Evidence for Pro-Inflammatory Activity of LTα3 on Macrophages: Significance for Experimental Arthritis and for Therapeutic Switching in Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Results
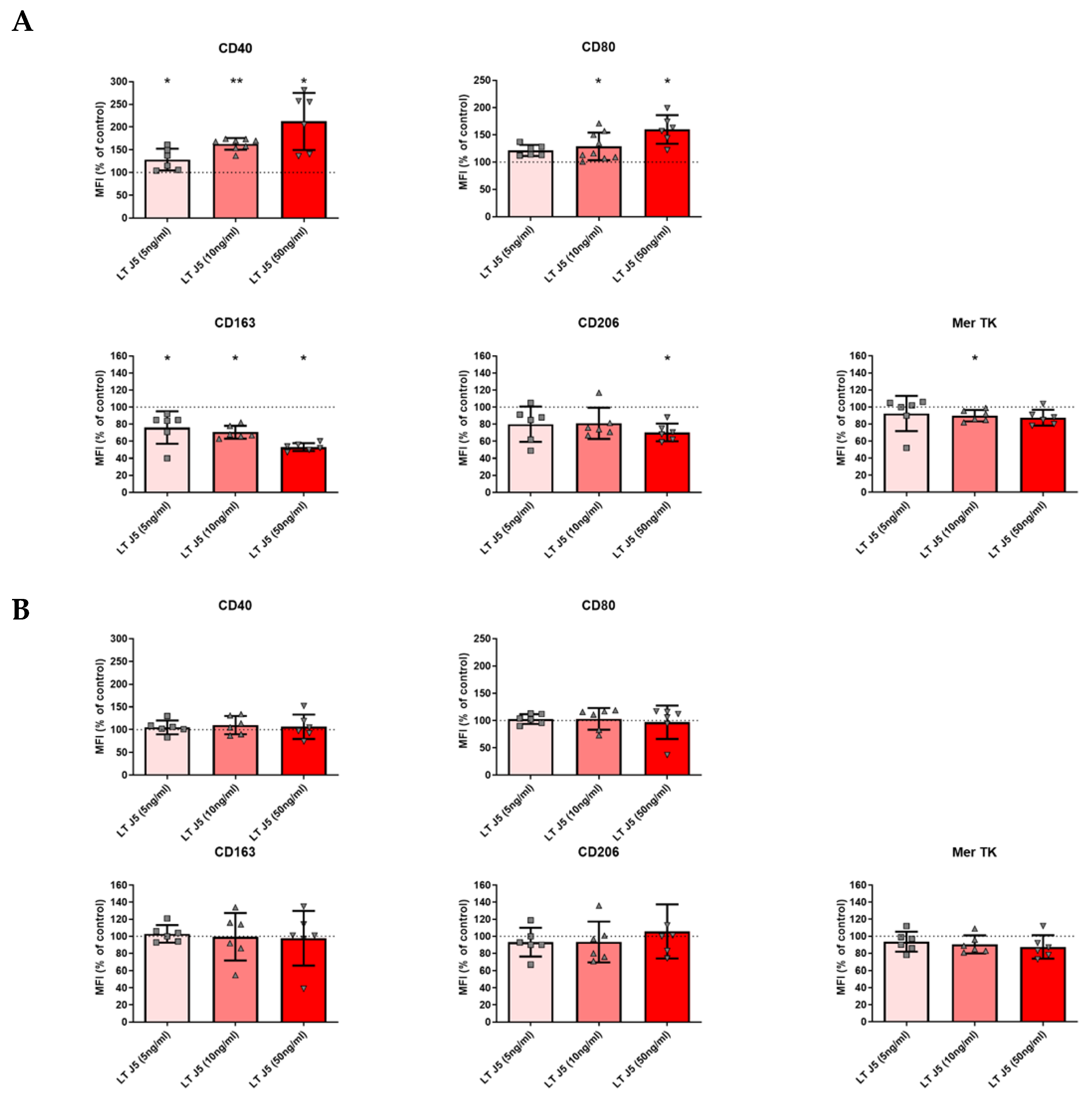
2.1. Induction of CD40 in Mouse Macrophages by LTα3
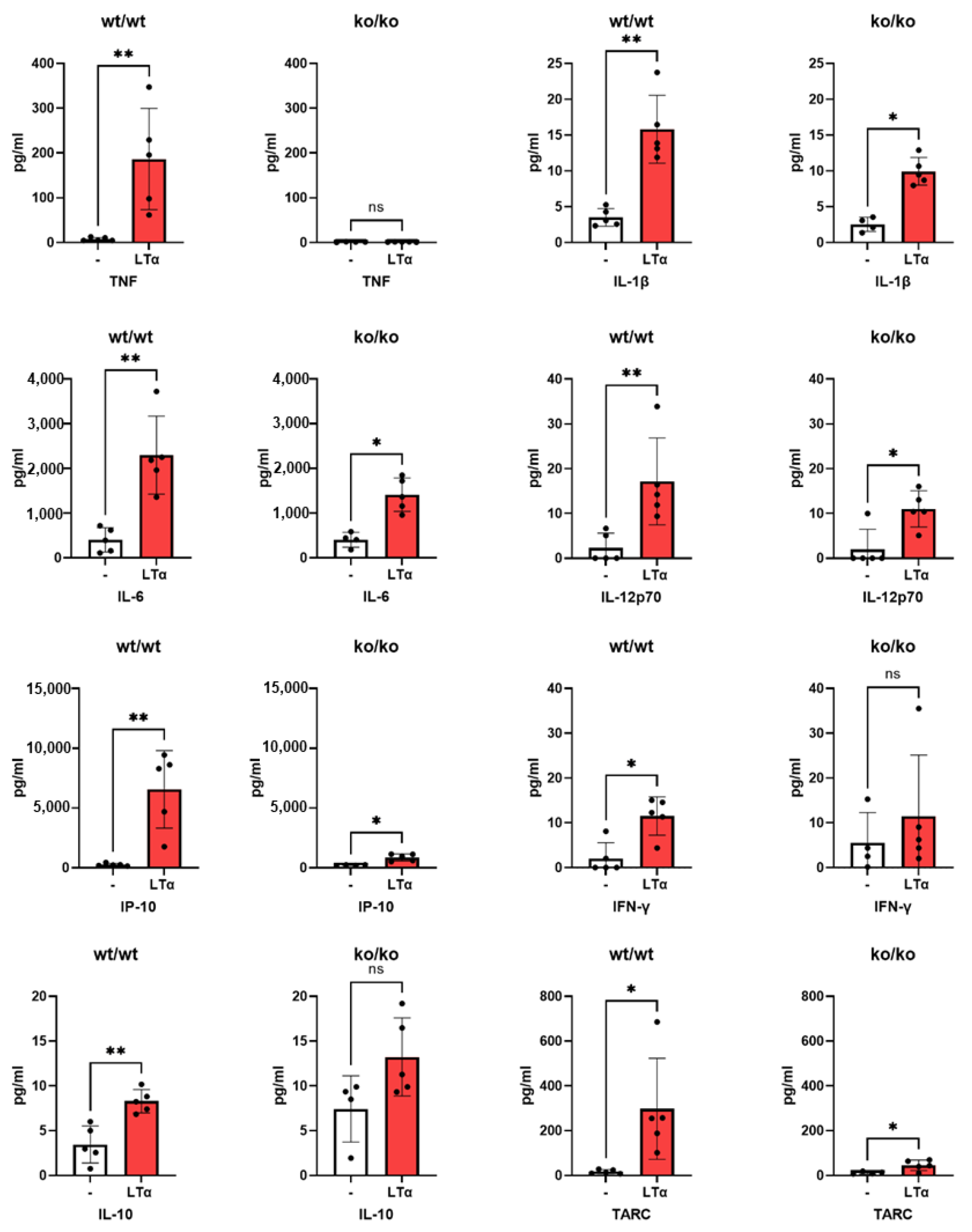
2.2. Induction of Mouse Pro-Inflammatory Cytokines by LTα3
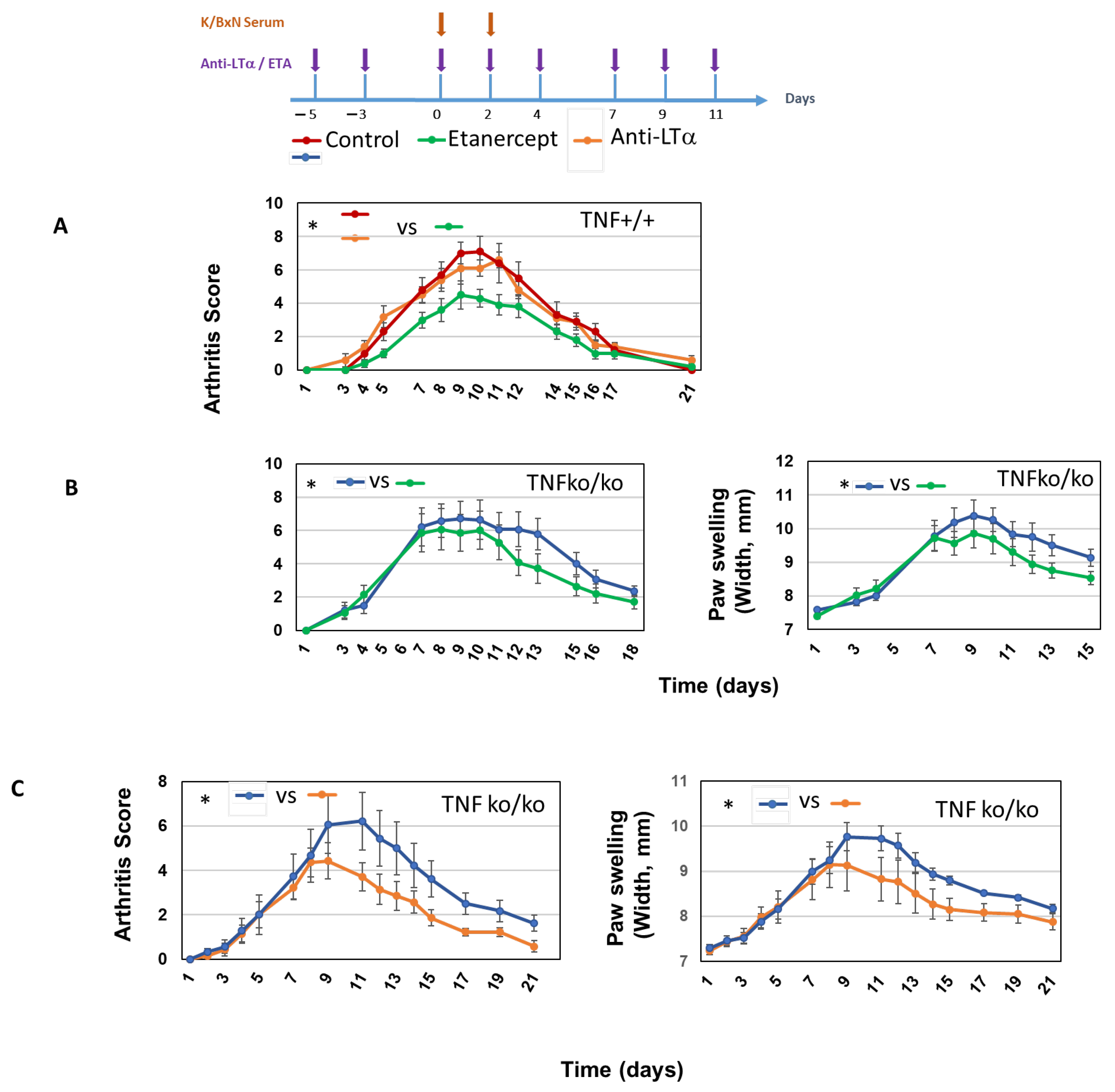
2.3. Role of LTα3 in K/BxN STA Arthritis
2.4. Induction of Human M1 Phenotype and Decrease in M2 Markers by LTα3 in the Absence of TNF
2.5. RA Patients from the “ROC” Registry Did Not Benefit from Switch to ETA
3. Discussion
4. Materials and Methods
4.1. Ethics Approval of the Procedure
4.2. Generation of Human Macrophages
4.3. Mouse Cells
4.4. Flow Cytometry Analysis
4.5. Cytokine Measurements
4.6. K/BxN Serum-Transferred Arthritis
4.7. Patients
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N. Anti-TNF therapy, from rationale to standard of care: What lessons has it taught us? J. Immunol. 2010, 185, 791–794. [Google Scholar] [CrossRef]
- Kishimoto, T.; Kang, S. IL-6 Revisited: From Rheumatoid Arthritis to CAR T Cell Therapy and COVID-19. Annu. Rev. Immunol. 2022, 40, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.-E.; Brocq, O.; Perdriger, A.; Lassoued, S.; Berthelot, J.-M.; Wendling, D.; Euller-Ziegler, L.; Soubrier, M.; Richez, C.; Fautrel, B.; et al. Non-TNF-Targeted Biologic vs a Second Anti-TNF Drug to Treat Rheumatoid Arthritis in Patients With Insufficient Response to a First Anti-TNF Drug: A Randomized Clinical Trial. JAMA 2016, 316, 1172–1180. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Polinova, A.I.; Petropavlovskiy, M.M.; Namakanova, O.A.; Medvedovskaya, A.D.; Zvartsev, R.V.; Telegin, G.B.; Drutskaya, M.S.; Nedospasov, S.A. Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice. Cancers 2021, 13, 1775. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Fukuma, Y.; Takeshita, A.; Nishida, K. The role of lymphotoxin-α in rheumatoid arthritis. Inflamm. Res. 2018, 67, 495–501. [Google Scholar] [CrossRef]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular mechanisms of action of anti-TNF-α agents-Comparison among therapeutic TNF-α antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef]
- Neregård, P.; Krishnamurthy, A.; Revu, S.; Engström, M.; Af Klint, E.; Catrina, A. Etanercept decreases synovial expression of tumour necrosis factor-α and lymphotoxin-α in rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 85–90. [Google Scholar] [CrossRef]
- Calmon-Hamaty, F.; Combe, B.; Hahne, M.; Morel, J. Lymphotoxin α stimulates proliferation and pro-inflammatory cytokine secretion of rheumatoid arthritis synovial fibroblasts. Cytokine 2011, 53, 207–214. [Google Scholar] [CrossRef]
- Calmon-Hamaty, F.; Combe, B.; Hahne, M.; Morel, J. Lymphotoxin α revisited: General features and implications in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β (lymphotoxin α) can activate the inflammatory environment in human chondrocytes. Arthritis Res. Ther. 2013, 15, R202. [Google Scholar] [CrossRef] [PubMed]
- Kuprash, D.V.; Alimzhanov, M.B.; Tumanov, A.V.; Grivennikov, S.I.; Shakhov, A.N.; Drutskaya, L.N.; Marino, M.W.; Turetskaya, R.L.; Anderson, A.O.; Rajewsky, K.; et al. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: Mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol. Cell. Biol. 2002, 22, 8626–8634. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.Y.; Kolumam, G.A.; Yu, X.; Francesco, M.; Ivelja, S.; Peng, I.; Gribling, P.; Shu, J.; Lee, W.P.; Refino, C.J.; et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 2009, 15, 766–773. [Google Scholar] [CrossRef]
