absolute numbers, proportions, and means (standard deviations) were used. For inferential statistics, a correlation matrix was performed to analyze the associations between IL-6, hs-CRP, ACER scores, and clinical variables (Pearson correlation test). IL-6 and hs-CRP means were compared according to high or low neurocognitive performance categorized according to the ACER-T mean (Student’s t-test); likewise, the means of ACER scores were compared according to groups divided by values below or above the mean of IL-6 and hs-CRP. Multiple linear regression models were performed to explore the influence of inflammation variables on neurocognitive performance and clinical outcomes. A significance level of 5% was used. Data were analyzed in Stata 17 (Statacorp, College Station, TX, USA).
5. Conclusions
The findings of this study support the hypothesis that IL-6 levels correlate inversely with cognitive functioning in patients with euthymic BD, allowing prediction of dimensions such as memory, language, visuospatial abilities, and orientation/attention. In consideration of the clinical outcomes, elevated IL-6 levels were associated with an increase in the NH (Figure 1). Different mechanisms related to the effect of I-L6 on central brain structures could be involved, such as the inflammatory effect on telomere structure, dysfunction at the synaptic level, activation of apoptotic pathways, and morphofunctional impact on specific regions. All these mechanisms have been linked to the pathophysiology of BD. Nevertheless, BD poses the challenge of studying biological mechanisms by means of biomarkers of a different nature from a fluctuating perspective according to the mood phase considered. Studies employing prospective designs, incorporating neurocognition and inflammation measurements from an evolutionary perspective, and at different mood stages of the disease, will help to elucidate the causality hypotheses discussed in this article. More importantly, these studies will enable the development of more precise interventions that extend beyond the recovery from euthymia.
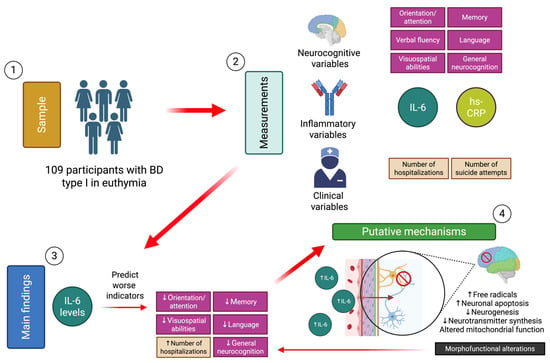
Figure 1.
Synthesis of the study methods, main findings, and proposed mechanisms [74]. Down arrows symbolize a decrease and up arrows an increase.
Author Contributions
Conceptualization, U.R. and M.A.; methodology, U.R., S.P., C.M., P.R.M. and M.A.; formal analysis, U.R., P.R.M. and M.A.; investigation, U.R., S.P., C.M., P.R.M. and M.A.; resources, U.R.; data curation, U.R., P.R.M. and M.A.; writing—original draft preparation, U.R., S.P., C.M., P.R.M. and M.A.; writing—review and editing, U.R., S.P., C.M., P.R.M. and M.A.; visualization, U.R., S.P., C.M. and M.A.; supervision, U.R. and M.A.; project administration, U.R. and M.A.; funding acquisition, U.R., P.R.M. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID Chile, FONDECYT Regular Grant Nº 1231012 (P.R.M) and Universidad de Valparaíso. CIDI Grant Nº 003 “Centro de Estudios Traslacionales en Estrés y Salud Mental (C-ESTRES)”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical-Scientific Committee of the Valparaíso-San Antonio Health Service (04/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BD | Bipolar disorder |
| hs-CRP | High-sensitivity C-reactive protein |
| IL-6 | Interleukin-6 |
| ACER-T | Addenbrooke’s Cognitive Examination Revised—Total |
| ACER-L | Addenbrooke’s Cognitive Examination Revised—Language |
| ACER-VE | Addenbrooke’s Cognitive Examination Revised—Visuospatial abilities |
| ACER-OA | Addenbrooke’s Cognitive Examination Revised—Orientation/attention |
| ACER-M | Addenbrooke’s Cognitive Examination Revised—Memory |
