Genes That Associated with Action of ACTH-like Peptides with Neuroprotective Potential in Rat Brain Regions with Different Degrees of Ischemic Damage
Abstract
1. Introduction
2. Results
2.1. Histological Examination of Rat Brain Samples
2.2. RNA-Seq Analysis of the Effect of Semax and ACTH(6-9)PGP Peptide on the Striatum of Rats at 24 h After tMCAO
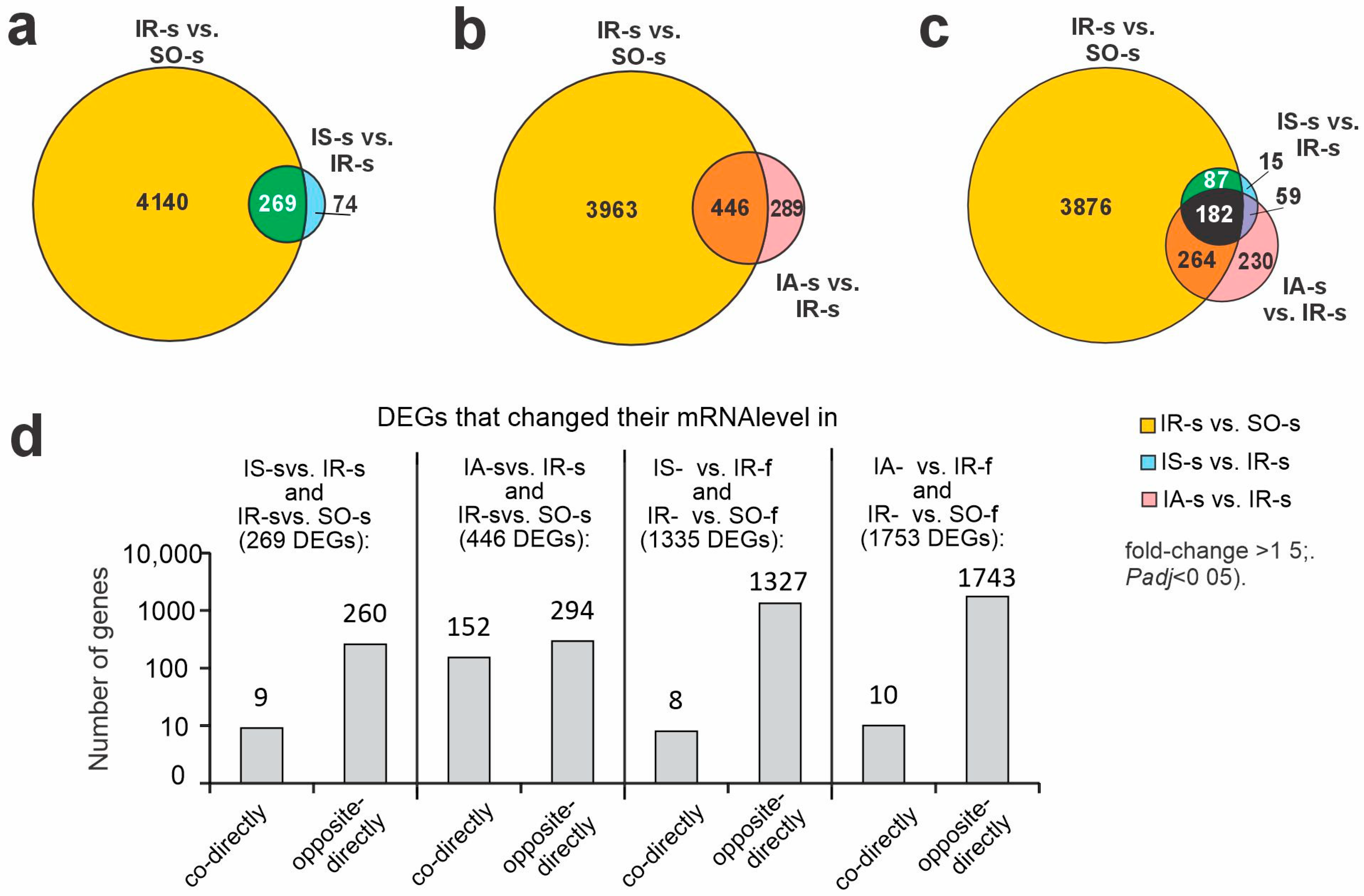
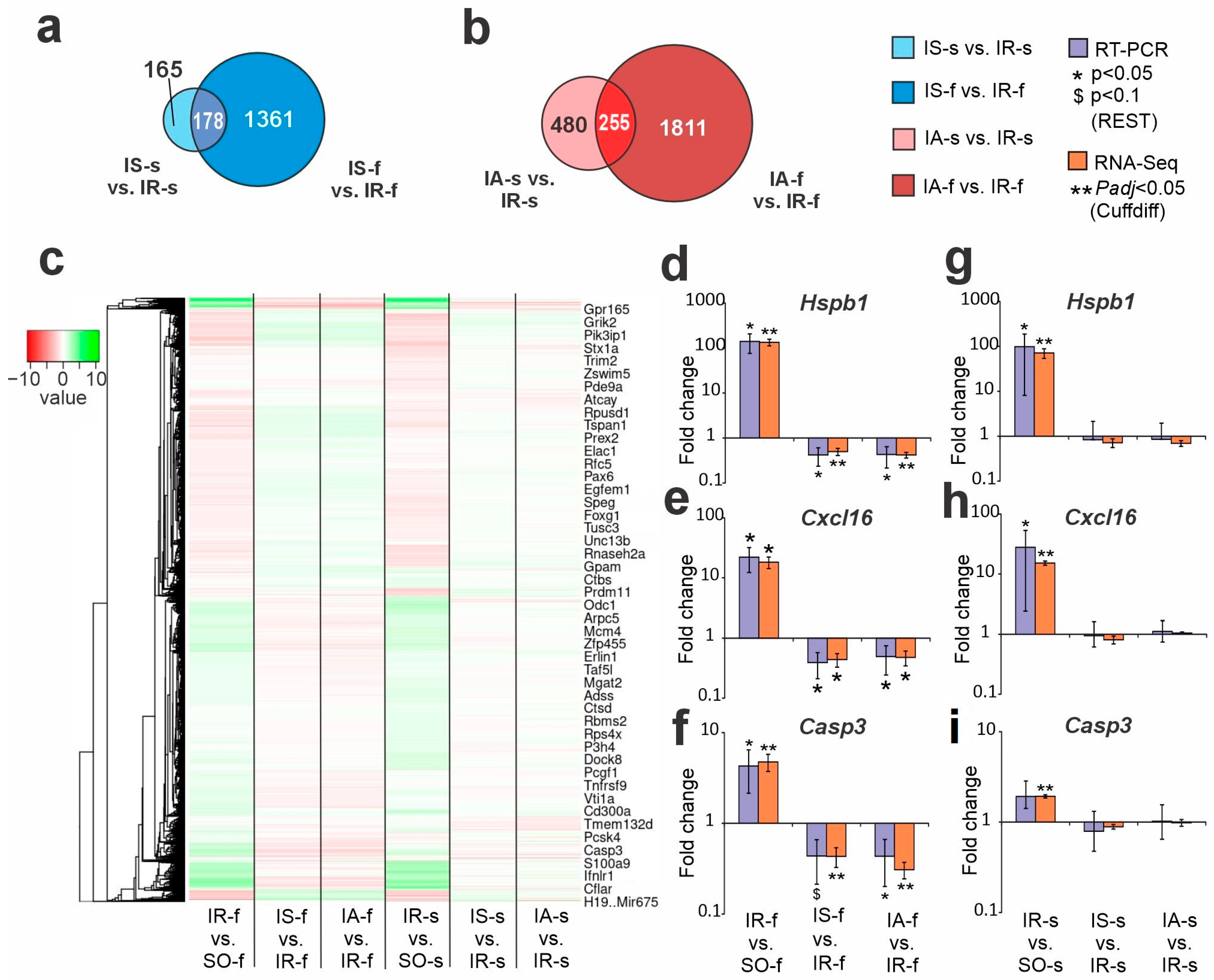
2.3. Differences in Rat Brain Transcriptomes Following Ischemia and After Peptide Administration in Striatum and FC at 24 h After tMCAO
2.4. Comparison of RNA-Seq Results of Semax and ACTH(6-9)PGP Action in Striatum and FC at 24 h After tMCAO
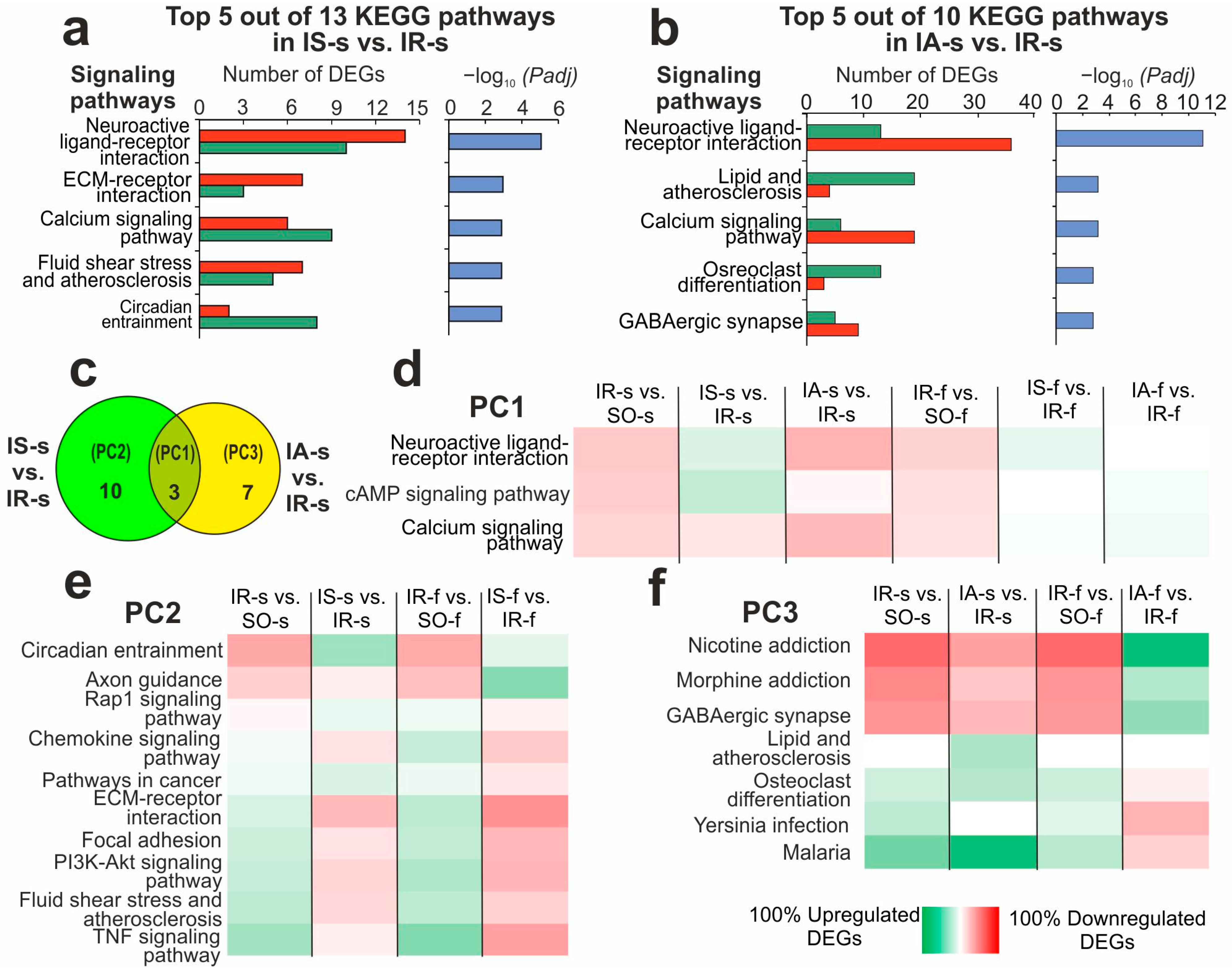
2.5. Functional Annotations of DEGs Altered in Different Comparison Groups
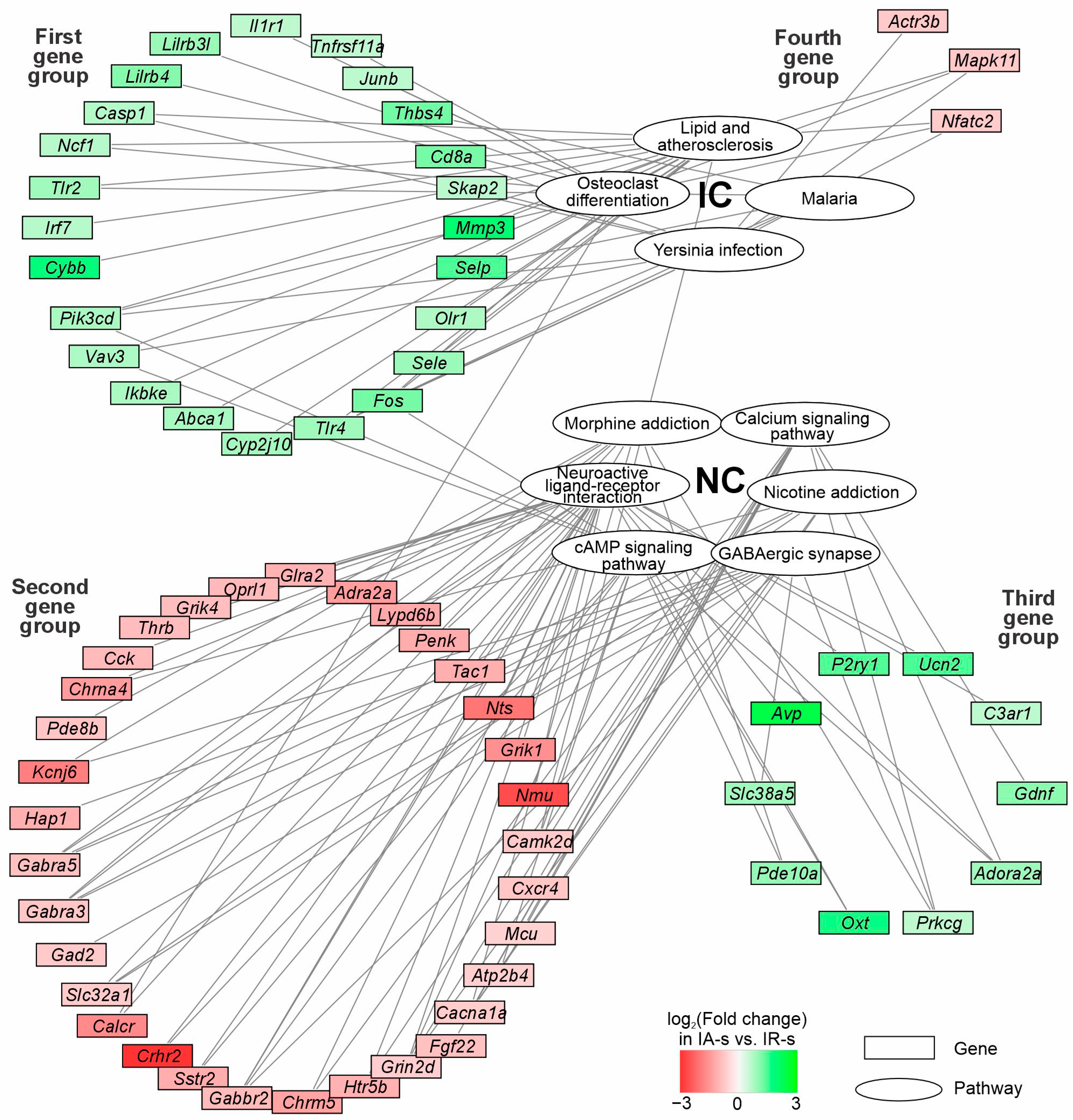
2.6. The Involvement of the DEGs of NC and IC Associated with Effects of ACTH(6-9)PGP Peptides a Day After IR Conditions in Striatum
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. tMCAO Model
4.3. Sample Collection and RNA Isolation
4.4. RNA-Seq
4.5. cDNA Synthesis and Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.6. RNA-Seq Data Analysis
4.7. Real-Time RT-PCR Data Analysis
4.8. Functional Analysis
4.9. Availability of Data and Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capirossi, C.; Laiso, A.; Renieri, L.; Capasso, F.; Limbucci, N. Epidemiology, organization, diagnosis and treatment of acute ischemic stroke. Eur. J. Radiol. Open 2023, 11, 100527. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; Abd ElHafeez, S.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systema. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef] [PubMed]
- Tymianski, M. Role of Neuroprotective Approaches in the Recanalization Era. Stroke 2024, 55, 1927–1931. [Google Scholar] [CrossRef]
