Using Gut Microbiota Modulation as a Precision Strategy Against Obesity
Abstract
1. Introduction
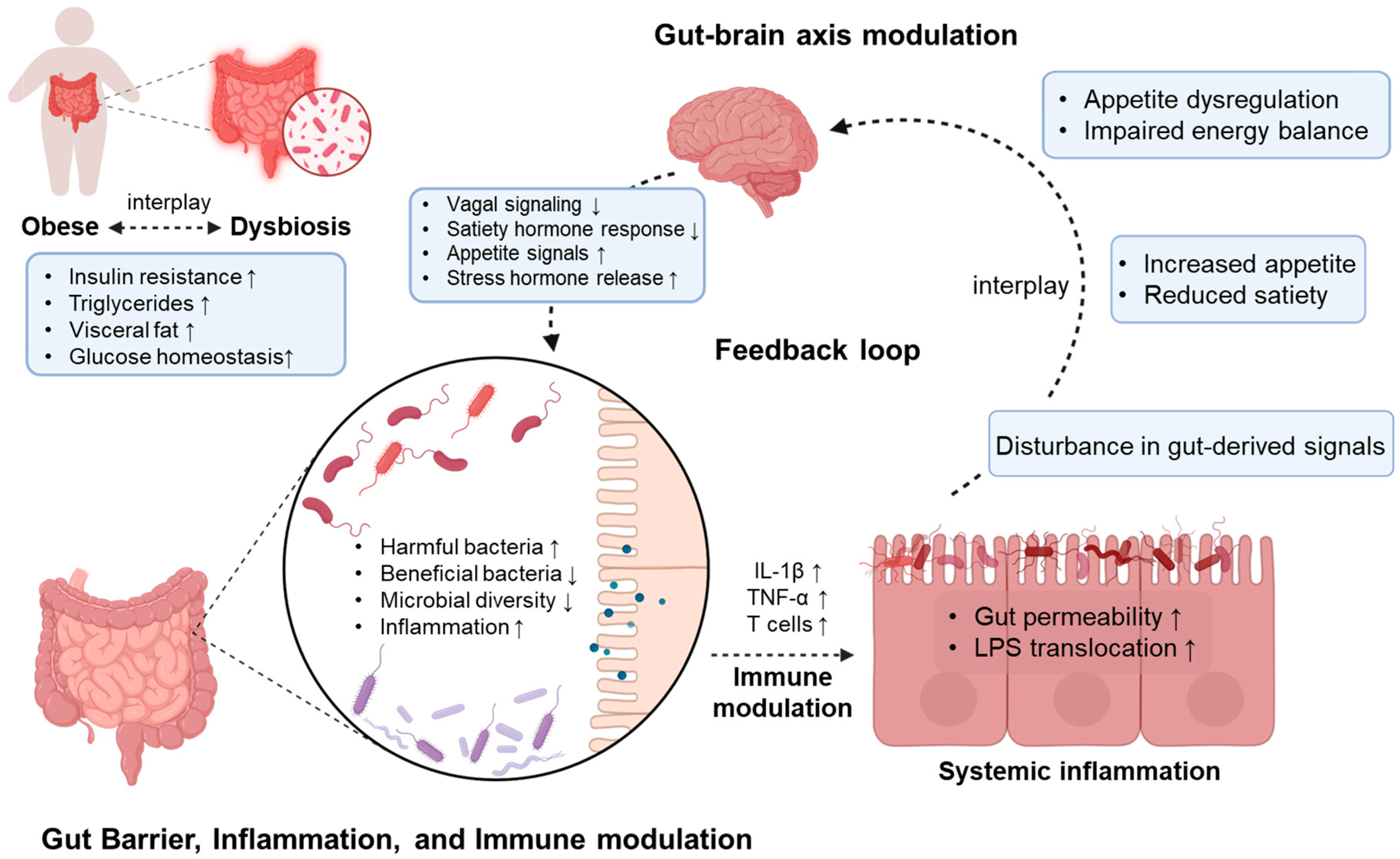
2. The Relationship Between the Gut Microbiome and Obesity
2.1. Dysbiosis of Gut Microbiome
2.2. SCFA Production by Gut Microbiome
2.3. Hormonal Modulation by Gut Microbiome
2.4. Increased Lipopolysaccharide (LPS) Levels
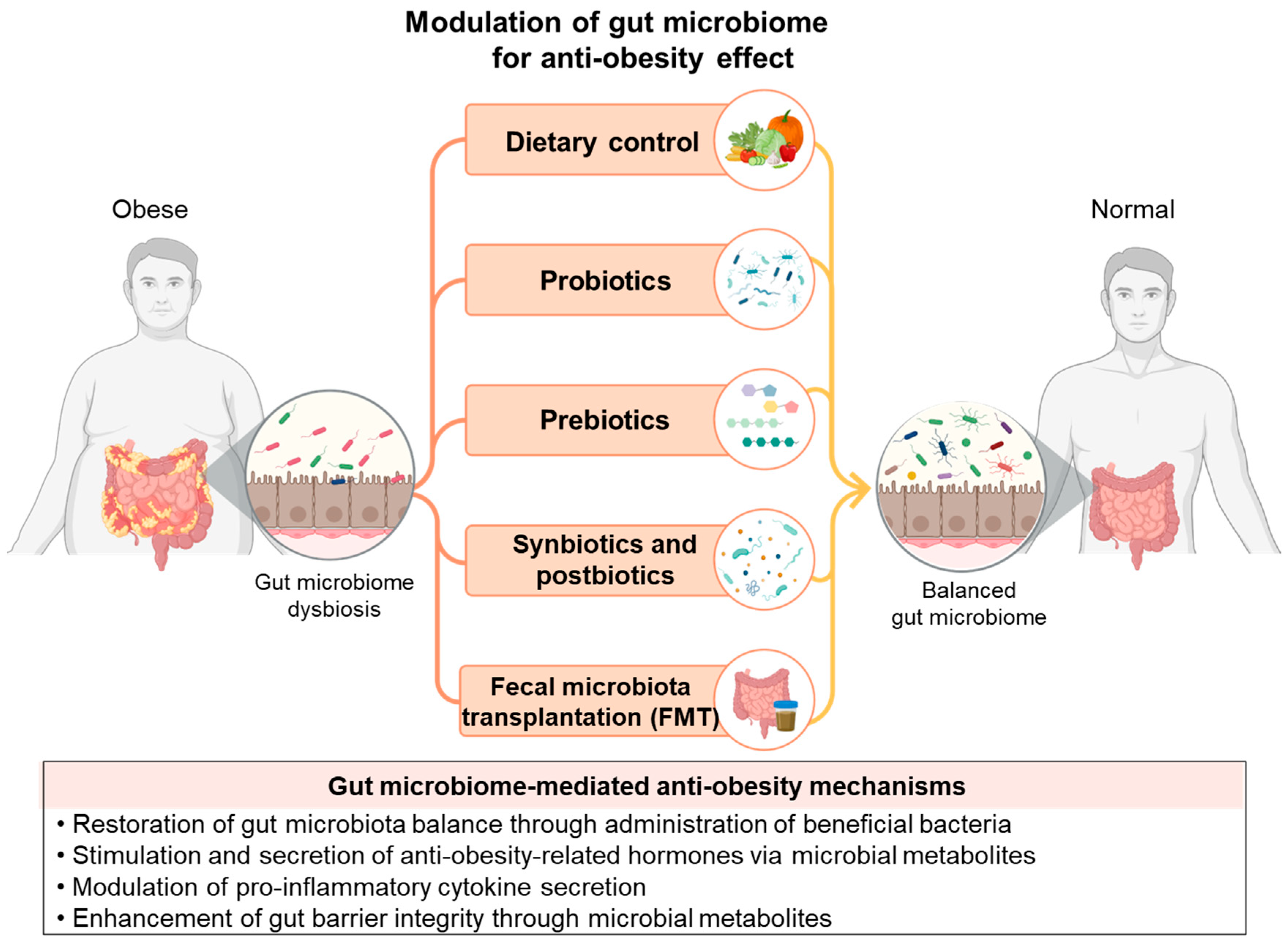
3. Current Anti-Obesity Strategies Used by Modulating Gut Microbiome
3.1. Dietary Control
3.2. Probiotics
3.3. Prebiotics
3.4. Synbiotics and Postbiotics
3.5. FMT
4. Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar]
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ärnlöv, J.; Collaborators, G.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA receptor GPR43 and energy metabolism. Front. Endocrinol. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Joyce, S.A.; Gahan, C.G. Bile acid modifications at the microbe-host interface: Potential for nutraceutical and pharmaceutical interventions in host health. Annu. Rev. Food Sci. Technol. 2016, 7, 313–333. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Wu, C.-Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, 7353642. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Bäckhed, F. Assessing the Human Gut Microbiota in Metabolic Diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef]
- Castaner, O.; Goday, A.; Park, Y.-M.; Lee, S.-H.; Magkos, F.; Shiow, S.-A.T.E.; Schröder, H. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Borgo, F.; Garbossa, S.; Riva, A.; Severgnini, M.; Luigiano, C.; Benetti, A.; Pontiroli, A.E.; Morace, G.; Borghi, E. Body mass index and sex affect diverse microbial niches within the gut. Front. Microbiol. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Muheyati, D.; Han, J.; Lv, M.; Jielili, M.; Jing, Z.; Zaibibuli, K.; Aisike, G.; Aihemaiti, A.; Yu, Y.; Kaliszewski, C.R. Composition of gut microbiota in obese and normal-weight Uygur adults and its association with adipocyte-related factors. Sci. Rep. 2024, 14, 24649. [Google Scholar] [CrossRef]
- He, B.; Xu, S.; Schooling, C.M.; Leung, G.M.; Ho, J.W.K.; Au Yeung, S.L. Gut microbiome and obesity in late adolescence: A case-control study in “Children of 1997” birth cohort. Ann. Epidemiol. 2025, 101, 58–66. [Google Scholar] [CrossRef]
- Salazar-Jaramillo, L.; Cuesta-Zuluaga, J.d.l.; Chica, L.A.; Cadavid, M.; Ley, R.E.; Reyes, A.; Escobar, J.S. Gut microbiome diversity within Clostridia is negatively associated with human obesity. mSystems 2024, 9, e00627-24. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- McNeil, N.I.; Cummings, J.H.; James, W.P. Short chain fatty acid absorption by the human large intestine. Gut 1978, 19, 819–822. [Google Scholar] [CrossRef]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 12515. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Neyrinck, A.M.; Bäckhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-h.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.-S.; Hasani-Ranjbar, S. Neuromodulatory effect of microbiome on gut-brain axis; new target for obesity drugs. J. Diabetes Metab. Disord. 2019, 18, 263–265. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lee, Y.S.; Ooi, D.S.Q. Engineering the gut microbiome for treatment of obesity: A review of current understanding and progress. Biotechnol. J. 2020, 15, 2000013. [Google Scholar] [CrossRef]