| ACER-VF | Addenbrooke’s Cognitive Examination Revised—Verbal fluency |
| TE | Time of evolution |
| NH | Number of hospitalizations |
| SA | Number of suicide attempts |
| GWAS | Genome-wide association study |
| SMD | Standardized mean difference |
| CI | Confidence interval |
References
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar Disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Gordovez, F.J.A.; McMahon, F.J. The Genetics of Bipolar Disorder. Mol. Psychiatry 2020, 25, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, R.; Miskowiak, K.W.; Hasler, G. Evaluating Endophenotypes for Bipolar Disorder. Int. J. Bipolar. Disord. 2021, 9, 17. [Google Scholar] [CrossRef]
- Baena-Oquendo, S.; Valencia, J.G.; Vargas, C.; López-Jaramillo, C. Neuropsychological Aspects of Bipolar Disorder. Rev. Colomb. Psiquiatr. 2022, 51, 218–226. [Google Scholar] [CrossRef]
- Bourne, C.; Aydemir, O.; Balanzá-Martínez, V.; Bora, E.; Brissos, S.; Cavanagh, J.T.O.; Clark, L.; Cubukcuoglu, Z.; Dias, V.V.; Dittmann, S.; et al. Neuropsychological Testing of Cognitive Impairment in Euthymic Bipolar Disorder: An Individual Patient Data Meta-Analysis. Acta. Psychiatr. Scand. 2013, 128, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Özerdem, A. Meta-Analysis of Longitudinal Studies of Cognition in Bipolar Disorder: Comparison with Healthy Controls and Schizophrenia. Psychol. Med. 2017, 47, 2753–2766. [Google Scholar] [CrossRef]
- Ehrlich, T.J.; Ryan, K.A.; Burdick, K.E.; Langenecker, S.A.; McInnis, M.G.; Marshall, D.F. Cognitive Subgroups and Their Longitudinal Trajectories in Bipolar Disorder. Acta. Psychiatr. Scand. 2022, 146, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Simjanoski, M.; McIntyre, A.; Kapczinski, F.; de Azevedo Cardoso, T. Cognitive Impairment in Bipolar Disorder in Comparison to Mild Cognitive Impairment and Dementia: A Systematic Review. Trends Psychiatry Psychother. 2022, 44, e20210300. [Google Scholar] [CrossRef]
- Douglas, K.M.; Gallagher, P.; Robinson, L.J.; Carter, J.D.; McIntosh, V.V.W.; Frampton, C.M.A.; Watson, S.; Young, A.H.; Ferrier, I.N.; Porter, R.J. Prevalence of Cognitive Impairment in Major Depression and Bipolar Disorder. Bipolar Disord. 2018, 20, 260–274. [Google Scholar] [CrossRef]
- Martino, D.J. Neurodevelopment as an Alternative to Neuroprogression to Explain Cognitive Functioning in Bipolar Disorder. Psychol. Med. 2024, 54, 4469–4474. [Google Scholar] [CrossRef]
- Strejilevich, S.A.; Samamé, C.; Martino, D.J. The Trajectory of Neuropsychological Dysfunctions in Bipolar Disorders: A Critical Examination of a Hypothesis. J. Affect. Disord. 2015, 175, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.J.; Strejilevich, S.A.; Manes, F. Neurocognitive Functioning in Early-Onset and Late-Onset Older Patients with Euthymic Bipolar Disorder. Int. J. Geriatr. Psychiatry 2013, 28, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Konarski, J.Z.; McIntyre, R.S.; Kennedy, S.H.; Rafi-Tari, S.; Soczynska, J.K.; A Ketter, T. Volumetric Neuroimaging Investigations in Mood Disorders: Bipolar Disorder versus Major Depressive Disorder. Bipolar Disord. 2008, 10, 1–37. [Google Scholar] [CrossRef]
- Sæther, L.S.; Ueland, T.; Haatveit, B.; Maglanoc, L.A.; Szabo, A.; Djurovic, S.; Aukrust, P.; Roelfs, D.; Mohn, C.; Ormerod, M.B.E.G.; et al. Inflammation and Cognition in Severe Mental Illness: Patterns of Covariation and Subgroups. Mol. Psychiatry 2022, 28, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Brietzke, E.; Mansur, R.B.; Maruschak, N.A.; Lee, Y.; McIntyre, R.S. Inflammation as a Neurobiological Substrate of Cognitive Impairment in Bipolar Disorder: Evidence, Pathophysiology and Treatment Implications. J. Affect. Disord. 2015, 188, 149–159. [Google Scholar] [CrossRef]