- Young, J.; Yu, X.; Wolslegel, K.; Nguyen, A.; Kung, C.; Chiang, E.; Kolumam, G.; Wei, N.; Wong, W.L.; DeForge, L. Lymphotoxin-αβ heterotrimers are cleaved by metalloproteinases and contribute to synovitis in rheumatoid arthritis. Cytokine 2010, 51, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.P.; Simon, J.A.; Offutt, C.; Horn, P.; Herman, A.; Townsend, M.J.; Tang, M.T.; Grogan, J.L.; Hsieh, F.; Davis, J.C. Efficacy and safety of pateclizumab (anti-lymphotoxin-α) compared to adalimumab in rheumatoid arthritis: A head-to-head phase 2 randomized controlled study (The ALTARA Study). Arthritis Res. Ther. 2014, 16, 467. [Google Scholar] [CrossRef]
- Degboé, Y.; Rauwel, B.; Baron, M.; Boyer, J.-F.; Ruyssen-Witrand, A.; Constantin, A.; Davignon, J.-L. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front. Immunol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Kollias, G.; Papadaki, P.; Apparailly, F.; Vervoordeldonk, M.J.; Holmdahl, R.; Baumans, V.; Desaintes, C.; Di Santo, J.; Distler, J.; Garside, P.; et al. Animal models for arthritis: Innovative tools for prevention and treatment. Ann. Rheum. Dis. 2011, 70, 1357–1362. [Google Scholar] [CrossRef]
- Korganow, A.S.; Ji, H.; Mangialaio, S.; Duchatelle, V.; Pelanda, R.; Martin, T.; Degott, C.; Kikutani, H.; Rajewsky, K.; Pasquali, J.L.; et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity 1999, 10, 451–461. [Google Scholar] [CrossRef]