- Parekh, P.J.; Balart, L.A.; Johnson, D.A. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin. Transl. Gastroenterol. 2015, 6, e91. [Google Scholar] [CrossRef]
- Rastelli, M.; Cani, P.D.; Knauf, C. The gut microbiome influences host endocrine functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef]
- Li, R.; Andreu-Sánchez, S.; Kuipers, F.; Fu, J. Gut microbiome and bile acids in obesity-related diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101493. [Google Scholar] [CrossRef]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut–microbiota–brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Shen, Y.L.; Zhang, L.Q.; Yang, Y.; Yin, B.C.; Ye, B.C.; Zhou, Y. Advances in the role and mechanism of lactic acid bacteria in treating obesity. Food Bioeng. 2022, 1, 101–115. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; Ten Hove, A.S.; Brul, S.; de Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.G.; Møller, P.; Loft, S.J.O.R. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Barra, N.G.; Cavallari, J.F.; Henriksbo, B.D.; Schertzer, J.D. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 2021, 36, 109691. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; De Castro, C.; Silipo, A.; Molinaro, A. Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol. Rev. 2019, 43, 257–272. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef]
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.l.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R.m. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 28, 9–17. [Google Scholar] [CrossRef]
- Walker, A.W.; Parkhill, J. Fighting obesity with bacteria. Science 2013, 341, 1069–1070. [Google Scholar] [CrossRef]
- Xu, J.; Mahowald, M.A.; Ley, R.E.; Lozupone, C.A.; Hamady, M.; Martens, E.C.; Henrissat, B.; Coutinho, P.M.; Minx, P.; Latreille, P. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007, 5, e156. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, V.; Ait-Belgnaoui, A.; Agostini, S.; Eutamene, H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 2014, 5, 430–629. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.-F.; He, S.; Chen, X.; Wang, J.; Li, J.; Zhu, Q.; Zhang, Z.; Li, L.; Alam, M.S. Effects of high carbohydrate diet-modulated microbiota on gut health in Chinese perch. Front. Microbiol. 2020, 11, 575102. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, S.-E.; Kim, A.; Kang, S.; Park, M.-Y.; Sung, M.-K. Dietary fat intake and age modulate the composition of the gut microbiota and colonic inflammation in C57BL/6J mice. BMC Microbiol. 2019, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-glucose or-fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev. Nutr. Food Sci. 2020, 25, 113. [Google Scholar] [CrossRef]