- Dammavalam, V.; Lin, S.; Nessa, S.; Daksla, N.; Stefanowski, K.; Costa, A.; Bergese, S. Neuroprotection during Thrombectomy for Acute Ischemic Stroke: A Review of Future Therapies. Int. J. Mol. Sci. 2024, 25, 38255965. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mato, M.; López-Arias, E.; Bugallo-Casal, A.; Correa-Paz, C.; Arias, S.; Rodríguez-Yáñez, M.; Santamaría-Cadavid, M.; Campos, F. New Perspectives in Neuroprotection for Ischemic Stroke. Neuroscience 2024, 550, 30–42. [Google Scholar] [CrossRef]
- Ghozy, S.; Reda, A.; Varney, J.; Elhawary, A.S.; Shah, J.; Murry, K.; Sobeeh, M.G.; Nayak, S.S.; Azzam, A.Y.; Brinjikji, W.; et al. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front. Neurol. 2022, 13, 35711268. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Che, X.; Huang, J.; Zhang, X.; Fu, X.; Cai, J.; Yao, Y.; Zhang, H.; Cai, R.; et al. Agonistic analog of growth hormone-releasing hormone promotes neurofunctional recovery and neural regeneration in ischemic stroke. Proc. Natl. Acad. Sci. USA 2021, 118, 34782465. [Google Scholar] [CrossRef] [PubMed]
- Kgosidialwa, O.; Hakami, O.; Zia-Ul-Hussnain, H.M.; Agha, A. Growth hormone deficiency following traumatic brain injury. Int. J. Mol. Sci. 2019, 20, 31284550. [Google Scholar] [CrossRef]
- Datta, A.; Saha, C.; Godse, P.; Sharma, M.; Sarmah, D.; Bhattacharya, P. Neuroendocrine regulation in stroke. Trends Endocrinol. Metab. 2023, 34, 260–277. [Google Scholar] [CrossRef]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar] [CrossRef]
- Tian, R.; Mao, G. Ghrelin reduces cerebral ischemic injury in rats by reducing M1 microglia/macrophages. Eur. J. Histochem. 2022, 66, 35016495. [Google Scholar] [CrossRef]
- Yuan, L.B.; Dong, H.L.; Zhang, H.P.; Zhao, R.N.; Gong, G.; Chen, X.M.; Zhang, L.N.; Xiong, L. Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology 2011, 114, 340–354. [Google Scholar] [CrossRef]
- Ding, H.; Yan, C.Z.; Shi, H.; Zhao, Y.S.; Chang, S.Y.; Yu, P.; Wu, W.S.; Zhao, C.Y.; Chang, Y.Z.; Duan, X.L. Hepcidin is involved in iron regulation in the ischemic brain. PLoS ONE 2011, 6, 21957487. [Google Scholar] [CrossRef]
