Long-Term Hypoxia Upregulates Wnt and TGFβ1 Signaling in Eccrine Sweat Gland Cells In Vitro
Abstract
1. Introduction
2. Results
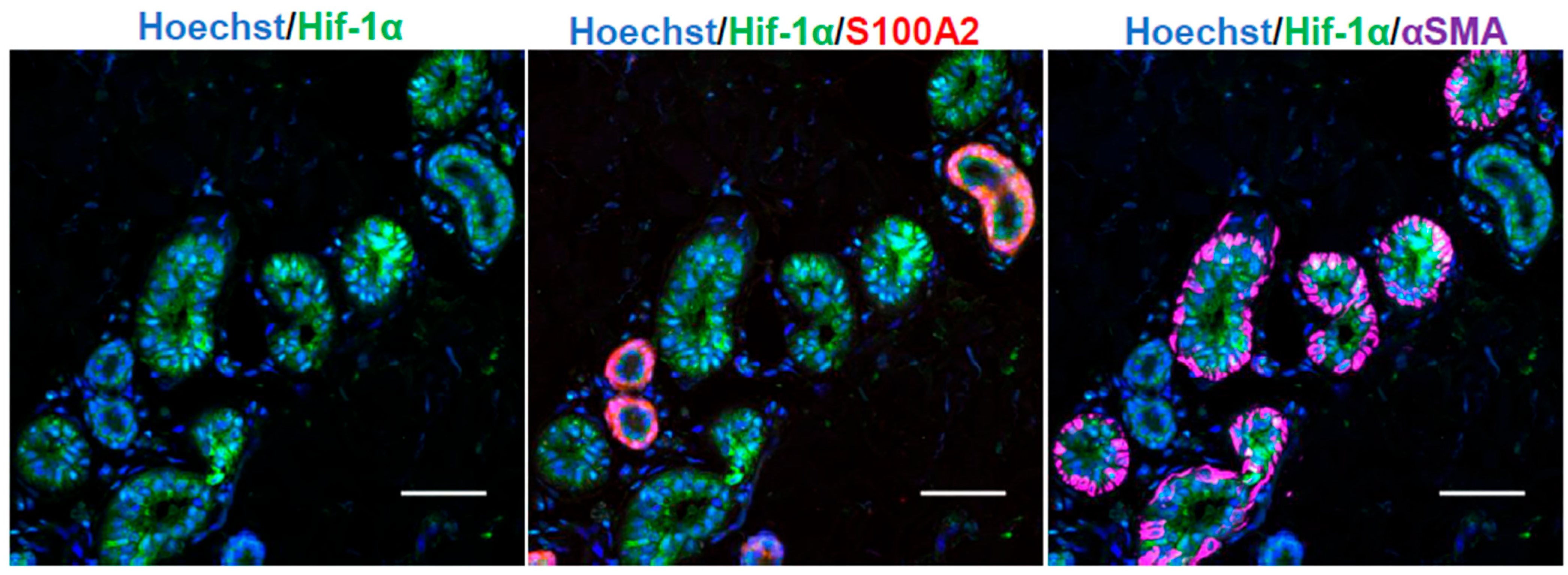
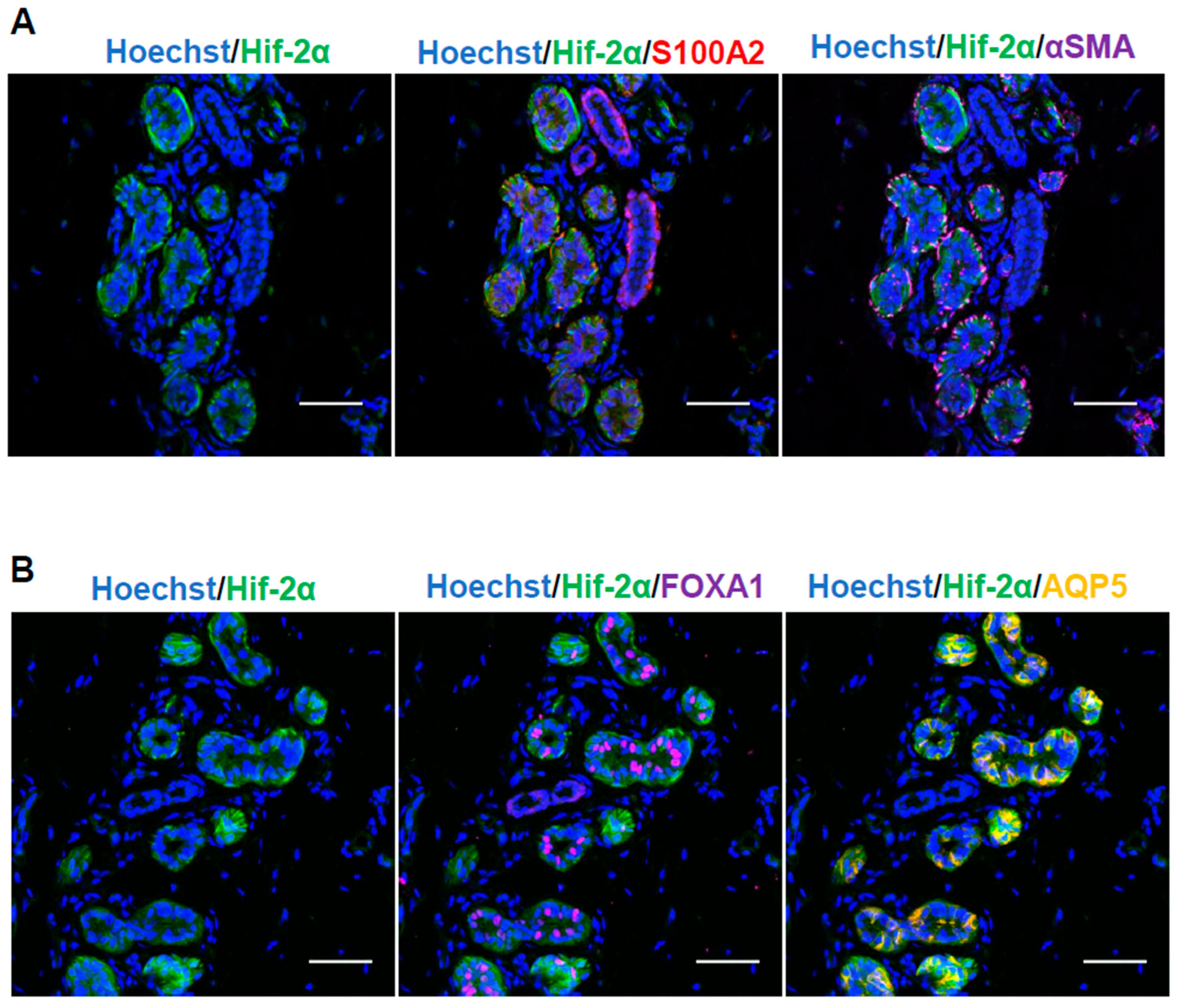
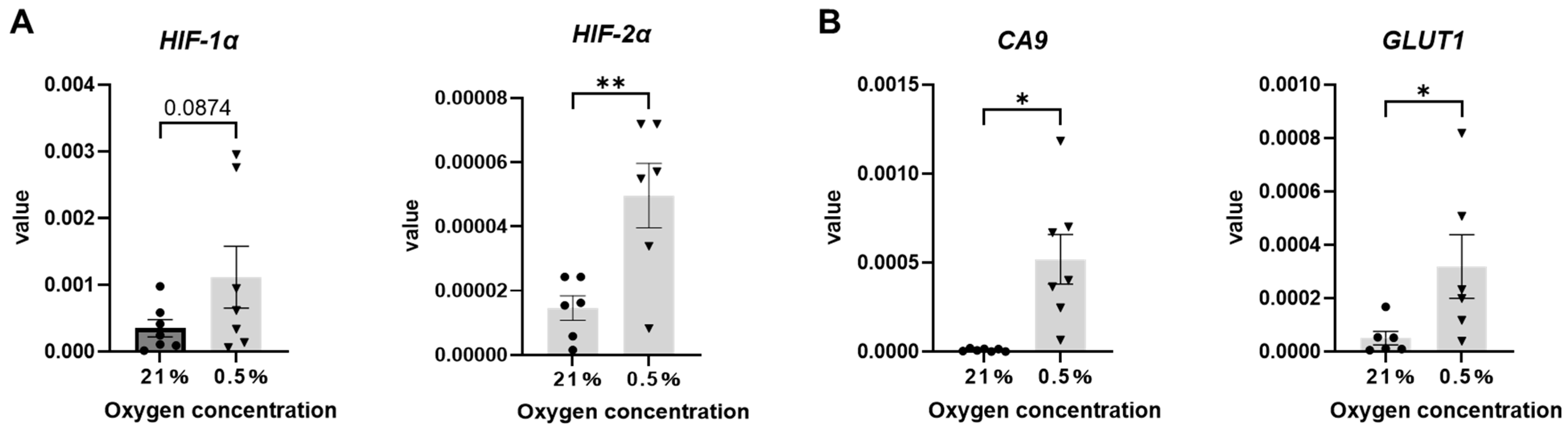
2.1. Human Sweat Glands and Primary Cultured Sweat Gland Cells Express HIF-1α and HIF-2α Under Hypoxia
2.2. Hypoxic Culture Enhances Myoepithelial Cell Marker Expression as Well as Stem Cell Maintenance-Associated Markers in Sweat Gland Cells
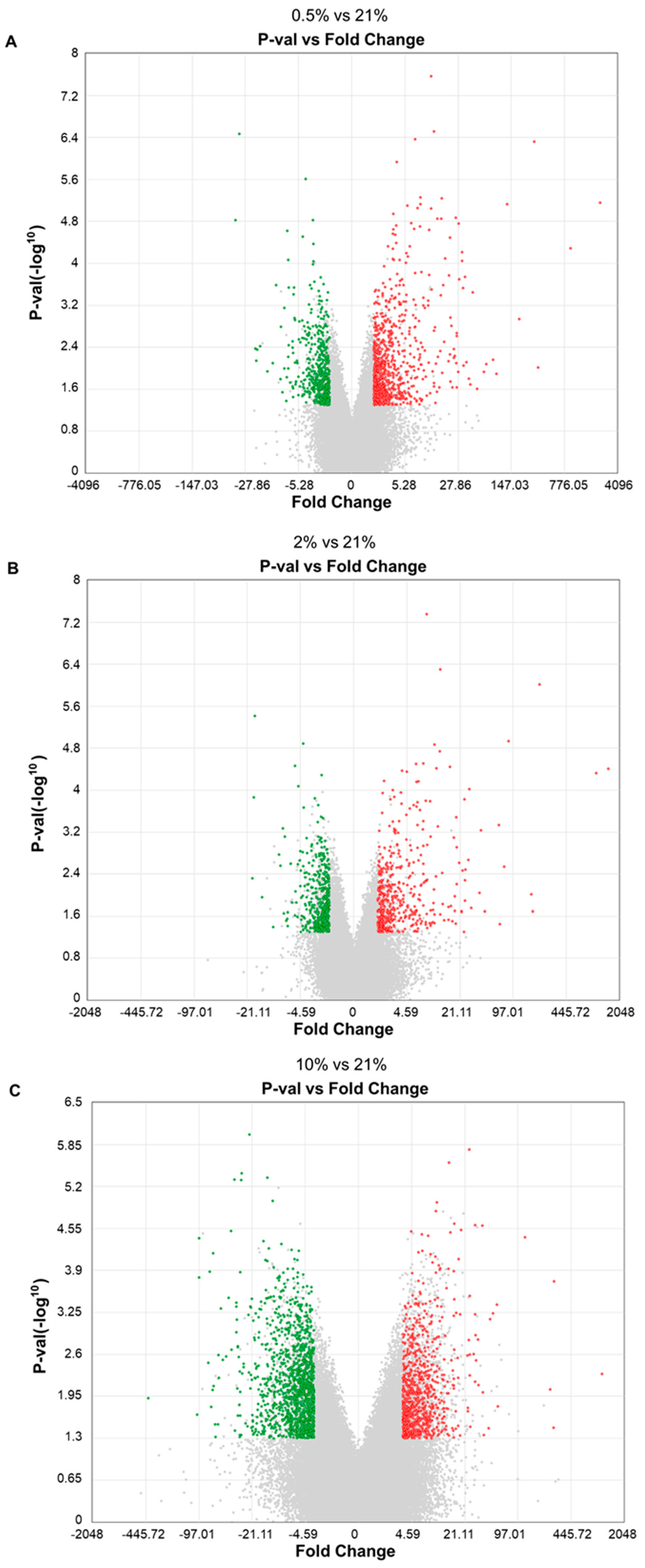
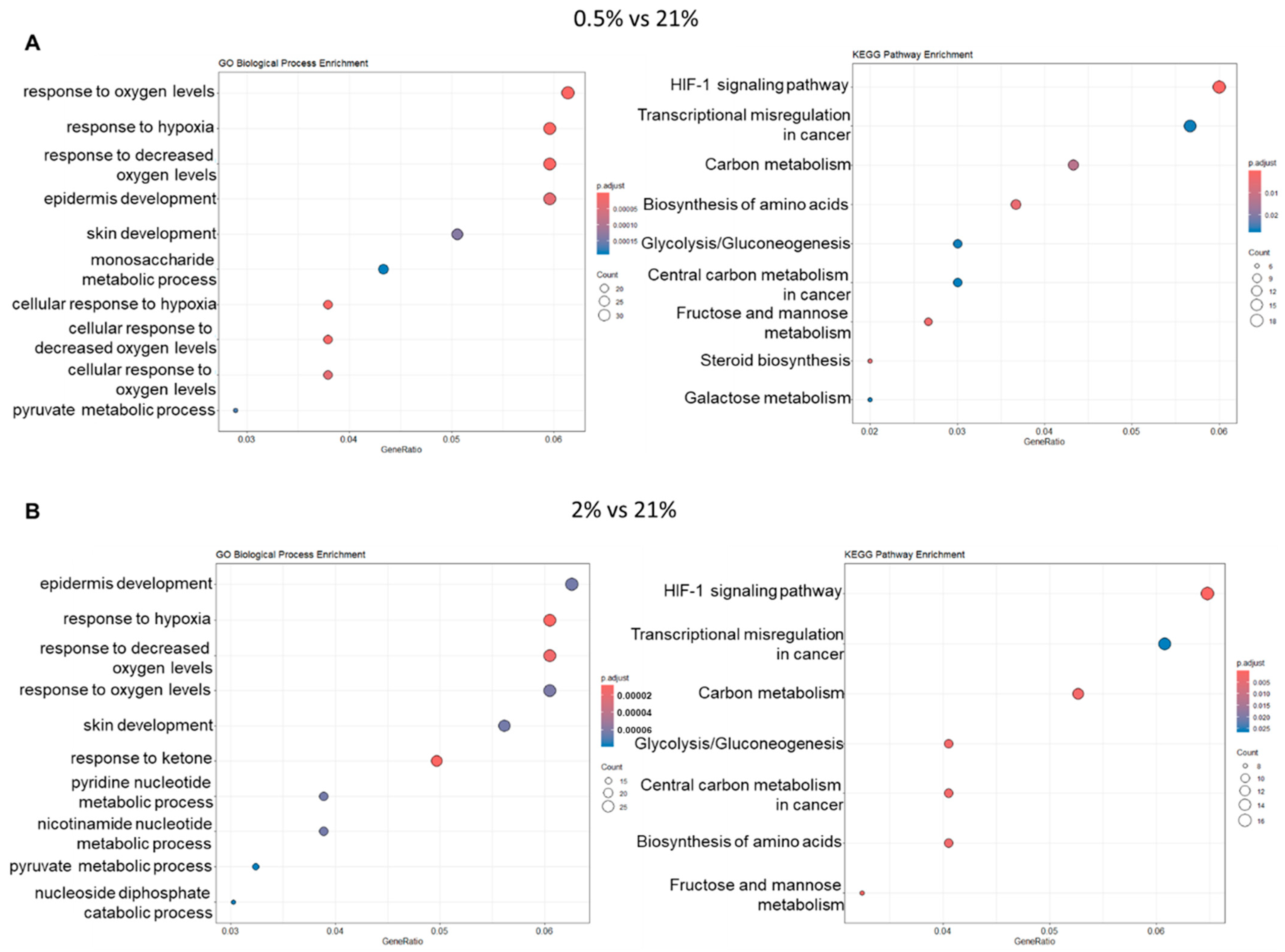
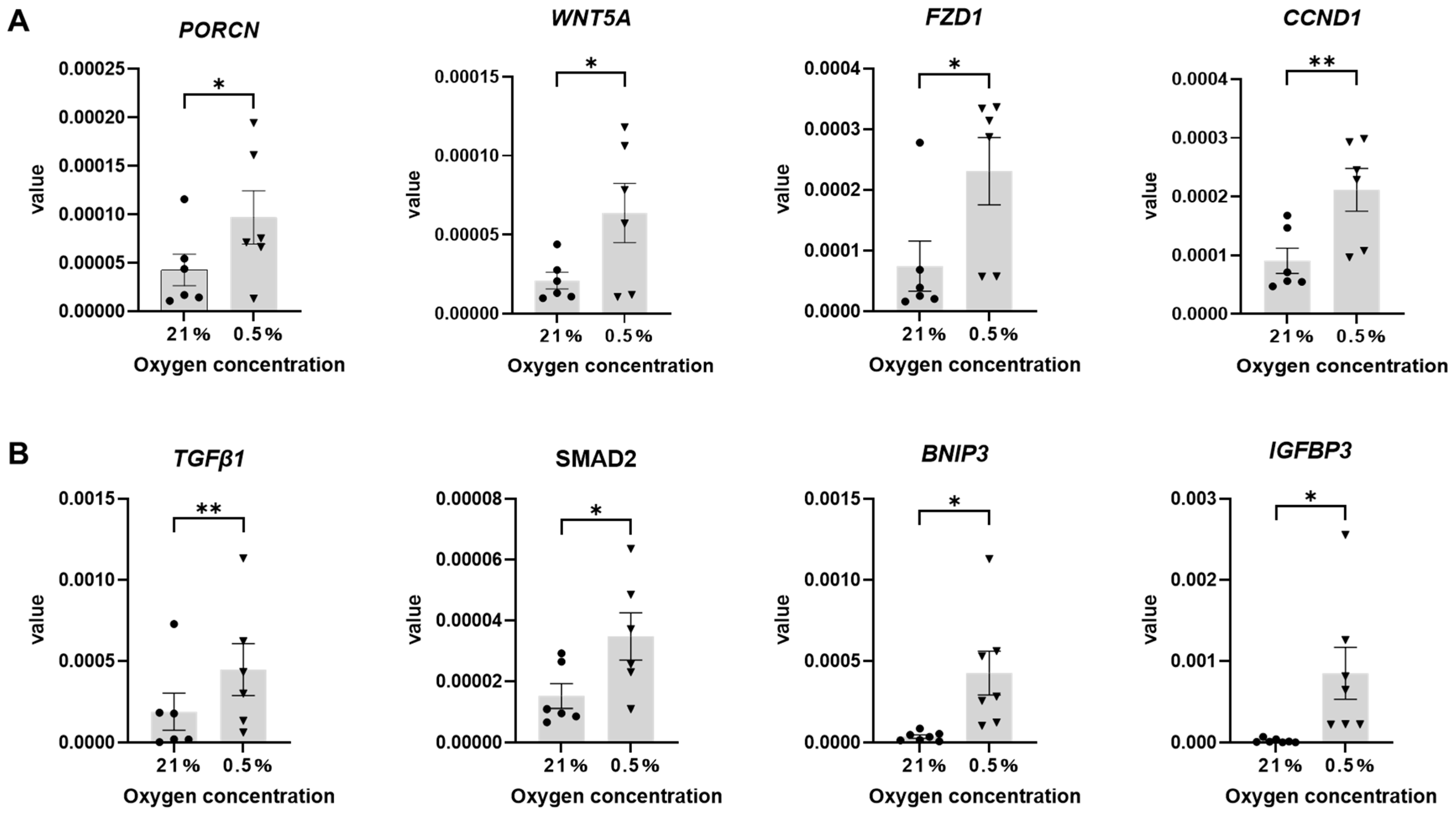
2.3. Hypoxic Culture Potentially Activates Wnt and TGFβ1 Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Primary Sweat Gland Cell Spheroid Culture
4.3. Immunohistochemical Analysis
4.4. RNA Extraction
4.5. DNA Microarray
4.6. RT-qPCR
4.7. Western Blotting
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, L.B. Physiology of Sweat Gland Function: The Roles of Sweating and Sweat Composition in Human Health. Temp. Multidiscip. Biomed. J. 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Honda, Y.; Phung, V.L.H.; Hijioka, Y. Prediction of Climate Change Impacts on Heatstroke Cases in Japan’s 47 Prefectures with the Effect of Long-Term Heat Adaptation. Environ. Res. 2023, 232, 116390. [Google Scholar] [CrossRef] [PubMed]
- Millyard, A.; Layden, J.D.; Pyne, D.B.; Edwards, A.M.; Bloxham, S.R. Impairments to Thermoregulation in the Elderly During Heat Exposure Events. Gerontol. Geriatr. Med. 2020, 6, 2333721420932432. [Google Scholar] [CrossRef]
- Kurosumi, K.; Kurosumi, U. Electron Microscopy of the Mouse Plantar Eccrine Sweat Glands. Arch. Histol. Jpn. 1970, 31, 455–475. [Google Scholar] [CrossRef]
- Bovell, D.L. The Evolution of Eccrine Sweat Gland Research towards Developing a Model for Human Sweat Gland Function. Exp. Dermatol. 2018, 27, 544–550. [Google Scholar] [CrossRef]