- Wang, H.; Hong, T.; Li, N.; Zang, B.; Wu, X. Soluble dietary fiber improves energy homeostasis in obese mice by remodeling the gut microbiota. Biochem. Biophys. Res. Commun. 2018, 498, 146–151. [Google Scholar] [CrossRef]
- Zhao, Q.; Hou, D.; Fu, Y.; Xue, Y.; Guan, X.; Shen, Q. Adzuki bean alleviates obesity and insulin resistance induced by a high-fat diet and modulates gut microbiota in mice. Nutrients 2021, 13, 3240. [Google Scholar] [CrossRef]
- Wang, B.; Yu, H.; He, Y.; Wen, L.; Gu, J.; Wang, X.; Miao, X.; Qiu, G.; Wang, H. Effect of soybean insoluble dietary fiber on prevention of obesity in high-fat diet fed mice via regulation of the gut microbiota. Food Funct. 2021, 12, 7923–7937. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, N.; Yang, L.; Hong, Z.; Cai, B.; Le, Q.; Yang, T.; Shi, L.; He, J.; Cui, C.-B.; et al. Insoluble dietary fiber derived from brown seaweed laminaria japonica ameliorate obesity-related features via modulating gut microbiota dysbiosis in high-fat diet–fed mice. Food Funct. 2021, 12, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ren, X.; Du, B.; Niu, K.; Yu, Z.; Yang, Y. Insoluble dietary fiber of pear fruit pomace (Pyrus ussuriensis Maxim) consumption ameliorates alterations of the obesity-related features and gut microbiota caused by high-fat diet. J. Funct. Foods 2022, 99, 105354. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Ji, K.; Zhang, P. Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci. Rep. 2016, 6, 32953. [Google Scholar] [CrossRef]
- Sun, H.; Wang, N.; Cang, Z.; Zhu, C.; Zhao, L.; Nie, X.; Cheng, J.; Xia, F.; Zhai, H.; Lu, Y. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes. Facts 2016, 9, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Zhao, Q.; Yousaf, L.; Xue, Y.; Shen, Q. Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice. Eur. J. Nutr. 2020, 59, 3617–3634. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-R.; Lin, C.-S.; Chang, C.-J.; Lin, T.-L.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Lu, C.-C.; Young, J.D.; Lai, H.-C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef]
- Kim, M.-J.; Shin, S.-K.; Han, J.-W.; Kim, J.E.; Lee, M.J.; Bae, H.R.; Kwon, E.-Y. Lactobacillus paragasseri SBT2055 attenuates obesity via the adipose tissue-muscle-gut axis in obese mice. Microbiol. Res. 2025, 290, 127972. [Google Scholar] [CrossRef]
- Kang, J.H.; Yun, S.I.; Park, M.H.; Park, J.H.; Jeong, S.Y.; Park, H.O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef]
- Weng, B.B.-C.; Yuan, H.-D.; Chen, L.-G.; Chu, C.; Hsieh, C.-W. Soy yoghurts produced with efficient GABA (γ-aminobutyric acid)-producing Lactiplantibacillus plantarum ameliorate hyperglycaemia and re-establish gut microbiota in streptozotocin (STZ)-induced diabetic mice. Food Funct. 2023, 14, 1699–1709. [Google Scholar] [CrossRef]
- Effects of viable Lacticaseibacillus paracasei K56 and its derived postbiotics on obesity and inflammation induced by a high-fat diet. Food Biosci. 2025, 65, 105999. [CrossRef]
