Circadian Clock Deregulation and Metabolic Reprogramming: A System Biology Approach to Tissue-Specific Redox Signaling and Disease Development
Abstract
1. Introduction
2. Circadian Rhythms and Neuroendocrine Adaptation to Environmental Challenges
2.1. Circadian Organization as an Adaptive Feature of Mammalian Physiology
2.2. Cyclic Rhythmicity of Neuroendocrine Secretions
2.3. Circadian Clocks and Their Importance to Mammalian Homeostasis
2.4. Core Elements of the Circadian Clock at the Cellular Level
3. Chrono-Endocrinology: Limbic Hypothalamic–Pituitary–Adrenal (LHPA)—Pineal Axis and Metabolic Dysfunction
3.1. Glucocorticoid Dysregulation and Circadian Metabolic Imbalance
3.2. Circadian Rhythm Desynchronization and Metabolic Disease Predisposition
4. Circadian Regulation of Adipose Tissue Metabolic and Endocrine Function
4.1. Adipose Tissue Diversity and Circadian-Regulated Adipokine Secretion
4.2. Circadian Rhythms Disruption as a Precondition for Obesity and Health Disorders
4.3. Oxidative Stress, Obesity, and Adipose Tissue Secretion
4.4. Kisspeptin as a Link Between Circadian Activity, Reproduction, and Metabolism
5. Circadian Control of Cellular Proteostasis and Redox Balance
5.1. The Proteasome-Circadian Interface in Metabolic Regulation
5.2. Circadian Inflammasome Activation and Metabolic Inflammation
6. Tissue-Specific Metabolic Clocks: Beyond the Liver and White Adipose Tissue
6.1. Circadian Control of Bone Marrow Adipose Tissue and Pink Adipose Tissue
6.2. Circadian Epigenetic Reprogramming in Liver Metabolic Disorders
7. Translational and Clinical Implications: Future Research Directions
7.1. Chrono-Pharmacology and Metabolic Disease Treatment
7.2. Circadian Nutritional Programming: The Role of Meal Timing in Redox Homeostasis
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AIM2 | Absent in melanoma 2 |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| AVPV | Hypothalamic anteroventral periventricular nucleus |
| ASC | Apoptosis-associated Speck-like protein containing a CARD |
| ATP | Adenosine triphosphate |
| BAT | Brown adipose tissue |
| BMAL1 | Brain and muscle ARNT-like 1 |
| BMAT | Bone marrow adipose tissue |
| BMI | Body mass index |
| CLOCK | Circadian locomotor output cycles kaput |
| GSK3β | Glycogen synthase kinase-3β |
| HDAC | Histone deacetylase |
| HFD | High-fat diet |
| HPG | Hypothalamic–pituitary–gonadal |
| HSC | Hematopoietic stem cell |
| hsCRP | High-sensitivity C-reactive protein |
| IL | Interleukin |
| LHPA | Lymbic hypothalamic–pituitary–adrenal |
| Kiss1 | Kisspeptin gene in mammals |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| MAsH | Metabolic dysfunction-associated steatohepatitis |
| MASL | Metabolic dysfunction-associated liver disease |
| MT2 | Melatonin type-2 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| NRF1 | Nuclear respiratory factor 1 |
| NRF2 | Nuclear factor erythroid 2–related factor 2 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PKC | Protein kinase C |
| REV-ERBα | Nuclear receptor subfamily 1 group D member 1 |
| ROS | Reactive oxygen species |
| SCN | Suprachiasmatic nucleus |
| TNF-α | Tumor necrosis factor-α |
| TRF | Time-restricted feeding |
| TXNIP | Thioredoxin-interacting protein |
| UPS | Ubiquitin–proteasome system |
| WAT | White adipose tissue |
References
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef]
- Opperhuizen, A.-L.; van Kerkhof, L.W.M.; Proper, K.I.; Rodenburg, W.; Kalsbeek, A. Rodent models to study the metabolic effects of shiftwork in humans. Front. Pharmacol. 2015, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The circadian syndrome: Is the metabolic syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Circadian clock control of liver metabolic functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.-K. Feeding rhythms and the circadian regulation of metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Zhang, E.E.; Liu, A.C.; Hirota, T.; Miraglia, L.J.; Welch, G.; Pongsawakul, P.Y.; Liu, X.; Atwood, A.; Huss, J.W.; Janes, J.; et al. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell 2009, 139, 199–210. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef]
- Betts, J.A.; Bowden Davies, K.A.; Smith, H.A.; Hawley, J.A. Physiological rhythms and metabolic regulation: Shining light on skeletal muscle. Exp. Physiol. 2025, in press. [CrossRef]
- Procopio, S.B.; Esser, K.A. Clockwork conditioning: Aligning the skeletal muscle clock with time-of-day exercise for cardiometabolic health. J. Mol. Cell. Cardiol. 2025, 198, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, A.; Seinkmane, E.; O’Neill, J.S. Understanding circadian regulation of mammalian cell function, protein homeostasis, and metabolism. Curr. Opin. Syst. Biol. 2021, 28, 100391. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M.; Weaver, D.R. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001, 30, 525–536. [Google Scholar] [CrossRef]
- Liu, A.C.; Welsh, D.K.; Ko, C.H.; Tran, H.G.; Zhang, E.E.; Priest, A.A.; Buhr, E.D.; Singer, O.; Meeker, K.; Verma, I.M.; et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007, 129, 605–616. [Google Scholar] [CrossRef]
- Crown, A.; Lightman, S. Management of patients with glucocorticoid deficiency. Nat. Clin. Pract. Endocrinol. Metab. 2005, 1, 62–63. [Google Scholar] [CrossRef]
- Stratmann, M.; Schibler, U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J. Biol. Rhythms 2006, 21, 494–506. [Google Scholar] [CrossRef]
- McMahon, D.G.; Iuvone, P.M.; Tosini, G. Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog. Retin. Eye Res. 2014, 39, 58–76. [Google Scholar] [CrossRef]
- Pevet, P.; Challet, E. Melatonin: Both Master Clock Output and Internal Time-Giver in the Circadian Clocks Network. J. Physiol. Paris 2011, 105, 170–182. [Google Scholar] [CrossRef]
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Rivera-Bermudez, M.A.; Gerdin, M.J.; Masana, M.I. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, d1093–d1108. [Google Scholar] [CrossRef]
