Trabecular Titanium Architecture Drives Human Mesenchymal Stem Cell Proliferation and Bone Differentiation
Abstract
1. Introduction
2. Results
2.1. Cell Proliferation and Osteogenic Differentiation
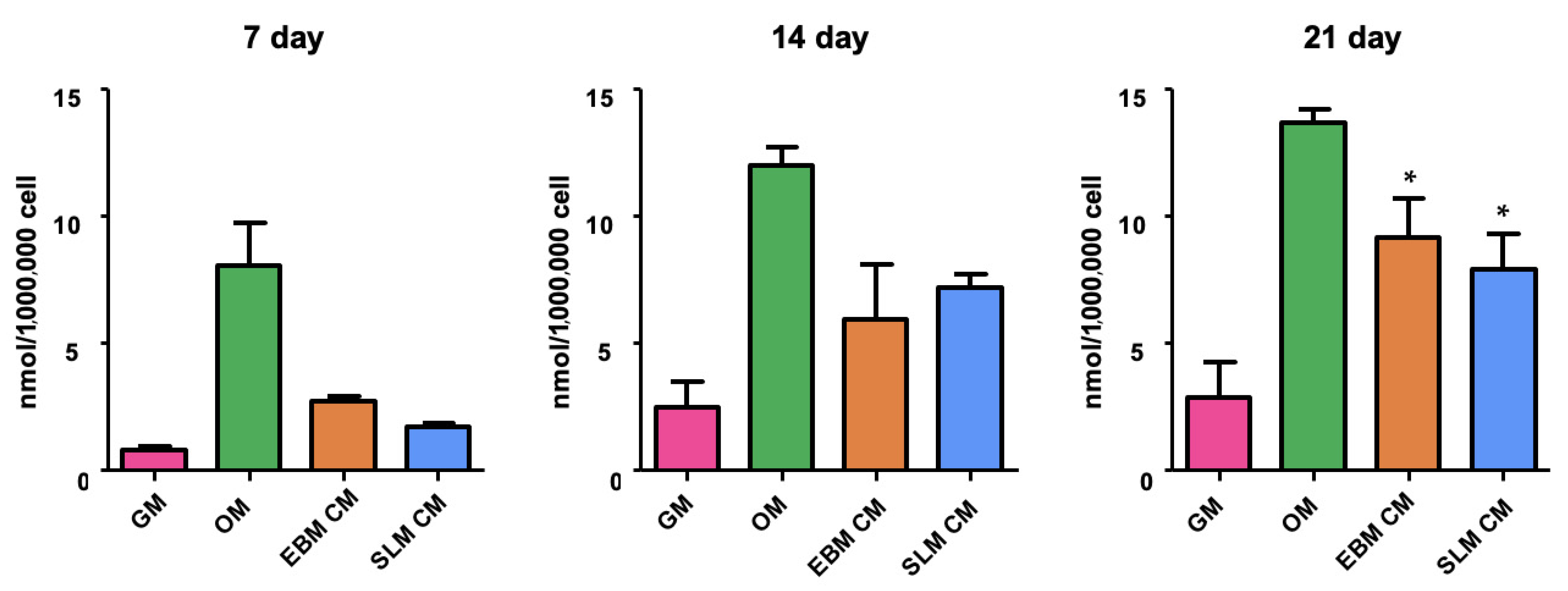
2.1.1. Adhesion and Proliferation Assay
2.1.2. Alkaline Phosphatase Activity (ALP)
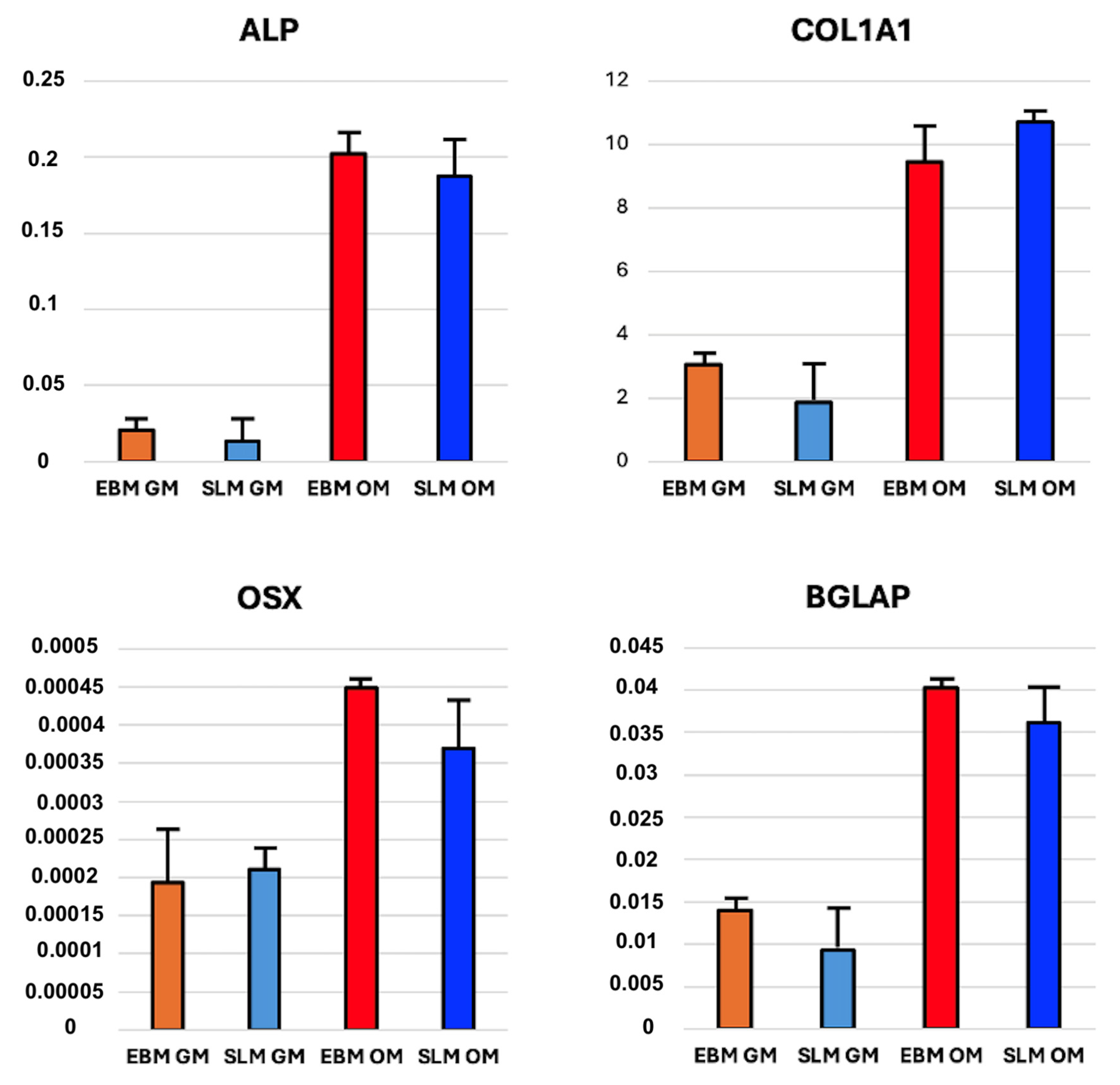
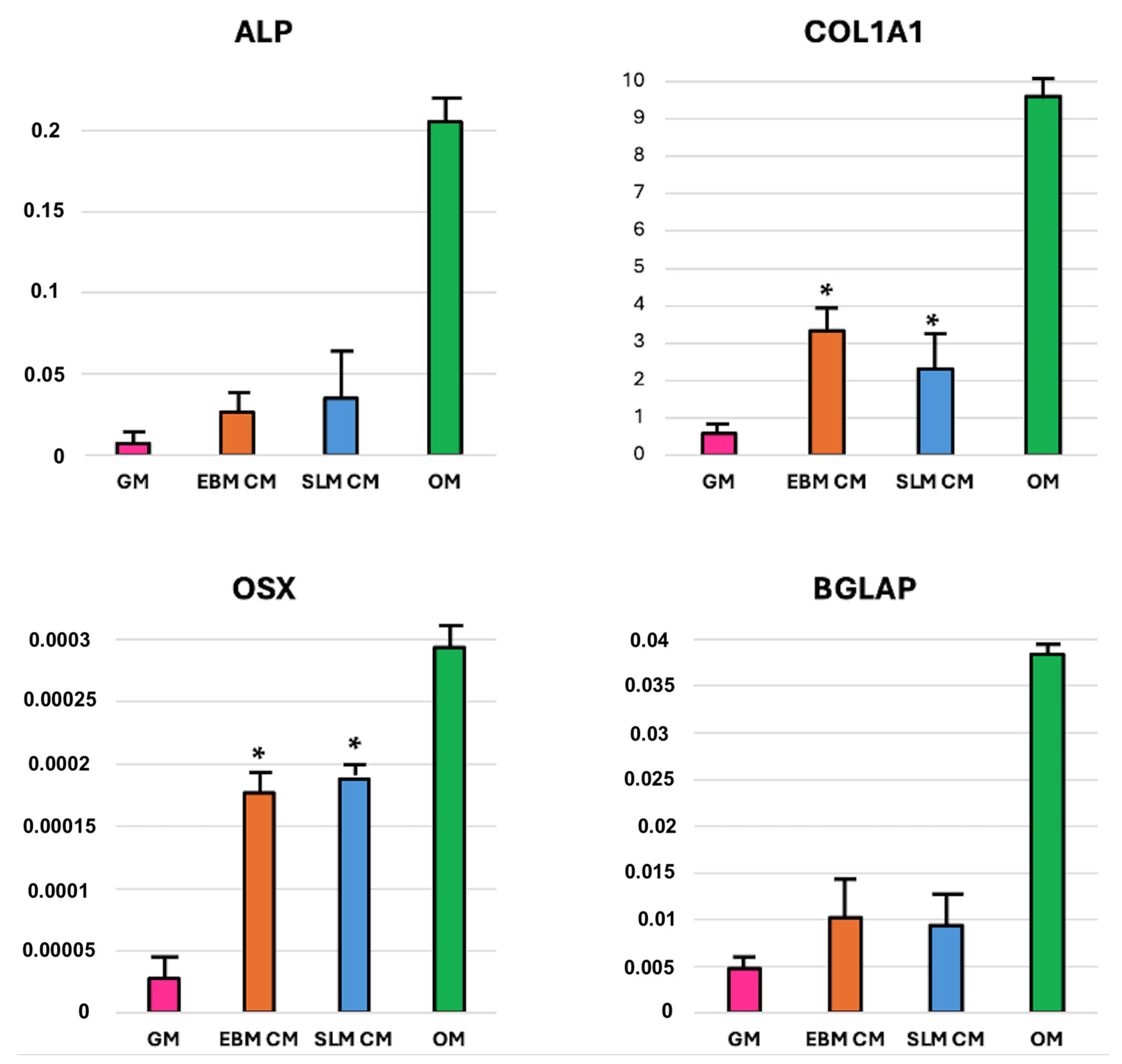
2.1.3. mRNA Expression
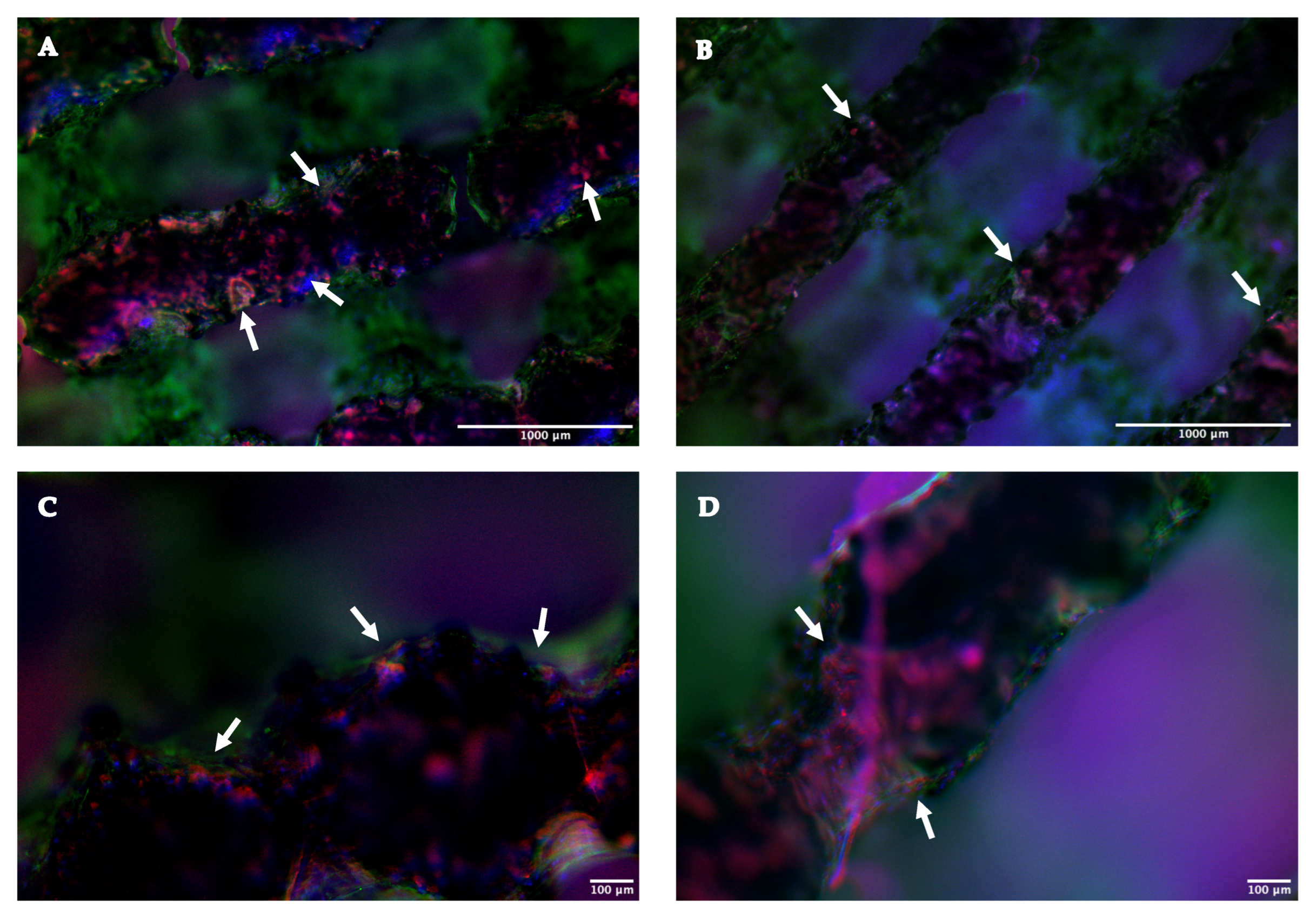
2.1.4. Immunofluorescence Assay
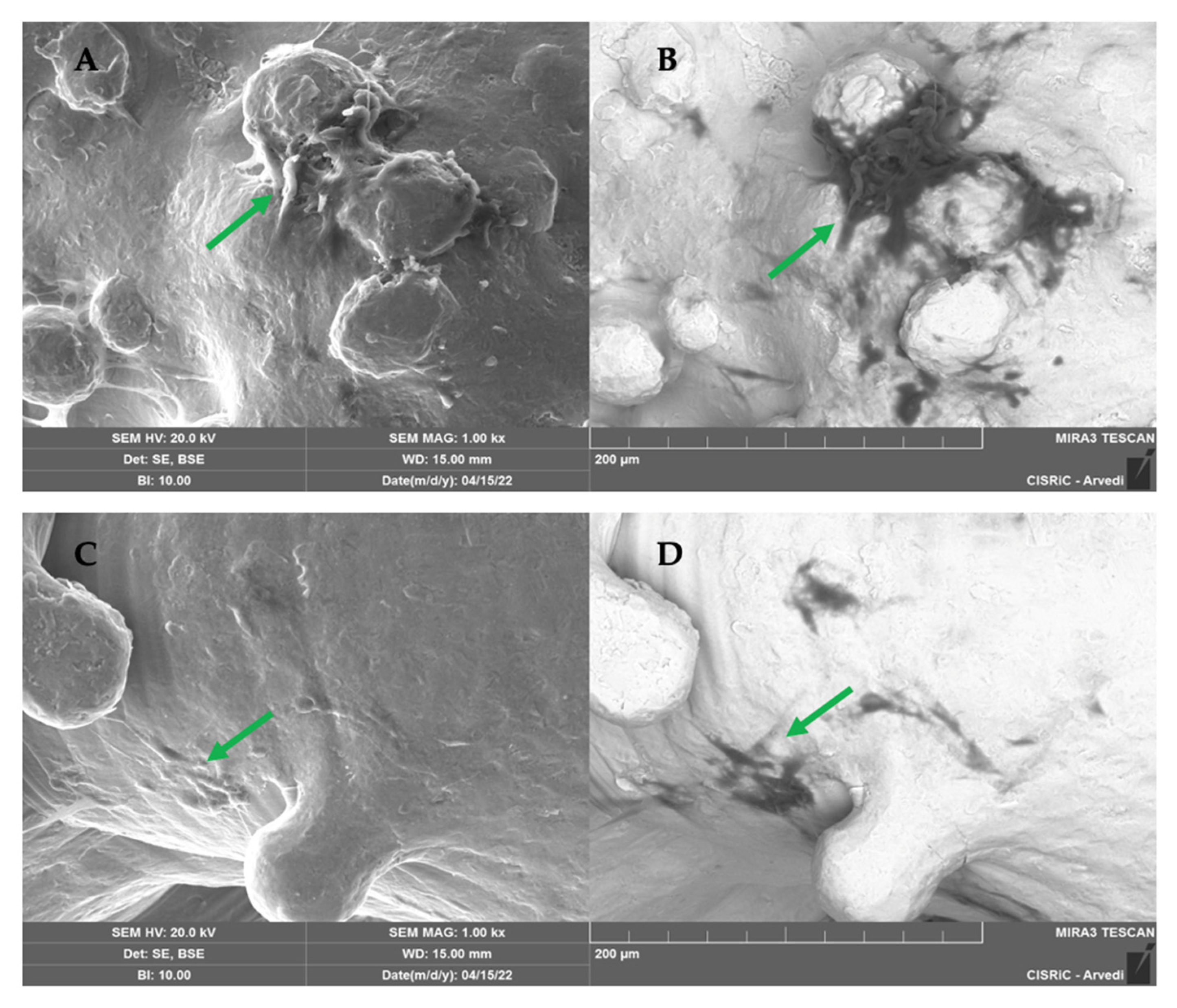
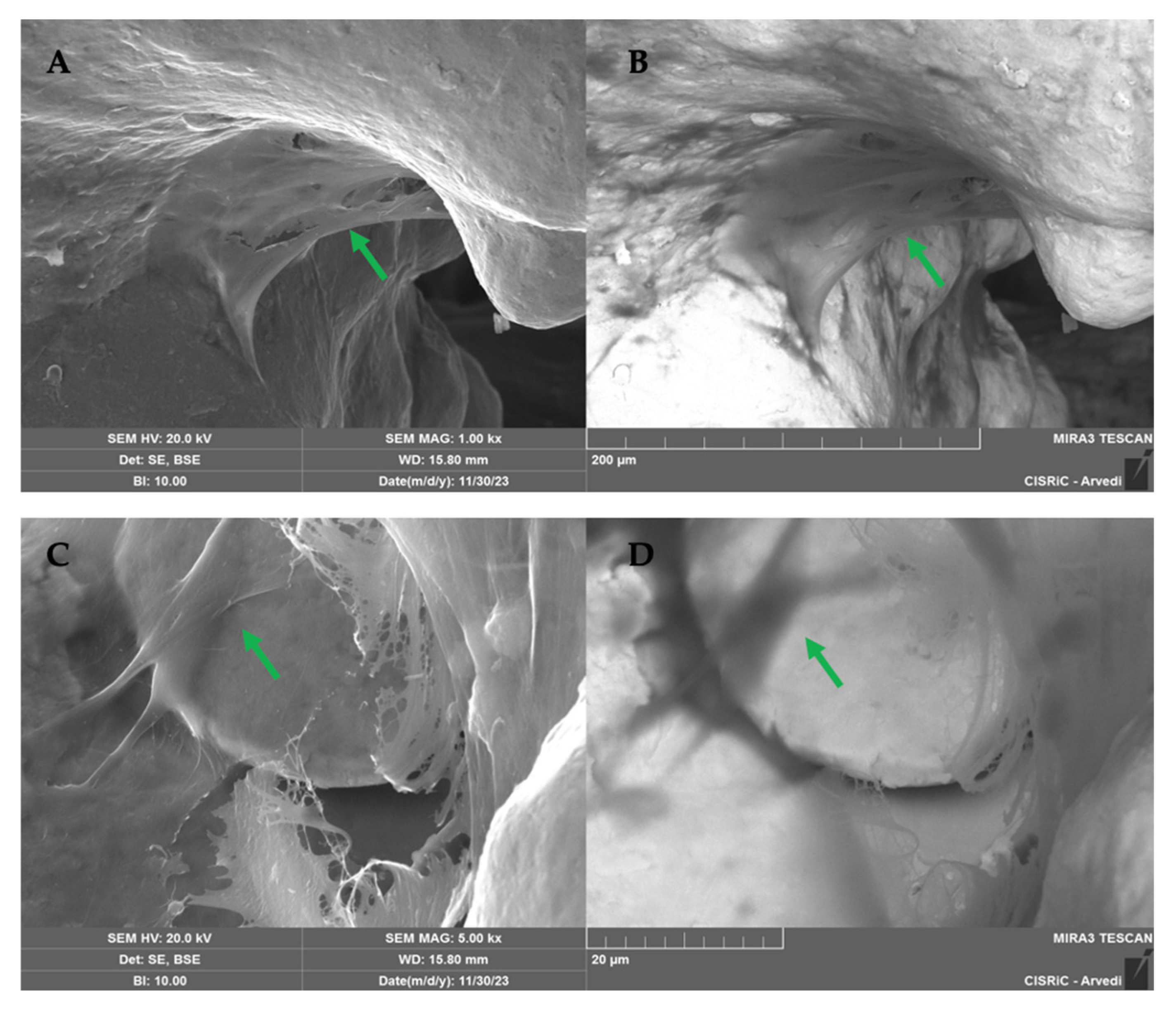
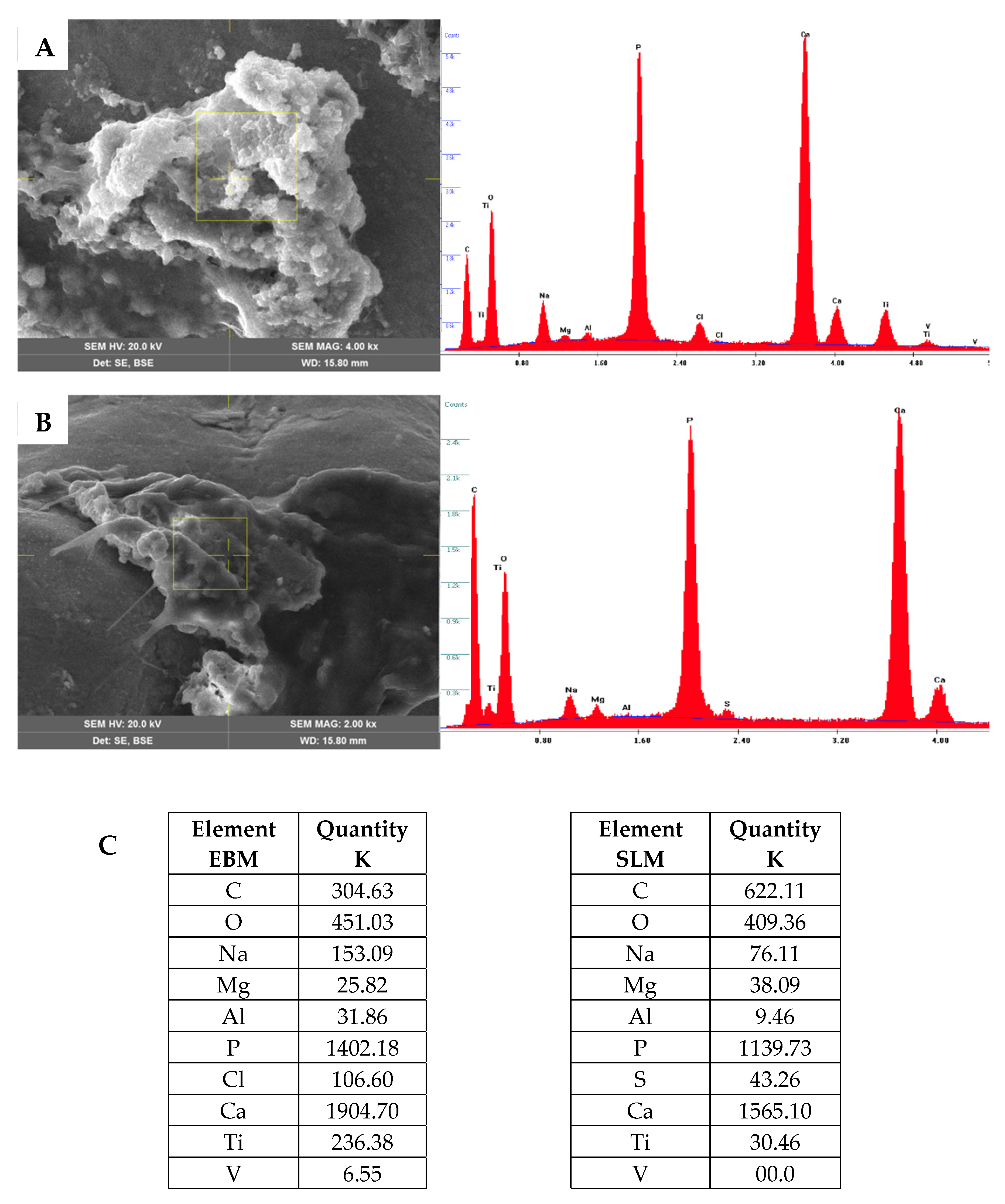
2.1.5. SEM Analysis
3. Discussion
4. Materials and Methods
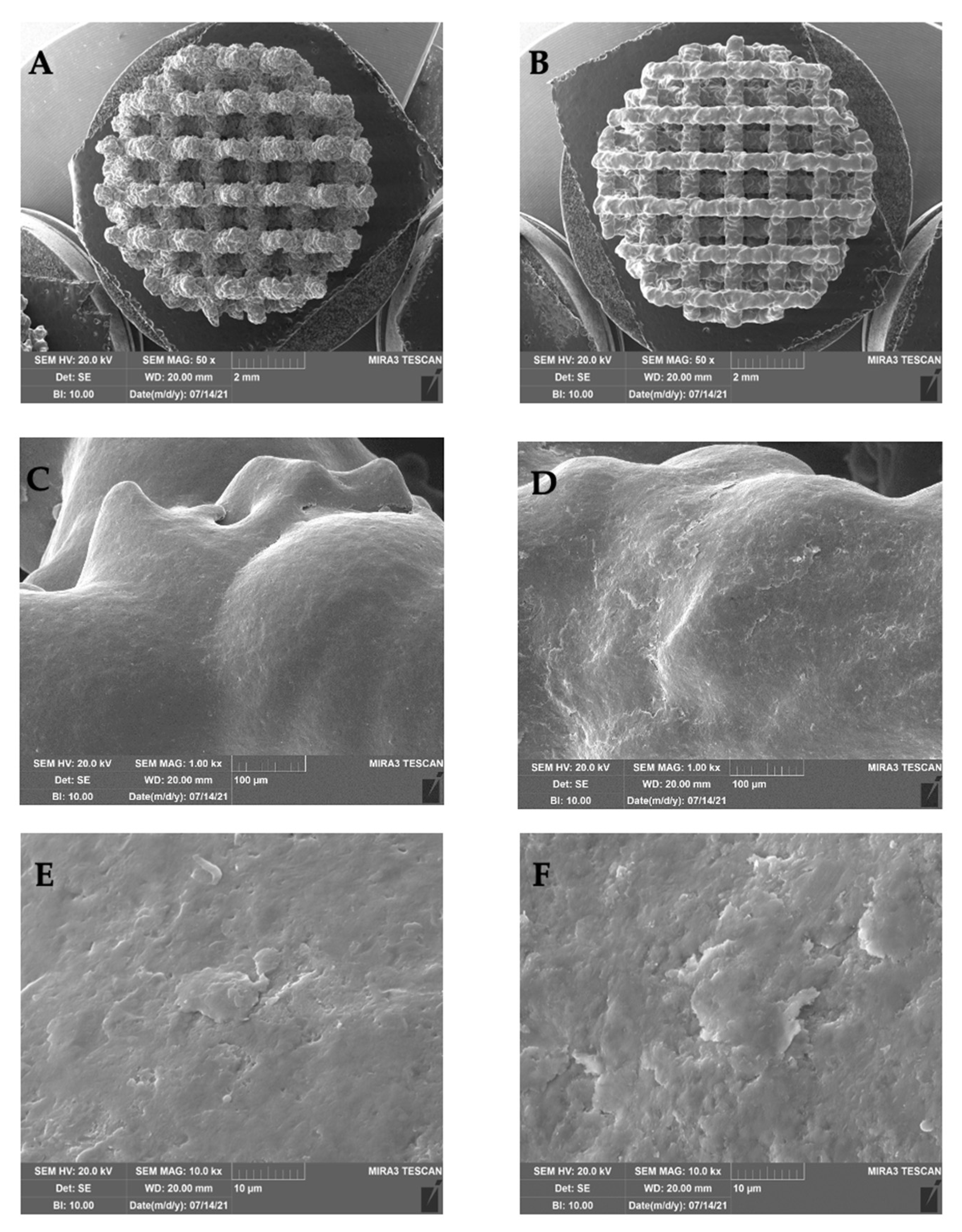
4.1. Trabecular Titanium Scaffolds
4.2. Isolation of Human Adipose-Derived Stem Cells (hASCs)
4.3. Cell Seeding and Culture on the Scaffold
4.4. Conditioned Medium (CM)
4.5. Cell Seeding on Monolayer
4.6. Proliferation Assay (WST)
4.7. ALP Activity
4.8. RNA Isolation and Reverse Transcriptase Quantitative Real-Time PCR (qRT-PCR)
4.9. Immunofluorescence Assay