- Davaanyam, D.; Lee, H.; Seol, S.I.; Oh, S.A.; Kim, S.W.; Lee, J.K. HMGB1 induces hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain. Exp. Mol. Med. 2023, 55, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.; Wang, H.; Ji, A.; Li, Y. Protective roles of bioactive peptides during ischemia-reperfusion injury: From bench to bedside. Life Sci. 2017, 180, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Goit, R.K.; Ng, T.C.; Tam, K.C.; Tsang, J.K.W.; Taylor, A.W.; Lo, A.C.Y. Neuropeptide α-Melanocyte-Stimulating Hormone Promotes Neurological Recovery and Repairs Cerebral Ischemia/Reperfusion Injury in Type 1 Diabetes. Neurochem. Res. 2022, 47, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhi, D.; Wang, H.; Ru, Y.; Ren, H.; Wang, N.; Liu, Y.; Li, Y.; Li, H. TAT-HSA-α-MSH fusion protein with extended half-life inhibits tumor necrosis factor-α in brain inflammation of mice. Appl. Microbiol. Biotechnol. 2016, 100, 5353–5361. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef]
- Yakovleva, E.V.; Kuzenkov, V.S.; Fedorov, V.N.; Skvortsova, V.I.; Koshelev, V.B.; Gusev, E.I.; Ashmarin, I.P. In vivo efficiency of semax in global cerebral ischemia. Bull. Exp. Biol. Med. 1999, 128, 806–807. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Myasoedov, N.F. Synacton and individual activity of synthetic and natural corticotropins. J. Mol. Recognit. 2017, 30, e2597. [Google Scholar] [CrossRef]
- Levitskaya, N.G.; Glazova, N.Y.; Sebentsova, E.A.; Manchenko, D.M.; Andreeva, L.A.; Kamensky, A.A.; Myasoedov, N.F. Nootropic and anxiolytic effects of heptapeptide ACTH6-9Pro-Gly-Pro. Russ. J. Physiol. 2019, 6, 761–770. [Google Scholar]