- Kurata, R.; Futaki, S.; Nakano, I.; Tanemura, A.; Murota, H.; Katayama, I.; Sekiguchi, K. Isolation and Characterization of Sweat Gland Myoepithelial Cells from Human Skin. Cell Struct. Funct. 2014, 39, 101–112. [Google Scholar] [CrossRef][Green Version]
- Lotz, S.; Goderie, S.; Tokas, N.; Hirsch, S.E.; Ahmad, F.; Corneo, B.; Le, S.; Banerjee, A.; Kane, R.S.; Stern, J.H.; et al. Sustained Levels of FGF2 Maintain Undifferentiated Stem Cell Cultures with Biweekly Feeding. PLoS ONE 2013, 8, e56289. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, M.W.; Lee, T.-H.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Cell Culture Density Affects the Stemness Gene Expression of Adipose Tissue-Derived Mesenchymal Stem Cells. Biomed. Rep. 2017, 6, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Inglés, M.; Gimeno-Mallench, L.; El Alami, M.; Viña-Almunia, J.; Gambini, J.; Viña, J.; Borrás, C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1195. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, M.; Mauro, A.; Citeroni, M.R.; Dufrusine, B.; Peserico, A.; Russo, V.; Berardinelli, P.; Dainese, E.; Cimini, A.; Barboni, B. Insight into Hypoxia Stemness Control. Cells 2021, 10, 2161. [Google Scholar] [CrossRef] [PubMed]
- Nombela-Arrieta, C.; Silberstein, L.E. The Science behind the Hypoxic Niche of Hematopoietic Stem and Progenitors. Hematology 2014, 2014, 542–547. [Google Scholar] [CrossRef]
- Jeon, S.; Jeon, M.; Choi, S.; Yoo, S.; Park, S.; Lee, M.; Kim, I. Hypoxia in Skin Cancer: Molecular Basis and Clinical Implications. Int. J. Mol. Sci. 2023, 24, 4430. [Google Scholar] [CrossRef]
- Rosenberger, C.; Solovan, C.; Rosenberger, A.D.; Jinping, L.; Treudler, R.; Frei, U.; Eckardt, K.-U.; Brown, L.F. Upregulation of Hypoxia-Inducible Factors in Normal and Psoriatic Skin. J. Investig. Dermatol. 2007, 127, 2445–2452. [Google Scholar] [CrossRef]