- Déchelotte, P.; Breton, J.; Trotin-Picolo, C.; Grube, B.; Erlenbeck, C.; Bothe, G.; Fetissov, S.O.; Lambert, G. The Probiotic Strain H. alvei HA4597(®) Improves Weight Loss in Overweight Subjects under Moderate Hypocaloric Diet: A Proof-of-Concept, Multicenter Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2021, 13, 1902. [Google Scholar] [CrossRef]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Reidelberger, R.D.; Chelikani, P.K. Inulin fiber dose-dependently modulates energy balance, glucose tolerance, gut microbiota, hormones and diet preference in high-fat-fed male rats. J. Nutr. Biochem. 2018, 59, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.H.; Liu, F.; Borewicz, K.; Lohuis, M.A.M.; Smidt, H.; Verkade, H.J.; Tietge, U.J.F. Long-Term β-galacto-oligosaccharides Supplementation Decreases the Development of Obesity and Insulin Resistance in Mice Fed a Western-Type Diet. Mol. Nutr. Food Res. 2020, 64, e1900922. [Google Scholar] [CrossRef]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef]
- Safavi, M.; Farajian, S.; Kelishadi, R.; Mirlohi, M.; Hashemipour, M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: A randomized triple-masked controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 687–693. [Google Scholar] [CrossRef]
- Khanna, S.; Bishnoi, M.; Kondepudi, K.K.; Shukla, G. Synbiotic (Lactiplantibacillus pentosus GSSK2 and isomalto-oligosaccharides) supplementation modulates pathophysiology and gut dysbiosis in experimental metabolic syndrome. Sci. Rep. 2021, 11, 21397. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Navarro, V.; Álvarez, B.; Chenoll, E.; Ramón, D.; Tortajada, M.; Martorell, P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb. Biotechnol. 2022, 15, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Mao, B.; Zhang, Q.; Tang, X.; Yang, B.; Zhao, J.; Cui, S.; Zhang, H. Postbiotics Prepared Using Lactobacillus paracasei CCFM1224 Prevent Nonalcoholic Fatty Liver Disease by Modulating the Gut Microbiota and Liver Metabolism. Int. J. Mol. Sci. 2022, 23, 13522. [Google Scholar] [CrossRef]
- Shimizu, H.; Masujima, Y.; Ushiroda, C.; Mizushima, R.; Taira, S.; Ohue-Kitano, R.; Kimura, I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci. Rep. 2019, 9, 16574. [Google Scholar] [CrossRef]
- Amiri, P.; Hosseini, S.A.; Saghafi-Asl, M.; Akbari-Naserkiadeh, A.; Zavieh, S.A.J.; Arefhosseini, S.; Asghari, S.; Roshanravan, N. Expression of lipid metabolism- related genes, body composition and inflammation: Effects of sodium butyrate supplementation in subjects with obesity. J. Funct. Foods 2025, 124, 106645. [Google Scholar] [CrossRef]