- Filippenkov, I.B.; Remizova, J.A.; Stavchansky, V.V.; Denisova, A.E.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; Dergunova, L.V. Synthetic Adrenocorticotropic Peptides Modulate the Expression Pattern of Immune Genes in Rat Brain following the Early Post-Stroke Period. Genes 2023, 14, 1382. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Shpetko, Y.Y.; Stavchansky, V.V.; Denisova, A.E.; Gubsky, L.V.; Andreeva, L.A.; Myasoedov, N.F.; Limborska, S.A.; Dergunova, L. V ACTH-like Peptides Compensate Rat Brain Gene Expression Profile Disrupted by Ischemia a Day After Experimental Stroke. Biomedicines 2024, 12, 2830. [Google Scholar] [CrossRef]
- Stavchansky, V.V.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Fomina, N.K.; Koretskaya, A.E.; Denisova, A.E.; Mozgovoy, I.V.; Gubsky, L.V.; Filippenkov, I.B.; Myasoedov, N.F.; et al. Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia. Curr. Issues Mol. Biol. 2024, 46, 2071–2092. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Shpetko, Y.Y.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Fomina, N.K.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L. V Differentially Expressed Genes in Rat Brain Regions with Different Degrees of Ischemic Damage. Int. J. Mol. Sci. 2025, 26, 2347. [Google Scholar] [CrossRef]
- Di Castro, M.A.; Trettel, F.; Milior, G.; Maggi, L.; Ragozzino, D.; Limatola, C. The chemokine CXCL16 modulates neurotransmitter release in hippocampal CA1 area. Sci. Rep. 2016, 6, 27721466. [Google Scholar] [CrossRef]
- Li, L.; Lou, W.; Li, H.; Zhu, Y.; Huang, X. Upregulated C-C Motif Chemokine Ligand 2 Promotes Ischemic Stroke via Chemokine Signaling Pathway. Ann. Vasc. Surg. 2020, 68, 476–486. [Google Scholar] [CrossRef]
- Rosito, M.; Deflorio, C.; Limatola, C.; Trettel, F. CXCL16 orchestrates adenosine A 3 receptor and MCP-1/CCL2 activity to protect neurons from excitotoxic cell death in the CNS. J. Neurosci. 2012, 32, 3154–3163. [Google Scholar] [CrossRef]
- Pluta, R.; Bogucka-Kocka, A.; Bogucki, J.; Kocki, J.; Czuczwar, S.J. Apoptosis, Autophagy, and Mitophagy Genes in the CA3 Area in an Ischemic Model of Alzheimer’s Disease with 2-Year Survival. J. Alzheimers. Dis. 2024, 99, 1375–1383. [Google Scholar] [CrossRef]