- Maleki, F.; Ovens, K.; Hogan, D.J.; Kusalik, A.J. Gene Set Analysis: Challenges, Opportunities, and Future Research. Front. Genet. 2020, 11. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; Di Serio, C.; Danza, G.; Rocca, B.J.; Ginori, A.; Prudovsky, I.; Marchionni, N.; del Vecchio, M.T.; Tarantini, F. Carbonic Anhydrase IX Is a Marker of Hypoxia and Correlates with Higher Gleason Scores and ISUP Grading in Prostate Cancer. Diagn. Pathol. 2016, 11, 45. [Google Scholar] [CrossRef]
- Benej, M.; Pastorekova, S.; Pastorek, J. Carbonic Anhydrase IX: Regulation and Role in Cancer. Subcell. Biochem. 2014, 75, 199–219. [Google Scholar] [CrossRef]
- Chung, F.-Y.; Huang, M.-Y.; Yeh, C.-S.; Chang, H.-J.; Cheng, T.-L.; Yen, L.-C.; Wang, J.-Y.; Lin, S.-R. GLUT1 Gene Is a Potential Hypoxic Marker in Colorectal Cancer Patients. BMC Cancer 2009, 9, 241. [Google Scholar] [CrossRef]
- Fujino, M.; Aishima, S.; Shindo, K.; Oda, Y.; Morimatsu, K.; Tsutsumi, K.; Otsuka, T.; Tanaka, M.; Oda, Y. Expression of Glucose Transporter-1 Is Correlated with Hypoxia-Inducible Factor 1α and Malignant Potential in Pancreatic Neuroendocrine Tumors. Oncol. Lett. 2016, 12, 3337–3343. [Google Scholar] [CrossRef][Green Version]
- Zhao, A.; Yang, L.; Ma, K.; Sun, M.; Li, L.; Huang, J.; Li, Y.; Zhang, C.; Li, H.; Fu, X. Overexpression of Cyclin D1 Induces the Reprogramming of Differentiated Epidermal Cells into Stem Cell-like Cells. Cell Cycle 2016, 15, 644–653. [Google Scholar] [CrossRef]
- Segeritz, C.-P.; Vallier, L. Chapter 9—Cell Culture: Growing Cells as Model Systems In Vitro. In Basic Science Methods for Clinical Researchers; Jalali, M., Saldanha, F.Y.L., Jalali, M., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 151–172. ISBN 978-0-12-803077-6. [Google Scholar]
- Rittié, L.; Sachs, D.L.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Eccrine Sweat Glands Are Major Contributors to Reepithelialization of Human Wounds. Am. J. Pathol. 2013, 182, 163–171. [Google Scholar] [CrossRef]