- Kim, T.T.; Parajuli, N.; Sung, M.M.; Bairwa, S.C.; Levasseur, J.; Soltys, C.M.; Wishart, D.S.; Madsen, K.; Schertzer, J.D.; Dyck, J.R.B. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E511–E519. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, L.; Lei, L.; Zhu, Y.; Xu, J.; Liu, L. Fecal microbiota transplantation ameliorates abdominal obesity through inhibiting microbiota-mediated intestinal barrier damage and inflammation in mice. Microbiol. Res. 2024, 282, 127654. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents With Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Del Pulgar, E.M.G.; Kjølbæk, L.; Brahe, L.K.; Astrup, A.; Larsen, L.; Sanz, Y. Impact of dietary fiber and fat on gut microbiota re-modeling and metabolic health. Trends Food Sci. Technol. 2016, 57, 201–212. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Ma, S.; Ye, J.; Zhang, H.; Li, Y.; Sair, A.T.; Pan, J.; Liu, X.; Li, X.J.; et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: Roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation—Current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, L.; Ke, H.; Jiang, S.; Zeng, M. Oyster (Crassostrea gigas) polysaccharide ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat diet fed mice. Int. J. Biol. Macromol. 2022, 216, 916–926. [Google Scholar] [CrossRef]
- Borgeraas, H.; Johnson, L.; Skattebu, J.; Hertel, J.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Ghalichi, F.; Ahrabi, S.S.; Jamilian, P.; Jamilian, P.; Ghoreishi, Z. Anti-obesity properties of probiotics; a considerable medical nutrition intervention: Findings from an umbrella meta-analysis. Eur. J. Pharmacol. 2022, 928, 175069. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on body weight and body-mass index: A systematic review and meta-analysis of randomized, controlled trials. Int. J. Food Sci. Nutr. 2016, 67, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sheng, B.; Wu, Y.; Li, H.; Xu, D.; Nian, Y.; Mao, S.; Li, C.; Xu, X.; Zhou, G. Comparison of Free and Bound Advanced Glycation End Products in Food: A Review on the Possible Influence on Human Health. J. Agric. Food Chem. 2019, 67, 14007–14018. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Troise, A.D.; Fogliano, V.; de Vos, W.M. Anaerobic Degradation of N-ε-Carboxymethyllysine, a Major Glycation End-Product, by Human Intestinal Bacteria. J. Agric. Food Chem. 2019, 67, 6594–6602. [Google Scholar] [CrossRef]
- Jangra, S.; Pothuraju, R.; Sharma, R.K.; Bhakri, G. Co-administration of soluble fibres and Lactobacillus casei NCDC19 fermented milk prevents adiposity and insulin resistance via modulation of lipid mobilization genes in diet-induced obese mice. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2020, 20, 1543–1551. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, Z.; Xing, X.; Fan, Y.; Zhang, Y.; Nan, B.; Li, X.; Wang, Y.; Liu, J. The ameliorative effect of probiotics on diet-induced lipid metabolism disorders: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 3556–3572. [Google Scholar] [CrossRef]
- Slizewska, K.; Chlebicz-Wojcik, A. The in vitro analysis of prebiotic to be used as a component of a synbiotic preparation. Nutrients 2020, 12, 1272. [Google Scholar] [CrossRef]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a tool for the prevention and treatment of obesity and diabetes: Classification and ability to modulate the gut microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef]