- Banaeeyeh, S.; Razavi, B.M.; Hosseinzadeh, H. Neuroprotective Effects of Morin Against Cadmium- and Arsenic-Induced Cell Damage in PC12 Neurons. Biol. Trace Elem. Res. 2024, 203, 3272–3286. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Mozerov, S.A.; Gubsky, L.V.; Limborska, S.A. Genome-wide transcriptome analysis using RNA-Seq reveals a large number of differentially expressed genes in a transient MCAO rat model. BMC Genomics 2018, 19, 655. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Beltran-Lobo, P.; Sung, K.; Goldrick, C.; Croft, C.L.; Nishimura, A.; Hedges, E.; Mahiddine, F.; Troakes, C.; Golde, T.E.; et al. Reactive astrocytes secrete the chaperone HSPB1 to mediate neuroprotection. Sci. Adv. 2024, 10, 38507480. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Hu, L. HSPB1 overexpression improves hypoxic-ischemic brain damage by attenuating ferroptosis in rats through promoting G6PD expression. J. Neurophysiol. 2022, 128, 1507–1517. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wang, Z.; Chen, J.-W.; Yuan, S.-Y.; Zhao, L.; Zhong, J.-Y.; Chen, J.-J.; Lin, W.-J.; Wu, W.-S. The neuroprotective effect of near infrared light therapy in aged mice with postoperative neurocognitive disorder by upregulating IRF7. J. Affect. Disord. 2024, 349, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Jiang, X.M.; Ganea, D. The neuropeptides VIP and PACAP inhibit IL-2 transcription by decreasing c-Jun and increasing JunB expression in T cells. J. Neuroimmunol. 2000, 104, 68–78. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Lu, X.; Hao, Y.H.; Tang, L.; He, Z.Y. Oxidized low-density lipoprotein receptor 1: A novel potential therapeutic target for intracerebral hemorrhage. Neural Regen. Res. 2022, 17, 1795–1801. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Chen, J.; Xu, S.; Luo, Y. Luteolin Mitigates Dopaminergic Neuron Degeneration and Restrains Microglial M1 Polarization by Inhibiting Toll Like Receptor 4. J. Integr. Neurosci. 2024, 23, 39473153. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Patel, M.Y.; Wilder, D.M.; Krishnan, J.; LaValle, C.; Pandya, J.D.; Shear, D.A.; Hefeneider, S.H.; Long, J.B.; Arun, P. Upregulation of multiple toll-like receptors in ferret brain after blast exposure: Potential targets for treatment. Neurosci. Lett. 2023, 810, 37391063. [Google Scholar] [CrossRef]
- Queiroga, C.S.F.; Alves, R.M.A.; Conde, S.V.; Alves, P.M.; Vieira, H.L.A. Paracrine effect of carbon monoxide—Astrocytes promote neuroprotection through purinergic signaling in mice. J. Cell Sci. 2016, 129, 3178–3188. [Google Scholar] [CrossRef]
- Fujita, T.; Tozaki-Saitoh, H.; Inoue, K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia 2009, 57, 244–257. [Google Scholar] [CrossRef]
- Gavrish, M.S.; Urazov, M.D.; Mishchenko, T.A.; Turubanova, V.D.; Epifanova, E.A.; Krut’, V.G.; Babaev, A.A.; Vedunova, M.V.; Mitroshina, E.V. Overexpression of Brain- and Glial Cell Line-Derived Neurotrophic Factors Is Neuroprotective in an Animal Model of Acute Hypobaric Hypoxia. Int. J. Mol. Sci. 2022, 23, 36077134. [Google Scholar] [CrossRef]
- Wang, Y.C.; Sanchez-Mendoza, E.H.; Doeppner, T.R.; Hermann, D.M. Post-Acute delivery of memantine promotes post-ischemic neurological recovery, peri-infarct tissue remodeling, and contralesional brain plasticity. J. Cereb. Blood Flow Metab. 2017, 37, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Zhao, Y.; Zhao, T.; Wang, Z.; Tang, Q. Neuronal Injury after Ischemic Stroke: Mechanisms of Crosstalk Involving Necroptosis. J. Mol. Neurosci. 2025, 75, 15. [Google Scholar] [CrossRef]
- Cao, J.; Roth, S.; Zhang, S.; Kopczak, A.; Mami, S.; Asare, Y.; Georgakis, M.K.; Messerer, D.; Horn, A.; Shemer, R.; et al. DNA-sensing inflammasomes cause recurrent atherosclerotic stroke. Nature 2024, 633, 433–441. [Google Scholar] [CrossRef]