- Pontiggia, L.; Biedermann, T.; Böttcher-Haberzeth, S.; Oliveira, C.; Braziulis, E.; Klar, A.S.; Meuli-Simmen, C.; Meuli, M.; Reichmann, E. De Novo Epidermal Regeneration Using Human Eccrine Sweat Gland Cells: Higher Competence of Secretory over Absorptive Cells. J. Investig. Dermatol. 2014, 134, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Petrakova, O.S.; Ashapkin, V.V.; Voroteliak, E.A.; Bragin, E.Y.; Shtratnikova, V.Y.; Chernioglo, E.S.; Sukhanov, Y.V.; Terskikh, V.V.; Vasiliev, A.V. Effect of 3D Cultivation Conditions on the Differentiation of Endodermal Cells. Acta Naturae 2012, 4, 47–57. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Zhang, M.; Chen, L.; Zhang, B.; Tang, S.; Fu, X. Three-Dimensional Co-Culture of BM-MSCs and Eccrine Sweat Gland Cells in Matrigel Promotes Transdifferentiation of BM-MSCs. J. Mol. Histol. 2015, 46, 431–438. [Google Scholar] [CrossRef]
- Nakashima, K.; Kato, H.; Kurata, R.; Qianwen, L.; Hayakawa, T.; Okada, F.; Fujita, F.; Nakagawa, Y.; Tanemura, A.; Murota, H.; et al. Gap Junction-Mediated Contraction of Myoepithelial Cells Induces the Peristaltic Transport of Sweat in Human Eccrine Glands. Commun. Biol. 2023, 6, 1175. [Google Scholar] [CrossRef] [PubMed]
- Bruneel, K.; Verstappe, J.; Vandamme, N.; Berx, G. Intrinsic Balance between ZEB Family Members Is Important for Melanocyte Homeostasis and Melanoma Progression. Cancers 2020, 12, 2248. [Google Scholar] [CrossRef]
- Li, L.; Mignone, J.; Yang, M.; Matic, M.; Penman, S.; Enikolopov, G.; Hoffman, R.M. Nestin Expression in Hair Follicle Sheath Progenitor Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9958–9961. [Google Scholar] [CrossRef]
- Clavel, C.; Grisanti, L.; Zemla, R.; Rezza, A.; Barros, R.; Sennett, R.; Mazloom, A.; Chung, C.-Y.; Cai, X.; Cai, C.-L.; et al. Sox2 in the Dermal Papilla Niche Controls Hair Growth by Fine-Tuning Bmp Signaling in Differentiating Hair Shaft Progenitors. Dev. Cell 2012, 23, 981–994. [Google Scholar] [CrossRef]
- Cui, C.-Y.; Yin, M.; Sima, J.; Childress, V.; Michel, M.; Piao, Y.; Schlessinger, D. Involvement of Wnt, Eda and Shh at Defined Stages of Sweat Gland Development. Dev. Camb. Engl. 2014, 141, 3752–3760. [Google Scholar] [CrossRef]