- Maessen, S.E.; Derraik, J.G.B.; Binia, A.; Cutfield, W.S. Perspective: Human Milk Oligosaccharides: Fuel for Childhood Obesity Prevention? Adv. Nutr. 2020, 11, 35–40. [Google Scholar] [CrossRef]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. Gastrointestinal effects of prebiotics. Br. J. Nutr. 2002, 87, S145–S151. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Bioecologic control of the gastrointestinal tract: The role of flora and supplemented probiotics and synbiotics. Gastroenterol. Clin. N. Am. 2005, 34, 413–436. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Rioux, K.P.; Madsen, K.L.; Fedorak, R.N. The role of enteric microflora in inflammatory bowel disease: Human and animal studies with probiotics and prebiotics. Gastroenterol. Clin. N. Am. 2005, 34, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Alizadeh, K.; Hajianfar, H.; Mohammadi, H.; Miraghajani, M. Efficacy of synbiotic supplementation in obesity treatment: A systematic review and meta-analysis of clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Joung, J.Y.; Kang, D.-K.; Oh, N.S.; Yoon, Y. Anti-obesity effects of Lactobacillus rhamnosus 4B15, and its synergy with hydrolysed lactose skim milk powder. Int. Dairy J. 2021, 123, 104997. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Dinu, L.D.; Avram, I.; Pelinescu, D.R.; Vamanu, E. Mineral-Enriched Postbiotics: A New Perspective for Microbial Therapy to Prevent and Treat Gut Dysbiosis. Biomedicines 2022, 10, 2392. [Google Scholar] [CrossRef]
- Osman, A.; El-Gazzar, N.; Almanaa, T.N.; El-Hadary, A.; Sitohy, M. Lipolytic Postbiotic from Lactobacillus paracasei Manages Metabolic Syndrome in Albino Wistar Rats. Molecules 2021, 26, 472. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Lee, P.; Yacyshyn, B.R.; Yacyshyn, M.B. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes. Metab. 2019, 21, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Juuti, A.; Luostarinen, M.; Niskanen, L.; Liukkonen, T.; Tillonen, J.; Kössi, J.; Ilvesmäki, V.; Viljakka, M.; Satokari, R.; et al. Effectiveness of Fecal Microbiota Transplantation for Weight Loss in Patients With Obesity Undergoing Bariatric Surgery: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2247226. [Google Scholar] [CrossRef] [PubMed]
- Zecheng, L.; Donghai, L.; Runchuan, G.; Yuan, Q.; Qi, J.; Yijia, Z.; Shuaman, R.; Xiaoqi, L.; Yi, W.; Ni, M.; et al. Fecal microbiota transplantation in obesity metabolism: A meta analysis and systematic review. Diabetes Res. Clin. Pract. 2023, 202, 110803. [Google Scholar] [CrossRef]
- He, R.; Li, P.; Wang, J.; Cui, B.; Zhang, F.; Zhao, F. The interplay of gut microbiota between donors and recipients determines the efficacy of fecal microbiota transplantation. Gut Microbes 2022, 14, 2100197. [Google Scholar] [CrossRef]
| Anti-Obesity Strategy | Treatment | Study Model | Diet | Modulation | Anti-Obesity Effects | Ref. |
|---|---|---|---|---|---|---|