- Mullins, N.; Forstner, A.J.; O’Connell, K.S.; Coombes, B.; Coleman, J.R.I.; Qiao, Z.; Als, T.D.; Bigdeli, T.B.; Børte, S.; Bryois, J.; et al. Genome-Wide Association Study of More than 40,000 Bipolar Disorder Cases Provides New Insights into the Underlying Biology. Nat. Genet. 2021, 53, 817–829. [Google Scholar] [CrossRef]
- Leboyer, M.; Soreca, I.; Scott, J.; Frye, M.; Henry, C.; Tamouza, R.; Kupfer, D.J. Can Bipolar Disorder Be Viewed as a Multi-System Inflammatory Disease? J. Affect. Disord. 2012, 141, 1–10. [Google Scholar] [CrossRef]
- Altamura, M.; Leccisotti, I.; Mollica, A.; Maddalena, S.; Altamura, C.; Moretti, M.; Bellomo, A. Inflammatory biomarkers, cognitive functioning, and brain imaging abnormalities in bipolar disorder: A systematic review. Clin. Neuropsychiatry 2024, 21, 32–62. [Google Scholar]
- Cunha, Â.B.; Andreazza, A.C.; Gomes, F.A.; Frey, B.N.; Da Silveira, L.E.; Gonçalves, C.A.; Kapczinski, F. Investigation of Serum High-Sensitive C-Reactive Protein Levels across All Mood States in Bipolar Disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 300–304. [Google Scholar] [CrossRef]
- Chang, H.H.; Wang, T.Y.; Lee, I.H.; Lee, S.Y.; Chen, K.C.; Huang, S.Y.; Yang, Y.K.; Lu, R.B.; Chen, P.S. C-Reactive Protein: A Differential Biomarker for Major Depressive Disorder and Bipolar II Disorder. World J. Biol. Psychiatry 2017, 18, 63–70. [Google Scholar] [CrossRef]
- Modabbernia, A.; Taslimi, S.; Brietzke, E.; Ashrafi, M. Cytokine Alterations in Bipolar Disorder: A Meta-Analysis of 30 Studies. Biol. Psychiatry 2013, 74, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Dargél, A.A.; Godin, O.; Kapczinski, F.; Kupfer, D.J.; Leboyer, M. C-Reactive Protein Alterations in Bipolar Disorder: A Meta-Analysis. J. Clin. Psychiatry 2015, 76, 142–150. [Google Scholar] [CrossRef]
- Rowland, T.; Perry, B.I.; Upthegrove, R.; Barnes, N.; Chatterjee, J.; Gallacher, D.; Marwaha, S. Neurotrophins, Cytokines, Oxidative Stress Mediators and Mood State in Bipolar Disorder: Systematic Review and Meta-Analyses. Br. J. Psychiatry 2018, 213, 514–525. [Google Scholar] [CrossRef]
- Dickerson, F.; Stallings, C.; Origoni, A.; Vaughan, C.; Khushalani, S.; Yang, S.; Yolken, R. C-Reactive Protein Is Elevated in Schizophrenia. Schizophr. Res. 2013, 143, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Evers, A.K.; Veeh, J.; McNeill, R.; Reif, A.; Kittel-Schneider, S. C-Reactive Protein Concentration in Bipolar Disorder: Association with Genetic Variants. Int. J. Bipolar Disord. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Stallings, C.; Origoni, A.; Boronow, J.; Yolken, R. Elevated Serum Levels of C-Reactive Protein Are Associated with Mania Symptoms in Outpatients with Bipolar Disorder. Prog Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 952–955. [Google Scholar] [CrossRef]
- Wadee, A.A.; Kuschke, R.H.; Wood, L.A.; Berk, M.; Ichim, L.; Maes, M. Serological Observations in Patients Suffering from Acute Manic Episodes. Hum. Psychopharmacol. 2002, 17, 175–179. [Google Scholar] [CrossRef]