- Ingman, W.V.; Robertson, S.A. Mammary Gland Development in Transforming Growth Factor Beta1 Null Mutant Mice: Systemic and Epithelial Effects. Biol. Reprod. 2008, 79, 711–717. [Google Scholar] [CrossRef]
- Varela-Nallar, L.; Rojas-Abalos, M.; Abbott, A.C.; Moya, E.A.; Iturriaga, R.; Inestrosa, N.C. Chronic Hypoxia Induces the Activation of the Wnt/β-Catenin Signaling Pathway and Stimulates Hippocampal Neurogenesis in Wild-Type and APPswe-PS1ΔE9 Transgenic Mice in Vivo. Front. Cell. Neurosci. 2014, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Charbonneau, M.; Grandmont, S.; Richard, D.E.; Dubois, C.M. Transforming Growth Factor Β1 Induces Hypoxia-Inducible Factor-1 Stabilization through Selective Inhibition of PHD2 Expression. J. Biol. Chem. 2006, 281, 24171–24181. [Google Scholar] [CrossRef]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of Pluripotency in Human and Mouse Embryonic Stem Cells through Activation of Wnt Signaling by a Pharmacological GSK-3-Specific Inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Sokol, S.Y. Maintaining Embryonic Stem Cell Pluripotency with Wnt Signaling. Dev. Camb. Engl. 2011, 138, 4341–4350. [Google Scholar] [CrossRef]
- Hong, C.-F.; Chen, W.-Y.; Wu, C.-W. Upregulation of Wnt Signaling under Hypoxia Promotes Lung Cancer Progression. Oncol. Rep. 2017, 38, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, K.D.; Virshup, D.M. Precise Regulation of Porcupine Activity Is Required for Physiological Wnt Signaling. J. Biol. Chem. 2012, 287, 34167–34178. [Google Scholar] [CrossRef]
- Biechele, S.; Cockburn, K.; Lanner, F.; Cox, B.J.; Rossant, J. Porcn-Dependent Wnt Signaling Is Not Required Prior to Mouse Gastrulation. Development 2013, 140, 2961–2971. [Google Scholar] [CrossRef]
- van Amerongen, R.; Fuerer, C.; Mizutani, M.; Nusse, R. Wnt5a Can Both Activate and Repress Wnt/β-Catenin Signaling during Mouse Embryonic Development. Dev. Biol. 2012, 369, 101–114. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a Protein Activates or Inhibits β-Catenin–TCF Signaling Depending on Receptor Context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef]