- Hunt, A.E.; Al-Ghoul, W.M.; Gillette, M.U.; Dubocovich, M.L. Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am. J. Physiol. Cell Physiol. 2001, 280, C110–C118. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Rauch, A.; Korf, H.W.; von Gall, C. The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol. Int. 2012, 29, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef]
- Tomova, A.; Kumanov, P.; Robeva, R.; Manchev, S.; Konakchieva, R. Melatonin secretion and non-specific immune responses are differentially expressed in corticotropin-dependent and corticotropin-independent Cushing’s syndrome. Med. Sci. Monit. 2008, 14, CR327–CR332. [Google Scholar]
- Colombini, B.; Dinu, M.; Murgo, E.; Lotti, S.; Tarquini, R.; Sofi, F.; Mazzoccoli, G. Ageing and low-level chronic inflammation: The role of the biological clock. Antioxidants 2022, 11, 2228. [Google Scholar] [CrossRef]
- Cunningham, P.S.; Meijer, P.; Nazgiewicz, A.; Anderson, S.G.; Borthwick, L.A.; Bagnall, J.; Kitchen, G.B.; Lodyga, M.; Begley, N.; Venkateswaran, R.V.; et al. The circadian clock protein REV-ERBα inhibits pulmonary fibrosis development. Proc. Natl. Acad. Sci. USA 2020, 117, 1139–1147. [Google Scholar] [CrossRef]
- Joshi, A.; Sundar, I.K. Circadian disruption in night shift work and its association with chronic pulmonary diseases. Adv. Biol. 2023, 7, e2200292. [Google Scholar] [CrossRef]
- Kiessling, S. Circadian rhythms in health and disease. In Beyond Our Genes; Teperino, R., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Lee, J.H.; Sancar, A. Regulation of apoptosis by the circadian clock through NF-κB signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 12036–12041. [Google Scholar] [CrossRef]
- Welz, P.S.; Benitah, S.A. Molecular connections between circadian clocks and aging. J. Mol. Biol. 2020, 432, 3661–3679. [Google Scholar] [CrossRef]
- Okamoto-Uchida, Y.; Izawa, J.; Nishimura, A.; Hattori, A.; Suzuki, N.; Hirayama, J. Post-translational modifications are required for circadian clock regulation in vertebrates. Curr. Genom. 2019, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. MYC on the Path to Cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Bozek, K.; Relógio, A.; Kielbasa, S.M.; Heine, M.; Dame, C.; Kramer, A.; Herzel, H. Regulation of clock-controlled genes in mammals. PLoS ONE 2009, 4, e4882. [Google Scholar] [CrossRef]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A.; et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef]
- del Rey, A.; Chrousos, G.P.; Besedovsky, H.O. The Hypothalamus-Pituitary-Adrenal Axis. In NeuroImmune Biology; Berczi, I., Szentivanyi, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; Volume 7. [Google Scholar]
- Nicolaides, N.C.; Charmandari, E.; Chrousos, G.P.; Kino, T. Circadian endocrine rhythms: The hypothalamic-pituitary-adrenal axis and its actions. Ann. N. Y. Acad. Sci. 2014, 1318, 71–80. [Google Scholar] [CrossRef]
- Matthews, S.G.; McGowan, P.O. Developmental programming of the HPA axis and related behaviours: Epigenetic mechanisms. J. Endocrinol. 2019, 242, T69–T79. [Google Scholar] [CrossRef]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009, 200, 3–22. [Google Scholar] [CrossRef]
- Lamia, K.A.; Papp, S.J.; Yu, R.T.; Barish, G.D.; Uhlenhaut, N.H.; Jonker, J.W.; Downes, M.; Evans, R.M. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 2011, 480, 552–556. [Google Scholar] [CrossRef]
- Speksnijder, E.M.; Bisschop, P.H.; Siegelaar, S.E.; Stenvers, D.J.; Kalsbeek, A. Circadian desynchrony and glucose metabolism. J. Pineal Res. 2024, 76, e12956. [Google Scholar] [CrossRef]
- Whirledge, S.; DeFranco, D.B. Glucocorticoid signaling in heeremialth and disease: Insights from tissue-specific GR knockout mice. Endocrinology 2018, 159, 46–64. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Præstholm, S.M.; Correia, C.M.; Grøntved, L. Multifaceted control of GR signaling and its impact on hepatic transcriptional networks and metabolism. Front. Endocrinol. 2020, 11, 572981. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Xia, N.; Li, H. Circadian rhythm in adipose tissue: Novel antioxidant target for metabolic and cardiovascular diseases. Antioxidants 2020, 9, 968. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Konakchieva, R.; Mitev, Y.; Almeida, O.F.X.; Patchev, V. Chronic melatonin treatment counteracts glucocorticoid-induced dysregulation of the hypothalamic-pituitary-adrenal axis in the rat. Neuroendocrinology 1998, 67, 171–180. [Google Scholar] [CrossRef]
- Konakchieva, R.; Mitev, Y.; Almeida, O.F.X.; Patchev, V. Chronic melatonin treatment and the HPA axis in the rat: Attenuation of the secretory response to stress and effects on hypothalamic neuropeptide content and release. Biol. Cell 1997, 89, 587–596. [Google Scholar]
- Arendt, J.; Skene, D.J. Melatonin as a chronobiotic. Sleep Med. Rev. 2005, 9, 25–39. [Google Scholar] [CrossRef]
- Lund, J.; Arendt, J.; Hampton, S.M.; English, J.; Morgan, L.M. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J. Endocrinol. 2001, 171, 557–564. [Google Scholar] [CrossRef]
- Knutsson, A. Health disorders of shift workers. Occup. Med. 2003, 53, 103–108. [Google Scholar] [CrossRef]
- Karlsson, H.K.; Zierath, J.R.; Kane, S.; Krook, A.; Lienhard, G.E.; Wallberg-Henriksson, H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 2005, 54, 1692–1697. [Google Scholar] [CrossRef]
- Halberg, F.; Sothern, R.B.; Cornélissen, G.; Czaplicki, J. Chronomics, human time estimation, and aging. Clin. Interv. Aging 2008, 3, 749–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robeva, R.; Kirilov, G.; Tomova, A.; Kumanov, P. Melatonin–insulin interactions in patients with metabolic syndrome. J. Pineal Res. 2008, 44, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kondratova, A.A.; Kondratov, R.V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 2012, 13, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. White, brown, beige and pink: A rainbow in the adipose organ. Curr. Opin. Endocr. Metab. Res. 2019, 4, 29–36. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Tratwal, J.; Rojas-Sutterlin, S.; Bataclan, C.; Blum, S.; Naveiras, O. Bone marrow adiposity and the hematopoietic niche: A historical perspective of reciprocity, heterogeneity, and lineage commitment. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101564. [Google Scholar] [CrossRef]
