Shifting Shapes: The Endothelial-to-Mesenchymal Transition as a Driver for Cancer Progression
Abstract
1. Introduction
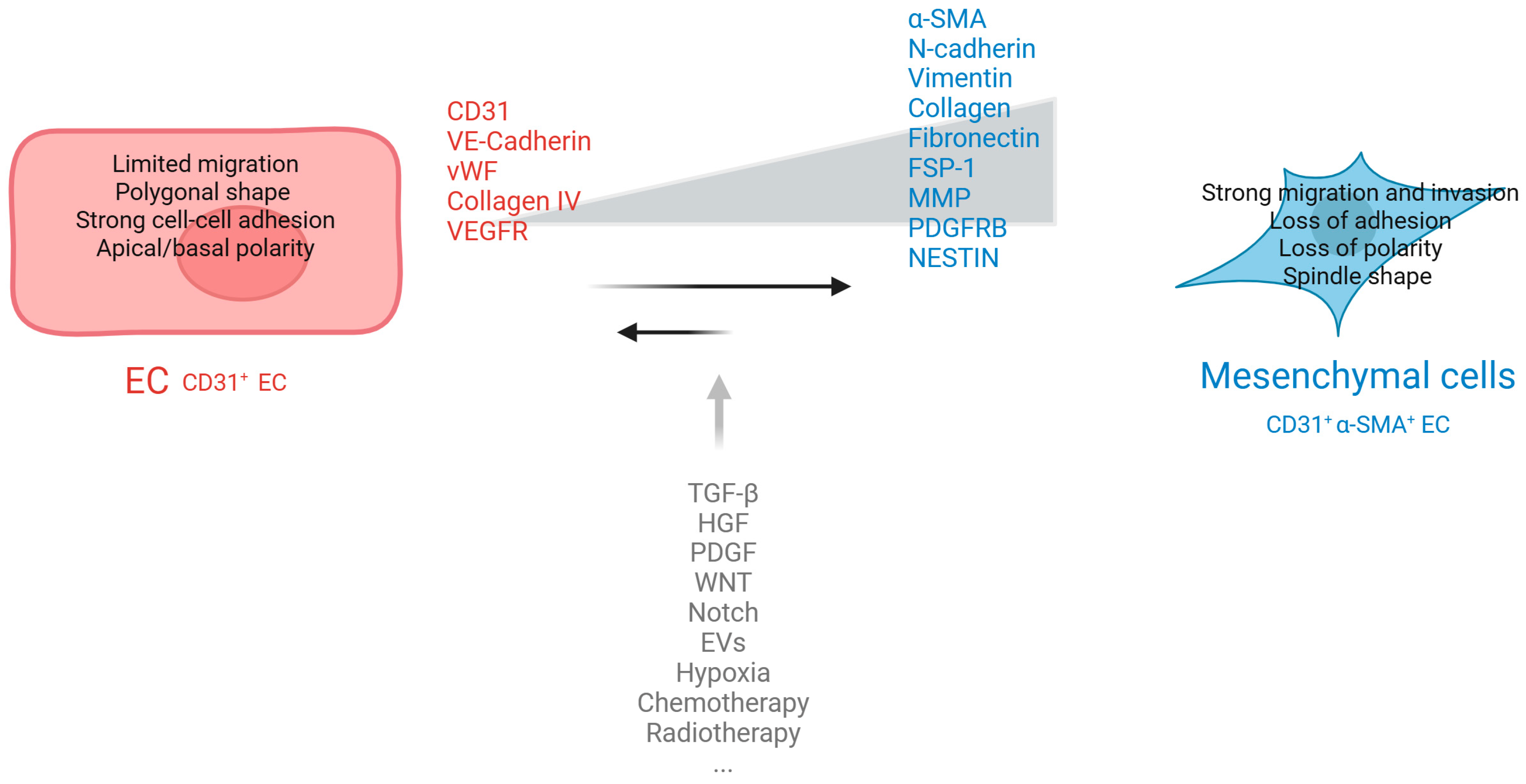
2. EMT and EndMT Interplay in Cancer Progression
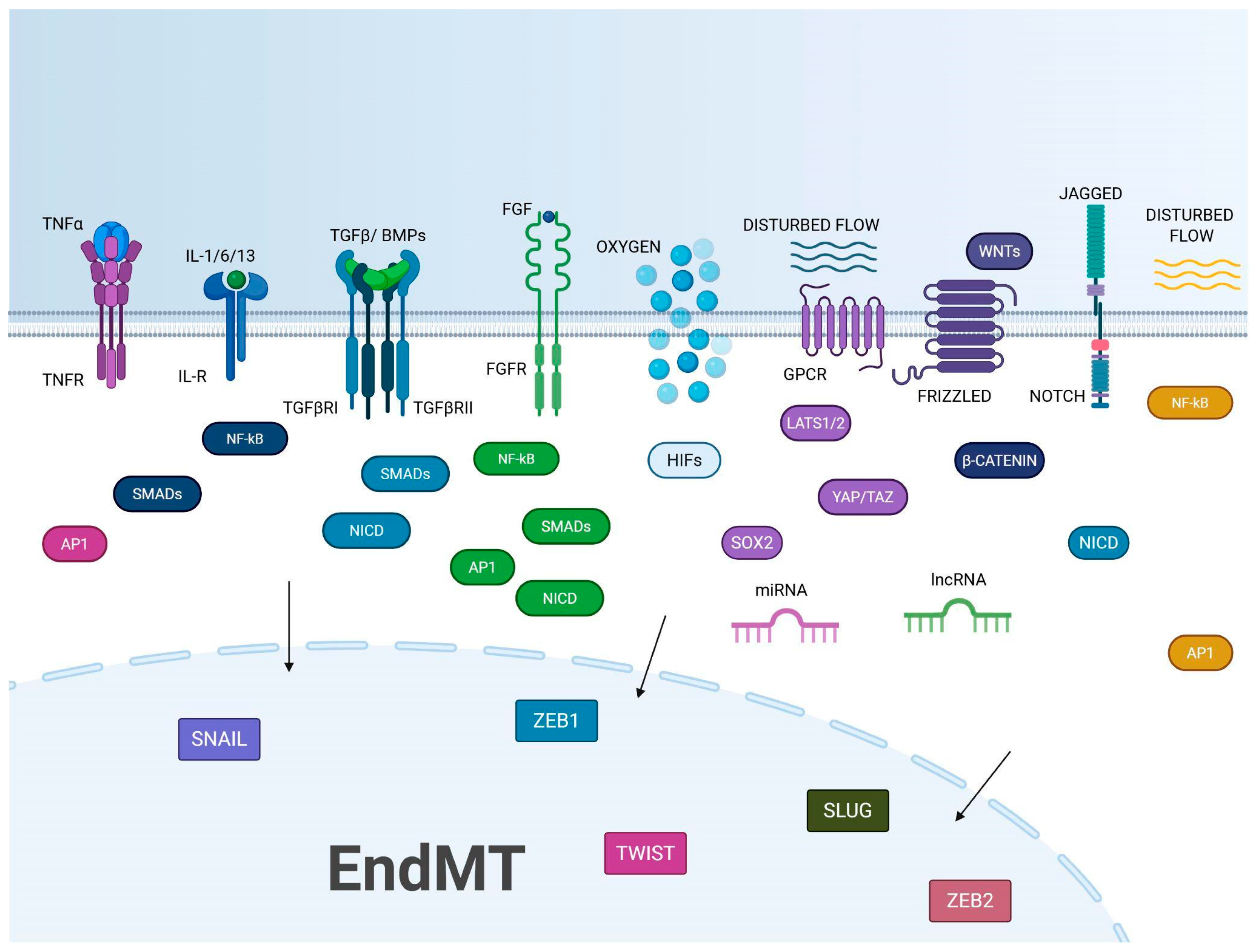
3. KEY Actors in EndMT
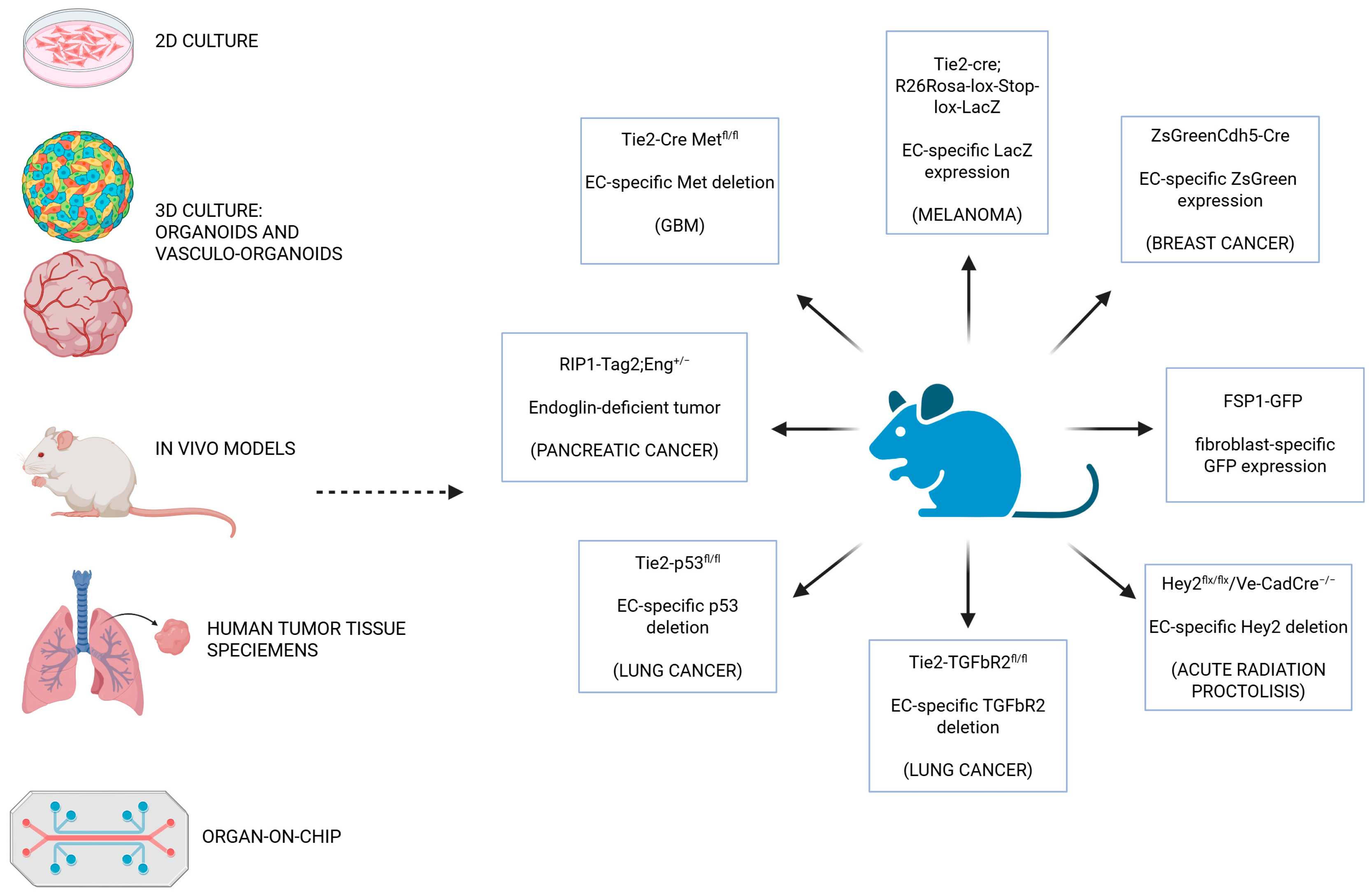
4. Models to Study EndMT
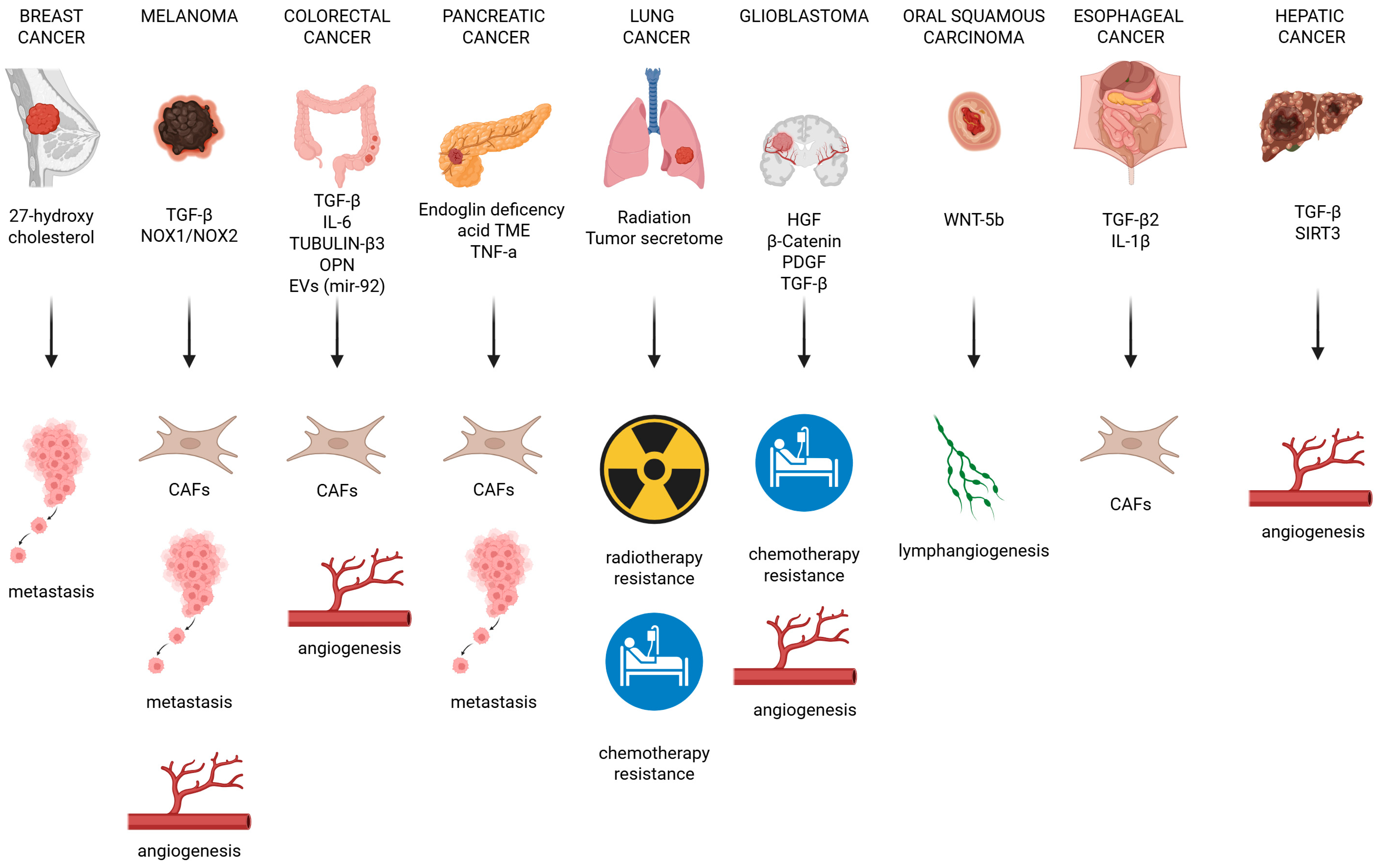
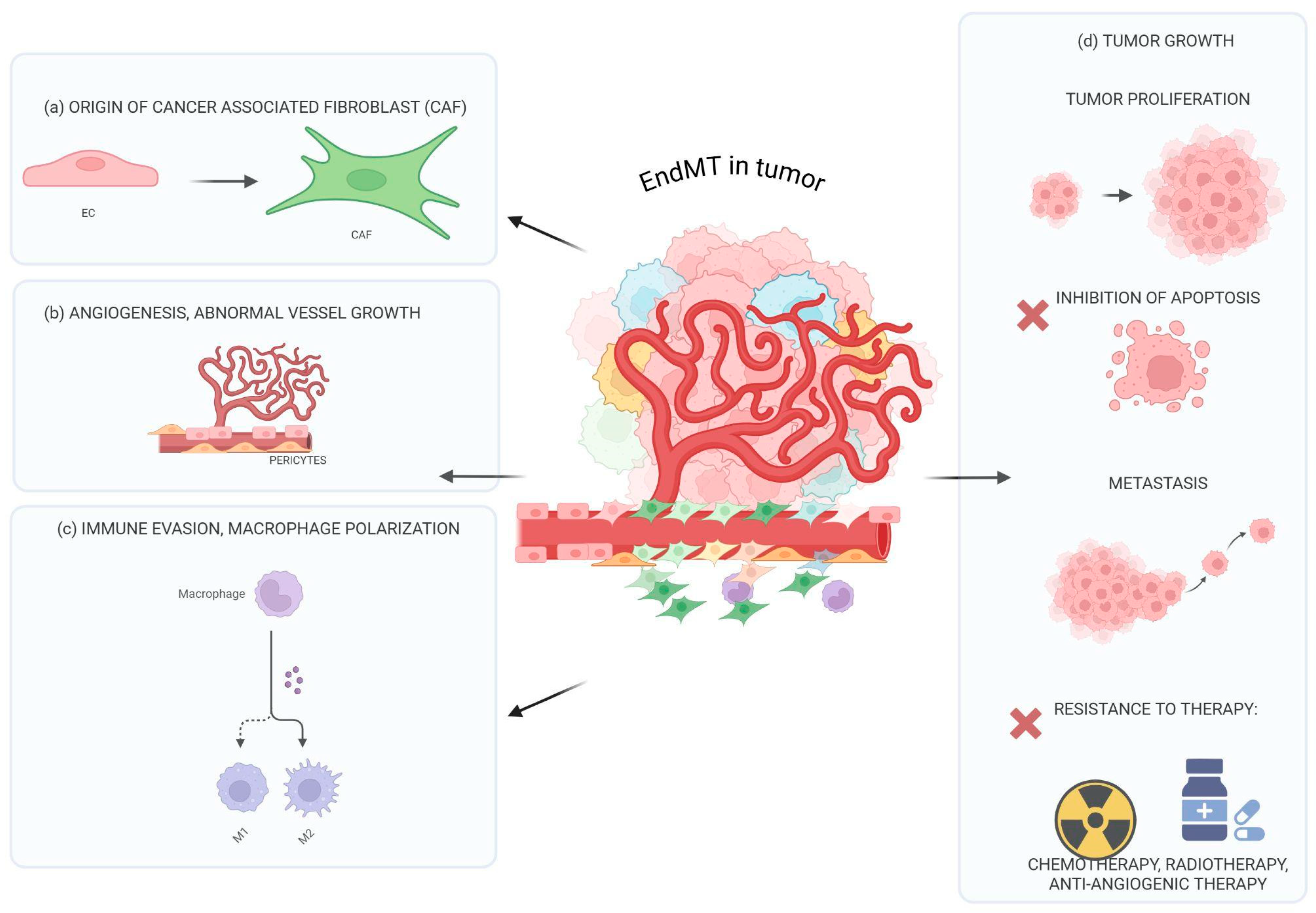
5. The Influence of EndMT on Tumor Progression
6. EndMT and Treatment Resistance
7. EndMT Inhibitors: Bench to Bedside Struggles
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 4, 374–403. [Google Scholar] [CrossRef]
- Potente, M.; Mäkinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 477–494. [Google Scholar] [CrossRef]
- Zeng, Q.; Mousa, M.; Nadukkandy, A.S.; Franssens, L.; Alnaqbi, H.; Alshamsi, F.Y.; Safar, H.A.; Carmeliet, P. Understanding tumour endothelial cell heterogeneity and function from single-cell omics. Nat. Rev. Cancer 2023, 23, 544–564. [Google Scholar] [CrossRef]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef]
- Platel, V.; Faure, S.; Corre, I.; Clere, N. Endothelial-to-mesenchymal transition (EndoMT): Roles in tumorigenesis, metastatic extravasation and therapy resistance. J. Oncol. 2019, 1, 8361945. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, L. Endothelial-to-mesenchymal transition in tumour progression and its potential roles in tumour therapy. Ann. Med. 2023, 55, 1058–1069. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Dejana, E.; Hirschi, K.K.; Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun. 2017, 8, 14361. [Google Scholar] [CrossRef]
- Ubil, E.; Duan, J.; Pillai, I.C.; Rosa-Garrido, M.; Wu, Y.; Bargiacchi, F.; Lu, Y.; Stanbouly, S.; Huang, J.; Rojas, M.; et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 2014, 514, 585–590. [Google Scholar] [CrossRef]
- Xiao, L.; Dudley, A.C. Intro Fine-tuning vascular fate during endothelial-mesenchymal transition. J. Pathol. 2017, 241, 25–35. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Huang, M.; Liu, T.; Ma, P.; Mitteer, R.A., Jr.; Zhang, Z.; Kim, H.J.; Yeo, E.; Zhang, D.; Cai, P.; Li, C.; et al. c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J. Clin. Investig. 2016, 126, 1801–1814. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, A.R.; Nam, J.K.; Kim, J.M.; Kim, J.Y.; Seo, H.R.; Lee, H.J.; Cho, J.; Lee, Y.J. Tumour-vasculature development via endothelial-tomesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nat. Commun. 2018, 9, 5108. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, L.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, P.; Espigol-Frigole, G.; McCormick, P.J.; Salvucci, O.; Maric, D.; Uldrick, T.S.; Polizzotto, M.N.; Yarchoan, R.; Tosato, G. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res. 2012, 72, 1157–1169. [Google Scholar] [CrossRef]
- Garcia, J.; Sandi, M.J.; Cordelier, P.; Binétruy, B.; Pouysségur, J.; Iovanna, J.L.; Tournaire, R. Tie1 deficiency induces endothelial-mesenchymal transition. EMBO Rep. 2012, 13, 431–439. [Google Scholar] [CrossRef]
- Zhu, K.; Pan, Q.; Jia, L.Q.; Dai, Z.; Ke, A.W.; Zeng, H.Y.; Tang, Z.Y.; Fan, J.; Zhou, J. MiR-302c inhibits tumor growth of hepatocellular carcinoma by suppressing the endothelial-mesenchymal transition of endothelial cells. Sci. Rep. 2014, 4, 5524. [Google Scholar] [CrossRef]
- Nie, L.; Lyros, O.; Medda, R.; Jovanovic, N.; Schmidt, J.L.; Otterson, M.F.; Johnson, C.P.; Behmaram, B.; Shaker, R.; Rafiee, P. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: Role of IL-1b and TGF-b2. Am. J. Physiol. Cell Physiol. 2014, 307, C859–C877. [Google Scholar] [CrossRef] [PubMed]
- Ghiabi, P.; Jiang, J.; Pasquier, J.; Maleki, M.; Abu-Kaoud, N.; Halabi, N.; Guerrouahen, B.S.; Rafii, S.; Rafii, A. Breast cancer cells promote a notch-dependent mesenchymal phenotype in endothelial cells participating to a pro-tumoral niche. J. Transl. Med. 2015, 13, 27. [Google Scholar] [CrossRef]
- Choi, S.H.; Nam, J.K.; Kim, B.Y.; Jang, J.; Jin, Y.B.; Lee, H.J.; Park, S.; Ji, Y.H.; Cho, J.; Lee, Y.J. HSPB1 inhibits the endothelial-to-mesenchymal transition to suppress pulmonary fibrosis and lung tumourigenesis. Cancer Res. 2016, 76, 1019–1030. [Google Scholar] [CrossRef]
- Wawro, M.E.; Chojnacka, K.; Wieczorek-Szukała, K.; Sobierajska, K.; Niewiarowska, J. Invasive Colon Cancer Cells Induce Transdifferentiation of Endothelium to Cancer-Associated Fibroblasts through Microtubules Enriched in Tubulin-β3. Int. J. Mol. Sci. 2018, 20, 53. [Google Scholar] [CrossRef]
- Fan, C.S.; Chen, W.S.; Chen, L.L.; Chen, C.C.; Hsu, Y.T.; Chua, K.V.; Wang, H.D.; Huang, T.S. Osteopontin-integrin engagement induces HIF-1α-TCF12-mediated endothelial-mesenchymal transition to exacerbate colorectal cancer. Oncotarget 2018, 9, 4998–5015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, L.; Gao, Y.; Wang, Y.; Liu, Y.; Zhang, H.; Wang, Q.; Hu, F.; Li, J.; Tan, J.; et al. Role of aneuploid circulating tumor cells and CD31(þ) circulating tumor endothelial cells in predicting and monitoring antiangiogenic therapy efficacy in advanced NSCLC. Mol. Oncol. 2021, 15, 2891–2909. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, S.; Garcia, E.; Jay, D.J.; Persad, S. Molecular Mechanisms Regulating Epithelial Mesenchymal Transition (EMT) to Promote Cancer Progression. Int. J. Mol. Sci. 2025, 26, 4364. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.M.; Yuan, H.Q.; Ren, Z.; Qu, S.L.; Liu, L.S.; Dang-Heng, W.; Yin, K.; Fu, M.; Jiang, Z.S. Endothelial to mesenchymal transition in atherosclerotic vascular remodeling. Clin. Chim. Acta 2019, 490, 34–38. [Google Scholar] [CrossRef]
- Fontana, R.; Mestre-Farrera, A.; Yang, J. Update on Epithelial-Mesenchymal Plasticity in Cancer Progression. Annu. Rev. Pathol. 2024, 19, 133–156. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; Dedhar, S.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Van Meeteren, L.A.; ten Dijke, P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2012, 347, 177–186. [Google Scholar] [CrossRef]
- Xiao, L.; Kim, D.J.; Davis, C.L.; McCann, J.V.; Dunleavey, J.M.; Vanderlinden, A.K.; Xu, N.; Pattenden, S.G.; Frye, S.V.; Xu, X.; et al. Tumour endothelial cells with distinct patterns of TGFβ-driven endothelial-to-mesenchymal transition. Cancer Res. 2015, 75, 1244–1254. [Google Scholar] [CrossRef]
- Pinto, M.T.; Ferreira Melo, F.U.; Malta, T.M.; Rodrigues, E.S.; Plaça, J.R.; Silva, W.A., Jr.; Panepucci, R.A.; Covas, D.T.; de Oliveira Rodrigues, C.; Kashima, S. Endothelial cells from different anatomical origin have distinct responses during SNAIL/TGF-β2-mediated endothelial-mesenchymal transition. Am. J. Transl. Res. 2018, 10, 4065–4081. [Google Scholar]
- Ferreira, F.U.; Eduardo Botelho Souza, L.; Hassibe Thomé, C.; Tomazini Pinto, M.; Origassa, C.; Salustiano, S.; Marcel Faça, V.; Olsen Câmara, N.; Kashima, S.; Tadeu Covas, D. Endothelial cells tissue-specific origins affects their responsiveness to TGF-β2 during endothelial-to-mesenchymal transition. Int. J. Mol. Sci. 2019, 20, 458. [Google Scholar] [CrossRef]