| Dietary control | Soluble dietary fiber Fibersol-2 | HFD-induced obese mouse | High-fat diet | F/B ratio ↓ | Body weight ↓ White fat accumulation ↓ | [69] |
| Soluble dietary fiber Adzuki bean | HFD-induced obese mouse | High-fat diet | F/B ratio ↓ Bifidobacterium ↑ | Lipid accumulation, serum lipids ↓ Improve insulin resistance | [70] | |
| Insoluble dietary fiber extracted from soybean | HFD-induced obese mouse | High-fat diet or HFD + orlistat treatment | Lactobacillales, Lactobacillus, Lachnospirace group ↑ Lachnospiraceae, Bacteroides acidifaciens ↓ | Body weight ↓ TC, TG, LDL-C ↓ HDL-C ↑ | [71] | |
| Insoluble dietary fiber extracted from brown seaweed | HFD-induced obese mouse | High-fat diet | Akkermansia muciniphila ↑ | Serum cholesterol and glucose ↓ | [72] | |
| Insoluble dietary fiber extracted from pear fruit pomace (Pyrus ussuriensis Maxim) | HFD-induced obese mouse | High-fat diet or HFD + orlistat treatment | F/B ratio ↓ Akkermansia, Parabacteroides, Alistipes, Alloprevotella ↑ | TC, TG, LDL-C ↓ HDL-C ↑ | [73] | |
| Bamboo shoot fiber | HFD-induced obese mouse | High-fat diet or HFD + cellulose treatment | Bacteroidetes ↑ Verrucomicrobia ↓ | Body weight, fat mass ↓ | [74] | |
| Herb component berberine | HFD-induced obese mouse | High-fat diet | F/B ratio ↓ SCFA-producing bacteria (Bacteriodes, Bilophila) ↑ | Body weight, plasma lipid levels, endogenous glucose production, lipolysis ↓ Improve insulin resistance | [75] | |
| Whole mung bean | HFD-induced obese mouse | High-fat diet | F/B ratio ↓ Akkermansia, Bifidobacterium ↑ | Body weight, fat accumulation, adipocyte size ↓ Improve glucose tolerance | [76] | |
| Hirsutella sinensis mycelium, a medicinal mushroom | HFD-induced obese mouse | High-fat diet | Parabacteroides goldsteinii ↑ | Body weight ↓ | [77] | |
| Probiotics | Bacteroides acidifaciens | HFD-induced obese mouse | High-fat diet | - | Body weight ↓ Fat mass ↓ PPARα activation DPP-4 ↓ | [44] |
| Lactobacillus paragasseri SBT2055 | HFD-induced obese mouse | High-fat diet | Bacteroidetes ↑ | Body weight ↓ Fat accumulation ↓ Intestinal fat absorption genes ↓ | [78] | |
| Lactobacillus gasseri BNR17 | HFD-fed mice | High-fat diet | - | Body weight ↓ Fat accumulation ↓ Leptin ↓ | [79] | |
| Lactiplantibacillus plantarum GA30 | STZ-induced diabetic mouse | Beneficial bacteria ↑ | Hyperglycemia↓ Body weight ↓ GABA ↑ | [80] | ||
| Lacticaseibacillus paracasei K56 | HFD-induced obese mouse | High-fat diet | - | Genes related to lipid synthesis (FAS, PPAR-γ, CEBP-α) ↓ Pro-inflammatory cytokines in the colon (IL-1β, TNF-α, IL-6) ↓ | [81] | |
| Hafnia alvei HA4597 | Overweight human (BMI 25–29.9 kg/m2) | Hypocaloric diet | - | Body weight ↓ BMI ↓ Hip circumference ↓ Satiety hormone ↑ | [82] | |
| Prebiotics | Inulin | HFD-induced obese mouse | High-fat diet | F/B ratio ↓ | Body weight ↓ Adiposity ↓ Improve glucose tolerance | [83] |
| Galactooligosaccharides (GOSs) | HFD-induced obese mouse | High-fat diet | F/B ratio ↓ | Body weight ↓ Adiposity ↓ Improve glucose tolerance | [84] | |