- Marinelli Busilacchi, E.; Morsia, E.; Poloni, A. Bone Marrow Adipose Tissue. Cells 2024, 13, 724. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Froy, O.; Garaulet, M. The circadian clock in white and brown adipose tissue: Mechanistic, endocrine, and clinical aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Emilova, R.; Dimitrova, D.Z.; Mladenov, M.; Hadzi-Petrushev, N.; Daneva, T.; Padeshki, P.; Schubert, R.; Chichova, M.; Lubomirov, L.; Simeonovska-Nikolova, D.; et al. Diabetes converts arterial regulation by perivascular adipose tissue from relaxation into H2O2-mediated contraction. Physiol. Res. 2016, 65, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kettner, N.M.; Mayo, S.A.; Hua, J.; Lee, C.; Moore, D.D.; Fu, L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015, 22, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef]
- Zvonic, S.; Ptitsyn, A.A.; Conrad, S.A.; Scott, L.K.; Floyd, Z.E.; Kilroy, G.; Wu, X.; Goh, B.C.; Mynatt, R.L.; Gimble, J.M. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 2006, 55, 962–970. [Google Scholar] [CrossRef]
- Paschos, G.K.; Ibrahim, S.; Song, W.L.; Kunieda, T.; Grant, G.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef]
- Sáinz, N.; Barrenetxe, J.; Moreno-Aliaga, M.J.; Martínez, J.A. Leptin resistance and diet-induced obesity: Central and peripheral actions of leptin. Metabolism 2015, 64, 35–46. [Google Scholar] [CrossRef]
- Qian, J.; Scheer, F.A.J.L. Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef]
- Perez, L.M.; Bernal, A.; de Lucas, B.; San Martin, N.; Mastrangelo, A.; García, A.; Sepúlveda, P. Altered metabolic and stemness capacity of adipose tissue-derived stem cells from obese mouse and human. PLoS ONE 2018, 13, e0195702. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Bravo Santos, R.; Fekry, B.; Tamim, Y.M.; Shivshankar, S.; Mohamed, A.M.T.; Baumgartner, C.; Kwok, C.; Gebhardt, C.; Rivera, A.; et al. Cellular and physiological circadian mechanisms drive diurnal cell proliferation and expansion of white adipose tissue. Nat. Commun. 2021, 12, 3482. [Google Scholar] [CrossRef]
- Cedernaes, J.; Waldeck, N.; Bass, J. Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Genes Dev. 2019, 33, 1136–1158. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zvonic, S.; Floyd, Z.E.; Kilroy, G.; Goh, B.C.; Hernandez, T.L.; Eckel, R.H.; Mynatt, R.L.; Gimble, J.M. Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity 2007, 15, 2560–2570. [Google Scholar] [CrossRef]
- Otway, D.T.; Mäntele, S.; Bretschneider, S.; Wright, J.; Trayhurn, P.; Skene, D.J.; Robertson, M.D.; Johnston, J.D. Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 2011, 60, 1577–1581. [Google Scholar] [CrossRef]
- Civelek, E.; Ozturk Civelek, D.; Akyel, Y.K.; Kaleli Durman, D.; Okyar, A. Circadian dysfunction in adipose tissue: Chronotherapy in metabolic diseases. Biology 2023, 12, 1077. [Google Scholar] [CrossRef]
- Froy, O. Metabolism and circadian rhythms—Implications for obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Marcheva, B.; Kohsaka, A.; Bass, J. The clockwork of metabolism. Annu. Rev. Nutr. 2007, 27, 219–240. [Google Scholar] [CrossRef]
- Albrecht, U. The circadian clock, metabolism, and obesity. Obes. Rev. 2017, 18, 915–929. [Google Scholar] [CrossRef]
- Barnea, M.; Madar, Z.; Froy, O. High-fat diet followed by fasting disrupts circadian expression of adiponectin signaling pathway in muscle and adipose tissue. Obesity 2010, 18, 230–238. [Google Scholar] [CrossRef]
- Pivovarova, O.; Jürchott, K.; Rudovich, N.; Hornemann, S.; Ye, L.; Möckel, S.; Murahovschi, V.; Kessler, K.; Seltmann, A.C.; Maser-Gluth, C.; et al. Changes of dietary fat and carbohydrate content alter central and peripheral clock in humans. J. Clin. Endocrinol. Metab. 2015, 100, 2291–2302. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Inoue, I.; Shinoda, Y.; Ikeda, M.; Hayashi, K.; Kanazawa, K.; Nomura, M.; Matsunaga, T.; Xu, H.; Kawai, S.; Awata, T.; et al. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J. Atheroscler. Thromb. 2005, 12, 169–174. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, J.; Zhou, M.; Huang, R.; Zhang, J.; Cui, Y.; Jiang, X.; Li, Y.; Zhou, R.; Xin, H.; et al. Dietary timing enhances exercise by modulating fat-muscle crosstalk via adipocyte AMPKα2 signaling. Cell Metab. 2025, 37, 1364–1380. [Google Scholar] [CrossRef]
- Mladenov, M.; Sazdova, I.; Hadzi-Petrushev, N.; Konakchieva, R.; Gagov, H. The Role of Reductive Stress in the Pathogenesis of Endocrine-Related Metabolic Diseases and Cancer. Int. J. Mol. Sci. 2025, 26, 1910. [Google Scholar] [CrossRef]
- Sazdova, I.; Hadzi-Petrushev, N.; Keremidarska-Markova, M.; Stojchevski, R.; Sopi, R.; Shileiko, S.; Mitrokhin, V.; Gagov, H.; Avtanski, D.; Lubomirov, L.T.; et al. SIRT-associated attenuation of cellular senescence in vascular wall. Mech. Ageing Dev. 2024, 220, 111943. [Google Scholar] [CrossRef]
- Keremidarska-Markova, M.; Sazdova, I.; Mladenov, M.; Pilicheva, B.; Zagorchev, P.; Gagov, H. Sirtuin 1 and Hormonal Regulations in Aging. Appl. Sci. 2024, 14, 12051. [Google Scholar] [CrossRef]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Shostak, A.; Meyer-Kovac, J.; Oster, H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013, 62, 2195–2203. [Google Scholar] [CrossRef]
- Unger, R.H.; Clark, G.O.; Scherer, P.E.; Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta 2010, 1801, 209–214. [Google Scholar] [CrossRef]
- Cano-Barquilla, P.; Jiménez-Ortega, V.; Fernández-Mateos, P.; Virto, L.; Maldonado Bautista, E.; Perez-Miguelsanz, J.; Esquifino, A.I. Daily lipolysis gene expression in male rat mesenteric adipose tissue: Obesity and melatonin effects. Int. J. Mol. Sci. 2025, 26, 577. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Fliers, E.; Romijn, J.A.; La Fleur, S.E.; Wortel, J.; Bakker, O.; Endert, E.; Buijs, R.M. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology 2001, 142, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Kantermann, T.; Juda, M.; Merrow, M.; Roenneberg, T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr. Biol. 2007, 17, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; VoPham, T.; Wright, K.P., Jr.; Vetter, C. A Chronobiological Evaluation of the Acute Effects of Daylight Saving Time on Traffic Accident Risk. Curr. Biol. 2020, 30, 729–735. [Google Scholar] [CrossRef]