- Gong, Z.; Guo, J.; Liu, B.; Guo, Y.; Cheng, C.; Jiang, Y.; Liang, N.; Hu, M.; Song, T.; Yang, L.; et al. Mechanisms of immune response and cell death in ischemic stroke and their regulation by natural compounds. Front. Immunol. 2023, 14, 38274789. [Google Scholar] [CrossRef] [PubMed]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef] [PubMed]
- Mannes, M.; Martin, C.; Menet, C.; Ballet, S. Wandering beyond small molecules: Peptides as allosteric protein modulators. Trends Pharmacol. Sci. 2022, 43, 406–423. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, K.; Zhao, C.; Chen, J.; Chen, L.; Zhang, Y.; Chen, T.; Yao, X.; Cai, Y.; Wu, J. Microglia LILRB4 upregulation reduces brain damage after acute ischemic stroke by limiting CD8+ T cell recruitment. J. Neuroinflammation 2024, 21, 39217343. [Google Scholar] [CrossRef]
- Koizumi, J.; Yoshida, Y.; Nakazawa, T.; Ooneda, G.; Koizumi, J.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. Nosotchu 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Ashmarin, I.; Nezavibatko, V.; Levitskaya, N.; Koshelev, V.; Kamensky, A. Design and Investigation of an ACTH(4-10) Analog Lacking D-Amino Acids and Hydrophobic Radicals. Neurosci. Res. Commun. 1995, 16, 105–112. [Google Scholar]
- Miasoedova, N.F.; Skvortsova, V.I.; Nasonov, E.L.; Zhuravleva, E.I.; Grivennikov, I.A.; Arsen’eva, E.L.; Sukhanov, I.I. [Investigation of mechanisms of neuro-protective effect of semax in acute period of ischemic stroke]. Zhurnal Nevrol. Psikhiatrii Im. S.S. Korsakova 1999, 99, 15–19. [Google Scholar]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- PRJNA1119923. Available online: https://dataview.ncbi.nlm.nih.gov/object/PRJNA1119923?reviewer=plp0lftpig94lmq8vmup84at3d (accessed on 25 June 2024).
- PRJNA1128447. Available online: https://dataview.ncbi.nlm.nih.gov/object/PRJNA1128447?reviewer=vkci32ofqame6mkpbivjt1pqn8 (accessed on 25 June 2024).