- Hsuchou, H.; Kastin, A.J.; Mishra, P.K.; Pan, W. C-Reactive Protein Increases BBB Permeability: Implications for Obesity and Neuroinfammation. Cell. Physiol. Biochem. 2012, 30, 1109–1119. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune Modulation of Learning, Memory, Neural Plasticity and Neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Berk, M.; Kapczinski, F.; Andreazza, A.C.; Dean, O.M.; Giorlando, F.; Maes, M.; Yücel, M.; Gama, C.S.; Dodd, S.; Dean, B.; et al. Pathways Underlying Neuroprogression in Bipolar Disorder: Focus on Inflammation, Oxidative Stress and Neurotrophic Factors. Neurosci. Biobehav. Rev. 2011, 35, 804–817. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder and Depression. In Molecular Psychiatry; Nature Publishing Group: London, UK, 2016; Volume 21, pp. 1696–1709. [Google Scholar]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis—Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Rose-John, S. Dissecting Interleukin-6 Classic- and Trans-Signaling in Inflammation and Cancer. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2018; Volume 1725, pp. 127–140. [Google Scholar]
- Fourrier, C.; Singhal, G.; Baune, B.T. Neuroinflammation and Cognition across Psychiatric Conditions. CNS Spectr. 2019, 24, 4–15. [Google Scholar] [CrossRef]
- Sundaresh, A.; Oliveira, J.E.; Chinnadurai, R.K.; Rajkumar, R.P.; Hani, L.; Krishnamoorthy, R.; Leboyer, M.; Singh Negi, V.; Tamouza, R.; Negi, V.S. IL6/IL6R Genetic Diversity and Plasma IL6 Levels in Bipolar Disorder: An Indo-French Study. Heliyon 2019, 5, e01124. [Google Scholar] [CrossRef]
- Bauer, I.E.; Pascoe, M.C.; Wollenhaupt-Aguiar, B.; Kapczinski, F.; Soares, J.C. Inflammatory Mediators of Cognitive Impairment in Bipolar Disorder. J. Psychiatr. Res. 2014, 56, 18–27. [Google Scholar] [CrossRef]
- Morrens, M.; Overloop, C.; Coppens, V.; Loots, E.; Van Den Noortgate, M.; Vandenameele, S.; Leboyer, M.; De Picker, L. The Relationship between Immune and Cognitive Dysfunction in Mood and Psychotic Disorder: A Systematic Review and a Meta-Analysis. Mol. Psychiatry 2022, 27, 3237–3246. [Google Scholar] [CrossRef] [PubMed]
- Brietzke, E.; Stertz, L.; Fernandes, B.S.; Kauer-Sant’Anna, M.; Mascarenhas, M.; Escosteguy Vargas, A.; Chies, J.A.; Kapczinski, F. Comparison of Cytokine Levels in Depressed, Manic and Euthymic Patients with Bipolar Disorder. J. Affect. Disord. 2009, 116, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Saccaro, L.F.; Crokaert, J.; Perroud, N.; Piguet, C. Structural and Functional MRI Correlates of Inflammation in Bipolar Disorder: A Systematic Review. J. Affect. Disord. 2023, 325, 83–92. [Google Scholar] [CrossRef]
- Long, J.Y.; Li, B.; Ding, P.; Mei, H.; Li, Y. Correlations between Multimodal Neuroimaging and Peripheral Inflammation in Different Subtypes and Mood States of Bipolar Disorder: A Systematic Review. Int. J. Bipolar. Disord. 2024, 12, 5. [Google Scholar] [CrossRef]
- Strawbridge, R.; Carter, R.; Saldarini, F.; Tsapekos, D.; Young, A.H. Inflammatory Biomarkers and Cognitive Functioning in Individuals with Euthymic Bipolar Disorder: Exploratory Study. BJPsych Open 2021, 7, e126. [Google Scholar] [CrossRef]
- D’arcangelo, G.; Tancredi, V.; Onofri, F.; D’antuono, M.; Giovedõ, S.; Benfenati, F. Interleukin-6 Inhibits Neurotransmitter Release and the Spread of Excitation in the Rat Cerebral Cortex. Eur. J. Neurosci. 2000, 12, 1241–1252. [Google Scholar] [CrossRef]
- Knight, E.L.; Engeland, C.G.; Yocum, A.K.; Abu-Mohammad, A.; Bertram, H.; Vest, E.; McInnis, M.G.; Saunders, E.F.H. Heightened Inflammation in Bipolar Disorder Occurs Independent of Symptom Severity and Is Explained by Body Mass Index. Brain. Behav. Immun. Health 2023, 29, 100613. [Google Scholar] [CrossRef] [PubMed]
- Coe, C.L.; Love, G.D.; Karasawa, M.; Kawakami, N.; Kitayama, S.; Markus, H.R.; Tracy, R.P.; Ryff, C.D. Population Differences in Proinflammatory Biology: Japanese Have Healthier Profiles than Americans. Brain. Behav. Immun. 2010, 25, 494. [Google Scholar] [CrossRef]