- Johnson, K.G.; Malow, B.A. Implications of Sleep Health Policy: Daylight Saving and School Start Times. Continuum 2023, 29, 1253–1266. [Google Scholar] [CrossRef]
- Mladenov, M.; Lubomirov, L.; Grisk, O.; Avtanski, D.; Mitrokhin, V.; Sazdova, I.; Keremidarska-Markova, M.; Danailova, Y.; Nikolaev, G.; Konakchieva, R.; et al. Oxidative Stress, Reductive Stress and Antioxidants in Vascular Pathogenesis and Aging. Antioxidants 2023, 12, 1126. [Google Scholar] [CrossRef]
- Trujillo-Rangel, W.Á.; Acuña-Vaca, S.; Padilla-Ponce, D.J.; García-Mercado, F.G.; Torres-Mendoza, B.M.; Pacheco-Moises, F.P.; Escoto-Delgadillo, M.; García-Benavides, L.; Delgado-Lara, D.L.C. Modulation of the Circadian Rhythm and Oxidative Stress as Molecular Targets to Improve Vascular Dementia: A Pharmacological Perspective. Int. J. Mol. Sci. 2024, 25, 4401. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Meng, Q.; Wu, X.; Bing, X.; Guo, N.; Zhao, X.; Hou, X.; Wang, B.; Xia, M.; et al. Circadian clock disruptions link oxidative stress and systemic inflammation to metabolic syndrome in obstructive sleep apnea patients. Front. Physiol. 2022, 13, 932596. [Google Scholar] [CrossRef]
- Deyurka, N.A.; Navigatore-Fonzo, L.S.; Coria-Lucero, C.D.; Ferramola, M.L.; Delgado, S.M.; Lacoste, M.G.; Anzulovich, A.C. Aging abolishes circadian rhythms and disrupts temporal organization of antioxidant-prooxidant status, endogenous clock activity and neurotrophin gene expression in the rat temporal cortex. Neuroscience 2024, 559, 125–138. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, N.; Zamaklar, M.; Lalic, K.; Jotic, A.; Lukic, L.; Milicic, T.; Singh, S.; Stosic, L.; Lalic, N.M. Relationship between Obesity, Adipocytokines and Inflammatory Markers in Type 2 Diabetes: Relevance for Cardiovascular Risk Prevention. Int. J. Environ. Res. Public Health 2014, 11, 4049–4065. [Google Scholar] [CrossRef] [PubMed]
- Budkowska, M.; Cecerska-Heryć, E.; Marcinowska, Z.; Siennicka, A.; Dołęgowska, B. The Influence of Circadian Rhythm on the Activity of Oxidative Stress Enzymes. Int. J. Mol. Sci. 2022, 23, 14275. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, H.G. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic adipose tissue inflammation: All immune cells on the stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef]
- Robertson, J.L.; Clifton, D.K.; de la Iglesia, H.O.; Steiner, R.A.; Kauffman, A.S. Circadian regulation of Kiss1 neurons: Implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 2009, 150, 3664–3671. [Google Scholar] [CrossRef]
- Ono, M.; Ando, H.; Daikoku, T.; Fujiwara, T.; Mieda, M.; Mizumoto, Y.; Iizuka, T.; Kagami, K.; Hosono, T.; Nomura, S.; et al. The Circadian Clock, Nutritional Signals and Reproduction: A Close Relationship. Int. J. Mol. Sci. 2023, 24, 1545. [Google Scholar] [CrossRef]
- Padda, J.; Khalid, K.; Moosa, A.; Syam, M.; Kakani, V.; Imdad, U.; Ismail, D.; Cooper, A.C.; Jean-Charles, G. Role of Kisspeptin on Hypothalamic-Pituitary-Gonadal Pathology and Its Effect on Reproduction. Cureus 2021, 13, e17600. [Google Scholar] [CrossRef]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The role of kisspeptin in the control of the hypothalamic-pituitary-gonadal axis and reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef]
- Neal-Perry, G.; Lebesgue, D.; Lederman, M.; Shu, J.; Zeevalk, G.D.; Etgen, A.M. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology 2009, 150, 3699–3708. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.N.; Nijher, G.M.; Comninos, A.N.; Abbara, A.; Januszewki, A.; Vaal, M.L.; Sriskandarajah, L.; Murphy, K.G.; Farzad, Z.; Ghatei, M.A.; et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J. Clin. Endocrinol. Metab. 2011, 96, E1963–E1972. [Google Scholar] [CrossRef] [PubMed]
- Chassard, D.; Bur, I.; Poirel, V.J.; Mendoza, J.; Simonneaux, V. Evidence for a Putative Circadian Kiss-Clock in the Hypothalamic AVPV in Female Mice. Endocrinology 2015, 156, 2999–3011. [Google Scholar] [CrossRef]
- Smarr, B.L.; Morris, E.; de la Iglesia, H.O. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology 2012, 153, 2839–2850. [Google Scholar] [CrossRef]
- Padilla, S.L.; Perez, J.G.; Ben-Hamo, M.; Johnson, C.W.; Sanchez, R.E.A.; Bussi, I.L.; Palmiter, R.D.; de la Iglesia, H.O. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr. Biol. 2019, 29, 592–604.e4. [Google Scholar] [CrossRef]
- Brown, R.E.; Imran, S.A.; Ur, E.; Wilkinson, M. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol. Cell. Endocrinol. 2008, 281, 64–72. [Google Scholar] [CrossRef]
- Hussain, M.A.; Song, W.J.; Wolfe, A. There is Kisspeptin—And Then There is Kisspeptin. Trends Endocrinol. Metab. 2015, 26, 564–572. [Google Scholar] [CrossRef]
- Song, W.J.; Mondal, P.; Wolfe, A.; Alonso, L.C.; Stamateris, R.; Ong, B.W.; Lim, O.C.; Yang, K.S.; Radovick, S.; Novaira, H.J.; et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014, 19, 667–681. [Google Scholar] [CrossRef]
- Tolson, K.P.; Garcia, C.; Yen, S.; Simonds, S.; Stefanidis, A.; Lawrence, A.; Smith, J.T.; Kauffman, A.S. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J. Clin. Investig. 2014, 124, 3075–3079. [Google Scholar] [CrossRef]
- Wahab, F.; Bano, R.; Jabeen, S.; Irfan, S.; Shahab, M. Effect of peripheral kisspeptin administration on adiponectin, leptin, and resistin secretion under fed and fasting conditions in the adult male rhesus monkey (Macaca mulatta). Horm. Metab. Res. 2010, 42, 570–574. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Pruszyńska-Oszmałek, E.; Kołodziejski, P.; Sassek, M.; Sliwowska, J.H. Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine 2017, 56, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.H.; Colledge, W.H. The Role of Kiss1 Neurons As Integrators of Endocrine, Metabolic, and Environmental Factors in the Hypothalamic-Pituitary-Gonadal Axis. Front. Endocrinol. 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.M.; Stincic, T.L. Estradiol and the control of feeding behavior. Steroids 2018, 133, 44–52. [Google Scholar] [CrossRef]