- Okamoto, M.; Udagawa, N.; Uehara, S.; Maeda, K.; Yamashita, T.; Nakamichi, Y.; Kato, H.; Saito, N.; Minami, Y.; Takahashi, N.; et al. Noncanonical Wnt5a Enhances Wnt/β-Catenin Signaling during Osteoblastogenesis. Sci. Rep. 2014, 4, 4493. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Flahaut, M.; Meier, R.; Coulon, A.; Nardou, K.A.; Niggli, F.K.; Martinet, D.; Beckmann, J.S.; Joseph, J.-M.; Mühlethaler-Mottet, A.; Gross, N. The Wnt Receptor FZD1 Mediates Chemoresistance in Neuroblastoma through Activation of the Wnt/β-Catenin Pathway. Oncogene 2009, 28, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.K.; Hubchak, S.; Hayashida, T.; Runyan, C.E.; Schumacker, P.T.; Schnaper, H.W. Interdependence of HIF-1α and TGF-β/Smad3 Signaling in Normoxic and Hypoxic Renal Epithelial Cell Collagen Expression. Am. J. Physiol.—Ren. Physiol. 2011, 300, F898–F905. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Hubchak, S.C.; Liang, X.; Rozen-Zvi, B.; Schumacker, P.T.; Hayashida, T.; Schnaper, H.W. Hypoxia-Inducible Factor-2α and TGF-β Signaling Interact to Promote Normoxic Glomerular Fibrogenesis. Am. J. Physiol.—Ren. Physiol. 2013, 305, F1323–F1331. [Google Scholar] [CrossRef]
- Mallikarjuna, P.; Raviprakash, T.S.; Aripaka, K.; Ljungberg, B.; Landström, M. Interactions between TGF-β Type I Receptor and Hypoxia-Inducible Factor-α Mediates a Synergistic Crosstalk Leading to Poor Prognosis for Patients with Clear Cell Renal Cell Carcinoma. Cell Cycle 2019, 18, 2141–2156. [Google Scholar] [CrossRef]
- Schedlich, L.J.; Yenson, V.M.; Baxter, R.C. TGF-β-Induced Expression of IGFBP-3 Regulates IGF1R Signaling in Human Osteosarcoma Cells. Mol. Cell. Endocrinol. 2013, 377, 56–64. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, Q.; Sun, H.; Liu, L.; Wang, C.; Li, Z.; Xu, Y.; Wang, L.; Zhang, L.; Zhang, H.; et al. BNIP3 (BCL2 Interacting Protein 3) Regulates Pluripotency by Modulating Mitochondrial Homeostasis via Mitophagy. Cell Death Dis. 2022, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, C.; Tang, P.; Zhang, H.; Yue, J.; Yu, Z. BNIP3 Induces Apoptosis and Protective Autophagy under Hypoxia in Esophageal Squamous Cell Carcinoma Cell Lines: BNIP3 Regulates Cell Death. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Glick, D.; Zhang, W.; Beaton, M.; Marsboom, G.; Gruber, M.; Simon, M.C.; Hart, J.; Dorn, G.W.; Brady, M.J.; Macleod, K.F. BNip3 Regulates Mitochondrial Function and Lipid Metabolism in the Liver. Mol. Cell. Biol. 2012, 32, 2570–2584. [Google Scholar] [CrossRef]
- Guo, K.; Searfoss, G.; Krolikowski, D.; Pagnoni, M.; Franks, C.; Clark, K.; Yu, K.T.; Jaye, M.; Ivashchenko, Y. Hypoxia Induces the Expression of the Pro-Apoptotic Gene BNIP3. Cell Death Differ. 2001, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yamada, P.M.; Mehta, H.H.; Hwang, D.; Roos, K.P.; Hevener, A.L.; Lee, K.W. Evidence of a Role for Insulin-Like Growth Factor Binding Protein (IGFBP)-3 in Metabolic Regulation. Endocrinology 2010, 151, 5741–5750. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, S.M.; Song, S.U.; Park, S.G.; Kim, W.-S.; Park, I.G.; Lee, J.; Sung, J.-H. Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of Mesenchymal Stem Cells via IGFBP3 Up-Regulation. Int. J. Mol. Sci. 2016, 17, 1389. [Google Scholar] [CrossRef]
- Hayakawa, T.; Fujita, F.; Okada, F.; Sekiguchi, K. Establishment and Characterization of Immortalized Sweat Gland Myoepithelial Cells. Sci. Rep. 2022, 12, 7. [Google Scholar] [CrossRef]


| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| 18S | CGGCTACCACATCCAAGGAA | AGCTGGAATTACCGCGGC |
| HIF-1α | TTAGAACCAAATCCAGAGTCAC | TATTCACTGGGACTATTAGGCT |
| HIF2α | ACCTGAAGATTGAAGTGATTGAG | GTGGCTGGAAGATGTTTGTC |
| CA9 | TTTGCCAGAGTTGACGAGGC | GCTCATAGGCACTGTTTTCTTCC |
| αSMA | ATAGAACATGGCATCATCACCAAC | GGGCAACACGAAGCTCATTGTA |
| Keratin 18 | CCCTGCTGAACATCAACCTCAA | GCTGTCCAAGGCATCACCAA |