- Ziegler, L.; Gajulapuri, A.; Frumento, P.; Bonomi Sc, A.M.; Wallén, H.; de Faire, U.; Rose-John, S.; Gigante, B. Interleukin 6 Trans-Signalling and Risk of Future Cardiovascular Events. Cardiovasc. Res. 2018, 115, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Saavedra Ramírez, P.G.; María Vásquez Duque, G.; Alonso González Naranjo, L. Interleucina-6: ¿amiga o Enemiga? Bases Para Comprender Su Utilidad Como Objetivo Terapéutico. Iatreia 2011, 24, 157–166. [Google Scholar] [CrossRef]
- Maes, M.; Carvalho, A.F. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol. Neurobiol. 2018, 55, 8885–8903. [Google Scholar] [CrossRef] [PubMed]
- Satizabal, C.L.; Zhu, Y.C.; Mazoyer, B.; Dufouil, C.; Tzourio, C. Circulating IL-6 and CRP Are Associated with MRI Findings in the Elderly: The 3C-Dijon Study. Neurology 2012, 78, 720–727. [Google Scholar] [CrossRef]
- Solmi, M.; Suresh Sharma, M.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral Levels of C-Reactive Protein, Tumor Necrosis Factor-α, Interleukin-6, and Interleukin-1β across the Mood Spectrum in Bipolar Disorder: A Meta-Analysis of Mean Differences and Variability. Brain. Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef]
- Sakrajda, K.; Szczepankiewicz, A. Inflammation-Related Changes in Mood Disorders and the Immunomodulatory Role of Lithium. Int. J. Mol. Sci. 2021, 22, 1532. [Google Scholar] [CrossRef]
- de Miranda, A.S.; de Miranda, A.S.; Teixeira, A.L. Lamotrigine as a Mood Stabilizer: Insights from the Pre-Clinical Evidence. Expert Opin. Drug Discov. 2019, 14, 179–190. [Google Scholar] [CrossRef]
- Squassina, A.; Pisanu, C.; Vanni, R. Mood Disorders, Accelerated Aging, and Inflammation: Is the Link Hidden in Telomeres? Cells 2019, 8, 52. [Google Scholar] [CrossRef]
- Vasconcelos-Moreno, M.P.; Fries, G.R.; Gubert, C.; Dos Santos, B.T.M.Q.; Fijtman, A.; Sartori, J.; Ferrari, P.; Grun, L.K.; Parisi, M.M.; Guma, F.T.C.R.; et al. Telomere Length, Oxidative Stress, Inflammation and BDNF Levels in Siblings of Patients with Bipolar Disorder: Implications for Accelerated Cellular Aging. Int. J. Neuropsychopharmacol. 2017, 20, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wiener, C.D.; Moreira, F.P.; Cardoso, T.A.; Mondin, T.C.; da Silva Magalhães, P.V.; Kapczinski, F.; de Mattos Souza, L.D.; da Silva, R.A.; Oses, J.P.; Jansen, K. Inflammatory Cytokines and Functional Impairment in Drug-Free Subjects with Mood Disorder. J. Neuroimmunol. 2017, 307, 33–36. [Google Scholar] [CrossRef]
- Barbosa, I.G.; de Almeida, R.F.; Rocha, N.P.; Mol, G.C.; da Mata Chiaccjio Leite, F.; Bauer, I.E.; Teixeira, A.L. Predictors of Cognitive Performance in Bipolar Disorder: The Role of Educational Degree and Inflammatory Markers. J. Psychiatr. Res. 2018, 106, 31–37. [Google Scholar] [CrossRef]
- Poletti, S.; Mazza, M.G.; Calesella, F.; Vai, B.; Lorenzi, C.; Manfredi, E.; Colombo, C.; Zanardi, R.; Benedetti, F. Circulating Inflammatory Markers Impact Cognitive Functions in Bipolar Depression. J. Psychiatr. Res. 2021, 140, 110–116. [Google Scholar] [CrossRef]
- Bradburn, S.; Sarginson, J.; Murgatroyd, C.A. Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-Demented Adults: A Meta-Analysis of Prospective Studies. Front. Aging Neurosci. 2018, 9, 438. [Google Scholar] [CrossRef]