- Allan, C.A.; McLachlan, R.I. Androgens and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 224–232. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef]
- Forbes, S.; Li, X.F.; Kinsey-Jones, J.; O’Byrne, K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci. Lett. 2009, 460, 143–147. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Zhong, K.; Tong, A.; Jia, D. Targeted protein degradation: Mechanisms, strategies and application. Signal Transduct. Target. Ther. 2022, 7, 113. [Google Scholar] [CrossRef]
- Desvergne, A.; Ugarte, N.; Radjei, S.; Gareil, M.; Petropoulos, I.; Friguet, B. Circadian modulation of proteasome activity and accumulation of oxidized protein in human embryonic kidney HEK 293 cells and primary dermal fibroblasts. Free Radic. Biol. Med. 2016, 94, 195–207. [Google Scholar] [CrossRef]
- Shi, S.-Q.; Ansari, T.S.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 2013, 23, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.S.; Humphrey, S.J.; Mann, M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Lee, J.; Moulik, M.; Fang, Z.; Saha, P.; Zou, F.; Xu, Y.; Nelson, D.L.; Ma, K.; Moore, D.D.; Yechoor, V.K. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol. Cell. Biol. 2013, 33, 2327–2338. [Google Scholar] [CrossRef]
- Mezhnina, V.; Ebeigbe, O.P.; Poe, A.; Kondratov, R.V. Circadian control of mitochondria in reactive oxygen species homeostasis. Antioxid. Redox Signal. 2022, 37, 647–663. [Google Scholar] [CrossRef]
- Su, K.-H.; Dai, C. Metabolic control of the proteotoxic stress response: Implications in diabetes mellitus and neurodegenerative disorders. Cell. Mol. Life Sci. 2016, 73, 4231–4248. [Google Scholar] [CrossRef]
- Bartelt, A.; Lemmer, I.; Willemsen, N.; Kotschi, S.; Toksöz, I.; Gjika, E.; Khani, S.; Haas, D.; Krahmer, N.; Rohm, M.; et al. Nfe2l1-Mediated Proteasome Function Controls Muscle Energy Metabolism in Obesity. Res. Sq. 2023. [CrossRef]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian Clock Protein BMAL1 Regulates IL-1β in Macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef]
- Proietti, S.; Cucina, A.; Dobrowolny, G.; D’Anselmi, F.; Dinicola, S.; Masiello, M.G.; Pasqualato, A.; Palombo, A.; Morini, V.; Reiter, R.J.; et al. Melatonin Down-Regulates MDM2 Gene Expression and Enhances P53 Acetylation in MCF-7 Cells. J. Pineal Res. 2014, 57, 120–129. [Google Scholar] [CrossRef]
- Hill, S.M.; Frasch, T.; Xiang, S.; Yuan, L.; Duplessis, T.; Mao, L. Molecular Mechanisms of Melatonin Anticancer Effects. Integr. Cancer Ther. 2009, 8, 337–346. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Silver, A.C.; Arjona, A.; Walker, W.E.; Fikrig, E. The Circadian Clock Controls Toll-Like Receptor 9-Mediated Innate and Adaptive Immunity. Immunity 2012, 36, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A Circadian Gene Expression Atlas in Mammals: Implications for Biology and Medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the Clock Components CLOCK and BMAL1 Leads to Hypoinsulinaemia and Diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota–Liver Axis in Hepatic Disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Pourcet, B.; Zecchin, M.; Ferri, L.; Beauchamp, J.; Sitaula, S.; Billon, C.; Delhaye, S.; Vanhoutte, J.; Mayeuf-Louchart, A.; Thorel, Q.; et al. NR1D1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce Severity of Fulminant Hepatitis in Mice. Gastroenterology 2018, 154, 1449–1464. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tarakcioglu, E.; Olcum, M.; Genc, S. The Role of Melatonin on NLRP3 Inflammasome Activation in Diseases. Antioxidants 2021, 10, 1020. [Google Scholar] [CrossRef]
- Garcia, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Disruption of the NF-κB/NLRP3 Connection by Melatonin Requires RORα and Blocks the Septic Response in Mice. FASEB J. 2015, 29, 3863–3875. [Google Scholar] [CrossRef]
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin Alleviates Cadmium-Induced Liver Injury by Inhibiting the TXNIP-NLRP3 Inflammasome. J. Pineal Res. 2017, 62, e12389. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, M.; Chu, Y.; Wang, W.; Chen, D.; Li, X.; Zhang, Z.; Zhang, D.; Fan, D.; Nie, Y.; et al. Gasdermin D Plays a Key Role as a Pyroptosis Executor of Non-Alcoholic Steatohepatitis in Humans and Mice. J. Hepatol. 2018, 68, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Khandaker, S.; Learman, B.S.; Cawthorn, W.P.; Anderson, L.M.; Pham, H.A.; Robles, H.; Wang, Z.; Li, Z.; Parlee, S.D.; et al. Bone Marrow Adipocytes Resist Lipolysis and Remodeling in Response to β-Adrenergic Stimulation. Bone 2019, 118, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; Parlee, S.D.; Pham, H.A.; Learman, B.S.; Redshaw, C.M.H.; Sulston, R.J.; Burr, A.A.; Das, A.K.; Simon, B.R.; et al. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated with Increased Circulating Glucocorticoids. Endocrinology 2016, 157, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Peña, G.; Bredella, M.A. Bone Marrow Adipose Tissue in Metabolic Health. Trends Endocrinol. Metab. 2022, 33, 401–408. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gaddameedhi, S.; Crooks, E.; Zhang, C.; Li, Y.; Qiao, Z.; Trzepizur, W.; Kay, S.A.; Andrade, J.; Satterfield, B.C.; et al. Circulating Exosomal miRNAs Signal Circadian Misalignment to Peripheral Metabolic Tissues. Int. J. Mol. Sci. 2020, 21, 6396. [Google Scholar] [CrossRef]
- Tencerova, M.; Kassem, M. The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis. Front. Endocrinol. 2016, 7, 127. [Google Scholar] [CrossRef]
- McGrath, C.; Sankaran, J.S.; Misaghian-Xanthos, N.; Sen, B.; Xie, Z.; Styner, M.A.; Zong, X.; Rubin, J.; Styner, M. Exercise Degrades Bone in Caloric Restriction, Despite Suppression of Marrow Adipose Tissue (MAT). J. Bone Miner. Res. 2020, 35, 106–115. [Google Scholar] [CrossRef]
- Cinti, S. The Adipose Organ at a Glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef]