- Li, Z.X.; Chen, J.X.; Zheng, Z.J.; Cai, W.J.; Yang, X.B.; Huang, Y.Y.; Gong, Y.; Xu, F.; Chen, Y.S.; Lin, L. TGF-β1 promotes human breast cancer angiogenesis and malignant behavior by regulating endothelial-mesenchymal transition. Front. Oncol. 2022, 12, 1051148. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Q.; Zhao, J.; Zheng, C.G.; Chun, J. Roles of notch signaling pathway and endothelial-mesenchymal transition in vascular endothelial dysfunction and atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6485–6491. [Google Scholar] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Liu, T.; Deng, S.H.; Han, R.; Zhang, T.; Li, J.; Xu, Y. Alpha-1 antitrypsin induces epithelial-to-mesenchymal transition, endothelial-to-mesenchymal transition, and drug resistance in lung cancer cells. Onco Targets Ther. 2020, 13, 3751–3763. [Google Scholar] [CrossRef]
- Liu, T.; Ma, W.; Xu, H.; Huang, M.; Zhang, D.; He, Z.; Zhang, L.; Brem, S.; O’Rourke, D.M.; Gong, Y.; et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun. 2018, 9, 3439. [Google Scholar] [CrossRef]
- Mahler, G.J.; Farrar, E.J.; Butcher, J.T. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 121–130. [Google Scholar] [CrossRef]
- Motallebnejad, P.; Rajesh, V.V.; Azarin, S.M. Evaluating the Role of IL-1β in Transmigration of Triple Negative Breast Cancer Cells Across the Brain Endothelium. Cell Mol. Bioeng. 2022, 15, 99–114. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Wakabayashi, I.; Kimuro, S.; Takahashi, N.; Takahashi, K.; Kobayashi, M.; Maishi, N.; Podyma-Inoue, K.A.; Hida, K.; Miyazono, K.; et al. TNF-α enhances TGF-β-induced endothelial-to-mesenchymal transition via TGF-β signal augmentation. Cancer Sci. 2020, 111, 2385–2399. [Google Scholar] [CrossRef]
- Adjuto-Saccone, M.; Soubeyran, P.; Garcia, J.; Audebert, S.; Camoin, L.; Rubis, M.; Roques, J.; Binétruy, B.; Iovanna, J.L.; Tournaire, R. TNF-α induces endothelial-mesenchymal transition promoting stromal development of pancreatic adenocarcinoma. Cell Death Dis. 2021, 12, 649. [Google Scholar] [CrossRef]
- Montorfano, I.; Becerra, A.; Cerro, R.; Echeverría, C.; Sáez, E.; Morales, M.G.; Fernández, R.; Cabello-Verrugio, C.; Simon, F. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-β1 and TGF-β2-dependent pathway. Lab. Investig. 2014, 94, 1068–1082. [Google Scholar] [CrossRef]
- Doerr, M.; Morrison, J.; Bergeron, L.; Coomber, B.L.; Viloria-Petit, A. Differential effect of hypoxia on early endothelial-mesenchymal transition response to transforming growth beta isoforms 1 and 2. Microvasc. Res. 2016, 108, 48–63. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, W.; Dong, H.Y.; Liu, M.L.; Luo, Y.; Li, Z.C. Hypoxia induces endothelial mesenchymal transition in pulmonary vascular remodeling. Int. J. Mol. Med. 2018, 42, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Barton, S.M.; Grabham, P.W.; Rumeld, A.L.; Okochi, S.; Street, C.; Kadenhe-Chiweshe, A.; Boboila, S.; Yamashiro, D.J.; Connolly, E.P. High-Dose Radiation Increases Notch1 in Tumor vasculature. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 857–866. [Google Scholar] [CrossRef]
- Bouten, R.M.; Dalgard, C.L.; Soltis, A.R.; Slaven, J.E.; Day, R.M. Transcriptomic profiling and pathway analysis of cultured human lung microvascular endothelial cells following ionizing radiation exposure. Sci. Rep. 2021, 11, 24214. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wu, Z.; Li, Z.; Huang, X.; Hu, Z.; Yu, H.; Yuan, Z.; Shi, J.; Hu, J.; Mulati, Y.; et al. Single-cell analysis extracted CAFs-related genes to established online app to predict clinical outcome and radiotherapy prognosis of prostate cancer. Clin. Transl. Oncol. 2024, 26, 1240–1255. [Google Scholar] [CrossRef]
- Wu, D.; Deng, S.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; Xu, Y. TGF-β1-mediated exosomal lnc-MMP2-2 increases blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis. 2021, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β signaling in the tumour metabolic microenvironment and targeted therapies. BioMed Central 2022, 15, 135. [Google Scholar]
- Merk, L.; Regel, K.; Eckhardt, H.; Evers, M.; El-Ayoubi, A.; Mittelbronn, M.; Krüger, M.; Gérardy, J.J.; Mack, A.F.; Naumann, U. Blocking TGF-β and Epithelial-to-Mesenchymal Transition (EMT)-mediated activation of vessel-associated mural cells in glioblastoma impacts tumour angiogenesis. Free Neuropathol. 2024, 4, 1–18. [Google Scholar]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Goumans, M.J.; van Zonneveld, A.J.; ten Dijke, P. Transforming growth factor β-induced endothelial-to-mesenchymal transition: A switch to cardiac fibrosis? Trends Cardiovasc. Med. 2008, 18, 293–298. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Yao, J.; Caruana, G.; Ricardo, S.D.; Yamamoto, Y.; Yamamoto, H.; Bertram, J.F. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 2010, 59, 2612–2624. [Google Scholar] [CrossRef]
- Medici, D.; Potenta, S.; Kalluri, R. Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem. J. 2011, 437, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, F.; Zha, S.; Cao, Q.; Sheng, J.; Chen, S. SIRT1 inhibits TGF-_-induced endothelial-mesenchymal transition in human endothelial cells with Smad4 deacetylation. J. Cell Physiol. 2018, 233, 9007–9014. [Google Scholar] [CrossRef]
- Lin, J.R.; Zheng, Y.J.; Zhang, Z.B.; Shen, W.L.; Li, X.D.; Wei, T.; Ruan, C.C.; Chen, X.H.; Zhu, D.L.; Gao, P.J. Suppression of Endothelial-to-Mesenchymal Transition by SIRT (Sirtuin) 3 Alleviated the Development of Hypertensive Renal Injury. Hypertension 2018, 72, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Roth, M.; Zhong, J.; Campregher, C.; Binder, P.; Burian, B.; Petkov, V.; Block, L.H. The interaction of endothelin-1 and TGF-beta1 mediates vascular cell remodeling. PLoS ONE 2013, 8, e73399. [Google Scholar] [CrossRef]
- Wermuth, P.J.; Li, Z.; Mendoza, F.A.; Jimenez, S.A. Stimulation of Transforming Growth Factor-β1-Induced Endothelial-To-Mesenchymal Transition and Tissue Fibrosis by Endothelin-1 (ET-1): A Novel Profibrotic Effect of ET-1. PLoS ONE 2016, 11, e0161988. [Google Scholar] [CrossRef]
- Pang, L.; Yang, S.; Dai, W.; Wu, S.; Kong, J. Role of caveolin-1 in human organ function and disease: Friend or foe? Carcinogenesis 2022, 43, 2–11. [Google Scholar] [CrossRef]
- Becerra, A.; Rojas, M.; Vallejos, A.; Villegas, V.; Pérez, L.; Cabello-Verrugio, C.; Simon, F. Endothelial fibrosis induced by suppressed STAT3 expression mediated by signaling involving the TGF-β1/ALK5/Smad pathway. Lab. Investig. 2017, 97, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Krizbai, I.A.; Gasparics, Á.; Nagy’’oszi, P.; Fazakas, C.; Molnár, J.; Wilhelm, I.; Bencs, R.; Rosivall, L.; Sebe, A. Endothelial-mesenchymal transition of brain endothelial cells: Possible role during metastatic extravasation. PLoS ONE 2015, 10, e0119655. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Dong, C.; Jiang, K.; Xu, Z.; Li, R.; Guo, K.; Shao, S.; Wang, L. Heterogeneity of cancer-associated fibroblasts and roles in the progression, prognosis, and therapy of hepatocellular carcinoma. J. Hematol. Oncol. 2019, 12, 101. [Google Scholar] [CrossRef]
- Wu, D.M.; Deng, S.H.; Zhou, J.; Han, R.; Liu, T.; Zhang, T.; Li, J.; Chen, J.P.; Xu, Y. PLEK2 mediates metastasis and vascular invasion via the ubiquitin-dependent degradation of SHIP2 in non-small cell lung cancer. Int. J. Cancer 2020, 146, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Song, Y.; Seo, H.R. GSK-3beta regulates the endothelialto-mesenchymal transition via reciprocal crosstalk between NSCLC cells and HUVECs in multicellular tumor spheroid models. J. Exp. Clin. Cancer Res. 2019, 38, 46. [Google Scholar] [CrossRef]