- Kyburz, D.; Corr, M. The KRN mouse model of inflammatory arthritis. Springer Semin. Immunopathol. 2003, 25, 79–90. [Google Scholar] [CrossRef]
- Christensen, A.D.; Haase, C.; Cook, A.D.; Hamilton, J.A. K/BxN Serum-Transfer Arthritis as a Model for Human Inflammatory Arthritis. Front. Immunol. 2016, 7, 213. Available online: http://journal.frontiersin.org/Article/10.3389/fimmu.2016.00213/abstract (accessed on 28 September 2022). [CrossRef] [PubMed]
- Hannemann, N.; Apparailly, F.; Courties, G. New insights into macrophage heterogeneity in rheumatoid arthritis. Jt. Bone Spine 2021, 88, 105091. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, N.; Cao, S.; Eriksson, D.; Schnelzer, A.; Jordan, J.; Eberhardt, M.; Schleicher, U.; Rech, J.; Ramming, A.; Uebe, S.; et al. Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis. J. Clin. Investig. 2019, 129, 2669–2684. [Google Scholar] [CrossRef] [PubMed]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef]
- Ji, H.; Pettit, A.; Ohmura, K.; Ortiz-Lopez, A.; Duchatelle, V.; Degott, C.; Gravallese, E.; Mathis, D.; Benoist, C. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J. Exp. Med. 2002, 196, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Diallo, K.; Simons, N.; Sayegh, S.; Baron, M.; Degboé, Y.; Boyer, J.-F.; Kruglov, A.; Nedospasov, S.; Novarino, J.; Aloulou, M.; et al. Evidence for tmTNF reverse signaling in vivo: Implications for an arginase-1-mediated therapeutic effect of TNF inhibitors during inflammation. iScience 2021, 24, 102331. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Edwards, S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 593–601. [Google Scholar] [CrossRef]
- Németh, T.; Nagy, G.; Pap, T. Synovial fibroblasts as potential drug targets in rheumatoid arthritis, where do we stand and where shall we go? Ann. Rheum. Dis. 2022, 81, 1055–1064. [Google Scholar] [CrossRef]