- Hardouin, P.; Rharass, T.; Lucas, S. Bone Marrow Adipose Tissue: To Be or Not to Be a Typical Adipose Tissue? Front. Endocrinol. 2016, 7, 85. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.; Sun, Z.; Bugge, A.; Mullican, S.E.; Alenghat, T.; Liu, X.S.; Lazar, M.A. A Circadian Rhythm Orchestrated by Histone Deacetylase 3 Controls Hepatic Lipid Metabolism. Science 2011, 331, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Yoo, S.-H.; Huang, H.-C.; Kumar, V.; Lee, C.; Kim, T.-K.; Takahashi, J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Morral, N.; Liu, S.; Conteh, A.M.; Chu, X.; Wang, Y.; Dong, X.C.; Liu, Y.; Linnemann, A.K.; Wan, J. Aberrant gene expression induced by a high-fat diet is linked to H3K9 acetylation in the promoter-proximal region. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194691. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, J.; Sahar, S.; Grimaldi, B.; Tamaru, T.; Takamatsu, K.; Nakahata, Y.; Sassone-Corsi, P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 2007, 450, 1086–1090. [Google Scholar] [CrossRef]
- Masri, S.; Rigor, P.; Cervantes, M.; Ceglia, N.; Sebastian, C.; Xiao, C.; Roqueta-Rivera, M.; Deng, C.; Osborne, T.F.; Mostoslavsky, R.; et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 2014, 158, 659–672. [Google Scholar] [CrossRef]
- Ding, L.; Liu, J.; Zhou, L.; Jia, X.; Li, S.; Zhang, Q.; Yu, M.; Xiao, X. A high-fat diet disrupts the hepatic and adipose circadian rhythms and modulates the diurnal rhythm of gut microbiota-derived short-chain fatty acids in gestational mice. Front. Nutr. 2022, 9, 925390. [Google Scholar] [CrossRef]
- Asif, S.; Morrow, N.M.; Mulvihill, E.E.; Kim, K.-H. Understanding dietary intervention-mediated epigenetic modifications in metabolic diseases. Front. Genet. 2020, 11, 590369. [Google Scholar] [CrossRef]
- Maude, H.; Sanchez-Cabanillas, C.; Cebola, I. Epigenetics of hepatic insulin resistance. Front. Endocrinol. 2021, 12, 681356. [Google Scholar] [CrossRef]
- Woller, A.; Duez, H.; Staels, B.; Lefranc, M. A mathematical model of the liver circadian clock linking feeding and fasting cycles to clock function. Cell Rep. 2016, 17, 1087–1097. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, B.; Emmett, M.J.; Damle, M.; Sun, Z.; Feng, D.; Armour, S.M.; Remsberg, J.R.; Jager, J.; Soccio, R.E.; et al. Discrete functions of nuclear receptor REV-ERBα couple metabolism to the clock. Science 2015, 348, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Patel, V.R.; Eckel-Mahan, K.L.; Peleg, S.; Forne, I.; Ladurner, A.G.; Baldi, P.; Imhof, A.; Sassone-Corsi, P. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 3339–3344. [Google Scholar] [CrossRef]
- Bass, J.; Lazar, M.A. Circadian time signatures of fitness and disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Smolensky, M.H.; Fernández, J.R.; Mojón, A.; Portaluppi, F. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens. Res. 2016, 39, 277–292. [Google Scholar] [CrossRef]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef]
- Reddy, A.B.; O’Neill, J.S. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010, 20, 36–44. [Google Scholar] [CrossRef]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behavior and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef]
- Wang, X.-L.; Kooijman, S.; Gao, Y.; Tzeplaeff, L.; Cosquer, B.; Milanova, I.; Wolff, S.E.C.; Korpel, N.; Champy, M.-F.; Petit-Demoulière, B.; et al. Microglia-specific knock-down of Bmal1 improves memory and protects mice from high-fat diet-induced obesity. Mol. Psychiatry 2021, 26, 6336–6349. [Google Scholar] [CrossRef]
- Skarke, C.; Lahens, N.F.; Rhoades, S.D.; Campbell, A.; Bittinger, K.; Bailey, A.; Hoffmann, C.; Olson, R.S.; Chen, L.; Yang, G.; et al. A pilot characterization of the human chronobiome. Sci. Rep. 2017, 7, 17141. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Prynne, C.J.; Hardy, R.; Stephen, A.M. Time-of-day and nutrient composition of eating occasions: Prospective association with the metabolic syndrome in the 1946 British birth cohort. Int. J. Obes. 2013, 37, 725–731. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef]
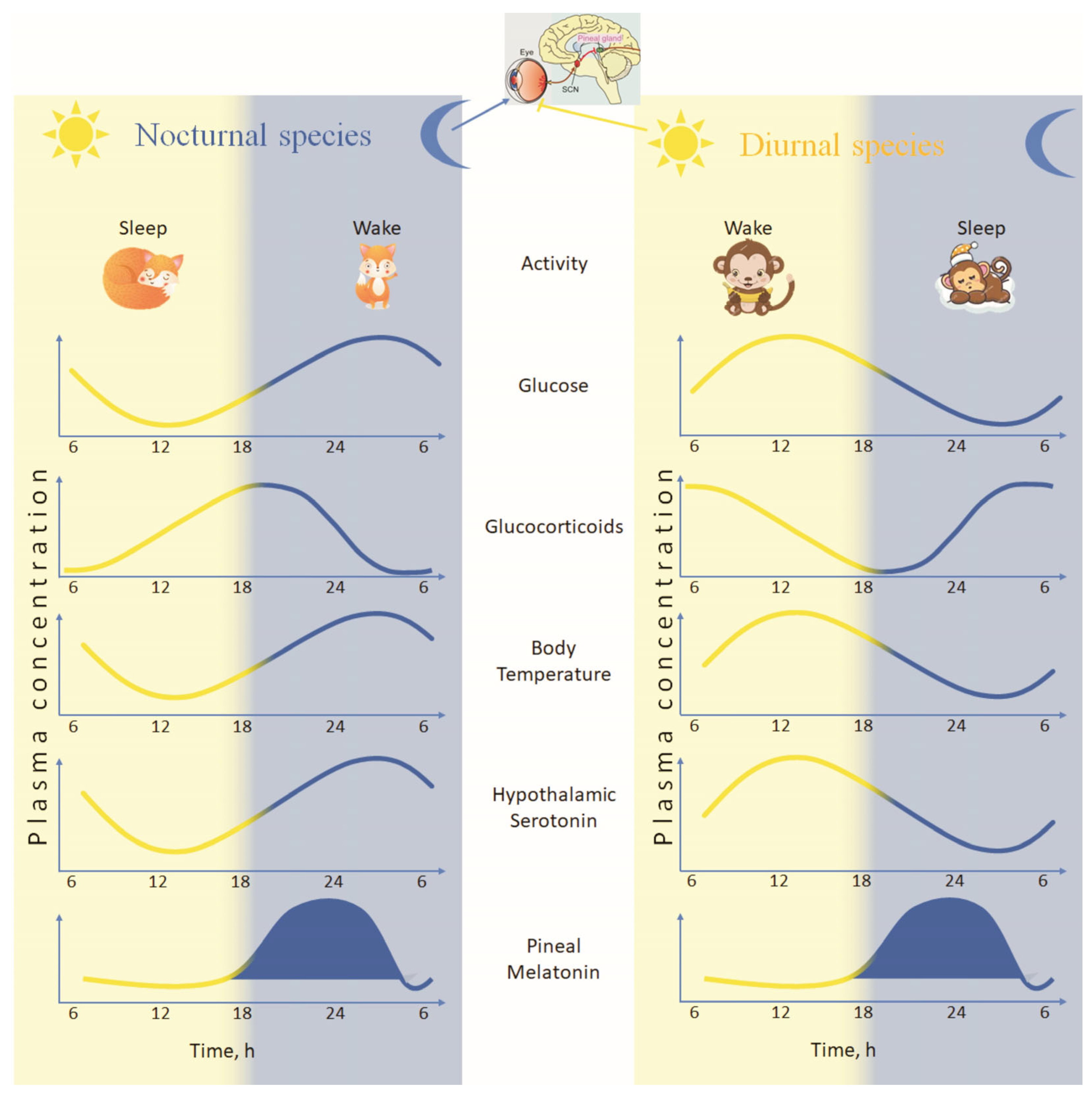

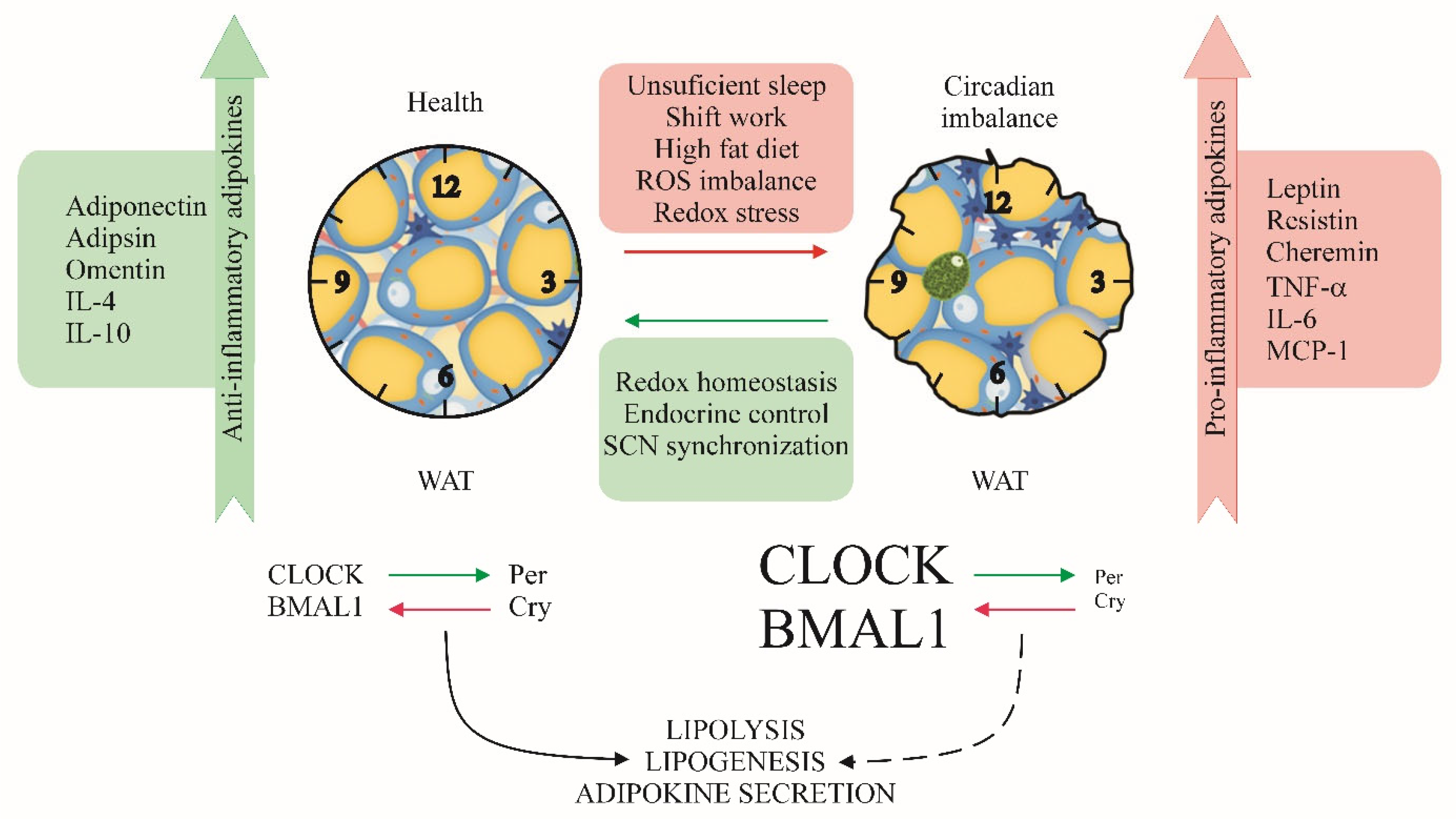
| Pathway/Process | Core Interaction | Effect on Systemic Physiology | References |
|---|---|---|---|
| Circadian regulation of GC secretion | GCs follow a circadian rhythm controlled by the SCN/PVN, regulating metabolism and immunity | Synchronizes peripheral clocks and regulates metabolism, immunity, and stress responses | [38] |
| BMAL1 and HPA axis control | BMAL1 modulates CRH, AVP, and REV-ERBα expression, linking circadian signals to GC secretion control | Ensures alignment between central clock and endocrine outputs | [39,40] |
| Chronic stress and Clock gene disruption | Chronic stress reprograms hepatic and systemic circadian rhythms, elevates fasting glucose, and dampens corticosterone rhythms | Disrupts glucose metabolism, immune regulation, and circadian gene expression | [41] |
| GR desensitization and metabolic dysfunction | Sustained GC exposure leads to GR downregulation and impaired circadian metabolic responses | Leads to insulin resistance, inflammation, and hepatic lipid accumulation | [42,43] |
| Tissue-specific GR activity in liver and adipose tissues | GCs regulate transcription and chromatin remodeling of metabolic genes (e.g., Pck1, G6pc, Fasn) in a tissue-specific manner | Coordinates metabolic adaptation in liver and adipose tissue; dysregulation contributes to disease | [44,45] |
| Chronotherapeutic targeting of GC rhythms | Selective GR modulators or timed inhibitors (e.g., metyrapone) restore rhythmic GC signaling and metabolic balance | Improves metabolic function and reduces effects of stress-induced circadian disruption | [42] |
| Lifestyle strategies to reinforce GC rhythms | Time-restricted feeding (TRF), light exposure, and structured activity reinforce endogenous GC oscillations | Boosts circadian alignment and enhances metabolic flexibility | [38] |
| Omics-guided chrono-endocrine precision medicine | Single-cell and time-series profiling help design personalized interventions based on GR sensitivity and circadian phase | Enables precision therapy for stress-related metabolic disorders like obesity and type 2 diabetes | [46] |