- Marin-Ramos, N.I.; Jhaveri, N.; Thein, T.Z.; Fayngor, R.A.; Chen, T.C.; Hofman, F.M. NEO212, a conjugate of temozolomide and perillyl alcohol, blocks the endothelial-to-mesenchymal transition in tumor-associated brain endothelial cells in glioblastoma. Cancer Lett. 2019, 442, 170–180. [Google Scholar] [CrossRef]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef]
- Guan, S.; Zhou, J. CXCR7 attenuates the TGF-_-induced endothelial-to-mesenchymal transition and pulmonary fibrosis. Mol. Biosyst. 2017, 13, 2116–2124. [Google Scholar] [CrossRef]
- Patel, J.; Baz, B.; Wong, H.Y.; Lee, J.S.; Khosrotehrani, K. Accelerated Endothelial to Mesenchymal Transition Increased Fibrosis via Deleting Notch Signaling in Wound Vasculature. J. Investig. Dermatol. 2018, 138, 1166–1175. [Google Scholar] [CrossRef]
- Wang, S.H.; Chang, J.S.; Hsiao, J.R.; Yen, Y.C.; Jiang, S.S.; Liu, S.H.; Chen, Y.L.; Shen, Y.Y.; Chang, J.Y.; Chen, Y.W. Tumour cell-derived WNT5B modulates in vitro lymphangiogenesis via induction of partial endothelial-mesenchymal transition of lymphatic endothelial cells. Oncogene 2017, 36, 1503–1515. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, D.; Wu, J.Y.; Xing, K.; Yeo, E.; Li, C.; Zhang, L.; Holland, E.; Yao, L.; Qin, L.; et al. Wnt mediated endothelial transformation into mesenchymal stem cell-like cells induces chemoresistance in glioblastoma. Sci. Transl. Med. 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Role of cellular senescence and NOX4-mediated oxidative stress in systemic sclerosis pathogenesis. Curr. Rheumatol. Rep. 2015, 17, 473. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, C.; Montorfano, I.; Sarmiento, D.; Becerra, A.; Nuñez-Villena, F.; Figueroa, X.F.; Cabello-Verrugio, C.; Elorza, A.A.; Riedel, C.; Simon, F. Lipopolysaccharide induces a fibrotic-like phenotype in endothelial cells. J. Cell Mol. Med. 2013, 17, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Ren, C.; Wu, X.; Zhang, Y.; Bai, X.; Lin, Y.; Li, M.; Fu, J.; Kopylov, P.; et al. Low-Intensity Pulsed Ultrasound Prevents the Oxidative Stress Induced Endothelial-Mesenchymal Transition in Human Aortic Endothelial Cells. Cell Physiol. Biochem. 2018, 45, 1350–1365. [Google Scholar] [CrossRef]
- Xu, X.; Tan, X.; Tampe, B.; Sanchez, E.; Zeisberg, M.; Zeisberg, E.M. Snail Is a Direct Target of Hypoxia-inducible Factor 1alpha (HIF1alpha) in Hypoxia-induced Endothelial to Mesenchymal Transition of Human Coronary Endothelial Cells. J. Biol. Chem. 2015, 290, 16653–16664. [Google Scholar] [CrossRef]
- Xu, X.; Tan, X.; Hulshoff, M.S.; Wilhelmi, T.; Zeisberg, M.; Zeisberg, E.M. Hypoxia-induced endothelial-mesenchymal transition is associated with RASAL1 promoter hypermethylation in human coronary endothelial cells. FEBS Lett. 2016, 590, 1222–1233. [Google Scholar] [CrossRef]
- Zhu, P.; Huang, L.; Ge, X.; Yan, F.; Wu, R.; Ao, Q. Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling. Int. J. Exp. Pathol. 2006, 87, 463–474. [Google Scholar] [CrossRef]
- Derada Troletti, C.; Fontijn, R.D.; Gowing, E.; Charabati, M.; van Het Hof, B.; Didouh, I.; van der Pol, S.M.A.; Geerts, D.; Prat, A.; van Horssen, J.; et al. Inflammation-induced endothelial to mesenchymal transition promotes brain endothelial cell dysfunction and occurs during multiple sclerosis pathophysiology. Cell Death Dis. 2019, 10, 45. [Google Scholar] [CrossRef]
- Pérez, L.; Muñoz-Durango, N.; Riedel, C.A.; Echeverría, C.; Kalergis, A.M.; Cabello-Verrugio, C.; Simon, F. Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor. Rev. 2017, 33, 41–54. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Yamakuchi, M.; Matsuyama, T.; Kondo, K.; Uchida, A.; Misono, S.; Hashiguchi, T.; Inoue, H. IL-13 enhances mesenchymal transition of pulmonary artery endothelial cells via down-regulation of miR-424/503 in vitro. Cell Signal 2018, 42, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, I.; Lenna, S.; Stawski, L.; Trojanowska, M. Interferon-alpha promotes vascular remodeling in human microvascular endothelial cells by upregulating endothelin (ET)-1 and transforming growth factor (TGF)beta2. J. Cell Physiol. 2013, 228, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, N.S.; Ying, R.; Xie, Y.; Chen, J.Y.; Wang, X.Q.; Gu, Z.J.; Mai, J.T.; Liu, W.H.; Wu, M.X.; et al. Macrophage-derived foam cells impair endothelial barrier function by inducing endothelial-mesenchymal transition via CCL-4. Int. J. Mol. Med. 2017, 40, 558–568. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fujiwara, K.; Takahashi, K.; Yoshioka, Y.; Ochiya, T.; Podyma-Inoue, K.A.; Watabe, T. Transforming growth factor-β-induced secretion of extracellular vesicles from oral cancer cells evokes endothelial barrier instability via endothelial-mesenchymal transition. Inflamm. Regen. 2022, 42, 38. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Barnes, C.; Charisse, K.; Yi, T.; Zhang, X.; Ali, R.; Medina, P.P.; Yu, J.; Slack, F.J.; et al. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012, 2, 1684–1696. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Tellides, G.; Simons, M. Fibroblast growth factor receptor 1 is a key inhibitor of TGFβ signaling in the endothelium. Sci. Signal 2014, 7, ra90. [Google Scholar] [CrossRef]
- Correia, A.C.; Moonen, J.R.; Brinker, M.G.; Krenning, G. FGF2 inhibits endothelial-mesenchymal transition through microRNA-20a-mediated repression of canonical TGF-β signaling. J. Cell Sci. 2016, 129, 569–579. [Google Scholar] [CrossRef]
- Wang, Z.; Calpe, B.; Zerdani, J.; Lee, Y.; Oh, J.; Bae, H.; Khademhosseini, A.; Kim, K. High-throughput investigation of endothelial-to-mesenchymal transformation (EndMT) with combinatorial cellular microarrays. Biotechnol. Bioeng. 2016, 113, 1403–1412. [Google Scholar] [CrossRef]
- Ichise, T.; Yoshida, N.; Ichise, H. FGF2-induced Ras-MAPK signalling maintains lymphatic endothelial cell identity by upregulating endothelial-cell-specific gene expression and suppressing TGFβ signalling through Smad2. J. Cell Sci. 2014, 127, 845–857. [Google Scholar]
- Yang, J.H.; Wylie-Sears, J.; Bischoff, J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem. Biophys. Res. Commun. 2008, 374, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Illigens, B.M.; Casar Berazaluce, A.; Poutias, D.; Gasser, R.; Del Nido, P.J.; Friehs, I. Vascular Endothelial Growth Factor Prevents Endothelial-to-Mesenchymal Transition in Hypertrophy. Ann. Thorac. Surg. 2017, 104, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Kanasaki, K.; Koya, D. Linagliptin but not Sitagliptin inhibited transforming growth factor-β2-induced endothelial DPP-4 activity and the endothelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2016, 471, 184–190. [Google Scholar] [CrossRef]
- Wang, Z.; Fei, S.; Suo, C.; Han, Z.; Tao, J.; Xu, Z.; Zhao, C.; Tan, R.; Gu, M. Antifibrotic Effects of Hepatocyte Growth Factor on Endothelial-to-Mesenchymal Transition via Transforming Growth Factor-Beta1 (TGF-β1)/Smad and Akt/mTOR/P70S6K Signaling Pathways. Ann. Transpl. 2018, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Yan, L.; Du, W.; Zhang, X.; Zhang, M.; Chen, H.; Zhang, Y.; Zhou, J.; Sun, H.; et al. Bone morphogenetic protein-7 inhibits endothelial-mesenchymal transition in pulmonary artery endothelial cell under hypoxia. J. Cell Physiol. 2018, 233, 4077–4090. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Liu, L. MiR-200a modulates TGF-beta1-induced endothelial-to-mesenchymal shift via suppression of GRB2 in HAECs. Biomed. Pharmacother. 2017, 95, 215–222. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, J.; Yu, S.; Chen, S.; Li, F.; Fan, C. MiR-630 Inhibits Endothelial-Mesenchymal Transition by Targeting Slug in Traumatic Heterotopic Ossification. Sci. Rep. 2016, 6, 22729. [Google Scholar] [CrossRef]