- Bioproject/916856. Available online: http://www.ncbi.nlm.nih.gov/bioproject/916856 (accessed on 5 January 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippenkov, I.B.; Shpetko, Y.Y.; Ales, D.A.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Fomina, N.K.; Gubsky, L.V.; Andreeva, L.A.; Myasoedov, N.F.; et al. Genes That Associated with Action of ACTH-like Peptides with Neuroprotective Potential in Rat Brain Regions with Different Degrees of Ischemic Damage. Int. J. Mol. Sci. 2025, 26, 6256. https://doi.org/10.3390/ijms26136256
Filippenkov IB, Shpetko YY, Ales DA, Stavchansky VV, Denisova AE, Yuzhakov VV, Fomina NK, Gubsky LV, Andreeva LA, Myasoedov NF, et al. Genes That Associated with Action of ACTH-like Peptides with Neuroprotective Potential in Rat Brain Regions with Different Degrees of Ischemic Damage. International Journal of Molecular Sciences. 2025; 26(13):6256. https://doi.org/10.3390/ijms26136256
Chicago/Turabian StyleFilippenkov, Ivan B., Yana Yu. Shpetko, Daria A. Ales, Vasily V. Stavchansky, Alina E. Denisova, Vadim V. Yuzhakov, Natalia K. Fomina, Leonid V. Gubsky, Lyudmila A. Andreeva, Nikolay F. Myasoedov, and et al. 2025. "Genes That Associated with Action of ACTH-like Peptides with Neuroprotective Potential in Rat Brain Regions with Different Degrees of Ischemic Damage" International Journal of Molecular Sciences 26, no. 13: 6256. https://doi.org/10.3390/ijms26136256
APA StyleFilippenkov, I. B., Shpetko, Y. Y., Ales, D. A., Stavchansky, V. V., Denisova, A. E., Yuzhakov, V. V., Fomina, N. K., Gubsky, L. V., Andreeva, L. A., Myasoedov, N. F., Limborska, S. A., & Dergunova, L. V. (2025). Genes That Associated with Action of ACTH-like Peptides with Neuroprotective Potential in Rat Brain Regions with Different Degrees of Ischemic Damage. International Journal of Molecular Sciences, 26(13), 6256. https://doi.org/10.3390/ijms26136256