| Pathway/Process | Core Interaction | Effect on Systemic Physiology | References |
|---|---|---|---|
| Adipocyte intrinsic clocks and gene rhythms | BMAL1, CLOCK, PER, and CRY drive rhythmic expression of lipolytic, lipogenic, and adipokine genes in adipocytes | Coordinates adipose metabolism with daily cycles and feeding cues | [67,68] |
| Depot-specific circadian regulation | WAT, BAT, and visceral fat show distinct circadian expression patterns and responsiveness to environmental cues | Enables tailored metabolic and immune responses in different fat depots | [65,66] |
| Adipokine secretion rhythmicity | Leptin and adiponectin are secreted in a diurnal pattern, modulated by the circadian clock | Maintains energy balance and insulin sensitivity; disruption leads to leptin resistance | [69,70] |
| Circadian disruption and metabolic inflexibility | Disruption by shift work, stress, or irregular meals blunts hormonal rhythms, leading to metabolic dysfunction | Promotes obesity and type 2 diabetes through hormonal and immune imbalance | [71] |
| Circadian regulation of adipose-derived stem cells | ASC differentiation, mitochondrial activity, and inflammation follow circadian patterns influenced by zeitgebers | The timing of ASC use may improve regenerative and metabolic therapeutic outcomes | [71,72] |
| Sympathetic nervous system-adipose axis | SNS regulates diurnal lipolysis and thermogenesis; its disruption impairs metabolic flexibility | Modulates thermogenesis and lipolysis, critical for energy homeostasis | [73] |
| Chronotherapeutic potential in adipose tissue | Timing interventions such as feeding schedules or ASC transplantation may restore circadian adipose function | Supports the treatment of obesity and insulin resistance through circadian alignment | [68,74] |
| Pathway/Process | Core Interaction | Effect on Metabolic Homeostasis | References |
|---|---|---|---|
| Proteasome–circadian interface | UPS activity shows circadian rhythmicity; CLOCK/BMAL1 regulate the expression of UPS components and protein turnover | Maintains temporal protein quality control in metabolically active tissues | [131,132,134,135] |
| BMAL1 and proteasome assembly | BMAL1 deficiency impairs proteasome assembly and degradation of oxidized/misfolded proteins | Loss of BMAL1 leads to proteotoxic stress and disrupted redox homeostasis | [24] |
| Circadian Control of Mitochondrial Proteostasis | Circadian degradation of PGC-1α and NRF1 coordinates mitophagy and mitochondrial biogenesis, linked to BMAL1 | Promotes mitochondrial efficiency and minimizes ROS during metabolic transitions | [136,137] |
| Proteasomal disruption in metabolic disease | Obesity and hyperglycemia suppress proteasome rhythmicity, causing impaired protein turnover and redox imbalance | Dysregulated proteostasis contributes to inflammation and oxidative stress in obesity | [138,139] |
| Circadian Control of Antioxidant Defense | BMAL1 modulates Nrf2 expression and degradation; links circadian rhythm to antioxidant gene regulation | Enhances antioxidant capacity and reduces inflammation through NRF2 stabilization | [35,140] |
| Pathway/ Process | Core Interaction | Effect on Metabolic Homeostasis | References |
|---|---|---|---|
| Circadian regulation of inflammasomes | NLRP3 and AIM2 inflammasome activity shows circadian rhythmicity controlled by BMAL1, CLOCK, and REV-ERBα | Coordinates immune surveillance with daily metabolic cycles; its disruption leads to chronic low-grade inflammation | [9,144] |
| Oxidative stress and NLRP3 activation | ROS generated by mitochondrial dysfunction due to circadian disruption enhances inflammasome activation | Promotes inflammation in metabolically active tissues like the liver and adipose through increased ROS and inflammasome activity | [6,136,145] |
| Temporal misalignment and inflammation | Disrupted circadian rhythms (e.g., jet lag, HFD during rest phase) increase NLRP3 expression and IL-1β production in liver and adipose tissues | Increases risk of insulin resistance, hepatic steatosis, and fibrosis by promoting immune cell infiltration | [146,147,148] |
| REV-ERBα and inflammasome inhibition | Pharmacological activation of REV-ERBα reduces NLRP3 activity and metabolic inflammation | Suppresses inflammasome-driven inflammation and improves metabolic outcomes in obesity models | [149] |
| BMAL1 and immune suppression | BMAL1 deletion in myeloid cells enhances inflammasome priming and cytokine production | Limits innate immune activation and supports circadian control of immune homeostasis | [9] |
| Pathway/Process | Core Interaction | Effect on Systemic Physiology | References |
|---|---|---|---|
| Circadian regulation of BMAT | BMAT expresses Bmal1, Per2, Rev-erb alpha with diurnal variation, suggesting the presence of an intrinsic clock | Links bone marrow metabolism to circadian rhythms, potentially affecting systemic energy balance | [145] |
| BMAT and hematopoietic crosstalk | BMAT may influence HSC maintenance and systemic inflammation through rhythmic secretion of lipids and adipokines | Coordinates metabolic and immune regulation within the bone marrow niche | [154,157] |
| BMAT exosomes and zeitgeber response | Exosomes from BMAT carry circadian-regulated miRNAs and proteins; their content is modulated by zeitgebers and stress | Disruption may promote inflammation, hematopoietic imbalance, and bone demineralization | [159] |
| BMAT and bone fragility | Circadian dysregulation of BMAT associated with impaired osteoblast differentiation and bone fragility | May contribute to osteoporosis pathogenesis under circadian disruption | [156,160] |
| Circadian role of pink adipose tissue | Pink adipose tissue emerges during lactation and may exhibit circadian plasticity in mitochondrial and secretory function | May play a role in thermogenic and secretory adaptation during specific physiological states | [161] |
| Pathway/Process | Core Interaction | Effect on Systemic Physiology | References |
|---|---|---|---|
| Circadian histone acetylation in liver | Histone marks like H3K9ac and H3K4me3 are rhythmically modified and regulate hepatic gene expression | Synchronizes hepatic metabolism with feeding-fasting cycles; disruption promotes steatosis and inflammation | [164,165] |