- Kanasaki, K.; Shi, S.; Kanasaki, M. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 2014, 63, 2120–2131. [Google Scholar] [CrossRef]
- Zhang, S.; Weinheimer, C.; Courtois, M. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J. Clin. Investig. 2003, 111, 833–841. [Google Scholar] [CrossRef]
- Ge, S.; Xie, J.; Liu, F.; He, J.; He, J. MicroRNA-19b reduces hepatic stellate cell proliferation by targeting GRB2 in hepatic fibrosis models in vivo and in vitro as part of the inhibitory effect of estradiol. J. Cell Biochem. 2015, 116, 2455–2464. [Google Scholar] [CrossRef]
- Papetti, M.; Shujath, J.; Riley, K.N.; Herman, I.M. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: A role for myf-5 and Smad-mediated signaling pathways. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4994–5005. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Becerril, C.; Montano, M. FGF-1 reverts epithelial-mesenchymal transition induced by TGF-{beta}1 through MAPK/ERK kinase pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L222–L231. [Google Scholar] [CrossRef]
- Miscianinov, V.; Martello, A.; Rose, L.; Parish, E.; Cathcart, B.; Mitic´, T.; Gray, G.A.; Meloni, M.; Al Haj Zen, A.; Caporali, A. MicroRNA-148b Targets the TGF-B Pathway to Regulate Angiogenesis and Endothelial-to-Mesenchymal Transition during Skin Wound Healing. Mol. Ther. 2018, 6, 1996–2007. [Google Scholar] [CrossRef]
- Lagendijk, A.K.; Goumans, M.J.; Burkhard, S.B. MicroRNA-23 restricts cardiac valve formation by inhibiting Has2 and extracellular hyaluronic acid production. Circ. Res. 2011, 109, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, A.S.; Teoh, J.P.; Aonuma, T. MicroRNA-532 protects the heart in acute myocardial infarction, and represses prss23, a positive regulator of endothelial-to-mesenchymal transition. Cardiovasc. Res. 2017, 113, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, R.; de Bruin, R.G.; van Solingen, C. MicroRNA-155 functions as a negative regulator of RhoA signaling in TGF-beta-induced endothelial to mesenchymal transition. Microrna 2012, 1, 2–10. [Google Scholar] [CrossRef]
- Feng, B.; Cao, Y.; Chen, S.; Chu, X.; Chu, Y.; Chakrabarti, S. miR-200b Mediates Endothelial-to-Mesenchymal Transition in Diabetic Cardiomyopathy. Diabetes 2016, 65, 768–779. [Google Scholar] [CrossRef]
- Geng, H.Z.; Guan, J. MiR-18a-5p inhibits endothelial mesenchymal transition and cardiac fibrosis through the Notch2 pathway. Biochem. Biophys. Res. Commun. 2017, 491, 329–336. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Volkmann, I.; Jazbutyte, V.; Dangwal, S.; Park, D.H.; Thum, T. Transforming Growth Factor-beta-Induced Endothelial-to-Mesenchymal Transition Is Partly Mediated by MicroRNA-21. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 361–369. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Nagpal, V.; Covington, J.W.; Michaels, M.A.; Vaughan, D.E. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): Differential expression of microRNAs during EndMT. Cell Signal 2012, 24, 1031–1036. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Katsura, A.; Mihira, H.; Horie, M.; Saito, A.; Miyazono, K. Regulation of TGF-beta-mediated endothelial-mesenchymal transition by microRNA-27. J. Biochem. 2017, 161, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, I.K.; Chiasson, V.; Chatterjee, P.; Gupta, S. NF-kappaB mediated miR-130a modulation in lung microvascular cell remodeling: Implication in pulmonary hypertension. Exp. Cell Res. 2017, 359, 235–242. [Google Scholar] [CrossRef]
- Xu, Y.P.; He, Q.; Shen, Z. MiR-126a-5p is involved in the hypoxia-induced endothelial-to-mesenchymal transition of neonatal pulmonary hypertension. Hypertens. Res. 2017, 40, 552–561. [Google Scholar] [CrossRef]
- Hulshoff, M.S.; Xu, X.; Krenning, G.; Zeisberg, E.M. Epigenetic regulation of endothelial-to-mesenchymal transition in chronic heart Disease. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1986–1996. [Google Scholar] [CrossRef]
- Wen, B.; Tao, R.; Liu, Y.; Zhang, Z. Investigating the role of exosomal microRNA-5703 in modulating tumor-associated endothelial cells in lung cancer. Cytojournal 2024, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.P.; Tingle, S.J.; Shuttleworth, V.G.; Cooke, K.; Redgrave, R.E.; Singh, E.; Glover, E.K.; Ahmad Tajuddin, H.B.; Kirby, J.A.; Arthur, H.M.; et al. MiR-126-3p Is Dynamically Regulated in Endothelial-to-Mesenchymal Transition during Fibrosis. Int. J. Mol. Sci. 2021, 22, 8629. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Jaé, N.; Knau, A.; Glaser, S.F.; Fouani, Y.; Rossbach, O.; Krüger, M.; John, D.; Bindereif, A.; Grote, P.; et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat. Commun. 2018, 9, 237. [Google Scholar] [CrossRef]
- Hu, J.X.; Zheng, Z.Q.; Kang, T.; Qian, W.; Huang, S.H.; Li, B.G. LncRNA LINC00961 regulates endothelial mesenchymal transition via the PTEN PI3K AKT pathway. Mol. Med. Rep. 2022, 26, 246. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, Y.; Tang, Y.; Li, Q. MALAT1 modulates tgf-beta1-induced endothelial-to-mesenchymal transition through downregulation of miR-145. Cell Physiol. Biochem. 2017, 42, 357–372. [Google Scholar] [CrossRef]
- Yu, X.; Huang, J.; Liu, X.; Li, J.; Yu, M.; Li, M.; Xie, Y.; Li, Y.; Qiu, J.; Xu, Z.; et al. LncRNAH19 acts as a ceRNA of let-7 g to facilitate endothelial-to-mesenchymal transition in hypoxic pulmonary hypertension via regulating TGF-β signalling pathway. Respir. Res. 2024, 25, 270. [Google Scholar] [CrossRef]
- Fan, C.S.; Chen, L.L.; Hsu, T.A.; Chen, C.C.; Chua, K.V.; Li, C.P.; Huang, T.S. Endothelial-mesenchymal transition harnesses HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2019, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Liu, B.; Tang, Y.; Li, F.; Qin, W.; Yuan, X. Irradiated Human Umbilical Vein Endothelial Cells Undergo Endothelial-Mesenchymal Transition via the Snail/miR-199a-5p Axis to Promote the Differentiation of Fibroblasts into Myofibroblasts. Biomed. Res. Int. 2018, 2018, 4135806. [Google Scholar] [CrossRef]
- Choi, S.H.; Hong, Z.Y.; Nam, J.K.; Lee, H.J.; Jang, J.; Yoo, R.J.; Lee, Y.J.; Lee, C.Y.; Kim, K.H.; Park, S.; et al. A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis. Clin. Cancer Res. 2015, 21, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Mintet, E.; Rannou, E.; Buard, V.; West, G.; Guipaud, O.; Tarlet, G.; Sabourin, J.C.; Benderitter, M.; Fiocchi, C.; Milliat, F.; et al. Identification of Endothelial-to-Mesenchymal Transition as a Potential Participant in Radiation Proctitis. Am. J. Pathol. 2015, 185, 2550–2562. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, W.M.; Sobierajska, K.; Wawro, M.E.; Klopocka, W.; Chefczyńska, N.; Muzyczuk, A.; Siekacz, K.; Wujkowska, A.; Niewiarowska, J. The ILK-MMP9-MRTF axis is crucial for EndMT differentiation of endothelial cells in a tumor microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2283–2296. [Google Scholar] [CrossRef]