4.10. Scanning Electron Microscope (SEM) Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frosch, K.; Barvencik, F.; Viereck, V.; Lohmann, C.H.; Dresing, K.; Breme, J.; Brunner, E.; Stürmer, K.M. Growth Behavior, Matrix Production, and Gene Expression of Human Osteoblasts in Defined Cylindrical Titanium Channels. J. Biomed. Mater. Res. 2004, 68A, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Habibovic, P.; Vandendoel, M.; Wilson, C.; Dewijn, J.; Vanblitterswijk, C.; Degroot, K. Bone Ingrowth in Porous Titanium Implants Produced by 3D Fiber Deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Biomaterials and Interface with Bone. Osteoporos. Int. 2011, 22, 2037–2042. [Google Scholar] [CrossRef]
- Marin, E.; Fusi, S.; Pressacco, M.; Paussa, L.; Fedrizzi, L. Characterization of Cellular Solids in Ti6Al4V for Orthopaedic Implant Applications: Trabecular Titanium. J. Mech. Behav. Biomed. Mater. 2010, 3, 373–381. [Google Scholar] [CrossRef]
- Pria, P.D.; Pressacco, M.; Veronesi, E. Nuove Frontiere nell’Osteointegrazione: Il Trabecular TitaniumTM. Sphera Med. J. 2008, 7, 46–50. [Google Scholar]
- Regis, M.; Marin, E.; Fedrizzi, L.; Pressacco, M. Additive Manufacturing of Trabecular Titanium Orthopedic Implants. MRS Bull. 2015, 40, 137–144. [Google Scholar] [CrossRef]
