Special Issue: “Gut Microbiota and Nutrition in Human Health”
1. Background
2. Key Themes and Insights
2.1. Diet as a Modulator of Gut Microbiota
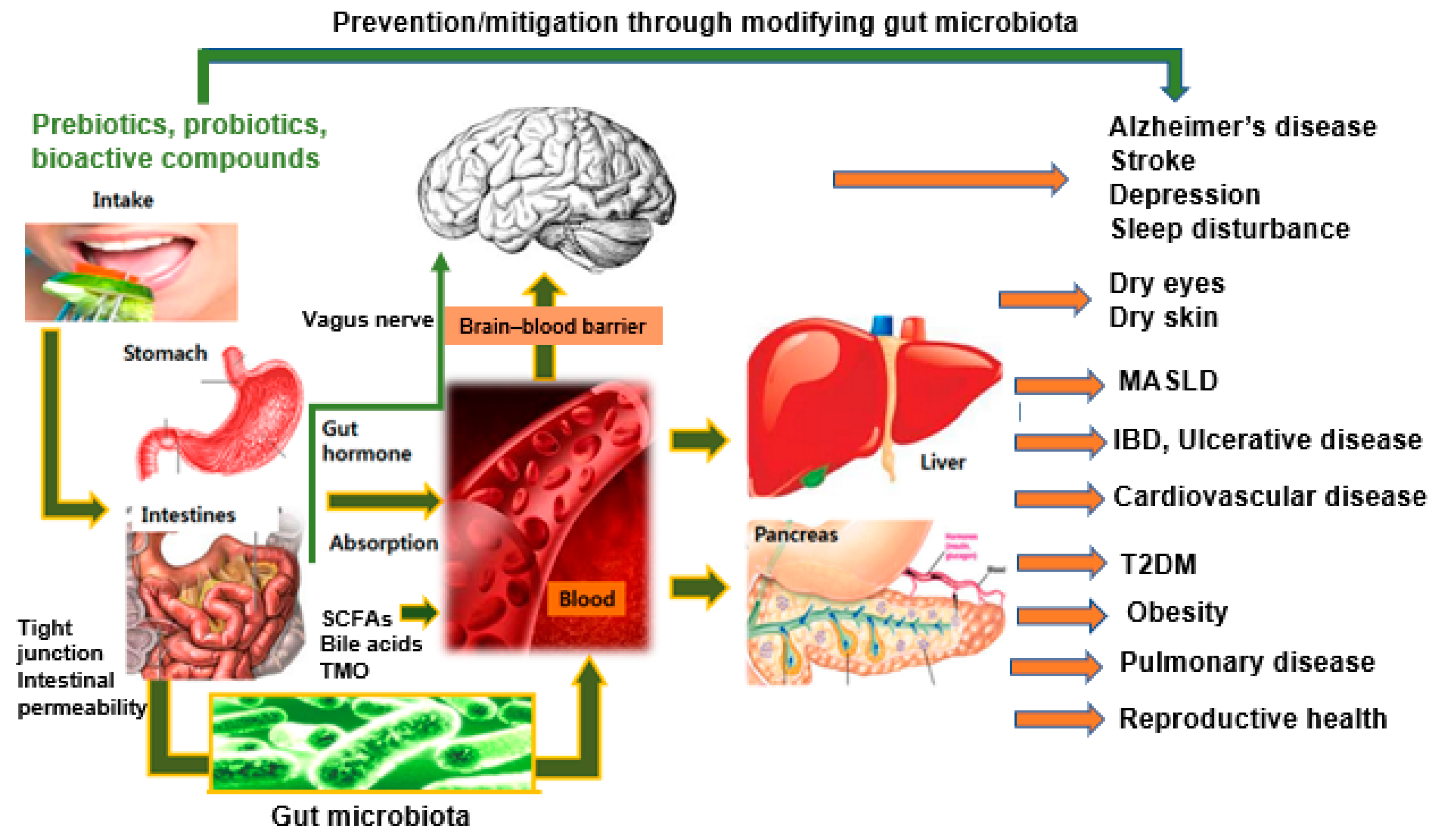
2.2. Beyond the Gut: Systemic Effects of Microbiota–Nutrition Interactions
2.3. Linking Food Components with Microbiota Metabolism
2.4. Therapeutic Potential of Microbiota Modulation
3. Overview of Featured Studies
4. Future Directions in Gut Microbiota and Nutrition Research
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Dorrestein, P.C.; Turnbaugh, P.J.; Knight, R. The intestinal metabolome: An intersection between microbiota and host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.S.; Chang, E.B. The microbiome: Composition and locations. Prog. Mol. Biol. Transl. Sci. 2020, 176, 1–42. [Google Scholar] [CrossRef]
- Song, H.S.; Lindemann, S.R.; Lee, D.Y. Editorial: Predictive Modeling of Human Microbiota and Their Role in Health and Disease. Front. Microbiol. 2021, 12, 782871. [Google Scholar] [CrossRef]
- Park, S.; Wu, X. Modulation of the Gut Microbiota in Memory Impairment and Alzheimer’s Disease via the Inhibition of the Parasympathetic Nervous System. Int. J. Mol. Sci. 2022, 23, 13574. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, Y.; Li, C.; Wu, X.; Park, S. Vagus Nerve Suppression in Ischemic Stroke by Carotid Artery Occlusion: Implications for Metabolic Regulation, Cognitive Function, and Gut Microbiome in a Gerbil Model. Int. J. Mol. Sci. 2024, 25, 7831. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Wiącek, J.; Podgórski, T.; Kusy, K.; Łoniewski, I.; Skonieczna-Żydecka, K.; Karolkiewicz, J. Evaluating the Impact of Probiotic Therapy on the Endocannabinoid System, Pain, Sleep and Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial in Dancers. Int. J. Mol. Sci. 2024, 25, 5611. [Google Scholar] [CrossRef]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors 2021, 47, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jung, E.S.; Chae, S.W.; Jung, S.J.; Daily, J.W.; Park, S. Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics. Nutrients 2024, 16, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, C.; Yue, Y.; Yang, H.-J.; Ryu, M.S.; Wu, X.; Jeong, D.Y.; Park, S. Fermented red pepper paste (kochujang) modulates glucose metabolism and gut microbiota in parasympathetic suppression: Network pharmacology and In vivo study. Food Biosci. 2024, 61, 104531. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Yue, Y.; Wu, X. Effects of Bile Acid Modulation by Dietary Fat, Cholecystectomy, and Bile Acid Sequestrant on Energy, Glucose, and Lipid Metabolism and Gut Microbiota in Mice. Int. J. Mol. Sci. 2022, 23, 5935. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Lee, K.; Gwon, H.; Shim, J.J.; Kim, J.Y.; Lee, J.H. Consumption of Limosilactobacillus fermentum Inhibits Corneal Damage and Inflammation in Dry Eye Disease Mouse Model through Regulating the Gut Microbiome. Int. J. Mol. Sci. 2024, 25, 3528. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Zhang, Y.; Wang, J.; Zheng, N. Strontium Chloride Improves Reproductive Function and Alters Gut Microbiota in Male Rats. Int. J. Mol. Sci. 2023, 24, 13922. [Google Scholar] [CrossRef]