- Zhen, J.; Jiao, K.; Yang, K.; Wu, M.; Zhou, Q.; Yang, B.; Xiao, W.; Hu, C.; Zhou, M.; Li, Z. The 14-3-3η/GSK-3β/β-catenin complex regulates EndMT induced by 27-hydroxycholesterol in HUVECs and promotes the migration of breast cancer cells. Cell Biol. Toxicol. 2021, 37, 515–529. [Google Scholar] [CrossRef]
- Chen, J.; Han, S.; Chen, J.; Hu, P.; Zeng, Z.; Hu, Y.; Xiong, H.; Ke, Z.; Zhang, Y.; Xu, F.; et al. reciprocal feedback of miR-548ac/YB-1/Snail induces EndMT of HUVECs during acidity microenvironment. Cancer Cell Int. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Dudley, A.C.; Khan, Z.A.; Shih, S.C.; Kang, S.Y.; Zwaans, B.M.; Bischoff, J.; Klagsbrun, M. Calcification of multipotent prostate tumor endothelium. Cancer Cell 2008, 14, 201–211. [Google Scholar] [CrossRef]
- Smeda, M.; Kieronska, A.; Adamski, M.G.; Proniewski, B.; Sternak, M.; Mohaissen, T.; Przyborowski, K.; Derszniak, K.; Kaczor, D.; Stojak, M.; et al. Nitric oxide deficiency and endothelial-mesenchymal transition of pulmonary endothelium in the progression of 4T1 metastatic breast cancer in mice. Breast Cancer Res. 2018, 20, 1–15. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Henke, C.A.; Horowitz, J.C.; Noble, P.W.; Roman, J.; Sime, P.J.; Zhou, Y.; Wells, R.G.; White, E.S.; Tschumperlin, D.J. Matrix biology of idiopathic pulmonary fibrosis: A workshop report of the national heart, lung, and blood institute. Am. J. Pathol. 2014, 184, 1643–1651. [Google Scholar] [CrossRef]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; de la Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-induced endothelial-to-mesenchymal transition: A novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, Q.; Li, M.; Zhang, Y.; Cai, Y.; Liang, X.; Fu, Y.; Xiao, Z.; Zhou, M.; Xie, Z.; et al. Quantitative proteomic profiling of tumor-associated vascular endothelial cells in colorectal cancer. Biol. Open 2019, 8, bio042838. [Google Scholar] [CrossRef]
- Wang, H.; Feng, C.; Lu, M.; Zhang, B.; Xu, Y.; Zeng, Q.; Xi, J.; Zhou, J.; Ying, X.; Zhang, J.; et al. Integrative single-cell transcriptome analysis reveals a subpopulation of fibroblasts associated with favorable prognosis of liver cancer patients. Transl. Oncol. 2021, 14, 100981. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Sun, X.; Jin, F.; Xiao, D.; Li, H.; Sun, H.; Wang, Y.; Lu, Y.; Liu, J.; Huang, C.; et al. PERK-eIF2α-ERK1/2 axis drives mesenchymal-endothelial transition of cancer-associated fibroblasts in pancreatic cancer. Cancer Lett. 2021, 515, 86–95. [Google Scholar] [CrossRef]
- Hashimoto, N.; Phan, S.H.; Imaizumi, K.; Matsuo, M.; Nakashima, H.; Kawabe, T.; Shimokata, K.; Hasegawa, Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2010, 43, 161–172. [Google Scholar] [CrossRef]
- Jonker, L.; Arthur, H.M. Endoglin expression in early development is associated with vasculogenesis and angiogenesis. Mech. Dev. 2002, 110, 193–196. [Google Scholar] [CrossRef]
- Bernabeu, C.; Conley, B.A.; Vary, C.P. Novel biochemical pathways of endoglin in vascular cell physiology. J. Cell Biochem. 2007, 102, 1375–1388. [Google Scholar] [CrossRef]
- Jerkic, M.; Rodríguez-Barbero, A.; Prieto, M.; Toporsian, M.; Pericacho, M.; Rivas-Elena, J.V.; Obreo, J.; Wang, A.; Pérez-Barriocanal, F.; Arévalo, M.; et al. Reduced angiogenic responses in adult Endoglin heterozygous mice. Cardiovasc. Res. 2006, 69, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, C.; Cunha, S.I.; Zhai, Z.; Cortez, E.; Pardali, E.; Johnson, J.R.; Franco, M.; Páez-Ribes, M.; Cordiner, R.; Fuxe, J.; et al. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. J. Exp. Med. 2013, 210, 563–579. [Google Scholar] [CrossRef]
- Chauhdari, T.; Zaidi, S.A.; Su, J.; Ding, Y. Organoids meet microfluidics: Recent advancements, challenges, and future of organoids-on-chip. In Vitro Models 2025, 4, 71–88. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y.; Chen, W.; Zhang, M.; Qin, J. Malignant Melanoma-Derived Exosomes Induce Endothelial Damage and Glial Activation on a Human BBB Chip Model. Biosensors 2022, 12, 89. [Google Scholar] [CrossRef]
- Jeong, K.; Yu, Y.J.; You, J.Y.; Rhee, W.J.; Kim, J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab. Chip. 2020, 20, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, J.; Arokiasamy, S.; Thompson, C.L.; Dufton, N.P. Novel application of live imaging to determine the functional cell biology of endothelial-to-mesenchymal transition (EndMT) within a liver-on-a-chip platform. In Vitro Models 2022, 1, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Plebani, R.; Potla, R.; Soong, M.; Bai, H.; Izadifar, Z.; Jiang, A.; Travis, R.N.; Belgur, C.; Dinis, A.; Cartwright, M.J.; et al. Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip. J. Cyst. Fibros. 2022, 21, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.; Corallo, C.; van den Heuvel, A.; Crawford, J.; Olivier, T.; Elstak, E.; Giordano, N.; Vulto, P.; Lanz, H.L.; Janssen, R.A.J.; et al. High-throughput 3D microvessel-on-a-chip model to study defective angiogenesis in systemic sclerosis. Sci. Rep. 2022, 12, 16930. [Google Scholar] [CrossRef]
- Hernández-Camarero, P.; Toledo, B.; Diaz-Ruano, A.B.; González-Titos, A.; García-Ortega, M.B.; Perán, M. What is the Impact of Endothelial-to-Mesenchymal Transition in Solid Tumours: A Qualitative Systematic Review and Quantitative Meta-Analysis. Int. J. Biol. Sci. 2025, 21, 2155–2178. [Google Scholar] [CrossRef]
- Welch-Reardon, K.M.; Ehsan, S.M.; Wang, K.; Wu, N.; Newman, A.C.; Romero-Lopez, M.; Fong, A.H.; George, S.C.; Edwards, R.A.; Hughes, C.C. Angiogenic sprouting is regulated by endothelial cell expression of Slug. J. Cell Sci. 2014, 127, 2017–2028. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar][Green Version]
- Gu, A.; Jie, Y.; Yao, Q.; Zhang, Y.; Mingyan, E. Slug Is Associated with Tumor Metastasis and Angiogenesis in Ovarian Cancer. Reprod. Sci. 2017, 24, 291–299. [Google Scholar] [CrossRef]
- Enholm, B.; Paavonen, K.; Ristimäki, A.; Kumar, V.; Gunji, Y.; Klefstrom, J.; Kivinen, L.; Laiho, M.; Olofsson, B.; Joukov, V.; et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 1997, 14, 2475–2483. [Google Scholar]
- Ferrari, G.; Cook, B.D.; Terushkin, V.; Pintucci, G.; Mignatti, P. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J. Cell Physiol. 2009, 219, 449–458. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Gasparics, Á.; Rosivall, L.; Krizbai, I.A.; Sebe, A. When the endothelium scores an own goal: Endothelial cells actively augment metastatic extravasation through endothelial-mesenchymal transition. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1055–H1063. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, I.; Scheffrahn, I.; Bartling, S.; Weis, J.; von Felbert, V.; Middleton, M.; Kato, M.; Ergün, S.; Augustin, H.G.; Schadendorf, D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010, 207, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, D.; Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011, 118, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Xu, J. Cancer-associated fibroblasts: A versatile mediator in tumor progression, metastasis, and targeted therapy. Cancer Metastasis Rev. 2024, 43, 1095–1116. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Natesh, N.R.; Mogha, P.; Chen, A.; Antonia, S.J.; Varghese, S. Differential roles of normal and lung cancer-associated fibroblasts in microvascular network formation. APL Bioeng. 2024, 8, 016120. [Google Scholar] [CrossRef]