- Toninato, R.; Cattano, G.; Salatin, E.; Pressacco, M. Qualification of Trabecular Titanium lattice structure made with Laser Powder Bed Fusion. In Proceedings of the International Conference on Advanced Manufacturing 2023, Washington, DC, USA, 30 October–3 November 2023. [Google Scholar]
- Zagra, L. Abstracts from the 10Th Congress of the European Hip Society. HIP Int. 2012, 22, 405–478. [Google Scholar] [CrossRef]
- Salatin, E.; Cattano, G.; Toninato, R.; Pressacco, M. ISTA—Preliminary Comparison between Trabecular Titanium Porous Structure Manufactured via EBM and SLM Technologies Friction and Primary Stability Performances. In Proceedings of the 33rd Annual Congress Emerging Technologies in Arthroplasty the Implant and Beyond, Maui, HI, USA, 31 August–3 September 2022. [Google Scholar]
- Devine, D.; Arens, D.; Burelli, S.; Bloch, H.R.; Boure, L. In vivo evaluation of the osteointegration of new highly porous trabecular titanium TM. Orthop. Proc. 2012, 97, 201. [Google Scholar]
- Dall’Ava, L.; Hothi, H.; Henckel, J.; Di Laura, A.; Tirabosco, R.; Eskelinen, A.; Skinner, J.; Hart, A. Osseointegration of Retrieved 3D-Printed, off-the-Shelf Acetabular Implants. Bone Jt. Res. 2021, 10, 388–400. [Google Scholar] [CrossRef]