- Miola, A.; Dal Porto, V.; Tadmor, T.; Croatto, G.; Scocco, P.; Manchia, M.; Carvalho, A.F.; Maes, M.; Vieta, E.; Sambataro, F.; et al. Increased C-Reactive Protein Concentration and Suicidal Behavior in People with Psychiatric Disorders: A Systematic Review and Meta-Analysis. Acta Psychiatr. Scand. 2021, 144, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kim, J.W.; Kim, S.W.; Han, J.S.; Lyoo, I.K.; Kim, J.M. Peripheral Markers of Suicidal Behavior: Current Findings and Clinical Implications. Clin. Psychopharmacol. Neurosci. 2023, 21, 650–664. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Tovilla-Zárate, C.A.; López-Narváez, M.L.; Genis-Mendoza, A.D.; Juárez-Rojop, I.E. Interleukin-6 Levels in Serum, Plasma, and Cerebral Spinal Fluid in Individuals with Suicide Behavior: Systematic Review and Meta-Analysis with Meta-Regression. J. Interferon Cytokine Res. 2021, 41, 258–267. [Google Scholar] [CrossRef]
- Wu, D.; Jin, Y.; Xing, Y.; Abate, M.D.; Abbasian, M.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abd-Allah, F.; Abdelmasseh, M.; Abdollahifar, M.A.; et al. Global, Regional, and National Incidence of Six Major Immune-Mediated Inflammatory Diseases: Findings from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 64, 102193. [Google Scholar] [CrossRef]
- Shao, X.; Xie, Z.; Zhu, L. Inflammatory Burden Index Is Correlated with Increased Depression: A Population-Based Study. BMC Psychiatry 2025, 25, 306. [Google Scholar] [CrossRef]
- Queissner, R.; Fellendorf, F.T.; Dalkner, N.; Bengesser, S.A.; Maget, A.; Birner, A.; Platzer, M.; Reininghaus, B.; Häussl, A.; Schönthaler, E.; et al. The Influence of Chronic Inflammation on the Illnesscourse of Bipolar Disorder: A Longitudinal Study. J. Psychiatr. Res. 2024, 174, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Lyra e Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.D.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-Inflammatory Interleukin-6 Signaling Links Cognitive Impairments and Peripheral Metabolic Alterations in Alzheimer’s Disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- Mekhora, C.; Lamport, D.J.; Spencer, J.P.E. An Overview of the Relationship between Inflammation and Cognitive Function in Humans, Molecular Pathways and the Impact of Nutraceuticals. Neurochem Int. 2024, 181, 105900. [Google Scholar] [CrossRef] [PubMed]
- Ríos, U. Evaluación de Un Modelo de Interacción Gen-Ambiente En Pacientes Con Trastorno Bipolar Tipo I En Eutimia: Asociación Entre Maltrato Infantil y Cognición Social, y Moderación de Polimorfismos Genéticos. Ph.D. Thesis, Pontificia Universidad Católica de Chile, Santiago, Chile, 2020. [Google Scholar]
- Bagarić, T.; Mihaljević-Peleš, A.; Skočić Hanžek, M.; Živković, M.; Kozmar, A.; Rogić, D. Serum Levels of Zinc, Albumin, Interleukin-6 and CRP in Patients with Unipolar and Bipolar Depression: Cross Sectional Study. Curr. Issues Mol. Biol. 2024, 46, 4533–4550. [Google Scholar] [CrossRef]
- Ozkaya, A.L.; Gürbüzer, N.; Tozoğlu, E.Ö.; Akyildirim, S.; Mercantepe, F. Serum Galectin-3 and IL-6 as Inflammatory Markers in Bipolar Disorder: Insights from Manic and Euthymic Episodes. J. Clin. Med. 2025, 14, 803. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR; American Psychiatric Association: Washington, DC, USA, 2000; ISBN 9780890423349. [Google Scholar]
- Young, R.; Biggs, J.; Ziegler, V.; Meyer, D. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Hamilton, R. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Muñoz-Neira, C.; Henríquez, F.; Ihnen, J.; Sánchez, M.; Flores, P.; Slachevsky, A. Psychometric Properties and Diagnostic Usefulness of the Addenbrooke’s Cognitive Examination-Revised in a Chilean Elderly Sample. Rev. Med. Chil. 2012, 140, 1006–1013. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma Concentration of Interleukin-6 and the Risk of Future Myocardial Infarction among Apparently Healthy Men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Arancibia, M. Created in BioRender. Available online: https://BioRender.com/lbr5yv1 (accessed on 25 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).