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: New insights and therapeutic implications. Cell Death Dis. 2023, 14, 586. [Google Scholar] [CrossRef]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by endothelial cells—Partnering up with the immune system? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef]
- Daum, S.; Decristoforo, L.; Mousa, M.; Salcher, S.; Plattner, C.; Hosseinkhani, B.; Trajanoski, Z.; Wolf, D.; Carmeliet, P.; Pircher, A. Unveiling the immunomodulatory dance: Endothelial cells’ function and their role in non-small cell lung cancer. Mol. Cancer 2025, 24, 21. [Google Scholar] [CrossRef]
- Goveia, J.; Rohlenova, K.; Taverna, F.; Treps, L.; Conradi, L.C.; Pircher, A.; Geldhof, V.; de Rooij, L.P.M.H.; Kalucka, J.; Sokol, L.; et al. An Integrated Gene Expression Landscape Profiling Approach to Identify Lung Tumor Endothelial Cell Heterogeneity and Angiogenic Candidates. Cancer Cell 2020, 37, 421. [Google Scholar] [CrossRef] [PubMed]
- Sozio, F.; Schioppa, T.; Laffranchi, M.; Salvi, V.; Tamassia, N.; Bianchetto-Aguilera, F.M.; Tiberio, L.; Bonecchi, R.; Bosisio, D.; Parmentier, M.; et al. CCRL2 Expression by Specialized Lung Capillary Endothelial Cells Controls NK-cell Homing in Lung Cancer. Cancer Immunol. Res. 2023, 11, 1280–1295. [Google Scholar] [CrossRef]
- Thompson, T.W.; Kim, A.B.; Li, P.J.; Wang, J.; Jackson, B.T.; Huang, K.T.H.; Zhang, L.; Raulet, D.H. Endothelial cells express NKG2D ligands and desensitize antitumor NK responses. Elife 2017, 6, e30881. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, E.; Rother, N.; Garsen, M.; Hofstra, J.M.; Satchell, S.C.; Hoffmann, M.; Loeven, M.A.; Knaapen, H.K.; van der Heijden, O.W.H.; Berden, J.H.M.; et al. Neutrophil Extracellular Traps Drive Endothelial-to-Mesenchymal Transition. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Z.; Peng, Z.P.; Liu, X.C.; Guo, H.F.; Zhou, M.M.; Jiang, D.; Ning, W.R.; Huang, Y.F.; Zheng, L.; Wu, Y. Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells. Oncoimmunology 2022, 111, 2052418. [Google Scholar] [CrossRef]
- Yang, S.; Sun, B.; Li, J.; Li, N.; Zhang, A.; Zhang, X.; Yang, H.; Zou, X. Neutrophil extracellular traps promote angiogenesis in gastric cancer. Cell Commun. Signal 2023, 21, 176. [Google Scholar] [CrossRef]
- Xie, L.; Yin, J.; Kong, H.; Yu, J.; Sun, M.; Wang, X.; Bai, C.; Song, Y.; Yang, D. The Involvement of Tumor Endothelial Cells in the Regulation of PD-L1 and Tregs in the Immune Microenvironment of Early-stage Lung Adenocarcinoma. J. Thorac. Oncol. 2023, 11, S444–S445. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. B7H3 As a Promoter of Metastasis and Promising Therapeutic Target. Front. Oncol. 2018, 8, 264. [Google Scholar] [CrossRef]
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235. [Google Scholar] [CrossRef]
- Bruno, T.C.; Ebner, P.J.; Moore, B.L.; Squalls, O.G.; Waugh, K.A.; Eruslanov, E.B.; Singhal, S.; Mitchell, J.D.; Franklin, W.A.; Merrick, D.T.; et al. Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non-Small Cell Lung Cancer Patients. Cancer Immunol. Res. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Yang, C.; Lee, H.; Pal, S.; Jove, V.; Deng, J.; Zhang, W.; Hoon, D.S.; Wakabayashi, M.; Forman, S.; Yu, H. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLoS ONE 2013, 8, e64159. [Google Scholar] [CrossRef]
- Sakano, Y.; Noda, T.; Kobayashi, S.; Sasaki, K.; Iwagami, Y.; Yamada, D.; Tomimaru, Y.; Akita, H.; Gotoh, K.; Takahashi, H.; et al. Tumor endothelial cell-induced CD8+ T-cell exhaustion via GPNMB in hepatocellular carcinoma. Cancer Sci. 2022, 113, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T.; Lu, S.; Ma, S.; Han, D.; Zhang, K.; Xu, C.; Liu, S.; Gan, L.; Wu, X.; et al. Single-cell analysis of multiple cancer types reveals differences in endothelial cells between tumors and normal tissues. Comput. Struct. Biotechnol. J. 2022, 21, 665–676. [Google Scholar] [CrossRef]
- Mori, M.; Sakamoto, A.; Kawakami, R.; Guo, L.; Slenders, L.; Mosquera, J.V.; Ghosh, S.K.B.; Wesseling, M.; Shiraki, T.; Bellissard, A.; et al. CD163+ Macrophages Induce Endothelial-to-Mesenchymal Transition in Atheroma. Circ. Res. 2024, 135, e4–e23. [Google Scholar] [CrossRef] [PubMed]
- Maleszewska, M.; Moonen, J.R.; Huijkman, N.; van de Sluis, B.; Krenning, G.; Harmsen, M.C. IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology 2013, 218, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Bronson, R.; Lyu, J.; Xiong, J. Transcriptome analysis reveals molecular signature and cell-type difference of Homo sapiens endothelial-to-mesenchymal transition. G3 2023, 13, jkad243. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Sun, H.; Breslin, J.W.; Zhu, J.; Yuan, S.Y.; Wu, M.H. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006, 13, 237–247. [Google Scholar] [CrossRef]
- Li, B.; Zhao, W.D.; Tan, Z.M.; Fang, W.G.; Zhu, L.; Chen, Y.H. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 2006, 580, 4252–4260. [Google Scholar] [CrossRef]
- Li, D.K.; Chen, X.R.; Wang, L.N.; Wang, J.H.; Li, J.K.; Zhou, Z.Y.; Li, X.; Cai, L.B.; Zhong, S.S.; Zhang, J.J.; et al. Exosomal HMGA2 protein from EBV-positive NPC cells destroys vascular endothelial barriers and induces endothelial-to-mesenchymal transition to promote metastasis. Cancer Gene Ther. 2022, 29, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Koyama, T.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Liu, T.; Xian, X.; Imai, A.; Zhai, L.; et al. The endothelial adrenomedullin-RAMP2 system regulates vascular integrity and suppresses tumour metastasis. Cardiovasc. Res. 2016, 111, 398–409. [Google Scholar] [CrossRef]
- Bhat, G.R.; Sethi, I.; Sadida, H.Q.; Rah, B.; Mir, R.; Algehainy, N.; Albalawi, I.A.; Masoodi, T.; Subbaraj, G.K.; Jamal, F.; et al. Cancer cell plasticity: From cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer Metastasis Rev. 2024, 43, 197–228. [Google Scholar] [CrossRef]
- Murugavel, S.; Bugyei-Twum, A.; Matkar, P.N.; Al-Mubarak, H.; Chen, H.H.; Adam, M.; Jain, S.; Narang, T.; Abdin, R.M.; Qadura, M.; et al. Valproic Acid Induces Endothelial-to-Mesenchymal Transition-Like Phenotypic Switching. Front. Pharmacol. 2018, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Hsu, C.C.; Pai, V.C.; Liao, W.Y.; Huang, S.S.; Tan, K.T.; Yen, C.J.; Hsu, S.C.; Chen, W.Y.; Shan, Y.S.; et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. J. Exp. Med. 2016, 213, 2967–2988. [Google Scholar] [CrossRef]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e16. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Park, S.Y.; Lee, G.K.; Lim, K.Y.; Kim, J.Y.; Hwang, J.A.; Yu, N.; Kang, E.H.; Hwang, M.; et al. Molecular Subtypes and Tumor Microenvironment Characteristics of Small-Cell Lung Cancer Associated with Platinum-Resistance. Cancers 2023, 15, 3568. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.S. Epithelial and endothelial cell plasticity in chronic obstructive pulmonary disease (COPD). Respir. Investig. 2017, 55, 04–113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhou, L.; Zhou, J.; Wan, H.; Li, Q.; Feng, Y. ACE2 overexpression inhibits acquired platinum resistance-induced tumor angiogenesis in NSCLC. Oncol. Rep. 2016, 36, 1403–1410. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.Y.; Kim, A.R.; Kim, S.; Heo, J.; Shum, D.; Kim, S.H.; Choi, I.; Lee, Y.J.; Seo, H.R. Identification of radiation-induced EndMT inhibitors through cell-based phenomic screening. FEBS Open Bio. 2018, 9, 82–91. [Google Scholar] [CrossRef]