- De Martino, I.; Sculco, P.; Meyers, K.; Nocon, A.; Wright, T.; Sculco, T. Initial Stability in Highly Porous Metal Acetabular Cups- A Biomechanical Study. In Proceedings of the 29th Annual Congress of the International Society for Technology in Arthroplasty (ISTA), Boston, MA, USA, 5–8 October 2016. [Google Scholar]
- Yoshimoto, K.; Nakashima, Y.; Wakiyama, M.; Hara, D.; Nakamura, A.; Iwamoto, M. Initial Stability of a Highly Porous Titanium Cup in an Acetabular Bone Defect Model. J. Orthop. Sci. 2018, 23, 665–670. [Google Scholar] [CrossRef]
- Gastaldi, G.; Asti, A.; Scaffino, M.F.; Visai, L.; Saino, E.; Cometa, A.M.; Benazzo, F. Human Adipose-derived Stem Cells (hASCs) Proliferate and Differentiate in Osteoblast-like Cells on Trabecular Titanium Scaffolds. J. Biomed. Mater. Res. 2010, 94A, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Benazzo, F.; Botta, L.; Scaffino, M.F.; Caliogna, L.; Marullo, M.; Fusi, S.; Gastaldi, G. Trabecular Titanium Can Induce in Vitro Osteogenic Differentiation of Human Adipose Derived Stem Cells without Osteogenic Factors. J. Biomed. Mater. Res. 2014, 102, 2061–2071. [Google Scholar] [CrossRef]
- Vincenzo, S.; Leo, M.; Furio, P.; Ambra, G.; Francesca, F.; Vincenzo, L.; Silvia, B.; Hans Rudolf, B.; Francesco, C. Genetic Effects of Trabecular Titanium on MG-63 Cell Line: A Genetic Profiling Evaluation. ISRN Mater. Sci. 2011, 2011, 392763. [Google Scholar] [CrossRef][Green Version]