| BMAL1 as a chromatin modifier | BMAL1 recruits p300 to enhancers, promoting circadian H3K9 acetylation at metabolic gene promoters | Coordinates transcription of genes involved in lipid metabolism and detoxification | [166,167] |
| Epigenetic disruption by HFD | HFD abolishes rhythmic histone modifications, disrupting transcriptional timing | Leads to liver susceptibility to MAFLD, inflammation, and fibrosis | [167,168] |
| DNA methylation and chromatin remodeling | Circadian disruption alters DNA methylation and chromatin structure, impairing hepatocyte function | Increases vulnerability to liver injury, OS, and functional decline | [169,170] |
| Chrono-epigenetics and therapeutic targeting | Chrono-pharmacology using HDAC inhibitors restores rhythmic gene expression and metabolism | Improves therapeutic outcomes in liver disease with time-optimized interventions | [167,171] |
| SIRT1 and time-dependent deacetylation | Targeting SIRT1 restores circadian chromatin dynamics and reduces hepatic steatosis | Enhances mitochondrial function and ameliorates metabolic liver disease | [89,172] |
| Behavioral restoration of circadian rhythms | TRF restores histone acetylation rhythmicity and protects against hepatic pathology | Entrains circadian rhythms independently of caloric restriction, protecting liver health | [173] |
| Pathway/Process | Core Interaction | Effect on Systemic Physiology | References |
|---|---|---|---|
| Circadian control of drug metabolism | Circadian rhythms regulate drug absorption, distribution, metabolism, and elimination | Improves drug safety and therapeutic efficacy via optimized timing | [177,178] |
| Chrono-pharmacology principles | Drug effectiveness and side effects vary depending on the circadian phase of administration | Aligns pharmacodynamics with physiological rhythms, minimizing toxicity | [70,177] |
| Time-dependent metformin efficacy | Metformin action enhanced during active phase; linked to BMAL1 and SIRT1 rhythms | Improves glucose regulation and reduces hepatic lipid content | [70,179] |
| SGLT2 inhibitor chrono dynamics | Efficacy of SGLT2 inhibitors varies with dosing time, reflecting renal circadian control | Reduces adverse events and enhances glucose excretion | [180] |
| Targeting the molecular clock (BMAL1/REV-ERBα) | BMAL1 and REV-ERBα modulate lipid metabolism, inflammation, and glucose homeostasis | Synchronizes metabolic pathways for homeostasis | [174] |
| Pharmacological activation of circadian targets | Synthetic REV-ERB agonists reduce adiposity and improve insulin sensitivity; BMAL1 activation enhances mitochondrial function | Ameliorates obesity and supports energy metabolism | [181,182] |
| Personalized chronotherapy with biomarkers | Time-resolved transcriptomics and metabolomics guide treatment aligned to individual circadian profiles | Enables precision medicine for metabolic disorders | [183] |
| Clinical potential and chronotherapy trials | Potential for improved therapy in patients with circadian disruption; requires more Randomized Controlled Trialsfor validation | Targets metabolic dysregulation in vulnerable populations (e.g., shift workers) | [177,178] |
| Pathway/Process | Core Interaction | Effect on Systemic Physiology | References |
|---|---|---|---|
| Chrono-nutrition and meal timing | Focuses on aligning eating patterns with circadian rhythms to optimize metabolic health | Improves glucose and lipid metabolism; supports redox balance in metabolic disease | [184] |
| Redox-circadian feedback loops | NAD+/NADH, ROS, and mitochondrial signals modulate clock gene expression and antioxidant defense | Coordinates mitochondrial function and antioxidant defenses | [5,6] |
| TRF | Restricting food intake to the active phase restores redox rhythms and circadian gene expression | Prevents diet-induced obesity, insulin resistance, and hepatic steatosis | [173,185] |
| TRF vs. intermittent fasting | Different fasting regimens vary in effect on redox enzymes and circadian alignment | TRF offers enhanced circadian benefits compared to other IF protocols | [186] |
| Gut microbiota rhythmicity | Microbial communities oscillate with feeding cycles and impact oxidative balance | Links circadian rhythms to gut health and inflammation control | [187] |
| TRF and gut-liver axis | TRF restores microbial oscillations, enhances SCFAs, and supports hepatic redox homeostasis | Modulates systemic inflammation and promotes mitochondrial efficiency | [188] |
| Nutrient timing and redox regulation | Feeding acts as a zeitgeber; proper timing enhances antioxidant capacity and metabolic resilience | Reduces metabolic stress and supports cardiometabolic health | [6,173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konakchieva, R.; Mladenov, M.; Konaktchieva, M.; Sazdova, I.; Gagov, H.; Nikolaev, G. Circadian Clock Deregulation and Metabolic Reprogramming: A System Biology Approach to Tissue-Specific Redox Signaling and Disease Development. Int. J. Mol. Sci. 2025, 26, 6267. https://doi.org/10.3390/ijms26136267
Konakchieva R, Mladenov M, Konaktchieva M, Sazdova I, Gagov H, Nikolaev G. Circadian Clock Deregulation and Metabolic Reprogramming: A System Biology Approach to Tissue-Specific Redox Signaling and Disease Development. International Journal of Molecular Sciences. 2025; 26(13):6267. https://doi.org/10.3390/ijms26136267
Chicago/Turabian StyleKonakchieva, Rossitza, Mitko Mladenov, Marina Konaktchieva, Iliyana Sazdova, Hristo Gagov, and Georgi Nikolaev. 2025. "Circadian Clock Deregulation and Metabolic Reprogramming: A System Biology Approach to Tissue-Specific Redox Signaling and Disease Development" International Journal of Molecular Sciences 26, no. 13: 6267. https://doi.org/10.3390/ijms26136267
APA StyleKonakchieva, R., Mladenov, M., Konaktchieva, M., Sazdova, I., Gagov, H., & Nikolaev, G. (2025). Circadian Clock Deregulation and Metabolic Reprogramming: A System Biology Approach to Tissue-Specific Redox Signaling and Disease Development. International Journal of Molecular Sciences, 26(13), 6267. https://doi.org/10.3390/ijms26136267