- Choi, K.J.; Nam, J.K.; Kim, J.H.; Choi, S.H.; Lee, Y.J. Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage. Exp. Mol. Med. 2020, 52, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Guo, H.; Shi, J.; Zhao, S.; Jia, Y.; Liu, X.; Liu, Y.; Cheng, L.; Zhao, C.; Li, X.; et al. Osimertinib in combination with antiangiogenesis therapy presents a promising option for osimertinib-resistant non-small cell lung cancer. BMC Med. 2024, 22, 174. [Google Scholar]
- Abbona, A.; Paccagnella, M.; Astigiano, S.; Martini, S.; Denaro, N.; Ruatta, F.; Barbieri, O.; Merlano, M.; Garrone, O. Effect of Eribulin on Angiogenesis and the Expression of Endothelial Adhesion Molecules. Anticancer. Res. 2022, 42, 2859–2867. [Google Scholar] [CrossRef]
- Masaki, N.; Wu, N.F.; Aoki, Y.; Yamamoto, J.; Miyazaki, J.; Hoffman, R.M. Osteosarcoma of the Breast in a Patient Derived Orthotopic Xenograft (PDOX) Mouse Model Is Arrested by both Cisplatinum and Eribulin. In Vivo 2021, 35, 3107–3110. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kikawa, Y.; Kashiwabara, K.; Taira, N.; Iwatani, T.; Shimozuma, K.; Ohtani, S.; Yoshinami, T.; Watanabe, J.; Kashiwaba, M.; et al. Eribulin versus S-1 as first or second-line chemotherapy to assess health-related quality of life and overall survival in HER2-negative metastatic breast cancer (RESQ study): A non-inferiority, randomised, controlled, open-label, phase 3 trial. EClinicalMedicine 2024, 74, 102715. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hamamichi, S.; Abe, T.; Akagi, T.; Shirota, H.; Kawano, S.; Asano, M.; Asano, O.; Yokoi, A.; Matsui, J.; et al. Antitumor effects of eribulin depend on modulation of the tumor microenvironment by vascular remodeling in mouse models. Cancer Sci. 2017, 108, 2273–2280. [Google Scholar] [CrossRef]
- Funahashi, Y.; Okamoto, K.; Adachi, Y.; Semba, T.; Uesugi, M.; Ozawa, Y.; Tohyama, O.; Uehara, T.; Kimura, T.; Watanabe, H.; et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014, 105, 1334–1342. [Google Scholar] [CrossRef]
- Zhuo, D.; Mei, Y.; Lin, C.; Wu, A.; Luo, Y.; Lu, H.; Fu, J. Nudifloside, a Secoiridoid Glucoside Derived from Callicarpa nudiflora, Inhibits Endothelial-to-Mesenchymal Transition and Angiogenesis in Endothelial Cells by Suppressing Ezrin Phosphorylation. J. Cancer 2024, 15, 2448–2459. [Google Scholar] [CrossRef]
- Calcabrini, A.; García-Martínez, J.M.; González, L.; Tendero, M.J.; Ortuño, M.T.; Crateri, P.; Lopez-Rivas, A.; Arancia, G.; González-Porqué, P.; Martín-Pérez, J. Inhibition of proliferation and induction of apoptosis in human breast cancer cells by lauryl gallate. Carcinogenesis 2006, 27, 1699–1712. [Google Scholar] [CrossRef]
- Chu, K.V.; Fan, C.S.; Chen, C.C.; Hsieh, S.C.; Huang, T.S. Octyl gallate induces pancreatic ductal adenocarcinoma cell apoptosis and suppresses endothelial-mesenchymal transition-promoted M2-macrophages, HSP90α secretion, and tumor growth. Cells 2019, 9, 91. [Google Scholar] [CrossRef]
- Wawro, M.E.; Sobierajska, K.; Ciszewski, W.M.; Niewiarowska, J. Nonsteroidal Anti-Inflammatory Drugs Prevent Vincristine-Dependent Cancer-Associated Fibroblasts Formation. Int. J. Mol. Sci. 2019, 20, 1941. [Google Scholar] [CrossRef] [PubMed]
- Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; Benhadji, K.A.; et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Devel Ther. 2015, 9, 4479–4499. [Google Scholar]
- Yu, J.; Du, X.; Zhang, S.; Long, J.; Wu, P.; Li, Z.; Lyu, X.; Hong, Q.; Chen, P.; Gao, B. Galunisertib promotes bevacizumab-induced vascular normalization in nasopharyngeal carcinoma: Multi-parameter MRI evaluation. Mol. Ther. Oncol. 2024, 32, 200858. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, D.S.; Li, M.; Zhang, Y.; Li, Q.; Li, T.T.; Ren, L.Q. Icariin attenuates endothelial-mesenchymal transition via H19/miR-148b-3p/ELF5 in ox-LDL-stimulated HUVECs. Mol. Ther. Nucleic Acids. 2020, 23, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, X.; Ji, X.B.; He, C.; Xu, S.; Xu, X. Biphasic function of GSK3β in gefitinib-resistant NSCLC with or without EGFR mutations. Exp. Ther. Med. 2023, 26, 488. [Google Scholar] [CrossRef]
- Kim, K.; Sohn, Y.J.; Lee, R.; Yoo, H.J.; Kang, J.Y.; Choi, N.; Na, D.; Yeon, J.H. Cancer-Associated Fibroblasts Differentiated by Exosomes Isolated from Cancer Cells Promote Cancer Cell Invasion. Int. J. Mol. Sci. 2020, 21, 8153. [Google Scholar] [CrossRef]
- Kovacs, R.J.; Maldonado, G.; Azaro, A.; Fernández, M.S.; Romero, F.L.; Sepulveda-Sánchez, J.M.; Corretti, M.; Carducci, M.; Dolan, M.; Gueorguieva, I.; et al. Safety of TGF-β Receptor I Kinase Inhibitor LY2157299 Monohydrate in Cancer Patients in a First-in-Human Dose Study. Cardiovasc. Toxicol. 2015, 15, 309–323. [Google Scholar] [CrossRef]
- Liu, D.; van der Zalm, A.P.; Koster, J.; Bootsma, S.; Oyarce, C.; van Laarhoven, H.W.M.; Bijlsma, M.F. Predictive biomarkers for response to TGF- β inhibition in resensitizing chemo(radiated) esophageal adenocarcinoma. Pharmacol. Res. 2024, 207, 107315. [Google Scholar] [CrossRef]
- Formenti, S.C.; Hawtin, R.E.; Dixit, N.; Evensen, E.; Lee, P.; Goldberg, J.D.; Li, X.; Vanpouille-Box, C.; Schaue, D.; McBride, W.H.; et al. Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. J. Immunother. Cancer 2019, 7, 177. [Google Scholar] [CrossRef]
- Pantziarka, P.; André, N. Editorial: Drug Repurposing. Front. Med. 2019, 6, 154. [Google Scholar] [CrossRef]
- Pantziarka, P.; Verbaanderd, C.; Huys, I.; Bouche, G.; Meheus, L. Repurposing drugs in oncology: From candidate selection to clinical adoption. Semin. Cancer Biol. 2021, 68, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Verbaanderd, C.; Meheus, L.; Huys, I.; Pantziarka, P. Repurposing Drugs in Oncology: Next Steps. Trends Cancer 2017, 3, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Wylie-Sears, J.; Levine, R.A.; Bischoff, J. Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-β-induced phosphorylation of ERK. Biochem. Biophys. Res. Commun. 2014, 446, 870–875. [Google Scholar] [CrossRef]
- Yao, J.; Guihard, P.J.; Blazquez-Medela, A.M.; Guo, Y.; Moon, J.H.; Jumabay, M.; Boström, K.I.; Yao, Y. Serine Protease Activation Essential for Endothelial-Mesenchymal Transition in Vascular Calcification. Circ. Res. 2015, 117, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, P.; Bledsoe, G.; Yang, Z.R.; Chao, L.; Chao, J. Kallistatin inhibits TGF-beta-induced endothelial-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression. Exp. Cell Res. 2015, 337, 103–110. [Google Scholar] [CrossRef]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Kozloff, M.; Simionato, F.; Cleverly, A.; et al. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother. Pharmacol. 2019, 83, 975–991. [Google Scholar] [CrossRef]
- Faivre, S.; Santoro, A.; Kelley, R.K.; Gane, E.; Costentin, C.E.; Gueorguieva, I.; Smith, C.; Cleverly, A.; Lahn, M.M.; Raymond, E.; et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019, 39, 1468–1477. [Google Scholar] [CrossRef]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef]