- Thelen, S.; Barthelat, F.; Brinson, L.C. Mechanics Considerations for Microporous Titanium as an Orthopedic Implant Material. J. Biomed. Mater. Res. 2004, 69A, 601–610. [Google Scholar] [CrossRef]
- Casagrande, S.; Tiribuzi, R.; Cassetti, E.; Selmin, F.; Gervasi, G.L.; Barberini, L.; Freddolini, M.; Ricci, M.; Schoubben, A.; Cerulli, G.G.; et al. Biodegradable Composite Porous Poly(DL -Lactide- Co -Glycolide) Scaffold Supports Mesenchymal Stem Cell Differentiation and Calcium Phosphate Deposition. Artif. Cells Nanomed. Biotechnol. 2018, 46, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Taher Mohamed, S.A.; Emin, N. Effects of Using Collagen and Aloe Vera Grafted Fibroin Scaffolds on Osteogenic Differentiation of Rat Bone Marrow Mesenchymal Stem Cells in SBF-Enriched Cell Culture Medium. Biomed. Mater. 2024, 19, 015011. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; Bidossi, A.; De Vecchi, E.; Francaviglia, N.; Romano, A.; Maestretti, G.; Tartara, F.; De Girolamo, L. Superior Osteo-Inductive and Osteo-Conductive Properties of Trabecular Titanium vs. PEEK Scaffolds on Human Mesenchymal Stem Cells: A Proof of Concept for the Use of Fusion Cages. Int. J. Mol. Sci. 2021, 22, 2379. [Google Scholar] [CrossRef]