- Park, S.; Li, C.; Wu, X.; Zhang, T. Gut Microbiota Alterations and Their Functional Differences in Depression According to Enterotypes in Asian Individuals. Int. J. Mol. Sci. 2023, 24, 13329. [Google Scholar] [CrossRef]
- Sato, S.; Iino, C.; Chinda, D.; Sasada, T.; Tateda, T.; Kaizuka, M.; Nomiya, H.; Igarashi, G.; Sawada, K.; Mikami, T.; et al. Effect of Liver Fibrosis on Oral and Gut Microbiota in the Japanese General Population Determined by Evaluating the FibroScan–Aspartate Aminotransferase Score. Int. J. Mol. Sci. 2023, 24, 13470. [Google Scholar] [CrossRef]
- Carnicero-Mayo, Y.; Sáenz de Miera, L.E.; Ferrero, M.Á.; Navasa, N.; Casqueiro, J. Modeling Dynamics of Human Gut Microbiota Derived from Gluten Metabolism: Obtention, Maintenance and Characterization of Complex Microbial Communities. Int. J. Mol. Sci. 2024, 25, 4013. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef]
- Olteanu, G.; Ciucă-Pană, M.-A.; Busnatu, Ș.S.; Lupuliasa, D.; Neacșu, S.M.; Mititelu, M.; Musuc, A.M.; Ioniță-Mîndrican, C.-B.; Boroghină, S.C. Unraveling the Microbiome–Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications. Int. J. Mol. Sci. 2024, 25, 5561. [Google Scholar] [CrossRef] [PubMed]
- Cisek, A.A.; Szymańska, E.; Wierzbicka-Rucińska, A.; Aleksandrzak-Piekarczyk, T.; Cukrowska, B. Methanogenic Archaea in the Pediatric Inflammatory Bowel Disease in Relation to Disease Type and Activity. Int. J. Mol. Sci. 2024, 25, 673. [Google Scholar] [CrossRef] [PubMed]
- Schupack, D.A.; Mars, R.A.T.; Voelker, D.H.; Abeykoon, J.P.; Kashyap, P.C. The promise of the gut microbiome as part of individualized treatment strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, T.; Zhang, T.; Park, S. The impact of gut microbiome enterotypes on ulcerative colitis: Identifying key bacterial species and revealing species co-occurrence networks using machine learning. Gut Microbes 2024, 16, 2292254. [Google Scholar] [CrossRef]
- Saxami, G.; Kerezoudi, E.N.; Eliopoulos, C.; Arapoglou, D.; Kyriacou, A. The Gut–Organ Axis within the Human Body: Gut Dysbiosis and the Role of Prebiotics. Life 2023, 13, 2023. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Wu, X.; Park, S. Fecal Bacterial Community and Metagenome Function in Asians with Type 2 Diabetes, According to Enterotypes. Biomedicines 2022, 10, 2998. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Kang, S. Fecal Microbiota Composition, Their Interactions, and Metagenome Function in US Adults with Type 2 Diabetes According to Enterotypes. Int. J. Mol. Sci. 2023, 24, 9533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S. Special Issue: “Gut Microbiota and Nutrition in Human Health”. Int. J. Mol. Sci. 2024, 25, 11589. https://doi.org/10.3390/ijms252111589
Park S. Special Issue: “Gut Microbiota and Nutrition in Human Health”. International Journal of Molecular Sciences. 2024; 25(21):11589. https://doi.org/10.3390/ijms252111589
Chicago/Turabian StylePark, Sunmin. 2024. "Special Issue: “Gut Microbiota and Nutrition in Human Health”" International Journal of Molecular Sciences 25, no. 21: 11589. https://doi.org/10.3390/ijms252111589
APA StylePark, S. (2024). Special Issue: “Gut Microbiota and Nutrition in Human Health”. International Journal of Molecular Sciences, 25(21), 11589. https://doi.org/10.3390/ijms252111589