- Matsumoto, I.; Staub, A.; Benoist, C.; Mathis, D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 1999, 286, 1732–1735. [Google Scholar] [CrossRef]
- Marino, M.W.; Dunn, A.; Grail, D.; Inglese, M.; Noguchi, Y.; Richards, E.; Jungbluth, A.; Wada, H.; Moore, M.; Williamson, B.; et al. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8093–8098. [Google Scholar] [CrossRef]


| Baseline Characteristics of Patients | |
| n | |
| Women, n (%) | 289 |
| Age, Mean (SD), y | 240 (83) |
| Disease Duration, Median (IQR), y | 56.5 (48.5–65.3) |
| Anti-CCP positive, n (%) | 225/276 (81.5) |
| Rheumatoid Factor positive, n (%) | 231/285 (81) |
| No. of Joints (28), Median (IQR) | |
| Tender | 7 (4–11) |
| Swollen | 5 (3–7) |
| ESR, Median (IQR), mm | 24 (11-44) |
| CRP Level, Median (IQR), mg/L | 8 (4–22.2) |
| DAS28, Mean (SD) | 5 (4.3–5.9) |
| Concomitant Treatment with a synthetic DMARD, n (%) | |
| First anti-TNF prescribed, n (%) | |
| Etanercept | 155 (53.6) |
| Adalimumab | 85 (29.4) |
| Infliximab | 41 (14.2) |
| Certolizumab pegol | 5 (1.7) |
| Golimumab | 3 (1.0) |
| Sequence, n (%) | |
| etanercept : : other class | 81 (28.0) |
| anti-TNF : : other class | 66 (22.8) |
| etanercept : : other anti-TNF | 74 (25.6) |
| anti-TNF : : etanercept | 53 (18.3) |
| anti-TNF : : other anti-TNF | 15 (5.2) |
| Sequence n (%) | Δ DAS28 (6-Months Minus Inclusion) | p | |
|---|---|---|---|
| Anti-TNF mAb : : Etanercept | 53 (18.3) | −1.1 (−1.9–−0.2) | - |
| Etanercept : : Anti-TNF mAb | 74 (26.6) | −1.2 (−1.9–−0.5) | 0.289 |
| Anti-TNF mAb : : Other anti-TNF mAb | 15 (5.2) | −1.1 (−1.8–0.4) | 0.178 |