- Mosconi, M.; Carlotto, E.; Caliogna, L.; Berni, M.; Gastaldi, G.; Conti, M.; Brancato, A.M.; Bina, V.; Minervini, D.; Malpede, S.; et al. Titanium Biohybrid Middle Ear Prostheses: A Preliminary In Vitro Study. J. Funct. Biomater. 2023, 14, 561. [Google Scholar] [CrossRef]
- Liu, J.C.; Lengner, C.J.; Gaur, T.; Lou, Y.; Hussain, S.; Jones, M.D.; Borodic, B.; Colby, J.L.; Steinman, H.A.; Van Wijnen, A.J.; et al. Runx2 Protein Expression Utilizes the Runx2 P1 Promoter to Establish Osteoprogenitor Cell Number for Normal Bone Formation. J. Biol. Chem. 2011, 286, 30057–30070. [Google Scholar] [CrossRef]
- Pham, Q.P.; Kurtis Kasper, F.; Scott Baggett, L.; Raphael, R.M.; Jansen, J.A.; Mikos, A.G. The Influence of an in Vitro Generated Bone-like Extracellular Matrix on Osteoblastic Gene Expression of Marrow Stromal Cells. Biomaterials 2008, 29, 2729–2739. [Google Scholar] [CrossRef]
- Subramaniam, M.; Pitel, K.S.; Withers, S.G.; Drissi, H.; Hawse, J.R. TIEG1 Enhances Osterix Expression and Mediates Its Induction by TGFβ and BMP2 in Osteoblasts. Biochem. Biophys. Res. Commun. 2016, 470, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. Signaling and Transcriptional Regulation in Osteoblast Commitment and Differentiation. Front. Biosci. 