| Fructooligosaccharides (FOSs) | HFD-induced obese mouse | High-fat diet | Akkermansia, Odoribacter, Roseburia, and Muribaculaceae ↑ | Body weight ↓ Fat accumulation ↓ Improve glucose tolerance | [85] | |
| Synbiotics and postbiotics | Synbiotic capsule, containing a combination of Lactobacillus spp., Streptococcus thermophilus, and Bifidobacterium spp. with FOSs | Overweight and obese children | Ad libitum diet | fecal LAB counts ↑ | BMI ↓ Serum triglyceride levels ↓ Total and LDL cholesterol levels ↓ | [86] |
| Lactiplantibacillus pentosus GSSK2 + isomalto-oligosaccharides | HFD-induced obese mouse | High-fat diet | F/B ratio, ↓ Enterobacteriaceae ↓ Lactobacillus spp., Akkermansia spp., Faecalibacterium spp., Roseburia spp. ↑ | Body weight ↓ Abdominal circumference ↓ BMI ↓ Visceral fat deposition ↓ | [87] | |
| Lipoteichoic acid derived from Bifidobacterium animalis subsp. lactis BPL1 | Caenorhabditis elegans N2 | Treatment with live probiotics or lipoteichoic acid extracts or heat-killed probiotics | - | Lipid storage ↓ TG levels ↓ | [88] | |
| Cell-free extract of Lactobacillus paracasei | HFD-induced obese rat | High-fat diet or HFD + ATOR | - | Total and LDL cholesterol levels ↓ Body weight ↓ | [89] | |
| SCFAs (acetate, propionate, and butyrate) | HFD-induced obese rat | High-fat diet | - | Body weight ↓ Blood sugar ↓ Fatty acid synthesis genes (Fas, Chrebp) ↓ | [90] | |
| Sodium butyrate | Obese adults (BMI 30–40 kg/m2) | Hypocaloric diet | - | Fat mass and visceral fat ↓ Lipid metabolism-related genes (ADIPOR1, UCP3) ↑ | [91] | |
| FMT | FMT + resveratrol | Male C57BL/6N mice | High-fat, high-sugar diet | - | Improve glucose homeostasis Intestinal inflammatory cytokines (TNF-α, IL-1 β) ↓ | [92] |
| FMT | Abdominal obesity (AO) mouse | Normal chow diet | Restoration of gut microbiota | Restore intestinal mucosal barrier Systemic inflammation ↓ Abdominal fat accumulation ↓ Body weight ↓ | [93] | |
| FMT | Adolescents with obesity | Usual diet | Microbial diversity ↑ | Improve glucose tolerance Improve insulin sensitivity Abdominal fat ↓ | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, K.-R.; Singh, S.; Hwang, H.-S.; Seo, S.-O. Using Gut Microbiota Modulation as a Precision Strategy Against Obesity. Int. J. Mol. Sci. 2025, 26, 6282. https://doi.org/10.3390/ijms26136282
Baek K-R, Singh S, Hwang H-S, Seo S-O. Using Gut Microbiota Modulation as a Precision Strategy Against Obesity. International Journal of Molecular Sciences. 2025; 26(13):6282. https://doi.org/10.3390/ijms26136282
Chicago/Turabian StyleBaek, Kwang-Rim, Saloni Singh, Hye-Seon Hwang, and Seung-Oh Seo. 2025. "Using Gut Microbiota Modulation as a Precision Strategy Against Obesity" International Journal of Molecular Sciences 26, no. 13: 6282. https://doi.org/10.3390/ijms26136282
APA StyleBaek, K.-R., Singh, S., Hwang, H.-S., & Seo, S.-O. (2025). Using Gut Microbiota Modulation as a Precision Strategy Against Obesity. International Journal of Molecular Sciences, 26(13), 6282. https://doi.org/10.3390/ijms26136282