| S100A2 | GCCAAGAGGGCGACAAGTT | AGGAAAACAGCATACTCCTGGA |
| S100P | AAGGATGCCGTGGATAAATGC | ACACGATGAACTCACTGAACTC |
| PORCN | CCCCTCCATCCTTGACC | CTCCCCTTCTCTGTTTCCC |
| WNT5A | CCTGAAGGAGAAGTACGACAG | GATGTAGACCAGGTCTTGTGTG |
| FZD1 | ACTCCCTTCTCCCACCTTAGTT | ATGCTTCTTCCCAAATCTCAGT |
| CCND1 | AGAGGCGGAGGAGAACAAAC | AAGCGTGTGAGGCGGTAGTA |
| TGFβ1 | ACAATTCCTGGCGATACCTCAGCA | TCTTCTCCGTGGAGCTGAAGCAAT |
| SMAD2 | GCCTTTCAGCTTCTCTGAACAA | ATGTGGCAATCCTTTTCGAT |
| IGFBP3 | GCTCTGCGTCAACGCTAGTG | GCTTCCTGCCTTTGGAAGGG |
| BNIP3 | CAGGGCTCCTGGGTAGAACT | CTACTCCGTCCAGACTCATGC |
| SOX2 | CCCCCGGCGGCAATAGCA | TCGGCGCCGGGGAGATACAT |
| SOX9 | AGCGAACGCACATCAAGAC | CTGTAGGCGATCTGTTGGGG |
| NESTIN | CTGCTACCCTTGAGACACCTG | GGGCTCTGATCTCTGCATCTAC |
| ZEB1 | CAGCTTGATACCTGTGAATGGG | TATCTGTGGTCGTGTGGGACT |
| GLUT1 | CTGCAACGGCTTAGACTTCGAC | TCTCTGGGTAACAGGGATCAAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Y.; Kato, H.; Luo, Q.; Otani, N.; Kubo, T.; Sekiguchi, K.; Fujita, F. Long-Term Hypoxia Upregulates Wnt and TGFβ1 Signaling in Eccrine Sweat Gland Cells In Vitro. Int. J. Mol. Sci. 2025, 26, 6664. https://doi.org/10.3390/ijms26146664
Lyu Y, Kato H, Luo Q, Otani N, Kubo T, Sekiguchi K, Fujita F. Long-Term Hypoxia Upregulates Wnt and TGFβ1 Signaling in Eccrine Sweat Gland Cells In Vitro. International Journal of Molecular Sciences. 2025; 26(14):6664. https://doi.org/10.3390/ijms26146664
Chicago/Turabian StyleLyu, Yanlin, Hiroko Kato, Qianwen Luo, Naoya Otani, Tateki Kubo, Kiyotoshi Sekiguchi, and Fumitaka Fujita. 2025. "Long-Term Hypoxia Upregulates Wnt and TGFβ1 Signaling in Eccrine Sweat Gland Cells In Vitro" International Journal of Molecular Sciences 26, no. 14: 6664. https://doi.org/10.3390/ijms26146664
APA StyleLyu, Y., Kato, H., Luo, Q., Otani, N., Kubo, T., Sekiguchi, K., & Fujita, F. (2025). Long-Term Hypoxia Upregulates Wnt and TGFβ1 Signaling in Eccrine Sweat Gland Cells In Vitro. International Journal of Molecular Sciences, 26(14), 6664. https://doi.org/10.3390/ijms26146664