2007, 12, 3068. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.-P. Cell Signaling and Transcriptional Regulation of Osteoblast Lineage Commitment, Differentiation, Bone Formation, and Homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Caliogna, L.; Bina, V.; Botta, L.; Benazzo, F.M.; Medetti, M.; Maestretti, G.; Mosconi, M.; Cofano, F.; Tartara, F.; Gastaldi, G. Osteogenic Potential of Human Adipose Derived Stem Cells (hASCs) Seeded on Titanium Trabecular Spinal Cages. Sci. Rep. 2020, 10, 18284. [Google Scholar] [CrossRef]
- Huang, X.; Das, R.; Patel, A.; Duc Nguyen, T. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018, 4, 216–237. [Google Scholar] [CrossRef]



| Gene | Target Transcript | Quantitect Primer Assay (Quiagen) | Amplicon Length |
|---|---|---|---|
| ALP | NM_000478 | QT00012957 | 110 bp |
| COL1A1 | NM_000088 | QT00037793 | 127 bp |
| OSX | NM_152860 | QT00213514 | 120 pb |
| BGLAP | NM_199173 | QT00232771 | 90 pb |
| β2M | NM_004048 | QT00088935 | 98 pb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caliogna, L.; Berni, M.; Gastaldi, G.; Grassi, F.A.; Jannelli, E.; Mosconi, M.; Salatin, E.; Burelli, S.; Toninato, R.; Pressacco, M.; et al. Trabecular Titanium Architecture Drives Human Mesenchymal Stem Cell Proliferation and Bone Differentiation. Int. J. Mol. Sci. 2025, 26, 6354. https://doi.org/10.3390/ijms26136354