- Morris, J.C.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Reiss, M.; Hsu, F.J.; Berzofsky, J.A.; Lawrence, D.P. Phase I study of GC1008 (fresolimumab): A human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE 2014, 9, e90353. [Google Scholar] [CrossRef]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L.; et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Investig. 2015, 125, 2795–2807. [Google Scholar] [CrossRef]
- Vincenti, F.; Fervenza, F.C.; Campbell, K.N.; Diaz, M.; Gesualdo, L.; Nelson, P.; Praga, M.; Radhakrishnan, J.; Sellin, L.; Singh, A.; et al. Focal Segmental Glomerulosclerosis Study Group. A Phase 2, Double-Blind, Placebo-Controlled, Randomized Study of Fresolimumab in Patients With Steroid-Resistant Primary Focal Segmental Glomerulosclerosis. Kidney Int. Rep. 2017, 2, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, K.; Collins, S.L.; Powell, J.D. Horton MR. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J. Clin. Investig. 2021, 131, e143226. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Li, T.; Sandy, L.; Newsom, C.; Petersen, B.; Godbold, J.; Hoffman, R. Anti-transforming growth factor-β therapy in patients with myelofibrosis. Leuk. Lymphoma 2014, 55, 450–452. [Google Scholar] [CrossRef] [PubMed]
| EMT | EndMT | References | |
|---|---|---|---|
| Cell of Origin | Epithelial cell | Endothelial cell | [4,5,6,7,8,9,10,11,24,25,26,27] |
| Polarity | Loss of apical–basal polarity | Loss of apical–luminal polarity | |
| Cell–Cell Junctions (loss) | E-cadherin, CD31, vWF, Tie2 | VE cadherin, CD31, vWF, Tie2 | |
| Cell–Cell Junctions (gained) | N-cadherin, vimentin, fibronectin, collagen type I/III | N-cadherin, vimentin, fibronectin, collagen type I/III | |
| Markers | α-SMA, FSP-1, FAP | α-SMA, FSP-1, FAP | |
| Signaling | Snail, Slug/SNAI2, Twist TGFB1 | Snail, Slug/SNAI2, Twist TGFB2 direct, TGFB1 -3 indirect, synergism with inflammatory cytokines (e.g., IL-1β) | |
| Endothelial-Specific Regulator | Sox9 Shear stress | Sox9 ETS transcription factors Shear stress (KLF2) | |
| Developmental Role | Embryogenesis, wound healing | Cardiogenesis, valvulogenesis, vasculogenesis, vascular remodeling | |
| Pathological Involvement | Loss of epithelial traits, pulmonary fibrosis, epithelial cancers, wound healing disorders, metastasis, chemoresistance | Loss of endothelial traits, fibroblast generation, cardiac and renal fibrosis, pulmonary hypertension, tumor microenvironment remodeling, Angiogenesis, metastasis, vascular permeability |
| miR | Target | Effect on EndMT | ECs | Ref |
|---|---|---|---|---|
| Let-7 | TGF-β | Inhibit | HUAEC, HUVEC | [87] |
| miR-200a | GRB2 | Inhibit | HAEC | [98,102] |
| miR-20a | TGF-βR1/2, SARA | Inhibit | HUVEC | [99] |
| miR-630 | SLUG | Inhibit | HD-MVEC | [100] |
| miR-29 | DPP4 | Inhibit | HMVEC | [101] |
| miR-23 | Has2 | Inhibit | MEEC | [108] |
| miR-200b | Smad, Snail, p300 | Inhibit | HRMEC, MHEC | [111] |
| miR-18a-5p | Notch2 | Inhibit | HAVEC | [112] |
| miR-21 | PTEN | Promote | HUVEC | [113] |
| miR-125b | p53 | Promote | MCEC | [114] |
| miR-27b | Elk1, Neuropilin2, PlexinA2, Plexind1 | Promote | MS-1 | [115] |
| mir-130a | BMPR2 | Promote | LMVEC | [116] |
| GATA6-AS | LOXL2 | Inhibit | HUVEC | [121] |
| LINC00961 | PTEN/PI3K/AKT | Promote | HCMEC | [122] |
| MALAT-1 | SMAD3, miR-145 | Promote | EPC | [123] |
| H19 | MAPK-ERK-1/2 TGFBR1 | Inhibit (glucose) Promote (hypoxia) | PAECS | [124,125] |
| (A) | ||||||
| Investigational Agent and Target/ Mechanism of Action | NCT Identifier | Title | Tumor Type | Phase | Status | Publications |
| Galunisertib TGFβRI inhibitor | NCT01373164 | A Study in Metastatic Cancer and Advanced or Metastatic Unresectable Pancreatic Cancer | Pancreatic cancer, advanced or metastatic | I–II | Completed | [217] |
| NCT01246986 | A Study of LY2157299 in Participants With Hepatocellular Carcinoma | Hepatocellular carcinoma, advanced | II | Completed | [218] | |
| NCT05700656 | Galunisertib Combined With Capecitabine in Advanced CRC With peritoneal metastasis | Advanced colorectal cancer | I–II | Recruiting | Not available | |
| NCT02452008 | Study of TGF-β Receptor Inhibitor Galunisertib (LY2157299) and Enzalutamide in Metastatic Castration-resistant Prostate Cancer | Metastatic castration resistant prostate cancer | II | Active, not recruiting | Not available | |
| Fresolimumab (GC1008) inhibits TGF-β activity by targeting all TGF-β isoforms | NCT01401062 | Fresolimumab and Radiotherapy in Metastatic Breast Cancer | Breast cancer, metastatic | II | Completed | [219] |
| NCT01112293 | Anti-TGF Monoclonal Antibody (GC1008) in Relapsed Malignant Pleural Mesothelioma | Relapsed malignant pleural mesothelioma | II | Completed | Not available | |
| NCT02581787 | SABR-ATAC: A Trial of TGF-beta Inhibition and Stereotactic Ablative Radiotherapy for Early Stage Non-small Cell Lung Cancer | Non-small cell lung cancer, early stage | I–II | Completed | Not available | |
| NCT00356460 | Safety and Efficacy Study of GC1008 to Treat Renal Cell Carcinoma or Malignant Melanoma | Advanced/metastatic renal cell carcinoma or melanoma | I | Completed | [220] | |
| (B) | ||||||
| Investigational agent and target/ mechanism of action | NCT identifier | Title | Disease/ condition | Phase | Status | Publications |
| Fresolimumab inhibits TGF-β activity by targeting all TGF-β isoforms | NCT01284322 | Fresolimumab in systemic sclerosis | Systemic sclerosis | I | Completed | [221] |
| NCT01665391 | A Study of Fresolimumab in Patients With Steroid-Resistant Primary Focal Segmental Glomerulosclerosis (FSGS) | Focal Segmental Glomerulosclerosis | II | Completed | [222] | |
| NCT00125385 | Study of GC1008 in Patients With Idiopathic Pulmonary Fibrosis (IPF) | Idiopathic pulmonary fibrosis | I | Completed | [223] | |
| NCT01291784 | Anti-TGF-beta Therapy in Patients With Myelofibrosis | Myelofibrosis | I | Completed | [224] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordanengo, L.; Proment, A.; Botta, V.; Picca, F.; Munir, H.M.W.; Tao, J.; Olivero, M.; Taulli, R.; Bersani, F.; Sangiolo, D.; et al. Shifting Shapes: The Endothelial-to-Mesenchymal Transition as a Driver for Cancer Progression. Int. J. Mol. Sci. 2025, 26, 6353. https://doi.org/10.3390/ijms26136353
Giordanengo L, Proment A, Botta V, Picca F, Munir HMW, Tao J, Olivero M, Taulli R, Bersani F, Sangiolo D, et al. Shifting Shapes: The Endothelial-to-Mesenchymal Transition as a Driver for Cancer Progression. International Journal of Molecular Sciences. 2025; 26(13):6353. https://doi.org/10.3390/ijms26136353
Chicago/Turabian StyleGiordanengo, Lucia, Alessia Proment, Virginia Botta, Francesca Picca, H. M. Waqas Munir, Jiahao Tao, Martina Olivero, Riccardo Taulli, Francesca Bersani, Dario Sangiolo, and et al. 2025. "Shifting Shapes: The Endothelial-to-Mesenchymal Transition as a Driver for Cancer Progression" International Journal of Molecular Sciences 26, no. 13: 6353. https://doi.org/10.3390/ijms26136353
APA StyleGiordanengo, L., Proment, A., Botta, V., Picca, F., Munir, H. M. W., Tao, J., Olivero, M., Taulli, R., Bersani, F., Sangiolo, D., Novello, S., Scagliotti, G. V., Merlini, A., & Doronzo, G. (2025). Shifting Shapes: The Endothelial-to-Mesenchymal Transition as a Driver for Cancer Progression. International Journal of Molecular Sciences, 26(13), 6353. https://doi.org/10.3390/ijms26136353