| Anti-TNF : : Other class | 66 (22.8) | −1.9 (−2.9–−1.0) | 0.0017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benezech, A.; Gottenberg, J.-E.; Degboé, Y.; Kruglov, A.; Grogan, J.; Briand-Mésange, F.; Cantagrel, A.; Ruyssen-Witrand, A.; Davignon, J.-L. Evidence for Pro-Inflammatory Activity of LTα3 on Macrophages: Significance for Experimental Arthritis and for Therapeutic Switching in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2025, 26, 6355. https://doi.org/10.3390/ijms26136355
Benezech A, Gottenberg J-E, Degboé Y, Kruglov A, Grogan J, Briand-Mésange F, Cantagrel A, Ruyssen-Witrand A, Davignon J-L. Evidence for Pro-Inflammatory Activity of LTα3 on Macrophages: Significance for Experimental Arthritis and for Therapeutic Switching in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2025; 26(13):6355. https://doi.org/10.3390/ijms26136355
Chicago/Turabian StyleBenezech, Ariane, Jacques-Eric Gottenberg, Yannick Degboé, Andrey Kruglov, Jane Grogan, Fabienne Briand-Mésange, Alain Cantagrel, Adeline Ruyssen-Witrand, and Jean-Luc Davignon. 2025. "Evidence for Pro-Inflammatory Activity of LTα3 on Macrophages: Significance for Experimental Arthritis and for Therapeutic Switching in Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 26, no. 13: 6355. https://doi.org/10.3390/ijms26136355
APA StyleBenezech, A., Gottenberg, J.-E., Degboé, Y., Kruglov, A., Grogan, J., Briand-Mésange, F., Cantagrel, A., Ruyssen-Witrand, A., & Davignon, J.-L. (2025). Evidence for Pro-Inflammatory Activity of LTα3 on Macrophages: Significance for Experimental Arthritis and for Therapeutic Switching in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences, 26(13), 6355. https://doi.org/10.3390/ijms26136355