Caliogna L, Berni M, Gastaldi G, Grassi FA, Jannelli E, Mosconi M, Salatin E, Burelli S, Toninato R, Pressacco M, et al. Trabecular Titanium Architecture Drives Human Mesenchymal Stem Cell Proliferation and Bone Differentiation. International Journal of Molecular Sciences. 2025; 26(13):6354. https://doi.org/10.3390/ijms26136354
Chicago/Turabian StyleCaliogna, Laura, Micaela Berni, Giulia Gastaldi, Federico Alberto Grassi, Eugenio Jannelli, Mario Mosconi, Elisa Salatin, Silvia Burelli, Riccardo Toninato, Michele Pressacco, and et al. 2025. "Trabecular Titanium Architecture Drives Human Mesenchymal Stem Cell Proliferation and Bone Differentiation" International Journal of Molecular Sciences 26, no. 13: 6354. https://doi.org/10.3390/ijms26136354
APA StyleCaliogna, L., Berni, M., Gastaldi, G., Grassi, F. A., Jannelli, E., Mosconi, M., Salatin, E., Burelli, S., Toninato, R., Pressacco, M., & Pasta, G. (2025). Trabecular Titanium Architecture Drives Human Mesenchymal Stem Cell Proliferation and Bone Differentiation. International Journal of Molecular Sciences, 26(13), 6354. https://doi.org/10.3390/ijms26136354








